Abstract
Despite remarkable effectiveness of reperfusion and drug therapies to reduce morbidity and mortality following myocardial infarction (MI), many patients have debilitating symptoms and impaired left ventricular (LV) function highlighting the need for improved post-MI therapies. A promising concept currently under investigation is intramyocardial injection of high-water content, polymeric biomaterial gels (e.g., hydrogels) to modulate myocardial scar formation and LV adverse remodeling. We propose a degradable, bioactive hydrogel that forms a unique microstructure of continuous, parallel capillary-like channels (Capgel). We hypothesize that the innovative architecture and composition of Capgel can serve as a platform for endogenous cell recruitment and drug/cell delivery, therefore facilitating myocardial repair after MI.
Introduction
Over the past few decades, advances in coronary reperfusion and drug therapies have been effective in limiting extension and expansion of ischemic injury, resulting in improved myocardial infarction (MI) survival rates. Among patients surviving acute MI, left ventricular (LV) remodeling attempts to compensate for tissue loss and maintain pump function. However, over time this may further impact global LV function leading to progressive heart failure (HF) [1]. Up to a third of MI patients develop HF at 5 years post-MI [2], and HF is the most frequent discharge diagnosis, with about 670,000 new HF patients identified yearly [3]. These patients have limited treatment options [4]; moreover, current post-MI treatments, though effective, have reached a therapeutic plateau. A goal of treatment is to delay progression of adverse remodeling that may exacerbate the systolic and diastolic dysfunction. Lifestyle changes and pharmacological interventions for risk factor control (e.g., hypertension, diabetes, hyperlipidemia) are recommended at all stages of HF. Angiotensin converting enzyme inhibitors (ACEIs) and β-blockers are the primary approach in patients at high risk for HF with or without structural heart disease [5,6].
Despite extensive research using therapeutic cells, growth factors, and other materials, there is currently no definitive therapy to regenerate myocardium; modulate scar tissue formation, structure, and composition; or prevent post-MI adverse remodeling.
A relatively new approach uses a combination of therapeutic cells with bioresorbable polymeric biomaterial hydrogels in an effort to improve their residence time in the targeted myocardial region [7]. A potential breakthrough post-MI therapy is intramyocardial injection of a novel, degradable, bioactive material that has a unique capillary-like microstructure of uniform channels (termed Capgel, Fig. 1) [8,9]. We hypothesize that intramyocardial injection of Capgel will modulate scar tissue formation and stimulate repair of ischemia-injured/infarcted myocardial tissue to help preserve cardiac contractile function.
Fig. 1.
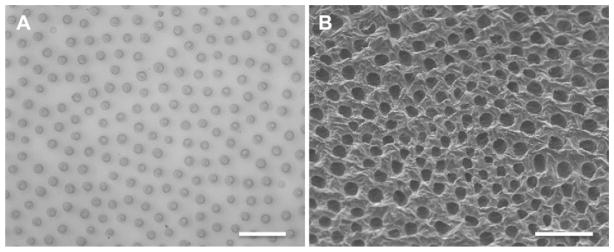
Optical microscope (A) and scanning electron microscope (B) images of the morphology of the capillary-like channel microstructure of Capgel in a section perpendicular to channel long axis. Average channel diameter ≈ 31 μm; average channel density ≈ 100/mm2. Scale bars = 200 μm.
Role of stem cells
Cell-based therapies delivered following MI are designed to improve long-term outcomes and represent a current focus of multiple clinical trials [10–12]. Circulating stem cells migrate into damaged tissues and, together with resident stem cells, contribute to tissue repair and regeneration or fibrosis with loss of pump function [13]. Each organ’s extracellular matrix is uniquely structured to maintain a milieu intérieur in which cell adhesion, differentiation, growth, and survival are supported and become pivotal for tissue regeneration and organ function [14]. Although the heart has been thought to be a post-mitotic organ, much evidence supports a certain degree of tissue plasticity and cellular dynamism [15]. However, cardiomyocytes have a very limited intrinsic capacity to regenerate after MI [16].
Once cardiomyocytes are lost and extracellular matrix damaged, there is limited cardiac regeneration [17,18]. The reasons are complex and could be due to a lack of growth factors and sufficient blood flow, as well as presence of inhibitory environmental factors/substances released by necrotic cells in the infarct zone, and/or inhibitory factors or matrix proteins secreted by scar-forming myofibroblast cells [19,20]. These and other reasons (e.g., stem cell lineage selection, timing, delivery modality, differences between animal models and humans) might explain some inconsistencies in results among studies.
