Abstract
Nephrolithiasis remains a major health problem in Western countries. Seventy to 80% of kidney stones are composed of calcium oxalate, and small changes in urinary oxalate affect risk of kidney stone formation. Intestinal oxalate secretion mediated by the anion exchanger SLC26A6 plays an essential role in preventing hyperoxaluria and calcium oxalate nephrolithiasis, indicating that understanding the mechanisms regulating intestinal oxalate transport is critical for management of hyperoxaluria. Purinergic signaling modulates several intestinal processes through pathways including PKC activation, which we previously found to inhibit Slc26a6 activity in mouse duodenal tissue. We therefore examined whether purinergic stimulation with ATP and UTP affects oxalate transport by human intestinal Caco-2-BBe (C2) cells. We measured [14C]oxalate uptake in the presence of an outward Cl− gradient as an assay of Cl−/oxalate exchange activity, ≥50% of which is mediated by SLC26A6. We found that ATP and UTP significantly inhibited oxalate transport by C2 cells, an effect blocked by the PKC inhibitor Gö-6983. Utilizing pharmacological agonists and antagonists, as well as PKC-δ knockdown studies, we observed that ATP inhibits oxalate transport through the P2Y2 receptor, PLC, and PKC-δ. Biotinylation studies showed that ATP inhibits oxalate transport by lowering SLC26A6 surface expression. These findings are of potential relevance to pathophysiology of inflammatory bowel disease-associated hyperoxaluria, where supraphysiological levels of ATP/UTP are expected and overexpression of the P2Y2 receptor has been reported. We conclude that ATP and UTP inhibit oxalate transport by lowering SLC26A6 surface expression in C2 cells through signaling pathways including the P2Y2 purinergic receptor, PLC, and PKC-δ.
Keywords: SLC26A6, PKC-δ, P2Y2 purinergic receptor, phospholipase C
after hypertension, kidney stone disease is the second-most-prevalent kidney disease in the United States. It is a major source of patient discomfort and disability, lost working days, and health-care expenditure, with an annual economic cost exceeding $2 billion. A history of a single kidney stone was recently shown to be associated with a significantly increased risk of poor renal outcome, including advanced chronic kidney disease and end-stage renal disease (1). Chronic kidney disease and end-stage renal disease are associated with remarkably higher long-term morbidity and mortality and tremendous economic burden. Seventy to 80% of kidney stones are composed of calcium oxalate (5), and mild hyperoxaluria is frequently seen in patients with recurrent calcium oxalate kidney stones (75). Minor changes in urinary oxalate levels affect the risk for stone formation, and urinary oxalate is a critical determinant of the level of calcium oxalate supersaturation (75). In addition, in men with recurrent calcium oxalate kidney stones, the amount and size of calcium oxalate crystals, as well as the severity of the disease, were shown to be highly related to their urinary oxalate excretion (75).
The mammalian intestine plays a crucial role in oxalate homeostasis by serving as a site for dietary oxalate absorption as well as an avenue, together with the kidneys, for oxalate excretion. Dietary intake of oxalate, net intestinal absorption, endogenous production, and renal clearance influence the amount of oxalate excreted in the urine. Intestinal oxalate transport takes place passively through the paracellular pathway or actively through the transcellular (transepithelial) pathway (41). The latter is mediated by apical and basolateral integral membrane proteins (anion exchangers) in intestinal cells and is of significant importance, as it is potentially regulated (41).
The essential role of the mammalian intestine in oxalate homeostasis is exemplified by the following observations. Hyperoxaluria and a high incidence of kidney stone formation are commonly seen in patients with inflammatory bowel disease (IBD) (17). Hyperoxaluria is also emerging as a major complication (developing in >50% of patients) following bariatric surgery for obesity (66). Moreover, mice lacking the anion exchanger Slc26a6 have a critical defect in intestinal oxalate secretion, resulting in enhanced net absorption of ingested oxalate, hyperoxalemia, hyperoxaluria, and a high incidence of calcium oxalate kidney stones (32, 45). Thus, Slc26a6-mediated intestinal oxalate secretion plays a major constitutive role in limiting net absorption of ingested oxalate, thereby preventing hyperoxaluria and calcium oxalate nephrolithiasis, indicating that defects in the function or regulation of this key transporter are potential molecular mechanisms predisposing to calcium oxalate nephrolithiasis in humans. Therefore, understanding the molecular mechanisms regulating SLC26A6 (and, hence, overall intestinal oxalate transport) is potentially very important for the management of hyperoxaluria and calcium oxalate nephrolithiasis. Better understanding of the physiological processes regulating intestinal oxalate transport, which are largely unknown, could yield valuable information that may lead to the design of new effective therapeutic approaches for prevention and/or treatment of hyperoxaluria and related calcium oxalate kidney stones.
Luminal extracellular nucleotides such as ATP and UTP have been shown to play an important role in the regulation of several intestinal processes (56) through pathways including PKC activation (12), which we previously found to negatively regulate Slc26a6 activity in mouse duodenal tissue under physiological conditions (39). Sustained supraphysiological levels of luminal extracellular nucleotides are highly expected to be seen under conditions of inflammation such as IBD [a disease associated with hyperoxaluria and a 10- to 100-fold increased risk of kidney stone formation (69)], where overexpression of the P2Y2 and P2Y6 purinergic receptors has been reported (36). In addition, many gut bacteria are known to alter intestinal oxalate transport through unknown mechanisms (40), which might involve extracellular nucleotides, given the fact that several bacteria enhance their survival by modulating nucleotide release and subsequent signaling by host cells (21). We therefore examined whether luminal ATP and UTP affect DIDS-sensitive Cl−/oxalate exchange activity, ≥50% of which is mediated by SLC26A6, in human intestinal Caco-2-BBe (C2) cells. We have found that ATP and UTP negatively regulate oxalate transport by lowering SLC26A6 surface expression in C2 cells through signaling pathways including the P2Y2 receptor, PLC, and PKC-δ.
MATERIALS AND METHODS
Cell culture.
Human intestinal (C2) cells (kindly provided by Dr. Jerrold Turner, University of Chicago) were grown under standard conditions in high-glucose DMEM supplemented with 10% FBS, 50 U/ml penicillin, and 50 mg/ml streptomycin at 37°C in 5% CO2-95% air. T84 cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in DMEM-Ham' F-12 culture medium supplemented with 5–10% FBS, 50 U/ml penicillin, and 50 mg/ml streptomycin at 37°C in 5% CO2-95% air. The oxalate flux and other studies described below were performed using confluent cells grown for 5–13 days postplating on 24-well plastic supports and/or on 0.4-μm collagen-coated polystyrene Transwell membrane filters (Corning, Corning, NY) in 12- and/or 24-mm inserts after overnight serum starvation (11, 13, 38, 76). C2 cells were similarly grown on 0.4-μm collagen-coated 12-mm Snapwell inserts (Corning) for the Ussing chamber studies. The transepithelial resistance (TER) was measured using an epithelial voltohmmeter (EVOM2, World Precision Instruments) to monitor the maturation of the monolayers grown on Transwell permeable supports. We also assessed villin protein expression (38), a marker of epithelial maturation (4, 30), to monitor differentiation of the monolayers. This was done by harvesting total protein lysates from C2 cells on days 1, 2, 3, 4, 5, 6, 8, 10, 12, and 14 postplating for determination of villin protein expression using immunoblotting. We noted that villin expression increased progressively from day 1 through day 5 and then remained stable through day 14 (i.e., we saw identical amounts of villin on days 5–14; data not shown). Equal loading is ensured by probing the lower half of the same gel with an anti-GAPDH antibody and observing identical protein expression on days 1–14. Villin plays a critical role in the maintenance of the enterocyte brush border architecture, and stable suppression of endogenous villin expression in Caco-2 cells remarkably alters brush border assembly (6, 22). In addition, the presence of villin correlates with the existence of a well-organized enterocyte brush border, as previously suggested (30).
Radioactive flux studies.
C2 and T84 cells were grown as described above. After aspiration of the culture medium, the cells were incubated in a Cl−-free solution (38) (in mM: 130 potassium gluconate, 5 glucose, and 20 HEPES, pH 7.4) containing 20 μM [14C]oxalate for 6 min. This 6-min period was based on our finding that it falls within the linear range of oxalate uptake by these cells. Uptake of [14C]oxalate was terminated by two to three rapid washes of the cell monolayers with ice-cold Cl−-free solution. The cells were then solubilized in 0.2 N NaOH and neutralized with an equivalent amount of 0.2 N HCl. The solubilized cells were transferred to vials with scintillation fluid (Opti-Fluor, Packard), and the radioactivity was measured by scintillation spectrometry. Flux studies on cells grown on Transwell inserts were performed in a manner similar to that described above, with the flux solution added to the apical side (top) of the insert for assessment of unidirectional apical influx. The influx of [14C]oxalate was terminated by two to three rapid washes of the cell monolayers with ice-cold Cl−-free solution, and the insert was then placed upside-down and allowed to dry for several minutes. Membrane filters containing the cells were cut from the support and placed into vials with scintillation fluid, and radioactivity was similarly measured after overnight solubilization. The presence of the filter in the counting vial did not have a significant effect on percent quench of 14C scintillation. The flux data are presented as a percentage of the respective control value, and the absolute magnitudes of oxalate fluxes, represented by “controls” of 100%, are provided. The oxalate flux studies were performed using confluent C2 cells of different passages grown for 5–13 days after plating. Although percent changes due to regulatory effects were very consistent, with small errors, baseline transport varied due to experiment-to-experiment variation in plating conditions.
