Abstract
The Na+/H+ exchanger NHE8 is expressed on the apical membrane of intestinal epithelial cells and is particularly abundant in the colon. Our previous study showed that Muc2 expression was significantly reduced in NHE8-knockout (NHE8−/−) mice, suggesting that NHE8 plays a role in mucosal protection in the colon. The current study confirms and extends our studies on the role of NHE8 in mucosal protection. The number of bacteria attached on the distal colon was significantly increased in NHE8−/− mice compared with their wild-type littermates. As expected, IL-4 expression was markedly increased in NHE8−/− mice compared with wild-type mice. Immunohistochemistry showed disorganization in the mucin layer of NHE8−/− mice, suggesting a possible direct bacteria-epithelia interaction. Furthermore, NHE8−/− mice were susceptible to dextran sodium sulfate-induced mucosal injury. In wild-type mice, dextran sodium sulfate treatment inhibited colonic NHE8 expression. In Caco-2 cells, the absence of NHE8 expression resulted in higher adhesion rates of Salmonella typhimurium but not Lactobacillus plantarum. Similarly, in vivo, S. typhimurium adhesion rate was increased in NHE8−/− mice compared with wild-type mice. Our study suggests that NHE8 plays important roles in protecting intestinal epithelia from infectious bacterial adherence.
Keywords: NHE8, mucosal layer, Salmonella
members of the Na+/H+ exchanger (NHE) family are key transporters in many physiological functions and are expressed in a broad range of tissues. NHE functions include intracellular pH homeostasis, cell volume regulation, and electroneutral NaCl absorption in the epithelia. Nine NHE isoforms have been cloned and identified. The isoforms differ by tissue distribution, membrane localization, inhibitor sensitivities, and physiological regulation (7, 24, 35, 43, 44). Four NHE isoforms have been identified in the intestinal tract. NHE1, a basolateral membrane protein in the intestinal epithelial cells, participates in intracellular pH regulation (5, 25). NHE2 and NHE3 are apical membrane proteins in the epithelial cells: NHE2 is crucial for parietal cell viability, and NHE3 plays important roles in Na+ absorption (28, 29). NHE8, the newest member of the NHE family, has been detected in liver, skeletal muscle, kidney, testes, and intestinal tract (8, 9, 36). In the intestinal tract, NHE8 expression level is the highest early in development, and its function is closely regulated in the course of development by EGF, glucocorticoids, and inflammatory cytokines (34, 39–41). Although NHE8 has been shown to play important roles in Na+ absorption, there is much to learn about this newest NHE. Earlier this year, we reported a possible role for NHE8 in gastric mucosal protection in mice (38).
Previously, we showed that ablation of NHE8 function in mice resulted in reduced expression of downregulated in adenoma (DRA) and Muc2 genes (42). Muc2, the major colonic secretory mucin in humans and mice, is a key structural component of the mucus layer (16, 33). The mucus layer covers the epithelial surface of the gastrointestinal tract and serves as the first line of defense against the luminal contents and plays an important role in preventing the adhesion of microorganisms to host surfaces (16, 19). In mice lacking Muc2 expression, the mucus layer was abolished, resulting in direct contact between bacteria and epithelial cells, which could trigger an inflammatory response (16, 32). Mice lacking NHE3 expression develop spontaneous colitis, and these animals have an inner mucus layer that is penetrated by bacteria (14, 18).
Because our newly established NHE8-knockout (NHE8−/−) mouse model has reduced Muc2 mRNA expression and is susceptible to gastric ulcer formation, we hypothesized that NHE8 protects the intestinal epithelium from bacterial adhesion and participates in mucus layer formation. Our results indicate that NHE8 indeed plays an important role in protecting the host from bacterial infection by hindering adherence of infectious bacteria to the intestinal epithelial surface.
MATERIALS AND METHODS
Animals.
NHE8−/− mice were generated from NHE8+/− breeding pairs with mixed genetic background (129/Swiss Webster), as previously described (38). Male 8- to 12-wk-old mice were used in this study. All animal work was approved by the University of Arizona Institutional Animal Care and Use Committee.
Bacterial culture.
Salmonella typhimurium ATCC 14028 (American Type Culture Collection, Manassas, VA) was cultured in tryptone soy broth at 37°C overnight with shaking. Lactobacillus plantarum JDM1 was obtained from Shanghai Jiao Tong University (Shanghai, China) and inoculated in De Man, Rogosa, and Sharpe (MRS) broth at 37°C for 24 h under anaerobic conditions (45, 46). The number of bacteria was estimated by measurement of the absorbance at 600 nm and correlation of the absorbance value to a standard curve of colony-forming units (CFU) on MRS agar (Merck) in preliminary experiments.
Cell culture.
Caco-2 cells (American Type Culture Collection) were cultured in MEM containing 20% fetal bovine serum, 1% nonessential amino acids, 100 U/ml penicillin, and 100 mg/ml streptomycin. Cells were grown at 37°C in a 5% CO2 atmosphere.
Fluorescence detection of mucosal structure and bacteria in mice.
Distal colon was collected from 8-wk-old mice and fixed in methanol-Carnoy's fixative solution, as previously reported (16). Sections were stained with anti-MUC2C3 antiserum with Alexa 488-conjugated anti-rabbit immunoglobulins (Life Technologies) and 4′,6-diamidino-2-phenylindole. Bacteria were detected by the fluorescent in situ hybridization (FISH) method using the general bacterial rRNA probe EUB338 conjugated with Alexa 555 (16). The images were acquired using a fluorescence microscope (Eclipse E1000) with a ×40/0.75 differential interference contrast objective (Nikon).
Quantification of a specific bacterial group in the distal colon.
