Abstract
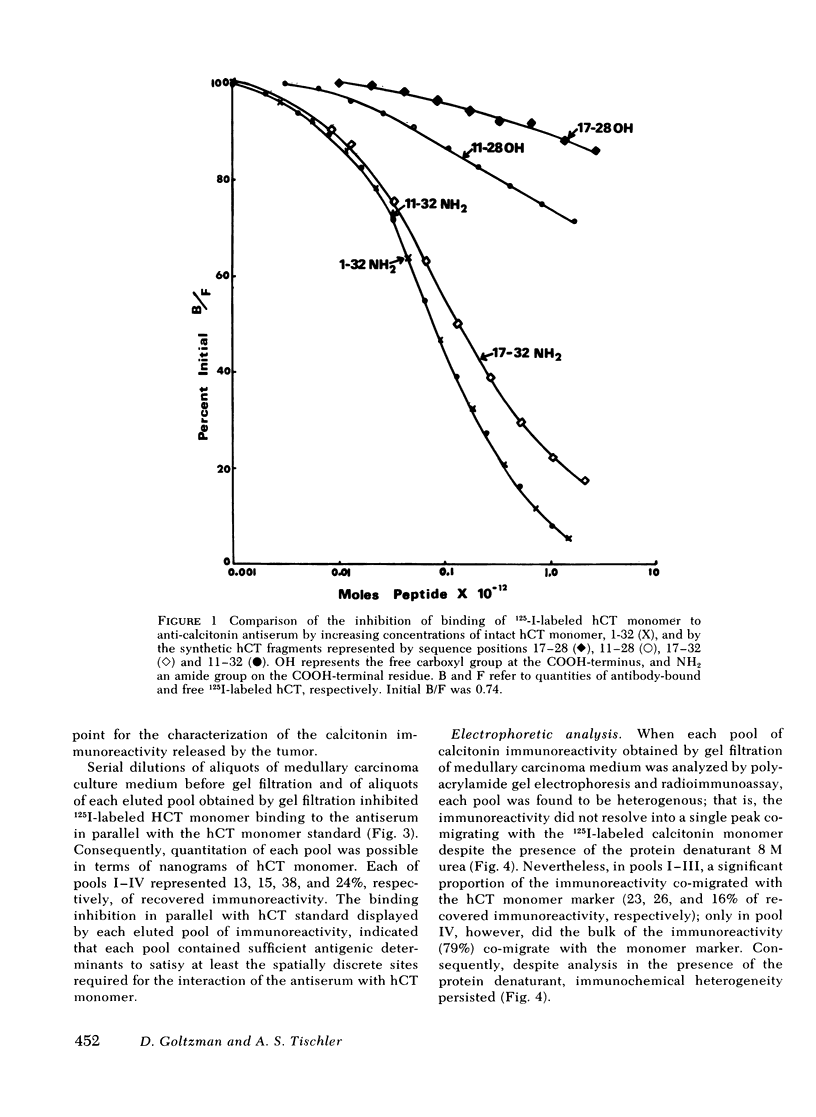
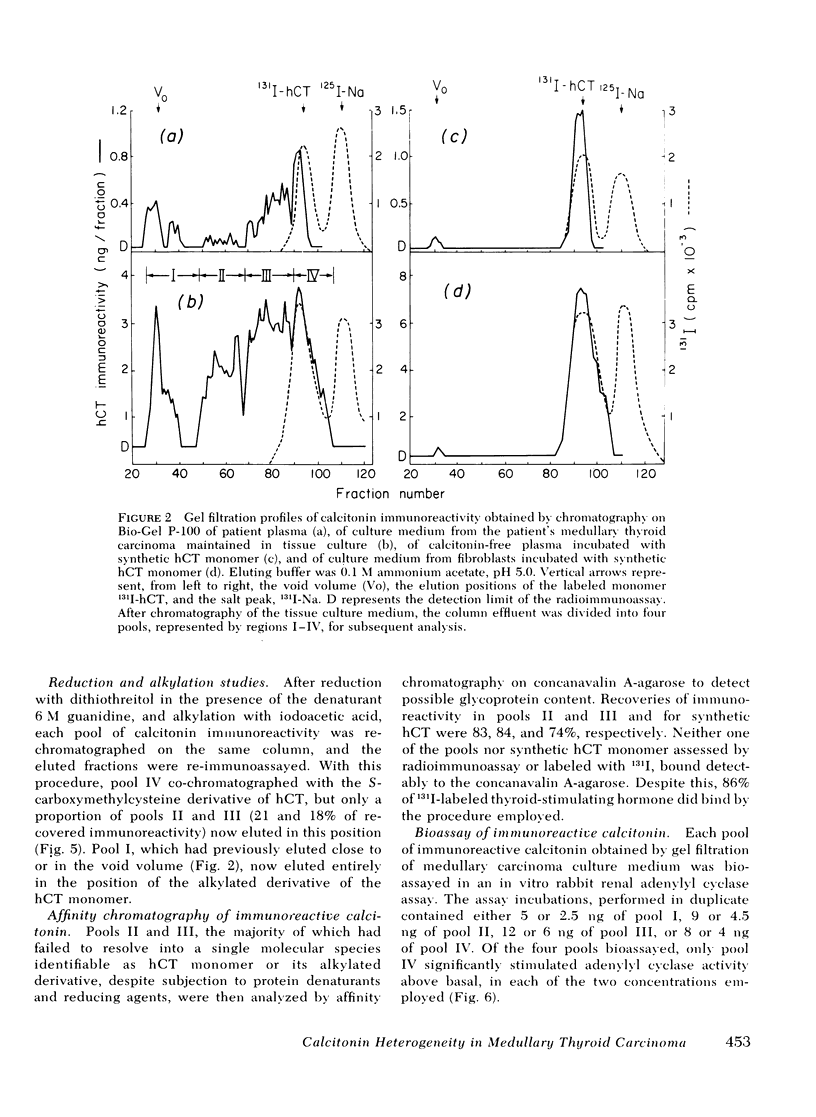
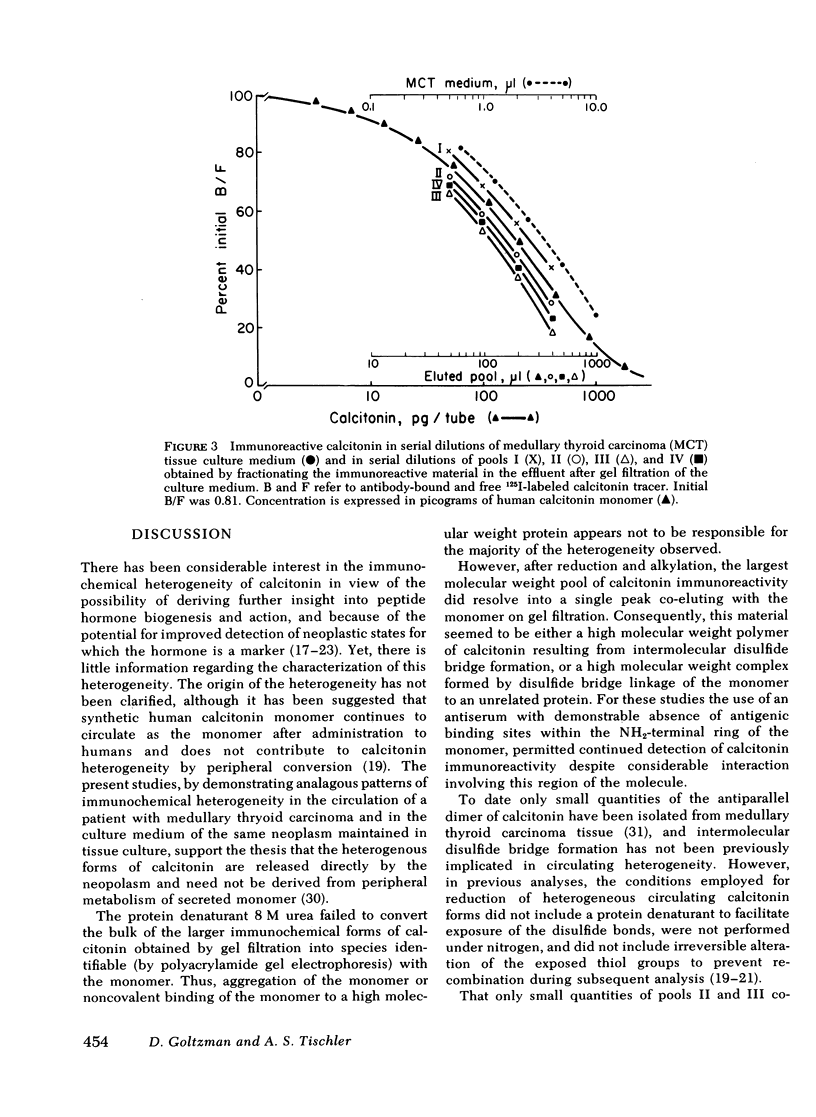
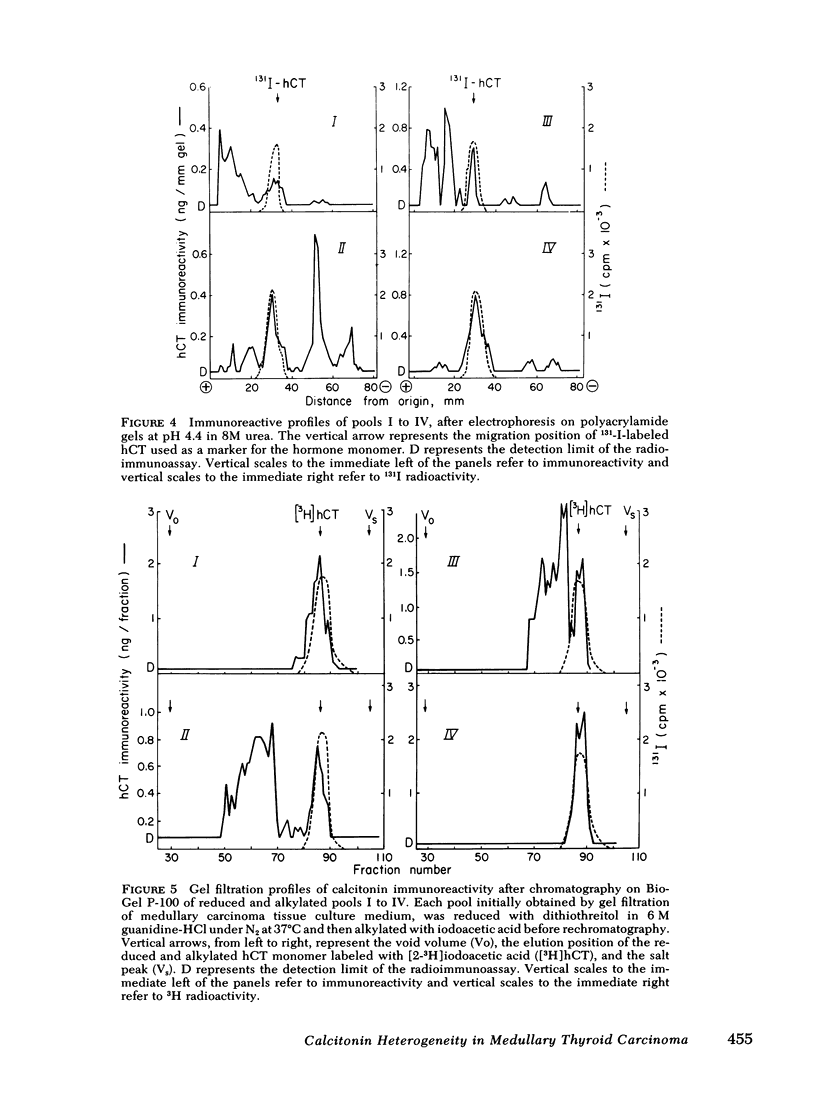
Immunoreactive calcitonin released by a medullary thyroid carcinoma in tissue culture has been found to exhibit heterogeneity when analyzed by gel chromatography and radioimmunoassay, in a pattern analogous to that seen in the circulation of the patient from whom the neoplasm was removed. To examine the cause of the heterogeneity, the immunoreactive material released by the tumor into tissue culture medium was further analyzed by gel electrophoresis in the presence of the protein denaturant 8 M urea, by gel chromatography after reduction and alkylation, by affinity chromatography on concanavalin A-agarose, and by bioassay in a renal adenylyl cyclase system of enhanced sensitivity. The results suggest that the larger immunochemical forms of calcitonin described in the circulation of patients with medullary thyroid carcinoma may be released directly from the neoplasm and need not derive from peripheral metabolism of the monomer. It could be demonstrated that a major proportion of the immunochemical enlargement is dependent upon intermolecular disulfide bridge formation whereas aggregation or non-convalent protein binding account for a smaller component of the heterogeneity. In view of the absence of binding of the immunoreactive material to the lectin agarose, carbohydrate side chains, at least of the α-d glucosyl variety, do not seem to contribute significantly to calcitonin enlargement. Additionally, the studies indicate that, at least by in vitro assay, the larger immunochemical forms of calcitonin, representing the majority of the immunoreactivity released by a medullary thyroid carcinoma, are biologically inactive.