Abstract
Blood glucose concentration is tightly regulated by the rate of insulin secretion and clearance, a process partially controlled by sensory neurons serving as metabolic sensors in relevant tissues. The activity of these neurons is regulated by the products of metabolism which regulate transmitter release, and recent evidence suggests that neuronally expressed ion channels of the transient receptor potential (TRP) family function in this critical process. Here, we report the novel finding that the cold and menthol-gated channel TRPM8 is necessary for proper insulin homeostasis. Mice lacking TRPM8 respond normally to a glucose challenge while exhibiting prolonged hypoglycemia in response to insulin. Additionally, Trpm8-/- mice have increased rates of insulin clearance compared with wild-type animals and increased expression of insulin-degrading enzyme in the liver. TRPM8 channels are not expressed in the liver, but TRPM8-expressing sensory afferents innervate the hepatic portal vein, suggesting a TRPM8-mediated neuronal control of liver insulin clearance. These results demonstrate that TRPM8 is a novel regulator of serum insulin and support the role of sensory innervation in metabolic homeostasis.
Keywords: insulin, clearance, TRPM8, neuronal, sensitivity
many TRP family cation channels are essential for the detection of environmental stimuli and their functional role in somatosensation is well established (2). However, these channels are also expressed in primary sensory neurons innervating internal tissues, where the environment changes little, and likely monitor the body's internal environment (30). For example, pancreatic sensory neurons expressing TRPV1, a noxious heat-gated ion channel that is the receptor for capsaicin, the “hot” ingredient in chili peppers, mediate insulin resistance and islet inflammation (24). Furthermore, TRPV1+ neurons innervate the hepatic portal vein (HPV) and are involved in the detection of hypoglycemia (9). Complicating the neuronal role of these channels, recent evidence suggests that many are also expressed in nonneuronal tissues and are involved in other cellular processes related to glucose metabolism. TRPV1 expression has been reported in pancreatic β-cell lines, where it may modulate insulin secretion (1), although it is not clear whether TRPV1 is expressed in native β-cells (30). Similarly, TRPA1, a broad-spectrum irritant receptor (12), is found in native β-cells, and its activation by endogenous ligands induces insulin release via an increase in intracellular calcium (7). Last, several TRPM channels mediate insulin secretion by sensing changes in intracellular second messengers such as Ca2+ and NAD metabolites and are integral in regulation through hormone receptors (30). Thus, TRP ion channels appear to be novel regulators of insulin secretion and pancreatic function, yet, mechanistically, their role in such processes is unclear.
Like TRPV1, TRPM8 is a temperature-gated ion channel activated by cool to cold temperatures and mediates the psychophysical sensation of cold associated with menthol, the active ingredient in mint (18, 19). TRPM8 is the predominant mediator of cold perception in mammals, owing to its robust expression in a subset of peripheral sensory neurons (26). However, TRPM8 has nonsomatosensory functions in tissues such as the bladder, where it has been implicated in the bladder micturition reflex and overactive bladder syndromes (16, 20). TRPM8 was initially identified in prostate, and its expression is androgen dependent and elevated in the initial stages of epithelial prostate malignancies, making the channel a potential marker for prostate cancer (28, 32). Nonetheless, a role for TRPM8 outside of the peripheral nervous system has yet to be established. Here, we report data suggesting that mice with a targeted mutation in the Trpm8 gene (Trpm8-/-) (3, 19) have heightened insulin sensitivity likely due to compensatory mechanisms related to enhanced insulin clearance, results demonstrating a novel role for this channel in insulin homeostasis.
MATERIALS AND METHODS
Breeding scheme.
Trpm8-/- mice are of the C57BL/6 genetic background and were obtained from The Jackson Laboratory. For weight tracking and food intake studies, Trpm8-/- and wild-type (both littermates and age-matched C57/Bl6 mice from Jackson) were used, generated from crosses of heterozygous animals (Trpm8+/-) as described (3, 13–15). All experiments were approved by the University of Southern California (USC) Institutional Animal Care and Use Committee and performed in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Streptozotocin experiments.
All animals used were males aged 8–12 wk of age (C57/BL6 background). Streptozotocin (STZ, 180 mg/kg) was administered by intraperitoneal injection (ip). Body weight and blood glucose were then monitored at varying intervals over a 2-wk period.
DAB labeling for islet size quantification.
Fixed/frozen pancreatic sections (10 μm) were thawed for 10 min and permeabilized in PBST (0.1 M PBS + 0.1% Triton X-100) for 30 min. Sections were pretreated in 1% H2O2 for 30 min, washed three times for 5 min in PBST, and blocked for 1 h in 5% normal goat serum (NGS)-PBST. Sections were then incubated overnight at 4°C in primary antibody solution containing 1:500-diluted guinea pig anti-insulin (AB7842, Abcam) in 1% NGS-PBST, washed as before, and incubated in 1:200-diluted biotinylated rabbit anti-guinea pig secondary antibody (Vectastain ABC kit) in 1% NGS-PBST for 1 h at room temperature. After three washes, slides were incubated in ABC reagent for 1 h at room temperature, washed again as before, and incubated first for 15 min in DAB solution (DAB in PBS) and then for 5 min in DAB solution + 0.003% H2O2. Slides were then washed three more times, coverslipped, and analyzed.
Islet isolation.
After clamping of the duodenum on both sides of the major duodenal papilla, the pancreas was inflated via the common bile duct with 3 ml of collagenase solution (1× HBSS, pH 7.4, 1 mM CaCl2, 1 mg/ml collagenase XI) using a 30½-G needle. The inflated pancreas was then removed and incubated in 2 ml of collagenase solution for 25 min at 37°C with frequent vigorous mixing by hand. Collagenase digestion was stopped by adding 25 ml of ice-cold resuspension solution (1× HBSS, pH 7.4, 1 mM CaCl2), and the cells were pelleted at 500 g for 30 s. The above steps were repeated two times to wash the pellet. The cell suspension was then filtered through a 70-μm nylon filter and washed over with 25 ml of resuspension solution. The nylon filter was then turned upside down, and any islets stuck to the filter surface were washed off using 10 ml of growth medium (RPMI 1640, 20 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% FBS). Islets were then picked by pipette and allowed to recover for 2 h in a 37°C 5% CO2 humidified incubator before use.
Static insulin secretion assay.
Similarly sized islets (∼100–200 μm in diameter) were grouped into sets of 10, added to 1ml of assay solution (10 mM HEPES, pH 7.4, 129 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 2 mM CaCl2, 5 mM NaHCO3, 2.8 mM glucose, 0.1% BSA) and allowed to equilibrate for 30 min in the incubator. Each set of 10 islets was then added to a baseline well of a 24-well plate containing 1 ml of assay solution. After 15 min, the islets were then promptly removed from the baseline well and added to an experimental well containing assay solution with 16.8 mM glucose or 40 mM KCl, depending on the experiment. After 15 min, the islets were removed from the experimental well, and insulin content was quantified using the Insulin (Mouse) Ultrasensitive ELISA kit (ALPCO).
Total pancreatic insulin content.
