Abstract
Objective
Contingency management (CM) is an evidence-based treatment, but few clinicians deliver this intervention in community-based settings.
Method
Twenty-three clinicians from three methadone maintenance clinics received training in CM. Following a didactics seminar and a training and supervision period in which clinicians delivered CM to pilot patients, a randomized trial evaluated the efficacy of CM when delivered entirely by clinicians. Sixteen clinicians treated 130 patients randomized to CM or standard care. In both conditions, urine and breath samples were collected twice weekly for 12 weeks. In the CM condition, patients earned the opportunity to win prizes ranging in value from $1 to $100 for submitting samples negative for cocaine and alcohol. Primary treatment outcomes were retention, longest continuous period of abstinence, and proportion of negative samples submitted.
Results
Patients randomized to CM remained in the study longer (9.5 ± 3.6 versus 6.7 ± 5.0 weeks), achieved greater durations of abstinence (4.7 ± 4.7 versus 1.7 ± 2.7 weeks), and submitted a higher proportion of negative samples (57.7% ± 40.0% versus 29.4% ± 33.3%) than those assigned to standard care.
Conclusions
These data indicate that, with appropriate training, community-based clinicians can effectively administer CM. This study suggests that resources ought to be directed toward training and supervising community-based providers in delivering CM, as patient outcomes can be significantly improved by integrating CM in methadone clinics.
Keywords: contingency management, therapist, community clinics, training
With the publication of the Institute of Medicine’s (IOM; 1998) report on bridging the gap between research and practice, moving evidence-based interventions into clinical settings has become a national priority. McGlynn et al. (2003) reviewed records from 6,000 patients to ascertain the quality of care received for 30 illnesses. Although 55% of patients overall received recommended care, only 11% of substance abusing patients received appropriate care. McGlynn et al. (2003) focused on mainstream health care delivery, yet available data suggest that the gap between research and practice is even larger in specialty sectors, such as drug abuse treatment (Greenlick, Lamb, & McCarty, 1998; Weisner & McLellan, 2004). Substance abuse treatment clinics are among the poorest in adopting empirically validated treatments (Brown, 2000; Carroll & Rounsaville, 2003; Compton et al., 2005; McLellan, Carise, & Kleber, 2003). Nowhere is this gap larger than with one specific evidence-based treatment—contingency management (McGovern, Fox, Xie, & Drake, 2004).
Contingency management (CM) is based on behavioral principles that a behavior that is positively reinforced will increase in frequency. Typically, in CM treatments for substance use disorders, urine samples are collected several times each week. Each time abstinence is noted, patients earn reinforcers in the form of vouchers worth escalating monetary amounts or the chance to win prizes ranging from $1 to $100 (Higgins, Wong, Badger, Ogden, & Dantona, 2000; Petry, 2000). In a meta-analysis of psychosocial treatments for substance use disorders, Dutra et al. (2008) concluded that CM was the intervention with the largest effect size.
In early CM studies (e.g., Milby, Garrett, Englih, Fritchi, & Clarke, 1978; Stitzer et al., 1977), methadone patients were reinforced for abstinence with take-home doses as the reinforcer; these studies ultimately led to the now common practice in methadone clinics of allowing take-home doses of methadone contingent upon abstinence. However, in this context, take-home doses are often not administered in accordance with behavioral principles such as frequent monitoring and reinforcement (Petry, 2000), and many methadone patients use drugs while maintained on methadone (e.g., Leri, Bruneau, & Stewart, 2003).
Although CM can be very efficacious when implemented according to behavioral principles, few clinicians are familiar with it. McGovern et al. (2004) surveyed 110 clinicians from 24 substance abuse treatment clinics about their awareness of and willingness to adopt evidence-based practices. CM was the intervention with which they were least familiar. Haug, Shopshire, Tajima, Gruber, and Guydish (2008) similarly noted that only a small number of 119 clinicians surveyed were familiar with CM. Cameron and Ritter (2007) conducted in-depth surveys with substance abuse clinicians and found that what knowledge they had about CM was inconsistent with its key principles.
Some efforts have been directed at increasing clinicians’ awareness of CM and disseminating it to clinical settings. In response to the IOM (1998) initiative, the National Institute on Drug Abuse’s Clinical Trials Network (CTN) conducted studies of CM (Peirce et al., 2006; Petry, Peirce et al., 2005) with over 800 stimulant abusing patients from 14 community clinics across the country. Patients were randomized to standard care plus frequent urine testing or that same treatment plus the chance to win prizes contingent upon abstinence. Among stimulant abusing patients in methadone maintenance settings (Peirce et al., 2006), effects were particularly pronounced, with the mean duration of stimulant abstinence increasing from 1.2 (standard deviation; SD = 1.9) weeks in standard care to 2.8 (SD = 3.9) weeks in CM, and the mean proportion of stimulant negative samples rising from 38.7% in standard care to 54.5% with CM. Although these studies point to the efficacy of CM in a range of community settings, in most clinics research assistants, not clinic counselors, administered the CM.
Some reports describe efforts targeted toward integrating CM in community settings. Kellogg et al. (2005) outline case reports of five substance abuse treatment clinics affiliated with the Hospital and Health Corporations in New York City that applied CM and reported highly positive effects among both patients and staff. In New England, Squires, Gumbley, and Storti (2008) provided day-long trainings in CM, followed by technical support for implementation, to providers in 28 community clinics, and 26 of the 28 (93%) clinics successfully implemented CM. In the State of South Carolina, Henggeler et al. (2007) noted that 432 of 543 (80%) clinicians who provide services to adolescents attended a workshop in CM. Henggeler et al. (2008) also found that 58% of clinicians with at least one substance abusing client reported adopting CM in the 6 months following the training. Lash et al. (2007) describe a study in which therapists delivered reinforcement to patients to increase attendance at aftercare, and Ledgerwood, Alessi, Hanson, Godley and Petry (2008) trained clinicians at four sites to implement prize CM for improving patient attendance. Across these projects, therapists were willing to implement CM, and most of the barriers to adoption related to practical issues, not philosophical ones.
However, for an intervention to be effective, clinicians need not only be trained about it, but also deliver it appropriately. The dissemination projects outlined above did not assess adherence to CM principles or competence in CM delivery, nor did most objectively measure patient outcomes. Competent delivery of treatments is associated with patient outcomes generally (Barber, Crits-Christoph, & Luborsky, 1996; Carroll et al., 2000; Martino, Ball, Nich, Fronkforter, & Carroll, 2008), and with CM in particular (Chapman, Sheidow, Henggeler, Halliday-Boykins, & Cunningham, 2008; Petry, Alessi, Ledgerwood & Sierra, 2010). In most research trials, CM administrators are carefully selected, highly trained, and closely supervised research assistants or research therapists (Budney, Moore, Rocha, & Higgins, 2006; Higgins et al., 2007; Petry et al., 2004; Petry, Alessi, Marx, Austin, & Tardiff, 2005; Petry, Alessi, Hanson, & Sierra, 2007). Principal investigators or project directors with extensive experience train and oversee CM administration and quickly correct problems that may arise. In contrast, in community settings, less emphasis is placed on fidelity of treatment administration. In the only known study evaluating adherence to CM implementation after initial training, Andrzejewski, Kirby, Morral, and Iguchi (2001) noted that without regular feedback from CM experts, administration of CM was suboptimal, with clinicians failing to monitor and reinforce behaviors according to pre-specified criteria. Even if clinicians are trained to administer CM competently, it is important to ascertain if patients improve along dimensions of clinical importance.
