Abstract
Mutations in inversin cause nephronophthisis type II, an autosomal recessive form of polycystic kidney disease associated with situs inversus, dilatation, and kidney cyst formation. Since cyst formation may represent a planar polarity defect, we investigated whether inversin plays a role in cell division. In developing nephrons from inv−/− mouse embryos we observed heterogeneity of nuclear size, increased cell membrane perimeters, cells with double cilia, and increased frequency of binuclear cells. Depletion of inversin by siRNA in cultured mammalian cells leads to an increase in bi- or multinucleated cells. While spindle assembly, contractile ring formation, or furrow ingression appears normal in the absence of inversin, mitotic cell rounding and the underlying rearrangement of the cortical actin cytoskeleton are perturbed. We find that inversin loss causes extensive filopodia formation in both interphase and mitotic cells. These cells also fail to round up in metaphase. The resultant spindle positioning defects lead to asymmetric division plane formation and cell division. In a cell motility assay, fibroblasts isolated from inv−/− mouse embryos migrate at half the speed of wild-type fibroblasts. Together these data suggest that inversin is a regulator of cortical actin required for cell rounding and spindle positioning during mitosis. Furthermore, cell division defects resulting from improper spindle position and perturbed actin organization contribute to altered nephron morphogenesis in the absence of inversin.
Keywords: actin, cytoskeleton, mitosis, mitotic spindle, polycystic kidney disease
inherited polycystic kidney disease (PKD) is a heterogeneous group of genetic diseases characterized by defects in the organization of the tubular epithelium that lead to expansion of the tubular lumen and cyst formation (4, 37). Nephronophthisis is an autosomal recessive form of chronic kidney disease that represents the leading genetic cause for kidney defects in children and young adults. Inversin (INVS) mutations have been linked to an infantile form of nephronophthisis (nephronophthisis type II), and loss of functional inversin in mice and zebrafish leads to the formation of renal cysts (10, 22, 28). Like most renal cyst-associated proteins, inversin has been shown to localize to primary cilia (19, 26, 28). The commonality of cilia localization for cyst-associated proteins supports the view that the common pathology of genetic defects in genes involved in different forms of PKD can be explained by their role in cilia formation or cilium-associated signaling (12, 40). While inversin does not appear to be required for cilia organization (30), a potential role for inversin during Wnt signaling has been reported (36).
The Wnt signaling pathway is an essential developmental pathway that branches into canonical and noncanonical signaling mechanisms. During canonical Wnt signaling, Wnt binding is sensed by cell surface receptors of the Frizzled family which leads to the activation of Disheveled (dsh in Drosophila, dvl in the mouse). This interaction prevents the degradation of β-catenin and induces β-catenin-dependent transcription of downstream TCF/LEF target genes (36). In contrast, during noncanonical Wnt signaling, Dvl signals to downstream effectors from the Rho GTPase family that induces the rearrangement of the actin cytoskeleton (9, 32). This later pathway is required for establishment of proper planar cell polarity (PCP). Remarkably, transgenic overexpression of β-catenin in mice leads to the formation of kidney cysts, supporting a potential role for the Wnt signaling cascade during kidney development and renal cyst formation (31).
Inversin is a member of the large family of ankyrin repeat-containing proteins, with the human isoform harboring 16 tandem ankyrin repeats as well as two D-Box destruction domains and two calmodulin binding IQ domains. Inversin binds to APC2, a component of the APC complex (APC/C) and Dvl. Inversin binding to APC2 is dependent on the first D-Box sequence, leading to APC/C-dependent degradation of Dvl. Furthermore, a patient with a mutation in this D-Box failed to properly degrade Disheveled. These observations led to the proposal that inversin is involved in the regulatory balance of canonical and noncanonical Wnt signaling by targeting a cytoplasmic pool of Disheveled for APC/C-dependent degradation (19, 36). This would place inversin at a central junction of canonical and noncanonical Wnt signaling, suggesting a potential role for inversin in regulating the balance between these two signaling cascades. However, the downstream mechanisms and potential targets of inversin-dependent Wnt signaling are largely unidentified.
We have previously reported that in addition to its association with primary cilia, inversin also localizes to the cell cortex in cultured cell monolayers and forms a complex with N-cadherin and the catenins, suggesting a role for inversin in the formation of cell adhesions (23, 34). Furthermore, inversin also localizes to centrosomes and spindle microtubules in mitotic cells and inversin can bind microtubules in vitro, thus indicating a potential role for inversin during mitosis (19, 26). To test whether inversin is involved in the regulation of mitotic processes, we investigated the role of inversin during mitotic events such as mitotic spindle assembly, contractile ring formation, rearrangement of the actin cytoskeleton and cell division. We find that loss of inversin leads to an increase of multinucleated cells. While contractile ring assembly and spindle organization or microtubule dynamics appear largely unperturbed, loss of inversin leads to a drastic reorganization of the cortical actin cytoskeleton that prevents cell rounding during mitosis and leads to spindle positioning defects.1
MATERIALS AND METHODS
Animal care and housing.
All animals were housed in the vivarium at Indiana University School of Medicine, and all experiments were performed with the approval of the Indiana University Animal Care and Use Committee.
Plasmids and RNAi.
Plasmids for ceRhoA green fluorescent protein (GFP) chimera, anillin-GFP, tubulin-GFP, and mlc-GFP were generously provided by A. Piekny (Concordia University, Montreal, CA). Plasmid containing EB-1 GFP and CLIP-170 GFP were a gift from H. Goodson (University of Notre Dame, South Bend, IN). Plasmid bearing eGFP-inversin chimeric protein was supplied by P. Overbeek (Baylor College of Medicine, Houston, TX). INVS siRNA was designed as suggested by the manufacturer (SASI_Hs01_00111914, Sigma-Aldrich, St. Louis, MO) (targets covered NM_014425.2, NM_183245.1, sense strand-GCACUAUGCUGCUCAGAGUdTdT, antisense strand ACUCUGAGCAGCAUAGUGCdTdT) and examined for possible cross-reactivity using NCBI-BLAST search.
Cell culture and transfection.
HeLa cells or HEK-293 cells were cultured using standard cell culture techniques. siRNA and scrambled siRNA control transfections were carried out using Generaser (Agilent Technologies, Santa Clara, CA) transfection reagent according to the manufacturer' instruction. Cells were washed in PBS 4 h posttransfection and cultured in fresh medium. Subsequently, cells were imaged live or fixed 48–58 h after transfection. For plasmid transfection, Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and Plus reagent (Invitrogen) were used using fivefold less reagent than recommended by the manufacturer for optimal results. When DNA was transfected in conjunction with siRNA treatment, cells were first transfected with siRNA using Generaser, and after 4 h the media were replenished and cells were transfected with DNA using Lipofectamine 2000.
Dermal fibroblasts were isolated from inv−/− pups or wild-type littermates by dissecting the skin from anesthetized pups before euthanasia. Skin was digested with collagenase type 2 (Worthington Biochemical, Freehold, NJ) dissolved in phosphate-buffered saline (PBS) for 30 min at 37°C. Cells were centrifuged and the pellet was suspended in DMEM media supplemented with 10% fetal calf serum. Cells were used between passages 1 and 3 for cell motility experiments.
Fixation and immunofluorescence.