Each mouse pancreas was incubated in 10 ml of acid-ethanol solution (1.5% HCl in 70% EtOH) overnight at -20°C, homogenized, and then incubated again overnight at -20°C. Extracts were centrifuged at ∼850 g at 4°C for 15 min, and samples were neutralized by adding an equal volume of 1 M Tris (pH7.5) solution. Insulin content was measured using the Insulin (Mouse) Ultrasensitive ELISA kit (ALPCO) and expressed as a function of total protein content found via Bradford assay.
qPCR/RT-PCR.
RNA was isolated from RNAlater ICE-stabilized tissue using the RNeasy minikit with in-column DNAse digestion (Qiagen). The iScript cDNA synthesis kit (Bio-Rad) was used for synthesizing cDNA from purified RNA samples, and qPCR was carried out using Ssofast EvaGreen supermix (Bio-Rad) and a Bio-Rad CFX96 detection system. RT-PCR experiments were carried out on the same cDNA used for qPCR, but a standard Taq polymerase was used in place of Ssofast EvaGreen. The primers used are listed below.
Insulin: FWD 5′-TCAGCAAGCAGGTCATTGTTTC-3′ (212 bp), REV 5′-CTTGTGGGTCCTCCACTTCA-3′; PDX1: FWD 5′-GAAACGTAGTAGCGGGACCC-3′(457 bp), REV 5′-CAGATCTGGCCATTCGCTTG-3′; GLUT2: FWD 5′-GATCACCGGAACCTTGGC-3′ (400 bp), REV 5′-GGGCTCCAGTCAATGAGAGG-3′; TRPM8 (5′): FWD 5′-GCTGCCTGAAGAGGAAATTG-3′(600 bp), REV 5′-GCCCAGATGAAGAGAGCTTG-3′; TRPM8 (3′) (pore): FWD 5′-CTTCCGCTCTGTCATCTATG-3′(127 bp), REV 5′-CACACACAGTGGCTTGGACT-3′; GAPDH: FWD 5′-TGTAGACCATGTAGTGAGGTCA-3′(123 bp), REV 5′-AGGTCGGTGTGAACGGATTTG-3′; α1-anti-trypsin: FWD 5′-CCTCTCCGGAATCACAGAGG-3′(327 bp), REV 5′-GGACTTGCTGTAGCATCAGG-3′.
Glucose tolerance/insulin tolerance tests.
GTT experiments were carried out on acute (5.5-h) or overnight (14- to 16-h) -fasted animals. Following 2 g/kg ip administration of glucose, blood was sampled from the tail vein every 30 min for 2 h, and glucose levels were measured using an AlphaTRAK (Abbot) blood glucose monitor. For serum insulin measurement, ∼20 μl of blood was sampled at each time point and allowed to clot in serum separator tubes (Sarstedt) for 20 min at room temperature. After clotting, serum was immediately isolated by spinning at 10,000 g for 5 min and stored on ice before being frozen at -20°C upon completion of the experiment. Serum insulin and C-peptide were then measured using the Insulin (Mouse) Ultrasensitive ELISA kit and (Mouse) C-peptide ELISA kit per manufacturer's instructions (ALPCO). ITT experiments were carried out on fed or acute-fasted animals. Following 0.75 U ip administration of recombinant Humulin (Lilly), blood glucose was measured as described previously, every 15 min for 1 h and every 30 min for an additional 1 h.
Core temperature monitoring and activity measurements.
Mice were implanted with G2 e-mitters (Mini Mitter, Bend, OR) as described (13) and allowed to recover from surgery for at least 1 wk to ensure the absence of infection and fever. On the day of experiments, animals were acclimated to the experiment room at least 1 h prior to the commencement of monitoring. Mice were placed in mouse cages containing food, water bottles, and bedding, with the cages placed on top of a telemeter receiver. Core temperature and gross motor activity (detected as a change in location by the telemetric receiver) were collected every 10 s for 24–48 h at ambient temperatures of 24°C. Studies began at 6:00 AM when the lights were turned on, and the lights were turned off at 6:00 PM. Data are represented as means ± SE in 10-min blocks, and statistical significance was determined using a Student's t-test.
Western blots and quantification.
Fresh tissue samples were isolated from wild-type and Trpm8-/- mice and immediately homogenized using a Tissue Tearor model 985-370 (Biospec Products) in RIPA buffer containing 0.5% sodium deoxycholate, 1% NP-40, and 0.1% SDS. Samples were then incubated for 1 h with agitation at 4°C to ensure complete lysis. After centrifuging at 18,000g for 20 min to remove cellular debris, supernatants were then flash frozen in liquid nitrogen and stored at -80°C. Total denatured protein (50 μg) was run on a 4%/10% polyacrylamide gel and transferred to a PVDF membrane. Membranes were blocked for 1 h at room temperature in 2.5% BSA and 2.5% normal donkey serum (NDS) in PBST (0.1% Tween 20). Primary antibody incubations were carried out overnight at 4°C at dilutions of 1:1,000 for each antibody in 1% BSA and 1% NDS in PBST. The primary antibodies used were as follows: chicken anti-β-actin (AB13822, Abcam), chicken anti-GAPDH (AB83956, Abcam), rabbit anti-INSR (SC-711, Santa Cruz Biotechnology), an d rabbit anti-IDE (AB32216, Abcam). After four 5-min washes in PBST, secondary antibody incubations were carried out for 30–60 min at room temperature at dilutions of 1:15,000 for each antibody in 1% BSA and 1% NDS in PBST plus 0.02% SDS. The secondary antibodies used were as follows: donkey anti-chicken 680 (926-68028, Li-Cor), and donkey anti-rabbit 800 (926-32213, Li-Cor). After four 5-min washes in PBST, membranes were then imaged using a Li-Cor model 9120 Odyssey imager.
Western band quantification was done using the Gel Analysis tool in ImageJ following standard methods (see http://lukemiller.org/index.php/2010/11/analyzing-gels-and-western-blots-with-image-j/). Band intensities for target proteins were normalized across each membrane and expressed as a percentage of summed intensities for each target. These values were then compared with loading control values from the same membrane to get relative protein expression values. For liver and kidney, β-actin was used as a loading control, and for muscle GAPDH was used.
Immunostaining.
Mice and rats were transcardially perfused with ice-cold 4% paraformaldehyde (PFA) solution in 0.1 M PBS. Tissues were carefully dissected and postfixed for 2 h on ice in 4% PFA and dehydrated in 30% sucrose solution in 0.1 M PBS overnight at 4°C. Pancreas tissue was quickly frozen in OCT on dry ice, sectioned with a cryostat at 10 μm onto Superfrost Plus slides (VWR), and stored at -80°C. HPV tissue was cut on one side to flatten and stored in cryoprotectant solution (30% sucrose, 30% ethylene glycol in 0.1 M PBS) at -20°C.
Cryosections were thawed at room temperature for 10 min, permeabilized in PBST for 30 min, washed three times in PBS for 5 min, and blocked for 1 h at room temperature in PBST + 5% NGS. Primary antibodies were diluted 1:500 in a working solution of PBST + 1% NGS and incubated on the slides overnight at 4°C in a humidified box. The primary antibodies used erre as follows: rabbit anti-GFP (A11122, Invitrogen), chicken anti-GFP (GFP1020, Aves), and guinea pig anti-PGP9.5 (AB5898, Millipore). Slides were washed three times in PBST for 5 min and incubated in secondary antibody solution (1:1,000 secondary antibody, PBST + 1% NGS) for 2 h at room temperature. The secondary antibodies used were fluorescently conjugated Alexa 488 or Alexa 594(Invitrogen). Slides were washed three times in PBST for 10 min and coverslipped with Vectorshield-DAPI (Vector Labs) or Prolong Gold (Invitrogen) mounting medium. Imaging was carried out on a Zeiss Axio Imager M2 with Apotome.