Distal colon from 8-wk-old mice was collected. Luminal contents were removed gently and rinsed with sterilized PBS. Total DNA and RNA were extracted using the AllPrep DNA/RNA Mini Kit (Qiagen, Valencia, CA). Quantitative PCR (qPCR) analysis was performed using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). The abundance of specific intestinal bacterial groups was detected using a real-time PCR detection system (model CFX96, Bio-Rad). All qPCR results were checked for specificity by melting-curve analysis. To determine the total amount of commensal bacteria in each intestinal segment, the 16S rRNA gene was amplified using the conserved 16S rRNA-specific primers EubacF and EubacR (Table 1). To determine the abundance of specific intestinal bacteria, group-specific 16S rRNA gene primers (Table 1) were used. All PCR results were normalized by the mouse GAPDH gene.
Table 1.
16S rRNA gene group-specific primers for quantitative PCR
| Group | Primer | Sequence (5′–3′) | Ref. No. |
|---|---|---|---|
| Eubacteria (all bacteria) | EubacF | TCCTACGGGAGGCAGCAGT | 23 |
| EubacR | GGACTACCAGGGTATCTAATCCTGTT | ||
| Firmicutes | Firm934F | GGAGYATGTGGTTTAATTCGAAGCA | 10 |
| Firm1060R | AGCTGACGACAACCATGCAC | ||
| Bacteroidetes | Bac934F | GGARCATGTGGTTTAATTCGATGAT | 10 |
| Bac1060R | AGCTGACGACAACCATGCAG | ||
| Lactobacillus | LactoF | GAGGCAGCAGTAGGGAATCTTC | 26 |
| LactoR | GGCCAGTTACTACCTCTATCCTTCTTC | ||
| Clostridium coccoides | gCcocF | AAATGACGGTACCTGACTAA | 22 |
| gCcocR | CTTTGAGTTTCATTCTTGCGAA | ||
| Segmented filament bacteria | SFB736F | GACGCTGAGGCATGAGAGCAT | 1 |
| SFB844R | GACGGCACGGATTGTTATTCA | ||
| Salmonella | MinF | ACGGTAACAGGAAGCAG | 31 |
| MinR | TATTAACCACAACACCT |
Dextran sodium sulfate treatment in wild-type and NHE8−/− mice.
Twelve-week-old mice were fed 3.0% (wt/vol) dextran sodium sulfate (DSS; Sigma-Aldrich, St. Louis, MO) for 6 days. Animals were observed daily for body weight, morbidity, stool consistency, and the presence of gross blood in the feces. Disease activity index (DAI) was determined from combined scores of body weight loss, stool consistency, and fecal hemoccult following the method described previously (27). For body weight loss score, 0 represents 0–9%, 1 represents 10–19%, and 2 represents ≥20%. Mice that lost >20% of their body weight before the end of DSS treatment were euthanized and excluded from further analysis. For stool consistency score, 0 represents no stools adherent to the anus and 1 represents diarrhea with liquid stools adherent to the anus. For hemoccult score, 0 represents no blood and 1 represents positive hemoccult (hemoccult kit, Beckman Coulter). On day 6 of DSS treatment, mice were euthanized, and the distal colon was collected for RNA and protein extraction.
NHE8 small interfering RNA transfection.
Caco-2 cells were seeded on six-well plates and grown to 80% confluence. Transfection was performed using HiPerFect transfection reagent (Qiagen) and NHE8 small interfering RNAs (siRNAs) at 5 nM (NM_015266.1_STEALTH_867 and NM_015266.1_STEALTH_738, Invitrogen, Carlsbad, CA). Silencing efficiency was evaluated by qPCR and Western blotting. siRNA assay was conducted in triplicate over three to five successive passages of Caco-2 cells.
Bacterial infection in Caco-2 cells.
Late exponential cultures of S. typhimurium and L. plantarum were adjusted with culture medium to an optical density at 600 nm corresponding to 1 × 108 cells/ml. Bacteria were washed twice with PBS and resuspended in MEM before use. Caco-2 cells were rinsed three times with PBS, and bacteria were added at multiplicity of infection of 100. Cells were incubated with bacteria for 2 h at 37°C in 5% CO2-95% air. Unattached bacteria were removed from the cells by four washes with PBS. qPCR was performed to determine cell-bound bacteria using genus-specific primers following a method described previously (3, 20). Briefly, 20 μl of cell suspension were incubated with 3.8 μl of trypsin inhibitor solution at room temperature for 10 min. PCR amplification was carried out in a 20-μl final volume containing 2 μl of cell suspension, 10 μl of iQ SYBR Green Supermix (Bio-Rad), and each primer at 0.2 μM. qPCR was performed in the CFX96 real-time PCR detection system, and SYBR Green I fluorophore was used to correlate the amount of PCR product with the fluorescent signal. The pCR 2.1 vector containing the genus-specific gene was constructed and used as an internal standard. Since the copy numbers of standard plasmid could be quantified, serial dilutions of the plasmid were PCR-amplified to represent the number of bacteria, which ranged from 1 × 107 to 1 × 103 CFU/μl.
S. typhimurium infection in mice.
Eight-week-old mice were housed individually under standard barrier conditions in cages equipped with steel-grid floors. At 4 h after water and food were withdrawn, mice were gavaged with streptomycin (20 mg in 100 μl of sterile water), and water and food were supplied again. At 24 h after streptomycin treatment, water and food were withdrawn again for 4 h. Then mice were gavaged with 108 CFU of S. typhimurium (100-μl suspension in PBS) or PBS. Drinking water was offered immediately, and food was supplied 2 h after gavage. Mice were euthanized 7 days after infection, and the distal colon was collected for Salmonella assay.
Quantification of Salmonella in distal colon.
Distal colon was collected and rinsed with sterilized PBS. Total DNA was extracted using the Allprep DNA/RNA Mini Kit. Semiquantitative PCR was used to amplify adhered Salmonella bacteria, as DNAs from commensal bacteria interfered with the qPCR. Salmonella species-specific 16S rRNA primers (MinF and MinR) were used to amplify Salmonella bacteria. The conserved 16S rRNA-specific primers (EubacF and EubacR) were used to amplify total bacteria. The amplicon was separated on 1.5% agarose gel and analyzed using Bio-Rad Quantity One software. The abundance of Salmonella was determined by comparing the density of the Salmonella-specific band with the density of the conserved 16S rRNA band.
