Abstract
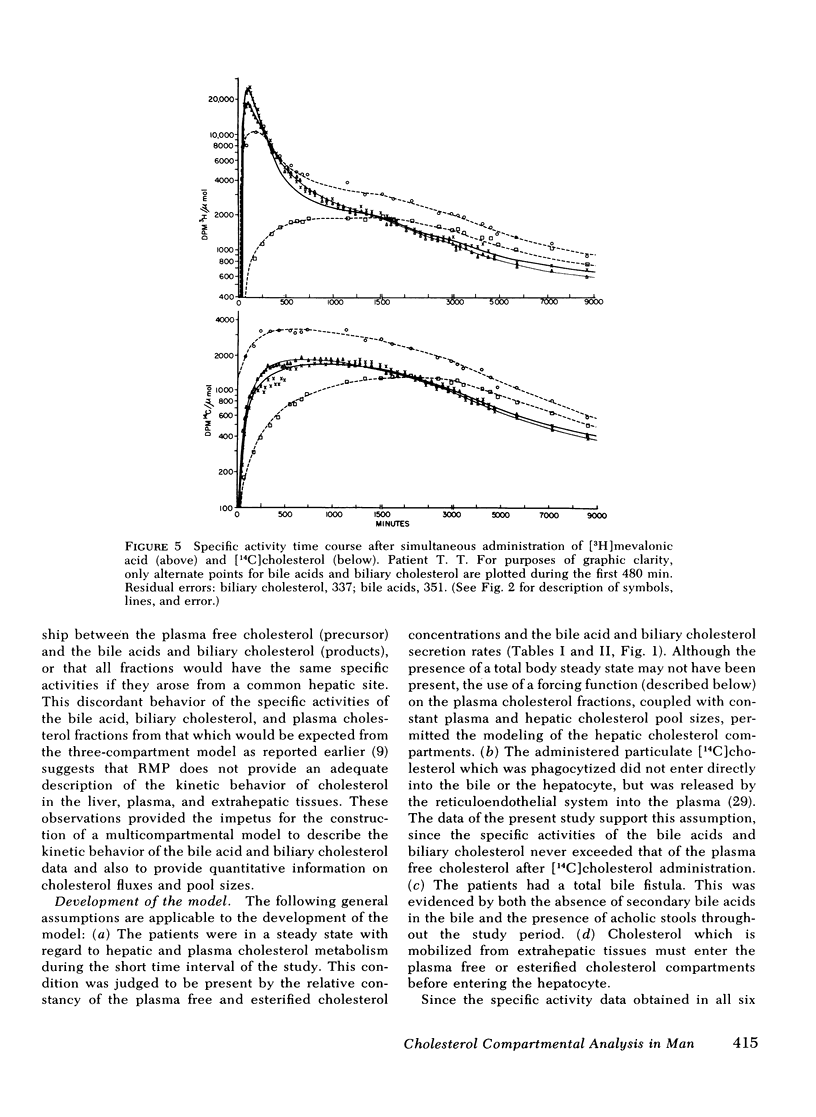
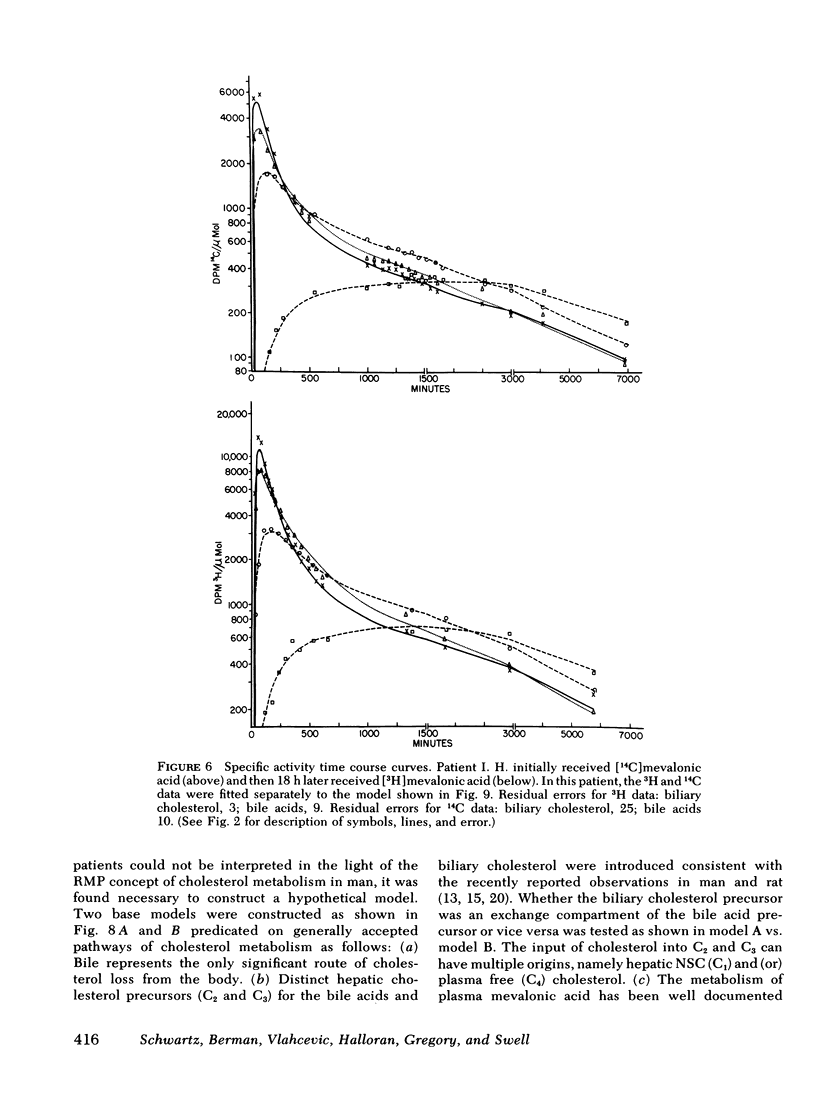
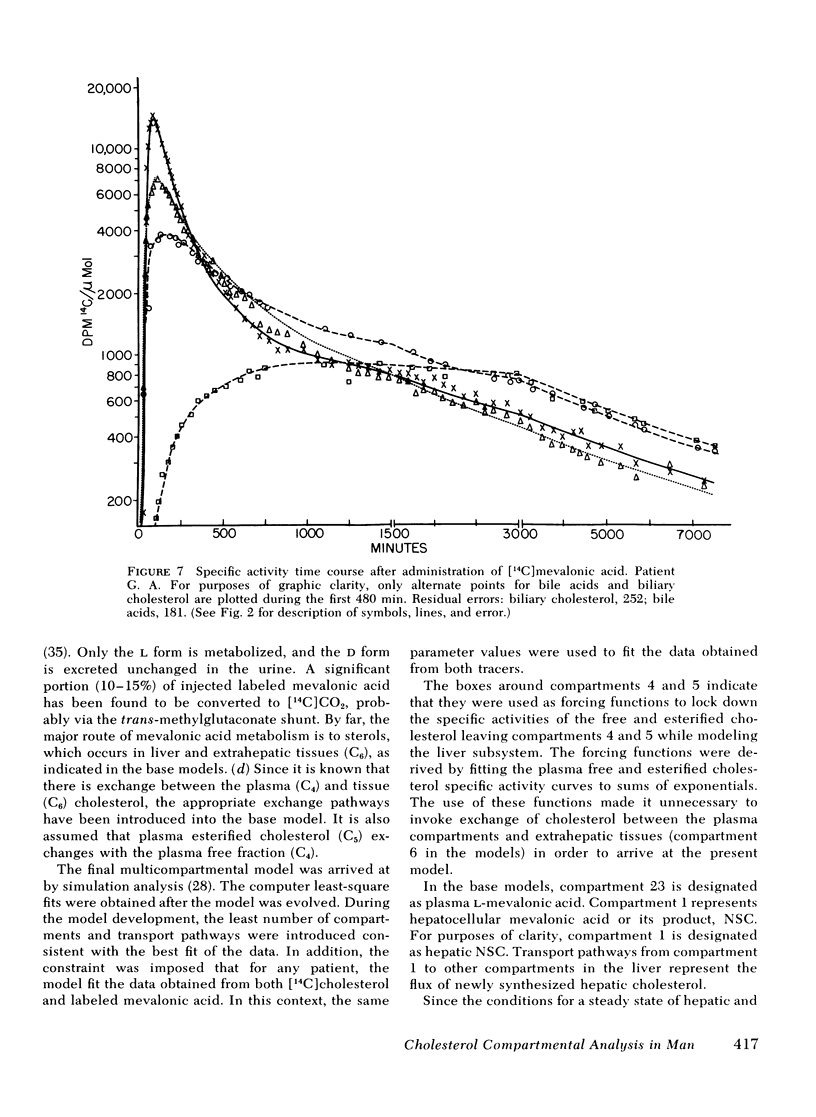
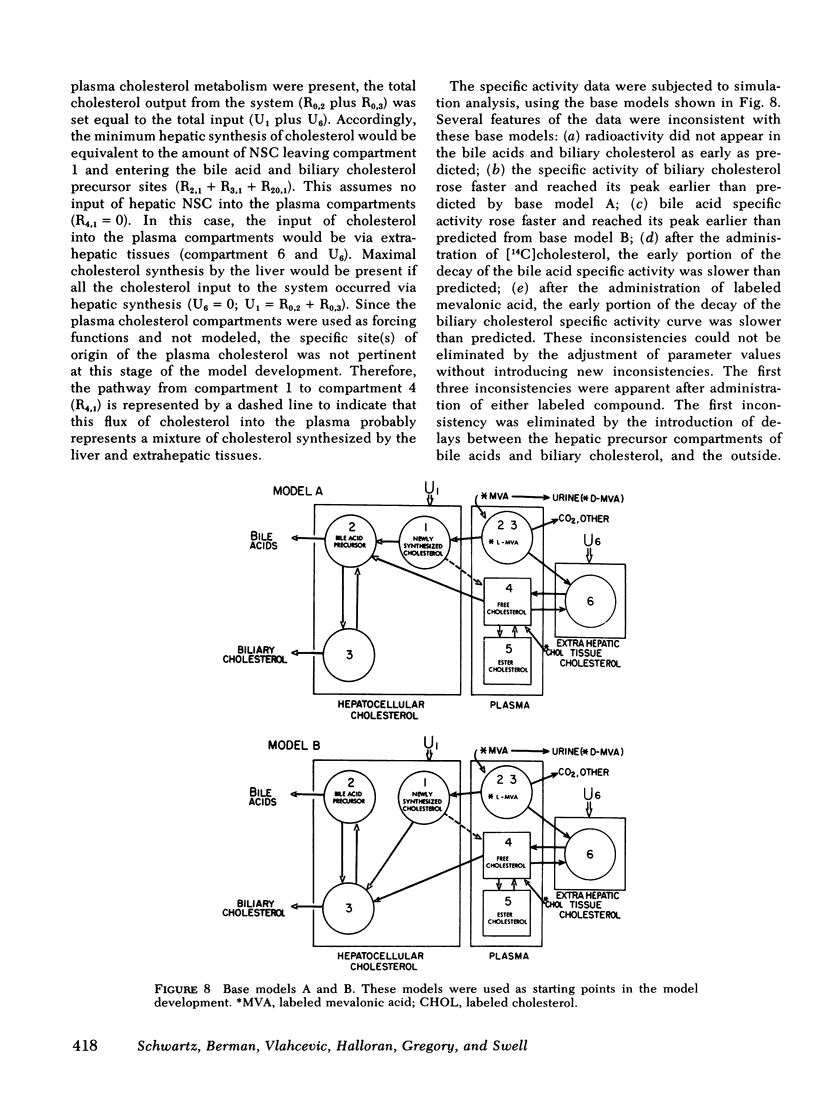
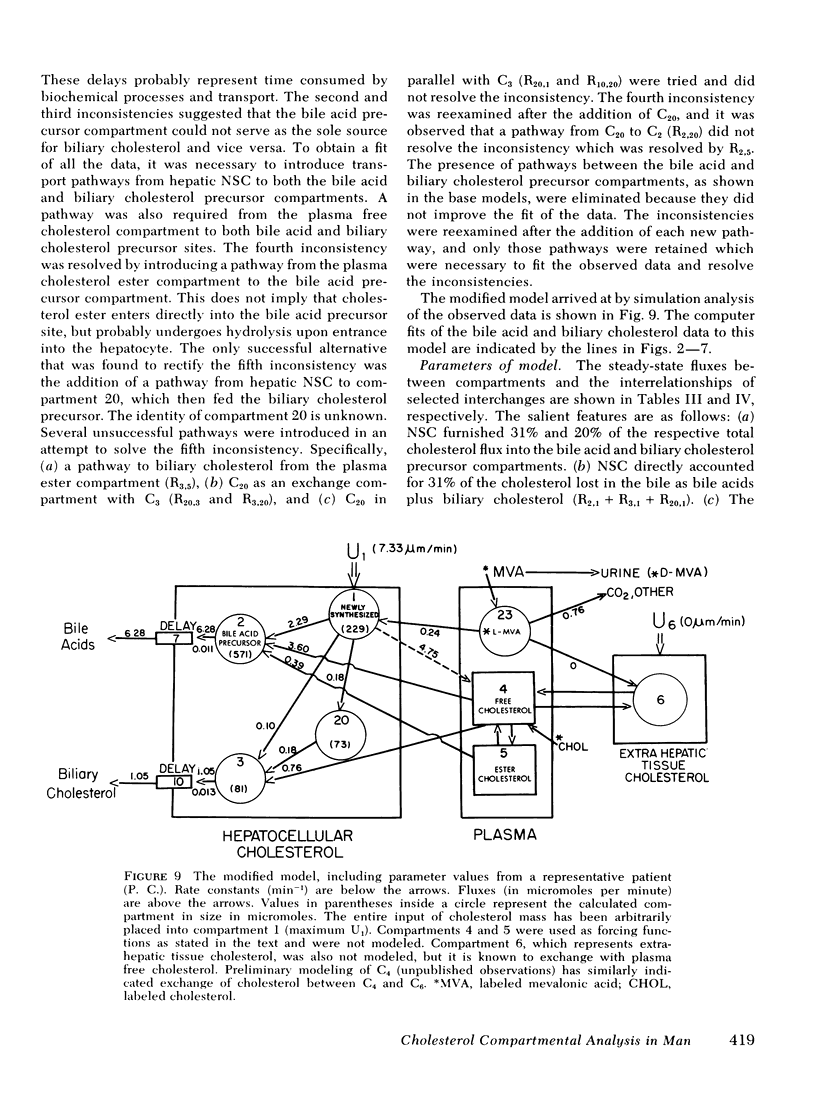
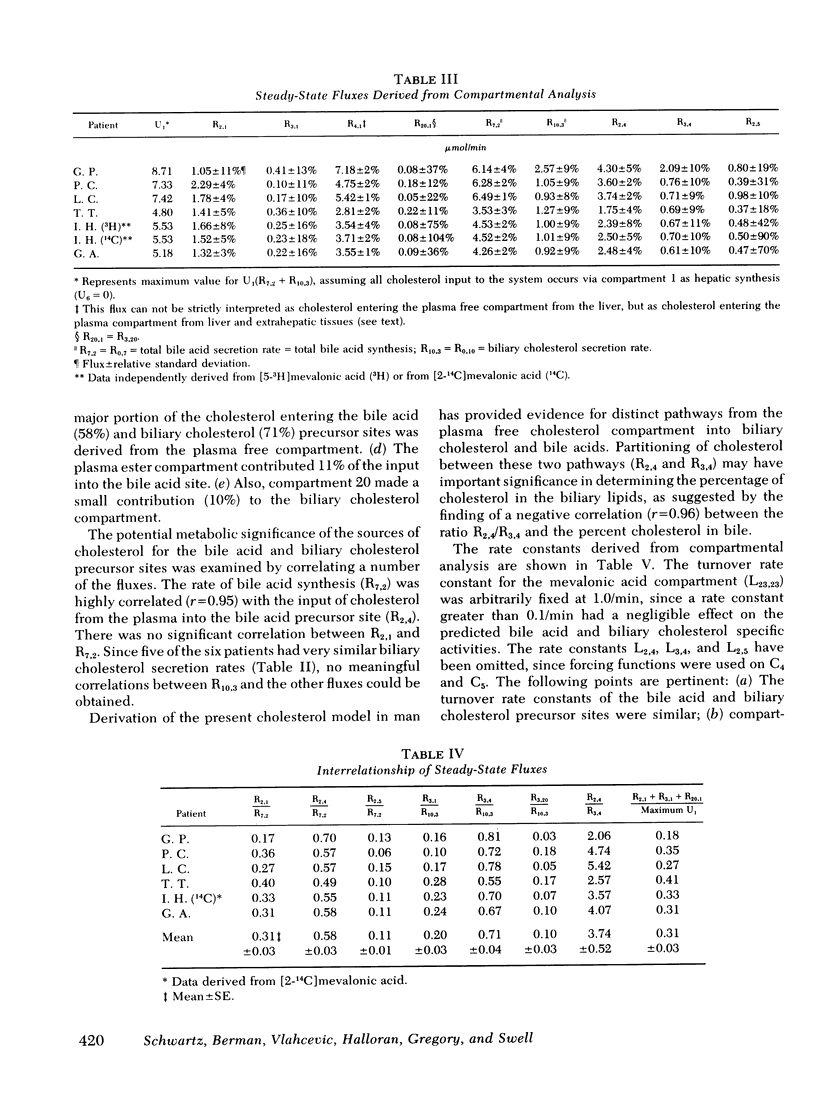
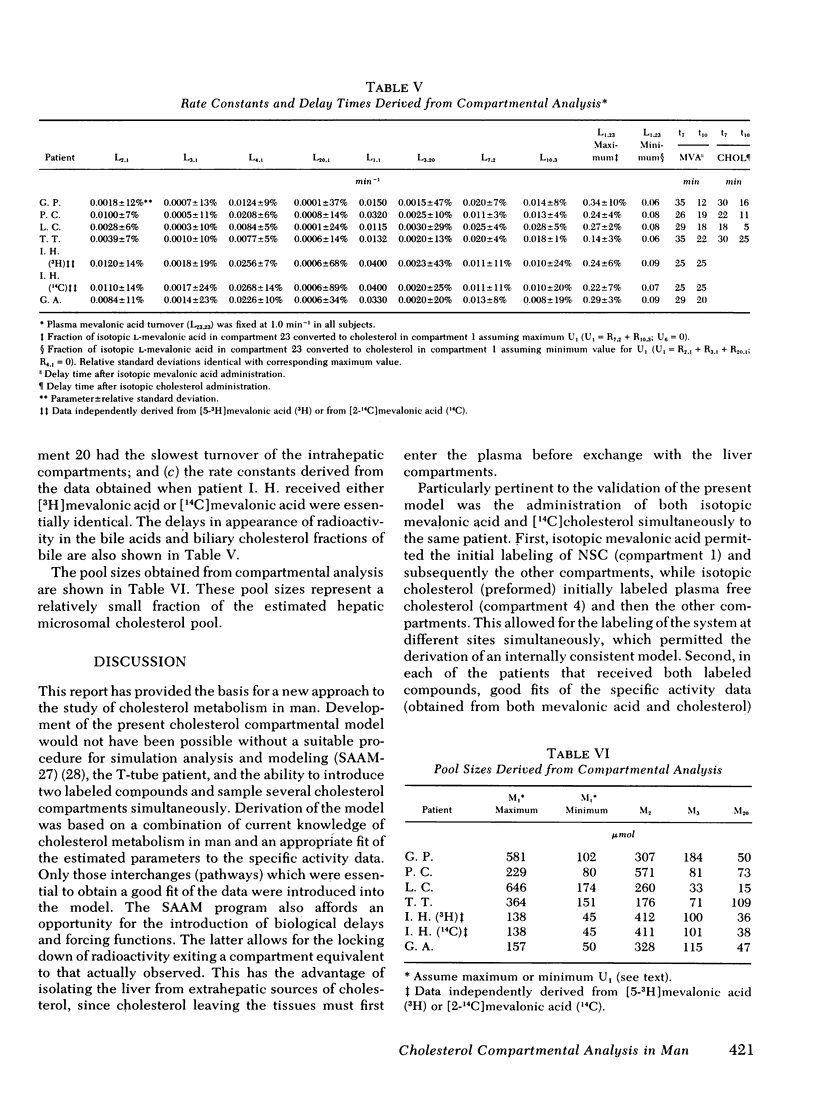
The present report has presented the first clear evidence in man for the existence of specific hepatic cholesterol precursor sites associated with the formation and secretion of bile acids and biliary cholesterol. These hepatic compartments derive virtually all their cholesterol from newly synthesized and lipoprotein free cholesterol. The model which is presented was formulated on current concepts of cholesterol metabolism in man and is concerned, at this initial stage, with the elucidation of the bile acid and biliary cholesterol compartments. The complexity of cholesterol metabolism in man necessitated an initial approach that would minimize the number of inputs of cholesterol into the system, allow for the sampling of several cholesterol compartments, and permit the simultaneous labeling of newly synthesized cholesterol and preformed cholesterol. To achieve these objectives, we studied the patient with a total bile fistula. Six patients were administered simultaneously pulse injections of labeled