The neurofibromatosis type 2 (NF2) tumor suppressor merlin plays an important role in contact inhibition of cell growth. It shows high similarity to the ezrin-radixin-moesin (ERM) proteins with which it shares three distinct structural domains: a highly conserved N-terminal FERM (Four-point-one, ERM) domain (residues 1-335), an α-helical domain (residues 336-505), and a C-terminal tail domain (CTT, residues 506-595) that can associate with the FERM domain to modulate the accessibility to its ligands, which include the scaffolding protein EBP50 (reviewed in (Fehon et al., 2010)). ERMs are activated in part by phosphorylation of a conserved threonine in the CTT that reduces the FERM/CTT association to convert the protein from a closed to a more open active state. Similar to ERMs, merlin is regulated by phosphorylation on a unique CTT residue (Ser518) (reviewed in (Bretscher et al., 2002)). Limited evidence suggests that similar to ERMs, phosphorylation of Ser518 reduces the FERM/CTT association (Rong et al., 2004; Shaw et al., 2001). However, in contrast to the ERMs, studies consistently show that this phosphorylation event leads to deactivation of merlin (Shaw et al., 2001; Surace et al., 2004) and a binary switch model has emerged that non-phosphorylated closed merlin represents the active state and phosphorylation opens the molecule to inactivate it. This model presents a paradox, as for example the interaction of merlin with growth factor receptors through EBP50 is required for growth regulation (Curto et al., 2007; James et al., 2004), yet would not be expected to occur with closed merlin.
We set out to rigorously test the current model by characterizing a spectrum of merlin variants with different degrees of association of the CTT with the FERM domain. Since the merlin FERM/CTT interaction is not as strong as in ezrin (Nguyen et al., 2001), we engineered (based on similarities in the CTT of merlin and EBP50; Figure S1A, B) a merlin mutant (designated ‘AR’) having a more stable closed form. As strongly open controls, we used a mutant missing the last two residues (ΔEL) and the splice variant isoform 2 (M2) with altered CTT (Nguyen et al., 2001). We compared these with wild type (WT) merlin, the phosphodeficient S518A mutant, the phosphomimetic S518D (Shaw et al., 2001) and S518E mutants, and a merlin fragment genuinely phosphorylated on Ser518.
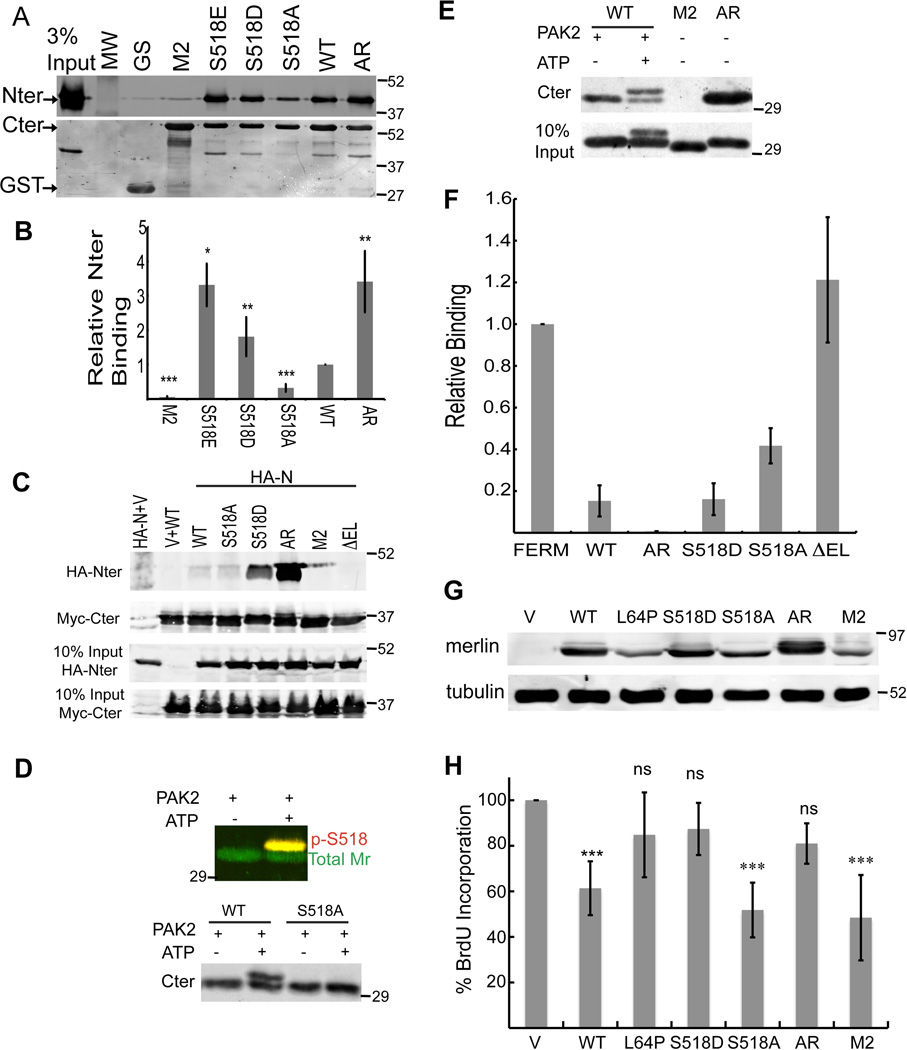
