Abstract
A clinical trial of a protein farnesyltransferase inhibitor (lonafarnib) for the treatment of Hutchinson-Gilford progeria syndrome (HGPS) was recently completed. Here, we discuss the mutation that causes HGPS, the rationale for inhibiting protein farnesyltransferase, the potential limitations of this therapeutic approach, and new potential strategies for treating the disease.
The specter of children aging prematurely has fascinated both biomedical scientists and the general public, as evidenced by the frequent newspaper articles about affected children and their families. The interest in Hutchinson-Gilford progeria syndrome (HGPS), the classical premature aging syndrome of children (1), has been particularly intense over the past several months with publication of the results of a clinical trial of the small-molecule drug lonafarnib for the treatment of HGPS (2). In this Perspective, we analyze HGPS pathophysiology and how it has driven the discovery and development of therapeutics.
HGPS, an extremely rare genetic disease caused by a point mutation in the LMNA gene (3), comes to medical attention early in life with reduced weight gain, loss of body fat, alopecia, and a variety of bone and dental abnormalities (1). When fully grown, affected patients weigh only ~12 to 15 kg and exhibit disease phenotypes that resemble premature aging, such as hair loss and thin skin. Children with HGPS also develop atherosclerosis and often succumb to myocardial infarction or stroke during their teenage years. However, some features of physiological aging—for example, dementia—are absent in HGPS.
The small-molecule drug lonafarnib is a protein farnesyltransferase inhibitor (FTI). Protein farnesyltransferase (FTase) is a cytosolic enzyme that adds a 15-carbon farnesyl lipid to a diverse group of protein substrates, including the Ras proteins, a family of small guanosine triphosphatases (GTPase) involved in normal signal transduction and cancer development. Ras proteins terminate with a CaaX motif—a cysteine, a pair of amino acids that are of en aliphatic, followed by one of many different amino acid residues (4)— that undergoes three sequential enzymatic modifications. The cysteine is farnesylated by FTase (in a process referred to as protein prenylation), which is followed by endoproteolytic release of the last three amino acids of the protein (aaX) and carboxyl methylation of the newly exposed farnesylcysteine (Fig. 1). These modifications render the carboxyl terminus more hydrophobic, facilitating interactions with membranes within cells. FTIs were initially developed as anticancer agents (5). The hope was that blocking protein farnesylation would interfere with the binding of activated Ras proteins to the plasma membrane and thereby block the constitutive signaling activity that fuels cell division.
Fig. 1. The nuclear lamina and prelamin A processing.
The nuclear lamina is an intermediate filament meshwork that lies beneath the inner nuclear membrane; the lamina serves as a scaffold for the cell nucleus and also affects many other functions in the cell nucleus. One of the main protein components of the nuclear lamina is mature lamin A. Prelamin A undergoes four posttranslational processing steps. First, the cysteine of the carboxyl-terminal CaaX motif (–CSIM) is farnesylated by FTase. Second, the last three amino acids (–SIM) are clipped off by either RCE1 or ZMPSTE24. Third, the newly exposed carboxyl-terminal farnesylcysteine is methylated by protein-S-isoprenylcysteine O-methyltransferase (ICMT). Fourth, the carboxyl-terminal 15 amino acids of the protein (the orange and yellow segments), including the farnesylcysteine methyl ester, are clipped off by ZMPSTE24, releasing mature lamin A (the blue and red segments). A deficiency of ZMPSTE24 prevents the last processing step, resulting in an accumulation of a farnesylated version of full-length prelamin A and a severe perinatal-lethal progeroid disorder, restrictive dermopathy. HGPS is caused by a point mutation in codon 608 of prelamin A that changes mRNA splicing, resulting in a 50–amino acid deletion in prelamin A (the red and orange segments). This deletion eliminates the ZMPSTE24 cleavage site, preventing the biogenesis of lamin A and resulting in the accumulation of a farnesylated, internally truncated prelamin A (progerin). A portion of the 15–amino acid segment that is normally released by ZMPSTE24 (the yellow segment) is retained at the carboxyl terminus of progerin.
NUCLEAR LAMINS AND PROGEROID DISORDERS
To understand the rationale for FTI treatment of HGPS, one must begin with the basics of prelamin A processing (6). LMNA produces transcripts for two major lamin isoforms: prelamin A (the precursor to lamin A) and lamin C. Two other genes encode lamins B1 and B2. Lamins A, C, B1, and B2 are intermediate filament proteins that form the nuclear lamina, a fibrous meshwork that serves as a scaffold for the cell nucleus. Like the Ras proteins, prelamin A, lamin B1, and lamin B2 terminate with a CaaX motif and undergo farnesylation, endoproteolytic clipping, and carboxyl methylation.
