Abstract
Type 2 diabetes (T2D) is caused by relative insulin deficiency, due in part to reduced β-cell mass (11, 62). Therapies aimed at expanding β-cell mass may be useful to treat T2D (14). Although feeding rodents a high-fat diet (HFD) for an extended period (3–6 mo) increases β-cell mass by inducing β-cell proliferation (16, 20, 53, 54), evidence suggests that adult human β-cells may not meaningfully proliferate in response to obesity. The timing and identity of the earliest initiators of the rodent compensatory growth response, possible therapeutic targets to drive proliferation in refractory human β-cells, are not known. To develop a model to identify early drivers of β-cell proliferation, we studied mice during the first week of HFD exposure, determining the onset of proliferation in the context of diet-related physiological changes. Within the first week of HFD, mice consumed more kilocalories, gained weight and fat mass, and developed hyperglycemia, hyperinsulinemia, and glucose intolerance due to impaired insulin secretion. The β-cell proliferative response also began within the first week of HFD feeding. Intriguingly, β-cell proliferation increased before insulin resistance was detected. Cyclin D2 protein expression was increased in islets by day 7, suggesting it may be an early effector driving compensatory β-cell proliferation in mice. This study defines the time frame and physiology to identify novel upstream regulatory signals driving mouse β-cell mass expansion, in order to explore their efficacy, or reasons for inefficacy, in initiating human β-cell proliferation.
Keywords: overnutrition, short-term high-fat diet, diet-induced obesity, islet replication, pancreatic β-cell mitosis
insulin deficiency in patients with type 2 diabetes (T2D) is due, in part, to a reduction in pancreatic β-cell mass (11, 62). Longitudinal studies in rodent models show that β-cell mass expands as insulin-secretory load increases, as in obesity, and subsequently declines as diabetes develops (16, 53, 54). β-Cell mass may increase due to β-cell proliferation, β-cell hypertrophy, or transdifferentiation from other cell types (23, 31). However, replication of existing β-cells is believed to be the dominant mechanism for increasing β-cell mass in adult rodents and, possibly, humans (13, 20, 38, 53). Therefore, strategies aimed at expanding β-cell mass through increased β-cell proliferation may be useful to prevent or treat T2D (14).
A first step toward developing a rational approach to expanding β-cell mass in humans is to understand which signals initiate the β-cell mass increase seen in rodents, so as to subsequently determine why these signals fail to expand β-cell mass in insulin-resistant prediabetic humans. An established, reliable model of increased rodent β-cell mass and proliferation is long-term exposure to high-fat diet (HFD). A number of signals, both extracellular and intracellular, have been implicated in rodent β-cell mass expansion in response to long-term HFD (12, 13, 53).
Interpretation of findings from extended exposure to HFD, such as the 3- to 6-mo period often used in mice, is complicated by the chronic changes related to obesity and insulin resistance. In obesity, islets are exposed to a markedly different environment, including alterations in circulating factors such as macronutrients, growth factors, adipokines, and inflammatory markers. Local islet changes may include chronic hyperfunction of the β-cell, amyloid deposition (32), altered islet vascularization (56), interaction with host exocrine pancreas (8), and neuronal signals from the central nervous system and other tissues (52). The number of uncontrolled variables, known and unknown, after extended exposure to the obesity milieu weakens the strength of conclusions regarding any individual component of β-cell mass accrual. In particular, the model is not well suited to search for the signals that initiate β-cell mass expansion.
We reasoned that, to develop potential therapeutic targets to drive proliferation of replication-refractory β-cells in adult humans, one ought to identify the earliest signals that initiate the proliferative response in rodents rather than the downstream late pathways engaged when proliferative β-cell mass expansion is under way. Prior studies have catalogued the earliest metabolic and physiological changes induced by HFD in rodents. In some cases, weight gain, insulin resistance, and glucose intolerance are present as early as 1 wk into HFD exposure (2, 33, 37, 44, 59), although the physiological changes in HFD exhibit inter- and even intrastrain variability (49). It remains unknown whether compensatory changes in β-cell mass and proliferation are initiated during this early time frame. Here, we carefully characterize the earliest onset of the β-cell proliferative response to HFD in C57BL/6J male mice, in parallel with metabolic analysis, and find that β-cell proliferation begins within the first 7 days of HFD exposure, concurrent with the onset of metabolic changes. Intriguingly, cyclin D2, a cell cycle regulator important for β-cell mass expansion in response to insulin resistance (21), is increased at the protein level in islets as early as 1 wk into HFD exposure.
EXPERIMENTAL PROCEDURES
Seven-day HFD feeding protocol.
All mouse studies were approved by both the University of Pittsburgh and the University of Massachusetts Medical School Institutional Animal Care and Use Committees. One day prior to the start of the HFD exposure, 10- to 12-wk-old male C57BL/6J (Charles River) mice were acclimatized in individual S-TANK cages (Instech) with free access to food and water. A total of 120 mice were studied. On day 0, mice were randomly assigned to either a normal chow diet (14% kcal from fat; Lab Diet, 5P76) or lard-based HFD (60% kcal from fat; Harlan, TD.06414). HFD pellets were stored in the dark at 4°C; mice received fresh food on days 0 and 3. Body weight and remaining chow weight were measured on days 0, 3, and 7; body weight is reported for all mice, food intake only for those that were individually housed, which excludes 22 mice. Nonfasting morning blood samples were collected for blood glucose, free fatty acids (FFA), and insulin on days 0 and 7. Tail blood samples were used for blood glucose measurement; submandibular samples were used for insulin and FFAs. A subset of mice received BrdU (0.8 mg/ml; Sigma) in the drinking water on days 3–7. A different subset of mice received a single intraperitoneal injection of BrdU (50 g/kg) on the morning of day 4. After 7 days, mice were euthanized, and pancreata were processed for histological studies or for islet isolation.
Biochemical assays.
Blood glucose was measured using an AlphaTRAK glucometer (Abbot Laboratories). Plasma insulin was measured by radioimmunoassay (Linco Ultra-Sensitive Rat Insulin RIA kit, Millipore) or by ELISA (CrystalChem). Plasma FFA were measured by colorimetric assay (Roche).
Insulin and glucose tolerance and in vivo and in vitro insulin secretion assays.
Intraperitoneal insulin (ITT) and glucose tolerance tests (GTT) were performed on day 5 or day 7. ITTs (1.5 U/kg or 0.75 U/kg, as specified; Humulin-R, Eli Lilly) were performed in the morning (fed state), whereas GTTs (2 g/kg; Hospira) were performed in the early afternoon after a 5-h fast. Blood glucose was measured at 0, 15, 30, and 60 min for ITTs and at 0, 15, 30, 60, and 120 min for GTTs. In vivo glucose-stimulated insulin secretion was performed on day 8 after a 5-h fast. After intraperitoneal injection of glucose (2 g/kg; Hospira), tail vein blood samples were obtained using chilled heparinized microcapillary tubes (Fisher). For in vitro glucose-stimulated insulin secretion, triplicate groups of 10 similarly sized islets isolated on day 7 were placed in cell culture inserts (12 μM; Millipore) and preincubated in Krebs buffer (10 mM HEPES, 1.19 mM MgSO4, 1.19 mM NaCl, 4.74 mM KCl, 1.19 mM KH2PO4, 2.54 mM CaCl2-2H2O, 25 mM NaHCO3, pH 7.4, 95% O2) with 3% BSA and 2.8 mM glucose for 60 min at 37°C. Islets were then incubated in Krebs buffer with 1% BSA and 3 mM glucose, followed by Krebs buffer with 1% BSA and 20 mM glucose, each for 30 min at 37°C. After each glucose exposure, the buffer was removed and frozen for insulin analysis. For insulin content, 20 islet equivalents were picked immediately after isolation, washed twice with PBS, lysed in extraction buffer (0.18 M HCl in 70% ethanol), and frozen for insulin analysis.