mevalonic acid and [14C]cholesterol. The qualitative features of the specific activity time course curves after labeled mevalonic acid revealed no precursor-product relationship between bile acid, biliary cholesterol, and plasma free cholesterol. The peak specific activity of the bile acids was reached in approximately 100 min and was higher than the biliary cholesterol, which was higher than the plasma free cholesterol. The plasma free cholesterol specific activity became higher than the other lipids after 12 h and remained higher throughout the period of study. Similar related observations were made with [14C]cholesterol. The data were then subjected to simulation analysis and modeling using the SAAM-27 computer program. Computer least-square fits of the data were obtained after the model was evolved. During the model development, the least number of compartments and transport pathways were introduced consistent with a good fit of the data. Of particular importance was the constraint that the model fit the data obtained from both [14C]cholesterol and labeled mevalonic acid. The same parameter values were used to fit the data from both tracers. The fluxes arrived at in the model indicate that 31% and 20%, respectively, of the cholesterol input into the bile acid and biliary cholesterol precursor sites were derived directly from the newly synthesized hepatic cholesterol. The remainder had its origin predominantly from lipoprotein free cholesterol. Plasma esterified cholesterol (as free) made a small contribution (11%) to the bile acid compartment. Similarly, 10% of the biliary cholesterol arose from an unknown hepatic site.
The present report has provided the basis for a new procedure for studying in vivo cholesterol metabolism in man. Examination of the derived cholesterol flux rates between the compartments suggests the presence of an important mechanism regulating the partitioning of lipoprotein free cholesterol between the bile acid and biliary cholesterol precursor sites. Aberrations in the proportioning of precursor cholesterol between these sites could be a causative factor precipitating the excessive secretion of biliary cholesterol and the production of lithogenic bile.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Balasubramaniam S., Mitropoulos K. A., Myant N. B. Evidence for the compartmentation of cholesterol in rat-liver microsomes. Eur J Biochem. 1973 Apr 2;34(1):77–83. doi: 10.1111/j.1432-1033.1973.tb02730.x. [DOI] [PubMed] [Google Scholar]
