Abstract
Purpose
This comparison employs mathematical disease progression models to identify a rat model of arthritis with the least inter-animal variability and features lending to better study designs.
Methods
Arthritis was induced with either collagen (CIA) or mycobacterium (AIA) in either Lewis or Dark Agouti (DA) rats. Disease progression was monitored by paw edema and body weight. Models with production, loss, and feedback components were constructed and population analysis using NONMEM software was employed to identify inter-animal variability in the various disease progression parameters.
Results
Onset time was the only parameter different within all four groups (DA–AIA 11.5 days, DA–CIA 16.5 days, Lewis–AIA 11.9 days, Lewis–CIA 13.9 days). The loss-of-edema rate constant was 20% slower in DA (0.362 h−1) than Lewis (0.466 h−1) rats. Most models exhibited peak paw edema 20 days post-induction. Edema in CIA returned to 150% of the initial value after the disease peaked. DA rats displayed more severe overall responses.
Conclusions
No statistical differences between groups were observed for inter-animal variation in disease onset, progression and severity parameters. Onset time varies and should be noted in the design of future studies. DA rats may offer a more dynamic range of edema response than Lewis rats.
Keywords: arthritis, disease, model, progression, rat
INTRODUCTION
Human rheumatoid arthritis (RA) is genetically complex and may originate for many reasons producing numerous variable disease pathologies. In animal models the disease is often induced by an antigen (e.g. heat-inactivated mycobacterium, type-II collagen, pristane, avridine) presented to the animal’s immune system in the dermis (1–7). Although induced by different means the animal disease may reflect a specific pathology of human RA. Additionally, adjuvant induced animal models have been valuable in discerning different pathways by which arthritis may develop. They provide an alternative when human tissue is unavailable and permit the investigator to design studies that best tests hypotheses of interest. Transgenic mouse models have been useful in identifying disease relevant genes, but successful mice adjuvant induced models (collagen, pristane) are few as mice generally do not respond well to adjuvants (3,7–10). Collagen-induced arthritis (CIA) and the mycobacterium adjuvant-induced arthritis (AIA) models in rats provide an inducible and severe arthritis, allow easier surgeries and tissue sampling and, in the case of CIA, provide a chronic relapsing animal model of RA (1).
Of the different adjuvant-induced arthritis models developed, the use of mycobacterium in mineral oil or Freund’s complete adjuvant appears to be the most common (1,7). Heat-inactivated mycobacterium are suspended in mineral oil and administered to the rat by intra-dermal injection at the base of the tail. Cell wall components of the mycobacterium are thought to be the antigenic factors initiating the inflammatory response (11,12). This model of inflammation was established in 1956 and has provided a robust animal model of arthritis/inflammation in peripheral joint tissue (3,6,7). Unlike CIA, AIA does not appear to be a chronic relapsing disease (13–16). Adjuvant induced arthritis arises in rats as early as 11 days and regression occurs as early as day 25 (1,13,16). Visible symptoms include redness and swelling in the paws and leg joints. Molecular pathology involves the up-regulation of many transcription factors, cyto/chemokines, proteases, and immune cell proliferation (7,17–19). AIA also appears to be more systemically involved and not localized to the peripheral joints. The liver, spleen, and organs other than joint tissue are also affected more in these animals (1).
Collagen-induced arthritis is a more recent model of human RA than AIA and appears to be more clinically relevant. Autologous or heterologous collagen type II is delivered in a dense emulsion of acetic acid and mineral oil by intradermal injection, similar to that done for AIA models (4,9,10,20,21). Paw swelling and molecular factors such as cytokines arise 2–5 days later in CIA than in AIA. With CIA, the disease does not regress as early or affect as many organs as extensively as AIA. Features of human RA present in CIA, but not in AIA include circulating cartilage oligomeric matrix protein and auto-immunity to cartilage driven by the presence of B-cells and auto-antibodies (2,4,14,20,22–25). Although CIA maintains a greater TH2-cell response than AIA, it is generally recognized that joint inflammation is TH1-cell driven and that these cells are the primary driving force of joint erosion and edema in CIA, AIA, and human RA.
Although CIA and AIA represent single disease pathologies, high inter-animal variability remains during the onset, progression, and in the overall severity of the inflammatory response. In the literature Lewis rats have appeared to show an incomplete susceptibility to disease induction whereas Dark–Agouti (DA) rats have exhibited a more complete incidence and pronounced severity, with the arthritis symmetrically appearing in all peripheral joints (1,26). Adjuvant-induced arthritis (AIA) has been assessed in our lab; however, collagen-induced arthritis (CIA) is expected to be a less variable model of RA in the onset, progression, and severity of the disease.