PKC-δ knockdown in C2 cells.
A PKC-δ-specific small interfering RNA (siRNA) oligonucleotide (Santa Cruz Biotechnology, Santa Cruz, CA) was used to knock down PKC-δ expression in C2 cells (84). A scrambled artificial sequence oligonucleotide was used as a negative control siRNA. C2 cells were grown as described above, and transfection of cells was begun 60 h before harvest, as previously reported (63). PKC-δ or control siRNA oligonucleotide was incubated in Opti-MEM (Life Technologies; 4 μl of a 10 μM stock solution in 100 μl of Opti-MEM) for 10 min. The lipid transfection reagent siLentFect (Bio-Rad Laboratories, Hercules, CA) was also incubated for 10 min in Opti-MEM (4 μl/100 μl). After 10 min, the two solutions were mixed to allow complex formation and allowed to sit for 10–15 min. The complete medium was replaced with 250 μl of Opti-MEM per well of 24-well plastic plates 30 min before addition of 50 μl of the complexed solution per well. The cells were allowed to incubate at 37°C for 3 h to allow for maximal uptake of siRNA; then medium containing 10% (vol/vol) FBS was reintroduced to bring the volume to 1 ml. To achieve sufficient knockdown of PKC-δ protein, this transfection procedure was repeated 12 h prior to experimental use (63). PKC-δ protein knockdown was assessed by immunoblotting.
SDS-PAGE and Western blotting.
C2 cells were scraped and lysed in lysis buffer (150 mM NaCl, 50 mM Tris·HCl, 5 mM EDTA, 50 mM sodium fluoride, 15 mM sodium pyrophosphate, 0.5% sodium deoxycholate, 1% Triton X-100, and 0.1% SDS, pH 7.4) supplemented with Complete Protease Inhibitor Cocktail (Roche Diagnostics). The lysate was centrifuged (14,000 g, 4°C, 10 min), and the supernatant was saved for gel electrophoresis. Total protein levels were assessed, and equal amounts of protein lysates were separated by SDS-PAGE using 7.5% polyacrylamide gels and then electrotransferred to polyvinylidene difluoride (Immobilon-P, Millipore). All immunoblots were stained with Ponceau S solution [0.1% Ponceau S (wt/vol) in 5% acetic acid (vol/vol)] following transfer. For Western blotting and blocking of nonspecific binding, polyvinylidene difluoride strips were first incubated in Blotto (5% nonfat dry milk and 0.1% Tween 20 in PBS) for 1 h. Immunoblots were incubated overnight at 4°C with anti-PKC-δ antibody (catalog no. sc-937-G, Santa Cruz Biotechnology; 1:400 dilution), anti-SLC26A6 antibody (catalog no. sc-26728, Santa Cruz Biotechnology; 1:100 dilution), anti-β-actin antibody (catalog no. A1978, Sigma; 1:50,000 dilution), anti-GAPDH antibody (catalog no. sc-32233, Santa Cruz Biotechnology; 1:1,000–5,000 dilution), or anti-P2Y2 antibody (catalog no. sc-15209, Santa Cruz Biotechnology; 1:400 dilution). Then the strips were washed in Blotto and incubated for 1 h with horseradish peroxidase-conjugated secondary antibody (donkey anti-rabbit, anti-mouse, or anti-goat IgG, Jackson Laboratory; 1:10,000 dilution). Antibody reactivity was detected with the enhanced chemiluminescence system (SuperSignal West, Thermo Scientific) according to the manufacturer' protocol.
Surface biotinylation.
C2 cells were grown and serum-starved as described above for the surface biotinylation studies. Cells were treated with vehicle (control) or 100 μM ATP for 30 min in the culture medium and then washed once in ice-cold PBS. C2 cells were subjected to two 30-min incubations in the biotinylation reagent Sulfo-NHS-SS-Biotin (Thermo Scientific; 2 mg/ml) in PBS (38, 39) to biotinylate apical membrane proteins (with all steps performed on ice and/or in a cold room) and washed twice in ice-cold PBS. The cells were then incubated in PBS containing 100 mM glycine for 15 min to quench excess biotin and washed twice with PBS. Total protein lysates were prepared as described above, and normalized samples were incubated overnight with 150–200 μl of streptavidin-agarose beads (Thermo Scientific). Biotinylated proteins were dissociated from the beads with sample buffer (10% SDS, 2% β-mercaptoethanol, 20% glycerol, and 5 mM Tris·HCl, pH 6.8) containing 100 mM DTT and boiled for 2 min. After separation by SDS-PAGE, proteins were transferred to immunoblots and probed with the anti-SLC26A6 antibody, as described above.
Short-circuit current measurement.
C2 cell monolayers, prepared as described above, were mounted in modified Ussing chambers (Physiological Instruments, San Diego, CA) for short-circuit current (Isc) measurement. The mucosal and serosal surfaces of the monolayer were bathed with 4 ml of warmed (37°C) Ringer buffer (140 mM Na+, 119.8 mM Cl−, 5.2 mM K+, 1.2 mM Mg2+, 1.2 mM Ca2+, 25 mM HCO3−, 2.4 mM HPO42−, and 0.4 mM H2PO4−, pH 7.4, gassed with 95% O2-5% CO2) containing 10 mM mannitol or glucose on the mucosal or serosal side, respectively. Transepithelial Isc, TER, and total tissue conductance across monolayers were continuously recorded (at 10-s intervals) using Acquire and Analyze software (Physiological Instruments). After a 15-min equilibration period, vehicle or the Cl− channel blocker CFTR inhibitor-172 (CFTR-172, 10 μM) was added to the mucosal side of matched pairs of monolayers (total tissue conductance ≤20%) for 15 min; then ATP (100 μM) was added to the mucosal side to stimulate Cl− secretion.
Materials.
UTP, U-73343, protein phosphatase type 2 (PP-2), SB-202190, CFTR-172, Gö-6976, and Gö-6983 were purchased from Calbiochem, ATP and DIDS from Sigma, the P2Y2 agonist 2-thiouridine-5′-triphosphate (2-thio-UTP) and the P2Y1 agonist MRS-2365 {[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl} from Tocris Bioscience, and U-73122 from Enzo Life Sciences. PP-2, U-73122, U-73343, CFTR-172, Gö-6983, and SB-202190 were dissolved in DMSO and stored at −20°C. Gö-6976 was dissolved in DMSO and stored at 4°C. DIDS was dissolved in H2O and made fresh prior to use. [14C]oxalate (specific activity 54 mCi/mmol) was purchased from Vitrax. Equivalent volumes of DMSO (0.1–0.2%) or H2O were added to control media. The concentration of each drug was based on published literature (see results), where for some drugs a wide range of concentration has been reported. In addition, we routinely test several concentrations and preincubation times in pilot experiments.
Statistical analysis.
Experimental data are presented as a percentage of the respective control (100%) value and are expressed as means ± SE. Data were analyzed by one-way ANOVA followed by Bonferroni' or Student-Newman-Keuls' post hoc test or by Student' t-test for paired or unpaired samples for comparison of two groups. P < 0.05 was considered statistically significant. The absolute flux values for the comparisons reported in Figs. 1–8 were subjected to statistical analysis utilizing mixed-effects ANOVA and paired t-test (using the experimental day for pairing) and were also found to be significant (data not shown).
Fig. 1.
Effect of ATP on [14C]oxalate uptake by Caco-2-BBE (C2) cells. A: C2 cells grown on Transwell inserts were preincubated apically with vehicle (control) or 100 μM ATP for 30 min in the culture medium, and [14C]oxalate uptake was measured. Values (means ± SE of 5 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 3.95 ± 0.78 pmol·cm−2·min−1). *P < 0.003 vs. control (by 2-tailed t-test). B: C2 cells grown on plastic supports (seeded at the same time and studied concurrently as in A) were preincubated with vehicle (control) or 100 μM ATP for 30 min in the culture medium, and [14C]oxalate uptake was measured. Values (means ± SE of 4 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 9.0 ± 1.08 pmol·cm−2·min−1). *P < 0.02 vs. control (by 2-tailed t-test).