- Bennion L. J., Grundy S. M. Effects of obesity and caloric intake on biliary lipid metabolism in man. J Clin Invest. 1975 Oct;56(4):996–1011. doi: 10.1172/JCI108180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronholm T., Burlingame A. L., Sjövall J. Utilization of the carbon and hydrogen atoms of ethanol in the biosynthesis of steroids and bile acids. Eur J Biochem. 1974 Dec 2;49(3):497–510. doi: 10.1111/j.1432-1033.1974.tb03854.x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fogelman A. M., Edmond J., Popjåk G. Metabolism of mevalonate in rats and man not leading to sterols. J Biol Chem. 1975 Mar 10;250(5):1771–1775. [PubMed] [Google Scholar]
- Goad L. J. Sterol biosynthesis. Biochem Soc Symp. 1970;29:45–77. [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P., Dell R. B. Three-pool model of the long-term turnover of plasma cholesterol in man. J Lipid Res. 1973 Mar;14(2):178–188. [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P. Turnover of plasma cholesterol in man. J Clin Invest. 1968 Feb;47(2):231–241. doi: 10.1172/JCI105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr Measurements of cholesterol turnover, synthesis, and absorption in man, carried out by isotope kinetic and sterol balance methods. J Lipid Res. 1969 Jan;10(1):91–107. [PubMed] [Google Scholar]
- HIRSCH J., AHRENS E. H., Jr The separation of complex lipide mixtures by the use of silicic acid chromatography. J Biol Chem. 1958 Aug;233(2):311–20. [PubMed] [Google Scholar]
