Abstract
There is a current biodefense interest in protection against Anthrax. Here we developed a new generation of stable and effective anthrax vaccine. We studied the immune response elicited by rPA delivered intranasally with a novel mucosal adjuvant, a mast cell activator Compound 48/80. The vaccine formulation was prepared in a powder form by spray-freeze-drying (SFD) under optimized conditions to produce particles with a target size of D50=25μm, suitable for delivery to the rabbit nasal cavity. Physicochemical properties of the powder vaccines were characterized to assess their delivery and storage potential. Structural stability of rPA was confirmed by CD and ATR-FTIR, while functional stability of rPA and C48/80 was monitored by cell-based assays. Animal study was performed using a unitdose powder device for direct nasal application. Results showed that C48/80 provided effective mucosal adjuvant activity in rabbits. Freshly prepared SFD powder vaccine formulations or powders stored for over two years at room temperature elicited significantly elevated serum PA-specific and lethal toxin neutralization antibody titers that were comparable to that induced by IM immunization with rPA. Nasal delivery of this vaccine formulation may be a viable alternative to the currently licensed vaccine, or an attractive vaccine platform for other mucosally transmitted diseases.
INTRODUCTION
Anthrax is a deadly disease caused by the rod-shaped, spore forming Gram-positive bacterium, Bacillus anthracis. Anthrax infection occurs mainly in herbivores, with the spores being present in different parts of infected animals as well as in soil. Human infections occur through contact with skin, the lungs and GI tract. Upon entry into the human body, the spores are taken up by macrophages and dendritic cells, and transported to lymph nodes where they germinate to become the vegetative cells, followed by bacillar multiplication, dissemination and toxin production. Eventually the bacteria can cause severe septicemia, followed by shock and death. The vegetative cell of the bacteria is known to secrete three proteins extracellularly, Protective antigen (PA), Lethal factor (LF) and edema factor (EF) which are all responsible for the killing mechanism. While LF & EF have effects on cAMP production and MAPK pathway, PA is essential to their cellular entry and endosome escape 1.
Most of the human cases in the US in recent decades have been sporadic and are limited to natural causes 2. However, the 2001 anthrax attack through the US postal system shortly after 911 was a reminder of the severity of this ancient disease. This marked the largest outbreak of inhalational anthrax in US history. This intentional distribution of anthrax spores emphasized the need for research on anthrax protection especially in the biodefense field. The unique characteristics of Bacillus anthracis, in particular the stability, ease of dispersion (aerosolization) of anthrax spores and the high morbidity and mortality rates have made the aerosol spore form of the bacterium a leading bioweapon threat 3,4.
The inhaled form of anthrax is difficult to diagnose and can be fatal without prompt antibiotic intervention. Prophylactic vaccination has become a critical measure for protection of individuals at risk of exposure. In the U.S., the currently licensed anthrax vaccine for human use is prepared from the sterile culture supernatant fraction of B. anthracis which is adsorbed on aluminum hydroxide (AVA: BioThrax) for intramuscular (IM) injection, while in the U.K. the supernatant is precipitated with aluminum phosphate (AVP) for subcutaneous (SC) injection. These vaccines contain varying amounts of natural PA with unknown amounts of LF and EF and rely on a complex six-dose primary vaccination schedule which requires annual booster vaccination to maintain immunity 5. As a result, unwanted side effects such as local injection-site reactions and systemic reactogenicity have been reported after repeated vaccination. There is an increasing need for more effective and tolerable anthrax vaccines able to elicit a rapid and durable response. A new anthrax vaccine would be suitable for mass vaccination in response to an outbreak as well as for vaccination in the developing world where anthrax epidemics are common.
Among many attempts to develop a second generation anthrax vaccine, purified recombinant PA (rPA) has been selected as the antigen component. PA itself is not toxic, and has been a major target for protective immunity against anthrax which is mainly antibody-based. It is known that anti-PA antibody specific immunity not only has anti-spore activity (to promote phagocytic uptake and suppress germination) 6, but also can prevent PA from binding to its cellular receptor and subsequently binding lethal factor (LF) and edema factor (EF) 7. Studies have shown that the rPA based-vaccine is capable of eliciting a comparable functional antibody response (toxin-neutralizing titer) as the commercially available vaccine AVA 8,9,10. Others have demonstrated administration of rPA can provide complete protection against aerosol anthrax spore challenge in murine models as well as in rabbits 9,10. Most encouragingly, rPA-based vaccines have been employed in clinical trials to evaluate safety and efficacy 8,11,12. However, like the AVA vaccine, some results with rPA-based vaccines indicate the requirement for an annual boost to ensure long-lasting protection. Nevertheless, it is believed that rPA based vaccines with the addition of an effective adjuvant warrant further investigation.
Vaccination via the nasal mucosa has been widely studied in animal models and is also in clinical use 13,14. Recently, Flumist™ an intranasal (IN) influenza vaccine was approved by FDA. The nasal mucosa is topically accessible and immunization via the nasal route may be suitable for mass vaccination. In addition to systemic immunity, mucosal immunity can be elicited 9,15-19 through nasal associated lymphoid tissue (NALT) where large amount of local lymphocytes are present. Vaccination at the mucosal sites where the pathogens usually initiate infection can be more efficacious than immunization by injection, since the pathogen may be neutralized at the mucosal surface before having any systemic effect.
It is known that a successful mucosal vaccine requires an effective mucosal adjuvant. In nasal vaccination formulations, the mucosal adjuvant enhances the antigen-specific mucosal immune response and may result in a dose-sparing effect. Although, cholera toxin (CT) and CpG are potent mucosal adjuvants in animal models 10,20-22 , concerns exist over the safety of CT used in human 23 and the possibility of eliciting an immune response to CpG oligonucleotide after multiple boosting. Most recently, supported by the recognition of mast cell’s immunomodulatory role in adaptive immune response 24-26, mast cell activators such as Compound 48/80 have been studied as a new class of vaccine adjuvants and appear to be highly effective and well-tolerated after nasal administration in mice and rabbits 27,28. It is proposed in this current study that Compound 48/80 will be an effective adjuvant for nasal vaccination when using a dry powder vaccine formulation containing rPA in a large animal model, the rabbit.
Intranasal delivery of vaccines has typically been achieved using liquid formulations, which requires a cold-chain for storage and transport to maintain vaccinepotency. There is a growing interest in powder formulations of vaccines, which offers the potential to eliminate preservatives and the cold-chain requirement for shipping and storage while maintaining long term stability for room temperature storage and shipping 13,29-32. Spray-Freeze-Drying (SFD) is a newly developed lab-scale process for dry powder preparation which is an improvement of conventional spray drying and freeze drying in terms of protein structure and activity preservation. Ge Jiang et al have demonstrated the preparation of a stable dry powder formulation for anthrax vaccine by SFD, where trehalose was added as a bulking agent and cryoprotectant 33. Trehalose is known for its ability to stabilize proteins under temperature and drying induced stress conditions. Furthermore, Garmise et al. has demonstrated that SFD process can be carried out under controlled conditions to produce particles in the desired size ranges suitable for direct nasal vaccine delivery without further size reduction 30. In the present study, we prepared rPA with the novel adjuvant C48/80 and trehalose into a simple three component SFD dry powder formulation suitable for direct nasal application. Physical properties of the powder formulation were characterized and the biophysical stability of rPA was closely monitored. In addition, functional stability of rPA and C48/80 were examined by in-vitro cell based assays following preparation and storage. Finally, the in vivo immunogenicity of the powder formulation was evaluated with rabbit nasal vaccination using a unitdose powder device (UnitDose, Aptargroup Inc. (formerly Pfeiffer GmbH), IL, USA). This is the first report of compound 48/80 being used as a vaccine adjuvant in a dry powder formulation for intranasal vaccination.