Polyclonal rabbit antibodies directed against inversin were purified as described previously (26). Microtubules were visualized using anti β-tubulin (Sigma-Aldrich), and actin staining was performed using rhodamine-conjugated phalloidin (Invitrogen). Anti-mouse IgG conjugated with Alexa 488 or Alexa 543 (Invitrogen) was used at a concentration of 1:300. Cells were routinely fixed using 3% formaldehyde in cytoskeleton stabilization buffer (80 mM K-PIPES pH 6.8, 2 mM MgCl2, 5 mM EDTA) and reaction was quenched with 100 mM NH4Cl in PBS, pH 7.2. For optimal visualization of microtubules, while retaining acceptable phalloidin staining, pH shift fixation method was used as described (2). In short the aforementioned initial fixation step was followed by a second fixation step with 3% formaldehyde in 100 mM sodium borate; pH 11 followed by two sequential incubations with 1 mg/ml sodium borohydride dissolved in PBS; pH 8. Images were collected with a Nikon Eclipse TE2000 microscope (Nikon Instruments, Melville, NY) equipped for epifluorescence using a Hamamatsu 1394 cooled CCD camera (Hamamatsu, Hamamatsu City, Japan) with a ×60 1.4 numerical aperture (NA) objective or a Zeiss UV LSM-510 Confocal Microscope equipped with a UV Argon Laser (351 nm and 364 nm excitation), a visible Argon laser (458 nm and 488 nm excitation), and two Helium-Neon Lasers (543 nm and 633 nm excitation) (Zeiss, Chester, VA), using a ×60 water objective, 1.45 NA. All images were acquired using the same acquisition setting for all experiments.
Immune blot.
Cell extracts from HEK-293 cells were harvested in 1 × RIPA buffer (25 mM Tris·HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). Total protein concentration was determined using Micro BCA Protein Assay Kit (Pierce Scientific, Rockford, IL), and equal amounts of protein were loaded for control and RNAi-treated samples. Following SDS-PAGE and transfer to nitrocellulose membrane, the membrane was probed with primary antibody at 4°C overnight, washed and probed with horseradish peroxidase-coupled secondary antibodies. HRP detection was carried out using Super Signal West Pico Chemiluminescent substrate (Thermo Fisher Scientific, Pittsburgh, PA). Blots were stripped using Restore Western Blot stripping buffer (Thermo Fisher Scientific) and reprobed with control antibody. Control β-tubulin antibody was used at a concentration of 1:1,000 for all immune blots (Sigma-Aldrich). HRP-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Live cell imaging.
Control or siRNA-treated cells were imaged between 40 h and 72 h posttransfection. Cells were kept at 37°C using a Warner DH-35 dish heater (Warner Instruments, Hamden, CT) and were maintained in DMEM media supplemented with 10 mM HEPES, pH 7.4. The CO2 concentration was ambient atmosphere. Images were acquired using a spinning disk confocal microscope equipped with an Andor iXon air cooled EMCCD camera (South Windsor, CT). Images were acquired every 10 s when imaging mitotic cells. Imaging of EB-1 GFP or CLIP-GFP in interphase cells was carried out by acquiring images every second for high temporal resolution of microtubule dynamics.
Two-photon confocal microscopy of embryonic kidneys.
Kidney sections were blocked with 3% bovine calf serum in PBS for 1 h, followed by a 2-h incubation with a α- and β-tubulin antibody mix in Tris-buffered saline with 0.5% Triton X-100. Hoescht 33342 was used to label DNA and rhodamine-phalloidin was used to label actin (Invitrogen). Images were acquired with either a Zeiss LSM-510 Meta Confocal/Multiphoton Microscope (Zeiss USA) or an Olympus FV1000-MPE Confocal/Multiphoton Microscope.
Image processing and quantification.
Image processing and quantification were carried out using ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD). For each figure, 16-bit images were scaled the same way and transformed into 8-bit images. For all quantification experiments, control or RNAi-treated cells were fixed and stained with rhodamine-phalloidin and α/β-tubulin. For total cell area measurements, the cell membrane of metaphase cells was outlined using an ImageJ freehand tool and the area was then quantified using ImageJ. To assess the extent of spindle elongation, the distance between the two spindle poles was measured. To determine spindle positioning during metaphase, the cell centroid was determined using ImageJ, and the distance between the centroid and the spindle midpoint (center of the axis connecting the two spindle poles) was measured. For spindle positioning during anaphase, the distance between spindle midpoint and cell center (center of the cell along the spindle axis) was measured.
Specimen preparation for electron microscopy.
The specimens were fixed in 2% glutaraldehyde (Polysciences, Warrington, PA) in 0.1 M phosphate buffer. After fixation the specimens were rinsed with PBS followed by postfixation with 1% osmium tetroxide in phosphate buffer for 1 h. After rinsing again with PBS the tissue specimens were dehydrated through a series of graded ethyl alcohols from 70 to 100%. After dehydration, specimens were infiltrated with two changes of 100% propylene oxide (PO) and a 50:50 mixture of PO and the embedding resin (Embed 812, Electron Microscopy Sciences, Hatfield, PA) overnight. Specimens were transferred to fresh 100% embedding media under vacuum for 6–8 h then embedded in a fresh change of 100% embedding media. Following polymerization overnight at 60°C, blocks were sectioned. Thin sections were cut (70–80 nm), stained with uranyl acetate, and viewed on a Tecnai BioTwin (FEI, Hillsboro, OR), and digital images were taken with an AMT (Advanced Microscope Techniques, Danvers, MA) CCD camera. For scanning electron microscopy (SEM) studies, tissue segments were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, postfixed in osmium tetroxide, dehydrated in a graded series of ethanol, critical point dried using a Tousimis Samdri 790 critical point dryer (Rockville MD), mounted on stubs, and sputter coated with Au/Pd using a Polaron Sputter Coater (Energy Beam Sciences, East Gramby, CT) before viewing on a JEOL 6390 SEM (JEOL USA, Peabody, MA).
Cell motility assay.
Dermal fibroblasts were grown to confluence in growth media after being plated on glass bottom dishes (MatTek, Ashland, MA). On the day of the experiment the media were changed to MEM with 0.25 g/l NaHCO3, 10 mM Na HEPES, pH 7.4 and 2% bovine serum albumin and 2% fetal bovine serum. The cells were scraped with a sterile pipette tip and imaged with a Nikon TE200 inverted microscope equipped for wide field phase contrast microscopy with a Hamamatsu Orca R2 camera (Hamamatsu Photonics, Bridgewater, NJ). Image collection was controlled by ImageJ software. Phase contrast images were collected every 5 min for 24 h using a ×60 oil immersion objective (1.4 NA) and Immersol oil (Thermo Fisher Scientific). Images were analyzed using ImageJ software to determine cell motility.
RESULTS
Embryonic kidneys from inv−/− mice show an increase in multinucleated cells and increased cell areas of tubular cells.