This study evaluated the efficacy of CM when delivered entirely by community-based clinicians. Methadone providers were chosen for this study because of the strong empirical background of CM in such settings (Griffith, Rowan-Szal, Roark, & Simpson, 2000; Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006) and the widespread use of toxicology testing in methadone clinics. Cocaine abusing methadone patients were included in the study because cocaine use occurs among 40–60% of methadone patients (Leri et al., 2003; Sees et al., 2000; Wasserman, Korcha, Havassy, & Hall, 1999), and it is related to psychiatric, legal and employment problems, HIV transmission, and attrition from treatment (Bandettini et al., 2006; DeMaria, Sterling, & Weinstein, 2000; Rothbard et al., 1999; Tross et al., 2009; Zanis, Metzger, & McLellan, 1994). Although methadone reduces heroin use (Council on Addiction Psychiatry, 1994), it has only marginal effects on cocaine use (Ball & Ross, 1991; Sees et al., 2000). No medication is reliably efficacious for treating cocaine abuse (Sofuoglu & Kosten, 2006), and enhanced psychosocial interventions such as CM are necessary to abate cocaine use in this population.
We describe a training program and methods to establish initial adherence and competence in CM delivery among 23 clinicians working at three methadone maintenance clinics. We then detail results from a randomized trial in which clinicians administered standard care or standard care plus CM to methadone-maintained cocaine abusing patients. We expected that once therapists were trained to standards of adherence and competence, CM would significantly reduce cocaine use in methadone patients.
Methods
Participants
Therapists were 23 counselors employed at three methadone clinics between 2005 and 2008. The clinics were all not-for-profit, had similar policies, and treated between 250 and 450 patients per day. All therapists were employed at the clinic for at least 6 months and had at least one year of experience with substance abusing patients. Therapists had to report sufficient time for training and supervision in CM delivery and willingness to allow random assignment of patients to CM and standard care conditions. All full time therapists at each clinic at the time of study initiation (7 to 8 per site) participated in the training. There were no significant differences in therapist characteristics across clinics. Nine (39.1%) of the therapists were women, and mean age was 45.7 (SD = 12.4) years. In terms of ethnicity, three (13.0%) were Hispanic American, two (8.7%) African American, one (4.3%) multiracial, and the remainder European American. One (4.3%) had a high school education, two (8.7%) an associate’s degree, eight (34.8%) a bachelor’s degree, and 10 (43.5%) a master’s degree, with the remaining two (8.7%) not indicating their educational background. Five (21.7%) had a license to practice. Five (21.7%) of the therapists were in recovery from substance use, and 10 (43.5%) said they were not in recovery, with the remainder choosing not to indicate their recovery status. On average, they worked at the clinics for 9.2 (SD = 7.6) months, with a range of experience treating substance abusers of 1.5 to 27 years. None had prior experience with CM.
Patients were 181 (51 pilot participants and 130 randomized participants) methadone maintained individuals recruited from one of three methadone clinics. Inclusion criteria were cocaine abusing or dependent, submitted one or more cocaine-positive urine samples on the usual clinic testing procedures in the past three months, maintained on a stable dose of methadone for at least one month, and age 18 years or older. Significant uncontrolled psychiatric conditions (e.g., suicidality, psychosis) and being in recovery from pathological gambling were exclusionary criteria. This latter criterion was used because of the potential similarity between the reinforcement system and gambling, although no increases in gambling have been reported among patients receiving prize CM (Petry & Alessi, 2010; Petry et al., 2006).
The University’s Institutional Review Board approved this study. Therapist and patient participants (including pilot participants, see below) signed informed consent for study procedures, including audiotaping of interactions. Sample size was determined based on medium effect sizes of CM when delivered by researchers (Lussier et al., 2006; Predergast et al., 2006).
Therapist training procedures
After signing informed consent, therapists participated in two half-day trainings led by a study author that included didactics, demonstration of onsite urine sample monitoring procedures, and practice with role plays (see below). Before and after the seminar, they completed a general CM knowledge test containing 20 items (available in Petry & Stitzer, 2002). They also completed a 20-item multiple choice knowledge test about details of CM procedures specific to this trial (e.g., how many draws should be provided given different urine sample results). In addition, therapists anonymously filled out a training satisfaction form.
After adequate scores of ≥ 16 correct were achieved on CM knowledge tests, therapists participated in at least three mock CM sessions in which they played the part of a therapist and a research assistant assumed the role of a patient. The role plays provided brief descriptions about scenarios in which therapists were expected to (1) describe CM treatment to a new CM patient, (2) provide increasing reinforcement to a patient testing negative, and (3) withhold reinforcement when a patient did not earn reinforcement. CM experts (study authors and research assistants with bachelor’s degrees) rated the role plays using the Contingency Management Competence Scale (CMCS; Petry et al., 2010). The CMCS contains 12-items, each rated on a 1 to 7 point scale (representing “poor” to “excellent,” respectively), and assesses issues central to competent CM administration. Internal consistency and descriptive, concurrent and predictive validity of the CMCS are good to excellent (Petry et al., 2010). Interrater reliability was 0.92. Total scores on the CMCS are derived by averaging scores on the 12 items, and range from 1–7, with higher scores reflecting greater competence in CM delivery. When therapists achieved average ratings of “4” (indicative of “acceptable” quality) or higher, they proceeded to a CM supervision phase.
In the supervision phase, therapists delivered CM (see treatment description below) to pilot patients. (Therapists were not provided pilot cases for the standard care condition because it was similar to usual clinical procedures, and only involved additional sample testing as described in the treatment section.) We created a 10-item CM checklist to rate therapists’ adherence to CM procedures. This CM adherence checklist was based on the written tracking procedures associated with CM administration. It included items such as: Did the therapist sign and date the form? Is the correct week of study participation indicated? Did the therapist indicate whether the patient attended the session? Did the therapist collect a sample or indicate why a sample was not collected that day? Are results of the sample recorded? Did the therapist award the correct number of draws? Did the therapist record the correct number of prize categories given draws awarded? Did the patient sign for prizes, if applicable? Did the therapist indicate the date of the next CM session? Did the therapist report the correct number of draws the patient is eligible for at the next session? Each item was scored 0 or 1 for “no” or “yes.” Scores ranged from 0 to 10, with 10 indicating perfect adherence. In the supervision phase, CM experts reviewed the therapists’ tracking forms with them, along with their scores on the CM adherence checklist.
In addition to rating therapists with respect to adherence to the CM protocol, we also rated them with respect to competence in CM delivery. CM interactions with patients were audiotaped twice weekly for up to 12 weeks for each patient, and CM experts reviewed each audiotape and provided graphical feedback to therapists weekly regarding their CM administration along the dimensions assessed by the CMCS. Selected audiotapes were reviewed in the typically 30 minute weekly supervision sessions to point out particularly good, and incorrect, CM administration. After therapists received overall ratings on the CMCS of “4” or higher for at least three sessions in a row with at least two different pilot patients and were achieving scores of “10” on the CM adherence checklists, they proceeded to the randomized phase of the study.
In the randomized phase, cocaine abusing methadone patients (N = 130) were assigned to standard care or CM (see below for further descriptions of treatments). The CM during the randomized phase was identical to that described in the training phase. Therapists treated patients in both conditions, and they audiorecorded all discussions regarding sample testing with patients assigned to both CM and standard care in the randomized phase. As in the pilot stage, research staff monitored therapists’ delivery of CM using the CM adherence checklist and by rating audiotapes of therapists’ interactions using the CMCS. However, research staff provided no feedback to therapists regarding CM delivery in the randomized phase.