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardaillou R., Isaac R., Nivez M. P., Kuhn J. M., Cazor J. L., Fillastre J. P. Effect of salmon calcitonin on renal excretion of adenosine 3', 5' monophosphate in man. Horm Metab Res. 1976 Mar;8(2):136–140. doi: 10.1055/s-0028-1093669. [DOI] [PubMed] [Google Scholar]
- Bell P. H., Barg W. F., Jr, Colucci D. F., Davis M. C., Dziobkowski C., Englert M. E., Heyder E., Paul R., Snedeker E. H. Purification and structure of porcine calcitonin-1. J Am Chem Soc. 1968 May 8;90(10):2704–2706. doi: 10.1021/ja01012a050. [DOI] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Ronan R. Amino acid sequence of bovine thyrocalcitonin. Proc Natl Acad Sci U S A. 1969 Jul;63(3):940–947. doi: 10.1073/pnas.63.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. B., Boyd G. W., Byfield P. G., Foster G. V. A radioimmunoassay for human calcitonin M. Lancet. 1969 Jul 12;2(7611):74–77. doi: 10.1016/s0140-6736(69)92389-7. [DOI] [PubMed] [Google Scholar]
- Coombes R. C., Hillyard C., Greenberg P. B., MacIntyre I. Plasma-immunoreactive-calcitonin in patients with non-thyroid tumours. Lancet. 1974 Jun 1;1(7866):1080–1083. doi: 10.1016/s0140-6736(74)90557-1. [DOI] [PubMed] [Google Scholar]
- Deftos L. J. Immunoassay for human calcitonin. I. Method. Metabolism. 1971 Dec;20(12):1122–1128. doi: 10.1016/0026-0495(71)90037-0. [DOI] [PubMed] [Google Scholar]
- Deftos L. J., Roos B. A., Bronzert D., Parthemore J. G. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocrinol Metab. 1975 Mar;40(3):409–412. doi: 10.1210/jcem-40-3-409. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Guenzi D. High molecular weight forms of adrenocorticotropic hormone are glycoproteins. J Biol Chem. 1976 Jul 10;251(13):4121–4126. [PubMed] [Google Scholar]