The biogenesis of lamin A from prelamin A is utterly dependent on protein prenylation; without this step, none of the other processing steps occur, leading to an accumulation of nonfarnesylated prelamin A in cells. In the case of prelamin A, one more endoproteolytic processing step follows: 15 additional amino acids, including the farnesylcysteine methyl ester, are cleaved from the carboxyl terminus, releasing mature lamin A (Fig. 1) (7). This cleavage step is mediated by the metallopeptidase ZMPSTE24 (8, 9). When ZMP-STE24 is absent, no lamin A is produced, and farnesyl–prelamin A accumulates at the nuclear rim. This event compromises the integrity of the nuclear lamina, which gives rise to misshapen nuclei in cultured fibroblasts. ZMPSTE24-deffcient mice (Zmpste24−/−) manifest many early hallmarks of HGPS— alopecia, slow growth, loss of subcutaneous fat, micrognathia (undersized jaw), osteolytic lesions in bones, and early death (8, 9). In humans, ZMPSTE24 deficiency causes restrictive dermopathy (10), a perinatal-lethal progeroid disorder that shares many features of HGPS.
The main HGPS-causing mutation in LMNA is a single-nucleotide substitution that changes splicing of the prelamin A transcript, resulting in an in-frame deletion of 50 amino acids (3). This deletion leaves the CaaX motif of prelamin A intact; hence, farnesylation and methylation proceed normally. However, the deletion eliminates the ZMPSTE24 cleavage site, preventing the conversion of the mutant prelamin A (generally called progerin) to lamin A. Progerin accumulation also leads to misshapen cell nuclei in cultured fibroblasts and is responsible for all of the disease phenotypes of HGPS. Rare point mutations that yield increased levels of progerin transcripts lead to particularly severe disease phenotypes (11).
CONCEPTUALIZING TREATMENTS
The notion that it might be possible to treat prelamin A–associated progeroid disorders gained support from studies of Zmpste24−/− mice carrying a single Lmna knockout allele (Zmpste24−/−Lmna+/−) (12). As expected, heterozygosity for Lmna deficiency reduced farnesyl–prelamin A levels by one half. However, the intriguing finding from these studies was that the Lmna knockout allele abolishes the disease phenotypes normally found in Zmpste24−/− mice. In considering this finding, the authors suggested that the farnesylated form of prelamin A might be toxic and the culprit in disease. The implications were clear: prelamin A–associated progeroid disorders might be treated by lowering farnesyl–prelamin A production or reducing the farnesylation of prelamin A.
TESTING FTI TREATMENT IN CULTURED CELLS
To test the importance of protein farnesylation in HGPS, Yang et al. (13) created an HGPS knock-in mouse model that expresses farnesylated progerin (LmnaHG/+) and then tested whether an FTI would reduce the frequency of misshapen nuclei in LmnaHG/+ fibroblasts. The rationale for these experiments was to mislocalize progerin. FTI treatment of cultured cells mislocalizes Ras oncoproteins away from the plasma membrane (4, 5), and published studies suggested that inhibition of protein prenylation in cultured cells leads to an accumulation of prelamin A in the nucleoplasm rather than at the nuclear rim (14). Any therapeutic strategy designed to mislocalize a toxic protein seemed to be a great idea (6). When LmnaHG/+ fibroblasts were treated with FTIs, the results were striking: The FTI inhibited protein prenylation and reduced the frequency of cells with misshapen nuclei (13). In differentiated LmnaHG/HG embryonic stem cells, FTI treatment led to clumps of progerin in the nucleoplasm (13). Within months, multiple laboratories, using human and mouse ZMPSTE24-deficient cells, human HGPS cell lines, and transfected cell lines, provided additional support for the ability of FTIs to ameliorate nuclear shape abnormalities in progeria (6).
INTERPRETING NUCLEAR SHAPE EFFECTS
The favorable effects of FTIs on nuclear shape in HGPS and ZMPSTE24-deficient cells generated considerable excitement (6) because there was already evidence suggesting that the frequency of misshapen nuclei in fibroblasts correlates with disease phenotypes in mice with progeria (12). From the beginning, however, it has been clear that the observed nuclear shape abnormalities are not specific for progeria and also occur in the setting of LMNA mutations that cause cardiomyopathy and muscular dystrophy, in which prelamin A processing is normal (15).