Biomaterial-based approaches
Intracardiac injection of biomaterials is a promising approach to modulate post-MI negative remodeling and prevent HF [7]. Considerable data have raised concerns about the very limited and short-lived cell retention after intracardiac stem/progenitor cell delivery [21] and about the unfavorable physical structural remodeling associated with infarct expansion [22]. Even if longer residence time and tissue retention could be achieved, there would be limited engraftment of implanted cells, basically due to loss of extracellular matrix and anoikis-induced apoptosis [19,20]. For these reasons, biomaterials have been combined with cells to improve their residence time and promote cellular engraftment [22]. Injection of biocompatible materials that structurally and functionally reproduce damaged extracellular matrix may reduce adverse environmental changes caused by ischemic injury and promote recruitment, integration, and growth of endogenous stem/progenitor cells [23,24]. Finding appropriate biomaterials for stem cell/growth factor delivery and/or cardiac remodeling prevention is a challenge. The opportunity to tailor the chemical, physical, and mechanical features of a certain biomaterial is of fundamental importance for therapeutic functionality.
Theoretically, the optimal biomaterial should be easy to manufacture, inexpensive to produce, biocompatible, biodegradable, non-toxic, and minimally immunogenic, and should possess mechanical properties similar to the target tissue [25]. Another critical property is low viscosity to permit the material to be injected. At the same time, in situ rheology of the injected material should exhibit viscoelastic properties to prevent washout from the target zone. Several compounds have been developed for cardiac tissue engineering applications.
Collagen is a group of insoluble proteins, principally found in connective tissue and the extracellular matrix, that confer tensile strength to tissues. Collagen type I is also the major component of post-ischemic myocardial scar. As a protein naturally occurring in animal and human tissues, collagen is non-toxic, minimally immunogenic and biodegradable and has been used as a platform for cell culture and delivery. In a rat hind-limb ischemic model, collagen-based matrices increased the residence time of transplanted stem/progenitor cells [26].
Alginate is a natural anionic polysaccharide present in cell walls of brown seaweed. One of alginate’s main uses is in food industry as a thickening and gelling agent. Alginate has also an extensive history of use in the pharmaceutical, dental, and medical industries. Calcium-crosslinked alginate scaffolds have been used as liquid-gel phase transition solutions for intramyocardial/intracoronary injection [27,28] or as monolayers for epicardial patches in both small and large animal models [29]. Their main beneficial effects are based on prevention of post-MI adverse remodeling and promotion of cellular retention when used to deliver cells [27–29].
Fibrin glue is an injectable biopolymer made of two separate components: fibrinogen and thrombin. When mixed with thrombin, fibrinogen is cleaved by thrombin to form fibrin, a fibrous protein involved in blood clotting, and the system changes from a liquid state to a solid state within seconds. Fibrin glue was approved by FDA in 1998 and, since then, has been used mainly and extensively as a surgical haemostatic agent. As a biomaterial for preventing cardiac remodeling after AMI, fibrin glue has been used by itself as an injectable compound [30] or an epicardial patch [31] as well as a delivery vehicle for stem cells, growth factors, proteins, plasmids, and other biologics [32,33].
Polyethylene glycol (PEG) is a non-toxic, non-degradable poly-ether compound with several medical applications, especially in the pharmaceutical industry where it is used as an excipient or a binder to prolong serum drug half-life. PEG is a bio-inert scaffold that prevents protein and cell adhesion, and has been used as an injectable biomaterial to provide passive mechanical support to the infarcted cardiac wall for prevention of post-MI LV dilation and dysfunction [34].
Self-assembling peptides are short peptides, typically between 8 and 16 amino acids in length, to be used as an injectable compound [35]. Self-assembling peptides are soluble at low pH and osmolarity but form nanofibers at physiological pH and osmolarity. The result is a nanostructured fibrous scaffold that allows cell and protein delivery and tissue integration [35] by promoting endogenous and exogenous cell adhesion, retention, and differentiation.
Capgel: hypothesis and preliminary studies
A promising post-MI treatment currently under investigation in clinical trials is the injection of alginate (brown sea algae–derived polysaccharide) gels directly into and around the scarred region of the LV [36–38]. In one approach, a low viscosity calcium alginate solution (IKA-5001, aka BL-1040) is injected into a recently-opened infarct-related coronary artery via a catheter; the solution penetrates into surrounding damaged heart muscle and forms an amorphous, bioresorbable gel [28,36]. Incorporation of this amorphous gel into the damaged myocardium serves as a passive mechanical support for the healing LV and thereby reduces LV wall stress and dilation. These reductions potentially stabilize post-MI cardiac function and architecture in the long term. In another trial, a bio-stable alginate-based gel (Algisyl-LVR) is injected intramyocardially in several spots around the infarcted region of a significantly dilated LV [37]. These injected gel boluses effectively change the shape of the LV, potentially resulting in an improvement of cardiac function.