HPV tissue was stained whole mount. HPV tissue was removed from cryoprotectant and washed six times in TBS for 5 min, blocked in TBST + 2% NGS for 2 h at room temperature, and then incubated for 48 h at 4°C with primary antibody diluted 1:1,000 in TBST + 2% NGS. The primary antibodies used were as follows: chicken anti-GFP (GFP1020, Aves), rabbit anti-TRPM8 (COOH terminus). Tissue was washed six times in TBS for 5 min, incubated for 24 h at 4°C with secondary antibody diluted 1:1,000 in TBST + 2% NGS, washed six times again with TBS for 5 min, mounted, coverslipped, and imaged as described.
Statistics.
All statistical analysis was carried out using Student's unpaired t-test, and means are expressed ± SE with P values < 0.05 considered statistically significant.
RESULTS
Streptozotocin sensitivity and decreased body weight and fasting insulin in TRPM8-null mice.
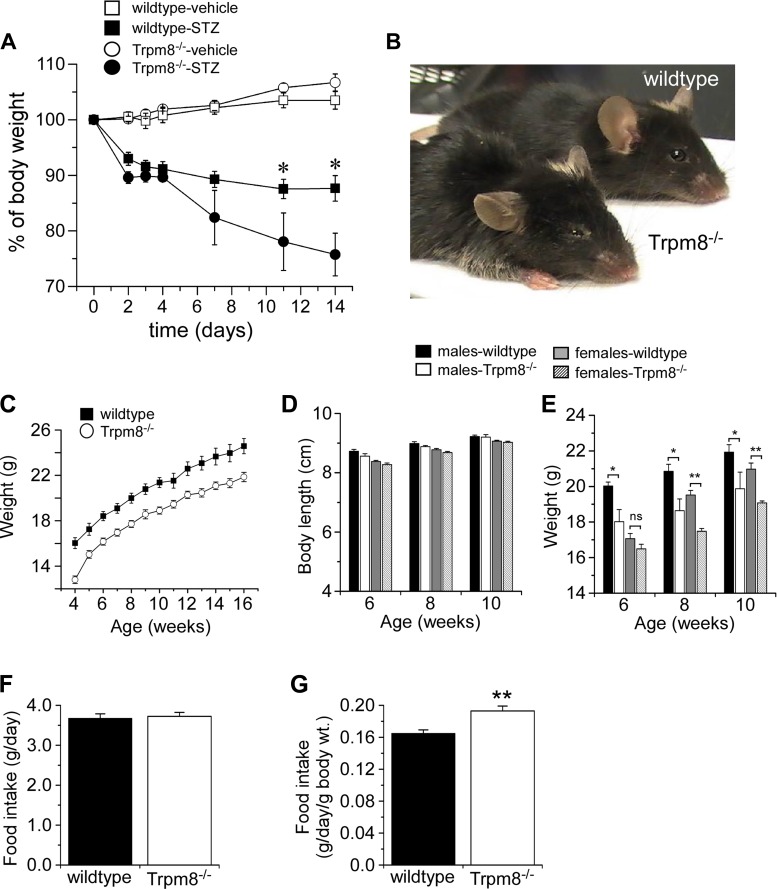
Streptozotocin (STZ), a glucosamine-nitrosourea compound that causes DNA alkylation and cell death, is commonly used to induce type 1 diabetes in rodents (6). The diabetogenicity of STZ comes from its structural similarity to glucose, allowing the compound to be exclusively taken up by insulin-secreting β-cells, resulting in their specific ablation. The ablation of these β-cells leads to a reduction in insulin secretion and the development of a type 1 diabetic/hyperglycemic state. After STZ (180 mg/kg) was administered by ip injection, we observed a rapid decline in body weight of Trpm8-/- mice (4 of 8 mice) compared with STZ-injected control littermates (Fig. 1A), even though these animals were eating equivalent amounts as their wild-type littermates (not shown). These mice were lethargic with matted fur and exhibited some rigidity (Fig. 1B), results suggesting that Trpm8-/- mice are highly sensitive to STZ in a model of type 1 diabetes. Next, we asked if Trpm8-/- mice display obvious metabolic abnormalities. On further examination, untreated Trpm8-/- mice were found to be more than 10% smaller than their wild-type littermates, examined up to 4 mo of age (Fig. 1C). This was not due to a developmental difference in body size, as body length was similar in both male and female wild-type and Trpm8-/- mice (Fig. 1D). Moreover, weights of Trpm8-/- mice of both sexes were significantly smaller than wild-type weights (Fig. 1E) despite their consuming equivalent amounts of food per day (Fig. 1F). However, when food intake was adjusted for body weight, Trpm8-/- mice ate ∼12% more per day than wild types (Fig. 1G).
Fig. 1.
Streptozotocin (STZ) sensitivity in Trpm8-/- mice. A: body weight tracking in wild-type and Trpm8-/- mice injected with 180 mg/kg STZ or vehicle (veh: n = 4–6, STZ: n = 4). B: representative picture of wild-type and Trpm8-/- mice 1 wk following STZ administration. C: weight tracking in male littermates (male and female) from 4–16 wk of age fed normal chow (wt: 24.1 ± 1.0; Trpm8-/-: 21.1 ± 0.3 g by 12 wk, P < 0.001, n = 6 each genotype). D: body length measurements of Trpm8-/- and wild-type mice of both sexes. E: weight tracking in mice from D; P > 0.05 NS, *P < 0.05, **P < 0.01, n = 6–8 mice. Food intake [wt: 3.68 ± 0.11; Trpm8-/-: 3.72 ± 0.09 g/day, P = 0.75 (F); wt: 0.17 ± 0.004, Trpm8-/-: 0.19 ± 0.01 g/day/g body wt, P < 0.01, n = 11–12 (G)].