- LINDSTEDT S. The turnover of cholic acid in man: bile acids and steroids. Acta Physiol Scand. 1957 Sep 17;40(1):1–9. doi: 10.1111/j.1748-1716.1957.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Liu G. C., Ahrens E. H., Jr, Schreibman P. H., Samuel P., McNamara D. J., Crouse J. R. Measurement of cholesterol synthesis in man by isotope kinetics of squalene. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4612–4616. doi: 10.1073/pnas.72.11.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos K. A., Myant N. B., Gibbons G. F., Balasubramaniam S., Reeves B. E. Cholesterol precursor pools for the synthesis of cholic and chemodeoxycholic acids in rats. J Biol Chem. 1974 Oct 10;249(19):6052–6056. [PubMed] [Google Scholar]
- Nestel P. J., Kudchodkar B. Plasma squalene as an index of cholesterol synthesis. Clin Sci Mol Med. 1975 Dec;49(6):621–624. doi: 10.1042/cs0490621. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M., Goodman D. S. Distribution and turnover of cholesterol in humans. J Clin Invest. 1969 Jun;48(6):982–991. doi: 10.1172/JCI106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A., Zilversmit D. B. Fate of intravenously administered particulate and lipoprotein cholesterol in the rat. J Lipid Res. 1972 Jan;13(1):32–38. [PubMed] [Google Scholar]