Fig. 8.
PKC-δ knockdown in C2 cells using small interfering RNA (siRNA). A: representative Western blot of PKC-δ protein expression in C2 cell lysate (30 μg protein/lane). UT, untransfected cells; CON, C2 cells transfected with negative control siRNA; PKC-δ, C2 cells transfected with siRNA targeting PKC-δ. Anti-β-actin antibody was used to normalize loading of protein in each lane. B: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values (means ± SE for 4 independent experiments) represent PKC-δ abundance relative to β-actin and are presented as percentage of UT value. *P < 0.001 and P < 0.01 vs. UT and CON, respectively (by ANOVA). C: effect of PKC-δ knockdown on ATP-induced inhibition of [14C]oxalate uptake by C2 cells. C2 cells transfected with negative control siRNA or PKC-δ siRNA were preincubated with vehicle (control) or 100 μM ATP [(control siRNA + ATP) or (PKC-δ siRNA + ATP)] for 30 min in the culture medium, and [14C]oxalate uptake was measured. Values (means ± SE of 6 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 9.13 ± 0.73 pmol·cm−2·min−1). *P < 0.01 and P < 0.05 vs. control and PKC-δ siRNA + ATP, respectively (by ANOVA).
RESULTS
We have used the well-characterized human intestinal C2 cells to examine whether intestinal oxalate transport is subject to regulation by luminal extracellular nucleotides. C2 cells closely resemble the native epithelium, and they express several purinergic receptors (23, 60). C2 cells express SLC26A6 on the apical surface, where ≥50% of Cl−/oxalate exchange has been shown using siRNA knockdown studies, resulting in >60% reduction of its protein expression (33), indicating that SLC26A6 mediates most of the Cl−/oxalate exchange activity in C2 cells. We assessed oxalate uptake by C2 cells by imposing an outward Cl− gradient by removing extracellular Cl− (intracellular Cl− > extracellular Cl−) and measuring DIDS (anion-exchange inhibitor)-sensitive influx of [14C]oxalate in exchange for intracellular Cl− (i.e., Cl−/oxalate exchange activity). We observed that luminal DIDS (100 μM) significantly inhibited apical [14C]oxalate uptake by C2 cells grown on Transwell inserts by >91% (7.51 ± 0.38 and 0.64 ± 0.08 pmol·cm−2·min−1 for control and DIDS, respectively). In addition, DIDS (100 μM) also significantly inhibited [14C]oxalate uptake by C2 cells grown on plastic supports by >93% (10.26 ± 2.07 and 0.69 ± 0.20 pmol·cm−2·min−1 for control and DIDS, respectively). These results strongly indicate that the oxalate uptake by C2 cells is an active transport process mediated by one or more of the involved anion exchanger(s), with SLC26A6 expected to be the main player, as described above (33).
Purinergic signaling modulates many intestinal processes through pathways including PKC activation (12), which we previously demonstrated to inhibit intestinal oxalate transport (38, 39). We therefore examined whether purinergic stimulation with the extracellular nucleotide ATP regulates oxalate transport by C2 cells. As shown in Fig. 1A, apical preincubation of C2 cells grown on Transwell inserts with ATP [100 μM (18, 51)] for 30 min caused significant inhibition of apical [14C]oxalate uptake (by ∼30%). In addition, ATP (100 μM for 30 min) also similarly inhibited (by ∼28%) [14C]oxalate uptake by C2 cells (Fig. 1B) grown on plastic supports (seeded at the same time and studied concurrently as in Fig. 1A). These results indicate that purinergic stimulation with ATP negatively regulates oxalate transport by C2 cells.
Since the response to ATP could be partially mediated by its degradation into adenosine and the activation of adenosine receptors (56), we similarly evaluated the effects of UTP, which is known to mediate its effects by mainly activating the P2Y2 and/or P2Y4 purinergic receptors (88), on oxalate transport by C2 cells. As seen in Fig. 2A, apical preincubation of C2 cells grown on Transwell inserts with UTP [100 μM (18, 51)] led to significant inhibition (by ∼26%) of apical [14C]oxalate uptake. Under the same conditions, UTP had no significant effect on TER (298 ± 26 and 286 ± 5 Ω·cm2, for control and UTP, respectively), indicating that it does not affect the paracellular permeability. UTP also similarly inhibited (by ∼30%) [14C]oxalate uptake by C2 cells grown on plastic supports (Fig. 2B). As ATP and UTP are equipotent (also see Fig. 5) in inhibiting oxalate transport by C2 cells, these results indicate that the ATP-induced inhibition of oxalate transport is most likely mediated by activation of one or more of the known purinergic receptor(s) and that potential degradation of ATP into adenosine and activation of adenosine receptors are unlikely to be contributing. Because we are interested in studying cellular accumulation of [14C]oxalate as a function of SLC26A6 and/or other involved anion exchanger(s) and since ATP and UTP inhibited oxalate transport by C2 cells grown on Transwell inserts or plastic supports to a similar extent, subsequent studies were performed on C2 cells grown on plastic supports (which is more convenient) for assessment of [14C]oxalate uptake, unless otherwise indicated.
Fig. 2.
Effect of UTP on [14C]oxalate uptake by C2 cells. A: C2 cells grown on Transwell inserts were preincubated apically with vehicle (control) or 100 μM UTP for 30 min in the culture medium, and [14C]oxalate uptake was measured. Values (means ± SE of 5 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 2.34 ± 0.19 pmol·cm−2·min−1). *P < 0.002 vs. control (by 2-tailed t-test). B: C2 cells grown on plastic supports were preincubated with vehicle (control) or 100 μM UTP for 30 min in the culture medium, and [14C]oxalate uptake was measured. Values (means ± SE of 5 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 6.84 ± 2.73 pmol·cm−2·min−1). *P < 0.0007 vs. control (by 2-tailed t-test).
Fig. 5.
Effect of P2Y1 and P2Y2 receptor agonists on [14C]oxalate uptake by C2 cells. C2 cells were preincubated with vehicle (control), 100 μM ATP, 100 μM UTP, 1 μM 2-thiouridine-5′-triphosphate (2-thio-UTP, a P2Y2 agonist), or 1 μM MRS-2365 (a P2Y1 agonist) for 30 min in the culture medium, and [14C]oxalate uptake was measured. Values (means ± SE of 5–8 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 3.66 ± 0.41 pmol·cm−2·min−1). *P < 0.001 vs. control (by ANOVA).
To ensure that the effects of extracellular nucleotides on intestinal oxalate transport are not cell line-specific, we also similarly evaluated the effects of ATP on oxalate transport by the human colonic cell line T84, which also expresses different purinergic receptors (81). After reaching confluence, C2 cells exhibit a small intestinal enterocyte-like phenotype (71), while differentiated T84 cells exhibit a colon crypt-like phenotype (27). Using short hairpin RNA knockdown studies, we previously demonstrated that endogenous SLC26A6 mediates most of the oxalate transport by T84 cells (38). As seen in Fig. 3 and similar to our observations in C2 cells, preincubation of T84 cells with ATP (100 μM for 30 min) also significantly inhibited oxalate transport by T84 cells (by ∼30%). In addition, we observed that preincubation of T84 cells with UTP (100 μM for 30 min) also similarly inhibited oxalate transport by T84 cells in two independent experiments (data not shown). These results indicate that the effects of the extracellular nucleotides ATP and UTP on oxalate transport by intestinal epithelial cells are not cell line-specific.
Fig. 3.
Effect of ATP on [14C]oxalate uptake by T84 cells. T84 cells were preincubated with vehicle (control) or 100 μM ATP for 30 min in the culture medium, and [14C]oxalate uptake was measured. Values (means ± SE of 6 independent experiments, each done in triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 3.71 ± 1.3 pmol·cm−2·min−1). *P < 0.002 vs. control (by 2-tailed t-test).
It is most likely that the ATP- and UTP-induced inhibition of Cl−/oxalate exchange activity is due to a direct effect on expression and/or function of the involved anion exchanger(s). However, since ATP and UTP are known to stimulate electrogenic Cl− secretion in intestinal cells (35, 81), they may have accelerated the dissipation of the outwardly directed Cl− gradient driving uptake of [14C]oxalate, thereby accounting for the observed inhibition. To exclude this possibility, we tested the effect of the Cl− channel blocker CFTR-172 [10 μM (2, 38)]. As shown in Fig. 4A, preincubation of C2 cells with CFTR-172 before incubation with ATP (ATP + CFTR-172) had no significant effect on the ATP-induced inhibition of [14C]oxalate uptake by C2 cells (by ∼31%) or on baseline transport. As a positive control, we similarly tested the effect of CFTR-172 on ATP-induced changes in Isc across C2 monolayers under the same conditions (seeded at the same time and studied concurrently as in Fig. 4A) mounted in Ussing chambers. Intestinal cells secrete Cl− and changes in Isc reflect Cl− secretion (15, 52), and ATP is known to stimulate Cl− secretion in T84 cells (28, 83). As shown in Fig. 4B, ATP significantly stimulated Isc across C2 monolayers, and CFTR-172 reduced the ATP-induced Cl− secretion by >58%. The observation that CFTR-172 significantly reduces ATP-induced Cl− secretion but has no effect on ATP inhibition of Cl−/oxalate exchange strongly argues that ATP must directly inhibit the activity of SLC26A6 and/or other involved anion exchanger(s).
