Abstract
Traumatic brain injury (TBI) -induced brain edema can be reduced by acute progesterone (PROG) treatment in young adult males and females, and in aged males. To extend these findings we tested these hypotheses: 1. Acute PROG treatment post-TBI will reduce cortical edema in aged females much as in young adults. 2. TBI will induce edema in sub-cortical structures (SCS): the thalamus (TH), hypothalamus (HT), brain stem (BS) and anterior pituitary (AP). 3. Acute, systemic PROG treatment post-TBI will reduce edema in SCS. Young adult (n=42) and aged (n=40), bilaterally ovariectomized rats were given medial frontal cortical (MFC) contusion injury, treated with PROG (16 mg/kg body weight) or vehicle at 1, 6 and 24 hours post-injury and killed at 6, 24 and 48 hours post-injury. Their brains were removed and the appropriate areas isolated and measured for water content. TBI induced cortical and delayed sub-cortical edema. Acute PROG treatment decreased this edema. At 5 hours post-TBI serum PROG levels were substantially elevated in both young and aged groups, but were higher in the latter. We conclude that acute PROG treatment post-TBI could prove an effective intervention to prevent or attenuate systemic, post-injury cortical and sub-cortical edema in young and aged females.
Keywords: Progesterone, TBI, Edema, Hypothalamus, Anterior Pituitary, Brain Stem
1. INTRODUCTION
Because of the high risk of morbidity and mortality that it can cause (Betz et al., 1989; Marmarou, 2007), controlling or reversing brain edema as soon as possible after TBI is a prime objective in the acute treatment of patients. Previous studies have established that bilateral contusion injury of the medial frontal cortex (MFC) in rat results in substantial edema in the cortex (CX) surrounding the site of injury (Galani et al., 2001; Roof et al., 1993; VanLandingham et al., 2006), and that the magnitude of post-TBI edema was less in females compared to males (Roof et al., 1992), an effect later shown to be due to higher levels of progesterone (PROG) in the females at the time of injury (Roof et al., 1993).
Exogenous PROG can also attenuate TBI-induced edema in young and aged males (Cutler et al., 2007; Roof et al., 1992) and in young females (intact cycling, induced-pseudopregnant and ovariectomized (OVX)) (Roof et al., 1993; Roof et al., 1992), but there are few data to show what might happen in senescent female rats whose endogenous PROG has tapered off. One of the three objectives of the present study was to examine the efficacy of PROG treatment in decreasing edema in the peri-contusion area post-TBI in aged OVX rats. We performed the OVXs to simulate the post-menopausal state of human females since ovary-intact rats between 15 and 22 months of age exhibit a persistent estrous state with continuously high levels of serum estradiol-17β and relatively decreased serum PROG compared to young OVX rats.
In addition to local pathological changes including edema and inflammation, trauma to the CX also produces pathological insults at more remote organs, and affects their function. In humans, TBI leads to long-lasting alterations in the endocrine system involving pathological changes at the level of the hypothalamo-pituitary (HP) axis (Behan et al., 2008). Consequently, victims of TBI suffer from hypopituitarism involving growth hormone (GH), luteinizing hormone (LH), thyroid hormones (T3/T4/Thyroid Stimulating Hormone, TSH), vasopressin, and cortisol (CORT) (Agha et al., 2004a., 2004b, 2004c; Aimaretti et al., 2004; Bondanelli et al., 2004; Kelly et al., 2000; Lieberman et al., 2001; Powner et al., 2006; Schneider et al., 2006). Recent reports also indicate that auto-antibodies against pituitary antigens (Tanriverdi et al., 2008) are present in the victims of TBI and may be responsible for a decline in HP axis activity in these subjects.
Studies from our laboratory at 2 months post-TBI suggest long-term reduction in the GH system in both the AP and serum and persistent inflammation-like gliotic changes in the CX, HT and AP of male rats (Kasturi and Stein, submitted). Others have observed a substantial inflammatory reaction in the gut and bowel (Chen et al., 2007a, 2007b; Hang et al., 2003). Hematomas have been observed in the HT and AP regions of human victims of TBI (Powner et al., 2006). In light of these facts and the role of edema in influencing recovery and treatment efficacy post-injury (Betz et al., 1989), it is important to investigate the possibility that water imbalance in the SCS is involved in post-injury neuroendocrine regulation. Our other objectives were to characterize injury-induced acute changes in water content in sub-cortical brain tissue such as the TH, HT, brain stem (BS) and AP, and to determine whether acute PROG treatment would have an effect on edema in these areas.
2. METHODS
2.1 Animals
Young adult (n=42; 3 months) and aged (n=40; 20 months) female Fischer-344 rats (Charles River Laboratories, Wilmington, MA, USA) were maintained on a 12:12 hour (light: dark) reverse lighting schedule, with lights on at 21:00 hrs. The animals were housed in a clean, temperature- (22+/−1°C) and humidity-controlled facility approved by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) in accordance with NIH guidelines, with food and water provided ad libitum both before and after surgery. All surgeries were carried out under aseptic conditions. The Emory University Institutional Animal Care and Use Committee (IACUC) approved all the animal care and experimental protocols (protocol #146-2005).
2.2 Experimental design
The rats were allowed to acclimatize to their quarters for 2 weeks, handled for another 2 weeks, and then subjected to bilateral OVX. Animals were assigned to two time-point groups (24 and 48 hours), each of which was subdivided into two age (young and aged) groups. Three groups of rats (n=3–5/group) for each age cohort and time point were used: sham-vehicle (SV), lesion-vehicle (LV) and lesion-PROG (16 mg/kg; LP) (Cutler et al., 2007). The SV group underwent sham surgery without craniotomy. The LV and LP groups received bilateral contusions of the MFC as described below. The LP group was injected with 0.1cc per 100 g body weight of 16 mg/ml PROG, while the SV and LV received injections of cyclodextrin (22.5%) solution at 0.1cc per 100 g body weight. Animals used for 6 hour study (for serum progesterone measures) received injection at 1 (IP) hour post-surgery and euthanized at 6 hours. Animals used for the 24-hour study were given injections post-surgery at 1 hour intraperitoneally (IP) and 6 hours subcutaneously (SC) and were euthanized at 24 hours. Rats in the 48-hour study received injections at 1 (IP), 6 (SC) and 24 (SC) hours post-surgery and were euthanized at 48 hours post-surgery.