RNA isolation and qPCR detection.
Total RNA was isolated from tissues and cells using TRIzol reagent (Invitrogen) or the RNeasy Mini Kit (Qiagen). RNA (2 μg) was reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). qPCR was performed to detect target genes using TaqMan probes (Applied Biosystems, Foster City, CA). Relative gene expression was determined by the 2−ΔΔCT method, as previously reported (21, 34). TATA-binding protein was used as an internal standard.
Protein preparation and Western blot detection.
Tissues and cells were homogenized in a small volume of RIPA buffer on ice. Resulting lysates were centrifuged at 15,000 rpm for 10 min at 4°C. Supernatants were collected and used for Western blotting. NHE8 antibody (1:2,500 dilution) (36), β-actin antiserum (1:20,000 dilution; Sigma-Aldrich), and TNFα antibody (1:1000 dilution; Santa Cruz Biotechnology) were used to detect NHE8, β-actin, and TNFα protein abundance, respectively. The ratio of target protein intensity to β-actin protein intensity was used for quantification of protein expression.
Statistical analysis.
Student's t-test was used to compare experimental data. P ≤ 0.05 was considered significant.
RESULTS
Abundance of bacterial groups attached on the surface of the colon in NHE8−/− mice.
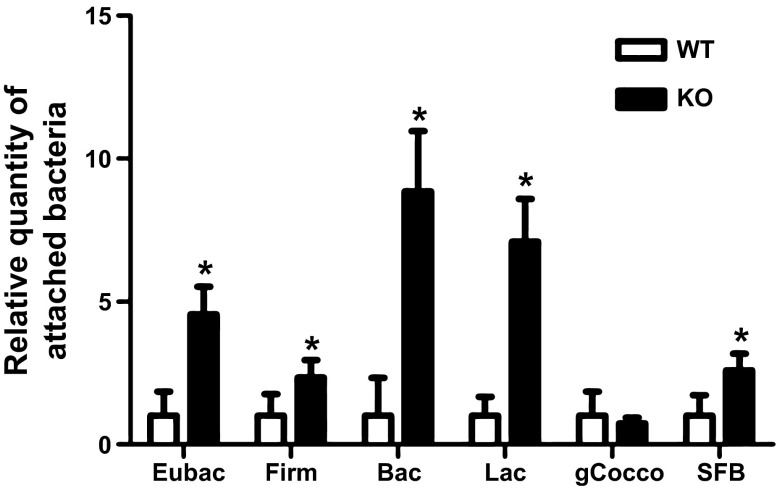
Distal colon was collected from 8-wk-old wild-type and NHE8−/− mice. DNA was isolated for PCR analysis. The major groups of commensal bacteria adhered to the mucosal surface were quantified by qPCR with group-specific primers. PCR results indicate a significant increase in total bacteria adhered at the mucosal surface in the distal colon of NHE8−/− mice (1.00 ± 0.26 and 4.55 ± 0.97 in wild-type and NHE8−/− mice, respectively, n = 30, P < 0.01; Fig. 1). The change was mainly associated with a significant increase in Bacteroidetes, Lactobacillus, and Firmicutes bacteria and in segmented filament bacteria. As shown in Fig.1, Bacteroidetes species increased from 1.00 ± 0.40 in wild-type mice to 8.85 ± 2.11 in NHE8−/− mice (n = 30, P < 0.01), Lactobacillus species increased from 1.00 ± 0.20 in wild-type mice to 7.09 ± 1.50 in NHE8−/− mice (n = 30, P < 0.01), Firmicutes species increased from 1.00 ± 0.23 in wild-type mice to 2.34 ± 0.61 in NHE8−/− mice (n = 30, P < 0.05), and segmented filament bacteria increased from 1.00 ± 0.22 in wild-type mice to 2.60 ± 0.58 in NHE8−/− mice (n = 30, P < 0.05).
Fig. 1.
Quantitative analysis of bacterial groups in the distal colon in wild-type (WT) and Na+/H+ exchanger isoform 8 (NHE8)-knockout (NHE8−/−) mice. Distal colon was collected, and luminal contents were removed immediately. Genomic DNA was extracted and used to quantify the relative abundance of major bacterial groups attached to the intestinal epithelial surface in the colon. Eubac, Eubacteria; Firm, Firmicutes; Bac, Bacteroidetes; Lac, Lactobacillus; gCocco, Clostridium coccoides; SFB, segmented filament bacteria. Values are means ± SE from 30 mice. *P ≤ 0.05.
Cytokine expression in the colon of NHE8−/− mice.
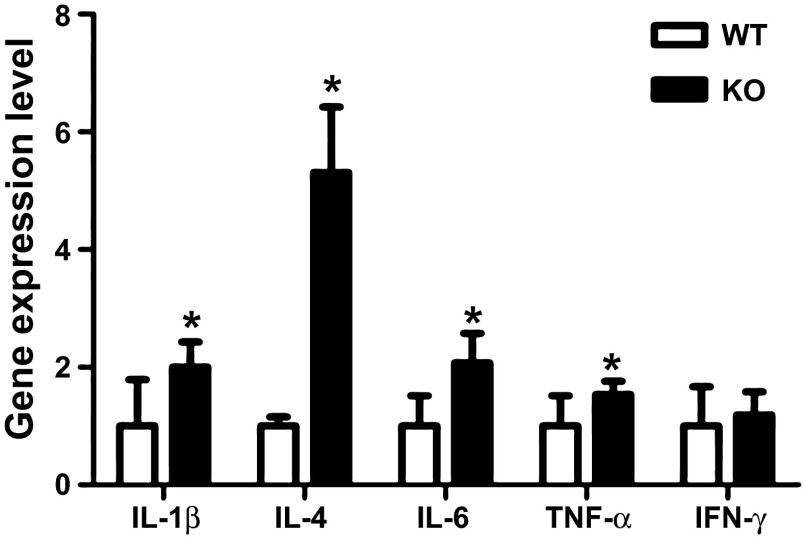
Because more bacteria attached to the colonic epithelial surface in NHE8−/− mice, we evaluated cytokine gene expression in the distal colon of NHE8−/− mice. As shown in Fig. 2, mRNA expression of IL-1β, IL-4, IL-6, and TNFα was markedly increased in the distal colon in NHE8−/− mice. Compared with wild-type mice, the expression of IL-1β, IL-4, IL-6, and TNFα in NHE8−/− mice was increased to 2.00 ± 0.43, 5.31 ± 1.11, 2.08 ± 0.50, and 1.54 ± 0.22, respectively (n = 16, P < 0.05). Interestingly, the expression of IFNγ remained unchanged (1.00 ± 0.24 and 1.19 ± 0.40 in wild-type and NHE8−/− mice, respectively, n = 16).