Fig. 4.
Effect of CFTR inhibitor-172 (CFTR-172) on ATP-induced inhibition of [14C]oxalate uptake by C2 cells. C2 cells were preincubated with vehicle (control), 100 μM ATP for 30 min, 10 μM CFTR-172 for 15 min followed by 100 μM ATP for 30 min (ATP + CFTR-172), or 10 μM CFTR-172 alone for 15 min, and [14C]oxalate uptake was measured. Values (means ± SE of 4 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 7.40 ± 0.93 pmol·cm−2·min−1). CFTR-172 had no significant effect on ATP-induced inhibition. B: effect of CFTR-172 on ATP-induced Cl− secretion [measured as changes in short-circuit current (ΔIsc)] across C2 cells. C2 cell monolayers (seeded at the same time and studied concurrently as in A) were mounted in Ussing chambers. After a 15-min equilibration period, vehicle (control) or 10 μM CFTR-172 was added to the mucosal side of matched pairs of monolayers for 15 min; then ATP (100 μM) was added to the mucosal side to elicit Cl− secretion. Values (means ± SE of 14 monolayers per group) are presented as peak Isc elicited by ATP. CFTR-172 significantly inhibited ATP-induced Cl− secretion (ΔIsc). *P < 0.001 vs. control (by ANOVA).
Extracellular nucleotides such as ATP and UTP modulate epithelial cell function via the activation of two classes of receptors: G protein-coupled P2Y receptors (1, 2, 4, 6, 11–14), which can mobilize intracellular Ca2+ and activate protein kinases and phosphatases, and P2X receptors (1–7), which have intrinsic activities of ion channels (85). As indicated above, UTP mediates its effects on target cells by mainly activating the P2Y2 and/or P2Y4 purinergic receptors (48). Our observation that ATP and UTP are equipotent in inhibiting oxalate transport in C2 cells suggests a role for the P2Y2, rather than the P2Y4, purinergic receptors, given the fact that the P2Y2 and the P2Y4 receptors respond to agonists with the following order of potency: UTP = ATP and UTP >> ATP, respectively (10). To provide additional evidence that the P2Y2 is the involved purinergic receptor, we tested the effect of the potent and selective P2Y2 receptor agonist 2-thio-UTP (44) on oxalate transport by C2 cells. C2 cells were preincubated with 2-thio-UTP (1 μM) for 30 min before measurement of [14C]oxalate uptake. As shown in Fig. 5, 2-thio-UTP caused significant inhibition of [14C]oxalate uptake by C2 cells, an effect that is similar to the effect induced by ATP or UTP (100 μM for 30 min). On the other hand, preincubation of C2 cells with 1 μM P2Y1 receptor agonist MRS-2365 for 30 min had no significant effect on oxalate transport, indicating that the 2-thio-UTP-induced inhibition of oxalate transport is specific and is due to activation of the P2Y2 receptor. These results strongly indicate that ATP and UTP signal through the P2Y2 purinergic receptor to inhibit oxalate transport by C2 cells. mRNA transcripts for several purinergic (P2) receptors, including P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11, have been reported in Caco-2 cells (23, 60). In addition, protein expression for P2Y1 and P2Y2 receptors has been shown immunohistochemically (23). We also verified the expression of the P2Y2 receptor in C2 cells by immunoblotting (data not shown).
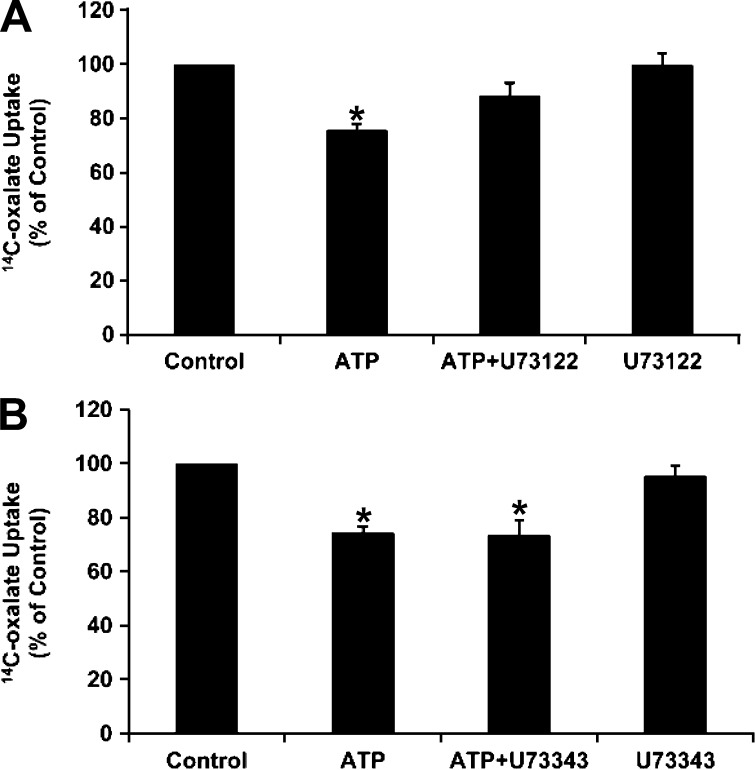
The P2Y2 receptor, a Gq protein-coupled receptor, is linked to activation of PLC (10, 73). We therefore examined whether PLC is involved in the signaling cascade leading to inhibition of oxalate transport by ATP. C2 cells were preincubated with the PLC inhibitor U-73122 [10 μM (7, 38)] before incubation with ATP, and [14C]oxalate uptake was measured. As shown in Fig. 6A, U-73122 significantly reduced the ATP-induced inhibition of [14C]oxalate uptake by C2 cells, whereas it had no significant effect on baseline transport. Importantly, under the same conditions, its inactive analog U-73343 (10 μM) had no significant effect on inhibition of [14C]oxalate uptake or on baseline transport (Fig. 6B). These results indicate that PLC is involved in the signaling cascade leading to inhibition of oxalate transport by ATP.
Fig. 6.
Effect of the PLC inhibitor U-73122 and its inactive analog U-73343 on ATP-induced inhibition of [14C]oxalate uptake by C2 cells. A: C2 cells were preincubated with vehicle (control), 100 μM ATP for 30 min, 10 μM U-73122 for 30 min followed by 100 μM ATP with continued presence of U-73122 (ATP + U-73122) for 30 min, or 10 μM U-73122 alone for 60 min, and [14C]oxalate uptake was measured. Values (means ± SE of 8 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 8.48 ± 1.55 pmol·cm−2·min−1). *P < 0.001 and P < 0.05 vs. control and ATP + U-73122, respectively (by ANOVA). B: C2 cells were preincubated with vehicle (control), 100 μM ATP for 30 min, 10 μM U-73343 for 30 min followed by 100 μM ATP with continued presence of U-73343 (ATP + U-73343) for 30 min, or 10 μM U-73343 alone for 60 min, and [14C]oxalate uptake was measured. Values (means ± SE of 7 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 6.20 ± 0.73 pmol·cm−2·min−1). U-73343 had no significant effect on inhibition induced by ATP. *P < 0.001 vs. control (by ANOVA).
As described above, purinergic signaling modulates several intestinal processes through pathways including PKC (12). To evaluate whether extracellular nucleotides inhibit [14C]oxalate uptake by C2 cells through PKC activation, we tested the effects of the PKC inhibitor Gö-6983 [1 μM (31, 68, 79)] on UTP-induced suppression of [14C]oxalate transport by C2 cells. As seen in Fig. 7, preincubation of C2 cells with Gö-6983 (1 μM for 30 min) before incubation with UTP completely blocked the UTP-induced inhibition of [14C]oxalate uptake by C2 cells, whereas it had no significant effect on baseline transport. Gö-6983 also completely blocked the ATP-induced inhibition of [14C]oxalate uptake by C2 cells (data not shown). On the other hand, the other PKC inhibitor, Gö-6976 [≤2 μM (55, 68, 70, 76)], had no significant effect on the ATP-induced inhibition of [14C]oxalate uptake by C2 cells (data not shown). These results indicate that the ATP/UTP-induced inhibition of [14C]oxalate uptake by C2 cells is due to activation of a Gö-6983-sensitive PKC signaling pathway, similar to previous observations of inhibition of Slc26a6 activity in Xenopus oocytes (39) and endogenous SLC26A6 activity in T84 cells (38) by the PKC activator PMA.