Now, 8 years after the initial observation that FTIs improve nuclear shape in cultured HGPS fibroblasts (6, 13, 16, 17), the relevance of these cell culture studies for mammalian tissues seems less certain. Also, recent findings with genetically modified mice have raised doubts about the underlying rationale of FTI treatment—that is, to mislocalize prelamin A and progerin away from the nuclear rim. By introducing a stop codon at the end of lamin A coding sequences, Coffinier et al. (18) generated lamin A–only knock-in mice in which lamin A is synthesized directly, completely bypassing prelamin A farnesylation and the other processing steps. In the tissues of lamin A–only mice, lamin A is positioned normally at the nuclear rim, and the mice exhibit no pathology. Misshapen nuclei were found in cultured fibroblasts, but their importance seems questionable, given the health and vitality of the mice. Also, nonfarnesylated prelamin A in nonfarnesylated prelamin A knock-in mice and nonfarnesylated progerin in nonfarnesylated HGPS mice were positioned normally at the nuclear rim in the tissues that were examined, as judged by immunofluorescence studies with commercial antibodies (19, 20). Similarly, nonfarnesylated prelamin A in FTase-deficient hepatocytes was found at the nuclear rim, not in the nucleoplasm (21). Thus, the importance of the farnesyl lipid for nuclear rim localization is not obvious, especially in the tissues of mice.
TESTING FTIS IN MICE
The next step was to test the efficacy of an FTI in mouse models of progeria. The initial study, by Fong et al. (22), tested the ability of a potent FTI to reduce disease phenotypes in Zmpste24−/− mice. As expected, FTI treatment led to accumulation of nonfarnesylated HDJ-2, a normally farnesylated CaaX protein that is used as a biomarker of FTI activity. The FTI improved body weight curves in Zmpste24−/− mice while reducing body weight in wild-type mice. Also, treatment substantially improved grip strength and reduced the numbers of osteolytic lesions and rib fractures. In subsequent studies, Yang et al. (17) showed that FTI treatment was efficacious in LmnaHG/+ mice, improving body weight curves, reducing rib fractures, and increasing adipose tissue mass. A subsequent study in LmnaHG/+ mice confirmed these findings and also showed that FTI treatment prolonged life (23). Thus, three FTI treatment studies showed substantial improvements in progeria-like disease phenotypes. However, although the results were consistent and highly statistically significant, one could argue that the big picture was more sobering: FTI-treated Zmpste24−/− and LmnaHG/+ mice still developed disease, the disease phenotypes still progressed during treatment, and the mice died from the disease.
An FTI was also tested in transgenic mice that harbored a human bacterial artificial chromosome (BAC) clone that spanned LMNA (engineered to contain the HGPS mutation) and three other genes (24). Unlike Zmpste24−/− and LmnaHG/+ mice, these mice did not display many of the early phenotypes of progeria found in Zmpste24−/− and LmnaHG/+ mice and children with HGPS. However, the mice did have disease in the medial and adventitial layers of large arteries, and that phenotype responded to FTI treatment (24). The BAC transgenic mice are sometimes referred to as a model of HGPS, but the absence of early hallmarks of progeria remains unexplained. Additional lines of human prelamin A and progerin transgenic mice, matched for expression levels, are needed to understand fully the biological features of this model.
INTERPRETING FTI TREATMENT IN MICE
Although FTI treatment ameliorated disease phenotypes in Zmpste24−/− and LmnaHG/+ mice (17, 22, 23), legitimate questions about underlying mechanisms remain. When thinking about FTI therapy for HGPS, the first issue to consider is the properties of the structurally abnormal lamin proteins that accumulate during FTI treatment. FTI treatment of LmnaHG/+ mice leads to an accumulation of nonfarnesylated prelamin A (from the wild-type Lmna allele) and nonfarnesylated progerin (from the LmnaHG allele). Are those nonfarnesylated lamins innocuous, or might they also promote disease?
To explore this issue, Yang et al. (25) generated nonfarnesylated progerin knock-in mice (LmnanHG/+ mice), which are identical to LmnaHG/+ mice except that progerin farnesylation was abolished by changing the CaaX motif from –CSIM to –SSIM. The progeria-like phenotypes in LmnanHG/+ mice were identical to, but slightly milder than, those in LmnaHG/+ mice. LmnanHG/+ mice lived several months longer but ultimately died from their disease. The Cys-to-Ser substitution in the progerin of LmnanHG/+ mice resulted in lower progerin levels in tissues, and it appeared likely that the change in progerin concentrations in cells, rather than any alteration in the intrinsic toxicity of progerin, accounts for the milder phenotypes. Additional studies showed that FTI treatment of LmnanHG/+ mice lowers steady-state levels of progerin (25). The discovery of severe disease in LmnanHG/+ mice was sobering because it implied that progerin can be toxic whether it is farnesylated or not.
Later, Yang et al. (20) created a second nonfarnesylated progerin allele, this time by changing the CaaX motif from –CSIM to –CSM. Remarkably, CSM-progerin was nontoxic; even mice homozygous for the LmnaCSM allele were free of disease, despite very high levels of progerin in their tissues. The implication of the LmnaCSM model seemed more upbeat for those interested in FTI treatment of children with HGPS. However, the real lesson of the LmnaCSM model is that extremely subtle structural changes in the carboxyl terminus of progerin can be associated with major differences in toxicity of the protein. That conclusion is not particularly surprising, given the wide range of diseases elicited by subtle missense mutations in lamin A/C (26).