- Goltzman D., Goltzmann D., Peytremann A., Callahan E., Tregear G. W., Potts J. T., Jr Analysis of the requirements for parathyroid hormone action in renal membranes with the use of inhibiting analogues. J Biol Chem. 1975 Apr 25;250(8):3199–3203. [PubMed] [Google Scholar]
- Goltzman D., Peytremann A., Callahan E. N., Segre G. V., Potts J. T., Jr Metabolism and biological activity of parathyroid hormone in renal cortical membranes. J Clin Invest. 1976 Jan;57(1):8–19. doi: 10.1172/JCI108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltzman D., Potts J. T., Jr, Ridgway R. C., Maloof F. Calcitonin as a tumor marker. Use of the radioimmunoassay for calcitonin in the postoperative evaluation of patients with medullary thyroid carcinoma. N Engl J Med. 1974 May 9;290(19):1035–1039. doi: 10.1056/NEJM197405092901901. [DOI] [PubMed] [Google Scholar]
- Heersche J. N., Marcus R., Aurbach G. D. Calcitonin and the formation of 3',5'-AMP in bone and kidney. Endocrinology. 1974 Jan;94(1):241–247. doi: 10.1210/endo-94-1-241. [DOI] [PubMed] [Google Scholar]
- Heynen G., Franchimont P. Human calcitonin radioimmunoassay in normal and pathological conditions. Eur J Clin Invest. 1974 Jun;4(3):213–222. doi: 10.1111/j.1365-2362.1974.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Kitabchi A. E. The biological and immunological properties of pork and beef insulin, proinsulin, and connecting peptides. J Clin Invest. 1970 May;49(5):979–987. doi: 10.1172/JCI106317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marx S. J., Fedak S. A., Aurbach G. D. Preparation and characterization of a hormone-responsive renal plasma membrane fraction. J Biol Chem. 1972 Nov 10;247(21):6913–6918. [PubMed] [Google Scholar]