MATERIALS AND METHODS
Materials
Anthrax recombinant protective antigen (rPA) and recombinant lethal factor (rLF) was obtained from List Biological Laboratories, Inc. (Campbell, CA). C48/80 and trehalose were purchased from Sigma-Aldrich (St. Louis).
Experimental Methods
Powder preparation by Spray-Freeze-Drying
Aqueous solutions containing one or more components of antigen, adjuvant and trehalose were prepared with an overall solute concentration of 10% w/v, which were passed through a two-fluid pneumatic spray nozzle (7mm diameter, Buchi Spray-Dryer B-191, Flawil, Switzerland) with a liquid feed rate of 10 ml/min. The typical batch size is 1-5 g of total powder in 10 - 50ml of aqueous solution. Nitrogen gas with a flow rate of 500L/min and a fixed back pressure of 3 bar was used to force the liquid into small droplets, which were collected in a stainless steel vessel containing liquid nitrogen. Upon evaporation of liquid nitrogen in a −80°C freezer, the frozen droplets were transferred to a pre-chilled jar and lyophilized for 72 hours with a manifold temperature of −55°C and a vacuum pressure of 20 mTorr (Kinetics Flexi-Dry, Kinetics Thermal Systems, Stone Ridge, NY).
Particle Morphology and Size Determination
The morphology and shape of the SFD powders were examined by Hitachi S-4700 Cold Cathode Field Emission Scanning Electron Microscope. Samples were loaded on SEM pin stubs with double-sided adhesive carbon conductive tab by dipping into dry powder stock, and sputter coated with Pd/Au with a thickness of 3 nm.
Particle size analysis by laser diffraction was performed using particle-in-liquid (pil) mode on Malvern 2600c (Malvern Instruments, Worcestershire, UK) with 100mm focal lens. A small amount of powder from each formulation was suspended in a 1% Span 80 in light mineral oil and sonicated for 10 seconds, which were added to a stirred sample cell (~15ml) until idea sample concentration was reached (obscuration level of 20-30%). Volume median diameter and span were measured.
Differential scanning calorimetry (DSC)
DSC (Perkin Elmer DSC 6, Wellesley, MA) was performed on powder samples. Known quantities of the powders were loaded in aluminum pans and differences in heat flow were measured against an empty reference pan. Analysis was performed with a ramp rate of 10°C/min.
Moisture Analysis
Coulometric Karl-Fischer titration (Model 270, Denver Instrument, Denver, CO.) was used to determine the moisture contents of the SFD powders. Approximately 20-25 mg of powder was added to the reaction cell directly.
Circular Dichroism (CD)
Far-UV CD spectra (190nm-260nm) were recorded at 25°C with an Applied Photophysics Pi-Star 180 circular dichroism spectrapolarimeter. Spectra were recorded in 0.5nm steps and averaged over 1.25s. All measurements were carried out in a 1-mm quartz cuvette. The powder vaccine formulation (SFD rPA formulation-2) was reconstituted with deionized water, and was concentrated by centrifugation filter using a Milllipore Centricon centrifugal filter device with an Ultracel® YM regenerated cellulose membrane with a molecular weight cut-off of 30KDa (15°C, 5000 × g, 30min), while removing interference from the adjuvant C48/80 at the same time. Protein retentate was then recovered in 10mM phosphate buffer by reverse spinning at 1000 × g for 3 mins. Final protein concentration for CD analysis is about 0.2 mg/ml. A blank spectrum with buffer only was subtracted. Molar ellipticity [θ] in the units of deg.cm2.dmol−1 is calculated based on the equation: [θ] = θ / (10 × C × l), where θ is the millidegree obtained from instrument, C is the molar concentration of the protein and l = 0.1cm is the path length of the cuvette.
Attenuated Total Reflectance (ATR)-FTIR
FT-IR measurements were conducted (FTIR-8400s, Shimadzu Corporation) in combination with attenuated total reflectance by MIRacle™ ATR accessory equipped with ZnSe crystal (PIKE technologies). SFD rPA formulation-2 was reconstituted and filtered through a Centricon® centrifulgal filter device with Ultracel® YM-30 membrane, the protein component was recovered in 10mM sodium phosphate buffer and loaded onto the crystal surface for direct measurements (scan range 1000cm−1-2000cm−1, 120scans, resolution 4cm−1). Buffer spectrum was subtracted from the sample spectrum using IRsolution software. Each spectrum was minimum-maximum normalized and the 2nd derivative was taken followed by curve smoothing (Savitzky-Golay method).
SDS-PAGE & Native PAGE
SDS-PAGE was performed with a precast NuPAGE 4-12% Bis-Tris gel (Invitrogen, Carlsbad, CA). Powder samples (reconstituted in deionized water) and standards ~10μg per band were mixed with NuPage LDS sample buffer (Novex) with addition of 5% (v/v) 2-Mercaptoethanol. Samples were incubated at 70°C for 10mins. Electrophoresis was performed with NuPAGE MOPS SDS running buffer (Invitrogen) at constant voltage of 200V for 50min. The resulted gel was stained with GelCode Blue Stain Reagent(Pierce Biotechnology, Rockford IL) and destained with distilled water.
Native-PAGE
A precast Native-PAGE™ Novex® 3-12% Bis-Tris Gel (Invitrogen) was used to perform native electrophoresis. Powder samples (reconstituted in deionzed water) ~10μg per band was mixed with NativePage™ sample buffer and kept on ice prior to loading. NativeMark™ Unstained protein standard was used as molecular weight reference. Eletrophoresis was performed with NativePAGE™ anode and cathode buffer with cathode additive (light blue) at constant voltage of 150V for 90min. Gel was stained with Colloidal Blue Staining Kit (Invitrogen) after separation and destained with distilled water.
In-vitro Stability of Antigen and Adjuvant
Macrophage Toxicity Assay
Mouse macrophages are sensitive to LeTx(the combination of PA and LF )-induced necrosis.. Here, rLF was added to the reconstituted powder formulation and the activity/stability of rPA was evaluated by measuring the PA dependent toxicity of rLF using mouse macrophage J774A.1 cells (ATCC, Manassas, VA). J774A.1 cells (6 ×104 cells/well) in 100μl Dulbecco’s Modified Eagles Medium (DMEM) phenol-red free with 10% fetal bovine serum, 4.5 g/L Glucose and 2mM L-glutamine were seeded in a flat-bottom 96-well microtiter plates and incubated for 24h at 37°C for cells to adhere at the bottom. rLF was added to the reconstituted powder formulation and control rPA in a 1:1 ratio with a final starting concentration of 1.5 μg/mL, followed by 2 fold serial dilution across the plate. After incubation at 37°C for one hour, 100μl of the rPA/rLF mixture was transferred to the plate containing the macrophage cells with media removed. The rPA/rLF mixture was incubated with cells for 4 hours, 20μl of CellTiter 96 Aqueous One solution (Promega, Madison, WI) was added and incubated for another 2 hrs before measuring the absorbance at 490 nm for cell viability.