2.2.2. Decapitation and tissue harvest for the edema measurement
Rats were anaesthetized using isoflurane (5%) for 4 minutes, followed by decapitation. Trunk blood was collected followed by brain extraction. The blood was allowed to stand for at least 30 minutes at room temperature before being spun at 14000 rpm for 5 minutes. The serum thus separated was saved in sterile Eppendorf tubes and stored at −80°C until the time of hormone analysis. The tissues harvested for edema measurement were rostral cortex (RC), caudal cortex (CC), HT, TH, BS and AP. Only one hemisphere of these tissues was used for the edema measurement.
2.2.2.1 Hypothalamus (−1.4 to 15.2 mm from bregma)
The HT was removed with the optic chiasm as the rostral boundary, the mammillary body as the posterior boundary, and both the lateral sulci as lateral boundaries. The HT was then placed on a glass plate and the two hemispheres separated using a surgical blade. One of the hemispheres was flash-frozen while the other was collected into pre-weighed Eppendorf tubes for edema measurement.
2.2.2.2 Brain stem ([−7.2 to −15 mm from bregma)
The BS was dissected at an angle dorso-ventrally (section from −9.2 (dorsal) to −7.2mm (ventral) from bregma) from the rostral boundary of the cerebellum-cortex junction, such that the entire BS was obtained. This tissue was later dissected from the cerebellum before being further separated into two hemispheres. Again, one half was flash-frozen on dry ice while the other half was dissected into 3 parts: rostral, medial and caudal (to increase surface area and ensure complete loss of water). Each of these 3 parts was collected onto different pre-weighed tubes for edema measurement.
2.2.2.3 Cortex
The remaining brain was dissected from the lateral aspect so that a dorsal and ventral half of the entire brain was obtained. The ventral half was discarded. Two rostral (RC1 and RC2; from one hemisphere) and two caudal sections of the CX (CC1 and CC2; from the same hemisphere) were used. Each section was collected into a separate pre-weighed tube. The lateral uninjured portion was discarded.
2.2.2.4 Thalamus
We included tissue just above the hypothalamic area and around the mid-central region of the brain. After coronal sectioning of the brain at the anterior and posterior border of the HT, we dissected the thalamic area out of the larger section.
2.2.2.5 Anterior Pituitary
The posterior pituitary situated on top of the AP as a small white matter was carefully removed and discarded. To prevent blood contamination which might cause a rise in water content, cotton-tipped applicators were used to absorb the blood that in some cases oozed from the severed venules on either side of the pituitary. The AP was then removed from the base of cranial cavity (sella tursica). The two hemispheres were dissected using a surgical blade. One half was flash-frozen on dry ice for assays, while the other half was collected on a pre-weighed tube for edema measurement.
2.3 Ovariectomy
Bilateral OVXs were performed through a mid-ventral approach, under mild isoflurane anesthesia. Briefly, animals were anaesthetized using isoflurane (5%) with oxygen as carrier (400 cu cm) during the first 5 minutes. The abdominal region was shaved using a hair clipper and the animals were maintained at 2.5% isoflurane. The abdomen was cleaned with iodine and alcohol and a mid-ventral incision was made. The uterus was located, retracted and ovaries exposed briefly and removed. In the case of aged rats, when excess bleeding was observed, cold cautery was used to stop it and any excess blood was gently blotted using sterile gauze. The uterus was placed back into the abdomen and the abdominal muscles sutured using Ethicon chromic gut – 4.0 (cat #635H, Henry Schein, Melville, NY, USA). Wound clips were used to staple the external skin. The whole procedure was completed in 15 to 20 minutes, in young and aged rats respectively. The animals were allowed to recover on a warm heating blanket, and then moved to their colony half an hour after the procedure. No analgesic or antibiotic was used. We had zero percent (0%) mortality with both young and aged rats due to OVX.
2.4 Preparation of cyclodextrin and progesterone solution
Cyclodextrin solution (22.5%, w/v; cat #H107-100G, Sigma-RBI, St. Louis, MO, USA) was freshly prepared using sterile distilled water (list #NDC 0409-4887-20, Hospira, Inc., Lake Forest, IL, USA). PROG (cat#P0130-25G, Sigma) was weighed and dissolved in the prepared cyclodextrin solution overnight to get a concentration of 16 mg/ml. Although 8 mg/ml PROG dissolved completely with overnight mixing on a magnetic stir plate, the 16 mg/ml solution was heated moderately to around 50–60°C for 30 minutes the next day to get it into solution. The final volumes were created to allow for evaporation and so reach the desired final concentration. All solutions were prepared using sterile glass/plastic ware. The PROG and cyclodextrin solutions were then stored at 4–8°C until use. Solutions older than 6 weeks were not used. On the day of surgery or treatment, these solutions were raised to room temperature for one hour before use and simultaneously mixed on magnetic stir plates.