Fig. 2.
Expression of inflammatory cytokine genes in the distal colon of NHE8−/− mice. Colonic tissue was collected and used for total RNA isolation. Real-time PCR was used to determine cytokine gene expression. Values are means ± SE from 16 mice. *P ≤ 0.05.
Immunohistochemical and FISH staining of the mucus layer in NHE8−/− mice.
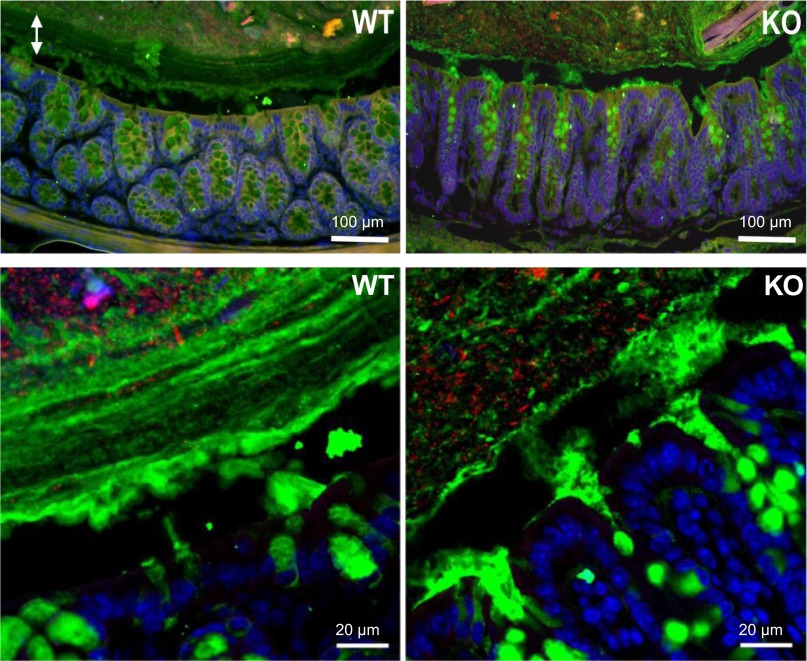
Immunohistochemical staining with anti-MUC2C3 antiserum was used to visualize the mucus layer of the distal colon fixed in methanol-Carnoy's solution. As shown in Fig. 3, colonic epithelial cells in the wild-type mice were covered by a stratified and well-organized inner mucus layer, whereas this mucin layer was less organized in the colon of the NHE8−/− mice. FISH staining with bacteria-specific probes showed that bacteria were only detected in the outer mucus layer and were excluded from the inner stratified layer in wild-type mice (Fig. 3, left). In NHE8−/− mice, the colonic epithelium was covered by a disorganized mucus layer and bacteria directly contacted the epithelial surface (Fig. 3, right).
Fig. 3.
Mucus and bacteria stain in the distal colon of NHE8−/− mice. Distal colon was sectioned and used for Muc2 and 4′,6-diamidino-2-phenylindole staining. Muc2-positive goblet cells and overlaying mucus layers were detected by anti-MUC2C3 antiserum (green). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). Bacteria were detected using the general bacterial probe EUB338-Alexa Fluor 555 (red). Top: low-power (×10) magnification; bottom: high-power (×40) magnification.
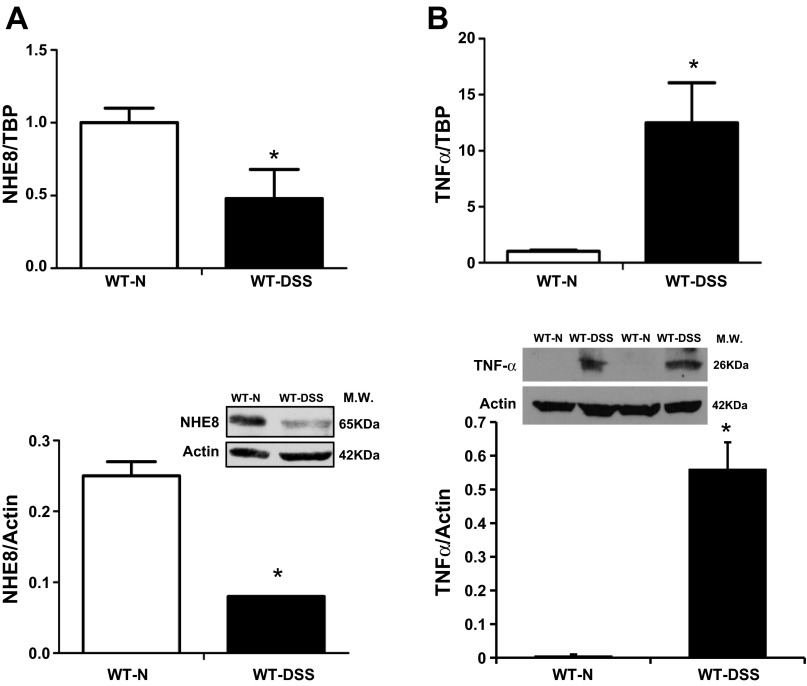
Colonic NHE8 and TNFα expression in DSS-treated wild-type mice.