β-Hexoaminidase Release for Mast Cell Degranulation
Mast cell degranulation activity of the adjuvant was examined by measuring the release of a granule marker, β-hexoaminidase 34. MC/9 cells (ATCC, Manassas, VA) were washed and plated in Tyrode’s buffer (135 mM NaCl, 5mM KCl, 1.8mM CaCl2, 1mM MgCl2, 5.6 mM glucose, 1mg/mL bovine serum albumin, and 20mM Hepes, pH 7.4) at concentration of 2 ×105 cells/well. Cells were spun down, and media was removed. Powder formulations reconstituted in Tyrode’s buffer with desired concentration of the stimulants were added to the plate, and incubated at 37°C for 1.5 hour. β-Hexoaminidase substrate, p-Nitrophenyl-N-acetyl-β-D-glucosainide (NAG, Sigma-Aldrich) 3.4 mg/mL was prepared in 0.1M citrate buffer (pH 4.5). After the incubation, 30μl of the supernatant were taken from the mixture and added to 10μl NAG in a separate plate which was further incubated for 1 hour. The reaction was then stopped with 100μl sodium carbonate buffer (0.1M, pH 10), and absorbance at 405nm was recorded. 0.1% Triton prepared in Tyrode’s buffer was used as positive control. Percent degranulation was calculated using the following formula ((Test–Media only) / (Triton–Media only)) × 100.
Storage Stability Studies of Anthrax Vaccine Formulations
The anthrax vaccine formulations were aliquotted into 2mL micro-centrifuge tubes (Fisherbrand). Each tube contained about 40-50 mg of SFD powder formulation, which was stored at 23°C, 40°C with different humidity levels (55% RH maintained with saturated sodium bromide solution or 75% RH with saturated sodium chloride solution). After storage powder samples were reconstituted in about 300-400μL of water for macrophage toxicity assay. Liquid formulation containing rPA (125 μg/mL), C48/80 (100 μg/mL) and Trehalose (123 mg/mL) in sterile water were also prepared, 320μl of which were aliquotted into 2mL micro-centrifuge tubes and stored at 4°C, 25°C and 40°C for in-vitro stability analysis.
Animal Study
Female New Zealand White rabbits ~2.5-3kg (Robinson Services, Mocksville, NC) were cared and used under conditions approved by Duke University’s Institutional Animal Care and Use Committee. Rabbits (4 rabbits/group) were anesthetized with acepromazine/butorphanol (1mg/kg each) prior to intranasal immunization. Rabbits were immunized three times on days 0, 21, and 42. Liquid formulations of rPA (30μg) with or without C48/80 (120μg) in 200μl PBS (100μl each nostril) were delivered intranasally to rabbits using a laboratory pipette or via IM injection. Powder vaccines of the same antigen and adjuvant dose (30μg rPA, 120μg C48/80 in 15mgs of powder) were delivered using a unitdose powder human device fitted with a tip (modified from P10000 pipette tip, with tip diameter of 2.33mm) for smaller openings of rabbit nasal cavity (6-9mg per nostril). Rabbits receiving liquid formulations were held on their back for 30s after nasal immunization before returning to their cage, whereas rabbits were immediate turned over after receiving nasal powder vaccination with Unitdose device. Blood samples were taken via marginal ear veins on days 7, 35, and 56 for ELISA and toxin neutralization analysis.
ELISA
A fluorescent ELISA was used to measure serum anti-PA immunoglobulin G (IgG) endpoint titers after each immunization. PA was coated onto black 384-well plates (Nuncbrand) at a final concentration of 2 μg/mL in carbonate-bicarbonate buffer (CBC). After incubation overnight at 4°C, Nonfat dry milk (3% w/v) in CBC buffer with Kathon (0.1% v/v) was added and incubated for at least 2 h. to block the non-specific binding sites. Plates were washed four times with ELISA wash buffer (0.1% kathon and 0.05% Tween20 in PBS) and diluted serum samples (1:32) were plated in complete sample diluents (1% w/v bovine serum albumin, 1% w/v non-fat dry milk, 5% normal goat serum, 0.05% Tween20, 0.1% Kathon in PBS) for overnight incubation at 4°C. Plates were washed with ELISA wash buffer and 20 antibody (goat anti-rabbit IgG, alkaline phosphatase conjugated, Southern Biotech) was added with 1:5000 dilution in secondary antibody diluent (0.5% w/v bovine serum albumin, 5% v/v normal goat serum, 0.05% v/v Tween20 and 0.1% v/v Kathon) and incubated for at least 2 h at room temperature. Plates were washed with ELISA wash buffer and 15 μl AttoPhos substrate (Promega) was added to each well and incubated for 15 min before reading at 440nm(excitation)/560nm (emission). The endpoint titer corresponds to the last serum sample dilution that gives a fluorescence value at least 3-fold higher than the value of the corresponding naive sample at the same dilution. The log2 endpoint titers were used for statistical analysis. Samples with no detectable anti-PA IgG titers were assigned a value of 1 for statistical analysis.
Anthrax lethal toxin neutralization assay
Mouse macrophages are sensitive to LeTx(the combination of PA and LF )-induced necrosis. An in vitro lethal toxin (LeTx) neutralization assay using J774A.1 mouse macrophage cells was performed to determine the functional titer of the anti-PA antibody present in rabbit serum. The details of the assay were reported previously 35. Briefly, rabbit sera were incubated with PA for 1 hr at 37°C to allow neutralization to occur, followed by incubation with added LF at room temperature for 1 hr. The serum-PA/LF mixture was then added to the plate containing macrophage cells. The starting serum dilution on the final plate is 1:128 or 1:27 with a final concentration of 0.1875 μg of LeTx (rPA+rLF) per ml. Percent neutralization was calculated based on (sample OD value – LeTx standard OD value)/(cells only OD value-LeTx standard OD value) 100. The percent neutralization was plotted vs serum log2 dilutions and fitted with a second order polynomial curve. The serum dilution able to neutralize 50% of LeTx (NT50) was calculated. Serum samples that had no detectable lethal toxin neutralization activity were assigned a log2 value of 1 for statistical analysis.
RESULTS AND DISCUSSION
Physicochemical properties of SFD powders
Different SFD powder formulations were prepared, including SFD powder prepared with trehalose alone, SFD C48/80 trehalose powder containing 12 μg of C48/80 per mg of trehalose, SFD rPA formulation-1 containing 0.67 μg of rPA plus 10 ug of C48/80 per mg of trehalose, and SFD rPA formulation-2 containing 2 μg of rPA plus 8 μg of C48/80 per mg of trehalose. The amount and ratio of rPA and compound 48/80 in the SFD powder formulations were adopted from liquid formulations optimized in the Staats lab.
Scanning electron micrographs (Figure 1) show that the particles prepared by SFD were spherical and very porous, which is consistent with their relatively low tapped density (Table 2). Low and high magnification of SFD trehalose formulation (Figure 1-A, B) demonstrated primary particles with sizes consistent with LD measurements. SEM image of C48/80 (Figure 1-C) showed spherical particle appears to have smaller pores and more materials inside. The addition of protein to the formulation has yielded particles with hollow porous structure as shown in image D and E. It seems that the particle backbone structure becomes fragile as additional protein content is introduced to the trehalose bulk.
Figure 1.
SEM images of SFD particles.
A) SFD trehalose low magnification, B) SFD trehalose high magnification, C) SFD trehalose C48/80, D) SFD rPA formulation-1, E) SFD rPA formulation-2.
Table 2.