What one would really like to define, of course, is the intrinsic toxicity of the non-farnesylated progerin that would accumulate during FTI therapy (that is, progerin terminating with –CSIM). Unfortunately, there are no obvious ways to address that issue. We had hoped to examine this issue by knocking out FTase in selected tissues of LmnaHG/+ mice, but the approach is not feasible because the elimination of FTase activity elicits severe tissue pathology in mice (21, 27).
FTI treatment of LmnaHG/+ mice also leads to an accumulation of nonfarnesylated, full-length prelamin A (from the wild-type Lmna allele). Normally, prelamin A is virtually undetectable in tissues because the conversion of prelamin A to lamin A is so efficient, but small amounts of prelamin A accumulate with FTI treatment. There is no evidence that nonfarnesylated prelamin A accumulation causes toxicity in humans. However, caution is warranted, particularly with long-term FTI treatment; knock-in mice that produce nonfarnesylated full-length prelamin A (by changing the carboxyl-terminal –CSIM motif to –SSIM) develop lethal cardiomyopathy (19).
A second issue is whether the favorable effects of FTI treatment on disease phenotypes in Zmpste24−/− and LmnaHG/+ mice are caused by blocking the farnesylation of prelamin A and progerin or are secondary— in some unanticipated way—to blocking the farnesylation of many other CaaX protein substrates within cells. To address this issue, Yang et al. (28) examined the ability of FTIs to improve disease phenotypes in LmnanHG/+ mice, in which progeria disease phenotypes are elicited by a nonfarnesylated version of progerin (SSIM-progerin). FTI treatment did not improve disease phenotypes in LmnanHG/+ mice, suggesting that the beneficial effects of FTI treatment in Zmpste24−/− and LmnaHG/+ mice result from blocking prelamin A farnesylation.
A third issue surrounding FTI treatment in mouse models is the limited extent of inhibition of protein farnesylation in vivo. In LmnaHG/+ mice, FTIs block HDJ-2 farnesylation by 30 to 80% (17, 22, 23), and the amount of nonfarnesylated prelamin A in LmnaHG/+ tissues is markedly increased, as judged by Western blots with a prelamin A–specific antibody (17, 23). Levels of mature lamin A were also reduced. However, the amount of nonfarnesylated prelamin A in tissues, relative to mature lamin A, was low (~10 to 15%) (17), indicating that the overall impact of the FTI on lamin A biogenesis was small. Because inhibition of HDJ-2 farnesylation was incomplete, it seems most likely that the less-than-complete blockade of lamin A biogenesis by the FTI results from incomplete inhibition of FTase and the ability of the residual enzyme activity to carry out prelamin A farnesylation.
A fourth issue to consider is the possibility that prelamin A and progerin are geranylgeranylated by GGTase-I in the setting of FTI treatment. Prelamin A terminates with a methionine, and it is well established that other CaaX proteins that terminate in methionine (for example, K-Ras) are geranylgeranylated in the setting of FTI treatment (29). If prelamin A and progerin are alternately prenylated by GGTase-I, it could explain both the limited ability of an FTI to interfere with lamin A biogenesis and the limited capacity of FTI treatment to ameliorate disease phenotypes. Does alternate prenylation of prelamin A occur during FTI treatment? And is alternate prenylation efficient? Varela et al. (30) concluded that the answer to both questions is yes. The authors immunoprecipitated prelamin A and progerin from FTI-treated cells and showed mass spectra of tryptic peptides that implied that the carboxyl-terminal cysteine is geranylgeranylated. However, other studies argue against substantial alternate prenylation. When FTase expression is inactivated in keratinocytes or hepatocytes, large amounts of nonfarnesylated prelamin A accumulate (21, 27). If alternate prenylation were truly efficient, one would have predicted that this prelamin A would be geranylgeranylated and then converted to mature lamin A. Also, the amount of prelamin A accumulation is no greater in hepatocytes that lack both FTase and GGTase-I than in hepatocytes lacking only FTase (21). If alternate prenylation were quantitatively important, one might expect to find more nonfarnesylated prelamin A in the FTase/GGTase-I–deficient hepatocytes. Last, researchers in the laboratory of Francis Collins showed that the electrophoretic mobility of a green fluorescent protein (GFP)–progerin fusion protein is retarded when protein farnesylation is inhibited with an FTI (31). If alternate prenylation were robust, one would not have expected a substantial change in electrophoretic mobility.