Of note, all existing alginate-based gel technologies currently in clinical testing for post-MI treatment are passive, unstructured, amorphous gels that only thicken the myocardium and mechanical support or augment the LV wall. The fundamental biology of alginate-based gels in the healing myocardium is understudied, and a recent review also raises the concern that the benefits observed could be temporary and lost once all gel has cleared from the healing myocardium [7].
We therefore propose for use post-MI an innovative biomaterial hydrogel (Capgel) that is both structured and bioactive [9]. This self-assembled material is composed of low Mw gelatin (hydrolyzed collagen I) and alginate [a negatively charged, linear polysaccharide composed of β-1,4-linked D-mannuronic acid (M) and α-1,4-linked L-guluronic acid (G) monosaccharides]. The hydrogel possesses a regular capillary-like channel morphology (channel diameter ≈ 31 μm; channel density ≈ 100/mm2) formed by a convection-like process that involves Cu2+ ions when the alginate polymer chains contract to the forming gel front [39]. The unidirectional diffusion of Cu2+ ions into the viscous solution of alginate tunnels parallel and regular channels, leading to the unique 3-dimensional microstructure of this gelatin/alginate-based hydrogel scaffold. The diameter and density of the gel channels can be tailored to facilitate host-cell ingrowth, vascular formation, and tissue integration. Growth factors and stem-cell recruitment moieties can be potentially loaded in and coupled to the gel. Capgel was developed by our group for use both as a stem-cell tissue scaffold and an injectable stem-cell delivery system.
We previously conducted a preliminary in-vitro study to investigate the morphology and growth of mouse embryonic stem (ES) cells, NIH-3T3 fibroblasts, and mouse multipotent astrocytic stem cells (MASC) [9] on and within Capgel slices. All three cell types were successfully cultured on the gelatinized alginate gel for 2 weeks. ES cells attached and proliferated on Capgel and formed regular cords of healthy cells spreading into the uniform capillary-like channels. The fibroblast-seeded Capgel scaffold maintained viability over 15 days in culture and enabled attachment and proliferation (Fig. 2A).
Fig. 2.
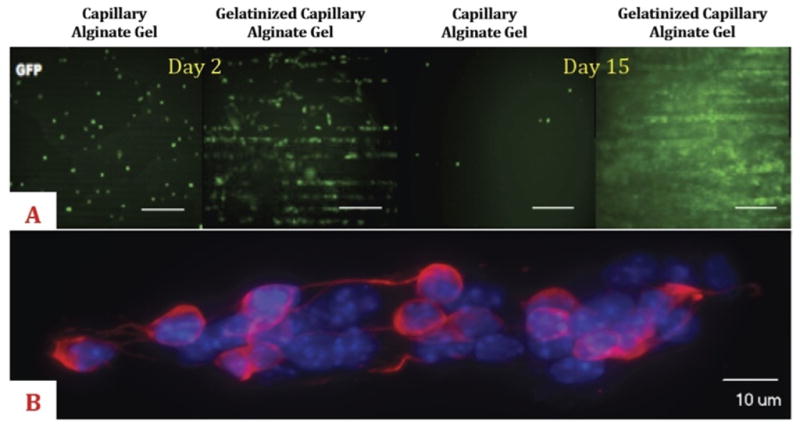
(A) Green fluorescent protein (GFP)-labeled fibroblast (NIH-3T3) culture on capillary alginate gel and gelatinized capillary alginate gel (Capgel) slices at 2 and 15 days. Compared to the alginate gel, incorporation of gelatin into the capillary-like channel microstructured alginate gel enhances cell adhesion and proliferation. Scale bars = 300 μM. (B) Spinning disc confocal microscopy images of Multipotent Astrocytic Stem Cells (MASC) oriented along the long axis of a channel of Capgel. Blue = 4′,6-diamidino-2-phenylindole (DAPI); red = β-III tubulin.
Because hydrogels and scaffolds made purely of alginate [8] allow for only limited cell adhesion, we incorporated gelatin [9] in an effort to enable adhesion, retention, and growth of endogenous and transplanted cells. Compared to an alginate gel, incorporation of gelatin into the channel-microstructured alginate scaffold [9] significantly enhanced cell adhesion and proliferation in vitro (Fig. 2A).