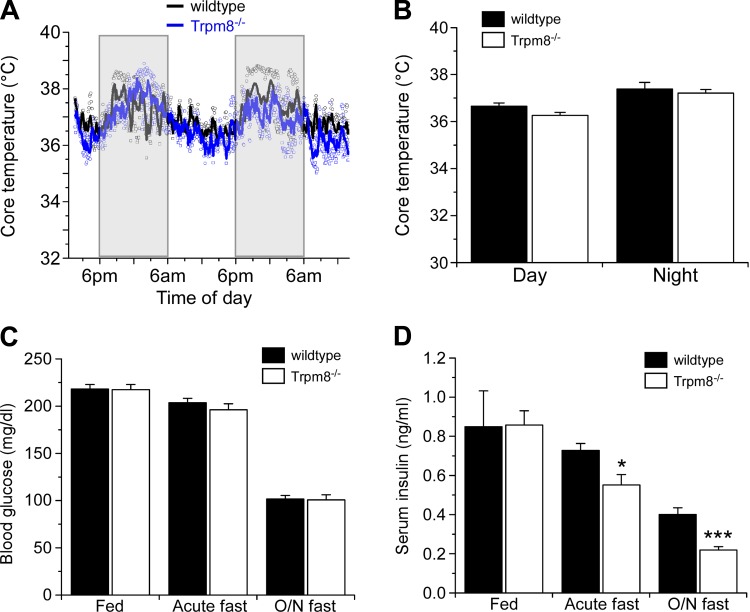
TRPM8 is a cold-gated ion channel that is known to be involved in hypothermia (13). Therefore, we asked whether Trpm8-/- mice had distinct differences in thermoregulation under controlled, nonstimulating environments. Wild-type and Trpm8-/- littermates were monitored for diurnal changes in core body temperatures with implantable internal telemetric monitors. Over a 48-h period, we observed no differences between the genotypes in core body temperature (Fig. 2A), nor were they statistically different (P > 0.05) between day and night temperatures (Fig. 2B). Next, we examined resting blood glucose and serum insulin levels, finding the former comparable in wild-type and Trpm8-/- mice under fed and fasting conditions (Fig. 2C). Similarly, serum insulin levels were equivalent in both genotypes under fed conditions but were significantly lower in Trpm8-/- mice after either acute (5.5 h) or overnight fasts (Fig. 2D). After an acute fast, serum insulin was 0.73 ± 0.4 and 0.55 ± 0.05 mg/dl (P < 0.05) in wild-type and Trpm8-/- mice, respectively, and 0.40 ± 0.03 and 0.22 ± 0.02 mg/dl (P < 0.001) after an overnight fast in wild-type and Trpm8-/- mice, respectively (Fig. 2D). Thus, in addition to differences in body weight and food intake, Trpm8-/- mice are deficient in insulin homeostasis when food restricted.
Fig. 2.
Metabolic characterization of Trpm8-/- mice. A: telemetric monitoring of core body temperature in wild-type and Trpm8-/- mice. Scatter plot and averaged date are shown over a 48-h period (n = 2). B: mean core body temperature during light (day: 6 AM-6 PM) and dark cycles (night: 6 PM-6 AM) for wild-type and Trpm8-/- mice (n = 4). Blood glucose (C) and serum insulin (D) levels under fed and fasting conditions (n = 7–16). Values are expressed as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 by Student's unpaired t-test.
Normal morphology and function in pancreatic β-cells in Trpm8-/- mice.
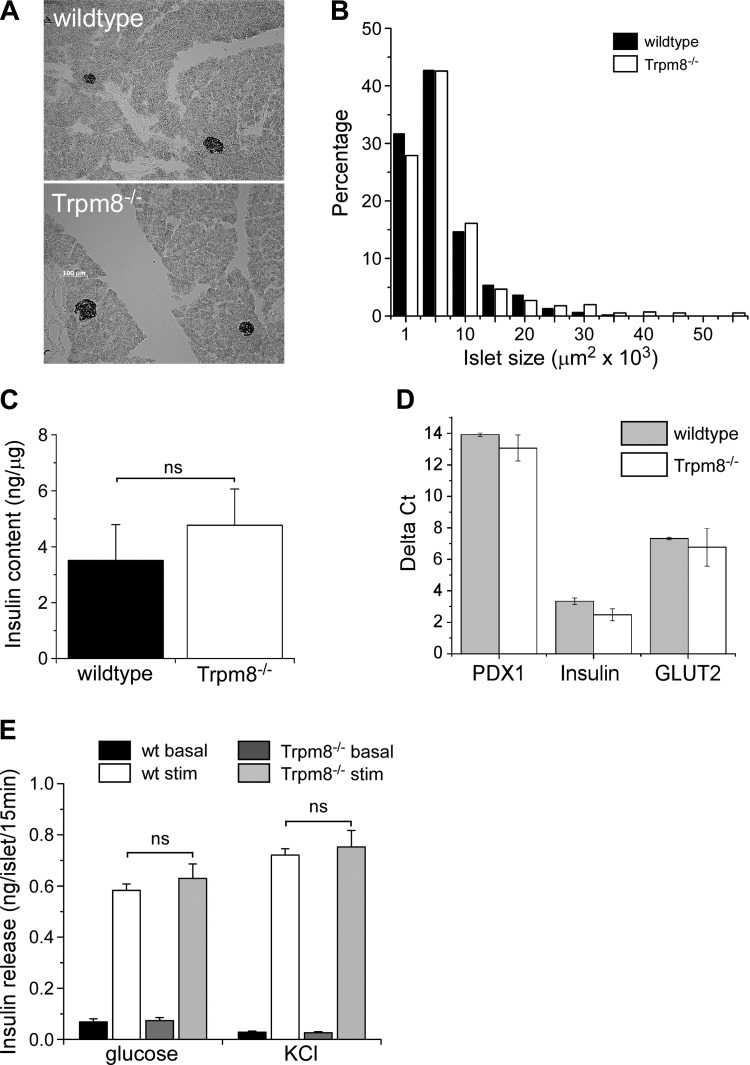
Since serum insulin levels were reduced in fasted animals, we determined whether this phenotype was due to altered pancreatic β-cell physiology and function. Gross pancreas morphology, based on islet shape and size distribution (Fig. 3, A and B), was normal in Trpm8-/- mice, as was total pancreas insulin content (Fig. 3C). Additionally, there was no difference in expression of common β-cell-specific transcripts, measured by qPCR, between wild-type and Trpm8-/- mice (Fig. 3D). To determine whether the decrease in serum insulin levels observed in Trpm8-/- mice was a result of a deficiency in insulin release mechanisms, we performed in vitro analyses of stimulus-evoked insulin release from isolated pancreatic islets. Isolated islets, stimulated with 16.8 mM glucose or depolarized with 40 mM KCl, released equivalent amounts of insulin from islets isolated from both wild-type and Trpm8-/- mice (P = 0.4–0.8; Fig. 3E).
Fig. 3.
Pancreatic β-cell function in Trpm8-/- mice. A: representative immunostaining for insulin in mouse pancreatic islets. B: distribution of pancreatic islet sizes in Trpm8-/- compared with wild-type mice (n = 2,076 islets measured from 3 mice of each genotype). C: whole pancreatic insulin content expressed as ng insulin/μg total protein (wt: 3.5 ± 1.3ng/μg, Trpm8-/-: 4.8 ± 1.3 ng/μg protein, P = 0.51, n = 3). D: gene expression analysis of common β-cell-specific transcripts in isolated pancreatic islets by qPCR. Values expressed as ΔCT relative to GAPDH expression (n = 3). E: insulin secretion stimulated by 16.8 mM glucose (wt: 0.58 ± 0.02, Trpm8-/-: 0.63 ± 0.06 ng/islet/15 min), or 40 mM KCl (wt: 0.72 ± 0.03, Trpm8-/-: 0.75 ± 0.06 ng/islet/15 min, P > 0.05, n = 3) from isolated islets (baseline: 2.8 mM glucose). All experiments were carried out on adult male mice aged 8–12 wk old unless otherwise noted. Values are expressed as means ± SE; P > 0.05 NS by Student's unpaired t-test.