Histological analyses.
Pancreata were fixed in Bouin's solution (Sigma) for 4 h, embedded in paraffin, and sectioned (5 μM). BrdU (Abcam), PCNA (Santa Cruz), and insulin (Invitrogen) staining were performed as previously described (47); images were acquired using Olympus Provis standard or Olympus Fluoview confocal microscopes. 1,271 ± 216 (days 3–7 BrdU), 2,041 ± 294 (day 4 BrdU), and 1,282 ± 141 (PCNA) cells per animal were counted by an individual who was blinded to experimental group. For β-cell mass measurements, sections were stained for insulin (Invitrogen) and hematoxylin by immunohistochemistry as previously described (5). Stained sections were scanned in their entirety using a Plustek slide scanner. To obtain β-cell mass, the ratio of cross-sectional β-cell area to total pancreatic area, obtained using Adobe Photoshop (Adobe) and Image J (NIH), was multiplied by the wet weight of the pancreas. β-Cell size was measured from sections stained for insulin by immunofluorescence, using Image J to calculate insulin-positive area per β-cell (5).
Islet isolation.
Islets were isolated as described (47), using ductal injection of collagenase and Ficoll gradient separation. Islets were plated in RPMI containing 1% (vol/vol) FBS and 5.5 mM glucose, hand-picked using a stereomicroscope, washed in phosphate-buffered saline containing 100 μM sodium orthovanadate (Sigma), and stored at −80°C for subsequent RNA and protein analysis.
qPCR.
RNA was isolated from frozen islets using the RNeasy Kit (Qiagen). cDNA was synthesized using the iScript kit (Bio-Rad). qPCR was performed as previously described (47), using a Realplex Thermalcycler (Eppendorf). Primer sequences are listed in Table 1. Data are expressed as ΔΔCT per the method described (35).
Table 1.
Primer sequences
| Gene | Forward | Reverse |
|---|---|---|
| Cyclin D1 | GCGTACCCTGACACCAATCT | CACAACTTCTCGGCAGTCAA |
| Cyclin D2 | GCTATGGAGCTGCTGTGCT | CCAAGAAACGGTCCAGGTAA |
| Cyclin D3 | GGAAGCTATGGACCAGCAAG | TTTGCACGCACTGGAAGTAG |
| Cyclin A2 | TCCTTGCTTTTGACTTGGCT | ATGACTCAGGCCAGCTCTGT |
| Cyclin E1 | GCTTCTAGACCTGTGCGTCC | CTTTCTTTGCTTGGGCTTTG |
| Cyclin G1 | AGGTCTGCGGCTTGAAACTA | ATTCGGATCAAATCAGTCGC |
| CDK2 | GTTGACGGGAGAAGTTGTGG | TGATGAGGGGAAGAGGAATG |
| CKD4 | TATGAACCCGTGGCTGAAAT | CCTTGATGTCCCGATCAGTT |
| CDK6 | GCCTATGGGAAGGTGTTCAA | GGGCTCTGGAACTTTATCCA |
| p18 | AATGGATTTGGGAGAACTGC | TGACAGCAAAACCAGTTCCA |
| p21 | TCCAGACATTCAGAGCCACA | GACCCAGGGCTCAGGTAGA |
| p27 | GATACGAGTGGCAGGAGGTG | TTCTGTTCTGTTGGCCCTTT |
| p57 | CACTCTGTACCATGTGCAAGGAGTA | TTTCTCTTTTTGTTTTGCACTGAGA |
| Menin | TTCAGCTTCATCACAGGCAC | ACCACCCAAGCATGATCTTC |
| KI67 | CTGCCTGCGAAGAGAGCATC | AGCTCCACTTCGCCTTTTGG |
| PCNA | ACCTGCAGAGCATGGACTCG | GCAGCGGTATGTGTCGAAGC |
| INS1 | ACCTTTGTGGTCCTCACCTG | AGCTCCAGTTGTGGCACTTG |
| INS2 | TGTGGTTCTCACTTGGTGGA | CTCCAGTTGTGCCACTTGTG |
| PDX1 | CTCCGGACATCTCCCCATAC | ACGGGTCCTCTTGTTTTCCT |
| GK | AAACTACCCCTGGGCTTCAC | CCACGATGTTGTTCCCTTCT |
| GLUT2 | GGCACAGACACCCCACTTAC | GCCAACATTGCTTTGATCCT |
| Actin | AGCCATGTACGTAGCCATCC | CTCTCAGCTGTGGTGGTGAA |
Immunoblots.
Islets were sonicated in lysis buffer containing 125 mM Tris, pH 6.8, 2% SDS, 1 mM DTT, 20 μg/ml APMSF, and protease inhibitors (Roche), separated by SDS-PAGE, transferred to nitrocellulose membrane, and blocked in 5% (wt/vol) nonfat dry milk in PBS containing 0.1% Tween 20. Antibodies used were cyclin D1 and cyclin D2 (Neomarkers), cyclin A (Sigma), and tubulin (Calbiochem). Data were collected on film using ECL or ECL Prime (Amersham) and quantified using Image J software.
Statistical analysis.
Data are expressed as means ± SE. P values were determined by two-tailed Student's t-test or ANOVA (one-way or two-way) with Bonferroni posttest for multiple comparisons. P < 0.05 was considered significant; P > 0.1 was considered nonsignificant. P values between 0.05 and 0.1 are reported.
RESULTS
Mice consumed more kilocalories and began to gain weight during the first 7 days of HFD exposure.
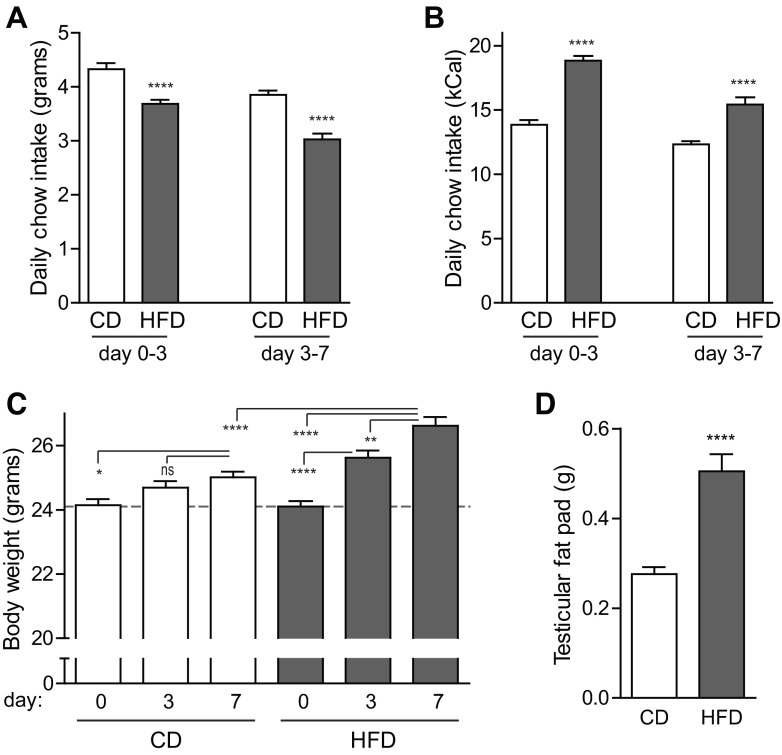
Mice fed HFD consumed fewer grams of chow per day than mice fed control diet (CD; Fig. 1A). However, due to the increased caloric density of the HFD chow, mice on HFD ingested 30% more kilocalories than mice on CD over the course of the 7-day exposure (Fig. 1B). This nutrient excess resulted in a small but significant weight gain as early as day 3, which was further increased by day 7 (10% increase in HFD mice at day 7 vs. day 0 vs. a 4% increase in CD mice; Fig. 1C). Caloric excess was disproportionately stored in adipose tissue; testicular fat pad weight on day 7 was 83% greater in HFD mice than that of CD mice (Fig. 1D).