If prelamin A and progerin undergo alternate prenylation during FTI treatment, it would be useful to consider strategies to block prenylation by both FTase and GGTase-I. Certain bisphosphonates, such as alendronate or zoledronic acid, inhibit protein prenylation by interfering with the production of the isoprenyl lipid substrates used in the reaction. Toth et al. (16) showed that alendronate causes an accumulation of nonprenylated prelamin A in both wild-type human cells and cells from HGPS patients, although the amount of accumulation is lower than in cells treated with an FTI alone. Alendronate also reduces the frequency of misshapen cell nuclei in restrictive dermopathy fibroblasts (16). Because bone abnormalities are prominent features of HGPS, they suggested that bisphosphonates might develop into an alternative treatment strategy (6, 16). Several years later, Varela et al. (30) administered both a bisphosphonate and a statin drug (which limits synthesis of isoprenyl lipids) to Zmpste24−/− mice and found that this drug combination ameliorates disease phenotypes. These findings are difficult to interpret, however, because the authors reported no evidence suggesting that the drug combination affects the processing of prelamin A (or any other prenylated protein) in the tissues of the mice. At this point, it is unclear whether a statin-bisphosphonate combination would be superior to an FTI alone in blocking prelamin A prenylation in the tissues of mice or whether the statin-bisphosphonate regimen would be more effective than an FTI alone in ameliorating disease.
In summary, FTI treatment results in reproducible improvements in disease phenotypes in mouse models of progeria (17, 22, 23), but the treatment falls far short of a cure. There are lingering uncertainties about the relevance of protein farnesylation for the toxicity of progerin, the relevance of protein farnesylation for targeting of prelamin A to the nuclear rim, and why FTIs are not more effective in blocking the biogenesis of lamin A and preventing disease.
FTI CLINICAL TRIAL
Lonafarnib was recently tested in 25 children with HGPS (2). The trial was open-label, meaning that the children and their families knew that they were taking an FTI. The investigators quantified annual body weight gain and other disease phenotypes during FTI therapy and compared those measurements with observations recorded before the initiation of drug treatment. When the children were treated with FTI, body weight gain was somewhat greater, arteries appeared to be less stiff (as judged via measurements of carotid-femoral pulse-wave velocity), and bone rigidity measurements and bone mineral density were improved relative to before drug treatment. The efficacy of the drug in inhibiting protein farnesylation, as judged by accumulation of nonfarnesylated HDJ-2, was lower in the children with HGPS than in the mouse studies (17, 22, 23), and in some patients an accumulation of nonfarnesylated HDJ-2 was absent or inconsistent. There was no correlation between inhibition of HDJ-2 farnesylation and clinical response (2).
Assembling 25 children with this rare disease for a clinical trial represents a remarkable achievement. As a result of these efforts, a great deal has been learned about disease phenotypes in HGPS. On the other hand, the absence of a double-blind or crossover design makes it difficult to be confident that the FTI was efficacious. These concerns are amplified by inconsistent effects on HDJ-2 farnesylation and the absence of a correlation between the inhibition of protein farnesylation and clinical response. One authority worried that the beneficial changes in body weight might fall into the realm of “noise” (32). In any case, documenting an improvement in children with HGPS is a tough challenge because of the small numbers of study participants and because each child had prominent disease phenotypes when the treatment was initiated. This situation is different from preclinical studies with Zmpste24−/− and LmnaHG/+ mice—in which the FTI was initiated early in life before disease phenotypes were evident.
TREATMENT STRATEGIES: WHERE DO WE STAND?
The recent clinical trial showed that FTI treatment is safe. Thus, it seems reasonable to treat children with HGPS with an FTI, particularly because the disease is severe and there are no proven alternatives. However, we are skeptical that long-term FTI treatment will prove to be a major step toward a cure. Attention should remain focused on finding more effective therapies.
One approach, now being tested in 45 children with HGPS, is treatment with a combination of an FTI (lonafarnib), a bisphosphonate (zoledronic acid), and a statin (pravastatin), with the underlying hope that this regimen will be more efficacious in inhibiting protein prenylation than an FTI alone. In this trial, it will be absolutely essential to determine whether the drug combination is more effective than an FTI alone in interfering with prelamin A processing in blood leukocytes. However, even if the drug combination proves to be ineffective in blocking prelamin A prenylation, the overall strategy might be beneficial. The cholesterol-lowering effects of the statin might retard atherogenesis in the arterial intima, and the bone-strengthening effects of the bisphosphonate could serve to ameliorate bone disease.
Efforts to substantially increase the blockade of prelamin A prenylation by use of more potent inhibitors of FTase, GGTase-I, or both are unlikely to be successful. Eliminating either protein farnesylation or protein geranylgeranylation leads to severe pathology in tissues (21, 27), so any attempt to push the blockade of protein prenylation will likely run into limiting side effects. And even if one were to identify strategies to specifically inhibit prelamin A prenylation, one would still need to worry about the possibility of unwanted side effects—for example, cardiomyopathy from an accumulation of nonfarnesylated prelamin A (19).