2.5 Traumatic brain injury
All the surgeries were performed between 08:00 and 14:00 hrs. The procedures followed for the TBI were as described elsewhere (Cutler et al., 2007). Briefly, rats were weighed and subjected to anesthesia for 3 minutes and 45 seconds (isoflurane 5% (cat #NDC 0409-3292-49, Hospira) and nitrous oxide 700 cc/min) in the presence of 400 cc/min of oxygen during the induction phase. Then the animals were removed from the anesthesia chamber and their heads shaved clean with hair clippers. They were maintained at 3% isoflurane, 300 cc/min nitrous oxide and 800–1000 cc/min oxygen, so that blood oxygen saturation was at or above 90 mm Hg as monitored by the hemoximeter. The surgery lasted 20–25 minutes. During and post-surgery, the subjects were kept on a heated blanket. Rectal temperature and blood flow/oxygen were monitored during the surgery. The animals were mounted in a stereotaxic frame and their heads cleaned with povidone-iodine scrub (cat #NDC 52380-1855-4, Aplicare Inc., Branford, CT, USA) and alcohol pads. A midline incision was made and the skin and fascia cleared. Measurements for the craniotomy site were taken from 3 mm anterior to bregma. The brain surface was exposed and any bleeding was stopped. The impact was made at a velocity of 2.5 m/s, a depth of 2 mm with an impact time of 0.5 msec before the piston was retracted from the dural surface. After the impact, most of the animals suffered a respiratory arrest for 30 seconds to one minute. Therefore, immediately after the injury, the isoflurane was adjusted to “0%” (not “off”) while still allowing oxygen to flow, followed by simulated breathing until breathing was re-established, when the isoflurane was switched back to 2.0% and the skin sutured. Failure to do these steps resulted in 100% mortality, irrespective of age. All the rats underwent the same procedure through the marking for the craniotomy sites, at which point rats were randomly selected for TBI or sham surgery. Rats selected for TBI endured the process described above, while shams were sutured and maintained under anesthesia for 20 minutes before being removed from the stereotaxic device. Following TBI, the bleeding was stopped and the skin sutured. The rats were allowed to awaken in the recovery cage on heated blankets and then transferred to clean cages with their food placed directly on the cage floor.
2.6 Edema measurement
2.6.1 Tissue processing for edema measurement
Before tissue harvesting, Eppendorf tubes were labeled and weighed (tube weight, W1). The tubes containing the tissues were weighed again (pre-weight, W2) and the lids were left open and kept in a vacuum oven maintained at 60°C and 15 in Hg Vac., and left for 24 hours. The tubes were removed from the oven and allowed to cool to room temperature for 30 minutes in an enclosed space to prevent any accidental gross contamination that might affect the weight. The lids were closed and tubes weighed again (post-weight, W3).
2.6.2 Calculation for edema
The overall calculation for the edema measurement is as described elsewhere (Roof et al., 1993). The tube weight (W1) was subtracted from both the pre-weight (W2) and the post-weight (W3) to get the wet weight (WW) and dry weight (DW) respectively. The % water content (WC) (in mg) was calculated as follows for the following regions: AP, HT, TH, BS and CX?
The relative water content (edema) of the rostral cortex was expressed as percent change with respect to CC (uninjured) from the same animal and was arrived at as follows:
2.7 Progesterone Assay
The PROG assay was carried out by an independent laboratory (Yerkes Biomarker Core Lab Facility) blinded to the details of the experiments. Briefly, the serum samples were extracted for PROG followed by a radioimmunoassay using Siemens Kit (Los Angeles, CA, USA; Cat. #TKPG5). Samples that had higher levels of PROG were subjected to a re-run after appropriate dilution. The values were adjusted for 3H-P4 recovery of 95.81%. The values are expressed as ng/ml of serum.
2.8 Statistics
Two-way ANOVA was used to study the effects of treatment (SV, LV and LP) and the effects of age (young vs. aged) on the outcomes. Bonferroni post-hoc tests were used to determine significant differences among all the groups at p<0.05, as were t-test comparisons between select groups (Table 1).
Table 1.
Serum PROG levels at 5 hours post-treatment
| ID | Groups Compared | Mean +/− SD | p value | df |
|---|---|---|---|---|
| 1 | SV (Young) vs SV (Aged) | 14.526 ± 9.35 vs 18.894 ± 4.31 | 0.1852208 | 8 |
| 2 | LP (Young) vs LP (Aged) | 62.544 ± 16.10 vs 86.46 ± 22.99 | 0.0466028 | 8 |
| 3 | SV (Young) vs LV (Young) | 14.526 ± 9.35 vs 31.19 ± 9.76 | 0.0123915 | 8 |
| 4 | SV (Aged) vs LV (Aged) | 18.894 ± 4.31 vs 17.974 ± 4.71 | 0.3777047 | 8 |
One-tailed t-test comparison of serum PROG levels between select groups at 6 hours after the surgery (5 hours after the first injection of PROG or vehicle). ID #1 comparison shows no significant difference in serum PROG levels between young SV and aged SV groups. ID #2 comparison reveals the possibility of altered drug clearance in aged subjects after PROG injection. ID #3 comparison reveals a robust increase in PROG secretion from adrenals in response to TBI (LV) compared to sham (SV) in young subjects, and this response to TBI is completely eliminated in aged subjects (ID #4 comparison).