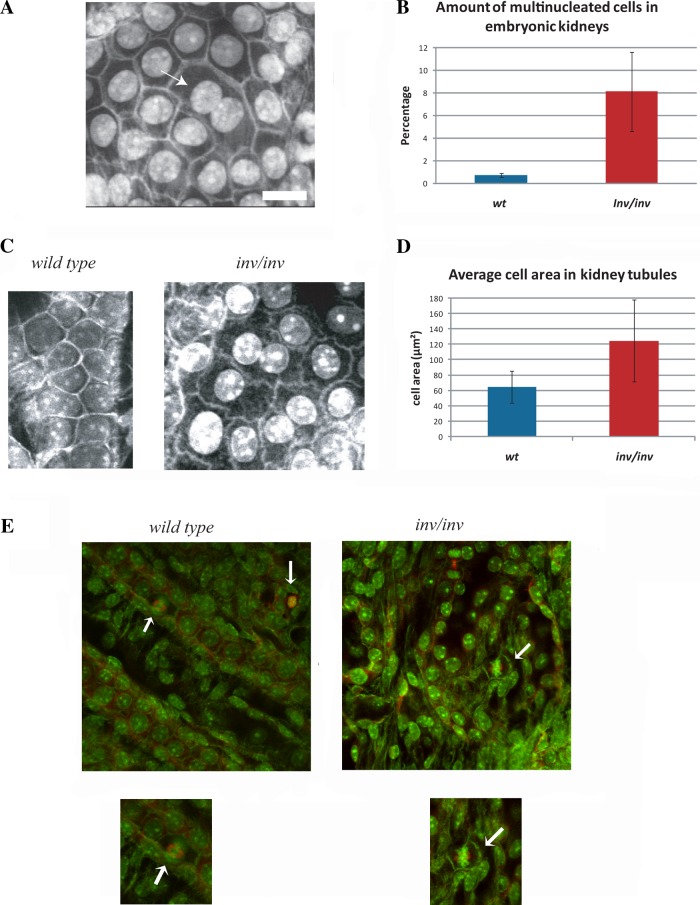
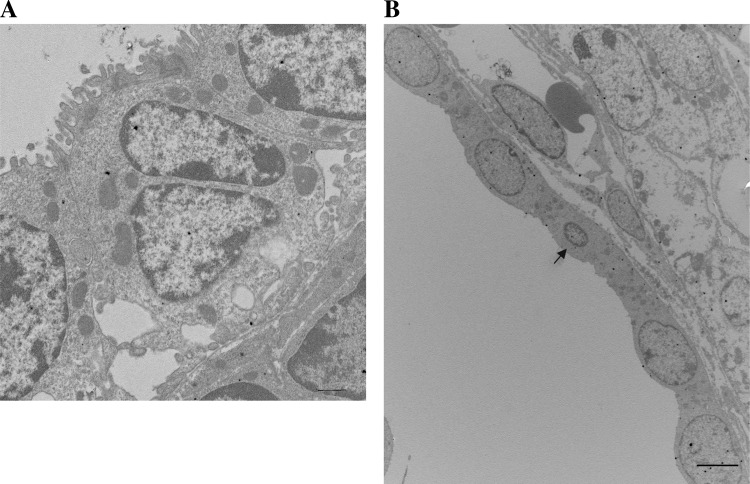
To identify phenotypes associated with loss of inversin, we used two-photon confocal microscopy to determine the cellular morphology of interphase and mitotic cells in the kidneys from inv−/− mice. As described previously, day 1 kidneys from inv−/− embryos show highly disorganized embryonic tubules with increased tubular diameter (30). Interestingly, we observed a significant increase in binuclear tubular epithelial cells (Fig. 1, A and D). Quantification of the number of binucleated tubular epithelial cells revealed a 10-fold increase over tubular cells from control kidneys (Fig. 1B). In addition, tubular epithelial cells in inv−/− kidneys had increased cell area as compared with wild-type controls (Fig. 1, C and D). The assessment of the number of multinucleated cells and cell area probably underestimated the extent of cellular changes because some tubular regions were severely disorganized in inv−/− kidneys. These regions were omitted from the quantification because confident identification of binucleated cells was difficult particularly since a significant number of cells appeared apoptotic. This observation was corroborated by ultrastructural analysis, revealing multinucleated cells as a readily observed phenotype in these kidneys (Fig. 2A) and cells with micronuclei (Fig. 2B).
Fig. 1.
Embryonic kidneys from inv−/− mice had increased numbers of multinucleated tubular cells with larger cell area and misplaced metaphase spindles. A: two-photon microscope image of a P1 embryonic kidney from inv−/− mouse. Arrow indicates multinucleated cell. All two-photon images in this figure are from kidneys fixed with 3% paraformaldehyde pH shift method and stained with rhodamine-phalloidin and Hoescht 33342. Bar, 10 µm. B: quantification of the amount of multinucleated cells in tubules from inversin-knockout kidneys compared with control kidneys. C: two-photon microscope images of tubular cells from control and inv−/− kidneys. Note the increase in cell area in the absence of inversin. Punctate staining in the middle of the control cells is due to nucleoli staining by Hoechst 33342. D: quantification of average cell area of tubular kidney cells of control or inv−/− kidneys. WT, wild type. E: representative images from control (left) and inv−/− kidneys (right) stained with rhodamine-phalloidin, Hoescht 33342, and α/β-tubulin antibodies. Note that there are spindle positioning alterations relative to the cell centroid in metaphase cells in kidneys from inv−/− mice (arrows). Insets: higher magnification of mitotic cells shown below the control (left) and inv−/− kidney (right).
Fig. 2.
Multinucleated cells are frequently observed in EM micrographs of kidney cysts from inv−/− mice. A: electron micrograph of inv−/− kidney showing multiple nuclei in the central cell. The nuclear morphology was not consistent with apoptotic changes. B: transmission electron micrograph of an inv−/− kidney showing a micronucleus in a field of cystic epithelial cells (arrow). Bar, 10 μm.
We additionally observed that tubular cells from inv−/− kidneys showed increased total cell area. Cell area measurements confirmed this observation showing a twofold increase in total cell area in tubular cells from inv−/− mice over control wild-type cells (Fig. 1, C and D). Furthermore, we observed that cell-cell contacts appeared abnormal in inv−/− kidneys. Instead of tight uniform contacts observed in wild-type cells we often observed that cell-cell contacts showed a ruffled pattern, suggesting that loss of inversin affected cell-cell contacts. Finally, we also observed that metaphase spindles of tubular cells were frequently displaced relative to the cell centroid in tubular cells from inv−/− embryos similar to the spindle positioning defect observed in cultured mammalian cells treated with inversin siRNA (Fig. 1E). To further evaluate inversin function, we characterized the cellular phenotype associated with inversin knockdown using RNAi in cell culture.
Knockdown of inversin by RNAi leads to an increase in multinucleated cells.