SFD powder physical properties (Mean (SD), n=3).
| Powders | Moisture Content (% w/w) |
Bulk Density (g/ml) |
Tapped Density (g/ml) |
Carr’s Compress. Index (CCI) |
|---|---|---|---|---|
| SFD Trehalose | 2.4 (0.16) | 0.05 (0.02) | 0.08 (0.03) | 38.1 (3) |
|
SFD C48/80
Trehalose |
1.5 (0.14) | 0.16 | 0.24 | 34.9 |
|
SFD rPA
formulation-1 |
5.2 (0.15) | 0.12 | 0.18 | 37.8 |
|
SFD rPA
formulation-2 |
4.3 (0.17) | 0.06 | 0.09 | 32.5 |
The volume median diameter of the SFD particles prepared from trehalose alone and protein containing trehalose formulations were very close to the target size D50=25μm (Table 2) when prepared under controlled SFD process optimized with trehalose alone. However, for SFD powders prepared with just C48/80 and Trehalose, the overall particle size was significantly smaller than those measured of SFD trehalose powder in terms of D10, D50 and D90 (P < 0.05). Nevertheless, SFD formulations prepared with both adjuvant C48/80 and antigen rPA had a similar D50 compared to SFD formulation made of pure trehalose. Particle size distribution of these SFD particles appear to be bimodal or multimodal (Figure 2).
Figure 2.
Particle size distributions of SFD powder formulations.
Powder flow properties influence the aerosol delivery efficiency of powder products. In the current project, a simple CCI index based on powder bulk and tapped densities was used to evaluate powder flow property. The bulk (0.05-0.16 g/ml) and tapped (0.08-0.24 g/ml) densities of the SFD powders were significantly lower than bulk trehalose (~0.5 and 0.6 g/ml). The bulk and tap densities of the SFD formulation-2 were similar to SFD trehalose (Table 2). The SFD C48/80 particles have larger bulk and tap densities (0.16 and 0.24 g/m, respectively) than other SFD formulations listed in the table. This observation is consistent with reduction in particle size measured by SEM analysis and LD, suggesting the particles are no longer maintaining bulk properties of SFD trehalose which could be a result of introducing excess hydrophobic content to the trehalose formulation. The CCI indices for all SFD powders were high (between 32-38%), suggesting the powders are easily compressible, and indicating large cohesive forces acting on the SFD powders. This can be attributed to the large porous structure of the SFD particle, the brittleness of the structure. The ambient moisture levels during the experiment could influence the cohesive forces between the particles due to the moisture sensitive nature of SFD trehalose. As mentioned in the introduction, a free-flowing powder has a CCI less than ~20% to 21%. None of the SFD powders would be considered free flowing based on their CCI index.
However, the flow property of the SFD powders as determined by CCI index did not seem to dictate the performance of the final product. The powder device was able to deliver almost all of SFD powders packed (loading amount 6-9mg). When the device was fitted with an extra tip for nasal delivery to rabbits, the emitted dose for SFD powders decreased slightly with overall efficiency to be over 98% (Table 3). Aerosols delivered from the device showed similar particle size distribution with powders measured in oil suspension (Figure 3). The overall volume diameters of emitted powders appear to be very close to SFD powders measured by PIL. The volume median diameters observed were larger for powder aerosols in PIA measurements, which may be explained by a modest aggregation phenomenon. Nevertheless, these results indicate that SFD powders tested showed good dispersion properties and delivery efficiency (complete dispersion) with the Unitdose powder device.
Table 3.
Powder delivery efficiency with Unitdose device +/− tips (Mean (SD), n=3).
| SFD particles | LD mode | D50 (μm) | Span | Emit Dose % |
Emitted Dose (w tip) % |
|---|---|---|---|---|---|
| SFD Trehalose | PIL | 27.48 (1.41) | 2.32 (0.11) | 99 (1.7) | 100 (0) |
| Device PIA | 37.3 (2.73) | 2.14 (0.29) | |||
|
| |||||
| SFD C48/80 Trehalose | PIL | 20.15 (1.58) | 1.62 (0.07) | 99 (1) | 99.7 (3.5) |
| Device PIA | 29.4 (1.95) | 2.1 (0.28) | |||
|
| |||||
| SFD rPA formulation-2 | PIL | 24.37 (1.37) | 1.72 (0.09) | 99 (1.3) | 98.5 (3.2) |
| Device PIA | 33.9 (1.60) | 2.52 (0.32) | |||
Figure 3.
Particle size distributions of SFD powder formulations measured as powder spray from Pfeiffer Unitdose device (PIA measurements) as determined by laser diffraction measurement.
Thermal properties and moisture content
The moisture content of the SFD powders was around 2-5% (Table 1) following lyophilization and storage at room temperature with desiccant. To determine whether the formulation components and the residual water content would affect the Tg of amorphous trehalose, which, might subsequently, influence the optimum storage temperature, DSC scans of SFD powders were performed at 10°C/min (Figure 4). Typical glass transition peaks for SFD trehalose, SFD rPA-1 and SFD rPA-2 formulations were not detected in the initial scan due to interference from other thermal transitions that occur at the same temperature range, namely the preceding broad dehydration process, and the onset of an accompanying relaxation endotherm at Tg (also called enthalpic recovery) 36. Since these formulations were handled under ambient conditions during the one year storage before DSC measurements, it is likely that residual water content in these powders acted as a plasticizer and introduced increased molecular mobility of the amorphous trehalose matrix which lead to structural relaxation. This enthalpic recovery was found overlapping the glass transition peak, which prevented identification of the Tg in the first scan. Nevertheless, after subjecting the sample to a heat-cool cycle, the second DSC scan showed fully resolved glass transition, with a Tg of 106°C (see Figure 4 insert), which is consistent with literature reports (106-120°C) 30,37,38. DSC results demonstrated that the addition of vaccine components and the presences of residual water content did not change the matrix Tg substantially, and these formulations may be stored at room temperature.
Table 1.
Particle sizes of SFD formulations (Mean (SD), n=3).
| Powders | D90 (μm) | D50 (μm) | D10 (μm) | Span |
|---|---|---|---|---|
| SFD Trehalose | 69.32 (1.77) | 27.48 (1.41) | 5.50 (1.17) | 2.32 (0.11) |
| SFD C48/80 Trehalose | 37.09 (0.89) | 20.15 (1.58) | 4.45 (0.80) | 1.62 (0.07) |
| SFD rPA formulation-1 | 72.95 (2.48) | 28.32 (2.52) | 10.92 (1.32) | 2.19 (0.10) |
| SFD rPA formulation-2 | 50.13 (1.96) | 24.37 (1.37) | 8.28 (0.74) | 1.72 (0.06) |
Figure 4.
Differential scanning calorimetry of SFD powders and pure C48/80.
Structural stability and integrity of rPA
CD measures the protein secondary structure in the far-UV region. It is important to remove any interference due to the buffer or any other component in the sample solution that has high UV absorption that could mask the CD signal at lower wavelength. In the present study, the reconstituted SFD rPA formulation-2 was subjected to ultrafiltration to eliminate the UV absorbing C48/80 prior to CD analysis. Figure 5 shows the molar ellipticity of rPA at the Far-UV region. The CD spectrum of rPA in the SFD formulation appears to be very close to the unprocessed rPA protein. Both CD spectra display a broad negative absorption with a single dominating minima at around 208nm, suggesting a mixture of α-helical and β-sheet component, which is comparable to CD spectra of PA reported previously in the literature 33. The positive α-helical signal near 195 nm (π0→π*) was not observed, which may be due to interaction from certain aromatic amino acid side chain in PA 39.
Figure 5.
CD spectrum of rPA in Far-UV region.