We believe that HGPS investigators need to shift from the focus on protein prenylation to the development of new therapeutic approaches. A seemingly ideal therapy would be to interfere with the abnormal splicing event underlying progerin synthesis, either with a small-molecule modulator of splicing or an antisense oligonucleotide. With the oligonucleotide strategy, both encouraging and discouraging results have been reported (33–35), and this approach needs to be pursued further. A recent report suggested that rapamycin, an immunosuppressant drug, reduces progerin concentrations in cultured cells by increasing the removal of progerin (36). It will be interesting to determine whether rapamycin is capable of reducing disease phenotypes and extending life in Zmpste24−/−, LmnaHG/+, and LmnanHG/+ mice.
Another potential therapeutic strategy is to alter LMNA splicing so as to reduce lamin A transcripts and increase lamin C transcripts. The rationale for this strategy appears strong for two reasons. First, Fong et al. (37) showed that knock-in mice that produce exclusively lamin C are free of disease and that the frequency of nuclear shape abnormalities in Zmpste24−/− fibroblast scan be reduced by lowering prelamin A transcripts with a prelamin A–specific antisense oligonucleotide. The second reason relates to a new discovery regarding lamins A and C in the brain. Earlier, we noted that children with HGPS do not manifest progerin toxicity in the central nervous system. That finding likely relates, at least in part, to low levels of prelamin A transcripts in the brain (38). A brain-specific microRNA, miR-9, binds to a single site in the 3′ UTR of the prelamin A transcript, lowering prelamin A transcripts and markedly reducing lamin A (and progerin) production in the mouse brain (Fig. 2) (38). The expression of miR-9 also lowers progerin expression in human HGPS fibroblasts (39). We suggest that what the brain accomplishes with a microRNA could be mimicked in peripheral tissues with antisense oligonucleotides or small-molecule modulators of prelamin A/lamin C splicing. This concept needs to be tested.
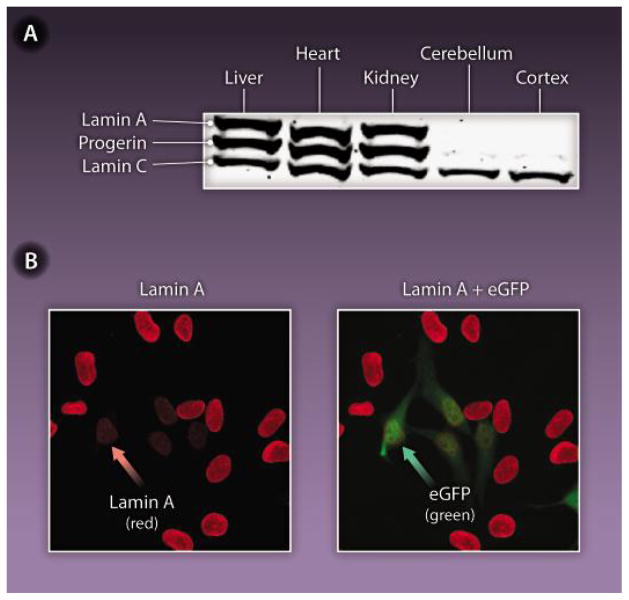
Fig. 2. Hints from the brain on a potential HGPS therapy.
The expression of lamin A in the mouse brain is inhibited by miR-9, a brain-specific microRNA. (A) Western blot analysis of lamin A, lamin C, and progerin in the tissues of a knock-in mouse model of HGPS. These mice have one Lmna allele yielding progerin and one wild-type Lmna allele producing lamin A and lamin C. There were high levels of lamin A, lamin C, and progerin in the liver, heart, and kidney but negligible amounts of lamin A and progerin in the brain. (B) Reduced expression of lamin A in HeLa cells transfected with miR-9. Cells transfected with miR-9 also expressed green fluorescent protein (right, green arrow). Those very same cells expressed reduced amounts of lamin A (left, red arrow).
Acknowledgments
Funding: This work was supported by U.S. National Institutes of Health grants AG035626, HL086683, and HL089781.