3. RESULTS
3.1 Cortex
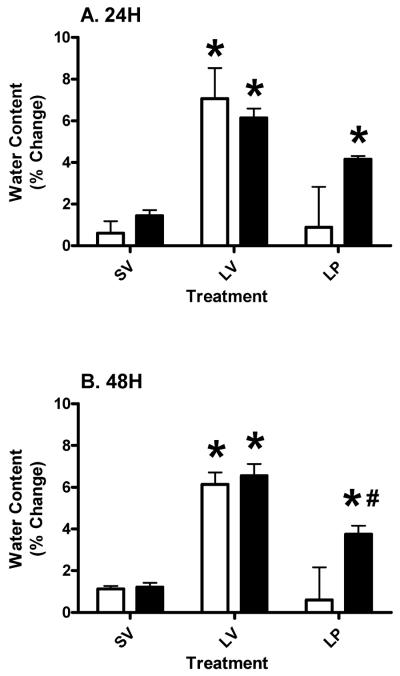
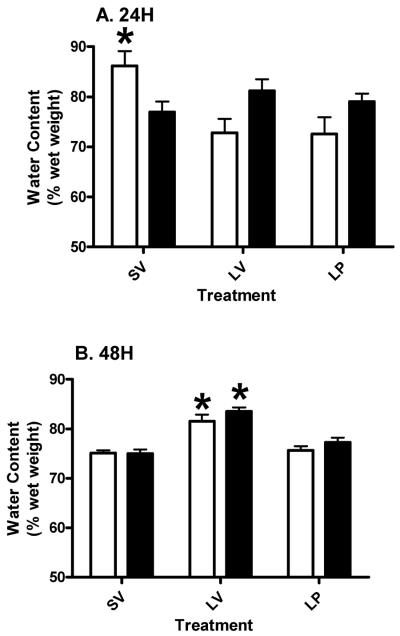
We tested the hypothesis that acute PROG treatment would reduce TBI-induced edema in the peri-contusion area in aged rats, as previously observed in young subjects. We found a significant (p<0.05) reduction in the percent of edema in the injured CX in both young and aged rats at both 24 (Figure 1A) and 48 (Figure 1B) hours post-injury compared to the LV group. At 24 hours post-injury (A) treatment had a significant (p<0.0021; F (2, 18) = 8.84) effect on outcome. Neither age (p>0.3547; F (1, 18) = 0.90) nor the interaction (p>0.3335; F (2, 18) = 1.17) had any significant effect on outcome. The mean edema of the aged LP group was significantly (p<0.05) higher compared to the aged SV group and significantly (p<0.05) lower compared to the aged LV group at both 24 (Figure 1A) and 48 (Figure 1B) hours post-injury. However, the younger lesion subjects treated with PROG had significantly (p<0.001) less edema compared to aged lesion subjects at 48 hours (Figure 1B). The main effects at 48 hours post-injury (B) were as follows: treatment had a significant (p<0.0001; F (2, 18) = 26.86) effect on outcome. The effects of interaction (p>0.1072; F (2, 18) = 2.53) and age (p>0.0608; F (1, 18) = 4) on outcome was not significant.
Figure 1.
Percent increase in water content in tissue from the peri-contusion (rostral cortex) area compared to non-injured, distal cortical area from the same animals at 24 (A) and 48 (B) hours post-TBI in young (clear bars) and aged (gray bars) rats with cortical injury (L), sham surgery (S) followed by vehicle (SV and LV) or progesterone (LP) injections. Two-way ANOVA results are represented as mean ± SEM. Asterisk (*) indicates significant difference from other treatment groups within the same AGE group. Pound (#) denotes significant difference between young and aged subjects within the same treatment.
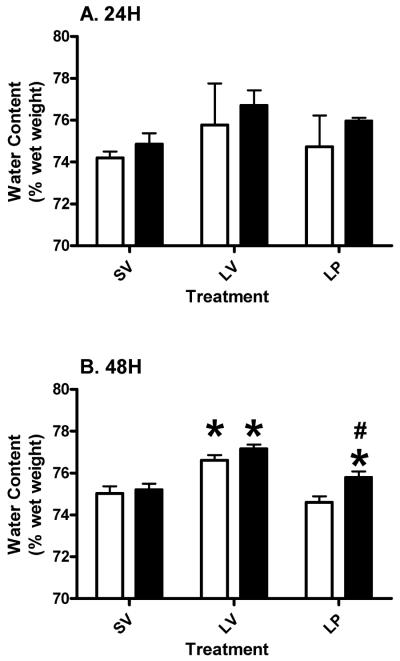
3.2 Thalamus
At 24 hours post-injury, we did not find any significant changes in water content in TH (Figure 2A). Two-way ANOVA revealed no overall significant effects of age (p>0.3701; F (1, 15) = 0.85), treatment (p>0.4113; F (2, 15) = 0.94) or interaction (p>0.9728; F (2, 15) = 0.03) between them. At 48 hours post-injury there was a significant main effect for treatment (p<0.0001; F (2, 18) = 25.52) and age (p<0.0115; F (1, 18) = 7.922). The interaction between them was not significant (p>0.2086; F (2, 18) = 1.712). The lesion subjects had significantly higher water content in the TH which was reduced (p<0.05) by acute PROG treatment in both the young and aged subjects (Figure 2B). The aged LP group had a mean water content significantly (p<0.05) higher than aged shams and significantly (p<0.05) lower than the aged LV group. Aged lesion subjects treated with PROG had significantly (p<0.05) higher water content compared to their younger counterparts at 48 hours post-injury (Figure 2B).
Figure 2.
Thalamic edema at 24 (A) and 48 (B) hours post-TBI in young and aged Ovx rats. Two-way ANOVA results are represented as mean ± SEM. Asterisk (*) indicates significant difference between treatment within the same age group. Pound (#) denotes significant difference between young and aged subjects within the same treatment. Clear bars represent young, and gray bars represent aged groups.
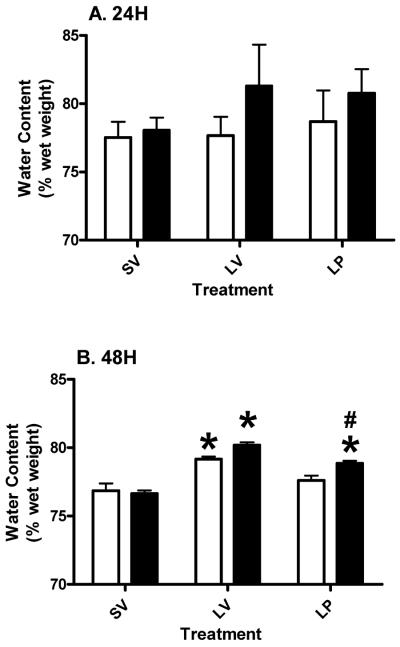
3.3 Hypothalamus
There was no significant change in the water content of the HT in the lesion subjects (both young and aged) treated with either vehicle or PROG at 24 hours post-injury (Figure 3A). At 48 hours post-injury there was significant (p<0.05) edema formation in both the young and aged subjects that was reduced (p<0.05) by acute PROG treatment (Figure 3B; P<0.0001; F (2, 18) = 44.13). Aged lesion subjects treated with PROG had a mean water content intermediate to the LV and SV, and significantly (p<0.05) different from both those groups, at 48 hours. Two-way ANOVA revealed a significant (p<0.0137; F (1, 18) = 7.47) effect of age on the outcome of PROG treatment at 48 hours post-injury. The interaction (p>0.0649; F (2, 18) = 3.20) between age and treatment was not significant
Figure 3.