MASC retained differentiation capacities and spread on Capgel slices, consistently infiltrating and proliferating within capillaries in an oriented manner driven by the 3-dimensional microstructure of the scaffold (Fig. 2B).
We have also recently demonstrated that Capgel can be loaded with therapeutic cells and used as an injectable tissue scaffolding system for use in regenerative medicine/tissue engineering [9]. In this study, neonatal rat pups underwent unilateral common carotid artery ligation to cause a hypoxic-ischemic brain injury. After 24 h, GFP+ MASC-loaded Capgel (≈1 μL) was injected into target area via a 30G needle. One week following the procedure, Capgel injection did not lead to extensive reactive gliosis; viable GFP+ cells expressing astrocyte-like morphologies were found within the gel implant and in the surrounding brain areas.
In light of these findings, we hypothesize that the chemical and structural properties of Capgel will display a positive synergistic effect on (1) stem cell recruitment, retention, and engraftment and (2) dynamics and features of wound healing formation after MI.
This may result in
angiogenesis, preservation, and formation of viable myocardium at the infarct border zone,
scar shrinkage and mechanical scaffolding to provide structural support and minimize dilation at the infarct core zone,
there by limiting the loss of pump efficacy (systolic dysfunction) and the maladaptive pathway characterized by a decrease in LV compliance and dilation (diastolic dysfunction) that lead to HF and predispose to sudden cardiac death.
The semi-solid structure and viscoelastic properties of the alginate component of Capgel could provide the cardiac scar tissue with better structural properties and compliance, consequently reducing ventricular wall stress that promotes wall thinning and cardiac dilation. The incorporation of gelatin could enable and enhance endogenous (local and/or circulating) stem cell adhesion, proliferation, and differentiation as well as delivery of therapeutic vectors, drugs, and/or cells.
We further posit that Capgel may serve as a robust platform that provides guidance for 3-dimensional matrix reestablishment, cell orientation, and vascular formation.
Conclusions
Capgel is an innovative bioactive and microstructured hydrogel that enables new modalities for cell/drug delivery treatments of the infarcted heart. Evidence from our studies suggests that this biomaterial is biocompatible and injectable via a fine needle (30G), therefore enabling catheter-based intracardiac delivery. Intramyocardial injection of Capgel may also provide some mechanical support to the infarcted area, resulting in wall thickening, reduced LV wall stress, and limited cavity dilation. The alginate/gelatin matrix in combination with the unique 3-dimensional microstructure of regular capillary-like channels may provide a favorable extracellular environment for cell recruitment, adhesion, and ingrowth; vascular formation, and tissue integration.
Acknowledgments
Drs. Della Rocca and Willenberg contributed equally to this work.
Dr. Steindler receives grant support from NIH/NINDS NS055165 and the Maren, McKinney and Thompson Regenerative Medicine funds to DAS.
Dr. Carl Pepine is principal investigator for the University of Florida Regional Clinical Center of the NHLBI-sponsored Cardiovascular Cell Therapy Research Network (2UM1 HL087366-06). Dr. Pe-pine also receives support from an NIH and NCRR Clinical and Translational Science Award to the University of Florida (UL1 TR000064).