ATR-FTIR experiments were carried out to confirm the result from CD experiments. Figure 6 shows the second derivative spectra of the two samples in the Amide I region (1600-1700cm−1), which indicates similarity of the peak shape, position and intensity. Different components of the secondary structure can be assigned according to the literature 40. One major band at 1652 cm−1 has been assigned to α–helical component of the structure. Several other band components at 1680cm−1, 1634cm−1 and 1618cm−1 are attributable to formation of β-sheet in the protein structure. Finally, the small band component at 1670 cm−1 is indicative of turn structure. These secondary structure components seems to correspond to the structure element of PA as reported in protein data bank (PDB: 1ACC).
Figure 6.
Second derivative FT-IR spectra of rPA in the Amide I region.
Top: unprocessed rPA control (0.25mg/ml), Bottom: rPA concentrated from reconstituted SFD rPA formulation-2 (0.24mg/ml)
Note: secondary structure element was assigned according to Table 2 in Schüle et al Eur J Pharm Biopharm. 2007, 65(1):1-9)
Both CD and FTIR spectroscopy confirmed the structural stability of PA in the SFD formulation. The integrity of the protein was further examined by gel electrophoresis (Figure 7). SDS-PAGE (left image) under reducing conditions shows that powders stored for 1.5 years (lane 2) and freshly prepared powders (lane 3), have similar migration patterns to unprocessed rPA control, which means the rPA is maintaining its molecular size and integrity. This result is also true for samples prepared and analyzed under non-reducing and native conditions, as shown in the right image. This result indicates the absence of antigen aggregation due to oxidation.
Figure 7.
SDS and Native PAGE of reconstituted rPA formulations.
LEFT: SDS-PAGE under reducing conditions. Lane 1, MW marker; Lane 2, SFDrPA formulation-1 upon 1.5 year of storage; Lane 3, SFD rPA formulation22 freshly prepared; Lane 4, rPA control; Lane 5, SFD C48/80 formulation freshly prepared.
RIGHT: Native-PAGE under non-reducing conditions. Lane 1, MW marker; Lane 2, SFD rPA formulation-1 upon 1.5 year of storage; Lane 3, SFD rPA formulation-2 freshly prepared; Lane 4, rPA control; Lane 5, SFD C48/80 formulation freshly prepared.
Functional stability of rPA and compound 48/80
The biological functions of the adjuvant and antigen may be employed to examine their functional stability in-vitro with cell-based assays. Compound 48/80 is a polymer prepared from a condensation reaction of N-methyl-p-methoxyphenethylamine with formaldehyde 41. A mixture of oligomers is thought to be formed, from trimer to octamer, with hexamer being the most potent 42,43. It has been studied since the 1950’s as a potent mast cell activator that promotes release of histamine44,45. Here the functional stability of C48/80 was examined by mast cell degranulation assay. SFD rPA formulation-2 (stored at room temperature over dessicant for 1.5 years) showed comparable mast cell degranulation activity with respect to control C48/80 at different concentration dilutions Figure 8. Slight variability in the degranulation activity among different formulation groups might be explained by the difference in polymer distribution between batches of commercially available C48/80 42,46. This result demonstrates functional stability of C48/80 after SFD process and upon 1.5 years of storage.
Figure 8.
Mast cell degranulation activity of SFD powder formulations. (Mean±SD, n=3).
In addition, the functional stability of rPA was assessed using a macrophage toxicity assay. A 1:1 ratio of LF was added to reconstituted SFD formulations, where the cellular toxicity of LeTx to macrophage cells was an indirect measurement for PA functionality since the killing mechanism of LF can only occur with the assistance of functional PA for its cell entry and endosomal escape 47. Figure 9 shows the cell viability across the PA/LF concentration dilutions. Low optical density corresponds with few viable cells which, in turn indicates PA is still active. The toxicity profile across PA/LF dilutions of LF+SFD rPA formulation-2 prepared two years previously with room temperature storage over desiccant and freshly prepared SFD rPA formulation-2+LF are very close to unprocessed control rPA+rLF. When percent activity were calculated based on the log dilution level that can cause 50% cell death, the rPA within SFD rPA formulation-2 powder (2 yrs) and freshly prepared SFD powders had a relative activity of 96% and 94%, respectively, compared to the standard. Whereas, when LF was added alone, high optical density are observed across its concentration dilutions, suggesting LF itself exerts no cellular toxicity to the macrophages. In addition, when trehalose was added to the PA/LF standard, the relative PA activity (104%) was also comparable to the PA/LF standard alone, suggesting trehalose did not affect the in-vitro function of rPA+LF. This macrophage toxicity assay was a useful tool to monitor the functional stability of PA after formulation, manufacturing process and storage.
Figure 9.
Antigen functional stability determined by macrophage toxicity assay.
Storage stability of powder formulations
SFD trehalose powder formulation appears to be highly moisture sensitive. Rapid moisture sorption can reduce powder flowability, lead to powder collapse and dose delivery failure. To gain a better understanding of the effect of moisture uptake on the stability of antigen, and to identify an optimum storage condition for both the powder and liquid formulations, liquid and powder formulations containing rPA, C48/80 and trehalose were prepared and stored under different temperature and different humidity levels (−80°C control, 4°C/liquid only, 25°C/with desiccant, 25°C/ambient RH (~30-40% RH), 25°C/55% RH, 40°C/75%RH) with either closed or open top vials. Instead of measuring physicochemical stability of rPA, the storage stability of each formulation was estimated by rPA functional activity based on macrophage toxicity assay.
Results (Figure 10-A) of SFD powder vaccine formulations stored at different conditions show that the rate of rPA degradation increases as humidity level increases for storing SFD powder formulations. SFD powder formulations stored with desiccant were the most stable in terms of PA functional activity preservation (Figure 10-A, C). For SFD powders stored at 25°C at ambient humidity levels (~ 30-40% RH), initial fast degradation was observed during the first week, then a steady PA activity level was reached . This might be due to an initial recrystallization process that prevented further moisture uptake to the crystal core which leads to a possible stabilizing effect, as indicated by DSC examination of SFD powder vaccines stored under different conditions (data not shown). SFD powders stored in a regular screw top plastic vial did not prevent moisture uptake and appeared to have a similar degradation profile as powders stored in an open top vial. SFD powders stored at 55% RH were more susceptible to degradation. The rate was faster and similar to liquid formulations stored at room 25°C (Figure 10-C).
Figure 10.
Powder Storage Stability Study.
A) SFD rPA powder storage stability at different temperature and relative humidity (%RH), B) rPA liquid formulation stability at different temperature, C) powder liquid comparison at 25°C, D) powder liquid comparison at 40°C.
The rate of degradation of the liquid formulation increased as temperature was elevated (Figure 10-B). For liquid formulations stored at 40°C, PA activity was lost completely within 1 day (Figure 10-B, D). However, the SFD powder formulation stored at the same temperature and 75% RH level extended the PA activity for a longer period compared to liquid formulations. Furthermore, when SFD powder formulation was stored over desiccant at 40°C, 80% of the PA activity remained after two weeks (Figure 10-D).
Results from the previous function stability study showed that the SFD powder vaccine formulations were stable upon 2 years of storage at room temperature (Figure 9). However, the formulation was susceptible to degradation even at ambient humidity levels. Consequently, SFD powders should be either stored using special sealed device or stored with desiccant to limit moisture uptake. The upper limit of storage temperature can be increased to 40°C for up to two weeks as long as the SFD has limited exposure to moisture, which allows temperature fluctuation during storage and shipping.