Edema in the hypothalamus at 24 (A) and 48 (B) hours post-TBI in young and aged Ovx rats. Two-way ANOVA results are represented as mean ± SEM. Asterisk (*) indicates significant difference of treatment within the same age group. Pound (#) denotes significant difference between young and aged subjects within the same treatment. Clear bars represent young, and gray bars represent aged groups.
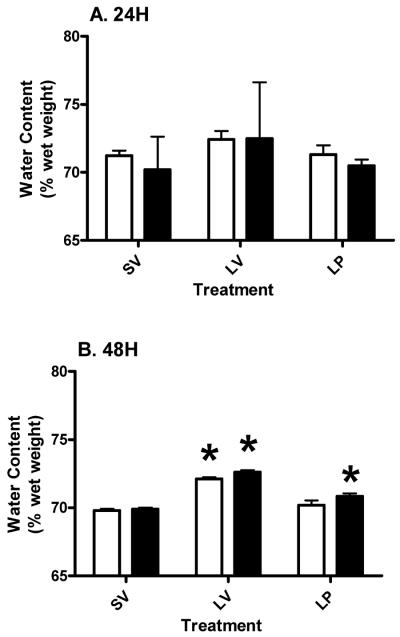
3.4 Brain stem
The cortical injury did not induce edema in the BS at 24 hours in either the young or aged subjects, and there was no overall effect of either age (p>0.6385; F (1, 18) = 0.23) or treatment (p>0.4786; F (2, 18) = 0.77) as analyzed by two-way ANOVA at 24 hours (Figure 4A). There was substantial variability in the aged lesion group at 24 hours compared to 48 hours (interaction effect (p>0.9343; F (2, 18) = 0.07)). At 48 hours post-injury, there was a significant (p<0.05) increase in water content in the LV group compared to SV in both young and aged subjects (Figure 4B). Acute PROG treatment significantly (p<0.05) reduced edema in both young and aged rats (p<0.0001; F (2, 18) = 89.84). The water content in the aged LP group was significantly (p<0.05) higher compared to the aged SV group and significantly (p<0.05) lower than in the aged LV group (Figure 4B). Although we did not find any significant difference between the two age groups at 48 hours, two-way ANOVA revealed an overall effect of age (p<0.0181; F (1, 18) = 6.75). No significant (p>0.3787; F (2, 18) = 1.03) effect of interaction was observed at 48 hours.
Figure 4.
Water content in the brain stem of young and aged Ovx rats at 24 (A) and 48 (B) hours post-injury. Two-way ANOVA results are represented as mean ± SEM. Asterisk (*) indicates a significant difference between treatment within the same age group. Pound (#) denotes significant difference between young and aged subjects within the same treatment. Clear bars represent young, and gray bars represent aged groups.
3.5 Anterior Pituitary
At 24 hours post-injury (Figure 5A) young LV and LP groups exhibited reduction in the mean water content compared to SV, which was not observed in the aged animals. While neither treatment (p>0.0892; F (2, 19) = 2.75) nor age (p>0.3912; F (1, 19) = 0.77) had a significant influence on outcome, there was a significant interaction (p<0.0054; F (2, 19) = 6.95) between age and treatment. The decrease in the mean water content in the young lesion groups (LV and LP) compared to SV was found to be significant (p<0.01) through post-hoc analysis. At 48 hours post-injury (Figure 5B), we observed significant (p<0.05) edema formation in both young and aged LV rats compared to shams, and this effect was (p<0.05) reduced by acute PROG treatment (p<0.0001; F (2, 18) = 37.58) in both age groups. Neither the overall effect of age (p>0.1358; F (1, 18) = 2.44) nor of interaction (between age and treatment; p>0.4796; F (2, 18) = 0.77) was found to be significant (p>0.1358) at 48 hours post-injury.
Figure 5.
Water content in the anterior pituitary at 24 (A) and 48 (B) hours post-injury in young (clear bars) and aged (gray bars) Ovx rats. Two-way ANOVA results are represented as mean ± SEM. Asterisk (*) indicates significant difference between treatments within the same age group. Pound (#) denotes significant difference between young and aged subjects within the same treatment.
3.6 Serum progesterone levels
At 6 hours post-injury (5 hours after the first PROG injection) PROG levels were significantly (p<0.05) higher in both the young and aged subjects compared to the SV and LV groups (Figure 6). Two-way ANOVA demonstrated a significant main effect of treatment (p<0.0001; F (2, 24) = 58.29) and of the interaction (p<0.0141; F (2, 24) = 5.12) between the treatment and age. However, it did not demonstrate any significant effect of age (p>0.2999; F (1, 24) = 1.12).
Figure 6.