Inter-animal variability in each treatment group and strain was assessed by population disease progression modeling of paw swelling in order to discern differences in inter-animal variability for each strain (i.e. Lewis, DA) and each treatment (i.e. CIA, AIA). The resulting measures of variation for CIA were compared with those from AIA in Lewis and DA rats for statistical significance. The least variable rat model of arthritis was sought for future pharmacokinetic and pharmacodynamic (PK/PD) experiments and features of the disease time course relevant to the design of these studies. This work not only tests inter-animal variability in disease response but is also a direct comparison of the time course of arthritis progression in the four most common rat models of arthritis, highlighting the onset, progression, and peak changes in paw edema and body weight.
METHODS
Materials/Reagents
Heat-inactivated mycobacterium butyricum was purchased from DIFCO (Detroit, MI, USA). Type II porcine collagen in acetic acid was purchased from Chondrex, Inc. (Redmond, WA, USA). Heavy mineral oil and incomplete Freund’s adjuvant were purchased from Sigma Aldrich (St. Louis, MO, USA).
Animals
Male Lewis and Dark Agouti rats, age 6 to 9 weeks, were purchased from Harlan Sprague Dawley, Inc. weight matched to approximately 150 g. Animals were housed individually in the University Laboratory Animal Facility and acclimatized for 1 week under constant temperature (22°C), humidity (72%) and 12 h light/12 h dark cycle. Rats had free access to rat chow and water. All protocols followed Principles of Laboratory Animal Care (National Institute of Health publication 85–23, revised 1985) and were approved by the University at Buffalo Institutional Animal Care and Use Committee.
Induction of Adjuvant-induced Arthritis
Male Dark Agouti and Lewis Rats were anesthetized with ketamine/xylazine (75/10 mg/kg). Four rats from each strain each received 0.5 mg of mycobacterium suspended in 0.1 mL heavy mineral oil by intra-dermal injection at the base of the tail. Suspension of the mycobacterium was achieved by passing the mixture of mineral oil and mycobacterium between two syringes through an 18 gauge needle. After the initial injection, animals were returned to their housing and monitored daily for signs of arthritis.
Induction of Collagen-Induced Arthritis
The induction of CIA in Dark Agouti and Lewis Rats followed protocols supplied by Chondrex, Inc. Porcine collagen type II (2 mg/mL) in 0.05 M acetic acid was emulsified with incomplete Freund’s adjuvant using an electric homogenizer (Virtis) equipped with a blade of 10 mm in diameter. Equal amounts of collagen (2 mg/mL) and Incomplete Freund’s Adjuvant (IFA) were mixed in an ice water bath, adding the collagen drop-wise to the IFA at low speed. The Virtis speed was increased to 30,000 rpm for 2.5 min, then 0 rpm for 2.5 min, and a final mix at 30,000 rpm for 2.5 min. The emulsion was ready when it appeared to be a stiff white substance that congealed instead of dissipating when dropped in water. Ensuring proper time for the solution to cool in the ice bath is critical to prevent collagen degradation. Four rats from each strain were anesthetized with ketamine/xylazine (75/10 mg/kg) and received 0.2 mL of collagen emulsion by intra-dermal injection at the base of the tail. Booster injections were administered on the seventh day of the study with 0.1 mL emulsion at the same injection site.
Measurement of Edema
Edema was evaluated as swelling of the rat’s hind paws. Two cross-sectional areas were determined with digital calipers, one on the forefoot (paw) and the other at the ankle (Fig. 1). Two measurements were made on each section, perpendicular to each other, to define the length and height of the ellipse from which the circumference was determined. Measurements were made side-to-side (a) and top-to-bottom (b) across the paw at the base of the last foot pad. Measurements on the ankle were made side-to-side and front-to-back at a 45° angle across the ankle. The ellipse area was determined by
Fig. 1.
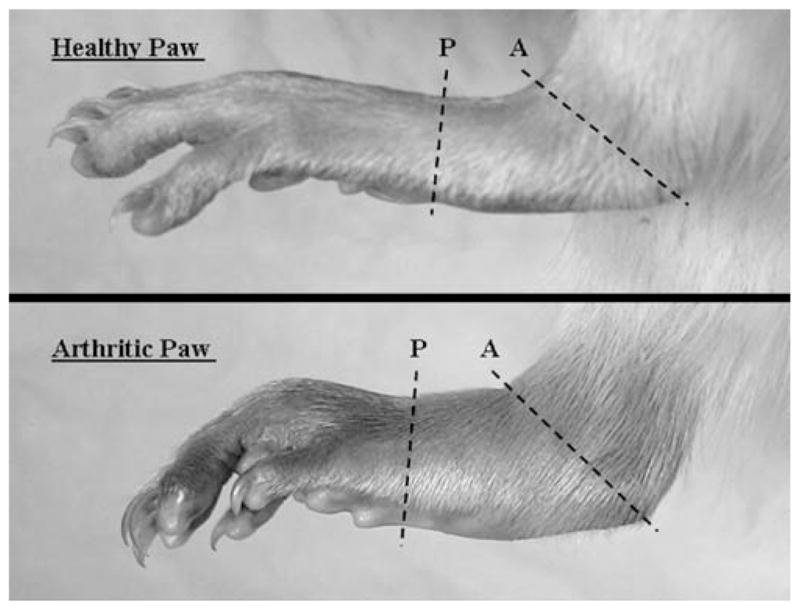
Diagram for determination of paw swelling circumference. Each panel indicates paw (P) and ankle (A) measurement locations to determine the approximate circumferences on study day 20.