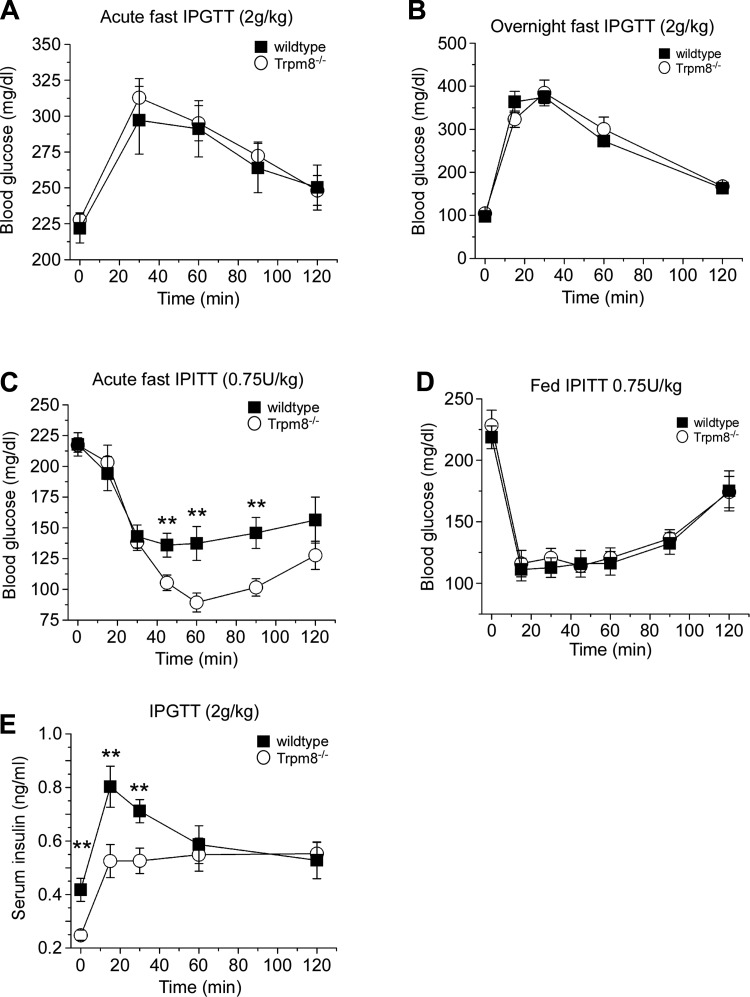
Trpm8-/- mice show prolonged hypoglycemia in response to insulin.
The above results prompted us to examine the response of Trpm8-/- mice to metabolic challenges to determine whether the STZ, weight, food intake, and fasted serum insulin phenotypes we observe in these animals are a result of altered metabolism of glucose or insulin. To test insulin function in vivo, we utilized both the glucose (GTT) and insulin tolerance tests (ITT), induced by bolus (ip) injections of either substance under fed and fasting conditions. Consistent with normal basal glucose levels observed in these animals when both fed and fasted (Fig. 2C), we found that Trpm8-/- mice responded similarly to wild-type mice to an injection of glucose (2 g/kg) after acute and overnight fasts (Fig. 4, A and B). However, when given a bolus ip injection of insulin (0.75 U/kg) after an acute fast, Trpm8-/- mice had a prolonged hypoglycemia with blood glucose levels dropping significantly to 137.3 ± 13.8 and 89.3 ± 7 mg/dl for wild-type and Trpm8-/- mice, respectively, by 60 min postinjection (P < 0.01; Fig. 4C). These results are consistent with the lowered basal insulin levels observed in fasted mice (Fig. 2D). Of note, fed Trpm8-/- mice responded similarly to wild types (Fig. 4D), in line with their normal serum insulin levels when fed (Fig. 2D). To test the regulation of insulin secretion in Trpm8-/- mice, we monitored serum insulin levels following a glucose challenge in mice fasted overnight. After a 2.0 g/kg glucose injection, spiking serum insulin levels were significantly lower in Trpm8-/- mice than in wild-type littermates, peaking at 15 min postinjection to 0.53 ± 0.06 and 0.80 ± 0.08 ng/ml, respectively (P < 0.01; Fig. 4E). Interestingly, although these and starting serum insulin levels in Trpm8-/- mice were reduced, they recovered to values similar to those in wild-type mice by 60 min after the injection.
Fig. 4.
Trpm8-/- mice exhibit heightened insulin sensitivity in vivo. Intraperitoneal glucose tolerance test (IPGTT) performed on acute- (5.5 h; A) and overnight-fasted (14–16 h; B) mice by injecting 2.0 g/kg body wt ip glucose (n = 14–16 for acute and n = 18–19 for overnight). C: blood glucose concentrations in an insulin tolerance (IPITT) test performed on acute-fasted mice by injecting 0.75 U/kg body wt ip insulin (n = 17–18). D: insulin tolerance test performed on fed mice by injecting 0.7 5 U/kg insulin ip (n = 5–6). E: serum insulin (basal: wt: 0.42 ± 0.04, Trpm8-/-: 0.24 ± 0.01 ng/ml, P < 0.01; at 60 min: wt: 0.59 ± 0.07, Trpm8-/-: 0.55 ± 0.06 ng/ml, P = 0.68, n = 14–17) following 2.0 g/kg body wt ip glucose to overnight-fasted mice. Values are expressed as means ± SE. **P < 0.01 by Student's unpaired t-test.
Enhanced insulin clearance in Trpm8-/- mice.
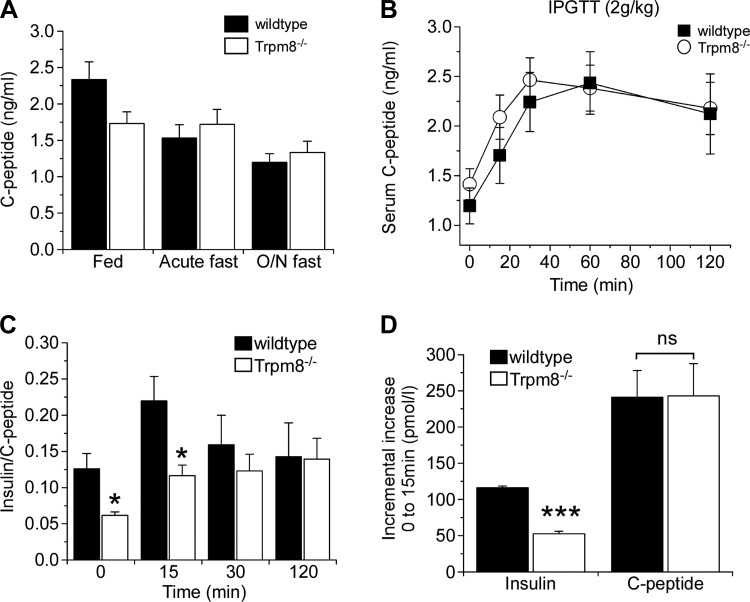
As Trpm8-/- mice have normal insulin content and β-cell function in vitro (Fig. 3, C and E), and reduced spiking serum insulin levels following glucose challenge in vivo, we examined insulin clearance in these animals. To test this, we examined the content and release of C-peptide in Trpm8-/- mice (17, 23). The mature form of insulin results from the cleavage of proinsulin into insulin and C-peptide in the secretory granules of the β-cell. Since the content of C-peptide and insulin is 1:1 as a result of this process, when the secretory granules of the β-cell release their contents into the blood upon stimulation, they release equimolar concentrations of insulin and C-peptide. Due to the half-life of C-peptide being 10-fold longer than that of insulin in the bloodstream, serum C-peptide levels are an accurate measure of insulin secretion following glucose challenge. The ratio of C-peptide to insulin following such a challenge is often used as a measure of insulin clearance as C-peptide clearance rates remain constant whereas insulin clearance rates can vary (17, 23). Resting levels of C-peptide in fed as well as acute- and overnight-fasted mice were similar in both genotypes (Fig. 5A), unlike the significant decrease in serum insulin under similar fasting conditions (Fig. 2D). Moreover, distinct from the significant reduction in the spike in serum insulin after a glucose challenge in Trpm8-/- vs. wild-type animals (Fig. 4E), there was no difference in C-peptide release in the IPGTT assay (Fig. 5B). As serum insulin concentration is the net of the rates of release vs. clearance, we measured the molar insulin-to-C-peptide ratio, a measure of insulin clearance, after glucose challenge, observing significantly lowered values in Trpm8-/- mice at both baseline and 15 min post-glucose challenge (P < 0.05; Fig. 5C). Last, in a further measurement of clearance, we compared the incremental increase in both peptides at 15 min following glucose challenge, finding no differences in the molar amount of C-peptide (P = 0.97), but a significantly decreased level of insulin in Trpm8-/- mice (P < 0.001; Fig. 5D), evidence demonstrating enhanced insulin clearance in these animals.
