Abstract
To investigate mechanisms of extrathyroidal thyroid hormone metabolism, conversion of thyroxine (T4) to 3,5,3′-triiodothyronine (T3) and degradation of 3,3′,5′-triiodothyronine (rT3) were studied in rat liver homogenates. Both reactions were enzymatic. For conversion of T4 to T3, the Km of T4 was 7.7 μM, and the Vmax was 0.13 pmol T3/min per mg protein. For rT3 degradation, the Km of rT3 was 7.5 nM, and the Vmax was 0.36 pmol rT3/min per mg protein. Production of rT3 or degradation of T4 or T3 was not detected under the conditions employed. rT3 was a potent competitive inhibitor of T4 to T3 conversion with a Ki of 4.5 nM; 3,3′-diiodothyronine was a less potent inhibitor of this reaction. T4 was a competitive inhibitor of rT3 degradation with a Ki of 10.2 μM. Agents which inhibited both reactions included propylthiouracil, which appeared to be an allosteric inhibitor, 2,4-dinitrophenol, and iopanoic acid. Sodium diatrizoate had a weak inhibitory effect. No inhibition was found with α-methylparatyrosine, Fe+2, Fe+3, reduced glutathione, β-hydroxybutyrate, or oleic acid.
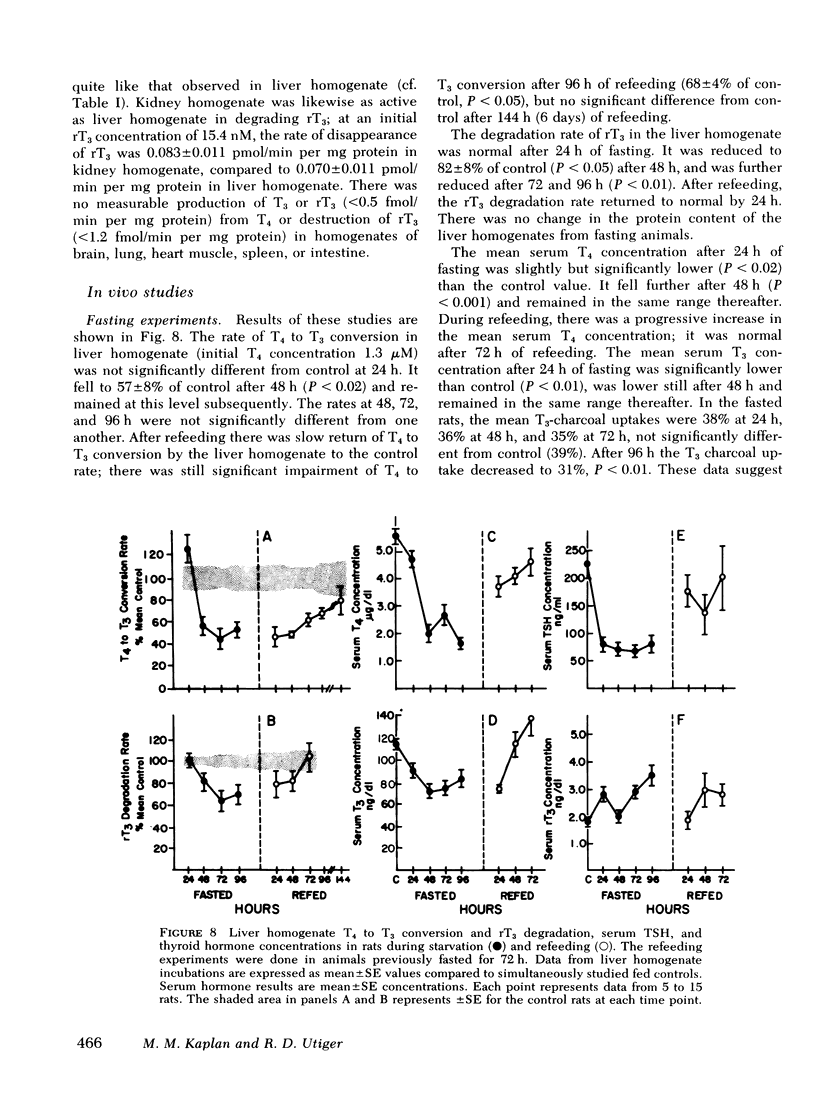
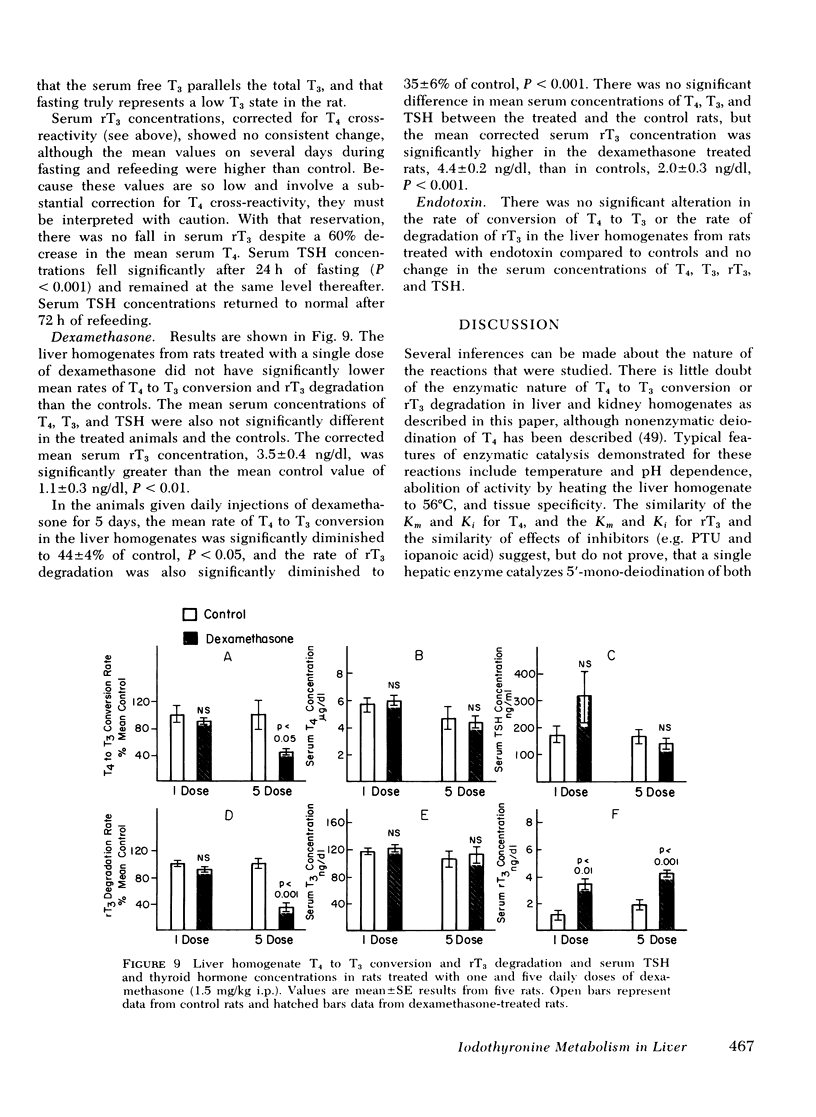
Fasting resulted in inhibition of T4 to T3 conversion and of rT3 degradation by rat liver homogenates which was reversible after refeeding. Serum T4, T3, and thyrotropin concentrations fell during fasting, with no decrease in serum protein binding as assessed by a T3-charcoal uptake. There was no consistent change in serum rT3 concentrations. Dexamethasone had no effect in vitro. In vivo dexamethasone administration resulted in elevated serum rT3 concentrations after 1 day, and after 5 days, in inhibition of T4 to T3 conversion and rT3 degradation without altering serum T4, T3, or thyrotropin concentrations. Endotoxin treatment had no effect of iodothyronine metabolism in liver homogenates. In kidney homogenates the reaction rates and response to propylthiouracil in vitro were similar to those in liver. No significant T4 to T3 conversion or rT3 production or degradation could be detected in other tissues.