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Aoyagi T, Fujii AM, Flanagan MF, et al. Transition from compensated hypertrophy to intrinsic myocardial dysfunction during development of left ventricular pressure-overload hypertrophy in conscious sheep. Systolic dysfunction precedes diastolic dysfunction. Circulation. 1993;88:2415–25. doi: 10.1161/01.cir.88.5.2415. [DOI] [PubMed] [Google Scholar]
- 2.Hellermann JP, Goraya TY, Jacobsen SJ, et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157(12):1101–7. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistic–2011 update: a report from the American Heart Association. Circulation. 2011;123:E18–E209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg B, Quinones MA, Koilpillai C, et al. Effects of long-term therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995;91:2573–81. doi: 10.1161/01.cir.91.10.2573. [DOI] [PubMed] [Google Scholar]
- 6.Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J. 1975;37:1022–36. doi: 10.1136/hrt.37.10.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segers VFM, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109:910–22. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 8.Willenberg BJ, Hamazaki T, Meng FW, Terada N, Batich C. Self-assembled copper-capillary alginate gel scaffolds with oligochitosan support embryonic stem cell growth. J Biomed Mater Res A. 2006;79(2):440–50. doi: 10.1002/jbm.a.30942. [DOI] [PubMed] [Google Scholar]
- 9.Willenberg BJ, Zheng T, Meng FW, et al. Gelatinized copper-capillary alginate gel functions as an injectable tissue scaffolding system for stem cell transplants. J Biomater Sci Polym Ed. 2011;22(12):1621–37. doi: 10.1163/092050610X519453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307(16):1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Kørbling M, Estrov Z. Adult stem cells for tissue repair – a new therapeutic concept? N Engl J Med. 2003;349(6):570–82. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 14.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36(6):1031–7. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–7. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 16.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Takagawa J, Lam VC, et al. Donor myocardial infarction impairs the therapeutic potential of bone marrow cells by an interleukin-1-mediated inflammatory response. Sci Transl Med. 2011;3:100ra90. doi: 10.1126/scitranslmed.3002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel JB. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol. 2003;23(12):2146–54. doi: 10.1161/01.ATV.0000099882.52647.E4. [DOI] [PubMed] [Google Scholar]
- 20.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48(3):504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle B, Kemp BJ, Chareonthaitawee P, et al. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med. 2007;48:1708–14. doi: 10.2967/jnumed.107.042838. [DOI] [PubMed] [Google Scholar]
- 22.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94(12):1543–53. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 23.Singelyn JM, Sundaramurthy P, Johnson TD, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59(8):751–63. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao ZQ, Puskas JD, Xu D, et al. Improvement in cardiac function with small intestine extracellular matrix is associated with recruitment of C-kit cells, myofibroblasts, and macrophages after myocardial infarction. J Am Coll Cardiol. 2010;55(12):1250–61. doi: 10.1016/j.jacc.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 25.Ma PX. Scaffolds for tissue fabrication. Mater Today. 2004;7:30–40. [Google Scholar]
- 26.Zhang Y, Thorn S, DaSilva JN, et al. Collagen-based matrices improve the delivery of transplanted circulating progenitor cells: development and demonstration by ex vivo radionuclide cell labeling and in vivo tracking with positron-emission tomography. Circ Cardiovasc Imaging. 2008;1(3):197–204. doi: 10.1161/CIRCIMAGING.108.781120. [DOI] [PubMed] [Google Scholar]
- 27.Landa N, Miller L, Feinberg MS, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 28.Leor J, Tuvia S, Guetta V, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009;54:1014–23. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Leor J, Aboulafia-Etzion S, Dar A, et al. Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation. 2000;102(19 Suppl 3):III56–61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 30.Danoviz ME, Nakamuta JS, Marques FL, et al. Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS One. 2010;5:e12077. doi: 10.1371/journal.pone.0012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Hu Q, Wang Z, et al. Autologous stem cell transplantation for myocardial repair. Am J Physiol Heart Circ Physiol. 2004;287:H501–11. doi: 10.1152/ajpheart.00019.2004. [DOI] [PubMed] [Google Scholar]
- 32.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10(3–4):403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 33.Kipshidze N, Chekanov V, Chawla P, et al. Angiogenesis in a patient with ischemic limb induced by intramuscular injection of vascular endothelial growth factor and fibrin platform. Tex Heart Inst J. 2000;27:196–200. [PMC free article] [PubMed] [Google Scholar]
- 34.Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. J Card Fail. 2009;15:629–36. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Davis ME, Motion JP, Narmoneva DA, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111(4):442–50. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikaria Holdings, Inc. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2012. IK-5001 for the Prevention of Remodeling of the Ventricle and Congestive Heart Failure After Acute Myocardial Infarction (PRESERVATION I) [cited 2012 Jan 22]. Available from: < http://www.clinicaltrials.gov/ct2/show/NCT01226563 NLM Identifier: NCT01226563>. [Google Scholar]
- 37.LoneStar Heart, Inc. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2011. A Randomized, Controlled Study to Evaluate Algisyl-LVR™ as a Method of Left Ventricular Augmentation for Heart Failure (AUGMENT-HF) [cited 2012 Jan 22]. Available from: < http://www.clinicaltrials.gov/ct2/show/NCT01311791 NLM Identifier: NCT01311791>. [Google Scholar]
- 38.LoneStar Heart, Inc. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2011. Safety and Feasibility of Algisyl-LVR™ as a Method of Left Ventricular Restoration in Patients with DCM Undergoing Open-Heart Surgery. [cited 2012 Jan 22]. Available from: < http://www.clinicaltrials.gov/ct2/show/NCT00847964 NLM Identifier: NCT00847964>. [Google Scholar]
- 39.Thumbs J, Kohler HH. Capillaries in alginate gel as an example of dissipative structure formation. Chem Phys. 1996;208:9–24. [Google Scholar]