| (1) |
The cross-sectional slices are shown in Fig. 1 for healthy and arthritic rat paws.
Edema measurements were made just prior to disease induction and every 2 days thereafter until day 23 for rats with AIA and day 34 for rats with CIA. Paw and ankle edema areas were added to produce one overall measure for the rat’s hind paw. These values were normalized by their respective start values at day zero to reduce variation from natural growth differences in paw size. Positive deviations from 1.0 indicate swelling.
Pharmacodynamic Disease Progression Model
A pharmacodynamic model was constructed to characterize the time course of paw or ankle edema in AIA or CIA in Lewis or Dark Agouti Rats. Fig. 2 is a schematic of the model. Despite differences between groups in the progression of the disease the base model was designed to capture the delay in disease onset, the rate of disease onset, the peak edema, and rate of remission. Paw edema was described by
Fig. 2.
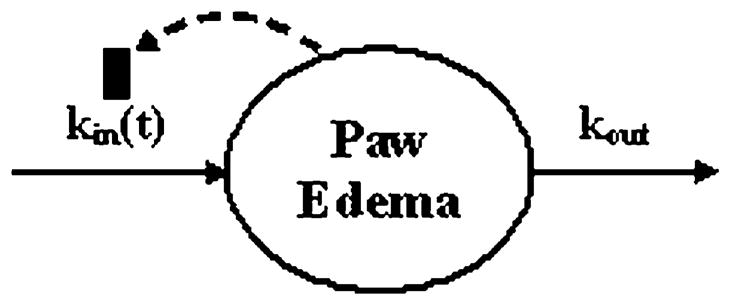
Schematic of disease progression model components. The model is a modified basic indirect response model for the production and loss of paw or ankle swelling. The production component [kin(t)] is both time dependent and inhibited by the degree of swelling to account for disease remission in the later portion of the time course. The loss component (kout) is a first-order stationary rate constant.
| (2) |
where Paw is the circumference of the measured cross section of paw divided by the value of the circumference at time zero for each specific rat paw in the study. The rate constant kout describes the loss of edema. The production rate kin, however, is a function of time starting at the onset time and dependent upon the degree of swelling as denoted in:
| (3) |
This equation was necessary to describe the remission of the disease post day 21 in most animals. The first-order rate constant Rdeg describes the rate at which the production of response declines in the presence of edema, a negative feedback loop.
Statistical Data Analysis
Variability in progression between animal groups was determined by pharmacodynamic disease progression modeling. Nonlinear mixed effects modeling was accomplished using the FOCE module of Nonmem V (GloboMax, San Francisco, CA, USA) for the time course of paw and ankle swelling for each arthritis type and animal strain. Population analysis permitted assessment of the variation of each parameter within each treatment and animal strain. Inter-animal variation was assessed by the estimated variance of the distributions:
| (4) |
where ‘P’ is the typical value of the ith model parameter, θi is the expected value of Pi and ηi is the measure of inter-animal variability inherent to all groups for the ith parameter. Differences in inter-individual variation of the parameter estimates of disease onset and progression between groups were modeled with the inclusion of a parameter- and group-specific ηi,j where i refers to the parameter and j refers to the group (animal strain and arthritis type). Random effects were included by a constant coefficient of variation model that was not group specific.
Data from each group were fitted simultaneously to determine the disease progression parameters and the degree of inter-animal variation for each model parameter and animal strain/disease. Initially variances for four additional η parameters were estimated in each group model, one for each of the four disease progression parameters to account for differences in the variability between groups. Using backwards elimination and the chi-squared test with the minimum value of the objective function (MVOF) the η distributions with the least influence on the model were removed sequentially if they did not test to have a statistically significant impact on the model fitting (i.e. change in the minimum value of the objective function was less than 3.84) (27,28). Differences in variability between groups would be concluded if an η remained in the final model for that group.