Patient assessments
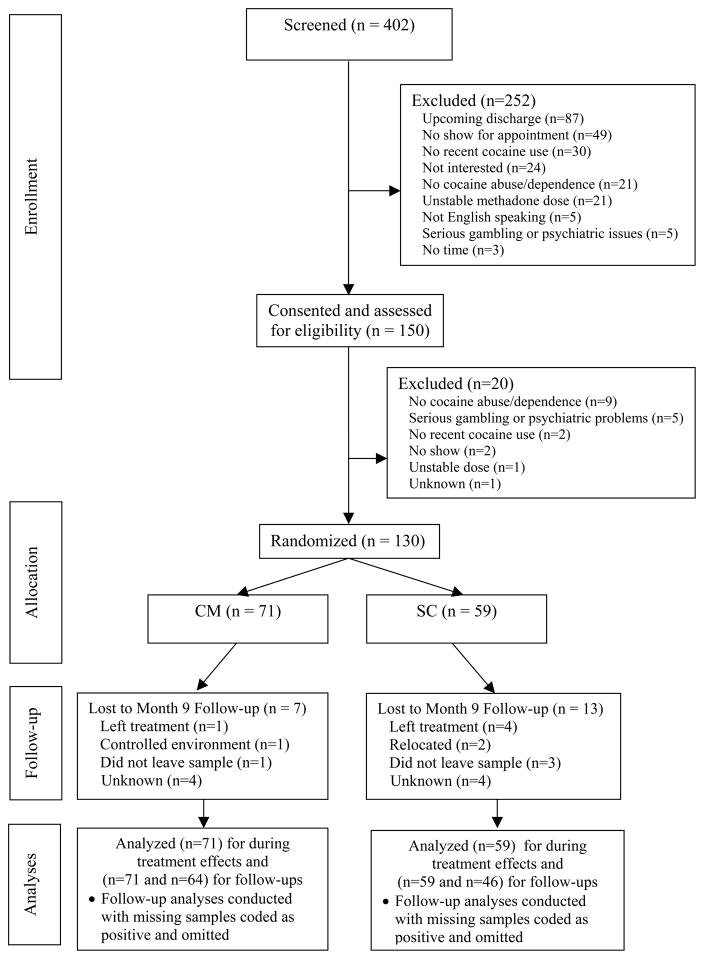
After signing informed consent, patients completed a baseline assessment. It consisted of a demographics questionnaire, modules adapted from the Structured Clinical Interview for the DSM-IV for assessing substance use diagnoses and pathological gambling (First, Spitzer, Gibbon, & Williams, 1996), and the Addiction Severity Index (ASI) (McLellan et al., 1985). Patients in the randomized phase also submitted a breath sample that was tested for the presence of recent alcohol use (Intoximeters, St. Louis, Mo), and a urine sample that was tested for cocaine metabolites using onsite Teststicks (Varian, Palo Alto, CA). Pilot patients completed abbreviated versions of the assessments that contained only information necessary to determine inclusion and exclusion criteria. The flow of patients through the study is shown in Figure 1. A follow-up evaluation, in which patients submitted urine and breath samples, was scheduled for nine months after randomization for those in the randomized phase. Patients received $25 for completing the baseline assessment and $30 for the follow-up.
Figure 1.
Flowchart of participants.
Treatments
Participants were randomized to one of two conditions using a computerized urn program (Stout, Wirtz, Carbonari, & Del Boca, 1994) that balanced patients on therapist and study intake sample result (positive for either alcohol or cocaine, or negative for both). Due to the behavioral nature of the treatments, it was not possible to blind individuals to study treatments.
Standard care (SC) was similar in all three methadone programs and consisted of daily methadone doses and a minimum of weekly group sessions and once per month individual sessions. Sessions addressed issues related to relapse prevention, coping and life skills training, depression, anxiety, and drug use, AIDS education, and daily planning. Standard care at each clinic also consisted of monthly urine testing, and samples were sent to outside laboratories for testing of opioids, cocaine, benzodiazepines and marijuana. These tests continued during the course of study participation for patients, but had no bearing on study participation.
Patients in the study submitted additional breath and urine samples twice weekly (e.g., Mondays-Thursday, Mondays-Fridays, or Tuesdays-Fridays) that were screened for cocaine and alcohol onsite using procedures described earlier. Sample submission was observed by a same-gender staff member whenever possible. Patients earned $1 in gift cards or bus tokens for each sample submitted. Therapists were instructed to congratulate SC patients when they tested negative and to discuss positive samples as clinically indicated and encourage abstinence.
Contingency management treatment consisted of the same treatment as SC, including $1 per sample submitted, regardless of results. Patients in this condition (in both the supervision and randomized phases) also earned the chance to win prizes for submission of negative samples. Each scheduled testing day that patients provided a breath sample that was alcohol negative (<.003 g/dl) and a urine sample that was negative for cocaine according to the onsite testing procedures, they earned at least one draw from an urn, each of which provided the chance of winning a prize. The number of draws increased by one for each consecutive set of negative samples submitted, such that the second consecutive day samples tested negative for cocaine and alcohol resulted in two draws, the third three draws, and so on up to a maximum of 10 draws per day. Patients could earn up to 195 draws if they submitted all cocaine and alcohol negative specimens throughout the 12-week study period. All draws were based upon results from onsite testing; standard monthly offsite testing did not impact reinforcement earned.
Number of draws earned decreased back to one if a positive sample was submitted or if a patient refused to submit a scheduled sample or had an unexcused absence on a testing day (excused absences include court appearance, family emergencies, commitments cleared 24 hours in advance by the therapist). Number of draws remained at reduced values (although they still escalated) until three consecutive negative samples were submitted, at which time the number of draws earned returned to the highest level previously attained.
The urn contained 500 cards, and 50% of them were winning cards: 216 were small prizes (e.g., patient’s choice of $1 fast food coupons, food items, toiletries or bus tokens) worth up to $1. Thirty-three were large prizes, worth up to $20 (e.g., choice of movie theater tickets, CDs, phone cards, art and crafts supplies, watches, etc.), and one was a jumbo prize worth up to $100 (e.g., choice of small stereo, television, or DVD player). A variety of prizes in each category was available from which patients could choose, and patients were also encouraged to suggest prizes in each category. The prizes were kept in a locked cabinet, and when they won a drawing, patients selected a prize from that category: small, large, or jumbo. The average expected cost per draw was about $1.95, and research staff purchased prizes.
Data analysis
We first provide descriptive information with respect to how therapists met the training criteria and their responses to the training satisfaction questionnaire. Paired t-tests compared therapists’ scores pre- and post-training on the CM knowledge test. During the pilot phase, paired t-test compared therapists’ performance on adherence and competence indices during three initial sessions of CM administration to three sessions that occurred at the end of the pilot phase, when therapists were achieving adherence and competence criteria.
We compared outcomes of patients participating in the randomized phase. We employed an intent-to-treat approach, including all 130 randomized patients. Initially, t-tests and chi-square tests compared baseline characteristics between patients randomized to the two treatment groups. Although not all continuous variables were normally distributed, these tests are robust to departures from normality when the sample size is large (Lumley, Diehr, Emerson, & Chen, 2002), and non-parametric tests yielded similar results.
Primary outcomes were available from 100% of patients: weeks remained in study treatment, longest duration of abstinence from cocaine and alcohol, and proportion of samples that tested negative for cocaine and alcohol. A week of abstinence was defined as two consecutively scheduled samples spanning a week that tested negative for both substances. If a patient did not attend a scheduled session or refused to provide or missed a sample because of an unexcused absence, we coded the string of abstinence as broken. Consistent with the reinforcement schedule, excused missed sessions did not break a string of abstinence if they were preceded and followed by negative samples. Excused absences were rare, with means of less than 1 per group. Because continuous abstinence was impacted by treatment drop-out and missed samples, we also analyzed proportions of negative samples. This measure was less impacted by missed samples, as the denominator consisted of the total number of samples submitted. A multivariate analysis evaluated group differences, with the three primary outcomes as dependent variables (Tabachnick & Fidell, 2007). Treatment condition, clinic, and the interaction between treatment condition and clinic were included as independent variables.
Logistic regression identified predictors of submission of samples negative for both cocaine and alcohol at the Month 9 follow-up. In step 1, clinic and baseline urine and breath results were included as categorical variables as these variables have been associated with outcomes in CM studies (Peirce et al., 2006; Stitzer et al., 2007). In step 2, time until follow-up completion (in days from study randomization), treatment condition, longest duration of abstinence achieved during treatment, and the interaction of these later two variables were entered. Because not all patients completed the follow-up, analyses were conducted twice—initially excluding non-followed up patients, and then including them as positive.