- Marx S. J., Woodward C. J., Aurbach G. D. Calcitonin receptors of kidney and bone. Science. 1972 Dec 1;178(4064):999–1001. doi: 10.1126/science.178.4064.999. [DOI] [PubMed] [Google Scholar]
- Melvin K. E., Tashjian A. H., Jr, Miller H. H. Studies in familial (medullary) thyroid carcinoma. Recent Prog Horm Res. 1972;28:399–470. [PubMed] [Google Scholar]
- Melvin K. E., Tashjian A. H., Jr The syndrome of excessive thyrocalcitonin produced by medullary carcinoma of the thyroid. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1216–1222. doi: 10.1073/pnas.59.4.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhaud G., Calmettes C., Raymond J. P., Bignon J., Moukhtar M. S. Carcinoïde sécrétant de la thyrocalcitonine. C R Acad Sci Hebd Seances Acad Sci D. 1970 May 4;18:2195–2198. [PubMed] [Google Scholar]
- Milhaud G., Tubiana M., Parmentier C., Coutris G. Epithélioma de la thyroïde sécrétant de la thyrocalcitonine. C R Acad Sci Hebd Seances Acad Sci D. 1968 Feb 5;266(6):608–610. [PubMed] [Google Scholar]
- Moya F., Nieto A., R-Candela J. L. Calcitonin biosynthesis: evidence for a precursor. Eur J Biochem. 1975 Jul 1;55(2):407–413. doi: 10.1111/j.1432-1033.1975.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Neher R., Riniker B., Rittel W., Zuber H. Menschliches Calcitonin. 3. Struktur von Calcitonin M un. Helv Chim Acta. 1968;51(8):1900–1905. doi: 10.1002/hlca.19680510811. [DOI] [PubMed] [Google Scholar]
- Neher R., Riniker B., Zuber H., Rittel W., Kahnt F. W. Thyrocalcitonin. II. Struktur von alpha-Thyrocalcitonin. Helv Chim Acta. 1968 May 31;51(4):917–924. doi: 10.1002/hlca.660510430. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Keutmann H. T., Copp D. H., Potts J. T., Jr Amino acid sequence of salmon ultimobranchial calcitonin. Proc Natl Acad Sci U S A. 1969 Oct;64(2):771–778. doi: 10.1073/pnas.64.