Fig. 5.
Enhanced insulin clearance in Trpm8-/- mice. A: resting serum C-peptide levels in fed and fasted mice (n = 7–10). B: serum C-peptide following 2.0 g/kg body wt ip glucose to overnight-fasted mice (n = 6). C: serum insulin-to-C-peptide ratios during different intervals following glucose injection. D: incremental increase of insulin (wt: 116.1 ± 2.6, Trpm8-/-: 52.4 ± 3.5 pmol/l) compared with C-peptide (wt: 249.1 ± 38.2, Trpm8-/-: 251.1 ± 46.0 pmol/l) from 0–15 min following glucose challenge. Values are expressed as means ± SE. *P < 0.05, ***P < 0.001, NS P > 0.05 by Student's unpaired t-test.
TRPM8-expressing afferent fibers innervate the hepatic portal vein.
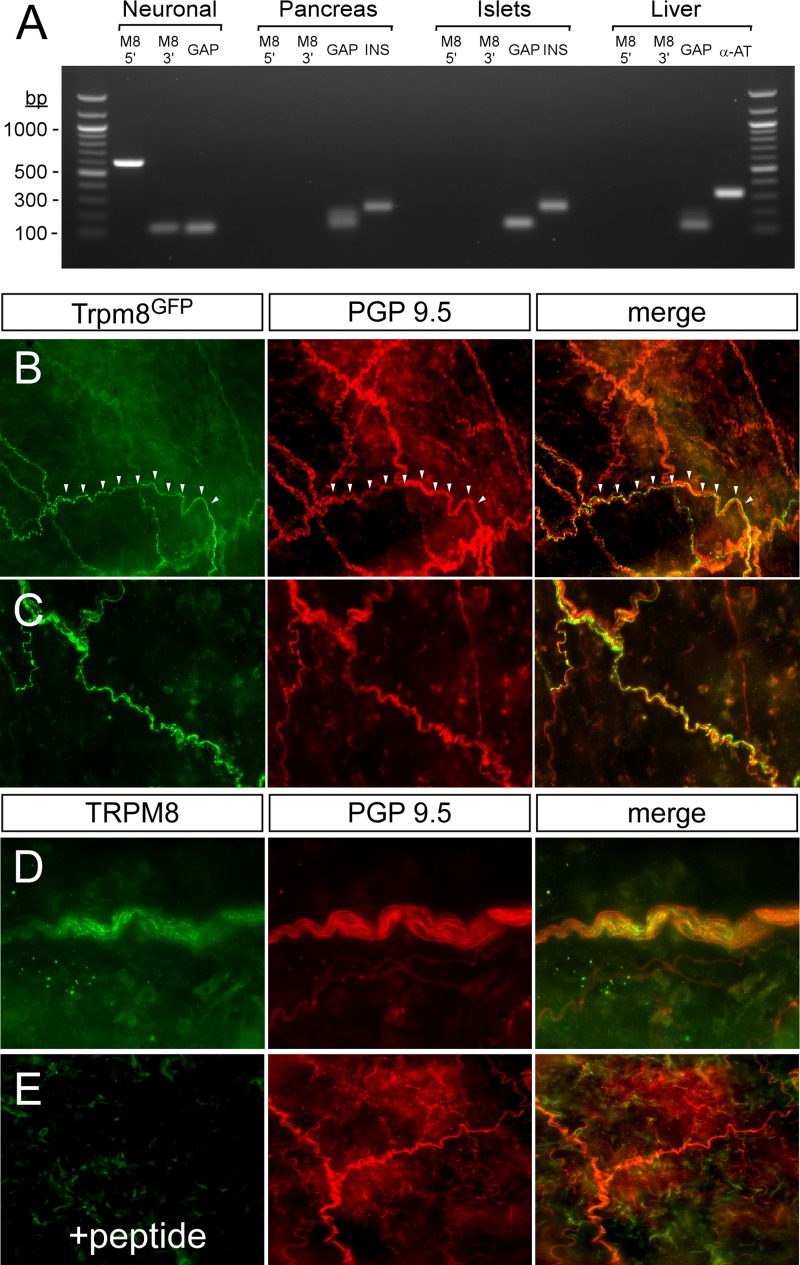
Pancreatic expression of TRPM8 has not been reported (28), and consistent with these data we did not detect TRPM8 transcripts in this tissue by RT-PCR (Fig. 6A). The liver is the primary organ involved in insulin clearance, responsible for removing over 50% of blood insulin after one pass through the hepatic network (31). As in the pancreas, Trpm8 transcripts were undetectable in the liver (Fig. 6A), results consistent with previous expression analysis in nonneuronal tissues (28). However, using a TRPM8 reporter line (26), we do find TRPM8-expressing afferent fibers innervating the HPV (Fig. 6, B and C). This is of note, as it is well established that afferent innervation of the HPV is important for proper glucose/insulin homeostasis (9). As TRPM8 antibodies are not reliable in mouse tissue staining, we confirmed that TRPM8 was being expressed in these axonal projections in the HPV by immunolabeling rat HPV tissue directly with an antibody raised against the COOH terminus of TRPM8 (Fig. 6D). Consistent with mouse reporter expression, we found TRPM8 immunolabeling in the rat HPV, immunoreactivity that was absent when the antibody was preincubated with the antigen peptide (Fig. 6E). These results confirm TRPM8 expression in neurons innervating the HPV, results consistent with previous reports showing TRPV1 expression in this tissue (30).
Fig. 6.