- Normann P. T., Norum K. R. Newly synthesized hepatic cholesterol as precursor for cholesterol and bile acids in rat bile. Scand J Gastroenterol. 1976;11(4):427–432. [PubMed] [Google Scholar]
- Ogura M., Shiga J., Yamasaki K. Studies on the cholesterol pool as the precursor of bile acids in the rat. J Biochem. 1971 Dec;70(6):967–972. doi: 10.1093/oxfordjournals.jbchem.a129726. [DOI] [PubMed] [Google Scholar]
- Quarfordt S. H., Greenfield M. F. Estimation of cholesterol and bile acid turnover in man by kinetic analysis. J Clin Invest. 1973 Aug;52(8):1937–1945. doi: 10.1172/JCI107378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- STAPLE E., GURIN S. The incorporation of radioactive acetate into biliary cholesterol and cholic acid. Biochim Biophys Acta. 1954 Nov;15(3):372–376. doi: 10.1016/0006-3002(54)90039-6. [DOI] [PubMed] [Google Scholar]
- Samuel P., Lieberman S. Improved estimation of body masses and turnover of cholesterol by computerized input--output analysis. J Lipid Res. 1973 Mar;14(2):189–196. [PubMed] [Google Scholar]
- Samuel P., Perl W. Long-term decay of serum cholesterol radioactivity: body cholesterol metabolism in normals and in patients with hyperlipoproteinemia and atherosclerosis. J Clin Invest. 