To test whether DSS-induced mucosal injury affected colonic NHE8 expression, wild-type mice were fed 3% DSS water for 6 days and the distal colon was collected for NHE8 and TNFα expression assay. As indicated in Fig. 4, NHE8 mRNA expression was decreased from 1.00 ± 0.10 in control mice to 0.48 ± 0.20 in DSS-treated mice, and NHE8 protein expression was reduced from 0.25 ± 0.02 in control mice to 0.08 ± 0.001 in DSS-treated mice (n = 20, P < 0.01; Fig. 4A). Interestingly, DSS-induced inhibition of NHE8 expression was not detected in the small intestine (data not shown). DSS treatment also dramatically increased TNFα expression in the distal colon. TNFα mRNA expression was increased from 1.04 ± 0.09 in control mice to 12.47 ± 3.60 in DSS-treated mice, and TNFα protein expression was increased from barely detectable (0.005 ± 0.005) in control mice to a very strong signal (0.56 ± 0.08) in DSS-treated mice (n = 16, P < 0.05; Fig. 4B).
Fig. 4.
Expression of NHE8 and TNFα in dextran sodium sulfate (DSS)-treated WT mice. Distal colon was collected and used for RNA isolation and tissue lysate preparation. NHE8 and TNFα mRNAs were detected by real-time PCR. NHE8 and TNFα proteins were analyzed by Western blotting. A: NHE8 mRNA expression (top) and protein abundance (bottom). B: TNFα mRNA expression (top) and protein abundance (bottom). WT-N, control mice; WT-DSS, DSS-treated mice; MW, molecular weight; TBP, TATA-binding protein. Values are means ± SE from 20 mice. *P ≤ 0.05.
Susceptibility of NHE8−/− mice to DSS treatment.
To test whether NHE8 participates in mucosal protection, NHE8−/− mice were fed 3% DSS water for 6 days. DSS-treated NHE8−/− mice showed severe symptoms compared with DSS-treated wild-type mice. Body weight loss was more dramatic in NHE8−/− than wild-type mice. NHE8−/− mice showed measurable body weight loss at day 2 of DSS treatment, but wild-type mice showed measurable body weight loss at day 4 of DSS treatment. By day 6, NHE8−/− mice lost 23% of body weight, while wild-type mice only lost 12% of body weight (n = 16, P < 0.05; Fig. 5A). Survival rate after DSS treatment was also lower in NHE8−/− mice: at the end of DSS treatment, only 40% of NHE8−/− mice survived, while all wild-type mice survived (Fig. 5B). DAI also reflected the severity of the response of NHE8−/− mice over the course of DSS treatment. DAI was significantly higher in NHE8−/− mice at day 3 of DSS treatment and remained higher than in wild-type mice throughout the rest of the treatment (Fig. 5C). In wild-type and NHE8−/− mice, TNFα expression was elevated and at a similar level in the two DSS-treated groups (data not shown).
Fig. 5.
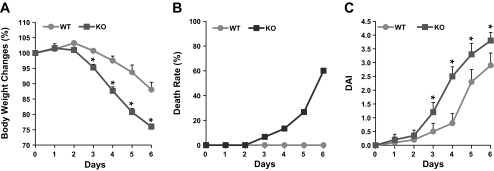
Susceptibility of NHE8−/− mice to DSS treatment. NHE8−/− mice were fed DSS water for 6 days, and body weight (A), death rate (B), and disease activity index (DAI; C) were recorded. Values are means ± SE from 20 mice. *P ≤ 0.05.
NHE8 siRNA transfection and bacterial adhesion assay in Caco-2 cells.
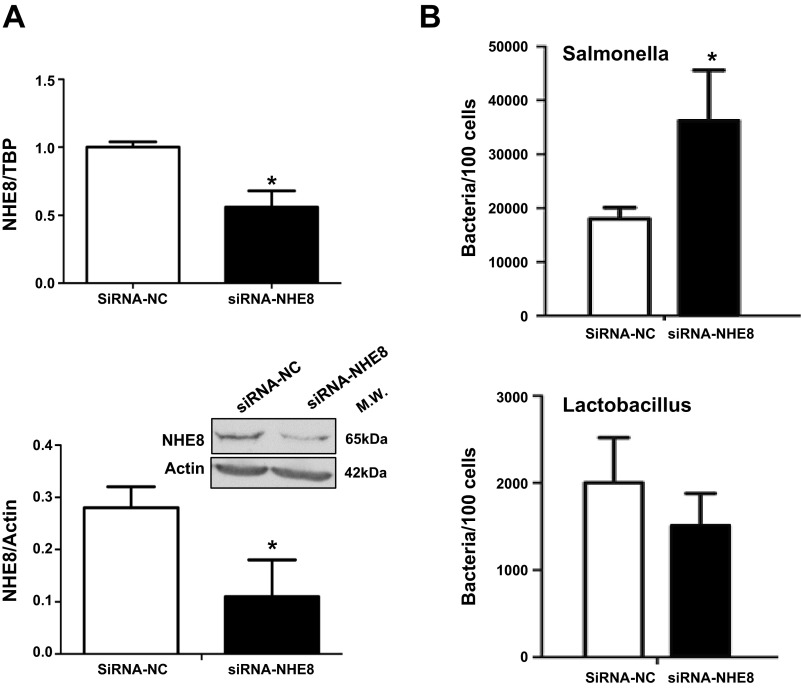
To further investigate the role of NHE8 in bacterial adhesion in the intestinal epithelial cells, NHE8 siRNA was used to silence NHE8 gene expression in Caco-2 cells. Caco-2 cells were transfected with NHE8 siRNAs, and knockdown efficiency was analyzed by measurement of NHE8 expression. As shown in Fig. 6A, NHE8 mRNA expression was reduced to 56% in siRNA-transfected cells 48 h after transfection (n = 3, P < 0.05), and NHE8 protein production was decreased to 40% in siRNA-transfected cells 72 h after transfection (n = 3, P < 0.05). NHE8 siRNA-induced inhibition of NHE8 expression was very specific, because NHE8 siRNAs had no effect on other NHEs (data not shown). To test the role of NHE8 protein in bacterial adhesion, normal Caco-2 cells or NHE8 siRNA-transfected Caco-2 cells were treated with S. typhimurium and L. plantarum for 2 h and adhered bacteria were determined by qPCR. As shown in Fig. 6B, S. typhimurium and L. plantarum could effectively adhere to normal and NHE8 siRNA-transfected Caco-2 cells. In nontransfected cells, the adhesion rate of S. typhimurium and L. plantarum was 18,000 ± 2,100 and 1,500 ± 380 per 100 cells, respectively. In siRNA-transfected cells, the adhesion rate of S. typhimurium was significantly increased to 36,200 ± 9,400 per 100 cells (n = 4, P < 0.05). However, no difference in L. plantarum adherence was seen in NHE8 siRNA-transfected cells (1,500 ± 380 per 100 cells).