Fig. 1.
C57BL/6J mice consumed more kilocalories and gained weight in the first 7 days of high-fat feeding. A and B: mice given high-fat diet (HFD) ate fewer grams of chow per day (A), which nonetheless translated to more kilocalories per day (B) than mice fed control chow (CD) (n = 51–53). C: young adult mice fed CD gained a small amount of body weight over the 7-day intervention, whereas mice fed HFD gained weight as early as day 3, with a 10% weight gain by day 7 (n = 59–60). D: testicular fat pad mass was doubled on day 7 in mice fed HFD (n = 6–7). Data are expressed as means ± SE; P values were calculated by Student's t-test (A, B, D) or ANOVA (C). *P < 0.05, **P < 0.01, ****P < 0.0001; ns, nonsignificant.
Fed-state hyperglycemia and hyperinsulinemia began within 1 wk of HFD exposure.
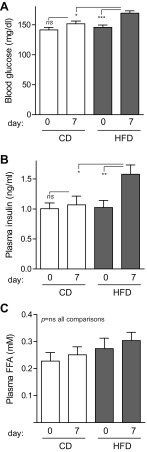
Nonfasting morning blood glucose measurements in CD mice were similar on days 0 and 7. However, mice given HFD showed a slight (16%) but significant increase in blood glucose on day 7 (Fig. 2A). This increase in blood glucose was accompanied by a 54% increase in nonfasting plasma insulin on day 7 in HFD mice (Fig. 2B). Nonfasting plasma FFA levels were not increased by HFD over the course of the experiment (Fig. 2C). In contrast to the fed-state results, 5-h-fasted blood glucose levels were not different between the two groups on either day 5 or day 7 (Fig. 3, A and B).
Fig. 2.
Nonfasting blood glucose and insulin levels increased after 7 days of HFD. Morning tail blood samples were obtained on days 0 and 7 to assess blood glucose, insulin, and free fatty acid (FFA) levels. A: blood glucose increased by day 7 in mice fed HFD, whereas mice fed CD maintained similar glucose levels throughout the experiment (n = 39–40). B: by day 7, HFD mice had nonfasting hyperinsulinemia (n = 22–31). C: plasma FFAs were not altered by either diet (n = 22–32). Data are expressed as means ± SE; P values were calculated by ANOVA with Bonferroni correction for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001.
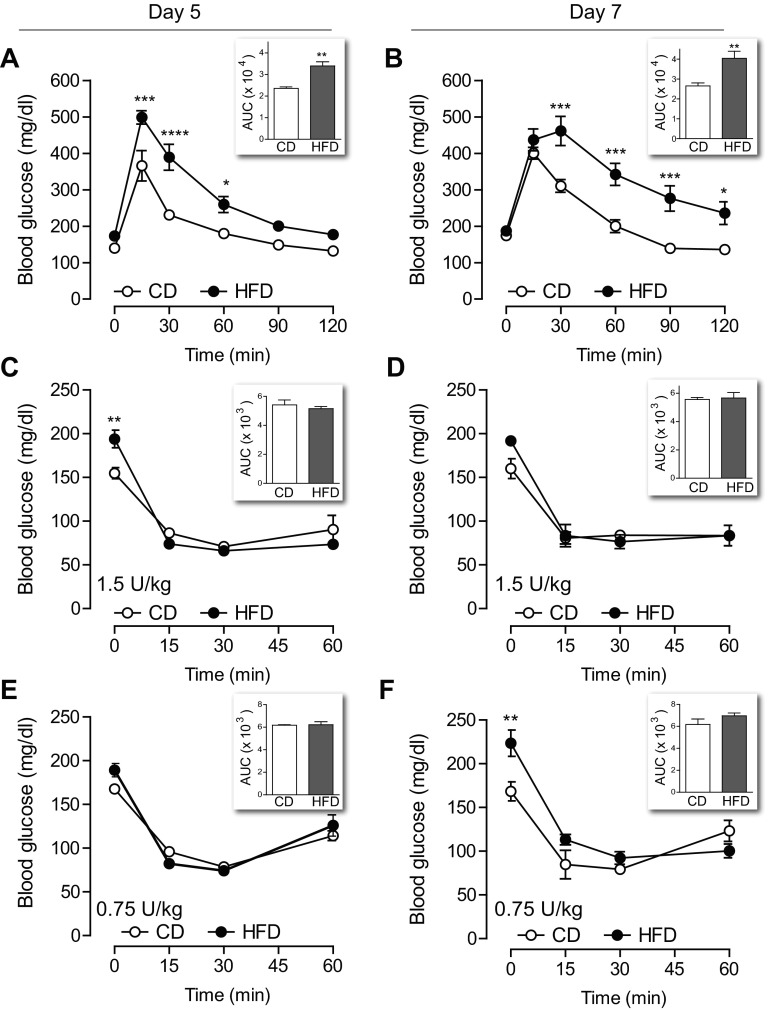
Fig. 3.
Glucose intolerance began within the 1st wk of high-fat feeding without evidence of insulin resistance. A and B: mice given HFD were intolerant to glucose challenge when tested on day 5 (A, n = 4) and day 7 (B, n = 8). C and D: intraperitoneal challenge with 1.5 U/kg insulin revealed no difference in insulin sensitivity between CD and HFD mice when tested on day 5 (C, n = 7–8) or day 7 (D, n = 4). E and F: ITT with a lower dose of insulin (0.75 U/kg) also showed no decrease in insulin sensitivity in mice on HFD at either time point (n = 4). Insets: area under the curve (AUC) for 120-min glucose tolerance test (GTT) or 60-min insulin tolerance test (ITT). Data are expressed as means ± SE; P values by two-way ANOVA with Bonferroni correction for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Glucose intolerance developed within 5 days of exposure to HFD without a measurable decline in insulin sensitivity.
Despite normal fasting blood glucose, the ability to clear an intraperitoneal glucose challenge was frankly impaired after both 5 (Fig. 3A) and 7 (Fig. 3B) days of HFD. On the basis of a prior study showing that insulin resistance develops within 3 days of HFD exposure (33), we expected to see a reduced response to insulin challenge after HFD treatment. Surprisingly, insulin sensitivity was normal on day 5 (Fig. 3C) and also on day 7 (Fig. 3D). To determine whether the ITT lacked sensitivity for subtle insulin resistance due to the relatively high dose of insulin used, we repeated the ITT using a lower dose. Intraperitoneal challenge with 0.75 U insulin/kg body wt also revealed normal insulin sensitivity in mice on HFD at both day 5 and day 7 (Fig. 3, E and F). Consistent with our previous data, nonfasting blood glucose levels were higher in the HFD group on days 5 and 7 (Fig. 3, C–F). Thus, in vivo dynamic testing revealed glucose intolerance in these mice that was not explained by insulin resistance detectable by ITT.