Powder vaccine immunogenicity study in rabbits
Rabbit nasal SFD powder vaccination using Unitdose powder device
SFD vaccine formulation prepared with trehalose appeared to be moisture sensitive and required an appropriate device to prevent moisture uptake and ensure efficient and consistent delivery of the powder dose. An animal study was carried out to further examine the in-vivo immunogenicity of the SFD powder vaccine formulations using the Unitdose powder device (Table 4). Two SFD powder formulations containing rPA + C48/80 were prepared with trehalose under the same optimized SFD process, and had been stored in a dessicator for either 2.5 years or 3 months before packing into the Unitpowder device (packing condition: RT, RH level under 40%). SFD powder loaded devices were stored in a ziploc-type bag with dessicant for 2-4 months before final testing in animal study.
Table 4.
Vaccine formulations prepared to test the potency of dry powder rPA vaccines.
| Groups | rPA Dose (μg) |
Route | Adjuvant | |
|---|---|---|---|---|
| 1 | liquid, rPA alone | 30 | Nasal | NONE |
| 2 | liquid, rPA and C48/80 | 30 | Nasal | C48/80, 120 μg |
| 3 | Powder (prepared Aug.09) | 30 | Nasal | C48/80, 120 μg |
| 4 | Powder (prepared Sep.07) | 30 | Nasal | C48/80, 120 μg |
| 5 | liquid, IM | 30 | IM | C48/80, 120 μg |
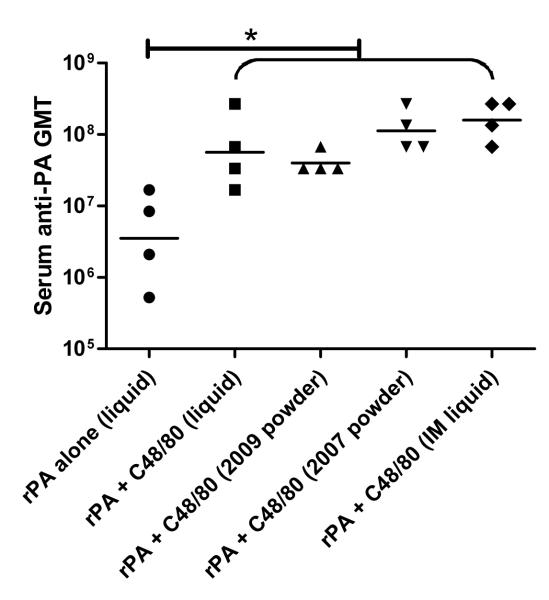
Rabbits were immunized on days 0, 21 and 42 (see Materials and Methods). After the initial immunization, no detectable serum anti-PA IgG was observed in rabbits vaccinated with liquid formulation. However, rabbits in the two IN powder groups and IM liquid control group showed 50% and 75% seropositive response, respectively, as determined by ELISA analysis of serum collected on day 7 (Figure 11-A). Although there was no statistical difference inthe anti-PA IgG titers between the any of the three seropositive groups was noted,our data demonstrates that a single nasal dose of powder vaccine was similar to liquid IM immunization for eliciting an early vaccine-specific IgG titer, whereas nasal vaccination with the liquid formulations failed to induce measurable serum antibody responses after the first immunization. The fact that the powder vaccine formulation was able to induce measurable immune responses after only one immunization suggests that powder nasal vaccines are superior to liquid nasal vaccines and comparable to IM immunization for rapid induction of vaccine-specific antibody response. This property of powder nasal vaccine may be especially valuable if used in the event of emergency.
Figure 11.
Animal study serum ELISA results.
(A) day 7 seropositive results. (B) day 35 serum anti-rPA IgG GMT titers. (C) day 56 serum anti-rPA IgG GMT titers. A one-way ANOVA followed by Tukey’s post test were performed on log2 transformed ELISA titer. A statistical significance was revealed between the adjuvanted groups and rPA alone group. (n=4, *, P < 0.05). Data are presented as ELISA titers with geometric mean.
Following the second immunization, a substantial increase in IgG titers (on day 35) was observed for all groups of rabbits (Figure 11-B) with the lowest being the group vaccinated with liquid rPA lone which had a geometric mean anti-PA IgG titer of 1:92,682. All adjuvanted groups including nasal liquid, nasal powder and IM liquid group had serum anti-rPA GMT that were significantly greater than in rabbits immunized with rPA alone, with titers in the range of 1:107. In addition, no significant difference was demonstrated between the serum anti-rPA IgG GMT in any of the adjuvanted groups. Most notably, the powder vaccine formulation stored for more than 2 years at room temperature was as effective as any of the adjuvanted vaccines (liquid or powder) in eliciting systemic anti-PA IgG response. It should be noted that rabbits were supine for 30s after vaccination with liquid formulations in order to maximize its nasal residence time, while rabbits receiving powder spray were immediately returned to their cages. The systemic IgG response may have been much lower for nasal liquid groups if the rabbits were immediately returned to the upright position following immunization due to liquid drainage 48.
Similar trends were seen in the day 56 ELISA results showing a continuous systemic immune response with moderate increase in overall anti-PA IgG titer among all groups (in the range of 1:106-1:108) (Figure 11-C). Additionally, the powder vaccine formulation stored for more than 2 years at room temperature was as potent as any of the adjuvanted vaccines and induced the highest serum anti-rPA IgG GMT of any of the nasal immunization groups with a titer of 1:1.1 × 108.
Serum samples collected on day 35 and 56 were also tested for their ability to neutralize anthrax lethal toxin (LeTx) using an in vivo macrophage toxicity assay. LeTx neutralization titers are presented as the serum dilution required to neutralize 50% of the LeTx (NT50). At day 35, all adjuvanted vaccine formulations, delivered IN or IM, had serum LeTx NT50 titers that were significantly greater than the LeTx NT50 in rabbits immunized IN with rPA alone, which further demonstrates that C48/80 provided significant adjuvant activity (Figure 12-A). There were no significant differences in the LeTx NT50 between any of the adjuvanted groups (nasal or IM delivery), although the LeTx NT50 induce by IM immunization was the highest. It is important to point out that only 75% of the animals in the nasal liquid rPA + C48/80 group had measurable LeTx NT50 while the two powder groups and the IM group had measureable LeTx NT50 in all animals after only two immunizations. Similar to ELISA results, the LeTx NT50 results demonstrates that powder formulations induced serum antibody responses at a faster rate than the liquid IN formation and similar to the IM rPA vaccine. As mentioned in the introduction, the serum toxin neutralization ability in rabbits correlates very well to the protection against anthrax spore. We believe the long-term storage stability of the powder vaccine formulation (at least 2 years room temp) and the ability of only 2 doses of powder vaccine to induce functional toxin-neutralizing antibody responses makes this an ideal vaccine formulation for biodefense applications offering the advantage of stockpiling and shipping of the vaccines in the absence of a cold chain. Additionally, nasal vaccination with powder vaccne formulations may provide a superior, needle-free vaccination regimen for the rapid induction of protective immunity, in case of emergency.
Figure 12.
Animal study serum lethal toxin neutralization results.
(A) day 35 serum LeTx NT50 titer. (B) day 56 serum LeTx NT50 titer.