Fig. 6.
NHE8 knockdown and bacterial adhesion assay in Caco-2 cells. A: Caco-2 cells were transfected with 5 nM NHE8 small interfering RNA (siRNA). Total RNA was isolated 48 h after transfection, and real-time PCR was performed to determine NHE8 mRNA expression (top). Total protein was extracted 72 h after transfection, and Western blotting was used to evaluate NHE8 protein expression (bottom). siRNA-NC, negative control siRNA; siRNA-NHE8, NHE8 siRNA. Values are means ± SE from 3 separate experiments. *P ≤ 0.01. B: NHE8 siRNA-transfected Caco-2 cells were infected with Salmonella typhimurium and Lactobacillus plantarum. Number of bacteria attached to cells was calculated by quantitative PCR using species-specific primers. Values are means ± SE from 5 separate experiments. *P ≤ 0.05.
Abundance of S. typhimurium attached on the colonic surface in NHE8−/− mice.
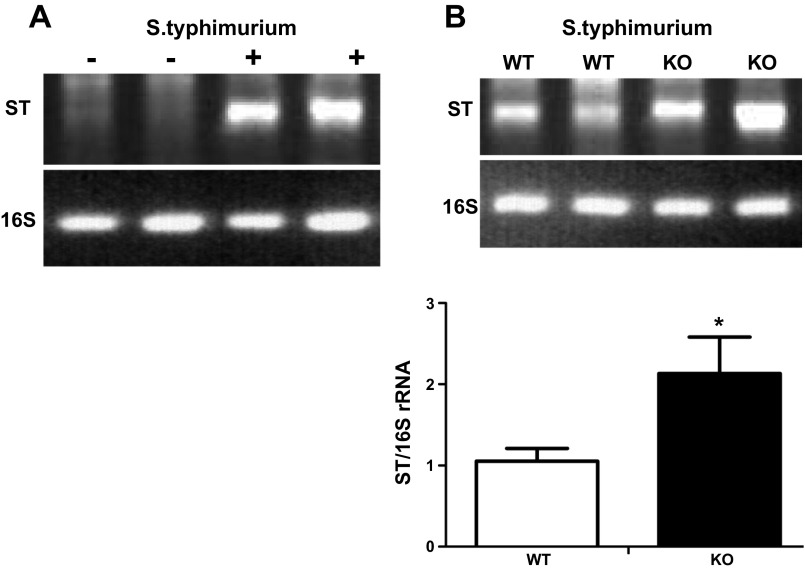
To test whether S. typhimurium has a similar adhesion pattern in vivo, wild-type and NHE8−/− mice were gavaged with S. typhimurium. At 7 days after gavage, the mice were euthanized and the distal colon was collected. Because the abundance of bacteria on the mucosal surface of the colon interfered with the qPCR, semiquantitative PCR using S. typhimurium-specific primers and conserved bacterial 16S rRNA primers was performed to determine the abundance of S. typhimurium adhered to the colonic surface. As shown in Fig. 7, S. typhimurium DNA was detected in mice gavaged with S. typhimurium, but not in mice gavaged with PBS (Fig. 7A). Furthermore, S. typhimurium adhesion on the mucosal surface of the distal colon was significantly increased in NHE8−/− mice compared with wild-type mice (1.05 ± 0.16 and 2.13 ± 0.45 S. typhimurium-specific primers/16S rRNA-specific primers in wild-type and NHE8−/− mice, respectively, n = 16, P < 0.05; Fig. 7B).
Fig. 7.
In vivo S. typhimurium adhesion assay. Wild-type and NHE8−/− mice were gavaged with S. typhimurium. Distal colon was collected 7 days after infection. Number of S. typhimurium attached to the epithelial surface was calculated by semiquantitative PCR using S. typhimurium-specific primers (ST) and general bacterial 16S rRNA primers (16S). A: PCR amplification of S. typhimurium and 16S rRNA in control (−) and infected (+) wild-type mice. B: relative changes of S. typhimurium bound to colonic mucosal surface in wild-type and NHE8−/− mice. Values are means ± SE from 16 mice. *P ≤ 0.05.