Model simulations were conducted for 10,000 Lewis rats with CIA using NONMEM. Data from an additional 34 Lewis rats with CIA were plotted and visually compared to the 10th, 50th, and 90th percentiles of these simulations as an indication of overall predictive performance.
RESULTS
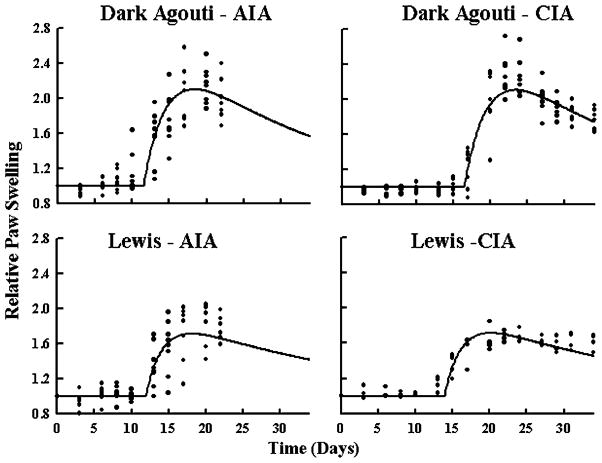
The observed time courses and model fittings of paw swelling for all four groups are shown in Fig. 3. Individual data points help indicate any visual differences in data variation between the four groups. All data points were normalized to the value at time zero to reduce variation in the disease progression due to differences in natural paw size and growth. The disease was monitored from prior to induction at day zero until day 23 in the animals with AIA and for 34 days for rats with CIA. Signs of the disease were not apparent until as early as day 12. Even though paw swelling may not have increased dramatically on day 12 it was apparent in some animals that they were no longer using their paws to move around the cage and their activity levels were somewhat diminished. Paw swelling was apparent in most animals that would develop the disease by day 15, when an increase in measured response was observed (Fig. 3). In the group of Lewis rats with AIA one animal of the four did not develop the disease as severely as the other animals. The time of onset was delayed and the severity of edema was reduced throughout this rat’s time course of disease progression. Two Lewis rats of the four total Lewis rats with CIA treatment died before developing the disease and were not included in the model fitting.
Fig. 3.
Arthritis disease progression as indicated by changes in paw swelling. Circles depict observed paw size relative to baseline values. The solid line is the population prediction for all points in each group, assuming no residual error. There are data from four rats (eight paws) in all plots except for the Lewis rats with CIA, n=2 (four paws).
The swelling appeared to peak at day 17 for DA rats with AIA, day 22 for DA rats with CIA, day 20 for Lewis rats with AIA, and day 20 for Lewis rats with CIA. It is possible that because no measurements were made for the DA/AIA, DA/CIA and Lewis/AIA groups on days 18 and 19 that the times of the true peaks for paw swelling were missed. Once edema peaked, there was an apparent remission in the DA/AIA, DA/CIA and Lewis/CIA groups. Lewis rats with AIA were sacrificed before a decline in the time course of response could be observed.
Observed time course and model fittings of disease progression as measured by ankle edema are shown in Fig. 4. The onset of the disease was readily detected as early as day 10 in the DA/AIA group, where there appeared to be a delay in onset of edema for the CIA model of arthritis as indicated by the paw swelling. Peaks were measured at day 20 for the DA rats and between days 17 and 20 for Lewis/AIA rats and on day 20 for Lewis/CIA rats.
Fig. 4.
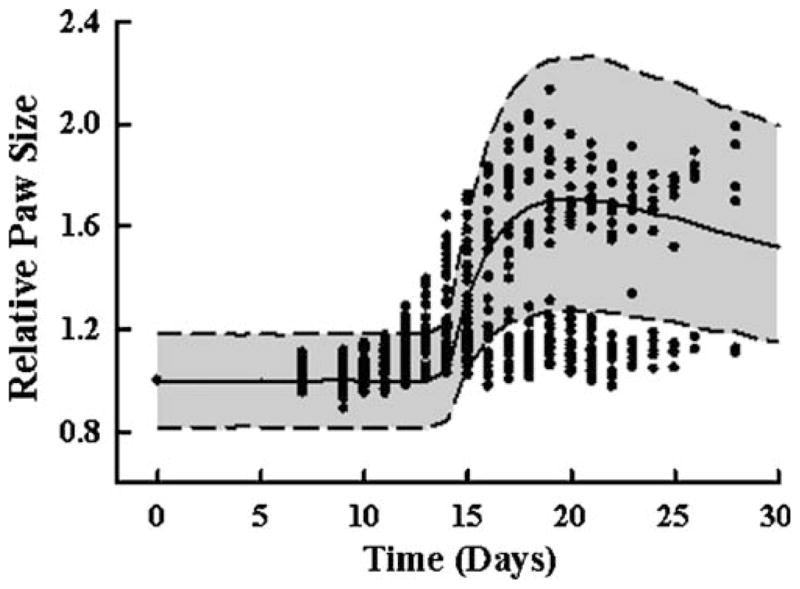
Arthritis disease progression model prediction of paw and ankle edema. Additional paw measurements were available from a time course of disease progression in 34 Lewis rats with CIA. Circles depict observed paw sizes. The solid line is the median prediction for each time point from a simulation of 10,000 rats, applying the model developed for Lewis rats with CIA. The shaded area is the 10th to 90th confidence interval.