Fig. 7.
Effect of the PKC inhibitor Gö-6983 on UTP-induced inhibition of [14C]oxalate uptake by C2 cells. C2 cells were preincubated with vehicle (control) or 100 μM UTP for 30 min in the culture medium, and [14C]oxalate uptake was measured. [14C]oxalate uptake was also measured in the presence of Gö-6983 (1 μM) for 30 min followed by 100 μM UTP with continued presence of Gö-6983 (UTP + Gö-6983) or 1 μM Gö-6983 alone for 60 min. Values (means ± SE of 6 independent experiments, each done in duplicate or triplicate) were normalized to the respective control value ([14C]oxalate uptake rate = 2.61 ± 0.50 pmol·cm−2·min−1). Gö-6983 completely and significantly reduced the inhibition induced by UTP: *P < 0.01 and P < 0.05 vs. control and UTP + Gö-6983, respectively (by ANOVA).
Analysis of the sensitivity profiles of Gö-6983 and Gö-6976, as we previously reported (38, 39), suggests a potential role for PKC-δ in the ATP/UTP-induced inhibition of [14C]oxalate transport by C2 cells. In addition, using the relatively selective PKC-δ inhibitor rottlerin and PKC translocation studies, we recently demonstrated that PMA and the cholinergic receptor agonist carbachol inhibit SLC26A6 activity in T84 cells through PKC-δ activation (38). To evaluate whether extracellular nucleotides negatively regulate [14C]oxalate uptake by C2 cells through PKC-δ activation, PKC-δ expression in C2 cells was knocked down using siRNA. C2 cells were untransfected or transfected with a negative control siRNA or a PKC-δ-specific siRNA. A representative immunoblot is shown in Fig. 8A, and the scanned data from four experiments are presented in Fig. 8B. The data are shown as the ratio of the densitometry of the PKC-δ protein band to the β-actin protein band from the same lane of the same gel and then presented as a percentage of the untransfected (100%) value. As shown in Fig. 8, A and B, the siRNA targeting PKC-δ reduced PKC-δ protein expression by ∼62% compared with untransfected cells and cells transfected with the control siRNA. Equal loading was verified by probing the lower half of the same blot with an anti-β-actin antibody and observing no significant difference (Fig. 8A). Interestingly, and as seen in Fig. 8C, silencing PKC-δ significantly reduced the ATP-induced inhibition of [14C]oxalate uptake by C2 cells. These results provide direct evidence that extracellular nucleotides negatively regulate [14C]oxalate uptake by C2 cells through PKC-δ activation.
ATP (acting via P2Y receptors) activates the MAPKs ERK1/2 and p38 in Caco-2 cells through signaling pathways involving Src family tyrosine kinases, PKC, and PKA (12). To evaluate whether one or more of these signaling pathways might be involved in the ATP-induced inhibition of oxalate transport by C2 cells, C2 cells were preincubated with the PKA inhibitor H-89 [10–40 μM (2, 65, 82)], the ERK1/2 inhibitor U-0126 [10–20 μM (90)], the p38 inhibitor SB-202190 [10 μM (72)], and the specific Src family kinase inhibitor PP-2 [10 μM (19, 38)] before incubation with ATP, and [14C]oxalate uptake was measured. We observed that these inhibitors [used at concentrations and for time periods known to effectively block these signaling pathways in Caco-2 and T84 intestinal cells (2, 19, 82, 90); 3 independent experiments for each inhibitor] had no significant effect on the ATP-induced inhibition of oxalate transport by C2 cells (data not shown). These findings indicate that the MAPKs ERK1/2 and p38, PKA, and Src family tyrosine kinases are unlikely to be involved in the ATP-induced signal cascade leading to inhibition of [14C]oxalate uptake by C2 cells.
To elucidate the molecular mechanism(s) underlying purinergic-mediated inhibition of oxalate transport by C2 cells, we carried out surface biotinylation studies to examine whether the inhibitory regulation is due to ATP/UTP-induced reduction in surface expression of SLC26A6, as previously seen with PKC and carbachol inhibition of Slc26a6 expressed in Xenopus oocytes and endogenous SLC26A6 in T84 cells, respectively (38, 39). Control or ATP-treated cells were incubated with the surface biotinylation reagent Sulfo-NHS-SS-Biotin, biotinylated proteins were precipitated with streptavidin, and immunoblots were prepared and probed with an anti-SLC26A6 antibody to assess surface SLC26A6 expression. In addition, immunoblots of C2 cell lysates were prepared and probed to assess total SLC26A6 expression. A representative immunoblot is shown in Fig. 9A, and the scanned data from cells grown on plastic supports and Transwell inserts are presented in Fig. 9, B and C. The data are shown as the ratio of the densitometry of the total SLC26A6 protein band to the respective GAPDH protein band from the same lane of the same gel and then presented as a percentage of the control (100%) value. The total SLC26A6, when normalized to GAPDH, did not change and, therefore, can be used to normalize the biotinylated SLC26A6. Biotinylated SLC26A6 was normalized to the total SLC26A6 in the cell lysate under each condition and then presented as a percentage of the control (100%) value. As seen in Fig. 9, ATP caused a significant decrease in the amount of surface SLC26A6 protein available to biotinylation in C2 cells grown on plastic supports or Transwell inserts, which is in general agreement with the ATP-induced inhibition of oxalate transport by C2 cells. However, ATP had no significant effect on total SLC26A6 abundance in cell lysate. Equal loading was verified by probing the lower half of the same blot with an anti-GAPDH antibody and observing no significant difference (Fig. 9A). Collectively, these results strongly support redistribution of SLC26A6 from the surface membrane without a change in total protein expression as the molecular mechanism underlying purinergic-mediated inhibition of oxalate transport by C2 cells.
Fig. 9.
Effect of ATP on SLC26A6 (A6) protein expression. A: representative Western blot of total and surface biotinylated SLC26A6. C2 cells were preincubated with vehicle (CON) or 100 μM ATP for 30 min, and SLC26A6 protein expression was evaluated in cell lysate [total (50 μg protein/lane)] and after streptavidin precipitation of surface biotinylated proteins from 2,000 μg of initial cell lysate (surface). Anti-GAPDH antibody was used to verify equal loading of protein in each lane. B and C: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values (means ± SE for 5 independent experiments for cells grown on plastic supports and 6 independent experiments for cells grown on Transwell inserts) represent total SLC26A6 abundance relative to GAPDH and surface biotinylated SLC26A6 relative to total SLC26A6 and are presented as a percentage of the respective control value. *P < 0.003 vs. CON (by paired t-test).
DISCUSSION
We used the human intestinal epithelial cell line C2 as a model in this study to examine whether luminal ATP and UTP affect intestinal oxalate transport. We measured DIDS-sensitive [14C]oxalate uptake in the presence of an outward Cl− gradient as an assay of Cl−/oxalate exchange activity in C2 cells, ≥50% of which is mediated by SLC26A6 (33). SLC26A6 operates in the direction of exchanging intracellular oxalate for mucosal Cl− during transepithelial oxalate secretion. However, SLC26A6 can operate in either direction (46); therefore, we measured its activity by the more convenient assay of cellular oxalate uptake. We found that [14C]oxalate uptake by C2 cells is negatively regulated by ATP and UTP. The observation that ATP and UTP are equipotent in inhibiting oxalate transport, complemented with experiments using the potent and selective P2Y2 receptor agonist 2-thio-UTP, strongly indicates that ATP and UTP negatively regulate [14C]oxalate uptake by C2 cells, likely through stimulation of the P2Y2 purinergic receptor. Utilizing pharmacological inhibitors as well as PKC-δ silencing studies, we demonstrated that ATP signals through activation of PLC and PKC-δ to suppress [14C]oxalate uptake by C2 cells. Moreover, using biotinylation studies to assess SLC26A6 localization, we showed reduction in surface membrane expression of the transporter to be the molecular mechanism underlying purinergic-mediated inhibition of oxalate transport by C2 cells.