First we compared the interaction of the variant Cter fragments (residues 342-end) with the Nter (residues 1-341) in vitro. As predicted, the AR Cter bound Nter most effectively (Figure 1A, B). Surprisingly and in contrast to earlier findings, S518A Cter showed reduced Nter binding, whereas Cter containing S518D or S518E mutations exhibited increased binding. Similar results were obtained with shorter fragments containing only the CTT and FERM domains (Figure S1C, D) and when the Cter fragments were used to recover Nter expressed in cells (Figure S1E). Moreover, when these fragments were co-expressed in 293T cells, Cter containing S518D and AR mutations efficiently recovered the Nter, but WT, S518A, M2, and ΔEL constructs recovered little or none (Figure 1C). To directly determine the effect of S518 phosphorylation on the Nter/Cter interaction, WT Cter was phosphorylated in vitro. Phosphorylation (~30% yield) resulted in an ATP-dependent shifted band specifically recognized by a S518-phosphomerlin antibody and not seen with S518A (Figure 1D). Immobilized Nter bound the phosphorylated Cter about 10-fold more effectively, binding about 11% of the input compared to 1% for the unphosphorylated species (Figure 1E), directly demonstrating that phosphorylation of S518 enhances the FERM/CTT interaction.
Figure 1.
A. GST-Cter fragments bound to glutathione-Sepharose beads were used to recover untagged Nter expressed in bacteria. B. Quantification of Nter binding (mean ± SD). C. 293 cells expressing HA-Nter and either vector (V) or Myc-tagged Cter (Myc-Cter). Myc immunoprecipitates were immunoblotted as indicated. D. Untagged Cter incubated with p21 activated kinase (PAK2) in the presence (+) or absence (−) of ATP, resolved on 15% SDS-PAGE gel and immunoblotted with anti-phospho-merlin (p-S518) (red) and anti-merlin (green) (Upper panel) or total merlin (lower panel). E. Immobilized Nter was used to recover phosphorylated WT Cter or mutated Cter fragments. F. Relative binding by GST-EBP50 to full-length mutants expressed in 293T cells (mean ± SD). G. Merlin levels in HEI-193 cells stably expressing merlin variants. H. HEI-193 cells were serum starved, restimulated with serum, and examined for relative BrdU incorporation (mean ± SD).
Since the binding site for EBP50 on the FERM domain is masked by bound CTT (Bretscher et al., 2002), the accessibility of this site reflects full-length merlin’s conformation. Full-length AR mutant expressed in 293 cells fails to bind immobilized EBP50 and is therefore highly closed, whereas ΔEL bound as effectively as the free FERM domain. WT (which is subject to phosphorylation in vivo) and S518D bound poorly, whereas S518A bound more efficiently (Figure 1F). Similar results were obtained with bacterially purified proteins (Figure S1F). Taken together, our results document a hierarchy of closure, with the AR mutant the most tightly closed, followed by S518D, which is more closed than unmodified WT, then S518A, and finally M2 and ΔEL which are fully open.