Model fittings with additional group specific η values to account for between group differences in data variation showed no significant improvement in model fittings. All group specific η distributions were included to start and each was removed one at a time and the differences between the MVOF before and after removal were compared. In no case did the group-specific η variances have a significant impact on the model. Given the limited size of each group (n=8 paws), this suggested that no differences in variation of the parameters were detected for any of the groups. This is confirmed visually when observing the data as the distribution of points for each group is very similar. Additionally two ηs for between-rat variation were removed from TOnset and kout to improve model stability as they had the least impact on model fitting compared to the ηs on kin0 and Rdeg.
Table I shows the parameter estimates and relative standard error of the final model fittings presented in Fig. 3. Even though no difference in variation between groups was noted, several other points may be noted. TOnset was the only parameter to change between all four groups. The model fitting for DA rats with CIA had a much later TOnset (16.5 days) when compared to the other groups (DA AIA–11.5 days, Lewis AIA–11.9 days, Lewis CIA–13.9 days). Additionally kout was different between DA (0.362 h−1) and Lewis (0.466 h−1) rats. The model appeared to fit the data sufficiently for comparison of variation of swelling measurements and magnitude of the model structural parameters between animal groups.
Table I.
Model Parameter Estimates for Rat Strain/Disease Group
| Parameter | Description | Estimate | % RSE |
|---|---|---|---|
| Group-independent parameters | |||
| Rdeg, per day | Loss of production rate constant | 0.0169 | 5.29 |
| kin0, per day | Disease production rate at TOnset | 0.855 | 30.8 |
| DA—adjuvant arthritis | |||
| TOnset, day | Time of disease onset | 11.5 | 52.0 |
| kout, per day | Loss of swelling rate constant | 0.362 | 20.4 |
| DA—collagen II arthritis | |||
| TOnset, day | Time of disease onset | 16.5 | 39.4 |
| kout, per day | Loss of swelling rate constant | 0.362 | 20.4 |
| Lewis—adjuvant arthritis | |||
| TOnset, day | Time of disease onset | 11.9 | 39.6 |
| kout, per day | Loss of swelling rate constant | 0.466 | 23.5 |
| Lewis—collagen II arthritis | |||
| TOnset, day | Time of disease onset | 13.9 | 46.6 |
| kout, per day | Loss of swelling rate constant | 0.466 | 23.5 |
| Inter-animal variation | |||
| ηRdeg, % CV | Between-rat variation for Rdeg | 27.3 | 85.4 |
| ηkin0, % CV | Between-rat variation for kin0 | 12.0 | 160 |
| CV of σ, % | Coefficient of variation for ε distribution | 9.35 | 4.53 |
The 10th, 50th, and 90th percentiles for 10000 model simulation of paw edema for the additional 34 Lewis rats with CIA is shown in Fig. 4. Of the total animals in this group, nine did not develop any sign of disease (25%), only six developed the disease in one paw and three other rats developed the disease, but with a slower and less severe progression. Animals that did not develop the disease are seen as points that continue along the baseline path and do not rise upward abruptly between study day 12 and 20. The model simulation for paw edema captures the general trend of all animals that developed the disease fairly well.
DISCUSSION
Four animal models of arthritis were induced and monitored side-by-side to compare variation in the primary disease endpoint between groups, paw and ankle swelling. Producing a model with least variation was important to future animal work. The most reproducible model reduces waste of animals that do not develop the disease. The model with least variation would also minimize the number of rats required in study designs reducing overall cost and time. This comparison study attempted to identify the least variable arthritis model and strain for future PK/PD experiments.
The disease progression modeling to identify group specific variation in paw and ankle swelling indicated that no additional group-specific parameters were necessary to account for greater or lesser variation between groups. This comparison was limited by the number of animals present in each group (four animals, eight hind paws per group except Lewis–CIA which had two rats and four hind paws) and variation was only assessed in paw and ankle swelling. No other biomarkers were used for this analysis. There were only four animals present in the DA/CIA, DA/AIA, and Lewis/AIA groups and only data from two animals in the Lewis/CIA model because two rats died early. One animal’s death was attributed to a heart attack by veterinary autopsy. The other rat died from unknown causes.