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth D. N., Nicholson W. E. High molecular weight forms of human ACTH are glycoproteins. J Clin Endocrinol Metab. 1977 Jan;44(1):214–217. doi: 10.1210/jcem-44-1-214. [DOI] [PubMed] [Google Scholar]
- Parthemore J. G., Deftos L. J., Bronzert D. The regulation of calcitonin in normal human plasma as assessed by immunoprecipitation and immunoextraction. J Clin Invest. 1975 Oct;56(4):835–841. doi: 10.1172/JCI108162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peytremann A., Goltzman D., Callahan E. N., Tregear G. W., Potts J. T., Jr Metabolism and biological activity of proparathyroid hormone and synthetic analogues in renal cortical membranes. Endocrinology. 1975 Nov;97(5):1270–1280. doi: 10.1210/endo-97-5-1270. [DOI] [PubMed] [Google Scholar]
- Potts J. T., Jr, Niall H. D., Keutmann H. T., Brewer H. B., Jr, Deftos L. J. The amino acid sequence of porcine thyrocalcitonin. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1321–1328. doi: 10.1073/pnas.59.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riniker B., Neher R., Maier R., Kahnt F. W., Byfield P. G., Gudmundsson T. V., Galante L., MacIntyre I. Menschliches Calcitonin. I. Isolierung und Charakterisierung. Helv Chim Acta. 1968 Oct 31;51(7):1738–1742. doi: 10.1002/hlca.19680510731. [DOI] [PubMed] [Google Scholar]
- Rittel W., Maier R., Brugger M., Kamber B., Riniker B., Sieber P. Struktur-Wirkungsbeziehungen beim menschlichen Calcitonin. III. Die biologische Aktivität verkürzter oder an den Kettenenden modifizierter, synthetischer Analoger. Experientia. 1976 Feb 15;32(2):246–248. doi: 10.1007/BF01937791. [DOI] [PubMed] [Google Scholar]