Extracellular nucleotides such as ATP and UTP signal through ubiquitously expressed membrane-associated purinergic receptors in an autocrine or paracrine fashion (10). They have been shown to play an important role in the regulation of epithelial ion transport (56). Sources for intestinal extracellular nucleotides include lysis of apoptotic enterocytes, intestinal bacterial invasion and metabolism, and mechanical stimulation by food, water, and stool (53). Enterocytes could also secrete nucleotides onto the luminal surface in a regulated nonlytic way (53). In addition, epithelial cells are able to release nucleotides in response to physiological stimuli such as membrane stress, changes in cell volume, and receptor stimulation (21). The release of extracellular nucleotides has been shown to occur preferentially onto the luminal side of epithelial cells (56). The P2Y2 purinergic receptor is a prominent luminal receptor in several epithelia including the intestine (56). Luminal extracellular nucleotides were shown to stimulate Cl−, K+, and HCO3− secretion and to inhibit epithelial Na+ channel-mediated Na+ absorption in the gastrointestinal tract (56). We now find that purinergic stimulation with ATP and UTP inhibits SLC26A6-mediated Cl−/oxalate exchange activity in C2 cells by reducing SLC26A6 surface membrane expression through signaling pathways including the P2Y2 receptor, PLC, and PKC-δ. Since absolute selectivity is always an issue with pharmacological agonists and antagonists, our relevant findings should be interpreted with caution. Given the fact that C2 cells are highly polarized and exhibit several functional properties resembling the native epithelium, including cell surface receptors and carrier-mediated transport systems (62, 71), we anticipate that similar regulation of oxalate transport by purinergic stimulation is likely to be active in native tissues in vivo. Since extracellular nucleotides are rapidly degraded (ATP → ADP → AMP → adenosine) by ectonucleotidases (56), it will be of significant physiological interest to examine in future studies whether intestinal oxalate transport is subject to regulation by one or more of these degradation products.
IBD patients have a 10- to 100-fold increased risk of kidney stones (69), with enteric hyperoxaluria, which is commonly seen in these patients (9, 17, 43), being the major risk factor. This hyperoxaluria has been largely attributed to malabsorption of fat and bile acids (16). Malabsorption occurs in Crohn' disease, but not in ulcerative colitis (UC); however, hyperoxaluria is also seen in UC (34). Therefore, malabsorption might not be the only important risk factor predisposing to hyperoxaluria and kidney stones in IBD, and it is possible that other mechanism(s) for the hyperoxaluria must be operative. As described above, Slc26a6-mediated intestinal oxalate secretion plays a major constitutive role in limiting net absorption of ingested oxalate, thereby preventing hyperoxaluria and calcium oxalate nephrolithiasis (45). A role for extracellular nucleotides in the regulation of intestinal oxalate transport had not been recognized previously. Our finding that ATP and UTP negatively regulate SLC26A6-mediated Cl−/oxalate exchange activity in C2 cells suggests a potential role for purinergic signaling in the regulation of oxalate homeostasis, as explained below. Large amounts of extracellular nucleotides are rapidly released into the extracellular environment (i.e., intestinal lumen in the setting of IBD) at sites of inflammation due to cell and tissue damage, stress factors such as hypoxia, and low pH (29, 58). In addition to intestinal epithelial cells, other cell types, such as activated leukocytes, platelets, and smooth muscle cells, also secrete nucleotides in IBD (29, 58). For example, a migrating neutrophil releases ATP from its leading edge, with 1,000-fold-higher ATP levels during inflammation (20, 49, 57). Therefore, it is highly possible that, as a result of being released from these multiple sources, sustained supraphysiological levels of luminal nucleotides could be seen in conditions associated with chronic inflammation such as IBD. Since the intracellular ATP levels are ∼5–10 mM (77), it is possible that relatively little cellular ATP must be released to reach a concentration significant enough to stimulate the purinergic receptors. Of remarkable interest in this regard is that the expression of the P2Y2 and P2Y6 purinergic receptors is significantly higher in colonic tissues isolated from a mouse model of colitis and from humans with IBD (i.e., patients with Crohn' disease and UC) (36). In view of our finding that ATP and UTP act through the P2Y2 receptor to inhibit SLC26A6-mediated oxalate transport by C2 cells, it is tempting to speculate that signaling through the overexpressed P2Y2 receptor might lead to sustained ATP/UTP-induced inhibition of SLC26A6-mediated intestinal oxalate secretion, thereby potentially contributing to the reported enteric hyperoxaluria and high incidence of related kidney stones in patients with IBD. Significantly reduced SLC26A6 mRNA expression was recently reported in a mouse model for mild ileocolonic inflammation (89). However, this study (89) did not examine SLC26A6 protein expression to see if there was an associated reduced protein expression. In addition, enteric hyperoxaluria has also been reported in the setting of chronic mesenteric ischemia (14), a situation where hypoxia can lead to sustained release of nucleotides, which might potentially contribute to the hyperoxaluria by inhibiting SLC26A6-mediated intestinal oxalate secretion.
As indicated above, bacterial metabolism and overgrowth within the intestinal lumen are among the sources of luminal ATP/UTP, which through stimulation of colonic K+ secretion and inhibition of Na+ absorption, as well as activation of small intestinal Cl− secretion, would lead to accumulation of large amounts of luminal fluid. This associated diarrhea response could serve as an epithelial defense mechanism by flushing away the bacteria-derived luminal irritant (56). Some pathogens strengthen their survival through modulation of extracellular nucleotide release and subsequent signaling by host cells (21). During infection with respiratory syncytial virus, the clearance of fluid by the bronchoalveolar epithelium was shown to be blocked by a mechanism involving respiratory syncytial virus-induced nucleotide release and subsequent stimulation of host P2Y receptors (25). It is possible that some pathogens in the gastrointestinal tract might use similar mechanisms, potentially leading to increased luminal nucleotides, which could inhibit SLC26A6-mediated intestinal oxalate secretion and predispose to hyperoxaluria in settings such as chronic infection and/or inflammation (e.g., IBD), as indicated above. Several gut bacteria, including Oxalobacter formigenes, Lactobacillus acidophilus, Bifidobacterium lactis, Eubacterium lentum, Enterococcus faecalis, and Providencia rettgeri, are capable of degrading intraluminal dietary oxalate (42). O. formigenes plays an important role in preventing recurrent calcium oxalate nephrolithiasis in animal and human studies (24, 78). In addition to degrading intraluminal dietary oxalate, O. formigenes also interacts with colonic epithelium by inducing enteric oxalate secretion and, hence, reduces urinary excretion via a potential secretagogue, which also increases net Cl− secretion (40). This is probably a survival strategy utilized by O. formigenes when oxalate, its sole energy source (required for ATP generation), is limited in the diet, as previously reported (40). However, the identity of this secretagogue remains unknown. Binding of flagellin of the gram-negative bacterium Pseudomonas aeruginosa to host cells was shown to induce cellular release of ATP (61). By releasing ATP and stimulating purinergic receptors (leading to MAPK activation), luminal exposure to flagellin was shown to inhibit epithelial Na+ channel-mediated Na+ absorption in mouse airways (10, 50). Since O. formigenes is an anaerobic gram-negative bacterium, it may similarly interact with host cells (colonocytes) by one or more of its components (perhaps flagellin), leading to cellular release of ATP. Although ATP is known to stimulate electrogenic colonic Cl− secretion (as observed with the secretagogue) (35, 81), it is unlikely that this potential secretagogue would be ATP, since we observed that ATP inhibits, rather than stimulates, SLC26A6-mediated oxalate transport by C2 cells.
Since activation of most purinergic receptors increases intracellular Ca2+ (8, 56), downstream stimulation of Ca2+-activated Cl− channels, rather than the cAMP-activated CFTR Cl− channel, would be expected. However, a role for CFTR in Ca2+-dependent purinergic-mediated Cl− secretion has recently been shown in human pancreatic duct epithelial cells (86). In addition, most of the UTP-induced Cl− secretion in primary cultures of human bronchial epithelial cells was shown to be mediated by CFTR by a mechanism involving Ca2+ activation of adenylyl cyclase I and cAMP/PKA signaling (64). Moreover, a role for CFTR in purinergic-mediated intestinal Cl− secretion is supported by the observation that UTP- and forskolin-induced jejunal Cl− secretion was decreased by >51% and 57%, respectively, in mice homozygous for the ΔF508 mutation of CFTR compared with wild-type littermates (35), as well as the fact that the jejunal Cl− secretory response to apical UTP was remarkably impaired in CFTR-null mice (54). Several potential mechanisms by which Ca2+-mobilizing agents (e.g., ATP and UTP) could stimulate CFTR have been reported (54, 64, 86), including PKC or PKG activation and prostaglandin-induced activation of adenylate cyclase-coupled prostaglandin E2 receptors. Caco-2 cells express CFTR (26, 67, 87); however, CFTR expression has not been studied in our C2 cells, other than to note effects of CFTR-172.