Data were also limited for the animals with AIA since our IACUC did not approve working with animals in this model beyond 23 days. However, because prior literature indicated that the disease arose later in animals with CIA, the studies were approved out to day 34 for the CIA model. As a result the decline phase was missed in the two groups with AIA. This hinders the ability to detect differences in both the variation and model parameters kout and Rdeg for these two groups. These data were analyzed after another study with CIA in Lewis rats had begun. This later study eventually provided additional measurements for model validation (Fig. 4).
Despite the fact that only two animals were used for model estimation of paw edema in Lewis rats with CIA, the median of the model simulations predicted the test group fairly well (Fig. 4). The model predicted the ankle data well although somewhat late. The paw data appeared to be under- and over-predicted in most cases nearing the average of the entire population response. Owing to differences in disease progression in individual rats, data with this degree of variation needs to be approached and analyzed with population modeling methods.
This approach assumes that paw edema as measured with digital calipers indicate the overall state of the arthritis in the animals. However, behavior and use of paws may also play a role in the severity of the developed arthritis (29). Three animals showed reduced activity, loss of body weight, and dragged their paws rather than using them to walk, yet the severity of their paw swelling was not near that of other rats in their arthritis group. However, in most cases the behavior correlates with the degree of swelling, in that as the swelling began to recede the animals began to gain body mass again. Observed behavioral differences between CIA and AIA were not noted. However, it did appear the DA rats were more subdued and less active than the Lewis rats. The DA animals did not move whereas the Lewis rats were still very curious and mobile. The redness and overall appearance of the paw was also more severe in the DA rats which was consistent with the peak response in the paw swelling data. Overall, paw swelling should be considered an endpoint relevant to edema in the arthritis disease etiology.
For the purpose of determining variation for each disease group (Lewis rats with AIA, Lewis rats with CIA, DA rats with AIA, DA rats with CIA) the model captured the data sufficiently well. However several limitations of this model should be noted. First, no other biomarkers were measured that would explain the ‘remission’ phase of the response and so the decline was correlated with the extent of swelling. When therapeutic drugs are added to this disease progression model to reduce swelling it may be impossible to discern between the contributions of the feedback and drug effect processes to resolve the pharmacological parameters of drug action. In future studies, measuring biomarkers that describe this ‘remission’ would help distinguish between these processes. Second, transduction can be incorporated into the time delay to better capture the early phase of the onset of the disease as the fitted curve for DA rats with AIA was a little late and too steep in capturing the disease onset in the observed data. Finally, the parameters Rdeg and kout exhibit some overlapping function in reducing the measured edema, limiting the ability to detect between-rat variation in all four structural parameters. While this model provided a tool indicating there were no differences in variation between groups, the final animal model was ultimately selected based on several factors.
The model indicated the parameter that varied the most between groups was the onset time (TOnset). This is in agreement with observations that not all animals appeared to develop the disease at the same time. The disease starts with antigen presentation within each animal. The time at which the epitope is sufficiently presented to systemic T-cells likely varies from one animal to the next. It is important then to recognize that variation may also be present in the time axis if time of injection is used versus time of disease onset. In future studies it may be useful to correct the timescale of each animal by subtracting each individual’s time of disease onset for a pooled analysis of all individual responses.
In addition to the lack of differences in variation of paw swelling between groups, the disease etiology for both AIA and CIA, and the differences between Lewis and DA rats need consideration when selecting an appropriate rat model of arthritis. Collagen-induced arthritis is preferred over AIA because of the wealth of literature indicating relevance of CIA to human RA (1). Of particular interest was that the CIA model appeared to develop rheumatoid factor antibodies and this humoral immune response was not apparent in the AIA model (1,7). Lewis rats are preferred over Dark–Agouti rats as a matter of availability and convenience. The Lewis rats are more readily obtained from the vendor and not in short supply like DA rats. This limited availability poses a problem for larger pharmacodynamic experiments. The DA but not Lewis rats are also known to develop arthritis from mineral oil alone, the vehicle used to administer the collagen (30–33). The choice of Lewis rats was made despite the visually severe nature of the disease in DA rats, because the paw swelling data did not indicate any differences between strains in severity of the disease. This modeling approach may have had more impact given a larger set of animals in each group; however it did outline various facets of the disease in the four models of arthritis necessary for future pharmacodynamic disease progression models—the time of onset, rate of disease progression, the time and peak value of paw edema, and the time course of swelling decline.