Innervation of TRPM8-expressing afferent fibers in hepatic portal vein (HPV). A: RT-PCR from cDNA from trigeminal/dorsal root ganglia, whole pancreas, isolated islets, or liver. Two primer sets were used to amplify both 5′- (M8 5′) and 3′- (M8 3′) regions of TRPM8. GAPDH (GAP) was used as a universal control for sample integrity, and insulin (INS) and α1-anti-trypsin (α-AT) were used as tissue-specific controls for pancreas and liver, respectively. All experiments were carried out on male mice age 8–12 wk. Values are expressed as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 by Student's unpaired t-test. B and C: Whole-mount staining shows TRPM8-expressing afferents (green) in the HPV of Trpm8GFP reporter mice vs. all afferent fibers labeled with the pan-neuronal marker PGP9.5 (red). Arrowheads demarcate axons, and all experiments were carried out on male mice aged 8–12 wk. D: immunoreactivity to TRPM8 (green) and PGP9.5 (red) from whole-mount rat HPV. E: TRPM8 immunoreacitivity was absent when tissue was probed with TRPM8 antibodies pretreated with the antigenic peptide.
Increased insulin-degrading enzyme expression in Trpm8-/- liver.
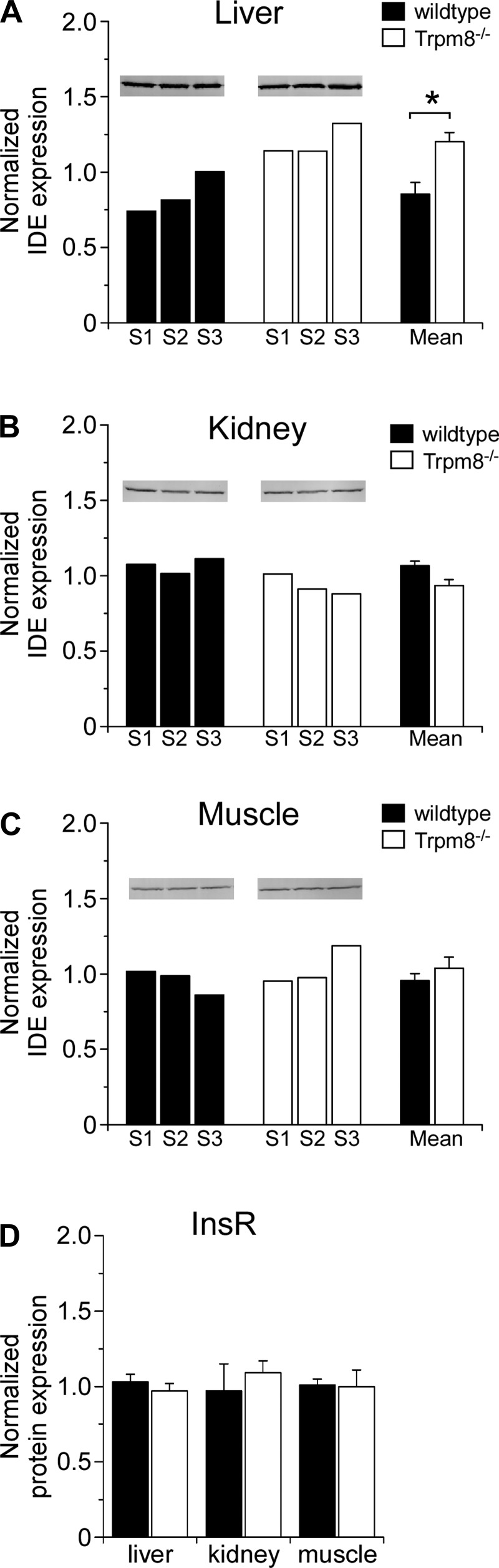
As Trpm8-/- mice clear insulin at a faster rate than wild-type mice, and TRPM8 afferent innervation was found in the HPV, we next determined whether liver function was altered in the absence of TRPM8 channels. Insulin clearance occurs in two main phases: 1) receptor binding and internalization and 2) degradation. As the insulin receptor (InsR) is required for the first phase of this process and insulin-degrading enzyme (IDE) is required for the second phase, we looked at expression levels of these proteins in relevant tissues regarding insulin clearance, the liver, kidney, and muscle. By quantitative Western blot analysis we observed that IDE expression levels in liver were significantly increased in Trpm8-/- mice (Fig. 7A) but unchanged in both kidney (Fig. 7B) and muscle (Fig. 7C), results consistent with the enhanced insulin clearance phenotype. In contrast, InsR was found to be expressed at statistically similar levels in all three tissues of wild-type and Trpm8-/- animals (Fig. 7D).
Fig. 7.
Insulin-degrading enzyme expression is increased in Trpm8-/- mouse livers. Semiquantitative Western blotting on whole tissue lysates isolated from overnight-fasted wild-type and Trpm8-/- mice. A–C: insulin-degrading enzyme (IDE) protein expression is quantified in liver (A), kidney (B), and muscle (C). Expression levels are shown by animal (left) and means ± SE (right) for each genotype relative to β-actin (liver and kidney) or GAPDH (muscle). D: insulin receptor (InsR) expression levels in the 3 aforementioned tissues expressed as means ± SE (n = 3). *P < 0.05, by Student's unpaired t-test.
DISCUSSION
Our studies show that TRPM8 plays a role in insulin homeostasis by influencing the regulation of insulin clearance and suggest a change in insulin sensitivity that may result from lower insulin levels under nonfed conditions. Our data show that TRPM8 is most likely not mediating these changes in insulin response via pancreatic β- or liver cell function directly, but support the growing evidence that sensory neuron innervation is critical for metabolic homeostasis (11, 30). Expression levels of IDE, the enzyme required for the degradation of insulin, were found to be higher in the Trpm8-/- liver relative to wild-type liver. Taken together with data showing TRPM8-expressing afferents innervating the HPV, these results suggest that TRPM8-expressing afferents regulate liver function in regard to insulin clearance. It remains to be determined whether this difference in insulin clearance occurs as a direct or indirect result of the loss of TRPM8 function, and future studies are warranted to determine the mechanistic function of TRPM8 in this regard. These results add to the newly appreciated role of TRP channels and their afferents in regulating the internal milieu in addition to their robust function in monitoring changes in the external environment (30).
Numerous nonselective cation channels of the TRP family have been implicated in the regulation of insulin. Their involvement has been shown at the cellular level in β-cells, as well as at the neuronal level via afferent innervation of the liver and pancreas (10, 11, 30). TRP channels of the TRPC (C1–6), TRPM (M3–5), TRPV (TRPV1–2, -V4), and TRPA (A1) subfamilies are reportedly expressed in mammalian pancreatic β-cells (7, 11). Although their roles in this context are not completely understood, evidence suggests they function in establishing excitability that is important for the switching of the β-cell from a basal to a stimulated state. For example, analyses of mice lacking functional TRPM2 and TRPM5 channels have shown that, when either gene is eliminated, glucose-induced insulin secretion is significantly reduced or abolished, solidifying an active role of TRP channels in insulin secretion (5, 8, 29).
Aside from the direct influence that various TRP channels have on β-cell function, and relevant to our own studies regarding TRPM8, TRPV1 has also been shown to be expressed in neurons innervating the pancreas, suggesting an indirect role in the regulation of insulin and glucose homeostasis. (24, 27). The pancreas is heavily innervated by both afferent and efferent neurons of both extrinsic and intrinsic origin, and as a result of this complexity our understanding of the pancreatic neural network is somewhat limited (21). TRPV1 afferents release calcitonin gene-related peptide (CGRP) and Substance P in response to robust afferent stimulation, and this release is involved in inflammatory responses and vasodilation (4). Recent studies have found that TRPV1-expressing afferents innervating the pancreas regulate insulin secretion and β-cell physiology through the release of Substance P, suggesting a negative feedback model in which insulin and Substance P are maintained at levels to preserve proper β-cell function (24). In agreement with this study, treatment with a TRPV1 antagonist has been shown to increase insulin secretion and sensitization in diabetic ob/ob mice and is believed to mediate this through suppressing TRPV1-mediated neuropeptide secretion (27). Given that ∼40% of TRPM8-expressing sensory neurons have been shown to colocalize with TRPV1, and TRPM8-expessing afferents were also found to innervate the pancreas (data not shown), it is plausible that the phenotype observed here could somehow be correlated with these pancreatic afferents, although with Trpm8-/- mice having no observable differences in pancreatic function, this is unlikely (26).