Results
Therapist training
Twenty-three therapists participated in the initial training. Twenty-two of them completed pre- and post-training knowledge tests about CM, and the other therapist ceased working at the clinic before completing the initial training. Mean correct scores pre-training were 11.2 (SD = 2.6) out of 20, and post-training they were 18.0 (SD = 1.7), t (21) = 10.37, p < .001. On the CM protocol test, which was only administered post-training, mean (SD) scores were 19.0 (0.9) out of 20. All therapists passed (≥ 16 of 20 items; 80% correct) both post-training tests on their first attempt. With respect to the role plays, only one therapist required more than three scored role play administrations to meet the competence criteria. Mean CMCS scores on the role plays were 6.3 (SD = 0.6), indicating “very good” competence in CM delivery. Training evaluations were available from 22 therapists. Responses are shown in Table 1.
Table 1.
Therapists’ (N = 22) Ratings on Training Experiences in Contingency Management
| Item | % (n) endorsing |
|---|---|
| How does this training rate to others you’ve received? | |
| Below average | 0.0 (0) |
| Average | 9.1 (2) |
| Above average | 27.3 (6) |
| Very good | 31.8 (7) |
| One of the best | 31.8 (7) |
| Would you recommend this training to others? | |
| No, definitely not | 0.0 (0) |
| No, I don’t think so | 0.0 (0) |
| Yes, I think so | 36.4 (8) |
| Yes | 9.1 (2) |
| Yes, enthusiastically | 54.5 (12) |
| How did this training’s materials relate to others? | |
| Much worse | 0.0 (0) |
| About the same | 9.2 (2) |
| Somewhat better | 22.7 (5) |
| Better | 40.9 (9) |
| Much better | 27.3 (6) |
| How often do you think contingency management will be helpful with your clients? | |
| Never | 0.0 (0) |
| Rarely | 4.5 (1) |
| Sometimes | 22.7 (5) |
| Often | 36.4 (8) |
| Very often | 36.4 (8) |
| How much conflict do you feel there is between contingency management and your belief about what is effective treatment for substance use? | |
| Extreme | 0.0 (0) |
| Considerable | 9.1 (2) |
| Moderate | 9.1 (2) |
| Slight | 22.7 (5) |
| None | 59.1 (13) |
In the supervision phase, 22 therapists treated a mean of 3.7 (SD = 2.0) pilot patients each. Demographic characteristics of the pilot patients are shown in Table 2, left-hand column. On average, therapists required 5.0 (SD = 3.7) weeks of supervision (range 3–19 weeks) to meet competence criteria, which did not vary by site, F (2,19) = 1.36, p = .28. Variability in duration of training was primarily related to some therapists working with only one pilot patient at a time, thereby requiring a longer time period to meet the criteria, which necessitated working with at least two pilot patients. Scores on the CM adherence checklist, while high during the initial sessions of CM delivery with a mean of 9.3 (SD = 0.9) out of 10 [range 7.33 to 10.0], rose significantly to 9.9 (0.2) [range 9.4 to 10.0] at the end of the pilot phase, t (15) = 2.79, p = .01. Nine of the 16 therapists showed improvement over time, and the remaining seven had perfect adherence scores with their initial pilot patients, which were retained over time. On average, therapists made 1.6 (SD = 2.6) errors with respect to miscalculating number of draws earned during the supervision phase.
Table 2.
Demographic and Baseline Characteristics
| Pilots | Randomized Patients | ||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Contingency management | Standard care | Contingency management | Statistical test value (df) | p |
| N | 51 | 59 | 71 | ||
| Clinic | χ2(2)=3.99 | .14 | |||
| Clinic A | 31.4 (16) | 42.4 (25) | 28.2 (20) | ||
| Clinic B | 29.4 (15) | 20.3 (12) | 33.8 (24) | ||
| Clinic C | 39.2 (20) | 37.3 (22) | 38.0 (27) | ||
| Age | 39.7 ± 7.2 | 36.4 ± 9.6 | 37.0 ± 9.2 | t (128) = 0.31 | .76 |
| Male, % (n) | 52.9 (27) | 57.6 (34) | 49.3 (35) | χ2 (1) = 0.90 | .34 |
| Years of education | 12.0 ± 1.3 | 11.7 ± 2.1 | 11.3 ± 1.6 | t (128) = −1.41 | .16 |
| Currently married, % (n) | 25.5 (13) | 13.6 (8) | 14.1 (10) | χ2(1) = 0.01 | .93 |
| Employed full-time, % (n) | 49.0 (25) | 54.2 (32) | 52.1 (37) | χ2 (1) = 0.06 | .81 |
| Past month income | $1481 ± $1429 | $717 ± 1,006 | $875 ± 1210 | t (235) = 1.29 | .20 |
| Ethnicity, % (n) | χ2 (3) = 4.30 | .23 | |||
| African American | 9.8 (5) | 10.2 (6) | 5.6 (4) | ||
| European American | 64.5 (33) | 62.7 (37) | 78.9 (56) | ||
| Hispanic American | 21.6 (11) | 23.7 (14) | 12.7 (9) | ||
| Other | 3.9 (2) | 3.4 (2) | 2.8 (2) | ||
| Alcohol dependent, % (n) | not assessed | 13.6 (8) | 14.1 (10) | χ2 (1) = 0.01 | .93 |
| Methadone dose (mg) | 84.9 ± 24.3 | 91.1 ± 29.0 | 87.2 ± 25.4 | t (125) = −0.82 | .41 |
| Intake sample cocaine and alcohol negative, % (n) | not assessed | 45.8 (27) | 52.1 (37) | χ2 (1) = 0.52 | .47 |
| Addiction Severity Index Scores | |||||
| Medical | not assessed | 0.31 ± 0.38 | 0.34 ± 0.38 | t (128) = 0.54 | .60 |
| Employment | not assessed | 0.57 ± 0.55 | 0.55 ± 0.27 | t (128) = −0.36 | .72 |
| Alcohol | not assessed | 0.05 ± 0.11 | 0.04 ± 0.10 | t (128) = −0.16 | .87 |
| Drug | not assessed | 0.19 ± 0.09 | 0.17 ± 0.08 | t (128) = −1.69 | .09 |
| Legal | not assessed | 0.10 ± 0.21 | 0.06 ± 0.13 | t (128) = −1.23 | .22 |
| Family/social | not assessed | 0.17 ± 0.20 | 0.20 ± 0.24 | t (128) = 0.96 | .34 |
| Psychiatric | not assessed | 0.30 ± 0.24 | 0.27 ± 0.20 | t (128) = −0.72 | .48 |
Note. Values are means and standard deviations unless otherwise noted. Statistical analyses refer to comparisons between patients randomized to the two treatment conditions.
In comparing CMCS scores from initial pilot interactions versus pilot interactions that occurred at the end of the supervision phase when therapists met competence criteria, overall scores rose significantly as shown in Table 3. Two CMCS items yielded significant differences over time: assessing clients’ desire for prizes, and praising clients for their efforts at abstinence. Sixteen therapists completed the supervision phase, with three therapists leaving the clinic, one retiring, and two not wanting to proceed in the study, primarily because of time requirements related to meeting with patients for sample collection.
Table 3.