ABBREVIATIONS
- AIA
adjuvant-induced arthritis
- CIA
collagen-induced arthritis
- CV
coefficient of variation
- FOCE
first order conditional estimation
- MVOF
minimum value of the objective function
- PD
pharmacodynamics
- PK
pharmacokinetics
- RA
rheumatoid arthritis
Glossary
- SYMBOLS
kout, loss of edema rate constant
- kin
production of edema rate constant
- Rdeg
loss of production of edema rate constant
- tOnset
disease onset time
- P
parameter
- θ
population mean of P
- η
measure of individual’s deviation from θ
References
- 1.Holmdahl R, Lorentzen JC, Lu S, Olofsson P, Wester L, Holmberg J, Pettersson U. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev. 2001;184:184–202. doi: 10.1034/j.1600-065x.2001.1840117.x. [DOI] [PubMed] [Google Scholar]
- 2.Kerwar SS, Bauman N, Oronsky AL, Sloboda AE. Studies on type II collagen induced polyarthritis in rats. Effect of complement depletion. J Immunopharmacol. 1981;3:323–337. doi: 10.3109/08923978109031065. [DOI] [PubMed] [Google Scholar]
- 3.Pearson CM. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956;91:95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- 4.Stuart JM, Cremer MA, Townes AS, Kang AH. Type II collagen-induced arthritis in rats. Passive transfer with serum and evidence that IgG anticollagen antibodies can cause arthritis. J Exp Med. 1982;155:1–16. doi: 10.1084/jem.155.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taurog JDA, Argentieri DC, McReynolds RA. Adjuvant arthritis. Methods Enzymol. 1956;162:339–355. doi: 10.1016/0076-6879(88)62089-1. [DOI] [PubMed] [Google Scholar]
- 6.Ward JR, Jones RS. Studies on adjuvant-induced polyarthritis in rats. I. Adjuvant composition, route of injection, and removal of depot site. Arthritis Rheum. 1962;5:557–564. doi: 10.1002/art.1780050604. [DOI] [PubMed] [Google Scholar]
- 7.Wooley PH. The usefulness and the limitations of animal models in identifying targets for therapy in arthritis. Best Pract Res Clin Rheumatol. 2004;18:47–58. doi: 10.1016/j.berh.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Akilesh S, Petkova S, Sproule TJ, Shaffer DJ, Christianson GJ, Roopenian D. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest. 2004;113:1328–1333. doi: 10.1172/JCI18838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 10.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohashi O, Aihara K, Ozawa A, Kotani S, Azuma I. New model of a synthetic adjuvant, N-acetylmuramyl-L-alanyl-D-isoglutamine- induced arthritis: clinical and histologic studies in athymic nude and euthymic rats. Lab Invest. 1982;47:27–36. [PubMed] [Google Scholar]
- 12.Kohashi O, Pearson CM, Watanabe Y, Kotani S. Preparation of arthritogenic hydrosoluble peptidoglycans from both arthritogenic and non-arthritogenic bacterial cell walls. Infect Immun. 1977;16:861–866. doi: 10.1128/iai.16.3.861-866.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colpaert FC, Meert T, De Witte P, Schmitt P. Further evidence validating adjuvant arthritis as an experimental model of chronic pain in the rat. Life Sci. 1982;31:67–75. doi: 10.1016/0024-3205(82)90402-7. [DOI] [PubMed] [Google Scholar]
- 14.Holmdahl R, Vingsbo C, Malmstrom V, Jansson L, Holmdahl M. Chronicity of arthritis induced with homologous type II collagen (CII) in rats is associated with anti-CII B-cell activation. J Autoimmun. 1994;7:739–752. doi: 10.1006/jaut.1994.1058. [DOI] [PubMed] [Google Scholar]
- 15.Larsson P, Kleinau S, Holmdahl R, Klareskog L. Homologous type II collagen-induced arthritis in rats. Characterization of the disease and demonstration of clinically distinct forms of arthritis in two strains of rats after immunization with the same collagen preparation. Arthritis Rheum. 1990;33:693–701. doi: 10.1002/art.1780330512. [DOI] [PubMed] [Google Scholar]
- 16.Pearson CMW, Wood FM. Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathologic characteristics and some modifying factors. Arthritis Rheum. 1959;2:440–459. doi: 10.1002/1529-0131(195910)2:5<440::AID-ART1780020510>3.0.CO;2-N. [DOI] [Google Scholar]