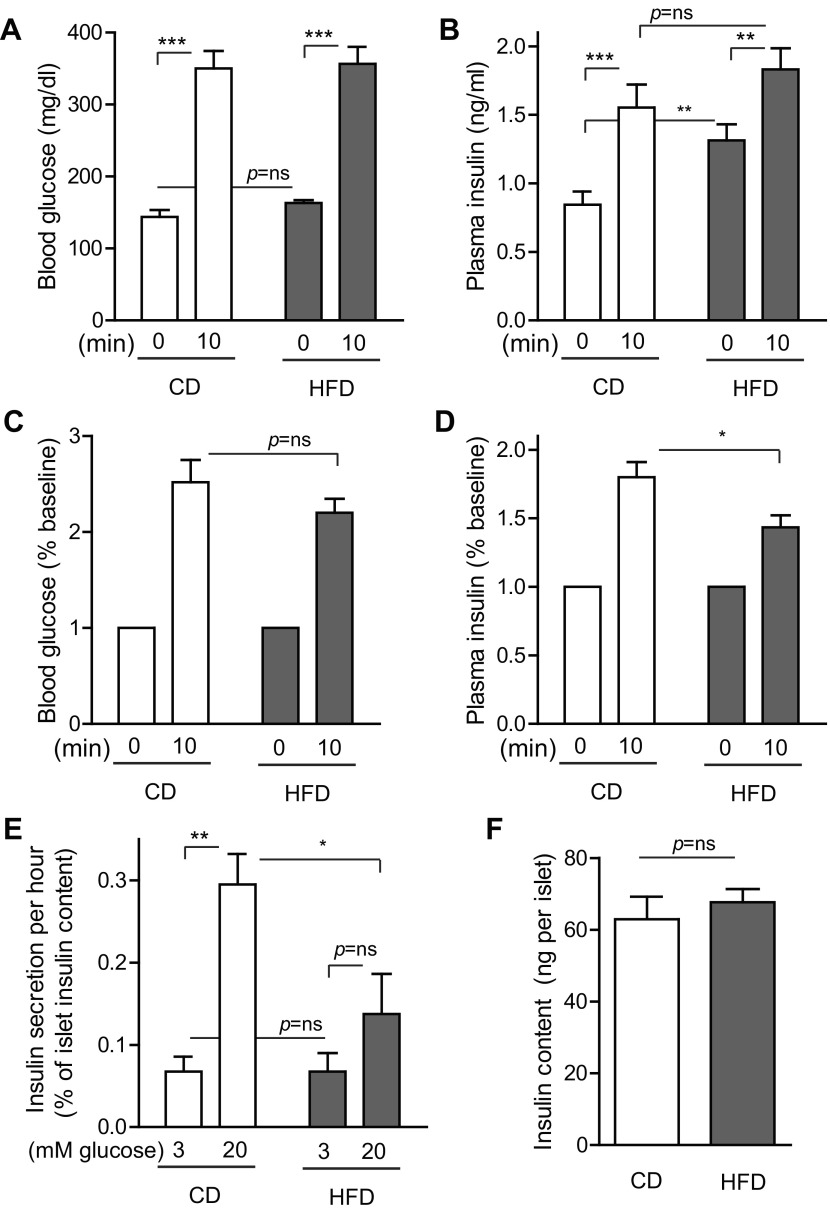
Insulin-secretory function was mildly impaired early in HFD exposure.
Since high-fat feeding did not appear to alter insulin sensitivity, we performed an in vivo insulin secretion assay to determine whether the observed glucose intolerance resulted from impaired insulin secretion. Tail vein blood samples were taken for glucose and insulin measurements before and after an intraperitoneal glucose challenge (Fig. 4, A–D). Consistent with prior results, baseline fasted blood glucose levels were similar between CD and HFD (Fig. 4A). Blood glucose was markedly increased 10 min after glucose challenge, to a similar degree in both CD and HFD mice (Fig. 4A). Fasted plasma insulin levels were higher at baseline in HFD mice, but postchallenge levels were similar between the groups (Fig. 4B). When analyzed as the fold change over baseline, despite the similar blood glucose dynamics between groups over this time frame (Fig. 4C), plasma insulin levels increased less in HFD mice (Fig. 4D). To determine whether impairment of insulin secretion was due to an intrinsic islet defect, islets were isolated after control or high-fat feeding, and insulin secretion was measured in vitro (Fig. 4E). Islets from CD mice showed a robust 4.7-fold increase in insulin secretion at 20 mM glucose [39.5 (3 mM) vs. 185.4 (20 mM) pg·islet−1·h−1]. Intriguingly, islets from HFD mice did not show elevated basal insulin secretion but did show reduced insulin release at high-glucose exposure, only 2.0-fold over baseline, confirming a defect in insulin secretion [44.5 (3 mM) vs. 90.6 (20 mM) pg·islet−1·h−1]. This defect was not due to reduced islet insulin content (Fig. 4F). Thus, 1 wk of HFD feeding resulted in a subtle defect in insulin secretion characterized by high circulating basal insulin levels in vivo with a reduced quantity of insulin secreted after glucose challenge.
Fig. 4.
Insulin-secretory response to glucose challenge was mildly impaired after 1 wk of high-fat feeding. A: blood glucose levels measured before and 10 min after intraperitoneal glucose injection were similar between CD and HFD mice on day 8. B: basal plasma insulin levels were higher in HFD mice, but insulin levels after glucose injection were similar between the groups. C and D: when considered as percent increase from baseline, blood glucose increased similarly in CD and HFD mice (C), but plasma insulin increased less in HFD mice (D). E: in vitro glucose-stimulated insulin secretion was impaired in islets isolated after 7 days of HFD exposure. F: islet insulin content was not altered by 7 days of HFD. Data are expressed as means ± SE; P values were calculated by Student's t-test; n = 12 for A–D, n = 4 for E–F. *P < 0.05, **P < 0.01, ***P < 0.001.
Compensatory β-cell mass expansion began within the first 7 days of high-fat feeding.
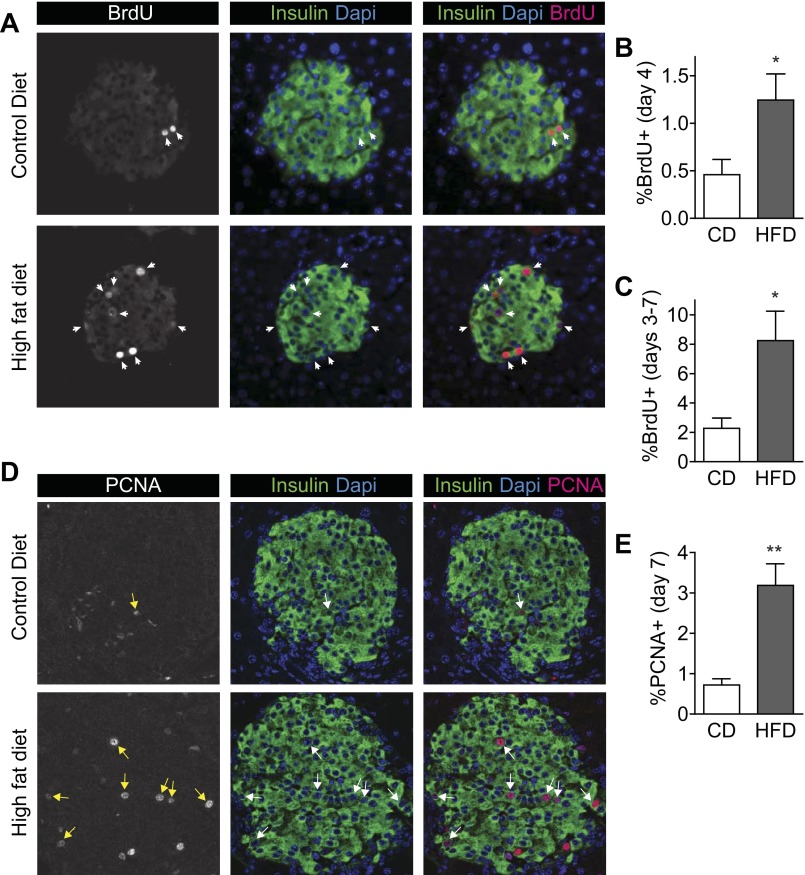
Having carefully characterized the metabolic state of these mice during the first week of HFD feeding, we asked whether the β-cell proliferative response also began during this early period. β-Cell proliferation events, as defined by BrdU incorporation in insulin-positive cells, were readily detectable in pancreas sections (Fig. 5A). Mice treated with a single injection of BrdU on the 4th day of HFD feeding, and killed on day 7, showed that β-cell proliferation was already increased by day 4 (Fig. 5B). When BrdU was added to the drinking water during the last 4 days of the 7-day diet exposure to measure cumulative proliferation events, mice fed HFD showed a marked increase in labeled β-cells (Fig. 5C). To verify the increased proliferation by a different method, sections were stained for PCNA and insulin (Fig. 5D). The percentage of β-cells staining for PCNA was increased in HFD mice on day 7 (Fig. 5E). Thus, the compensatory β-cell proliferative response to high-fat feeding began during the first week of HFD exposure.