Mean Ratings on Items of the Contingency Management Competence Scale
| Item | Early pilot (n = 20) | End of pilot (n = 20) | Statistical test value (df), p | Randomized phase (n = 820) |
|---|---|---|---|---|
| Overall score | 5.9 (0.7) | 6.2 (0.5) | t (20) = 2.10, p = .04 | 5.8 (0.8) |
| 1. To what extent did the therapist discuss outcomes of urine and breath sample monitoring? | 6.7 (0.5) | 6.8 (0.6) | t (20) = 0.65, p = .52 | 6.4 (1.1) |
| 2. To what extent did the therapist state how many draws were earned at this session? | 6.5 (0.5) | 6.5 (1.0) | t (20) = 0.09, p = .93 | 6.6 (1.2) |
| 3. To what extent did the therapist state how many draws would be earned at the next session if the client were abstinent? | 5.8 (1.1) | 6.1 (1.1) | t (20) = 0.92, p = .36 | 6.0 (2.1) |
| 4. To what extent did the therapist assess the client’s desire for items in the prize cabinet? | 4.8 (1.4) | 5.7 (1.2) | t (20) = 3.00, p = .007 | 4.9 (1.8) |
| 5. To what extent did the therapist discuss the client’s self-report of substance use? | 5.3 (0.6) | 5.9 (1.6) | t (9) = 1.15, p = .28 | 4.9 (1.8) |
| 6. If the client self-reported substance use, to what extent did the therapist relate self-reports of substance use to objective indicators of substance use? | 5.3 (0.7) | 5.8 (1.2) | t (9) = 0.98, p = .35 | 5.1 (1.9) |
| 7. If the client self-reported substance use, to what extent did the therapist relate self-report of substance use to consequences of positive samples? | 5.3 (1.0) | 5.9 (0.9) | t (20) = 1.85, p = .10 | 5.1 (1.7) |
| 8. To what extent did the therapist compliment/praise client’s efforts toward abstinence? | 5.7 (1.3) | 6.3 (0.6) | t (20) = 2.15, p = .04 | 5.5 (1.2) |
| 9. To what extent did the therapist communicate confidence that the client’s efforts will yield success in the future? | 5.7 (1.2) | 6.1 (0.6) | t (20) = 1.93, p = .07 | 5.3 (1.3) |
| 10. General skillfulness (expertise, competence and commitment, engages client in discussion, interventions made at appropriate times—not missed or made too early). | 5.9 (0.8) | 6.2 (0.6) | t (20) = 1.37, p = .19 | 5.7 (0.9) |
| 11. Maintaining session structure (maintains session focus, sets appropriate tone and structure, appropriate level of therapist activity/directiveness, duration). | 6.0 (0.5) | 6.2 (0.5) | t (20) = 1.92, p = .07 | 5.8 (0.9) |
| 12. Empathy (conveys warmth and sensitivity, demonstrates genuine concern and a non- judgmental stance, understands/expresses clients’ feelings and concerns). | 6.3 (0.6) | 6.3 (0.6) | t (20) = 0.08, p = .94 | 5.8 (0.9) |
Randomized phase
After successfully completing all stages of the training, 16 therapists participated in the randomized phase of the study. Together, they treated 130 patients (range 1–19), with 59 patients randomized to SC and 71 to CM. Demographic and baseline characteristics of the randomized patients are presented in Table 2. The two groups did not differ with respect to any baseline characteristics, as shown in the right-hand columns.
Main study outcomes were: time remained in the study, longest duration of abstinence achieved, and proportion of negative samples submitted. The multivariate analysis revealed significant effects of treatment condition with respect to these variables, F (3,122) = 8.03, p < .000, and subsequent univariate tests revealed significant effects on each measure as shown in Table 4. Patients randomized to the CM condition remained significantly longer in the study, achieved significantly longer durations of abstinence from cocaine and alcohol, and submitted significantly greater proportions of samples negative for cocaine and alcohol than those assigned to SC. The vast majority of the positive samples related to cocaine use; only 2.3% of submitted samples tested positive for alcohol. Overall effect sizes were in the medium to large range.
Table 4.
Retention and Abstinence Outcomes
| Variable | Standard care | Contingency management | Statistical test Value (df), p-value | Effect size Cohen’s d |
|---|---|---|---|---|
| N | 59 | 71 | ||
|
| ||||
| Weeks remained in study | 6.7 ± 5.0 | 9.5 ± 3.6 | F (1,124) = 17.86, p < .000 | 0.64 |
| Clinic A | 6.4 ± 4.8 | 8.7 ± 4.4 | t (43) = 1.58, p = .12 | 0.50 |
| Clinic B | 4.2 ± 4.8 | 10.4 ± 2.9 | t (34) = 4.88, p < .000 | 1.56 |
| Clinic C | 8.0 ± 5.0 | 9.3 ± 3.4 | t (47) = 1.04, p < .30 | 0.30 |
| Longest duration of cocaine and alcohol negative samples (in weeks) | 1.7 ± 2.7 | 4.7 ± 4.7 | F (1,124) = 19.31, p < .000 | 0.78 |
| Clinic A | 1.3 ± 2.0 | 4.1 ± 5.1 | t (43) = 2.57, p = .01 | 0.72 |
| Clinic B | 1.8 ± 2.8 | 6.8 ± 4.5 | t (34) = 3.55, p < .000 | 1.33 |
| Clinic C | 2.2 ± 3.2 | 3.3 ± 4.0 | t (47) = 1.08, p = .28 | 0.30 |
| Proportion of samples negative for cocaine and alcohol | 29.4 ± 33.3 | 57.7 ± 40.0 | F (1,124) = 17.52, p < .000 | 0.77 |
| Clinic A | 21.4 ± 26.2 | 48.1 ± 42.7 | t (43) = 2.58, p = .01 | 0.75 |
| Clinic B | 31.5 ± 39.8 | 75.8 ± 33.7 | t (34) = 3.50, p < .000 | 1.20 |
| Clinic C | 37.3 ± 36.0 | 48.8 ± 38.9 | t (47) = 1.06, p = .29 | 0.31 |
Note. Values represent means and standard deviations.
Means (SD) of outcome measures for patients in each clinic are also presented in Table 4, along with effect sizes comparing CM to SC within each clinic. Although effect sizes ranged from 0.3 to over 1.5 across the clinics, clinic was not significantly associated with any of the outcomes in the multivariate analyses (p > .08), and the clinic by treatment group interaction effect was also not significant (p > .19). Effects were always in the same direction, with CM patients having better outcomes than SC patients during the 12-week treatment period.
Follow-up analyses
Patients who were randomly assigned to CM were significantly more likely to submit samples at the Month 9 follow-up than those assigned to SC, 91.1% (n = 64) versus 78.0% (n = 46), χ2 (1) = 3.70, p = .05. In addition, time to follow-up completion was significantly shorter in patients who had been assigned to CM versus those who had received SC earlier, 274 (SD = 20) versus 285 (SD = 35) days on average, t (108) = 2.01, p < .05. The proportion of samples at follow-up that tested negative for cocaine and alcohol were numerically lower, albeit not significantly, in CM treated versus SC treated patients when including only patients who submitted samples at the follow-up, 54.7% (n = 35 of 64) versus 71.7% (n = 33 of 46), χ2 (2) = 3.30, p = .07. When missing samples were considered positive, the trend towards group differences disappeared, with 49.3% (n = 35 of 71) and 55.9% (n = 33 of 59) negative samples at follow-up in CM treated patients versus SC treated patients, χ2 (1) = 0.57, p = .45.
In evaluating predictors of abstinence at Month 9, Step 1 of the first logistic regression that included only patients who submitted samples at the follow-up (n = 110) was significant, χ2 (3) = 9.53, p = .02, with a negative sample at baseline significantly associated with a negative sample at Month 9, Beta (B) = 0.84, Standard error (SE) = 0.41, Wald = 4.08, p = .04. The odds ratio (OR) was 2.31, 95% confidence interval (CI) = 1.03 – 5.20. Step 2 was also significant, χ2 (4) = 18.60, p < 0.001, along with the model, χ2 (7) = 28.13, p < 0.001, and 70.0% of cases were correctly identified. Longer time to follow-up completion was significantly and positively associated with submission of a negative sample, B (SE) = 0.03 (0.01), Wald = 4.81, p = .03, OR (95% CI) = 1.03 (1.00 – 1.06), indicating that each additional day until completing the follow-up was associated with a 3% increased probability of submitting a negative sample. Clinic was also significantly related to submission of a negative sample at Month 9 when Step 2 was included in the model, with Clinics B and C having greater rates of abstinence at Month 9 than Clinic A, B (SE) = 1.63 (0.61), Wald = 7.24, p = .007, OR (95% CI) = 5.10 (1.56–16.68) and B (SE) = 1.28 (0.57), Wald = 5.04, p = .03, OR (95% CI) = 3.58 (1.18 – 10.89), respectively.