The influence of neural projections in the liver are perhaps more intriguing in this regard. TRPV1/CGRP-expressing afferents innervating the HPV have been shown to be required for the proper detection of hypoglycemic conditions (9). The elimination of TRPV1+ HPV neurons significantly suppresses the sympathoadrenal response to hypoglycemia, highlighting the importance of these neurons in glucose sensing (9). Furthermore, TRPV1-mediated regulation of liver-related paraventricular nucleus (PVN) neurons has also been shown, suggesting a direct influence of TRPV1 in the regulation of hepatic glucose production (10). Interestingly, TRPV1-mediated excitation of liver-related PVN neurons was found to be reduced in a mouse model of type 1 diabetes, and this activity was restored by insulin administration in a phosphatidylinositol 3-kinase/protein kinase C-dependent manner, illustrating the ability of insulin to control TRPV1 activity (10). This phenotype is reminiscent of human subjects who have undergone liver transplant and highlights the influence of hepatic innervation on insulin clearance. All hepatic neuronal connections are eliminated as a result of this transplant surgery, and two independent studies found that transplant patients cleared insulin at a faster rate than control patients in a manner that was independent of immunosuppressive treatment (22, 25). Thus, TRPM8-mediated neuronal signals may provide negative regulation of insulin clearance via hepatic neural innervations influencing local IDE expression levels. In such a scenario, hepatic insulin clearance is heightened due to disinhibition of this circuit in the absence of TRPM8 channels.
In summary, our studies suggest that TRPM8 plays an important role in insulin homeostasis by the regulation of insulin clearance. As the liver is essential for proper hypoglycemic detection and clearance of insulin, the presence of TRPM8 afferents in the HPV is intriguing. Given that TRP channels have been shown to be important in the regulation of insulin on both β-cell and neuronal levels, future studies are warranted in an effort to understand the mechanistic role of TRPM8 in insulin clearance. Understanding these pathways could shed new light on how insulin is regulated in the body, with the potential to yield new therapeutic targets for the treatment and prevention of diabetes and other metabolic disorders.
GRANTS
This work was supported by National Institutes of Health Grant NS-054069 to D. D. McKemy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.D. McCoy, A.G.W., C.M.D., and D.D. McKemy conception and design of research; D.D. McCoy, L.Z., and A.-K.N. performed experiments; D.D. McCoy, C.M.D., and D.D. McKemy analyzed data; D.D. McCoy, A.G.W., and D.D. McKemy interpreted results of experiments; D.D. McCoy and D.D. McKemy prepared figures; D.D. McCoy and D.D. McKemy drafted manuscript; A.G.W., C.M.D., and D.D. McKemy edited and revised manuscript; D.D. McKemy approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Robert Chow for assistance with islet preparation and members of the McKemy lab for support during this work.
REFERENCES
- 1. Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun 321: 219–225, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 139: 267–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol 9: 9–14, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Brixel LR, Monteilh-Zoller MK, Ingenbrandt CS, Fleig A, Penner R, Enklaar T, Zabel BU, Prawitt D. TRPM5 regulates glucose-stimulated insulin secretion. Pflügers Arch 460: 69–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brosky G, Logothetopoulos J. Streptozotocin diabetes in the mouse and guinea pig. Diabetes 18: 606–611, 1969 [DOI] [PubMed] [Google Scholar]
- 7. Cao DS, Zhong L, Hsieh TH, Abooj M, Bishnoi M, Hughes L, Premkumar LS. Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PLos One 7: e38005, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, Kokrashvili Z, Gilon P, Nilius B, Schuit FC, Vennekens R. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5-/- mice. Proc Natl Acad Sci USA 107: 5208–5213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujita S, Bohland M, Sanchez-Watts G, Watts AG, Donovan CM. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol Endocrinol Metab 293: E96–E101, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Gao H, Miyata K, Bhaskaran MD, Derbenev AV, Zsombok A. Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes 61: 1381–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Islam MS. TRP channels of islets. Adv Exp Med Biol 704: 811–830, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological Blockade of TRPM8 Ion Channels Alters Cold and Cold Pain Responses in Mice. PLos One 6: e25894, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knowlton WM, Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150: 340–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A Sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci 33: 2837–2848, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lashinger ES, Steiginga MS, Hieble JP, Leon LA, Gardner SD, Nagilla R, Davenport EA, Hoffman BE, Laping NJ, Su X. AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol 295: F803–F810, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Lebowitz MR, Blumenthal SA. The molar ratio of insulin to C-peptide. An aid to the diagnosis of hypoglycemia due to surreptitious (or inadvertent) insulin administration. Arch Intern Med 153: 650–655, 1993 [PubMed] [Google Scholar]
- 18. McCoy DD, Knowlton WM, McKemy DD. Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am J Physiol Regul Integr Comp Physiol 300: R1278–R1287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58., 2002 [DOI] [PubMed] [Google Scholar]
- 20. Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, Benham CD, Bountra C, Agarwal SK, Anand P. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol 6: 6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niebergall-Roth E, Singer MV. Central and peripheral neural control of pancreatic exocrine secretion. J Physiol Pharmacol 52: 523–538, 2001 [PubMed] [Google Scholar]
- 22. Perseghin G, Regalia E, Battezzati A, Vergani S, Pulvirenti A, Terruzzi I, Baratti D, Bozzetti F, Mazzaferro V, Luzi L. Regulation of glucose homeostasis in humans with denervated livers. J Clin Invest 100: 931–941, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes 33: 486–494, 1984 [DOI] [PubMed] [Google Scholar]
- 24. Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 127: 1123–1135, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Schneiter P, Gillet M, Chiolero R, Jequier E, Tappy L. Hepatic nonoxidative disposal of an oral glucose meal in patients with liver cirrhosis. Metab Clin Exper 48: 1260–1266, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci 27: 14147–14157, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka H, Shimaya A, Kiso T, Kuramochi T, Shimokawa T, Shibasaki M. Enhanced insulin secretion and sensitization in diabetic mice on chronic treatment with a transient receptor potential vanilloid 1 antagonist. Life Sci 88: 559–563, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 61: 3760–3769, 2001 [PubMed] [Google Scholar]
- 29. Uchida K, Dezaki K, Damdindorj B, Inada H, Shiuchi T, Mori Y, Yada T, Minokoshi Y, Tominaga M. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes 60: 119–126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Uchida K, Tominaga M. The role of thermosensitive TRP (transient receptor potential) channels in insulin secretion. Endocr J 58: 1021–1028, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Valera Mora ME, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr 22: 487–493, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer 13: 27–38, 2006 [DOI] [PubMed] [Google Scholar]