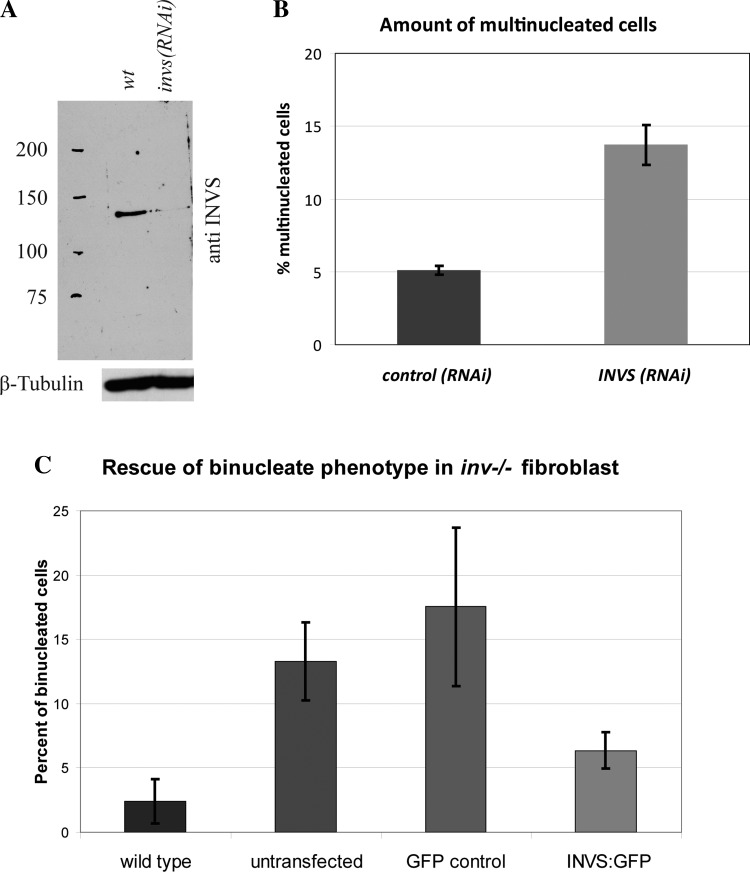
The proliferative defects associated with perturbed inversin function that lead to the formation of large kidney cysts, combined with the observation that inversin localizes to the mitotic spindle, suggested a potential role for inversin during mitosis. To investigate this possibility, we initially decided to knock down endogenous inversin in cultured cells to determine whether loss of inversin leads to mitotic defects. As evidenced by immune blots, we were able to reduce endogenous inversin levels by >90% in both HeLa and HEK-293 cells (Fig. 3A). We then quantified mitotic defects by counting the proportion of bi- or multinucleated cells in fixed samples after 48 h of RNAi treatment. We observe a 2.5-fold increase in multinucleated cells in cells treated with INVS RNAi as compared with control RNAi-treated cells (Fig. 3B, P < 0.03). Similarly, we found that 14% of mouse dermal fibroblasts isolated from inv−/− pups were multinucleated compared with 3% of wild-type dermal fibroblast (P < 0.002). Rescue experiments with full-length GFP-tagged inversin in embryonic fibroblast from inv−/− mice decreased the percentage of multinucleated cells to 6.5% close to wild-type levels and decreased the percentage of multinucleated cells in GFP-rescued inv−/− cells (Fig. 3C, P < 0.05). Taken together, these data show that inversin loss leads to an increase in mitotic defect frequency.
Fig. 3.
Loss of inversin leads to an increase in bi- and multinucleated cells. A: immune blot of extracts from control cells and cells treated with INVS siRNA for 48 h, probed with inversin antibody as well as β-tubulin (loading control). B: amount of bi- or multinucleated cells determined by immunofluorescence in control (scrambled RNAi) vs. INVS RNAi-treated cells. Averages of at least three independent experiments scoring at least 500 cells per experiment are shown. Error bars represent standard deviation in all figures. Difference in percentage of multinucleated cells in wild-type versus inv−/− cells was statistically significant (P = 0.00187). C: relative amount of bi- or multinucleated cells in wild-type dermal fibroblast, dermal fibroblasts from inv−/− embryos (untransfected group), inv−/− dermal fibroblast transfected with a green fluorescent protein (GFP)-only control plasmid, or transfected with GFP-tagged full-length inversin as determined by immunofluorescence. Note that dermal fibroblast exhibited a significant increase in bi- or multinucleated cells when compared with wild-type dermal fibroblast (P = 0.00187). Rescue experiments with GFP-tagged full-length inversin in inv−/− embryonic fibroblasts led to a significant reduction of bi- or multinucleated cells when compared with untransfected inv−/− cells (P = 0.0061) or inv−/− cells transfected with a GFP control plasmid (P = 0.0495).
Inversin is required for proper cell rounding during mitosis by regulating the organization of cortical actin.
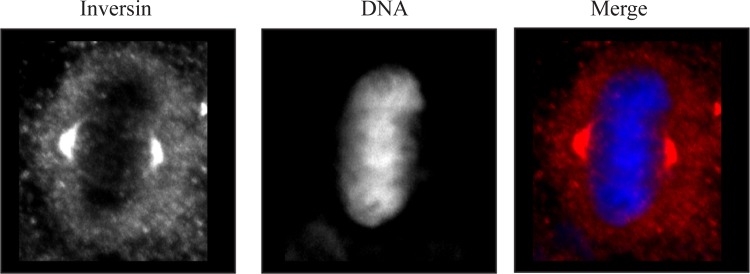
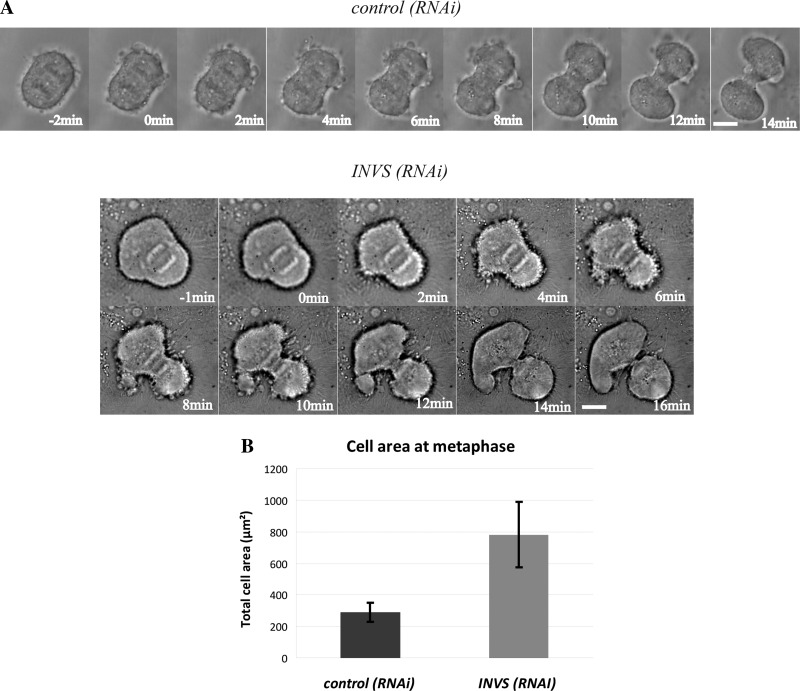
Prior studies have reported that inversin is recruited to the mitotic spindle (27). We confirmed this observation in HeLa cells by staining HeLa cells during mitosis with anti-inversin antibodies. In Fig. 4, inversin localized to the spindle poles and was also located in diffuse cytoplasmic puncta in a M phase HeLa cell. This finding is in agreement with prior published results (27). To determine the nature of the mitotic defect that led to an increase in multinucleated cells, we followed inversin-depleted mitotic cells using live cell microscopy. In contrast to control cells that acquired a spherical shape during metaphase, inversin-depleted metaphase cells remained partially flattened, exhibited significantly larger cell areas than control cells, and in some cases had drastically asymmetric shape. Although RNAi-treated cells partially rounded up upon entering anaphase, they still exhibited increased cell surface areas (Fig. 5A). We quantified the extent of the cell rounding defect by measuring total cell area of fixed inversin-depleted cells in metaphase and comparing it to control cells. Inversin-depleted cells show a 2.5-fold increase in total cell area illustrating defects in cell rounding (Fig. 5B, P < 0.001). Despite this cell rounding defect the cytokinetic furrow ingresses normally midway between the spindle poles. Interestingly, mitotic spindles were often misplaced leading to the formation of two nascent daughter cells of unequal size.
Fig. 4.
Immunofluorescence localization of inversin in a mitotic HeLa cell. Left: inversin staining showing localization at the spindle poles and in cytoplasmic puncta. Middle: Hoescht 33342 staining of metaphase chromosomes. Right: merged image from left and middle images. Inversin staining is shown in the red channel with Hoescht 33342 staining shown in the blue channel.
Fig. 5.