A second logistic regression included all randomized patients in the analyses (N = 130), with positive samples and average days to follow-up within group coded for patients who failed to complete the follow-up. Again, Step 1 was significant, χ2 (3) = 12.63, p < .01, with negative baseline sample results associated with abstinence at Month 9, B (SE) = 0.96 (0.37), Wald = 6.69, p = .01, OR (95% CI) = 2.62 (1.26 – 5.44). Step 2 was also significant and improved the model, χ2 (4) = 17.11, p < .002 for the step and χ2 (7) = 29.74, p < .001 for the model, correctly identifying 70.0% of the cases. Again, time until follow-up completion was significantly associated with submission of a negative sample, B (SE) = 0.02 (0.01), Wald = 5.00, p = .02, OR (95% CI) = 1.02 (1.00 – 1.04) as was clinic, with Clinic B having more abstinent patients at Month 9 than Clinic A, B (SE) = 1.30 (0.54), Wald = 5.85, p = .02, OR (95% CI) = 3.68 (1.28 – 10.61). In this analysis, longest duration of abstinence achieved during treatment was also significantly associated with abstinence at Month 9, B (SE) = 0.57 (0.28), Wald = 4.18, p = .04, OR (95% CI) = 1.76 (1.02 – 3.04). Thus, each continuous week of abstinence achieved during treatment was associated with a 76% increased chance of abstinence at Month 9.
Therapist performance in CM delivery during the randomized phase
During the randomized phase, therapists were provided no feedback regarding their administration of SC or CM treatments. However, fidelity to CM administration was measured in the same manner as during the pilot supervision phase. The mean CMCS score on 820 tapes rated from patients randomized to the CM condition was 5.8 (SD = 0.8), reflecting “very good” competence in CM delivery; mean scores on each items are shown in Table 3. On the 867 CM adherence checklists, the average score was 9.8 (SD = 0.6), indicating near perfect recording of results and awarding of prizes. Therapists made a mean of 0.2 (SD = 0.1) errors in terms of calculating draws earned during the randomized phase. The mean duration of a CM session, including awarding of prizes, was 6.0 (SD = 2.8) minutes.
Reinforcement earned and adverse events
On average, patients assigned to CM earned 72.6 (SD = 76.8) draws during the 12-week study. The mean number of small, large and jumbo prizes won were 32.2 (SD = 34.6), 5.5 (SD = 6.5), and 0.2 (SD = 0.6), respectively, resulting in an overall reinforcement cost of $160 (186) per patient. There were no study related adverse events, no patients filed complaints about study related procedures, and no patients experienced increases in gambling problems.
Discussion
Results from this study reveal that community-based therapists readily trained to high levels of adherence and competence in CM delivery. Ninety-six percent of therapists who began the training passed quizzes that assessed general and specific knowledge of CM, and the training was well received with modal ratings of “better than average” to “very good” on all indices. Therapists’ adherence to the CM protocol with respect to tracking of sample collection and number of draws was high following the initial training and at the beginning of the pilot supervision phase, and it rose to near perfect levels within an average of 5 weeks of supervision. Although fairly frequent mistakes in awarding reinforcement were noted initially, errors were quickly corrected with the weekly individual supervision sessions. These descriptive data are important from a dissemination standpoint because CM requires more (and different) paperwork than most psychotherapies, and inappropriate reinforcement parameters (e.g., too low magnitude, infrequent or delayed reinforcement, non-escalating reinforcers, failure to include resets) can adversely impact patient outcomes (Lussier et al., 2006; Roll, Higgins, & Badger, 1996; Roll, Reilly, & Johanson, 2000; Silverman, Chutuape, Bigelow, & Stitzer, 1999).
To be effective, not only must a CM protocol be delivered according to appropriate reinforcement parameters, but the quality of CM delivery also impacts outcomes. A therapist who administers the correct amount of reinforcement but who does so in an unenthusiastic or negative tone could undermine the patient-therapist relationship and the impact of reinforcement procedures on subsequent abstinence. The CMCS measures therapist performance with respect to delivery of CM, and scores on it are significantly associated with patient outcomes (Petry et al., 2010). These community-based therapists were readily able to administer CM competently. Following the didactics training, therapists’ delivery of CM during role-plays was rated “very good” on the CMCS. When provided with pilot patients, statistically significant (albeit minor in magnitude) increases in competence were noted over time when supervision was provided.
Once adequately trained, these community-based clinicians maintained high levels of adherence and competence in CM delivery. Scores on the CM adherence checklist were very high, and the number of mistakes in terms of draws awarded was very low. Average scores on the CMCS remained at levels of “very good” during the randomized phase, when clinicians were no longer receiving feedback about intervention delivery. Thus, with training and individual supervision, community-based clinicians can administer CM with high levels of adherence and very good competence. Because this study did not evaluate different training modalities or types, similar levels of adherence and competence may have been achieved with less intensive training. Future studies should evaluate other methods for training clinicians in CM treatments. However, most likely individualized performance feedback and coaching regarding CM delivery will be necessary to engender appropriate delivery (Andrejewski et al., 2001). Most studies evaluating training methods concur that manuals and workshops alone are insufficient in producing competent delivery of psychotherapies (IOM, 1998; Miller, Sorenson, Slezer, & Brigham, 2006).
Importantly, once therapists are trained to high levels of adherence and competence, CM is efficacious. The overall effect sizes of CM in this study were as high as-- and sometimes higher than-- effect sizes noted when research staff deliver CM (Lussier et al., 2006). Statistically significant benefits were noted in terms of longest duration of abstinence achieved and proportion of negative samples submitted. Although effects were not significant within each individual clinic, all clinics evidenced effect sizes of 0.3 or greater. Clinic differences in the efficacy of CM have been reported previously (Peirce et al., 2006) and are not surprising given potential differences in patient populations, therapists, and clinic policies and procedures that may impact retention and drug use. Nevertheless, the consistently beneficial effects of CM for reducing cocaine use in methadone patients point to its wide-scale usefulness.
In this study, significant effects on study continuation were also evident, with CM patients remaining in the study an average of 3.5 weeks longer than SC patients. In most prior CM studies conducted in methadone clinics (e.g., Peirce et al., 2006), differential study attrition was not noted, most likely because methadone itself is a strong reinforcer. Similarly, relatively few patients in this study-- 15 of 130 (11.5%)-- withdrew from methadone treatment, and methadone discontinuation rates did not differ by treatment condition. A small payment was provided for sample submission (regardless of results) to all patients in this study, but perhaps larger payments are necessary to keep patients engaged in frequent urine testing when such testing is not universally applied. Further, these clinicians may not have been as well trained as research staff in encouraging sample submission and continued study participation among patients randomized to SC. Regardless of the differential rates of study drop out during the study period, the significant benefit of CM on the proportion of negative samples submitted is unaffected by missing samples.
Some (e.g., Ghitza et al., 2007; Petry & Martin, 2002; Petry, Martin, & Simcic, 2005), but not all (e.g., Petry et al., 2007; Rawson et al., 2002; Silverman et al., 1999), studies of CM in methadone clinics found significant benefits of CM on abstinence after CM ended. This study found a trend for SC patients to submit more negative samples at follow-up than CM patients. However, this trend was likely related to differential rates of follow-up participation as significantly more CM patients completed the follow-up and they did so with less delay than SC patients. In the logistic regressions, time to follow-up completion was significantly associated with abstinence at the Month 9 follow-up, indicating that some patients may have delayed participating in the follow-up until they achieved a brief period of abstinence. When research staff administer all study interventions, differential rates of follow-up completion are not observed (Petry & Martin, 2002; Petry, Martin et al., 2005; Petry et al., 2007), perhaps because research staff establish strong relationships with both SC and CM patients, repeatedly emphasizing their role as research participants and the need for timely completion of follow-ups. Another explanation for differential attrition and follow-up may have been related to a perceived confound between study data and clinical care. In most CM trials (Petry & Martin, 2002; Petry, Martin, et al., 2005; Petry et al., 2007), results of study urinalysis testing are not shared with clinical staff; in the present study, clinical staff collected and tested study samples. Some patients may have perceived or experienced adverse effects related to submitting positive samples, leading to study attrition. Nevertheless, no patients voiced complaints about study procedures, and reviews of audiotapes did not reveal administration of adverse clinical consequences related to submission of positive samples. Patients in this study were not eligible for take-home methadone doses due to submission of positive samples prior to study participation, so take-home privileges were not suspended based on study urine sample results.