REFERENCES AND NOTES
- 1.DeBusk FL. The Hutchinson-Gilford progeria syndrome. Report of 4 cases and review of the literature. J Pediatr. 1972;80:697–724. doi: 10.1016/s0022-3476(72)80229-4. [DOI] [PubMed] [Google Scholar]
- 2.Gordon LB, Kleinman ME, Miller DT, Neuberg DS, Giobbie-Hurder A, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland R, Snyder BD, Fligor B, Bishop WR, Statkevich P, Regen A, Sonis A, Riley S, Ploski C, Correia A, Quinn N, Ullrich NJ, Nazarian A, Liang MG, Huh SY, Schwartzman A, Kieran MW. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2012;109:16666–16671. doi: 10.1073/pnas.1202529109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang FL, Casey PJ. Protein prenylation: Molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 5.James GL, Goldstein JL, Brown MS, Rawson TE, Somers TC, McDowell RS, Crowley CW, Lucas BK, Levinson AD, Marsters JC., Jr Benzodiazepine peptidomimetics: Potent inhibitors of Ras farnesylation in animal cells. Science. 1993;260:1937–1942. doi: 10.1126/science.8316834. [DOI] [PubMed] [Google Scholar]
- 6.Young SG, Fong LG, Michaelis S, Prelamin A. Zmpste24, misshapen cell nuclei, and progeria—New evidence suggesting that protein farnesylation could be important for disease pathogenesis. J Lipid Res. 2005;46:2531–2558. doi: 10.1194/jlr.R500011-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Sinensky M, Fantle K, Trujillo M, McLain T, Kupfer A, Dalton M. The processing pathway of prelamin A. J Cell Sci. 1994;107:61–67. doi: 10.1242/jcs.107.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Pendás AM, Zhou Z, Cadiñanos J, Freije JMP, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodríguez F, Tryggvason K, López-Otín C. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31:94–99. doi: 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- 9.Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, Mohr A, Meta M, Genant H, Jiang Y, Wisner ER, Van Bruggen N, Carano RAD, Michaelis S, Griffey SM, Young SG. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci USA. 2002;99:13049–13054. doi: 10.1073/pnas.192460799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moulson CL, Go G, Gardner JM, van der Wal AC, Smitt JH, van Hagen JM, Miner JH. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Invest Dermatol. 2005;125:913–919. doi: 10.1111/j.0022-202X.2005.23846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moulson CL, Fong LG, Gardner JM, Farber EA, Go G, Passariello A, Grange DK, Young SG, Miner JH. Increased progerin expression associated with unusual LMNA mutations causes severe progeroid syndromes. Hum Mutat. 2007;28:882–889. doi: 10.1002/humu.20536. [DOI] [PubMed] [Google Scholar]
- 12.Fong LG, Ng JK, Meta M, Coté N, Yang SH, Stewart CL, Sullivan T, Burghardt A, Majumdar S, Reue K, Bergo MO, Young SG. Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc Natl Acad Sci USA. 2004;101:18111–18116. doi: 10.1073/pnas.0408558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang SH, Bergo MO, Toth JI, Qiao X, Hu Y, Sandoval S, Meta M, Bendale P, Gelb MH, Young SG, Fong LG. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc Natl Acad Sci USA. 2005;102:10291–10296. doi: 10.1073/pnas.0504641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutz RJ, Trujillo MA, Denham KS, Wenger L, Sinensky M. Nucleoplasmic localization of prelamin A: Implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc Natl Acad Sci USA. 1992;89:3000–3004. doi: 10.1073/pnas.89.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muchir A, Medioni J, Laluc M, Massart C, Arimura T, van der Kooi AJ, Desguerre I, Mayer M, Ferrer X, Briault S, Hirano M, Worman HJ, Mallet A, Wehnert M, Schwartz K, Bonne G. Nuclear envelope alterations in fibroblasts from patients with muscular dystrophy, cardiomyopathy, and partial lipodystrophy carrying lamin A/C gene mutations. Muscle Nerve. 2004;30:444–450. doi: 10.1002/mus.20122. [DOI] [PubMed] [Google Scholar]
- 16.Toth JI, Yang SH, Qiao X, Beigneux AP, Gelb MH, Moulson CL, Miner JH, Young SG, Fong LG. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc Natl Acad Sci USA. 2005;102:12873–12878. doi: 10.1073/pnas.0505767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang SH, Meta M, Qiao X, Frost D, Bauch J, Coffinier C, Majumdar S, Bergo MO, Young SG, Fong LG. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J Clin Invest. 2006;116:2115–2121. doi: 10.1172/JCI28968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffinier C, Jung HJ, Li Z, Nobumori C, Yun UJ, Farber EA, Davies BS, Weinstein MM, Yang SH, Lammerding J, Farahani JN, Bentolila LA, Fong LG, Young SG. Direct synthesis of lamin A, bypassing prelamin a processing, causes misshapen nuclei in fibroblasts but no detectable pathology in mice. J Biol Chem. 2010;285:20818–20826. doi: 10.1074/jbc.M110.128835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies BS, Barnes RH, 2nd, Tu Y, Ren S, Andres DA, Spielmann HP, Lammerding J, Wang Y, Young SG, Fong LG. An accumulation of non-farnesylated prelamin A causes cardiomyopathy but not progeria. Hum Mol Genet. 