DISCUSSION
Mammalian NHE isoforms, even the four intestinal isoforms, vary in their functions, tissue distributions, and membrane localizations. NHE1 is located at the basolateral membrane and regulates intracellular pH and cellular volume. NHE2, NHE3, and NHE8 are located at the apical membrane in the intestinal epithelial cells and contribute to intestinal Na+ absorption. Na+ absorption early in development is primarily mediated by NHE8, whereas Na+ absorption in adults is primarily mediated by NHE2 and NHE3 (4, 35, 36). Loss of function for each individual NHE isoform showed various abnormalities in mice: growth retardation and epilepsy in NHE1−/− mice (2, 6), progressive loss of parietal cells in NHE2−/− mice (28), diarrhea, impaired acid-base balance, and a greater propensity to develop colon inflammation in NHE3−/− mice (18, 29), and decreased Muc2 gene expression in the colon and susceptibility to development of gastric ulcer in NHE8−/− mice (38, 42).
In the current study, we showed that loss of NHE8 expression resulted in a significant increase in the total number of attached bacteria in the distal colon in mice. Increased bacteria-epithelia interaction may trigger inflammatory responses. In fact, the expression of inflammatory cytokines, especially IL-4, was dramatically increased in the distal colon in NHE8−/− mice. For intestinal inflammation, IFNγ is a key effector of the T helper (Th) type 1 (Th1) response, and IL-4 is a major player in the Th type 2 (Th2) response (12, 13). Unlike NHE3−/− mice, which showed a dramatic increase in IFNγ expression (18), NHE8−/− mice showed significantly increased IL-4 expression. This suggests the presence of a Th2-like, rather than a Th1-like, response. Thus abnormal NHE8 function may contribute to gut inflammation.
Muc2 is the major colonic secretory mucin in humans and mice (16, 33). In the colon, Muc2 organizes the mucus into two layers, an inner layer and an outer layer. The inner layer, a well-organized, attached, and stratified mucus layer, is converted to a loose outer mucus layer (15, 16). The inner mucus layer is normally impenetrable to bacteria, whereas the outer is the habitat for the commensal bacteria (14–16). Loss of the inner mucus layer results in excessive bacteria on the surface of the epithelial cells and induces colitis (16). We reported previously that Muc2 gene expression was reduced in the colon in NHE8−/− mice (42). Recently, we showed that NHE3−/− mice have a mucus layer that is penetrable to bacteria (14). In the current study, we found that NHE8−/− mice have increased bacterial adhesion, elevated inflammatory cytokine expression, and disorganized mucin layer in the distal colon. Together, these observations suggest a role for NHE8 in colonic mucosal protection. Since NHE3 and NHE8 involve mucosal protection, it is possible that the absence of either of these transporters in the colon causes ion or pH alterations that result in a defective inner mucus layer. Future studies are needed to understand the mechanism(s) of NHE involvement in forming the mucin layer.
We previously reported that NHE8 expression in the small intestine was inhibited in 2,4,6-trinitrobenzene sulfonic acid-induced colitis and that TNFα inhibited NHE8 expression at the basal promoter activation level (34). Similar results were observed in mice treated with DSS. In the distal colon of DSS-treated wild-type mice, NHE8 expression was significantly reduced by 62% at the mRNA level and by 75% at the protein level. DSS treatment also stimulated colonic TNFα production by 12-fold at the mRNA level and by 110-fold at the protein level. Because TNFα production was increased by DSS treatment, the reduced NHE8 expression observed here is most likely due to the elevated TNFα level.
Our previous study showed that mice lacking NHE3 expression were susceptible to DSS treatment (17). In the current study, we showed that loss of NHE8 expression in mice also increased susceptibility to DSS-induced intestinal epithelial injury. Compared with wild-type mice, DSS-treated NHE8−/− mice developed more rapid body weight loss, accelerated mortality, and higher DAI. Thus normal NHE8 function is important for protecting the epithelia from injury in the colon. Since TNFα expression did not increase further in DSS-treated NHE8−/− mice, the low survival rate in DSS-treated NHE8−/− mice was unlikely the result of excessive TNFα production. The cause of high mortality in DSS-treated NHE8−/− mice remains unclear.
NHE8−/− mice displayed a disorganized mucus layer and increased bacterial adhesion in the distal colon, which suggests that NHE8 may play a role in regulating bacterial adhesion. To understand whether NHE8 participates in bacterial adhesion, we tested the attachment of S. typhimurium and L. plantarum to cultured Caco-2 cells. S. typhimurium, one of the most common food-borne enteropathogens, adheres to the brush border of intestinal cells to damage the tight junction and cause severe diarrhea (11, 30). L. plantarum, a nonpathogen bacterium, is widely used in the probiotic industry. Under normal conditions, S. typhimurium and L. plantarum could adhere to the surface of Caco-2 cells. In Caco-2 cells transfected with NHE8 siRNA, NHE8 expression was significantly reduced at mRNA and protein levels, and the number of attached bacteria was increased by twofold in S. typhimurium, but not in L. plantarum. Further in vivo study using S. typhimurium-infected mice confirmed this observation. The adhesion rate of S. typhimurium in the distal colon was twofold higher in NHE8−/− than wild-type mice. These findings indicate that NHE8 protein indeed protected the host from bacterial adhesion, and the protective role is likely via its selective effect on blocking infectious bacterial adherence to the intestinal epithelial cells. Future studies are needed to explore the mechanism of NHE8 against enteropathogenic bacteria, such as S. typhimurium infection.
In summary, in NHE8−/− mice, bacterial adhesion was increased in the disorganized mucus layer, which likely triggered a Th2-like inflammatory response. DSS treatment greatly reduced NHE8 expression, and loss of NHE8 increased susceptibility to DSS-induced mucosal injury in mice. Furthermore, lack of NHE8 expression in epithelial cells resulted in significantly enhanced adhesion of enteropathogenic S. typhimurium in cultured intestinal epithelial cells and in mice. We concluded that NHE8 plays important roles in preventing commensal bacteria from reaching the epithelial surface via its effect on mucus layer formation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-073638 and Swedish Research Council and Swedish Foundation for Strategic Research Grant U01AI095473.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L., H.X., B.Z., and F.K.G. are responsible for conception and design of the research; C.L., H.X., B.Z., M.E.J., and J.L. performed the experiments; C.L., H.X., B.Z., M.E.J., J.L., and G.C.H. analyzed the data; C.L., H.X., B.Z., M.E.J., J.L., and G.C.H. interpreted the results of the experiments; C.L., H.X., and M.E.J. prepared the figures; C.L. and H.X. drafted the manuscript; H.X. and F.K.G. edited and revised the manuscript; H.X. and F.K.G. approved the final version of the manuscript.