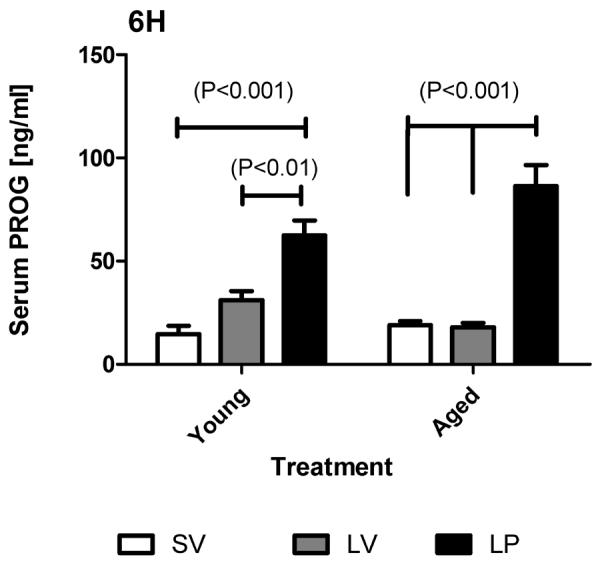
Serum PROG levels at 6 hours post-TBI (5 hours after 1st PROG/Vehicle injection) in young and aged rats. Sham (SV), lesion-vehicle (LV) and lesion-PROG (LP) groups are represented by white, grey and black bars respectively. Two-way ANOVA results are represented as mean ± SEM.
Since there appeared to be an induction of PROG secretion in response to injury in young adults compared to aged subjects, we did a t-test comparison (one-tailed) between the SV and LV groups in both young and aged subjects (Table 1). We found a significant increase in serum PROG levels in young (p<0.0124) subjects in response to injury which was absent in the aged subjects (p<0.3777).
We also performed a comparison between the LP (young) and LP (aged) groups (Table 1) to determine whether the serum PROG levels in the aged subjects were significantly higher compared to young animals. There was a significantly higher level of PROG (p<0.0466) in the aged subjects at 5 hours after the first PROG injection (6 hours after TBI). The basal PROG level in the young and aged SV groups was not significantly different (p>0.1852).
4. DISCUSSION
In this study we demonstrated that acute PROG treatment post-TBI is effective in reducing edema in aged and younger ovariectomized female subjects, a finding similar to that for aged male rats (Cutler et al., 2007). A novel aspect of this experiment is that, instead of examining edema only in the area surrounding the cortical contusion, we measured it in a number of sub-cortical areas relatively distal to the zone of injury and not immediately subject to the mechanical trauma itself. While there was no substantial edema in the SCS at 24 hours post-injury, we did note a delayed induction of swelling in the SCS including the AP at 48 hours post-TBI. Although PROG treatment substantially reduced edema in the young animals, this reversal was not complete in the aged subjects in the TH, HT and BS.
It is likely that PROG's mode of action involves not only the classic intranuclear PROG receptors, but also other non-genomic mechanisms. For instance, VanLandingham et al. (2006) recently reported that injections of the enantiomer of PROG can reduce edema even though it cannot bind to the PROG receptor. In addition, there is growing evidence for a class of PROG membrane receptors and binding proteins (e.g., 25-Dx) that may directly respond to PROG treatment (Ashley et al., 2006; Cai & Stocco, 2005; Guennoun et al., 2008; Pluchino et al., 2009), and modulate water balance. These receptor types are especially abundant in the hypothalamic and thalamic regions (Guennoun R., personal communication) where cortical contusion caused edema in this study. The observation that old rats have higher serum levels of PROG and do not show the same extent of edema reduction also points to the possibility that they have altered receptor and membrane sensitivity to PROG treatment, and thus might require larger doses to attain the same effects observed in young subjects.
Another reason for the decrease in the effectiveness of PROG in the aged subjects could be altered pharmacokinetics (PK). We demonstrated earlier that while PROG was effective at 16 mg/kg, it did not affect morphological and functional outcome after TBI at a higher dose of 32 mg/kg (Goss et. al., 2003). This altered PK could in turn be due to altered metabolism/clearance of PROG in the aged rats. In this study, while PROG levels were higher (than SV) in the LP group of younger subjects, in the aged rats, the mean level of PROG was higher in the LP group compared to younger animals at 5 hours after the first injection. Specific comparisons between the LP young subjects and LP aged subjects revealed the mean PROG levels in the aged subjects to be significantly higher compared to their younger counterparts. This indicates that there is a substantial age-related difference in hormonal metabolism in the older animals. Since we give a second injection of PROG at 5 hours after injury, we can assume that there would be a much more pronounced difference in PROG levels in the serum between young and aged subjects which could account for the altered PK of PROG in the aged rats.
It is worth noting that stress can also cause an increase in the blood levels of PROG (Persengiev et al., 1991; Andersen et al., 2004 and 2005; Romeo, et. al., 2004 and 2005). In the young OVXed females at 6 hours post-injury, we found elevated levels of PROG in the LV compared to the SV group; however, this response to TBI was absent in the aged rats. Comparisons between the LV and SV groups in young and aged subjects revealed that while younger subjects exhibited a robust rise in serum PROG levels in response to brain injury, aged subjects did not (Table 1). We speculate that the rise in serum PROG in the OVXed brain-injured rats treated with vehicle is the result of increased PROG secretion from the adrenals. This notion is consistent with the literature (Boehm, et al., 1982; Schaeffer and Aron, 1987; Budec, et al., 2002; Romeo et. al., 2004 and 2005) demonstrating the effects of stress on serum PROG (from the adrenals). It is likely that the increased PROG from the adrenals after brain injury (or stress in general) is a `coping response' to the trauma.
The substantial variability in tissue water content (edema) at 24 hours, but not at 48 hours, post-TBI is probably the effect of the many acute physiologic perturbations during the initial phase post-injury, all of which are probably more stabilized at a later time point (48 hours). Hence, we think that at least for the measurement of tissue edema in the SCS, 48 hours post-injury may be the best time to get a reliable indicator of the impact of injury on tissue water content. This is similar to the issue of whether the Glasgow Coma Scale should be measured immediately after injury, when the patients are drugged, and are under acute blood volume loss and shock, or whether the GCS should be measured once the patient is more stable.