SLC26A6 is capable of mediating electrogenic Cl−/oxalate exchange (46), which could be affected by changes in membrane potential. Significant ATP-induced Cl− secretion in C2 cells should lead to cellular depolarization, which would be expected to enhance electrogenic Cl−/oxalate exchange in the direction of oxalate uptake by C2 cells. However, luminal ATP and UTP rapidly induce K+ secretion via the P2Y2 receptors and the P2Y2 and P2Y4 receptors in rat distal colon and mouse colon, respectively (47, 59). This rapid K+ secretion could offset any cellular depolarization resulting from ATP-induced Cl− secretion; therefore, it is likely possible that no effect or a minimal effect on electrogenic Cl−/oxalate exchange would be seen in this setting in the absence of altered SLC26A6 activity. In addition, O. formigenes promotes enteric oxalate and Cl− secretion, as described above. The cellular depolarization accompanying Cl− secretion should inhibit, rather than stimulate, oxalate secretion by electrogenic Cl−/oxalate exchange. Since colonic secretagogues that trigger CFTR-dependent Cl− secretion at the same time also usually stimulate K+ secretion (37, 74, 80), it is possible that the O. formigenes-derived secretagogue, which promoted colonic oxalate and Cl− secretion, also activates K+ secretion. This could potentially offset any cellular depolarization accompanying Cl− secretion and, therefore, would be unlikely to impact colonic oxalate secretion by itself.
In summary, we have shown that the extracellular nucleotides ATP and UTP negatively regulate oxalate transport by lowering SLC26A6 surface expression in C2 cells through signaling pathways that likely include the P2Y2 purinergic receptor, PLC, and PKC-δ. These findings suggest that purinergic regulation of intestinal oxalate transport might play an important role in overall oxalate homeostasis and, thereby, could affect urinary oxalate excretion and risk of stone formation, including a potential relevance to the pathophysiology of IBD-associated hyperoxaluria and related kidney stones.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K08-DK-067245 (H. A. Hassan) and P30 DK-42086 (Digestive Disease Research Center, University of Chicago).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A., S.S., S.R., and H.A.H. performed the experiments; R.A., S.S., S.R., and H.A.H. analyzed the data; R.A., S.S., S.R., and H.A.H. interpreted the results of the experiments; R.A., S.S., S.R., and H.A.H. drafted the manuscript; R.A., S.S., S.R., and H.A.H. edited and revised the manuscript; R.A., S.S., S.R., and H.A.H. approved the final version of the manuscript; H.A.H. is responsible for conception and design of the research; H.A.H. prepared the figures.
ACKNOWLEDGMENTS
We thank Dr. Eugene Chang (Section of Gastroenterology, University of Chicago) and Dr. Peter Aronson (Departments of Internal Medicine and Cellular and Molecular Physiology, Yale University School of Medicine) for critical reading of the manuscript and helpful discussions, Kristen Wroblewski (University of Chicago) for help with statistical analysis of the data, and Sohee Jeon, Yong-chul Jung, and Rania Saleh for technical assistance.
REFERENCES
- 1. Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, Klarenbach SW, Curhan GC, Tonelli M, Alberta Kidney Disease Network Kidney stones and kidney function loss: a cohort study. Br Med J 345: e5287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl− secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci 56: 339– 351, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Arpin M, Blair L, Coudrier E, Dudouet B, Finidori J, Carcia A, Huet C, Pringault E, Robine S, Sahuguillo-Merino C, et al. Villin, a specific marker for some epithelia specialized in transport, to study the differentiation of intestinal and kidney cells in vivo and in a human colon adenocarcinoma line HT29 in culture. Mol Aspects Med 10: 257– 272, 1988 [DOI] [PubMed] [Google Scholar]
- 5. Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am 31: 927– 949, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Athman R, Louvard D, Robine S. The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells? Am J Physiol Gastrointest Liver Physiol 283: G496– G502, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Berna MJ, Hoffmann KM, Tapia JA, Thill M, Pace A, Mantey SA, Jensen RT. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-δ and PKC-independent pathways. Biochim Biophys Acta 1773: 483– 501, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boeynaems JM, Communi D, Gonzalez NS, Robaye B. Overview of the P2 receptors. Semin Thromb Hemostasis 31: 139– 149, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Bohles H, Beifuss OJ, Brandl U, Pichl J, Akcetin Z, Demling L. Urinary factors of kidney stone formation in patients with Crohn' disease. Klin Wochenschr 66: 87– 91, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Bucheimer RE, Linden J. Purinergic regulation of epithelial transport. J Physiol 555: 311– 321, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem 277: 28182– 28190, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Buzzi N, Bilbao PS, Boland R, de Boland AR. Extracellular ATP activates MAP kinase cascades through a P2Y purinergic receptor in the human intestinal Caco-2 cell line. Biochim Biophys Acta 1790: 1651– 1659, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Cammisotto PG, Bendayan M, Sane A, Dominguez M, Garofalo C, Levy E. Receptor-mediated transcytosis of leptin through human intestinal cells in vitro. Int J Cell Biol 2010: 928169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Canos HJ, Hogg GA, Jeffery JR. Oxalate nephropathy due to gastrointestinal disorders. Can Med Assoc J 124: 729– 733, 1981 [PMC free article] [PubMed] [Google Scholar]
- 15. Cartwright CA, McRoberts JA, Mandel KG, Dharmsathaphorn K. Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest 76: 1837– 1842, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caspary WF, Tonissen JI. [Enteric hyperoxaluria. Intestinal oxalate absorption in gastrointestinal diseases (author's transl)]. Klin Wochenschr 56: 607– 615, 1978 [DOI] [PubMed] [Google Scholar]
- 17. Caudarella R, Rizzoli E, Pironi L, Malavolta N, Martelli G, Poggioli G, Gozzetti G, Miglioli M. Renal stone formation in patients with inflammatory bowel disease. Scanning Microsc 7: 371– 380, 1993 [PubMed] [Google Scholar]
- 18. Chang SJ, Tzeng CR, Lee YH, Tai CJ. Extracellular ATP activates the PLC/PKC/ERK signaling pathway through the P2Y2 purinergic receptor leading to the induction of early growth response 1 expression and the inhibition of viability in human endometrial stromal cells. Cell Signal 20: 1248– 1255, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 282: 14– 28, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792– 1795, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3: re1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costa de Beauregard MA, Pringault E, Robine S, Louvard D. Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J 14: 409– 421, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira Souza C, Ojcius DM, Burnstock G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol 288: G1024– G1035, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Daniel SL, Hartman PA, Allison MJ. Microbial degradation of oxalate in the gastrointestinal tracts of rats. Appl Environ Microbiol 53: 1793– 1797, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 286: L112– L120, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Denning GM, Ostedgaard LS, Cheng SH, Smith AE, Welsh MJ. Localization of cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Invest 89: 339– 349, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol Gastrointest Liver Physiol 246: G204– G208, 1984 [DOI] [PubMed] [Google Scholar]
- 28. Dho S, Stewart K, Foskett JK. Purinergic receptor activation of Cl− secretion in T84 cells. Am J Physiol Cell Physiol 262: C67– C74, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97: 587– 600, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Dudouet B, Robine S, Huet C, Sahuquillo-Merino C, Blair L, Coudrier E, Louvard D. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29-18 clones. J Cell Biol 105: 359– 369, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ehre C, Zhu Y, Abdullah LH, Olsen J, Nakayama KI, Nakayama K, Messing RO, Davis CW. nPKCε, a P2Y2-R downstream effector in regulated mucin secretion from airway goblet cells. Am J Physiol Cell Physiol 293: C1445– C1454, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719– G728, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Freel RW, Morozumi M, Hatch M. Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1. Am J Physiol Gastrointest Liver Physiol 297: G918– G929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fukushima T, Ishiguro N, Matsuda Y, Takemura H, Tsuchiya S. Clinical and urinary characteristics of urolithiasis in ulcerative colitis. Am J Gastroenterol 77: 238– 242, 1982 [PubMed] [Google Scholar]
- 35. Ghanem E, Robaye B, Leal T, Leipziger J, Van Driessche W, Beauwens R, Boeynaems JM. The role of epithelial P2Y2 and P2Y4 receptors in the regulation of intestinal chloride secretion. Br J Pharmacol 146: 364– 369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grbic DM, Degagne E, Langlois C, Dupuis AA, Gendron FP. Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol 180: 2659– 2668, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Halm DR, Frizzell RA. Active K transport across rabbit distal colon: relation to Na absorption and Cl secretion. Am J Physiol Cell Physiol 251: C252– C267, 1986 [DOI] [PubMed] [Google Scholar]