Results from this study should be interpreted in the context of some limitations. The differential rates of follow-up completion likely biased long-term outcomes, and future studies should extend efforts to ensure high rates of follow-up participation among all randomized patients. This study was conducted with clinicians at methadone clinics and stimulant abusing and dependent methadone patients; these results may not generalize to other therapist and patient populations. Compared to therapists employed in non-opioid maintenance settings, therapists in methadone clinics may have educational and personal drug use backgrounds that may make them more or less willing and able to utilize CM. Therapist effects on outcomes were not examined because some therapists treated only a very small number of patients (range 1–19). All clinics showed effects in the same direction and effects of clinic were not significant in the multivariate analysis of during-treatment outcomes, but the clinic with the highest retention and lowest drug use rates among SC patients had lower effect sizes of CM, an effect noted previously (Peirce et al., 2006). Benefits of CM may vary based on clinic characteristics, and greater understanding of clinic effects is needed.
Other issues related to the study design should also be considered. As noted earlier, this study did not evaluate the efficacy of training and supervision methods for CM, and the training was individualized and rather intensive, albeit for relatively short durations. Methods for estimating training and supervision time and costs are outlined in Petry (2011). In this study, we did not collect detailed records on amounts of time therapists spent in training activities, but general estimates are two half days for didactics, one or two half days for reviewing materials and practicing role plays, and 30 minutes per week for individual supervision for an average of 5 weeks. If less personalized training procedures were utilized, beneficial effects of CM may not be as pronounced as those reported herein. Finally, this study provided financial resources and administrative oversight for the prizes, and none of the clinics implemented CM as described herein after study support ended. Management of prize CM systems adds to their costs (Olmstead, Sindelar & Petry, 2007), further impeding their uptake in practice settings. Additional work is necessary to address issues related to broad dissemination and sustainability of CM.
Despite these limitations, this is the first known study to train and evaluate the efficacy of CM as described in the research literature when delivered entirely by community-based clinicians. Conducting randomized trials in community clinics using existing clinical staff is a challenging endeavor (Carroll et al., 2002). Difficulties may be compounded when a relatively unrecognized and controversial intervention such as CM (Kirby, Benishek, Dugosh, & Kerwin, 2006) is the target of such investigation.
Strengths of this study are that it employed a randomized design, and three community-based clinics participated, along with 23 therapists, 16 of whom (70%) completed study procedures. Data from this study show that community-based clinicians are willing and able to implement CM with a high degree of adherence and competence when provided with appropriate CM protocols and training experiences. Importantly, patient outcomes improved along all domains during the period in which CM was implemented. Further investigation of long-term benefits of CM is needed, along with methods to extend the improvements gained when patients receive CM. Given the high rates of attrition from treatment and poor prognosis of cocaine-dependent methadone patients, training of community-based clinicians in this evidence-based treatment is justified.
Acknowledgments
Funding for this study and preparation of this report was provided by NIH Grants R01-DA016855, P30-DA023918, R01-DA13444, R01-DA018883, R01-DA021567, R01-DA022739, R01-DA024667, R01-DA027615, P50-DA09241, P60-AA03510, and M01-RR06192.
References
- Andrzejewski ME, Kirby KC, Morral AR, Iguchi MY. Technology transfer through performance management: The effects of graphical feedback and positive reinforcement on drug treatment counselors’ behavior. Drug Alcohol Dependence. 2001;63:179–186. doi: 10.1016/S0376-8716(00)00207-6. [DOI] [PubMed] [Google Scholar]
- Ball JC, Ross A. The effectiveness of methadone maintenance treatment: Patients, programs, services, and outcomes. New York: Springer-Verlag; 1991. [Google Scholar]
- Bandettini DPA, Fornai F, Paparelli A, Pacini M, Perugi G, Maremmani I. Comparison between heroin and heroin-cocaine polyabusers: a psychopathological study. Annals of the New York Academy of Sciences. 2006;1074:438–45. doi: 10.1196/annals.1369.044. [DOI] [PubMed] [Google Scholar]
- Barber JP, Crits-Christoph P, Luborsky L. Effects of therapist adherence and competence on patient outcome in brief dynamic therapy. Journal of Consulting and Clinical Psychology. 1996;64:619–622. doi: 10.1037/0022-006X.64.3.619. [DOI] [PubMed] [Google Scholar]
- Brown BS. From research to practice: The bridge is out and the water’s rising. Advances in Medical Sociology. 2000;7:345–365. [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. Journal of Consulting and Clinical Psychology. 2006;74:307–316. doi: 10.1037/0022-006X.74.2.307. [DOI] [PubMed] [Google Scholar]
- Cameron J, Ritter A. Contingency management: perspectives of Australian service providers. Drug and Alcohol Review. 2007;26:183–189. doi: 10.1080/09595230601184653. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Farentinos C, Ball SA, Crits-Christoph P, Libby B, Morgenstern J, Woody GE. MET meets the real world: design issues and clinical strategies in the Clinical Trials Network. Journal of Substance Abuse Treatment. 2002;23:73–80. doi: 10.1016/S0740-5472(02)00255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifry R, Nuro K, Frankforter T, Ball SA, Rounsaville BJ. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/S0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. Bridging the gap: A hybrid model to link efficacy and effectiveness research in substance abuse treatment. Psychiatric Services. 2003;54:333–339. doi: 10.1176/appi.ps.54.3.333. Retrieved from http://psychservices.psychiatryonline.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JE, Sheidow AJ, Henggeler SW, Halliday-Boykins C, Cunningham PB. Developing a measure of therapist adherence to contingency management: An application of the many-facet Rasch model. Journal of Child and Adolescent Substance Abuse. 2008;17:47–68. doi: 10.1080/15470650802071655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Stein JB, Robertson EB, Pintello D, Pringle B, Volkow ND. Charting a course for health services research at the National Institute on Drug Abuse. Journal of Substance Abuse Treatment. 2005;29:167. doi: 10.1016/j.jsat.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council on Addiction Psychiatry. Position statement on methadone maintenance treatment. American Journal of Psychiatry. 1994;151:792–794. doi: 10.1176/ajp.151.5.792. Retrieved from http://ajp.psychiatryonline.org. [DOI] [PubMed] [Google Scholar]
- DeMaria PA, Sterling R, Weinstein SP. The effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatment. American Journal on Addictions. 2000;9:145–153. doi: 10.1080/10550490050173217. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbin M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Ghitza UE, Epstein DH, Schmittner J, Vahabzadeh M, Lin JL, Preston KL. Randomized trial of prize-based reinforcement density for simultaneous abstinence from cocaine and heroin. Journal of Consulting and Clinical Psychology. 2007;75:765–774. doi: 10.1037/0022-006X.75.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlick M, Lamb S, McCarty D. Bridging the Gap Between Practice and Research: Foraging Partnerships with Community-based Drug and Alcohol Treatment. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug and Alcohol Dependence. 2000;58:55–66. doi: 10.1016/S0376-8716(99)00068-X. [DOI] [PubMed] [Google Scholar]