We assessed the ability of these mutants to control cell growth, using the nonfunctional L64P mutant (Gutmann et al., 1998) as a control. All constructs, except mutant L64P, localized mostly to the plasma membrane, indicating that both open and closed conformations of merlin associate with the plasma membrane (data not shown). Mutant L64P and the more closed mutants AR and S518D failed to block the proliferation of merlin-deficient human schwannoma cells, whereas WT merlin, and the more open S518A and M2 were proficient at growth control, even though their expression level was significantly lower compared to the closed mutants (Figures 1G-H). Consistent results were obtained with mouse 3T3 cells and primary human schwannoma cells. In all cases a more open conformation correlates with higher growth control activity and enhances association with growth factor receptors through EBP50 (Figure S1G-J).
In accord with the rheostat model of conformational regulation suggested for ERM proteins (Li et al., 2007), these results show that WT merlin tends not to be fully open or closed, but varies in its degree of openness depending on its circumstances. In contrast to previous work (Shaw et al., 2001; Surace et al., 2004), our studies support a model in which the conformationally open state of merlin is the one that favors growth control, with S518 phosphorylation being a central regulatory event enhancing the FERM/CTT association, tipping the balance toward a more (but not fully) closed molecule with reduced ability to control cell growth. Unmodified merlin (like S518A) is in a more open state and is therefore more active in growth control. Since the amount of active merlin necessary for growth control may vary between different experimental settings, it is not surprising to see the apparently variable results found in the field. That S518A is not as open as M2 or ΔEL and S518D is not as closed as AR implies that additional factors can further regulate the balance between open and closed states. With this new model in hand, and the AR and ΔEL mutants available as tightly closed and open forms of merlin, the stage is set for studies to identify and characterize in detail the effectors that selectively associate with merlin in its different conformations and how these impinge on the regulation of cell growth and other merlin functions.
Supplementary Material
Acknowledgments
We thank Prof. Lim from the House Ear Institute for HEI-193 cells and Prof. Cerione from Cornell University for Rac1-Q61L construct. We are grateful to Russell Carpenter (OSU) for preparing Figure S1B. Funded by US Army Department of Defense grant W81XWH-08-1-0052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;8:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J. Cell Biol. 2007;5:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010;4:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH, Geist RT, Xu H, Kim JS, Saporito-Irwin S. Defects in neurofibromatosis 2 protein function can arise at multiple levels. Hum. Mol. Genet. 1998;3:335–345. doi: 10.1093/hmg/7.3.335. [DOI] [PubMed] [Google Scholar]
- James MF, Beauchamp RL, Manchanda N, Kazlauskas A, Ramesh V. A NHERF binding site links the betaPDGFR to the cytoskeleton and regulates cell spreading and migration. J. Cell. Sci. 2004;(Pt 14):2951–2961. doi: 10.1242/jcs.01156. [DOI] [PubMed] [Google Scholar]
- Li Q, Nance MR, Kulikauskas R, Nyberg K, Fehon R, Karplus PA, Bretscher A, Tesmer JJ. Self-masking in an intact ERM-merlin protein: an active role for the central alpha-helical domain. J. Mol. Biol. 2007;365:1446–1459. doi: 10.1016/j.jmb.2006.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen R, Reczek D, Bretscher A. Hierarchy of merlin and ezrin N- and C-terminal domain interactions in homo- and heterotypic associations and their relationship to binding of scaffolding proteins EBP50 and E3KARP. J. Biol. Chem. 2001;10:7621–7629. doi: 10.1074/jbc.M006708200. [DOI] [PubMed] [Google Scholar]
- Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;52:8447–8454. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O'Bryan JP, Gupta V, Ratner N, Der CJ, Jacks T, McClatchey AI. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev. Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Surace EI, Haipek CA, Gutmann DH. Effect of merlin phosphorylation on neurofibromatosis 2 (NF2) gene function. Oncogene. 2004;2:580–587. doi: 10.1038/sj.onc.1207142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.