Fig. 5.
β-Cell proliferation increased within the 1st wk of HFD feeding. A: pancreas sections stained for insulin (green), BrdU (red), and DAPI (blue) show that proliferating β-cells were strongly labeled for accurate quantification. These images are from mice treated with BrdU in the drinking water on days 3–7. B: β-cell proliferation was increased on day 4 of HFD as detected by a single intraperitoneal injection of BrdU on the morning of day 4. C: β-cell proliferation was increased in HFD mice when measured after exposure to BrdU continuously on days 3–7. D: pancreas sections stained for insulin (green), PCNA (red), and DAPI (blue) show that proliferating β-cells were strongly labeled for accurate quantification. E: percent insulin-positive cells colabeled for PCNA was increased on day 7; n = 8 (B) and n = 4 (C and E). Data are expressed as means ± SE; P values were calculated by Student's t-test. *P < 0.05, **P < 0.01.
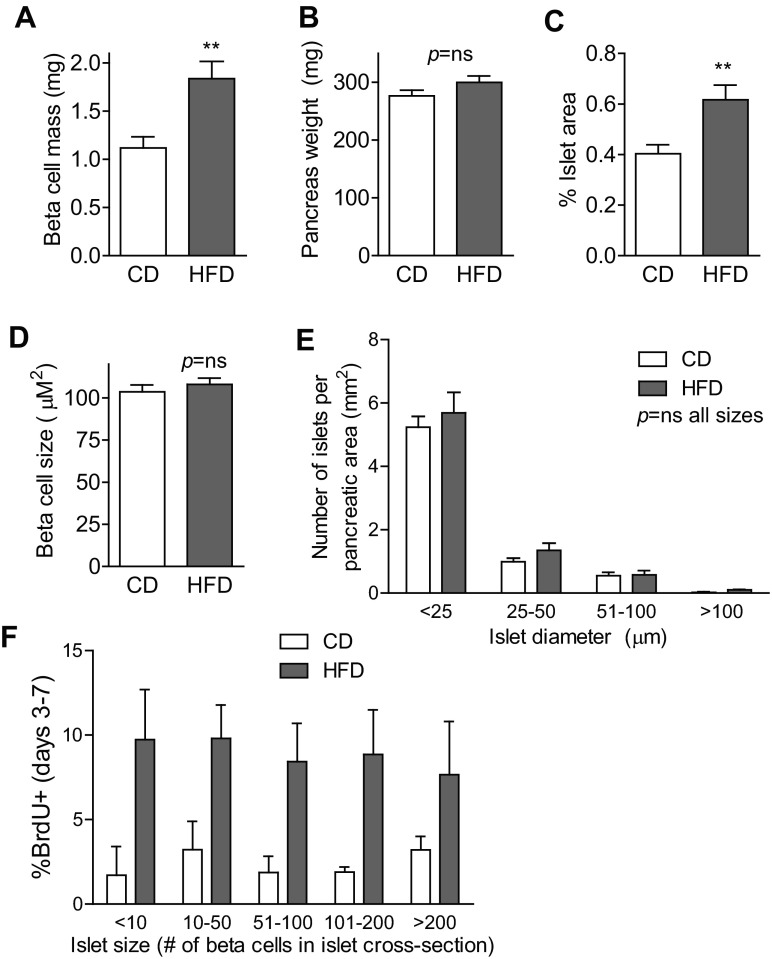
To assess whether the early increase in β-cell proliferation was sufficient to increase β-cell mass within the first week of HFD exposure, pancreas sections were quantitatively analyzed to determine the fractional area of β-cells. Remarkably, after only 7 days of high-fat feeding, β-cell mass had already begun to increase (Fig. 6A), which was driven not by alteration in pancreas weight (Fig. 6B) but by an increase in the percentage of pancreatic area that was composed of β-cells (Fig. 6C). To determine whether hypertrophy of individual β-cells contributed to the expanding β-cell mass, insulin area per β-cell was determined. β-Cell size was not altered after 7 days of HFD (Fig. 6D). Analysis of islet size distribution revealed that the number of very small islets, a possible marker of neogenesis events, was not increased by HFD (Fig. 6E). Finally, analysis of the β-cell proliferation rate as a function of islet size showed that high-fat feeding increased the rate of proliferation similarly across all islet sizes, from the smallest to the largest (Fig. 6F). In sum, during the first week of HFD consumption, these C57BL/6J mice had already begun to expand β-cell mass, due to an increase in proliferation of β-cells that began by day 4, without evidence for β-cell hypertrophy or new generation of small islets.
Fig. 6.
Compensatory β-cell mass expansion began during the 1st wk of HFD. A–C: β-cell mass had begun to increase after only 7 days of HFD (n = 12; A). This increase was not due to increased pancreas weight (n = 12; B) but instead to an increase in percent pancreatic area made up of islets (n = 12; C). D: size of individual β-cells was not altered by HFD treatment (n = 4). E: analysis of size distribution of pancreatic islets revealed no increase in the number of very small islets (n = 8). F: high-fat feeding increased the proliferation rate to a similar degree in islets of all sizes (n = 4). These data are from mice treated with BrdU in the drinking water on days 3–7. Data are expressed as means ± SE; P values were calculated by Student's t-test (A–D) or two-way ANOVA with Bonferroni correction for multiple comparisons (E: P < 0.0001 for islet size, P = ns for diet; F: P < 0.0001 for diet, P = ns for islet size). **P < 0.01.
Early compensatory β-cell proliferation was associated with increased expression of cyclin D2 protein.