Anthrax LeTx neutralizing antibody responses of day 56 samples had a similar trend to day 35 samples, which demonostrates long term stability of dry powder vaccines. All adjuvanted vaccine formulations, delivered IN or IM, had serum LeTx NT50 titers that were significantly greater than the LeTx NT50 in rabbits immunized IN with rPA alone, once again demonstrating that C48/80 provided significant adjuvant activity (Figure 12-B). There were no significant differences in the LeTx NT50 between any of the adjuvanted groups (nasal or IM delivery), although the LeTx NT50 induce by IM immunization was the highest. By day 56, all rabbits vaccinated with an adjuvanted vaccine formulation (IN or IM) had measurable anthrax toxin neutralizing antibody responses. The maximum serum LeTx NT50 was in the IM group (1:4,374) while the 2009 and 2007 powder nasal formulations had the next highest LeTx NT50 (1:1,122 and 1:1,085, respectively). The serum LeTx NT50 in the IN liquid rPA + C48/80 group was the lowest of the adjuvanted groups (1:607). It is important to mention that others use much lower doses of rPA and rLF in their anthrax lethal toxin neutralization (50 ng/ml rPA + 40 ng/ml rLF) 49 than our assay (187.5 ng/ml rPA + 187.5 ng/ml rLF) indicating that our calculated LeTx NT50 are likely under-estimates of the toxin neutralization capacity of our vaccine-induced anti-serum.
These in-vivo results not only showed that the powder device efficiently delivered the powders after two months of device storage time, but also confirmed results from in-vitro stability study that rPA in a powder formulation was stable at room temperature upon 2.5 years of storage and C48/80 and was an effective mucosal adjuvant in the rabbit model. No evident toxicity was observed for all the vaccine groups. All rabbits maintained continuous weight gain throughout the study and all survived after the study.
CONCLUSION
SFD powders containing rPA and C48/80 were successfully prepared under controlled SFD condition, with particle physicochemical properties appropriate for direct nasal application to the rabbit nasal cavity. Formulation with protein and adjuvant did not change the particle size distribution in comparison to SFD powders of pure trehalose. The spectral information obtained from CD and FT-IR measurements demonstrated the rPA in SFD formulation maintained their secondary structure after SFD process and storage. The retention of rPA integrity was confirmed by results from SDS-PAGE and Native PAGE. The mast cell degranulation assay and in-vitro macrophage toxicity assay were useful in determining the functional stability of adjuvant and antigen rPA. The effectiveness of the SFD powder formulation was further examined in-vivo in an animal study using Unitdose powder device for nasal delivery in rabbits. Nasal delivery of SFD powder formulation of rPA and C48/80 either freshly prepared or stored for 2.5 years at room temperature was as effective as IM injection of freshly prepared liquid formulation. This room temperature storage stability of the SFD powder vaccine was achieved by storing the SFD vaccine formulation with desiccant and packaging in a nasal delivery device to avoid moisture uptake.
Positive results from both in-vitro and in-vivo studies support the use of C48/80 as a new class of adjuvant for mucosal vaccination. Direct nasal delivery of dry powder vaccine formulation containing recombinant PA and an effective mucosal adjuvant may provide the ultimate approach for developing a stable and effective alternative to improve current available anthrax vaccines. In comparing with other complex vaccine delivery systems, the simple dry powder approach seems far more likely to be clinically/commercially viable the same required protection can be achieved.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Amar Kumbhar (CHANL, UNC) for help in the SEM imaging analysis; Afton Thompson and Dr. Kathleen Ashcraft (Department of Pathology, Duke University) for assistance with rabbit immunizations and blood sampling. The following reagents were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Anthrax Protective Antigen (PA), Recombinant from Bacillus anthracis, NR-140 and Anthrax Lethal Factor (LF), Recombinant from Bacillus anthracis, NR-570. This work was supported by NIH grants R01 AI064879 and R21 AI059591.
REFERENCES
- 1.Bhatnagar R, Batra S. Anthrax toxin. Crit Rev Microbiol. 2001;27(3):167–200. doi: 10.1080/20014091096738. [DOI] [PubMed] [Google Scholar]
- 2.Sternbach G. The history of anthrax. Journal of Emergency Medicine. 2003;24(4):463–467. doi: 10.1016/s0736-4679(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 3.Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Greene C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh WJ, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yeskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8(10):1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedlander AM. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr Clin Top Infect Dis. 2000;20:335–349. [PubMed] [Google Scholar]
- 5.CDC Use of Anthrax Vaccine in the United States: Recommendations of the Advisory Committee on Immunization Practices. 2000. [DOI] [PubMed]
- 6.Welkos S, Friedlander A, Weeks S, Little S, Mendelson I. In-vitro characterisation of the phagocytosis and fate of anthrax spores in macrophages and the effects of anti-PA antibody. J Med Microbiol. 2002;51(10):821–831. doi: 10.1099/0022-1317-51-10-821. [DOI] [PubMed] [Google Scholar]
- 7.Welkos S, Little S, Friedlander A, Fritz D, Fellows P. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology. 2001;147(Pt 6):1677–1685. doi: 10.1099/00221287-147-6-1677. [DOI] [PubMed] [Google Scholar]
- 8.Health NIo A New Anthrax Vaccine Administered by the Intramuscular (IM) Route in Healthy Adults. 2004.
- 9.Flick-Smith HC, Eyles JE, Hebdon R, Waters EL, Beedham RJ, Stagg TJ, Miller J, Alpar HO, Baillie LW, Williamson ED. Mucosal or parenteral administration of microsphere-associated Bacillus anthracis protective antigen protects against anthrax infection in mice. Infect Immun. 2002;70(4):2022–2028. doi: 10.1128/IAI.70.4.2022-2028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikszta JA, Sullivan VJ, Dean C, Waterston AM, Alarcon JB, Dekker JP, 3rd, Brittingham JM, Huang J, Hwang CR, Ferriter M, Jiang G, Mar K, Saikh KU, Stiles BG, Roy CJ, Ulrich RG, Harvey NG. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J Infect Dis. 2005;191(2):278–288. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- 11.Keitel WA. Recombinant protective antigen 102 (rPA102): profile of a second-generation anthrax vaccine. Expert Rev Vaccines. 2006;5(4):417–430. doi: 10.1586/14760584.5.4.417. [DOI] [PubMed] [Google Scholar]
- 12.Gorse GJ, Keitel W, Keyserling H, Taylor DN, Lock M, Alves K, Kenner J, Deans L, Gurwith M. Immunogenicity and tolerance of ascending doses of a recombinant protective antigen (rPA102) anthrax vaccine: a randomized, double-blinded, controlled, multicenter trial. Vaccine. 2006;24(33-34):5950–5959. doi: 10.1016/j.vaccine.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51(1-3):81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 14.Kemble G, Greenberg H. Novel generations of influenza vaccines. Vaccine. 2003;21(16):1789–1795. doi: 10.1016/s0264-410x(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 15.Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J Immunol. 2003;170(11):5636–5643. doi: 10.4049/jimmunol.170.11.5636. [DOI] [PubMed] [Google Scholar]