The sub-cortical regions where we measured water content--TH, HT, BS and AP—are involved in the control of homeostatic/autonomic functions. This includes the regulation of the endocrine system with the AP as the master gland under tight control of hypothalamic secretions (Nemeroff, 1992). The BS also harbors many of the noradrenergic, adrenergic and serotonergic nuclei, and sends ascending and descending projections to many areas including the CX and the HT (Muller & Nistico, 1989). This region is implicated in the regulation of many neuroendocrine events, including those involved in the stress and reproductive axis, circadian rhythmicity, maintenance of body temperature, feeding and drinking (water and ionic homeostasis), and growth and metabolism (Nemeroff, 1992). Also, many of the cranial nerves have their origin in the BS regions (Marieb et al., 2005). The largest cranial nerve, the vagus, innervates and regulates systemic structures including the heart, the stomach and intestines, liver, lungs and the pancreas (Marieb et al., 2005). It is now becoming more apparent that TBI patients suffer from numerous endocrine dysfunctions (Agha et al., 2004a, 2004b, 2004c; Aimaretti et al., 2004; Bondanelli et al., 2004; Kelly et al., 2000; Lieberman et al., 2001; Powner et al., 2006; Schneider et al., 2006) during the acute and chronic phases post-injury, including pathologic changes in the intestinal membranes (Chen et al., 2007a, 2007b; Hang et al., 2005a, 2005b). In light of our finding that the SCS tend to undergo edematous change after TBI, we think that the deregulation of water homeostasis in these diencephalic, BS and pituitary structures may disrupt their normal functioning and lead to further alterations in the endocrine and autonomic nervous system.
We are not completely certain of the signaling mechanisms leading to edema formation in distal structures to the injury. However, we can speculate that cytotoxic signaling factors are triggering edema by downstream induction of inflammatory cytokines. We recently demonstrated (Kasturi and Stein, submitted) the induction of interleukin-1β and GFAP in the HT and AP at 2 months after cortical TBI in male rats, and proposed the possibility of volume transmission similar to that described by Bach-Y-Rita (2003) in mediating persistent inflammation in these distal structures. Thus, it is likely that edema in the SCS might be a secondary effect of the spread of inflammatory reactions from the peri-contusion site, through the ventricular and extracellular pathways.
In summary, a short course of PROG treatment post-TBI is effective in preventing edema in aged and younger OVX females not only in the CX but also in the SCS. The induction of edema in the SCS also supports the possibility that edema plays a causative role in the disturbances in the endocrine system during the acute phase post-injury, leading to what is sometimes characterized as sickness behavior (Dantzer & Kelley, 2007). There is accumulating evidence that even circumscribed TBI can lead to substantial inflammatory and swelling reactions in liver and gut tissue, exacerbating the cycle of tissue damage and morbidity (Chen et al., 2007a, 2007b; Hang et al., 2005a, 2005b). Acute PROG treatment post-injury may help to prevent further long-term complications in the endocrine system.
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance provided by Craig Wirth for part of this study and to Ilana Garza of The Yerkes Biomarker Core Lab Facilities for providing the progesterone assays. We would also like to thank Leslie McCann for her help in the preparation of the manuscript. This study was supported by funding from NIH grant # 5RO1NS048451.
Footnotes
AUTHOR DISCLOSURE STATEMENT
Dr. Donald Stein is entitled to royalty derived from BHR Pharma sale of products related to the research described in this paper. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
REFERENCES
- Agha A, Phillips J, O'Kelly P, Tormey W, Thompson CJ. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118(12):1416. doi: 10.1016/j.amjmed.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Agha A, Rogers B, Mylotte D, Taleb F, Tormey W, Phillips J, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf) 2004a;60(5):584–591. doi: 10.1111/j.1365-2265.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- Agha A, Rogers B, Sherlock M, O'Kelly P, Tormey W, Phillips J, et al. Anterior pituitary dysfunction in survivors of traumatic brain injury. J Clin Endocrinol Metab. 2004b;89(10):4929–4936. doi: 10.1210/jc.2004-0511. [DOI] [PubMed] [Google Scholar]
- Agha A, Thornton E, O'Kelly P, Tormey W, Phillips J, Thompson CJ. Posterior pituitary dysfunction after traumatic brain injury. J Clin Endocrinol Metab. 2004c;89(12):5987–5992. doi: 10.1210/jc.2004-1058. [DOI] [PubMed] [Google Scholar]
- Aimaretti G, Ambrosio MR, Di Somma C, Fusco A, Cannavo S, Gasperi M, et al. Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin Endocrinol (Oxf) 2004;61(3):320–326. doi: 10.1111/j.1365-2265.2004.02094.x. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Bignotto M, Machado RB, Tufik S. Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res. 2004;37:791–797. doi: 10.1590/s0100-879x2004000600003. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Martins PJ, D'Almedia V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology. 2006;147(9):4151–4159. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P. Theoretical basis for brain plasticity after a TBI. Brain Inj. 2003;17(8):643–651. doi: 10.1080/0269905031000107133. [DOI] [PubMed] [Google Scholar]
- Behan LA, Phillips J, Thompson CJ, Agha A. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79(7):753–759. doi: 10.1136/jnnp.2007.132837. [DOI] [PubMed] [Google Scholar]
- Betz AL, Iannotti F, Hoff JT. Brain edema: a classification based on blood-brain barrier integrity. Cerebrovasc Brain Metab Rev. 1989;1(2):133–154. [PubMed] [Google Scholar]
- Boehm N, Plas-Roser S, Roos M, Aron C. How different procedures of blood removal affect blood progesterone concentrations in the cyclic female rat. J Steroid Biochem. 1982;16:339–342. doi: 10.1016/0022-4731(82)90187-x. [DOI] [PubMed] [Google Scholar]