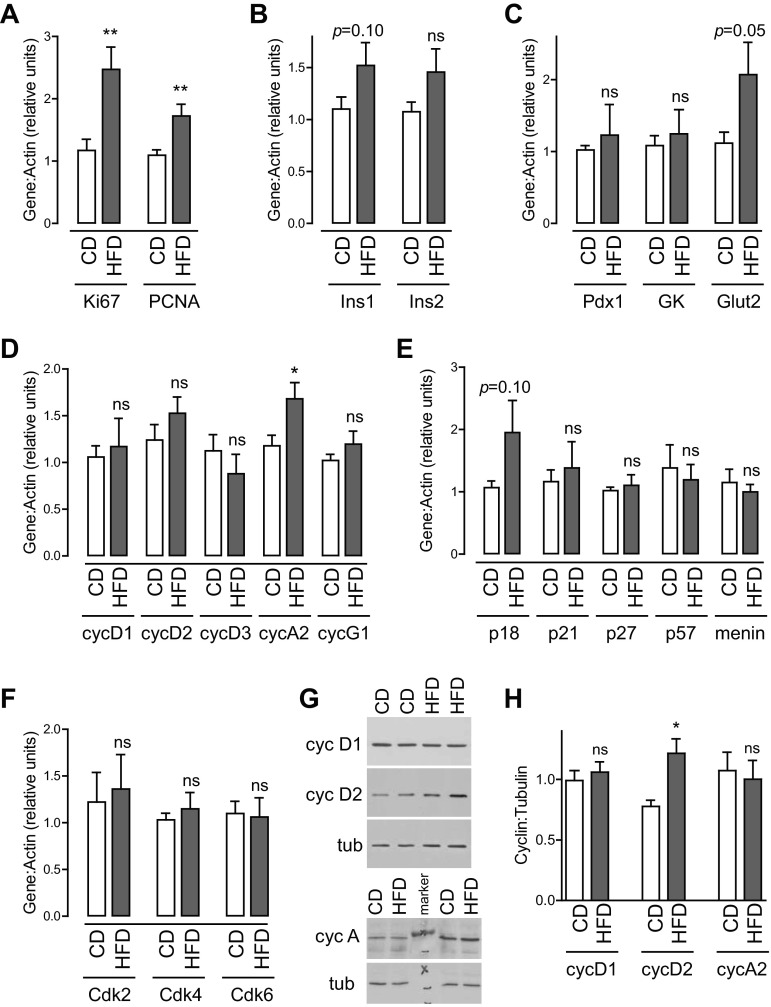
Real-time PCR was performed on islets isolated after 7 days of HFD to assess for changes in gene expression. Ki67 and PCNA mRNA levels were both increased by HFD, confirming the proliferative state in these islet samples (Fig. 7A). Assessment of genes associated with β-cell function revealed no significant change in insulin, Pdx1, glucokinase, or Glut2 (Fig. 7, B and C). Analysis of genes known to be important for controlling cell cycle entry revealed no change in mRNA expression of most relevant cyclins, cell cycle inhibitors, or cyclin-dependent kinases (Figs. 7, D–F). Only cyclin A2 mRNA levels were increased in mice fed HFD for 1 wk (Fig. 7D).
Fig. 7.
Early compensatory β-cell proliferation is associated with increased cyclin D2 protein expression. Real-time PCR was performed on islets isolated after 7 days of high-fat feeding. A: Ki67 and PCNA expression were increased by HFD, further supporting the conclusion that islet cell proliferation was increased. B and C: analysis of genes related to β-cell function revealed a trend toward an increase in Glut2 expression without alteration of other genes related to β-cell differentiation. D: cyclin A2 mRNA levels were increased after HFD exposure without change in cyclins D1, D2, D3, E1, or G1. E: no changes in G1-S inhibitors were observed between the two diets. F: expression of Cdk genes relevant to the G1-S transition were not altered. G and H: immunoblots showed that cyclin D2 protein was increased in islets after 7 days of HFD; cyclins D1 and A were not; n = 14–19 (A–F); n = 7 (G, H). Data are expressed as means ± SE; P values were calculated by Student's t-test. *P < 0.05, **P < 0.01.
To determine whether protein expression levels of key regulators were altered, immunoblots were performed on islets isolated from animals exposed to HFD for 7 days (Fig. 7, G and H). Intriguingly, cyclin D2 was increased by HFD; this finding was specific, as cyclin D1 expression was not altered in these samples. Despite the finding of increased cyclin A2 mRNA, cyclin A protein level was not increased by HFD.
DISCUSSION
To our knowledge, this study is the first work characterizing the onset of compensatory β-cell mass expansion in relation to the physiological changes that occur early in HFD exposure. Previous studies have examined the metabolic changes that occur early in high-fat feeding from a physiology perspective (2, 33, 37, 44, 59), but they did not correlate findings with parameters determining β-cell mass. In order to develop therapies with the potential to entice quiescent human β-cells toward proliferation in response to increased insulin-secretory need, it may be important to determine the earliest signals that initiate rodent β-cell proliferation. An important finding of this study is the observation that β-cell proliferation begins very early in HFD exposure, coincident with the onset of hyperglycemia, hyperinsulinemia, and glucose intolerance but, surprisingly, before the onset of frank insulin resistance. Our data do not support important roles for β-cell hypertrophy or generation of new small islets in early compensatory β-cell mass expansion in C57BL/6J mice. A directed screen of relevant distal cell cycle effectors revealed that cyclin D2 protein expression increased during this early time period, possibly implicating it as an early driver.
The current standard approach to study compensatory β-cell mass expansion in overnutrition is to feed rodents HFD for 8–24 wk. During this time, chronic obesity-related changes in nearly every organ occur, rendering experimental mice different from controls in myriad ways, most of which are unmeasured. In principle, factors initiating β-cell mass accrual are more likely to be altered early during this process and are less likely to be obscured by irrelevant late-stage changes. In addition, long-term mouse experiments with regular monitoring are expensive, labor intensive, and time consuming. Our finding that compensatory β-cell proliferation and mass expansion begin as early as the first week of HFD exposure has the potential to alter standard practice in β-cell mass homeostasis research.
β-Cell proliferation, measured using two different established and reliable techniques, increased within the first week of high-fat feeding. Given the rapidity with which other interventions increase mouse β-cell proliferation (5, 10, 15, 48, 58), this is perhaps not surprising, although it is in sharp contrast to the usual long-term high-fat feeding protocols used to study β-cell proliferation in mice. The timing of proliferation induction early in HFD exposure coincided with the onset of nonfasting hyperglycemia and hyperinsulinemia. Both glucose (5, 9, 10, 19, 25, 34, 40, 61) and insulin (6, 17, 26, 39, 43, 53) increase β-cell proliferation and mass in rodents and have been implicated in compensatory β-cell growth in the setting of chronic insulin resistance (43, 57). In vivo, continuous hyperglycemia increases β-cell proliferation within 2–4 days of exposure (5, 10, 34), consistent with the early time frame observed in this study. However, in the current study, nonfasting blood glucose was elevated but fasting was not; it is unknown whether episodic hyperglycemia increases β-cell proliferation.
β-Cell mass had already begun to increase by the 7th day of high-fat feeding. In addition to β-cell proliferation, β-cell size and islet size distribution were also measured. Despite the relationship between nutrient oversupply and mammalian target of rapamycin (mTOR) signaling, and the effect of mTOR to increase β-cell size (41, 51), no alteration in β-cell size was observed during the first week of HFD. One long-term study found that β-cell size was actually reduced after 3 mo of HFD but increased by 6 mo (3). Since β-cell size is positively correlated with increased insulin-secretory capacity (22), perhaps it is not surprising that β-cell size did not increase during the first week of HFD, since insulin secretion was mildly impaired in these mice. Analysis of islet size distribution revealed similar numbers of very small islets in CD and HFD mice, which does not support a model in which compensatory β-cell mass accrual early in high-fat feeding is dependent on generation of new small islets from other cell types. Although we did not measure β-cell death, the rate is so low in young healthy mice that it is unlikely that a further reduction could meaningfully impact β-cell mass accrual over this time frame.
These data revealed a surprising degree of glucose intolerance in healthy C57BL/6J mice after only 5–7 days of HFD. This phenomenon has been reported in other studies as well (2, 59). Further testing did not identify a clear cause for this glucose intolerance, as insulin sensitivity remained normal and insulin secretion was only mildly reduced. It is possible that high-fat feeding results in an early decline in insulin-independent glucose uptake (Sg), which was not measured in this study. Sg, which is thought by some to be the most important determinant of glucose disposal in rodents (36, 45), is reduced after long-term high-fat feeding and is inversely correlated with glucose disposal in obese mice (4). Intriguingly, human subjects consuming a HFD for 3 days had increased nonfasting, but not fasting, blood glucose along with impaired glucose clearance, which was due to impaired insulin secretion without evidence of insulin resistance (42), very similar to our current findings in mice. It is unknown whether rodents or humans with impaired fasting glucose have similar or different rates of β-cell proliferation than subjects with impaired glucose tolerance.