Depletion of inversin causes mitotic cell rounding defects leading to an increased cell area during metaphase but does not prevent furrow ingression. A: time series from DIC movies of control scrambled RNAi-treated HeLa cells (top) and HeLa cells treated with INVS siRNA for 48 h (bottom). Timing is relative to onset of furrow ingression. Inversin-depleted cells exhibited increased cell area but the cleavage furrow ingressed normally. Increased cortical blebbing and asymmetric spindle position were frequently observed. B: measurement of total cell area during metaphase. Cells were fixed and stained with rhodamine-phalloidin using actin as readout for the cellular cortex. Measurements represent averages of 40 or more individual cells.
Cortical actin dynamics and distribution of focal adhesions are perturbed in the absence of inversin.
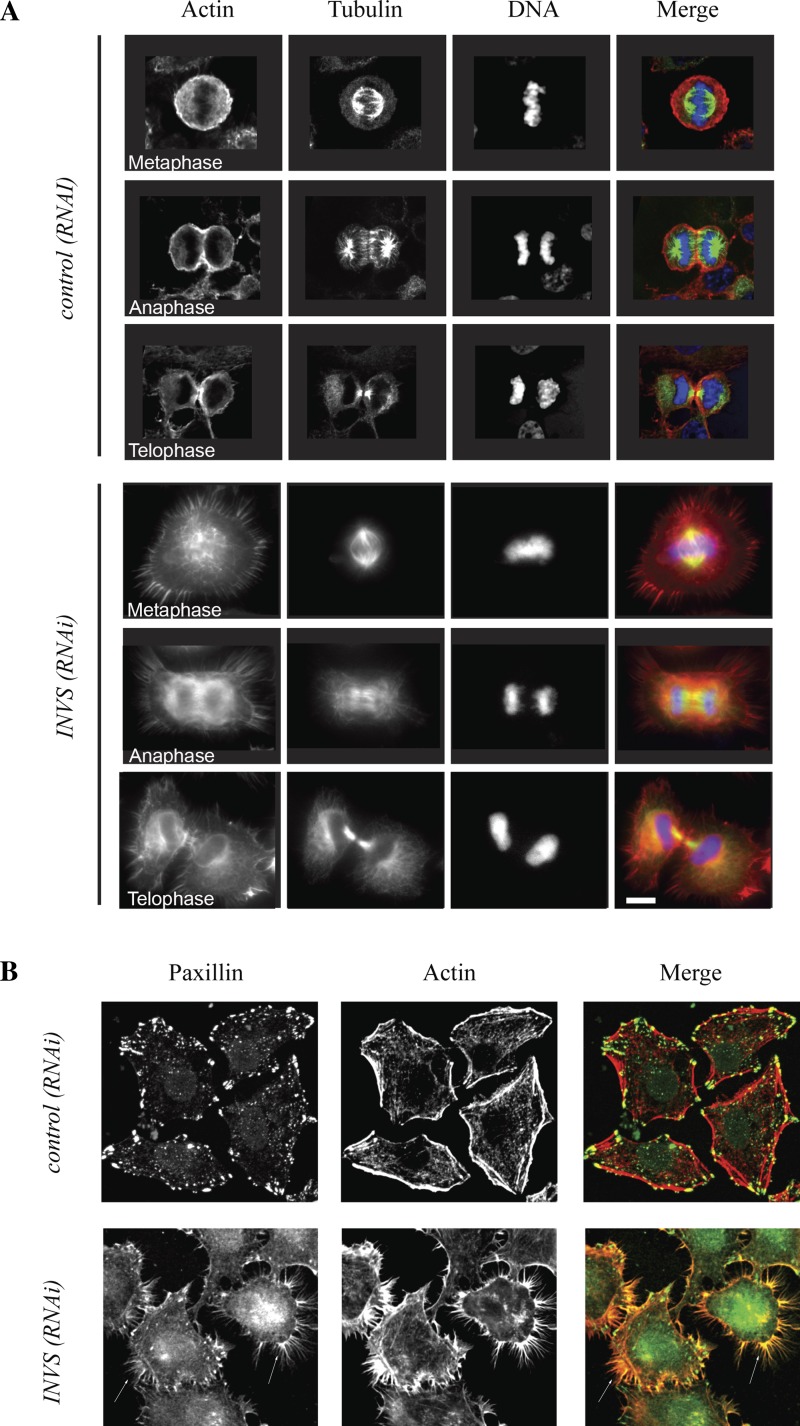
To test whether the change in cell shape and the failure of inversin-depleted cells to round up properly were a consequence of failure to reorganize the actin cytoskeleton, we used phalloidin labeling to visualize the organization of the actin cytoskeleton. In contrast to control cells, where actin accumulated uniformly around the cellular cortex during metaphase before becoming enriched in the equatorial plane and in the ingressing cleavage furrow, we observed large filopodia-like structures, reminiscent of retraction fibers, surrounding the entire cell cortex. These actin fibers persisted in cortical regions of anaphase cells and rapidly spread around the entire cortex during telophase when cells flattened out (Fig. 6A). Remarkably, we also observed a similar increase of cortical filopodia like structures in all interphase cells suggesting that the effect of inversin loss on the organization of the cortical actin cytoskeleton is not restricted to mitosis (Fig. 6A).
Fig. 6.
Inversin is required for cortical actin organization during mitosis. A: representative cells from different mitotic stages stained with α/β-tubulin, rhodamine-phalloidin, and Hoescht 33342. Control RNAi-treated cells had a rounded membrane without filopodia during mitosis. Inversin-depleted cells formed extensive filopodia-like structures throughout mitosis. B: control RNAi-treated and inversin-depleted fixed cells stained with anti-paxillin antibody and rhodamine-phalloidin. The cortical actin cytoskeleton was perturbed in interphase cells, and paxillin was redistributed to the cell periphery in the absence of inversin. Note that paxillin localized to filopodia-like structures in both mitotic and interphase cells (arrows).
We used antibodies against a filopodia component, paxillin, to determine whether the observed cortical actin fibers were filopodia (6, 17). Indeed, paxillin localized to these cortical actin-rich extensions in both interphase and mitotic cells (Fig. 6B). Furthermore, we also observed localization of α-actinin to the base of these filopodia (data not shown), confirming the identity of those actin extensions. Remarkably, we also observed a significant redistribution of focal adhesions from the cell body to the cell periphery in inversin-depleted cells (Fig. 6B), suggesting that inversin may be involved in regulating cellular adhesion. To test whether this translates into defects in cell motility, we performed scratch-wound assays on wild-type and mutant fibroblasts. Indeed, inv−/− fibroblasts showed significantly reduced motility (17.1 ± 8.3 nm/min) than control fibroblasts (5.4 ± 4.2 nm/min; P < 0.005).
Loss of inversin does not affect contractile ring assembly, furrow ingression, or spindle organization.