2010;19:2682–2694. doi: 10.1093/hmg/ddq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang SH, Chang SY, Ren S, Wang Y, Andres DA, Spielmann HP, Fong LG, Young SG. Absence of progeria-like disease phenotypes in knock-in mice expressing a non-farnesylated version of progerin. Hum Mol Genet. 2011;20:436–444. doi: 10.1093/hmg/ddq490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang SH, Chang SY, Tu Y, Lawson GW, Bergo MO, Fong LG, Young SG. Severe hepatocellular disease in mice lacking one or both CaaX prenyltransferases. J Lipid Res. 2012;53:77–86. doi: 10.1194/jlr.M021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong LG, Frost D, Meta M, Qiao X, Yang SH, Coffinier C, Young SG. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science. 2006;311:1621–1623. doi: 10.1126/science.1124875. [DOI] [PubMed] [Google Scholar]
- 23.Yang SH, Qiao X, Fong LG, Young SG. Treatment with a farnesyltransferase inhibitor improves survival in mice with a Hutchinson-Gilford progeria syndrome mutation. Biochim Biophys Acta. 2008;1781:36–39. doi: 10.1016/j.bbalip.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capell BC, Olive M, Erdos MR, Cao K, Faddah DA, Tavarez UL, Conneely KN, Qu X, San H, Ganesh SK, Chen X, Avallone H, Kolodgie FD, Virmani R, Nabel EG, Collins FS. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc Natl Acad Sci USA. 2008;105:15902–15907. doi: 10.1073/pnas.0807840105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang SH, Andres DA, Spielmann HP, Young SG, Fong LG. Progerin elicits disease phenotypes of progeria in mice whether or not it is farnesylated. J Clin Invest. 2008;118:3291–3300. doi: 10.1172/JCI35876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119:1825–1836. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee R, Chang SY, Trinh H, Tu Y, White AC, Davies BS, Bergo MO, Fong LG, Lowry WE, Young SG. Genetic studies on the functional relevance of the protein prenyltransferases in skin keratinocytes. Hum Mol Genet. 2010;19:1603–1617. doi: 10.1093/hmg/ddq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang SH, Chang SY, Andres DA, Spielmann HP, Young SG, Fong LG. Assessing the efficacy of protein farnesyltransferase inhibitors in mouse models of progeria. J Lipid Res. 2010;51:400–405. doi: 10.1194/jlr.M002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272:14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 30.Varela I, Pereira S, Ugalde AP, Navarro CL, Suárez MF, Cau P, Cadiñanos J, Osorio FG, Foray N, Cobo J, de Carlos F, Lévy N, Freije JM, López-Otín C. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med. 2008;14:767–772. doi: 10.1038/nm1786. [DOI] [PubMed] [Google Scholar]
- 31.Capell BC, Erdos MR, Madigan JP, Fiordalisi JJ, Varga R, Conneely KN, Gordon LB, Der CJ, Cox AD, Collins FS. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2005;102:12879–12884. doi: 10.1073/pnas.0506001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couzin-Frankel J. Medicine. Drug trial offers uncertain start in race to save children with progeria. Science. 2012;337:1594–1595. doi: 10.1126/science.337.6102.1594. [DOI] [PubMed] [Google Scholar]
- 33.Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med. 2005;11:440–445. doi: 10.1038/nm1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fong LG, Vickers TA, Farber EA, Choi C, Yun UJ, Hu Y, Yang SH, Coffinier C, Lee R, Yin L, Davies BS, Andres DA, Spielmann HP, Bennett CF, Young SG. Activating the synthesis of progerin, the mutant prelamin A in Hutchinson-Gilford progeria syndrome, with anti-sense oligonucleotides. Hum Mol Genet. 2009;18:2462–2471. doi: 10.1093/hmg/ddp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osorio FG, Navarro CL, Cadiñanos J, López-Mejía IC, Quirós PM, Bartoli C, Rivera J, Tazi J, Guzmán G, Varela I, Depetris D, de Carlos F, Cobo J, Andrés V, De Sandre-Giovannoli A, Freije JM, Lévy N, López-Otín C. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med. 2011;3:106ra107. doi: 10.1126/scitranslmed.3002847. [DOI] [PubMed] [Google Scholar]
- 36.Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- 37.Fong LG, Ng JK, Lammerding J, Vickers TA, Meta M, Coté N, Gavino B, Qiao X, Chang SY, Young SR, Yang SH, Stewart CL, Lee RT, Bennett CF, Bergo MO, Young SG. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest. 2006;116:743–752. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung HJ, Coffinier C, Choe Y, Beigneux AP, Davies BS, Yang SH, Barnes RH, 2nd, Hong J, Sun T, Pleasure SJ, Young SG, Fong LG. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc Natl Acad Sci USA. 2012;109:E423–E431. doi: 10.1073/pnas.1111780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nissan X, Blondel S, Navarro C, Maury Y, Denis C, Girard M, Martinat C, De Sandre-Giovannoli A, Levy N, Peschanski M. Unique preservation of neural cells in Hutchinson-Gilford progeria syndrome is due to the expression of the neural-specific miR-9 microRNA. Cell Reports. 2012;2:1–9. doi: 10.1016/j.celrep.2012.05.015. [DOI] [PubMed] [Google Scholar]