The observation that insulin secretion may be diminished during the first week of high-fat feeding is also somewhat surprising, but, in fact, has also been reported (1, 59). Long-term HFD is associated with increased insulin-secretory capacity in strains of mice not prone to diabetes (4). Our study suggests that β-cell functional compensation had not yet begun within the first week of HFD, without evidence for increased expression of glucose sensing or insulin genes, insulin content, or insulin secretion after glucose challenge. In fact, our results indicate that insulin-secretory function was actually impaired at this early time point of high-fat feeding both in vitro and in vivo. Whether this early decline in islet function parallels islet dysfunction in human prediabetes is unknown. Our data suggest the intriguing possibility that in people destined for diabetes the response to overnutrition might stall in this early stage marked by reduced islet function if compensation mechanisms fail to engage. The reason for basal hyperinsulinemia in the setting of reduced capacity for stimulated insulin secretion is unclear. Insulin clearance rates were not measured in this study, although one study found that insulin clearance was not altered in mice after 8 wk of HFD (60). It is possible that a circulating interfering substance, such as proinsulin, may have been measured along with insulin, influencing results; the Crystalchem ELISA used for the insulin secretion assay may cross-react with proinsulin (personal communication with the company). From a metabolic perspective, consuming a diet in which the majority of calories derive from fat may reduce the ability to secrete insulin in response to a pure glucose challenge. From a cell biology perspective it is possible that as the population of β-cells shifts toward a more proliferative phenotype it undergoes a subtle dedifferentiation. It remains unknown whether any given β-cell, when proliferating, retains insulin-secretory capacity.
Contrary to carefully performed studies using sophisticated techniques (2, 33), we did not detect insulin resistance within the first week of high-fat feeding in mice. Insulin tolerance testing may be less sensitive than clamp or intravenous glucose tolerance test. Fat mass increased out of proportion to lean body mass during the first week of HFD. Since glucose disposal after insulin injection in the mouse is predominantly into muscle, it is possible that insulin dosing based on lean body mass might reveal a subtle abnormality in insulin sensitivity (7). Body composition was not measured in these mice. On the other hand, one study measuring insulin sensitivity by euglycemic clamp found that insulin resistance developed between 1.5 and 3 wk of HFD (46), supporting the possibility that our data are correct. The observation that hyperglycemia was present only in the nonfasted state, and not in fasting conditions, suggests the absence of hepatic insulin resistance. The observation that the β-cell proliferative response to high-fat feeding preceded the onset of insulin resistance is intriguing and calls into question the concept that insulin resistance is the driver of β-cell mass expansion in HFD models.
Nonfasting FFA levels in circulating plasma were not increased after 1 wk of HFD. It has been previously observed that elevated FFAs are a late, and not early, finding in obesity (27). To the extent that FFAs are associated with insulin resistance, another relationship that has been questioned (27, 28), the absence of elevated FFAs in our study is consistent with the absence of insulin resistance. Since they were not increased, FFAs are not likely to be the cause of impaired insulin secretion in these mice (18, 50). FFA flux was not measured.
We have begun to explore islet gene expression changes during the earliest stages of the β-cell proliferative response to HFD, focusing first on relevant direct regulators of cell cycle progression (12, 29). The finding that cyclin D2 protein is increased within 7 days of high-fat feeding is novel. In fact, to the best of our knowledge, this may be the first report that cyclin D2 expression is upregulated by HFD at all. Cyclin D2 is required for β-cell proliferation in response to genetic forms of insulin resistance (21). Although commonly cited as the critical cyclin regulating mouse β-cell proliferation (21, 24, 30), one study has found that cyclin D2 was increased after partial pancreatectomy but not after other proliferative stimuli (exendin 4 or leptin deficiency) (24). Cyclin D2 is known to be upregulated in rodent islets in response to glucose (5, 55), although data are conflicting whether this regulation is due to transcriptional or posttranscriptional effects. In this study, cyclin A2 mRNA was increased after HFD; cyclin A protein was not increased, rendering the relevance of this finding uncertain.
In conclusion, in C57BL/6J mice, the β-cell proliferative response begins within the first week of high-fat feeding, when the metabolic physiology reflects early glucose intolerance, before the onset of frank insulin resistance. Many questions remain. Do human β-cells proliferate in response to HFD? Which upstream signals, secreted, membrane, or intracellular, initiate the rodent proliferative response? Are these signals active in human β-cells, and if not, why not? We hope, in future studies, to use this model to identify early drivers of β-cell proliferation, to seek novel approaches to initiate β-cell mass expansion. It is our hope that by identifying these early signals we may find new therapeutic targets that increase functional β-cell mass to treat, or even cure, diabetes.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-095140 (L. C. Alonso) and ADA-7-11-BS-04 (L. C. Alonso).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.E.S., R.B.S., and D.A.H. performed experiments; R.E.S. and L.C.A. drafted manuscript; R.E.S. and L.C.A. edited and revised manuscript; R.E.S., R.B.S., D.A.H., and L.C.A. approved final version of manuscript; L.C.A. conception and design of research; L.C.A. and R.E.S. analyzed data; L.C.A. interpreted results of experiments; L.C.A. prepared figures.
ACKNOWLEDGMENTS
We gratefully acknowledge the thoughtful input of Drs. Rupangi Vasavada, Adolfo Garcia-Ocana, and Andrew Stewart during the early stages of this project. We thank Gabriella Casinelli for insulin measurements.
REFERENCES
- 1. Ahren B, Gudbjartsson T, Al-Amin AN, Martensson H, Myrsen-Axcrona U, Karlsson S, Mulder H, Sundler F. Islet perturbations in rats fed a high-fat diet. Pancreas 18: 75–83, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Ahren B, Pacini G. Insufficient islet compensation to insulin resistance vs. reduced glucose effectiveness in glucose-intolerant mice. Am J Physiol Endocrinol Metab 283: E738–E744, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Ahren J, Ahren B, Wierup N. Increased beta-cell volume in mice fed a high-fat diet: a dynamic study over 12 months. Islets 2: 353–356, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Alonso LC, Watanabe Y, Stefanovski D, Lee EJ, Singamsetty S, Romano LC, Zou B, Garcia-Ocana A, Bergman RN, O'Donnell CP. Simultaneous measurement of insulin sensitivity, insulin secretion, and the disposition index in conscious unhandled mice. Obesity (Silver Spring) 20: 1403–1412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocana A. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 56: 1792–1801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol 29: 3219–3228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Barreto SG, Carati CJ, Toouli J, Saccone GT. The islet-acinar axis of the pancreas: more than just insulin. Am J Physiol Gastrointest Liver Physiol 299: G10–G22, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 51: 66–72, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes 38: 49–53, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 27: 356–370, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Davis DB, Lavine JA, Suhonen JI, Krautkramer KA, Rabaglia ME, Sperger JM, Fernandez LA, Yandell BS, Keller MP, Wang IM, Schadt EE, Attie AD. FoxM1 is up-regulated by obesity and stimulates beta-cell proliferation. Mol Endocrinol 24: 1822–1834, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Koning EJ, Bonner-Weir S, Rabelink TJ. Preservation of beta-cell function by targeting beta-cell mass. Trends Pharmacol Sci 29: 218–227, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Demirci C, Ernst S, Alvarez-Perez JC, Rosa T, Valle S, Shridhar V, Casinelli GP, Alonso LC, Vasavada RC, Garcia-Ocana A. Loss of HGF/c-Met signaling in pancreatic beta-cells leads to incomplete maternal beta-cell adaptation and gestational diabetes mellitus. Diabetes 61: 1143–1152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, Buckingham RE. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes 50: 1021–1029, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci USA 98: 7475–7480, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fontes G, Zarrouki B, Hagman DK, Latour MG, Semache M, Roskens V, Moore PC, Prentki M, Rhodes CJ, Jetton TL, Poitout V. Glucolipotoxicity age-dependently impairs β-cell function in rats despite a marked increase in β-cell mass. Diabetologia 53: 2369–2379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Futamura M, Yao J, Li X, Bergeron R, Tran JL, Zycband E, Woods J, Zhu Y, Shao Q, Maruki-Uchida H, Goto-Shimazaki H, Langdon RB, Erion MD, Eiki J, Zhou YP. Chronic treatment with a glucokinase activator delays the onset of hyperglycaemia and preserves β-cell mass in the Zucker diabetic fatty rat. Diabetologia 55: 1071–1080, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Georgia S, Bhushan A. Β-cell replication is the primary mechanism for maintaining postnatal β-cell mass. J Clin Invest 114: 963–968, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Georgia S, Hinault C, Kawamori D, Hu J, Meyer J, Kanji M, Bhushan A, Kulkarni RN. Cyclin D2 is essential for the compensatory beta-cell hyperplastic response to insulin resistance in rodents. Diabetes 59: 987–996, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giordano E, Cirulli V, Bosco D, Rouiller D, Halban P, Meda P. β-Cell size influences glucose-stimulated insulin secretion. Am J Physiol Cell Physiol 265: C358–C364, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Granger A, Kushner JA. Cellular origins of beta-cell regeneration: a legacy view of historical controversies. J Int Med 266: 325–338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He LM, Sartori DJ, Teta M, Opare-Addo LM, Rankin MM, Long SY, Diehl JA, Kushner JA. Cyclin D2 protein stability is regulated in pancreatic beta-cells. Mol Endocrinol 23: 1865–1875, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jetton TL, Everill B, Lausier J, Roskens V, Habibovic A, LaRock K, Gokin A, Peshavaria M, Leahy JL. Enhanced β-cell mass without increased proliferation following chronic mild glucose infusion. Am J Physiol Endocrinol Metab 294: E679–E687, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, Habibovic A, Peshavaria M, Leahy JL. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes 54: 2294–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab 292: E1590–E1598, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Kim SP, Ellmerer M, Van Citters GW, Bergman RN. Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Diabetes 52: 2453–2460, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human beta-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 61: 2205–2213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol 25: 3752–3762, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kushner JA, Weir GC, Bonner-Weir S. Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metab 11: 2–3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leckstrom A, Lundquist I, Ma Z, Westermark P. Islet amyloid polypeptide and insulin relationship in a longitudinal study of the genetically obese (ob/ob) mouse. Pancreas 18: 266–273, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60: 2474–2483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levitt HE, Cyphert TJ, Pascoe JL, Hollern DA, Abraham N, Lundell RJ, Rosa T, Romano LC, Zou B, O'Donnell CP, Stewart AF, Garcia-Ocana A, Alonso LC. Glucose stimulates human β-cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia 54: 572–582, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta CT) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 36. McArthur MD, You D, Klapstein K, Finegood DT. Glucose effectiveness is the major determinant of intravenous glucose tolerance in the rat. Am J Physiol Endocrinol Metab 276: E739–E746, 1999 [DOI] [PubMed] [Google Scholar]
- 37. McDonald SD, Pesarchuk E, Don-Wauchope A, El Zimaity H, Holloway AC. Adverse metabolic effects of a hypercaloric, high-fat diet in rodents precede observable changes in body weight. Nutr Res 31: 707–714, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57: 1584–1594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Merino JF, Nacher V, Raurell M, Aranda O, Soler J, Montanya E. Improved outcome of islet transplantation in insulin-treated diabetic mice: effects on beta-cell mass and function. Diabetologia 40: 1004–1010, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Metukuri MR, Zhang P, Basantani MK, Chin C, Stamateris RE, Alonso LC, Takane KK, Gramignoli R, Strom SC, O'Doherty RM, Stewart AF, Vasavada RC, Garcia-Ocana A, Scott DK. ChREBP mediates glucose-stimulated pancreatic beta-cell proliferation. Diabetes 61: 2004–2015, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mori H, Inoki K, Opland D, Munzberg H, Villanueva EC, Faouzi M, Ikenoue T, Kwiatkowski DJ, Macdougald OA, Myers MG, Jr, Guan KL. Critical roles for the TSC-mTOR pathway in β-cell function. Am J Physiol Endocrinol Metab 297: E1013–E1022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Numao S, Kawano H, Endo N, Yamada Y, Konishi M, Takahashi M, Sakamoto S. Short-term low carbohydrate/high-fat diet intake increases postprandial plasma glucose and glucagon-like peptide-1 levels during an oral glucose tolerance test in healthy men. Eur J Clin Nutr 66: 926–931, 2012 [DOI] [PubMed] [Google Scholar]
- 43. Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA 104: 8977–8982, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Omar B, Pacini G, Ahren B. Differential development of glucose intolerance and pancreatic islet adaptation in multiple diet induced obesity models. Nutrients 4: 1367–1381, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pacini G, Thomaseth K, Ahren B. Contribution to glucose tolerance of insulin-independent vs. insulin-dependent mechanisms in mice. Am J Physiol Endocrinol Metab 281: E693–E703, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 54: 3530–3540, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Pascoe J, Hollern D, Stamateris R, Abbasi M, Romano LC, Zou B, O'Donnell CP, Garcia-Ocana A, Alonso LC. Free fatty acids block glucose-induced beta-cell proliferation in mice by inducing cell cycle inhibitors p16 and p18. Diabetes 61: 632–641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peshavaria M, Larmie BL, Lausier J, Satish B, Habibovic A, Roskens V, Larock K, Everill B, Leahy JL, Jetton TL. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes 55: 3289–3298, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R, Pineda M, Jetton TL, Madiraju SR, Joly E, Prentki M. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 59: 2178–2187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontes G. Glucolipotoxicity of the pancreatic β-cell. Biochim Biophys Acta 1801: 289–298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, Chang-Chen KJ, Gambello MJ, Bernal-Mizrachi E. Disruption of Tsc2 in pancreatic β-cells induces β-cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci USA 105: 9250–9255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14: 45–54, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sachdeva MM, Stoffers DA. Minireview: meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion. Mol Endocrinol 23: 747–758, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saisho Y, Butler AE, Manesso E, Galasso R, Zhang L, Gurlo T, Toffolo GM, Cobelli C, Kavanagh K, Wagner JD, Butler PC. Relationship between fractional pancreatic β-cell area and fasting plasma glucose concentration in monkeys. Diabetologia 53: 111–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salpeter SJ, Klochendler A, Weinberg-Corem N, Porat S, Granot Z, Shapiro AM, Magnuson MA, Eden A, Grimsby J, Glaser B, Dor Y. Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic beta-cells through glycolysis and calcium channels. Endocrinology 152: 2589–2598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Svensson AM, Hellerstrom C, Jansson L. Diet-induced obesity and pancreatic islet blood flow in the rat: a preferential increase in islet blood perfusion persists after withdrawal of the diet and normalization of body weight. J Endocrinol 151: 507–511, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T. Glucokinase and IRS-2 are required for compensatory β-cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 117: 246–257, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult β-cells does not involve specialized progenitors. Dev Cell 12: 817–826, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, Suppl 3: S215–S219, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Winzell MS, Magnusson C, Ahren B. Temporal and dietary fat content-dependent islet adaptation to high-fat feeding-induced glucose intolerance in mice. Metab Clin Exper 56: 122–128, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Yokoe T, Alonso LC, Romano LC, Rosa TC, O'Doherty RM, Garcia-Ocana A, Minoguchi K, O'Donnell CP. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol 586: 899–911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus. Korea J Clin Endocrinol Metab 88: 2300–2308, 2003 [DOI] [PubMed] [Google Scholar]