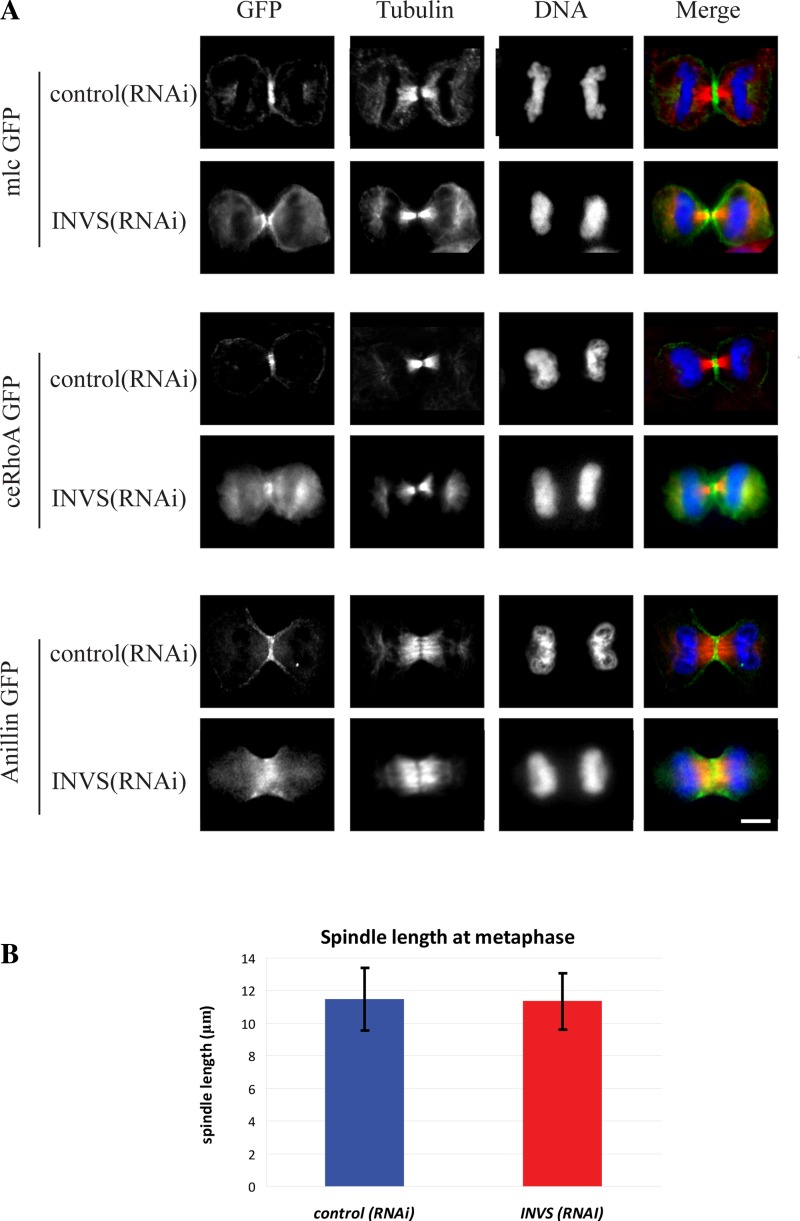
To determine whether inversin plays a role in other mitotic processes, we examined whether loss of inversin results in defects in contractile ring formation or spindle assembly. We found that loss of inversin did not affect contractile ring assembly or furrow ingression since contractile ring proteins RhoA, myosin light chain, and anillin all localized normally to the ingressing furrow (Fig. 7A). Since inversin binds to microtubules in vitro and to the mitotic spindle in cultured mammalian cells (19, 26, 28), we hypothesized that inversin regulates spindle assembly or microtubule dynamics during mitosis. However, inversin-depleted cells formed proper bipolar spindles, exhibited wild-type levels of spindle elongation, and had no apparent defects in microtubule dynamics as determined by measurements of mitotic spindle length at metaphase (Fig. 7B). We extended these data by examining CLIP-170 and EB-1 movement along microtubules and observed no obvious defect in movement of either protein along microtubule tracks in inversin-depleted cells (Supplemental Fig. S1; Supplemental Material for this article can be found online at the Journal website).
Fig. 7.
Inversin depletion does not prevent contractile ring assembly or proper mitotic spindle formation. A: HeLa cells transfected with either myosin light chain kinase GFP, ceRhoA:GFP, or anillin:GFP fixed with 3% paraformaldehyde to preserve the fluorescent tag and stained with anti-β-tubulin antibody and Hoescht 33342 DNA stain. Note that all three components of the contractile ring still accumulated normally at the equatorial plane and the ingressing furrow. B: measurement of spindle length in control RNAi-treated and inversin-depleted cells from fixed HeLa cells. Averages of at least 40 individual measurements are shown in the graph. There was no significant difference in spindle length between control RNAi-treated cells and inversin-depleted cells (P = 0.785).
Inversin is required for proper spindle positioning during mitosis.
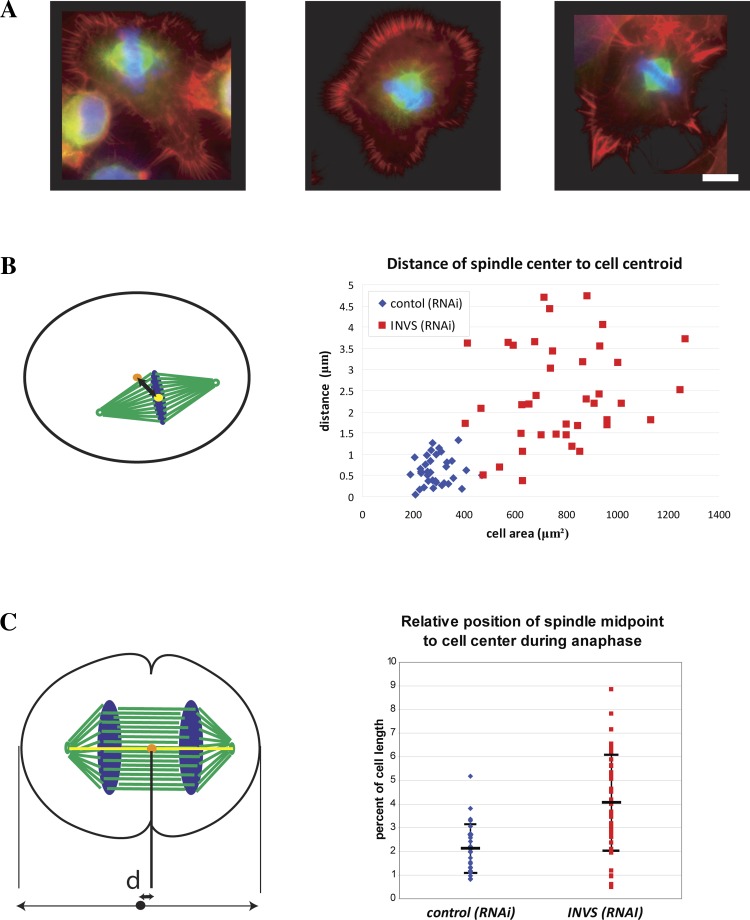
Since we observed unequal cell divisions in inversin depleted cells, we quantified spindle positioning during metaphase by measuring the distance between the cell centroid and the spindle midpoint, which we define as the midpoint between the two spindle poles (20, 27, 28). In contrast to control cells where mitotic spindles were in close proximity of the cell centroid, the majority of mitotic spindles were significantly displaced in inversin-depleted cells. These positioning defects are variable and can be quite dramatic as illustrated in Fig. 8A. Plotting the distance of the spindle midpoint relative to the cell centroid as a function of total cell area revealed a partial correlation of the spindle positioning defect with the extent of the defect in cell rounding, suggesting that spindle misalignment is a consequence of defective cell rounding (Fig. 8B). Furthermore, measuring the distance between the spindle midpoint and cell center along the spindle axis revealed marked asymmetry in positioning of the spindle as compared with control cells (Fig. 8C). This asymmetric position of the mitotic spindle results in the unequal division of the cell into two daughter cells of slightly different cell area.
Fig. 8.