- Haug NA, Shopshire M, Tajima B, Gruber V, Guydish J. Adoption of evidence-based practices among substance abuse treatment providers. Journal of Drug Educaiton. 2008;38:181–92. doi: 10.2190/DE.38.2.f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler SW, Chapman JE, Rowland M, Halliday-Boykins C, Randall J, Shackelford J, Schoenwald SK. Statewide adoption and initial implementation of contingency management for substance-abusing adolescents. Journal of Consulting and Clinical Psychology. 2008;76:556–567. doi: 10.1037/0022-006X.76.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler SW, Chapman JE, Rowland MD, Halliday-Boykins CA, Randall J, Shackelford J, Schoenwald SK. If you build it, they will come: statewide practitioner interest in contingency management for youths. Journal of Substance Abuse Treatment. 2007;32:121–131. doi: 10.1016/j.jsat.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DEH, Dantona RL. Contingency reinforcement increases cocaine abstinence during outpatient treatment and at 1 year follow-up. Journal of Consulting and Clinical Psychology. 2000;68:64–72. doi: 10.1037/0022-006X.68.1.64. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Bridging the Gap Between Practice and Research: Forging Partnerships with Community-Based Drug and Alcohol Treatment. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- Kellogg SH, Burns M, Coleman P, Stitzer M, Wale JB, Kreek MJ. Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. Journal of Substance Abuse Treatment. 2005;28:57–65. doi: 10.1016/j.jsat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: implications for dissemination. Drug and Alcohol Dependence. 2006;85:19–27. doi: 10.1016/j.drugalcdep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Lash SJ, Stephens RS, Burden JL, Grambow SC, DeMarce JM, Jones ME, Homer RD. Contracting, prompting, and reinforcing substance use disorder continuing care: A randomized clinical trial. Psychology of Addictive Behaviors. 2007;21:387–397. doi: 10.1037/0893-164X.21.3.387. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Alessi SM, Hanson T, Godley MD, Petry NM. Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. Journal of Applied Behavior Analysis. 2008;41:517–526. doi: 10.1901/jaba.2008.41-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health datasets. Annual Review of Public Health. 2002;23:151–169. doi: 10.1146/annurev.publheath.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil S, Mongeon J, Badger G, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Martino S, Ball S, Nich C, Frankforter TL, Carroll KM. Community program therapist adherence and competence in motivational enhancement therapy. Drug and Alcohol Dependence. 2008;96:37–48. doi: 10.1016/j.drugalcdep.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn EA, Asch SM, Adams I, Keesey J, Hicks J, DeChristofano A, Kara E. The quality of health care delivered to adults in the US. New England Journal of Medicine. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. Retrieved from http://www.nejm.org. [DOI] [PubMed] [Google Scholar]
- McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: dissemination research in an addiction treatment system. Journal of Substance Abuse Treatment. 2004;26:305–312. doi: 10.1016/j.jsat.2004.03.003. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Carise D, Kleber HD. Can the national addiction treatment infrastructure support the public’s demand for quality care? Journal of Substance Abuse Treatment. 2003;25:117–121. doi: 10.1016/S0740-5472(03)00156-9. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Milby JB, Garrett C, English C, Fritschi O, Clarke C. Take-home methadone – contingency effects on drug-seeking and productivity of narcotic addicts. Addictive Behaviors. 1978;3:215–220. doi: 10.1016/0306-4603(78)90022-9. [DOI] [PubMed] [Google Scholar]
- Miller WR, Sorensen JL, Selzer JA, Brigham GS. Disseminating evidence-based practices in substance abuse treatment: a review with suggestions. Journal of Substance Abuse Treatment. 2006;31:25–39. doi: 10.1016/j.jsat.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, Petry NM. Cost-effectiveness of prize-based incentives for stimulant abusers in outpatient psychosocial treatment programs. Drug and Alcohol Dependence. 2007;87:175–182. doi: 10.1016/j.drugalcdep.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. Retrieved from http://archpsyc.ama-assn.org. [DOI] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence. 2000;58:27–33. doi: 10.1016/S0376-8716(99)00071-X. [DOI] [PubMed] [Google Scholar]
- Petry NM. Contingency Management for Substance Abuse Treatment: A Guide to Implementing this Evidenced-based Practice. New York: Routledge; 2011. [Google Scholar]
- Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. Journal of Substance Abuse Treatment. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes vs. vouchers in cocaine-using methadone patients. Journal of Consulting and Clinical Psychology. 2007;75:983–991. doi: 10.1037/0022-006X.75.6.983. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the contingency management competence scale. Drug and Alcohol Dependence. 2010;109:167–174. doi: 10.1016/j.drugalcdep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, Tardiff M. Vouchers versus prizes: Contingency management treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology. 2005;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Peirce JM, Roll JM, Stitzer ML, Hamilton JA. Prize-based contingency management does not increase gambling. Drug and Alcohol Dependence. 2006;83:269–273. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology. 2002;70:398–405. doi: 10.1037/0022-006X.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68:250–257. doi: 10.1037/0022-006X.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic F., Jr Prize reinforcement contingency management for cocaine dependence: integration with group therapy in a methadone clinic. Journal of Consulting and Clinical Psychology. 2005;73:354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Li R. Effects of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment program: A National Drug Abuse Treatment Clinical Trials Network Study. Archives of General Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. Retrieved from http://archpsyc.ama-assn.org/ [DOI] [PubMed] [Google Scholar]
- Petry NM, Stitzer ML. Contingency Management: Using Motivational Incentives to Improve Drug Abuse Treatment. West Haven, CT: Yale University Psychotherapy Development Center; 2002. [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Archives of General Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Reilly MP, Johanson CE. The influence of exchange delays on cigarette versus money choice: a laboratory analog of voucher-based reinforcement therapy. Experimental and Clinical Psychopharmacology. 2000;8:366–70. doi: 10.1037/1064-1297.8.3.366. [DOI] [PubMed] [Google Scholar]
- Rothbard AB, Alterman A, Rutherford M, Liu F, Zelinski S, McKay J. Revisiting the effectiveness of methadone treatment on crime reductions in the 1990s. Journal of Substance Abuse Treatment. 1999;16:329–335. doi: 10.1016/S0740-5472(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, Hall SM. Methadone maintenance vs. 180-day psychosocially enriched detoxification for treatment of opioid dependence: A randomized controlled trial. Journal of the American Medical Association. 2000;283:1303–1310. doi: 10.1001/jama.283.10.1303. Retrieved from http://jama.ama-assn.org/ [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–38. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opinion on Emerging Drugs. 2006;11:91–98. doi: 10.1517/14728214.11.1.91. [DOI] [PubMed] [Google Scholar]
- Squires DD, Gumbley SJ, Storti S. Training substance abuse treatment organizations to adopt evidence-based practices: The Addiction Technology Transfer Center of New England Science to Service Laboratory. Journal of Substance Abuse Treatment. 2008;34:293–301. doi: 10.1016/j.jsat.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Stitzer M, Bigelow G, Lawrence C, Cohen J, Dlugoff B, Hawthorne J. Medication take-home as a reinforcer in a methadone-maintenance program. Addictive Behaviors. 1977;2:9–14. doi: 10.1016/0306-4603(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Peirce J, Petry NM, Kirby K, Roll J, Krasnansky J, Li R. Abstinence-based incentives in methadone maintenance: interaction with intake stimulant test results. Experimental and Clinical Psychopharmacology. 2007;15:344–350. doi: 10.1037/1064-1297.15.4.344. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol, Supplement. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. Retrieved from http://www.jsad.com/ [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics, Fifth Edition. Boston, Massachusettes: Allyn & Bacon; 2007. [Google Scholar]
- Tross S, Hanner J, Hu MC, Pavlicova M, Campbell A, Nunes EV. Substance use and high risk sexual behaviors among women in psychosocial outpatient and methadone maintenance treatment programs. American Journal of Drug and Alcohol Abuse. 2009;35:368–374. doi: 10.1080/00952990903108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DA, Korcha R, Havassy BE, Hall SM. Detection of illicit opioid and cocaine use in methadone maintenance treatment. American Journal of Drug and Alcohol Abuse. 1999;25:561–571. doi: 10.1081/ADA-100101879. [DOI] [PubMed] [Google Scholar]
- Weisner C, McLellan AT. Report of the Blue Ribbon Task Force on Health Services Research at the National Institute on Drug Abuse. Rockville, MD: National Institute on Drug Abuse; 2004. [Google Scholar]
- Zanis DA, Metzger DS, McLellan AT. Factors associated with employment among methadone patients. Journal of Substance Abuse Treatment. 1994;11:443–447. doi: 10.1016/0740-5472(94)90097-3. [DOI] [PubMed] [Google Scholar]