- Roos B. A., Okano K., Deftos L. J. Evidence for a pro-calcitonin. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1134–1140. doi: 10.1016/0006-291x(74)90430-6. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Samaan N. A., Hill C. S., Jr, Beceiro J. R., Schultz P. N. Immunoreactive calcitonin in medullary carcinoma of the thyroid and in maternal and cord serum. J Lab Clin Med. 1973 May;81(5):671–681. [PubMed] [Google Scholar]
- Silva O. L., Becker K. L., Primack A., Doppman J., Snider R. H. Ectopic secretion of calcitonin by oat-cell carcinoma. N Engl J Med. 1974 May 16;290(20):1122–1124. doi: 10.1056/NEJM197405162902007. [DOI] [PubMed] [Google Scholar]
- Silva O. L., Snider R. H., Becker K. L. Radioimmunoassay of calcitonin in human plasma. Clin Chem. 1974 Mar;20(3):337–339. [PubMed] [Google Scholar]
- Singer F. R., Habener J. F. Multiple immunoreactive forms of calcitonin in human plasma. Biochem Biophys Res Commun. 1974 Nov 27;61(2):710–716. doi: 10.1016/0006-291x(74)91015-8. [DOI] [PubMed] [Google Scholar]
- Sizemore G. W., Hpeath H., 3rd, Larson J. M. Immunochemical heterogeneity of calcitonin in plasma of patients with medullary thryoid carcinoma. J Clin Invest. 1975 May;55(5):1111–1118. doi: 10.1172/JCI108012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Wolfe H. J., Voelkel E. F. Human calcitonin. Immunologic assay, cytologic localization and studies on medullary thyroid carcinoma. Am J Med. 1974 Jun;56(6):840–849. doi: 10.1016/0002-9343(74)90813-4. [DOI] [PubMed] [Google Scholar]
- Wolfe H. J., Voelkel E. F., Tashjian A. H., Jr Distribution of calcitonin-containing cells in the normal adult human thyroid gland: a correlation of morphology with peptide content. J Clin Endocrinol Metab. 1974 Apr;38(4):688–694. doi: 10.1210/jcem-38-4-688. [DOI] [PubMed] [Google Scholar]