Inversin is required for proper spindle positioning during metaphase and anaphase. A: inversin-depleted cells stained with rhodamine-phalloidin (red), α/β-tubulin (green), and Hoescht 33342 (blue) illustrating the extent of spindle positioning defects observed during metaphase. B: quantification of the spindle positioning defect by measurement of the distance between the cell centroid (orange) and the spindle midpoint (yellow). Scatterplot shows values for individual measurements as a function of cell area. Note that mitotic spindles were significantly displaced in inversin-depleted cells and that the extent of displacement correlated with cell size (P = 6.008 E-12). C: quantification of spindle positioning defects during anaphase by measurement of the distance (d) between spindle midpoint (orange) and cell center (black dot). Scatterplot shows spindle displacement from the cell center relative to total cell length (P = 3.874 E-6).
DISCUSSION
Inversin is the product of the Invs gene first identified by a random insertion mutagenesis screen (18, 22, 43). It localizes to adherens junctions and the ciliary axoneme in epithelial cells and acts as a master switch for Wnt signaling by targeting Dsh for degradation (24, 27, 34, 36). The underlying mechanism(s) of kidney cyst formation as a consequence of inversin loss is still unidentified (38). In this study we show that inversin is a critical regulator of the cortical actin cytoskeleton required for proper cell rounding and spindle positioning during mitosis as well as maintenance of the tubular epithelia integrity.
In analyzing the cellular response to inversin knockdown we determined that inversin is a critical regulator of the cortical actin network. Notably, we observed formation of cortical filopodia in both mitotic and interphase cells when inversin was depleted. These phenotypes were accompanied by defects in cell rounding upon entry into mitosis. Mitotic cell rounding is an important step during cell division and has been shown to be dependent on actin-binding proteins such as moesin and is accompanied by cortical actin cytoskeleton remodeling (15, 39). In addition, there is a failure to suppress filopodia formation during entry into mitosis, a phenotype associated with a failure of paxillin degradation during entry into M phase (41, 42). Remarkably, focal adhesion proteins such as paxillin or α-actinin were partially redistributed from the cell body to filopodia at the cellular cortex in inversin-depleted cells, suggesting that inversin is involved in cell adhesion to the substratum. Consistent with these observations, inv−/− fibroblasts showed significantly reduced motility when compared with control fibroblasts.
The observed defects in mitotic cell rounding were accompanied by significant spindle positioning defects. Spindle asymmetry correlated with the cell rounding defect, suggesting that the positioning defect is related to a failure to correctly position aster microtubule contacts. This may be due to a failure to reorganize the cortical actin cytoskeleton. Similar positioning defects have been reported in the absence of other actin regulatory proteins such as cofilin or moesin (1, 5, 13, 16). However, we did not observe defects in mitotic spindle assembly, nor did we detect drastic changes in microtubule dynamics. It is possible that our assay sensitivity for microtubule dynamics, i.e., using CLIP-170 or EB-1 to examine microtubule ends, failed to reveal subtle changes in microtubule stability (Supplemental Fig. S1). Given the cross talk between microtubules, actin cytoskeleton, and the interaction of inversin with microtubules, further experiments may be needed to exclude a subtle role for inversin in regulating microtubule dynamics (20).
The cause of the defect leading to multinucleated or binucleated cells is not entirely clear. It is likely that cytokinetic defects are a consequence of cell rounding and/or spindle positioning defects since cytokinetic defects have been reported when either of these processes was compromised (11, 16). We did not observe failure of contractile ring assembly as evidenced by normal localization of key contractile ring proteins accompanied by normal furrow ingression. One possible cause for the multinuclear phenotype is that cell abscission is compromised as a consequence of asymmetric spindle positioning and subsequent unequal cell division. Another possibility is that the increase in total cell area, or changes in cortical rigidity due to the defects in cortical actin reorganization prevent proper abscission. Given that inversin binds to the APC2 subunit, another explanation is that cell cycle progression or cell cycle timing is defective due to alterations in anaphase promoting complex activity as a consequence of inversin depletion (20). Further work is needed to evaluate this possibility.
Most notably, mitotic phenotypes similar to those observed in cell culture system were also observed in kidney tissue derived from inv−/− mice, which provided insights into the mechanisms of cyst formation. We found a marked increase in multinucleated cells using both two-photon microscopy and SEM, suggesting that cytokinetic defects contribute to cyst formation. Interestingly, SEM micrographs reveal that multinucleated cells in kidney cysts from inv−/− mice frequently exhibit more than one primary cilium. This raises the interesting possibility that cyst formation in inv−/− mice is the consequence of aberrant primary cilium-associated signaling, due to the presence of additional primary cilia in tubular cells that fail to divide normally.
It has been suggested that alterations in planar polarity signaling are responsible for cyst formation (7, 8). One manifestation of planar polarity alterations is randomization of mitotic spindle orientation relative to the tubule lumen, an effect demonstrated in a PKD1 murine knockout model. Given the extent of the cystic phenotype in inv−/− mice we were unable to determine spindle orientation relative to the lumen as done by Fischer et al. (7) and Sugiyama et al. (38). Nevertheless, we observed a significant misalignment of metaphase spindles relative to the cell centroid in tubular kidney cells from inv−/− mice, suggesting that similar spindle positioning defects could cause extension of the tubular lumen and cyst formation. Additionally, Dvl-dependent regulation of PCP signaling has been frequently associated with modulation of the actin cytoskeleton (14). In this context, similar to what we observe in tissue culture, overall cell area of tubular epithelial cells was significantly increased and the lateral membrane of the epithelial cells lacked the smooth regular outline of renal epithelial cells observed in wild-type littermates. Since inversin interacts with N-cadherin at adherens junctions it is conceivable that inversin contributes to epithelial integrity by stabilizing cell-cell contacts directly through its interaction with N-cadherin or by regulating the cytoplasmic actin network through regulation of cellular levels of Dvl.
The current unifying model for PKD suggests that cyst formation is a consequence of defects in cilia-dependent processes. In this context, inversin, whose localization pattern includes prominent localization to the base of the ciliary axoneme (27, 34), has been proposed to be a master switch for canonical and noncanonical Wnt signaling by targeting Dsh for APC/C-dependent degradation by proteosomes (3, 36). Based on our findings we propose that inversin plays several roles during kidney development as a consequence of its role in regulating the actin cytoskeleton. First, inversin is responsible for actin cytoskeleton reorganization necessary for orienting spindle positioning to allow for convergent extension along the tubule axis in response to planar cell polarity signals. Second, inversin-dependent actin reorganization during mitosis is required for mitotic cell rounding and completion of cytokinesis. Third, inversin stabilizes focal adhesions and adherens junctions on the lateral and basal membrane to maintain epithelial integrity. Therefore, cystogenesis due to a lack of functional inversin is caused by a compromised regulation of cellular actin dynamics. This leads to multiple defects during kidney development such as defective PCP signaling due to misaligned spindles, cell rounding and cytokinesis defects as well as loss of epithelial integrity.
Finally, the observation that inversin inhibits filopodia formation and the severity of the inv−/− phenotype in mice suggest that inversin may be required for regulation of actin dynamics outside of its role in kidney development. It will be interesting to define inversin' role in other filopodia-dependent processes such as cell migration, wound healing, or axon guidance.