- 38. Hassan HA, Cheng M, Aronson PS. Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells. Am J Physiol Cell Physiol 302: C46– C58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hassan HA, Mentone S, Karniski LP, Rajendran VM, Aronson PS. Regulation of anion exchanger Slc26a6 by protein kinase C. Am J Physiol Cell Physiol 292: C1485– C1492, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 69: 691– 698, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res 33: 1– 16, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Hoppe B, von Unruh G, Laube N, Hesse A, Sidhu H. Oxalate degrading bacteria: new treatment option for patients with primary and secondary hyperoxaluria? Urol Res 33: 372– 375, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Hueppelshaeuser R, von Unruh GE, Habbig S, Beck BB, Buderus S, Hesse A, Hoppe B. Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn's disease. Pediatr Nephrol 27: 1103– 1109, 2012 [DOI] [PubMed] [Google Scholar]
- 44. Ivanov AA, Ko H, Cosyn L, Maddileti S, Besada P, Fricks I, Costanzi S, Harden TK, Calenbergh SV, Jacobson KA. Molecular modeling of the human P2Y2 receptor and design of a selective agonist, 2′-amino-2′-deoxy-2-thiouridine 5′-triphosphate. J Med Chem 50: 1166– 1176, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474– 478, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem 277: 33963– 33967, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Kerstan D, Gordjani N, Nitschke R, Greger R, Leipziger J. Luminal ATP induces K+ secretion via a P2Y2 receptor in rat distal colonic mucosa. Pflügers Arch 436: 712– 716, 1998 [DOI] [PubMed] [Google Scholar]
- 48. King BF, Wildman SS, Ziganshina LE, Pintor J, Burnstock G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br J Pharmacol 121: 1445– 1453, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol 294: G401– G410, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Kunzelmann K, Scheidt K, Scharf B, Ousingsawat J, Schreiber R, Wainwright B, McMorran B. Flagellin of Pseudomonas aeruginosa inhibits Na+ transport in airway epithelia. FASEB J 20: 545– 546, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Langlois C, Gendron FP. Promoting MPhi transepithelial migration by stimulating the epithelial cell P2Y2 receptor. Eur J Immunol 39: 2895– 2905, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Lau C, Lytle C, Straus DS, DeFea KA. Apical and basolateral pools of proteinase-activated receptor-2 direct distinct signaling events in the intestinal epithelium. Am J Physiol Cell Physiol 300: C113– C123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785– 795, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Lazarowski ER, Rochelle LG, O'Neal WK, Ribeiro CM, Grubb BR, Zhang V, Harden TK, Boucher RC. Cloning and functional characterization of two murine uridine nucleotide receptors reveal a potential target for correcting ion transport deficiency in cystic fibrosis gallbladder. J Pharmacol Exp Ther 297: 43– 49, 2001 [PubMed] [Google Scholar]
- 55. Lee YM, Li WH, Kim YK, Kim KH, Chung JH. Heat-induced MMP-1 expression is mediated by TRPV1 through PKCα signaling in HaCaT cells. Exp Dermatol 17: 864– 870, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419– F432, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Linden J. Cell biology. Purinergic chemotaxis. Science 314: 1689– 1690, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Luttikhuizen DT, Harmsen MC, de Leij LF, van Luyn MJ. Expression of P2 receptors at sites of chronic inflammation. Cell Tissue Res 317: 289– 298, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol 564: 269– 279, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McAlroy HL, Ahmed S, Day SM, Baines DL, Wong HY, Yip CY, Ko WH, Wilson SM, Collett A. Multiple P2Y receptor subtypes in the apical membranes of polarized epithelial cells. Br J Pharmacol 131: 1651– 1658, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McNamara N, Khong A, McKemy D, Caterina M, Boyer J, Julius D, Basbaum C. ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc Natl Acad Sci USA 98: 9086– 9091, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Merlin D, Steel A, Gewirtz AT, Si-Tahar M, Hediger MA, Madara JL. hPepT1-mediated epithelial transport of bacteria-derived chemotactic peptides enhances neutrophil-epithelial interactions. J Clin Invest 102: 2011– 2018, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Musch MW, Arvans DL, Walsh-Reitz MM, Uchiyama K, Fukuda M, Chang EB. Synaptotagmin I binds intestinal epithelial NHE3 and mediates cAMP- and Ca2+-induced endocytosis by recruitment of AP2 and clathrin. Am J Physiol Gastrointest Liver Physiol 292: G1549– G1558, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Namkung W, Finkbeiner WE, Verkman AS. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell 21: 2639– 2648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nduati V, Yan Y, Dalmasso G, Driss A, Sitaraman S, Merlin D. Leptin transcriptionally enhances peptide transporter (hPepT1) expression and activity via the cAMP-response element-binding protein and Cdx2 transcription factors. J Biol Chem 282: 1359– 1373, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Nelson WK, Houghton SG, Milliner DS, Lieske JC, Sarr MG. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis 1: 481– 485, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Novaira HJ, Ornellas DS, Ortiga-Carvalho TM, Zhang XM, Souza-Menezes J, Guggino SE, Guggino WB, Morales MM. Atrial natriuretic peptide modulates cystic fibrosis transmembrane conductance regulator chloride channel expression in rat proximal colon and human intestinal epithelial cells. J Endocrinol 189: 155– 165, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE, Shin JS. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol 182: 5800– 5809, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol 93: 504– 514, 1998 [DOI] [PubMed] [Google Scholar]
- 70. Pernas-Sueiras O, Alfonso A, Vieytes MR, Botana LM. PKC and cAMP positively modulate alkaline-induced exocytosis in the human mast cell line HMC-1. J Cell Biochem 99: 1651– 1663, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581– 600, 1992 [DOI] [PubMed] [Google Scholar]
- 72. Pham H, Vincenti R, Slice LW. COX-2 promoter activation by AT1R-Gq-PAK-p38β signaling in intestinal epithelial cells. Biochim Biophys Acta 1779: 408– 413, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413– 492, 1998 [PubMed] [Google Scholar]
- 74. Rechkemmer G, Frizzell RA, Halm DR. Active potassium transport across guinea-pig distal colon: action of secretagogues. J Physiol 493: 485– 502, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Robertson WG, Peacock M. The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron 26: 105– 110, 1980 [DOI] [PubMed] [Google Scholar]
- 76. Saksena S, Gill RK, Syed IA, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Inhibition of apical Cl−/OH− exchange activity in Caco-2 cells by phorbol esters is mediated by PKCε. Am J Physiol Cell Physiol 283: C1492– C1500, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 1615: 7– 32, 2003 [DOI] [PubMed] [Google Scholar]
- 78. Sidhu H, Schmidt ME, Cornelius JG, Thamilselvan S, Khan SR, Hesse A, Peck AB. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol 10 Suppl 14: S334– S340, 1999 [PubMed] [Google Scholar]
- 79. Sinnett-Smith J, Jacamo R, Kui R, Wang YM, Young SH, Rey O, Waldron RT, Rozengurt E. Protein kinase D mediates mitogenic signaling by Gq-coupled receptors through protein kinase C-independent regulation of activation loop Ser744 and Ser748 phosphorylation. J Biol Chem 284: 13434– 13445, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Smith PL, McCabe RD. Mechanism and regulation of transcellular potassium transport by the colon. Am J Physiol Gastrointest Liver Physiol 247: G445– G456, 1984 [DOI] [PubMed] [Google Scholar]
- 81. Smitham JE, Barrett KE. Differential effects of apical and basolateral uridine triphosphate on intestinal epithelial chloride secretion. Am J Physiol Cell Physiol 280: C1431– C1439, 2001 [DOI] [PubMed] [Google Scholar]
- 82. Srivastava V, Dey I, Leung P, Chadee K. Prostaglandin E2 modulates IL-8 expression through formation of a multiprotein enhanceosome in human colonic epithelial cells. Eur J Immunol 42: 912– 923, 2012 [DOI] [PubMed] [Google Scholar]
- 83. Stutts MJ, Lazarowski ER, Paradiso AM, Boucher RC. Activation of CFTR Cl− conductance in polarized T84 cells by luminal extracellular ATP. Am J Physiol Cell Physiol 268: C425– C433, 1995 [DOI] [PubMed] [Google Scholar]
- 84. Torgersen ML, Walchli S, Grimmer S, Skanland SS, Sandvig K. Protein kinase Cδ is activated by Shiga toxin and regulates its transport. J Biol Chem 282: 16317– 16328, 2007 [DOI] [PubMed] [Google Scholar]
- 85. von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110: 415– 432, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Wang J, Haanes KA, Novak I. Purinergic regulation of CFTR and Ca2+-activated Cl− channels and K+ channels in human pancreatic duct epithelium. Am J Physiol Cell Physiol 304: C673– C684, 2013 [DOI] [PubMed] [Google Scholar]
- 87. Ward CL, Krouse ME, Gruenert DC, Kopito RR, Wine JJ. Cystic fibrosis gene expression is not correlated with rectifying Cl− channels. Proc Natl Acad Sci USA 88: 5277– 5281, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol 140: 1177– 1186, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xiao F, Juric M, Li J, Riederer B, Yeruva S, Singh AK, Zheng L, Glage S, Kollias G, Dudeja P, Tian DA, Xu G, Zhu J, Bachmann O, Seidler U. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3− secretion in murine ileocolonic inflammation. Inflamm Bowel Dis 18: 101– 111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yoo BK, He P, Lee SJ, Yun CC. Lysophosphatidic acid 5 receptor induces activation of Na+/H+ exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol 301: C1008– C1016, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]