- 16.Debin A, Kravtzoff R, Santiago JV, Cazales L, Sperandio S, Melber K, Janowicz Z, Betbeder D, Moynier M. Intranasal immunization with recombinant antigens associated with new cationic particles induces strong mucosal as well as systemic antibody and CTL responses. Vaccine. 2002;20(21-22):2752–2763. doi: 10.1016/s0264-410x(02)00191-3. [DOI] [PubMed] [Google Scholar]
- 17.Gaur R, Gupta PK, Banerjea AC, Singh Y. Effect of nasal immunization with protective antigen of Bacillus anthracis on protective immune response against anthrax toxin. Vaccine. 2002;20(21-22):2836–2839. doi: 10.1016/s0264-410x(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 18.Rharbaoui F, Drabner B, Borsutzky S, Winckler U, Morr M, Ensoli B, Muhlradt PF, Guzman CA. The Mycoplasma-derived lipopeptide MALP-2 is a potent mucosal adjuvant. Eur J Immunol. 2002;32(10):2857–2865. doi: 10.1002/1521-4141(2002010)32:10<2857::AID-IMMU2857>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Staats HF, Bradney CP, Gwinn WM, Jackson SS, Sempowski GD, Liao HX, Letvin NL, Haynes BF. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J Immunol. 2001;167(9):5386–5394. doi: 10.4049/jimmunol.167.9.5386. [DOI] [PubMed] [Google Scholar]
- 20.Plant A, Williams NA. Modulation of the immune response by the cholera-like enterotoxins. Curr Top Med Chem. 2004;4(5):509–519. doi: 10.2174/1568026043451230. [DOI] [PubMed] [Google Scholar]
- 21.Holmgren J, Harandi AM, Czerkinsky C. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Expert Rev Vaccines. 2003;2(2):205–217. doi: 10.1586/14760584.2.2.205. [DOI] [PubMed] [Google Scholar]
- 22.Kodama S, Abe N, Hirano T, Suzuki M. Safety and efficacy of nasal application of CpG oligodeoxynucleotide as a mucosal adjuvant. Laryngoscope. 2006;116(2):331–335. doi: 10.1097/01.mlg.0000194222.93067.f7. [DOI] [PubMed] [Google Scholar]
- 23.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165(9):4778–4782. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 24.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 25.Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci U S A. 2005;102(18):6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzoni A, Siraganian RP, Leifer CA, Segal DM. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. J Immunol. 2006;177(6):3577–3581. doi: 10.4049/jimmunol.177.6.3577. [DOI] [PubMed] [Google Scholar]
- 27.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, Abraham SN. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14(5):536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 28.Staats HF, Fielhauer JR, Thompson AL, Tripp AA, Sobel AE, Maddaloni M, Abraham SN, Pascual DW. Mucosal targeting of a BoNT/A subunit vaccine adjuvanted with a mast cell activator enhances induction of BoNT/A neutralizing antibodies in rabbits. PLoS One. 6(1):e16532. doi: 10.1371/journal.pone.0016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson J, Fishbourne E, Corteyn A, Donaldson AI. Protection of cattle against rinderpest by intranasal immunisation with a dry powder tissue culture vaccine. Vaccine. 2000;19(7-8):840–843. doi: 10.1016/s0264-410x(00)00228-0. [DOI] [PubMed] [Google Scholar]
- 30.Garmise RJ, Staats HF, Hickey AJ. Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS PharmSciTech. 2007;8(4):E81. doi: 10.1208/pt0804081. [DOI] [PubMed] [Google Scholar]
- 31.LiCalsi C, Maniaci MJ, Christensen T, Phillips E, Ward GH, Witham C. A powder formulation of measles vaccine for aerosol delivery. Vaccine. 2001;19(17-19):2629–2636. doi: 10.1016/s0264-410x(00)00503-x. [DOI] [PubMed] [Google Scholar]
- 32.Garmise RJ, Mar K, Crowder TM, Hwang CR, Ferriter M, Huang J, Mikszta JA, Sullivan VJ, Hickey AJ. Formulation of a dry powder influenza vaccine for nasal delivery. AAPS PharmSciTech. 2006;7(1):E19. doi: 10.1208/pt070103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang G, Joshi SB, Peek LJ, Brandau DT, Huang J, Ferriter MS, Woodley WD, Ford BM, Mar KD, Mikszta JA, Hwang CR, Ulrich R, Harvey NG, Middaugh CR, Sullivan VJ. Anthrax vaccine powder formulations for nasal mucosal delivery. J Pharm Sci. 2006;95(1):80–96. doi: 10.1002/jps.20484. [DOI] [PubMed] [Google Scholar]
- 34.Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, Montecucco C. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur J Immunol. 2002;32(3):671–676. doi: 10.1002/1521-4141(200203)32:3<671::aid-immu671>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 35.Staats HF, Alam SM, Scearce RM, Kirwan SM, Zhang JX, Gwinn WM, Haynes BF. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infect Immun. 2007;75(11):5443–5452. doi: 10.1128/IAI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hancock BC, Shamblin SL, Zografi G. Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm Res. 1995;12(6):799–806. doi: 10.1023/a:1016292416526. [DOI] [PubMed] [Google Scholar]
- 37.Naini V, Byron PR, Phillips EM. Physicochemical stability of crystalline sugars and their spray-dried forms: dependence upon relative humidity and suitability for use in powder inhalers. Drug Dev Ind Pharm. 1998;24(10):895–909. doi: 10.3109/03639049809097269. [DOI] [PubMed] [Google Scholar]
- 38.Crowe LM, Reid DS, Crowe JH. Is trehalose special for preserving dry biomaterials? Biophys J. 1996;71(4):2087–2093. doi: 10.1016/S0006-3495(96)79407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krittanai C, Johnson WC. Correcting the circular dichroism spectra of peptides for contributions of absorbing side chains. Anal Biochem. 1997;253(1):57–64. doi: 10.1006/abio.1997.2366. [DOI] [PubMed] [Google Scholar]
- 40.Schule S, Friess W, Bechtold-Peters K, Garidel P. Conformational analysis of protein secondary structure during spray-drying of antibody/mannitol formulations. Eur J Pharm Biopharm. 2007;65(1):1–9. doi: 10.1016/j.ejpb.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Baltzly R, Dvorkovitz V, Phillips AP. Synthetic analogs of oxytocic drugs; phenethyl beta-alanine derivatives. J Am Chem Soc. 1949;71(4):1162–1164. doi: 10.1021/ja01172a009. [DOI] [PubMed] [Google Scholar]
- 42.Read GW, Lenney JF. Molecular weight studies on the active constituents of compound 48-80. J Med Chem. 1972;15(3):320–323. doi: 10.1021/jm00273a026. [DOI] [PubMed] [Google Scholar]
- 43.Ortner MJ, Sik RH, Chignell CF, Sokoloski EA. A nuclear magnetic resonance study of compound 48/80. Mol Pharmacol. 1979;15(1):179–188. [PubMed] [Google Scholar]
- 44.Paton WD. Compound 48/80: a potent histamine liberator. Br J Pharmacol Chemother. 1951;6(3):499–508. doi: 10.1111/j.1476-5381.1951.tb00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koibuchi Y, Ichikawa A, Nakagawa M, Tomita K. Histamine release induced from mast cells by active components of compound 48/80. Eur J Pharmacol. 1985;115(2-3):163–170. doi: 10.1016/0014-2999(85)90687-9. [DOI] [PubMed] [Google Scholar]
- 46.Hall JB, Hino GN, Inouye L, Nada A, Lau CK, Read GW. Antimicrobial action of compound 48/80--II mechanism of action. Biochem Pharmacol. 1983;32(3):449–453. doi: 10.1016/0006-2952(83)90522-1. [DOI] [PubMed] [Google Scholar]
- 47.Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 48.Gwinn WM, Kirwan SM, Wang SH, Ashcraft KA, Sparks NL, Doil CR, Tlusty TG, Casey LS, Hollingshead SK, Briles DE, Dondero RS, Hickey AJ, Foster WM, Staats HF. Effective induction of protective systemic immunity with nasally administered vaccines adjuvanted with IL-1. Vaccine. 28(42):6901–6914. doi: 10.1016/j.vaccine.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, Soroka SD, Taylor TH, Jr., Stamey KL, Stinson KW, Freeman AE, Abramson DR, Desai R, Cronin LX, Oxford JW, Caba J, Pleatman C, Pathak S, Schmidt DS, Semenova VA, Martin SK, Wilkins PP, Quinn CP. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J Immunol Methods. 2008;333(1-2):89–106. doi: 10.1016/j.jim.2008.01.007. [DOI] [PubMed] [Google Scholar]