- 17.Bush KA, Kirkham BW, Walker JS. The in vivo effects of tumour necrosis factor blockade on the early cell mediated immune events and syndrome expression in rat adjuvant arthritis. Clin Exp Immunol. 2002;127:423–429. doi: 10.1046/j.1365-2249.2002.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt DW, Corson L, Barker HD, Levy JG, Petty RE. Relationship between collagen-induced and adjuvant arthritis in the Lewis rat. J Autoimmun. 1993;6:691–700. doi: 10.1006/jaut.1993.1058. [DOI] [PubMed] [Google Scholar]
- 19.Panayi GS, Lanchbury JS, Kingsley GH. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992;35:729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- 20.Kerwar SS, Englert ME, McReynolds RA, Landes MJ, Lloyd JM, Oronsky AL, Wilson FJ. Type II collagen-induced arthritis. Studies with purified anticollagen immunoglobulin. Arthritis Rheum. 1983;26:1120–1131. doi: 10.1002/art.1780260910. [DOI] [PubMed] [Google Scholar]
- 21.Rioja I, Bush KA, Buckton JB, Dickson MC, Life PF. Joint cytokine quantification in two rodent arthritis models: kinetics of expression, correlation of mRNA and protein levels and response to prednisolone treatment. Clin Exp Immunol. 2004;137:65–73. doi: 10.1111/j.1365-2249.2004.02499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson E, Mussener A, Heinegard D, Klareskog L, Saxne T. Increased serum levels of cartilage oligomeric matrix protein and bone sialoprotein in rats with collagen arthritis. Br J Rheumatol. 1997;36:1258–1261. doi: 10.1093/rheumatology/36.12.1258. [DOI] [PubMed] [Google Scholar]
- 23.Stuart JM, Tomoda K, Yoo TJ, Townes AS, Kang AH. Serum transfer of collagen-induced arthritis. II. Identification and localization of autoantibody to type II collagen in donor and recipient rats. Arthritis Rheum. 1983;26:1237–1244. doi: 10.1002/art.1780261011. [DOI] [PubMed] [Google Scholar]
- 24.Takagishi K, Kaibara N, Hotokebuchi T, Arita C, Morinaga M, Arai K. Serum transfer of collagen arthritis in congenitally athymic nude rats. J Immunol. 1985;134:3864–3867. [PubMed] [Google Scholar]
- 25.Wernhoff P, Unger C, Bajtner E, Burkhardt H, Holmdahl R. Identification of conformation-dependent epitopes and V gene selection in the B cell response to type II collagen in the DA rat. Int Immunol. 2001;13:909–919. doi: 10.1093/intimm/13.7.909. [DOI] [PubMed] [Google Scholar]
- 26.Lorentzen JC, Klareskog L. Comparative susceptibility of DA, LEW, and LEW.1AV1 rats to arthritis induced with different arthritogens: mineral oil, mycobacteria, muramyl dipeptide, avridine and rat collagen type II. Transplant Proc. 1997;29:1692–1693. doi: 10.1016/S0041-1345(97)00018-3. [DOI] [PubMed] [Google Scholar]
- 27.Rubino CM, Capparelli EV, Bradley JS, Blumer JL, Kearns GL, Reed M, Jacobs RF, Cirincione B, Grasela DM. Population pharmacokinetic model for gatifloxacin in pediatric patients. Antimicrob Agents Chemother. 2007;51:1246–1252. doi: 10.1128/AAC.00685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wählby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001;28:231–252. doi: 10.1023/a:1011527125570. [DOI] [PubMed] [Google Scholar]
- 29.Dimitrijevic M, Laban O, Djuric VJ, Stanojevic S, Miletic T, Kovacevic-Jovanovic V, Todorovic C, Radulovic J. Behavior and severity of adjuvant arthritis in four rat strains. Brain Behav Immun. 2001;15:255–265. doi: 10.1006/brbi.2000.0599. [DOI] [PubMed] [Google Scholar]
- 30.Cannon GW, Woods ML, Clayton F, Griffiths MM. Induction of arthritis in DA rats by incomplete Freund’s adjuvant. J Rheumatol. 1993;20:7–11. [PubMed] [Google Scholar]
- 31.Carlsen S, Hansson AS, Olsson H, Heinegard D, Holmdahl R. Cartilage oligomeric matrix protein (COMP)-induced arthritis in rats. Clin Exp Immunol. 1998;114:477–484. doi: 10.1046/j.1365-2249.1998.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmdahl R, Goldschmidt TJ, Kleinau S, Kvick C, Jonsson R. Arthritis induced in rats with adjuvant oil is a genetically restricted, alpha beta T-cell dependent autoimmune disease. Immunology. 1992;76:197–202. [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinau S, Erlandsson H, Holmdahl R, Klareskog L. Adjuvant oils induce arthritis in the DA rat. I. Characterization of the disease and evidence for an immunological involvement. J Autoimmun. 1991;4:871–880. doi: 10.1016/0896-8411(91)90050-M. [DOI] [PMC free article] [PubMed] [Google Scholar]