GRANTS
Portions of this work were supported by generous contributions by the Bloch family. This work was also supported by National Institutes of Health Grants R21 DK-067246 and RO1 DK-50141. H. Ward was supported by fellowship support from the National Kidney Foundation. Microscopy images were collected at the Indiana Center for Biologic Microscopy, which was supported by a generous grant from INGEN and an O'Brien Center of Excellence award from the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK-079312).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.W., H.H.W., C.L.P., V.H.G., and R.L.B. conception and design of the research; M.W., H.H.W., C.M., and R.L.B. performed the experiments; M.W., H.H.W., C.L.P., C.M., V.H.G., and R.L.B. analyzed the data; M.W., H.H.W., V.H.G., and R.L.B. interpreted the results of the experiments; M.W., C.M., V.H.G., and R.L.B. prepared the figures; M.W. and R.L.B. drafted the manuscript; M.W., V.H.G., and R.L.B. approved the final version of the manuscript; C.L.P., V.H.G., and R.L.B. edited and revised the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Bruce Molitoris, Ken Dunn, and Simon Atkinson for advice and support.
Footnotes
This article is the topic of an Editorial Focus by Michael J. Caplan (4a).
REFERENCES
- 1. Amano T, Kaji N, Ohashi K, Mizuno K, Amano T, Kaji N, Ohashi K, Mizuno K. Mitosis-specific activation of LIM motif-containing protein kinase and roles of cofilin phosphorylation and dephosphorylation in mitosis. J Biol Chem 277: 22093– 22102, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bacallao R, Kiai K, Jesaitis L. Guiding principles of specimen preservation for confocal fluorescence microscopy. In: Handbook of Biological Confocal Microscopy, edited by Pawley JB. New York: Plenum, 1995, p. 311– 325 [Google Scholar]
- 3. Benzing T, Simons M, Walz G, Benzing T, Simons M, Walz G. Wnt signaling in polycystic kidney disease. [see comment] J Am Soc Nephrol 18: 1389– 1398, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bissler JJ, Dixon BP. A mechanistic approach to inherited polycystic kidney disease. Pediatr Nephrol 20: 558– 566, 2005 [DOI] [PubMed] [Google Scholar]
- 4a. Caplan MJ. An inversin convergence. Focus on “Inversin modulates the cortical actin network during mitosis.” Am J Physiol Cell Physiol ( May 15, 2013). 10.1152/ajpcell.00126.2013 [DOI] [PubMed] [Google Scholar]
- 5. Carreno S, Kouranti I, Glusman ES, Fuller MT, Echard A, Payre F, Carreno S, Kouranti I, Glusman ES, Fuller MT, Echard A, Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J Cell Biol 180: 739– 746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deakin NO, Turner CE, Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci 121: 2435– 2444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21– 23, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Germino GG. Linking cilia to Wnts. Nat Genet 37: 455– 457, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107: 843– 854, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Haider NB, Carmi R, Shalev H, Sheffield VC, Landau D. A Bedouin kindred with infantile nephronophthisis demonstrates linkage to chromosome 9 by homozygosity mapping. Am J Hum Genet 63: 1404– 1410, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgings J, Midgley C, Bergh AM, Bell SM, Askham JM, Roberts E, Binns RK, Sharif SM, Bennett C, Glover DM, Woods CG, Morrison EE, Bond J. Human ASPM participates in spindle organization, spindle orientation and cytokinesis. BMC Cell Biol 2: 85– 102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 6: 928– 940, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P, Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell 16: 649– 664, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim HY, Davidson LA. Punctuated actin contractions during convergent extension and their permissive regulation by the non-canonical Wnt-signaling pathway. J Cell Sci 124: 635– 646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kunda P, Baum B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol 19: 174– 179, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kunda P, Pelling AE, Liu T, Baum B, Kunda P, Pelling AE, Liu T, Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol 18: 91– 101, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Mattila PK, Lappalainen P, Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 9: 446– 454, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Mochizuki T, Saijoh Y, Tsuchiya K, Shirayoshi Y, Takai S, Taya C, Yonekawa H, Yamada K, Nihei H, Nakatsuji N, Overbeek PA, Hamada H, Yokoyama T. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature 395: 177– 181, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Morgan D, Eley L, Sayer J, Strachan T, Yates LM, Craighead AS, Goodship JA. Expression analyses and interaction with the anaphase promoting complex protein Apc2 suggest a role for inversin in primary cilia and involvement in the cell cycle. Hum Mol Genet 11: 3345– 3350, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Morgan D, Eley L, Sayer J, Strachan T, Yates LM, Craighead AS, Goodship JA, Morgan D, Eley L, Sayer J, Strachan T, Yates LM, Craighead AS, Goodship JA. Expression analyses and interaction with the anaphase promoting complex protein Apc2 suggest a role for inversin in primary cilia and involvement in the cell cycle. Hum Mol Genet 11: 3345– 3350, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, Gardner A, Schuster G, Vien L, Harrison W, Elder FF, Penman-Splitt M, Overbeek P, Strachan T. Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse.[erratum appears in Nat Genet 20: 312, 1998]. Nat Genet 20: 149– 156, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Nurnberger J, Bacallao RL, Phillips CL. Inversin forms a complex with catenins and N-cadherin in polarized epithelial cells. Mol Biol Cell 13: 3096– 3106, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nurnberger J, Bacallao RL, Phillips CL, Nurnberger J, Bacallao RL, Phillips CL. Inversin forms a complex with catenins and N-cadherin in polarized epithelial cells. Mol Biol Cell 13: 3096– 3106, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nurnberger J, Kribben A, Opazo Saez A, Heusch G, Philipp T, Phillips CL. The Invs gene encodes a microtubule-associated protein. J Am Soc Nephrol 15: 1700– 1710, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Nurnberger J, Kribben A, Opazo Saez A, Heusch G, Philipp T, Phillips CL, Nurnberger J, Kribben A, Opazo Saez A, Heusch G, Philipp T, Phillips CL. The Invs gene encodes a microtubule-associated protein. J Am Soc Nephrol 15: 1700– 1710, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. [see comment] Nat Genet 34: 413– 420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phillips CL, Miller KJ, Filson AJ, Nurnberger J, Clendenon JL, Cook GW, Dunn KW, Overbeek PA, Gattone VH, 2nd, Bacallao RL. Renal cysts of inv/inv mice resemble early infantile nephronophthisis. J Am Soc Nephrol 15: 1744– 1755, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20: 5972– 5981, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev 23: 265– 277, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Shiba D, Yamaoka Y, Hagiwara H, Takamatsu T, Hamada H, Yokoyama T. Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region. J Cell Sci 122: 44– 54, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. [see comment] Nat Genet 37: 537– 543, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int 70: 854– 864, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Sugiyama N, Tsukiyama T, Yamaguchi TP, Yokoyama T. The canonical Wnt signaling pathway is not involved in renal cyst development in the kidneys of inv mutant mice. Kidney Int 79: 957– 965, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Théry M, Bornens M. Cell shape and cell division. Curr Opin Cell Biol 18: 648– 657, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Watnick T, Germino G. From cilia to cyst. Nat Genet 34: 355– 356, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Yamaguchi R, Mazaki Y, Hirota K, Hashimoto S, Sabe H. Mitosis specific serine phosphorylation and downregulation of one of the focal adhesion protein, paxillin. Oncogene 15: 1753– 1761, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, Matsumura F. Dissociation of FAK/p130(CAS)/c-Src complex during mitosis: role of mitosis-specific serine phosphorylation of FAK. J Cell Biol 144: 315– 324, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yokoyama T, Copeland NG, Jenkins NA, Montgomery CA, Elder FF, Overbeek PA. Reversal of left-right asymmetry: a situs inversus mutation. [see comment] Science 260: 679– 682, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.