- Bondanelli M, De Marinis L, Ambrosio MR, Monesi M, Valle D, Zatelli MC, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21(6):685–696. doi: 10.1089/0897715041269713. [DOI] [PubMed] [Google Scholar]
- Budec M, Koko V, Milovanovic TLB-P, Petkovic A. Acute ethanol treatment increases level of progesterone in ovariectomized rats. Alcohol. 2002;26:173–178. doi: 10.1016/s0741-8329(02)00197-0. [DOI] [PubMed] [Google Scholar]
- Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146(12):5522–5532. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- Chen G, Shi J, Ding Y, Yin H, Hang C. Progesterone prevents traumatic brain injury-induced intestinal nuclear factor kappa B activation and proinflammatory cytokines expression in male rats. Mediators Inflamm. 2007a;2007:93431. doi: 10.1155/2007/93431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shi JX, Qi M, Wang HX, Hang CH. Effects of Progesterone on Intestinal Inflammatory Response, Mucosa Structure Alterations, and Apoptosis Following Traumatic Brain Injury in Male Rats. J Surg Res. 2007b doi: 10.1016/j.jss.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Cekic M, Miller DM, Wali B, Vanlandingham JW, Stein DG. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma. 2007;24(9):1475–1486. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani R, Hoffman SW, Stein DG. Effects of the duration of progesterone treatment on the resolution of cerebral edema induced by cortical contusions in rats. Restor Neurol Neurosci. 2001;18(4):161–166. [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic corellates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behavior. 2003;76(2):231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Meffre D, Labombarda F, Gonzalez SL, Deniselle MC, Stein DG, et al. The membrane-associated progesterone-binding protein 25-Dx: expression, cellular localization and up-regulation after brain and spinal cord injuries. Brain Res Rev. 2008;57(2):493–505. doi: 10.1016/j.brainresrev.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Hang CH, Shi JX, Li JS, Li WQ, Wu W. Expressions of intestinal NF-kappaB, TNF-alpha, and IL-6 following traumatic brain injury in rats. J Surg Res. 2005a;123(2):188–193. doi: 10.1016/j.jss.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Hang CH, Shi JX, Li JS, Li WQ, Yin HX. Up-regulation of intestinal nuclear factor kappa B and intercellular adhesion molecule-1 following traumatic brain injury in rats. World J Gastroenterol. 2005b;11(8):1149–1154. doi: 10.3748/wjg.v11.i8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang CH, Shi JX, Li JS, Wu W, Yin HX. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J Gastroenterol. 2003;9(12):2776–2781. doi: 10.3748/wjg.v9.i12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi BS, Stein DG. Traumatic brain injury causes long-term reduction in serum Growth Hormone and persistent gliosis in the cortical-hypothalamo-pituitary axis of adult male rats. J Neurotrauma. doi: 10.1089/neu.2008.0751. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DF, Gonzalo IT, Cohan P, Berman N, Swerdloff R, Wang C. Hypopituitarism following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a preliminary report. J Neurosurg. 2000;93(5):743–752. doi: 10.3171/jns.2000.93.5.0743. [DOI] [PubMed] [Google Scholar]
- Lieberman SA, Oberoi AL, Gilkison CR, Masel BE, Urban RJ. Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J Clin Endocrinol Metab. 2001;86(6):2752–2756. doi: 10.1210/jcem.86.6.7592. [DOI] [PubMed] [Google Scholar]
- Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22(5):E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- Marieb EN, Mallatt J, Wilhelm PB. Human Anatomy. 4th edition Pearson Benjamin Cummings; San Francisco, CA 94111: 2005. [Google Scholar]
- Muller EE, Nistico G. Brain Messengers and the Pituitary. Academic Press, Inc.; San Diego, California: 1989. [Google Scholar]
- Nemeroff CB. Neuroendocrinology. CRC Press; Boca Raton, Florida: 1992. [Google Scholar]
- Persengiev S, Kanchev L, Vezenkova G. Circadian patterns of melatonin, corticosterone, and progesterone in male rats subjected to chronic stress: Effect of constant illumination. J Pineal Res. 1991;11:57–62. doi: 10.1111/j.1600-079x.1991.tb00456.x. [DOI] [PubMed] [Google Scholar]
- Powner DJ, Boccalandro C, Alp MS, Vollmer DG. Endocrine failure after traumatic brain injury in adults. Neurocrit Care. 2006;5(1):61–70. doi: 10.1385/ncc:5:1:61. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80(6):387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, McEwen BS. Stress-induced progesterone secretion and progesterone receptor immunoreactivity in the paraventricular nucleus are modulated by pubertal development in male rats. Stress. 2005;8(4):265–271. doi: 10.1080/10253890500489320. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607(1–2):333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG. Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restorative Neurology and Neuroscience. 1992;4:425–427. doi: 10.3233/RNN-1992-4608. [DOI] [PubMed] [Google Scholar]
- Schaeffer C, Aron C. Stress-related effects on the secretion of progesterone by the adrenals in castrated male rats presented to a stimulus male. Involvement of oestrogen. Acta Endocrinol. 1987;114:440–445. doi: 10.1530/acta.0.1140440. [DOI] [PubMed] [Google Scholar]
- Schneider HJ, Schneider M, Saller B, Petersenn S, Uhr M, Husemann B, et al. Prevalence of anterior pituitary insufficiency 3 and 12 months after traumatic brain injury. Eur J Endocrinol. 2006;154(2):259–265. doi: 10.1530/eje.1.02071. [DOI] [PubMed] [Google Scholar]
- Tanriverdi F, De Bellis A, Bizzarro A, Sinisi AA, Bellastella G, Pane E, et al. Antipituitary antibodies after traumatic brain injury: is head trauma-induced pituitary dysfunction associated with autoimmunity? Eur J Endocrinol. 2008;159(1):7–13. doi: 10.1530/EJE-08-0050. [DOI] [PubMed] [Google Scholar]
- VanLandingham JW, Cutler SM, Virmani S, Hoffman SW, Covey DF, Krishnan K, et al. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51(6):1078–1085. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]