REFERENCES
- 1. Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun 76: 907– 915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol 276: C788– C795, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Candela M, Seibold G, Vitali B, Lachenmaier S, Eikmanns BJ, Brigidi P. Real-time PCR quantification of bacterial adhesion to Caco-2 cells: competition between bifidobacteria and enteropathogens. Res Microbiol 156: 887– 895, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937– C1946, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Ontogeny of basolateral membrane sodium-hydrogen exchange (NHE) activity and mRNA expression of NHE-1 and NHE-4 in rat kidney and jejunum. Biochim Biophys Acta 1369: 247– 258, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Cox GA, Lutz CM, Yang CL, Biemesderfer D, Bronson RT, Fu A, Aronson PS, Noebels JL, Frankel WN. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell 91: 139– 148, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Ghishan FK, Kiela PR. Small intestinal ion transport. Curr Opin Gastroenterol 28: 130– 134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol 288: F530– F538, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol 284: F467– F473, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol 47: 367– 373, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol 6: 53– 66, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Iijima H, Takahashi I, Kishi D, Kim JK, Kawano S, Hori M, Kiyono H. Alteration of interleukin 4 production results in the inhibition of T helper type 2 cell-dominated inflammatory bowel disease in T cell receptor α-chain-deficient mice. J Exp Med 190: 607– 615, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ince MN, Elliott DE. Immunologic and molecular mechanisms in inflammatory bowel disease. Surg Clin North Am 87: 681– 696, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Johansson ME, Gustafsson JK, Holmén-Larsson JM, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and in patients with ulcerative colitis. Gut. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA 108 Suppl 1: 4659– 4665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064– 15069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiela PR, Laubitz D, Larmonier CB, Midura-Kiela MT, Lipko MA, Janikashvili N, Bai A, Thurston R, Ghishan FK. Changes in mucosal homeostasis predispose NHE3 knockout mice to increased susceptibility to DSS-induced epithelial injury. Gastroenterology 137: 965– 975, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laubitz D, Larmonier CB, Bai A, Midura-Kiela MT, Lipko MA, Thurston RD, Kiela PR, Ghishan FK. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol 295: G63– G77, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linden SK, Florin TH, McGuckin MA. Mucin dynamics in intestinal bacterial infection. PLos One 3: e3952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu C, Zhang ZY, Dong K, Guo XK. Adhesion and immunomodulatory effects of Bifidobacterium lactis HN019 on intestinal epithelial cells INT-407. World J Gastroenterol 16: 2283– 2290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402– 408, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Matsuki T, Watanabe K, Fujimoto J, Miyamoto Y, Takada T, Matsumoto K, Oyaizu H, Tanaka R. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl Environ Microbiol 68: 5445– 5451, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148: 257– 266, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflügers Arch 447: 549– 565, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem 267: 9331– 9339, 1992 [PubMed] [Google Scholar]
- 26. Rinttila T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol 97: 1166– 1177, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Sasaki M, Mathis JM, Jennings MH, Jordan P, Wang Y, Ando T, Joh T, Alexander JS. Reversal of experimental colitis disease activity in mice following administration of an adenoviral IL-10 vector. J Inflamm 2: 13, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest 101: 1243– 1253, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282– 285, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Srikanth CV, Mercado-Lubo R, Hallstrom K, McCormick BA. Salmonella effector proteins and host-cell responses. Cell Mol Life Sci 68: 3687– 3697, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trkov M, Avgustin G. An improved 16S rRNA based PCR method for the specific detection of Salmonella enterica. Int J Food Microbiol 80: 67– 75, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117– 129, 2006 [DOI] [PubMed] [Google Scholar]
- 33. van Klinken BJ, Einerhand AW, Duits LA, Makkink MK, Tytgat KM, Renes IB, Verburg M, Buller HA, Dekker J. Gastrointestinal expression and partial cDNA cloning of murine Muc2. Am J Physiol Gastrointest Liver Physiol 276: G115– G124, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK. Tumor necrosis factor-α downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol 296: C489– C497, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109– 116, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36– G41, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Xu H, Li J, Chen H, Wang C, Ghishan FK. NHE8 plays important roles in gastric mucosal protection. Am J Physiol Gastrointest Liver Physiol 304: G257– G261, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu H, Li J, Chen R, Zhang B, Wang C, King N, Chen H, Ghishan FK. NHE2X3 DKO mice exhibit gender-specific NHE8 compensation. Am J Physiol Gastrointest Liver Physiol 300: G647– G653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu H, Zhang B, Li J, Chen H, Tooley J, Ghishan FK. Epidermal growth factor inhibits intestinal NHE8 expression via reducing its basal transcription. Am J Physiol Cell Physiol 299: C51– C57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu H, Zhang B, Li J, Chen H, Wang C, Ghishan FK. Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5. Am J Physiol Gastrointest Liver Physiol 299: G921– G927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu H, Zhang B, Li J, Wang C, Chen H, Ghishan FK. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol 303: G335– G343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1– G11, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411– 443, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Zhang ZY, Liu C, Zhu YZ, Wei YX, Tian F, Zhao GP, Guo XK. Safety assessment of Lactobacillus plantarum JDM1 based on the complete genome. Int J Food Microbiol 153: 166– 170, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Zhang ZY, Liu C, Zhu YZ, Zhong Y, Zhu YQ, Zheng HJ, Zhao GP, Wang SY, Guo XK. Complete genome sequence of Lactobacillus plantarum JDM1. J Bacteriol 191: 5020– 5021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]