1970 Feb;49(2):346–357. doi: 10.1172/JCI106243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. C., Cohen B. I., Vlahcevic Z. R., Gregory D. H., Halloran L. G., Kuramoto T., Mosbach E. H., Swell L. Quantitative aspects of the conversion of 5 beta-cholestane intermediates to bile acids in man. J Biol Chem. 1976 Oct 25;251(20):6308–6314. [PubMed] [Google Scholar]
- Schwartz C. C., Vlahcevic Z. R., Halloran L. G., Gregory D. H., Meek J. B., Swell L. Evidence for the existence of definitive hepatic cholesterol precursor compartments for bile acids and biliary cholesterol in man. Gastroenterology. 1975 Dec;69(6):1379–1382. [PubMed] [Google Scholar]
- Smith F. R., Dell R. B., Noble R. P., Goodman D. S. Parameters of the three-pool model of the turnover of plasma cholesterol in normal and hyperlipidemic humans. J Clin Invest. 1976 Jan;57(1):137–148. doi: 10.1172/JCI108253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swell L., Gregory D. H., Vlahcevic Z. R. Current concepts of the pathogenesis of cholesterol gallstones. Med Clin North Am. 1974 Nov;58(6):1449–1471. doi: 10.1016/s0025-7125(16)32083-1. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Miller J. R., Farrar J. T., Swell L. Kinetics and pool size of primary bile acids in man. Gastroenterology. 1971 Jul;61(1):85–90. [PubMed] [Google Scholar]