These data suggest that one iodothyronine 5′-deiodinase is responsible for both T4 to T3 conversion and rT3 degradation in liver and, perhaps, in kidney. Alterations in serum T3 and rT3 concentrations induced by drugs and disease states may result from decreases in both T3 production and rT3 degradation consequent to inhibition of a single reaction in the pathways of iodothyronine metabolism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A., KEATING F. R., Jr The role of the gastrointestinal tract, including the liver, in the metabolism of radiothyroxine. Endocrinology. 1952 Nov;51(5):427–443. doi: 10.1210/endo-51-5-427. [DOI] [PubMed] [Google Scholar]
- ALBRIGHT E. C., LARSON F. C. Metabolism of L-thyroxine by human tissue slices. J Clin Invest. 1959 Nov;38:1899–1903. doi: 10.1172/JCI103967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez F., Surks M. I., Oppenheimer J. H. High incidence of decreased serum triiodothyronine concentration in patients with nonthyroidal disease. J Clin Endocrinol Metab. 1975 Jul;41(1):27–40. doi: 10.1210/jcem-41-1-27. [DOI] [PubMed] [Google Scholar]
- Burger A., Dinichert D., Nicod P., Jenny M., Lemarchand-Béraud T., Vallotton M. B. Effect of amiodarone on serum triiodothyronine, reverse triiodothyronine, thyroxin, and thyrotropin. A drug influencing peripheral metabolism of thyroid hormones. J Clin Invest. 1976 Aug;58(2):255–259. doi: 10.1172/JCI108466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A., Nicod P., Suter P., Vallotton M. B., Vagenakis P., Braverman L. Reduced active thyroid hormone levels in acute illness. Lancet. 1976 Mar 27;1(7961):653–655. doi: 10.1016/s0140-6736(76)92774-4. [DOI] [PubMed] [Google Scholar]
- Bürgi H., Wimpfheimer C., Burger A., Zaunbauer W., Rösler H., Lemarchand-Béraud T. Changes of circulating thyroxine, triiodothyronine and reverse triiodothyronine after radiographic contrast agents. J Clin Endocrinol Metab. 1976 Dec;43(6):1203–1210. doi: 10.1210/jcem-43-6-1203. [DOI] [PubMed] [Google Scholar]
- Campbell G. A., Kurcz M., Marshall S., Meites J. Effects of starvation in rats on serum levels of follicle stimulating hormone, luteinizing hormone, thyrotropin, growth hormone and prolactin; response to LH-releasing hormone and thyrotropin-releasing hormone. Endocrinology. 1977 Feb;100(2):580–587. doi: 10.1210/endo-100-2-580. [DOI] [PubMed] [Google Scholar]
- Carter J. N., Eastman C. J., Corcoran J. M., Lazarus L. Effect of severe, chronic illness on thyroid function. Lancet. 1974 Oct 26;2(7887):971–974. doi: 10.1016/s0140-6736(74)92070-4. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A radioimmunoassay for measurement of thyroxine in unextracted serum. J Clin Endocrinol Metab. 1972 Jun;34(6):938–947. doi: 10.1210/jcem-34-6-938. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. An assessment of daily production and significance of thyroidal secretion of 3, 3', 5'-triiodothyronine (reverse T3) in man. J Clin Invest. 1976 Jul;58(1):32–40. doi: 10.1172/JCI108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J., Chopra U., Smith S. R., Reza M., Solomon D. H. Reciprocal changes in serum concentrations of 3,3',5-triiodothyronine (T3) in systemic illnesses. J Clin Endocrinol Metab. 1975 Dec;41(06):1043–1049. doi: 10.1210/jcem-41-6-1043. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Sack J., Fisher D. A. 3,3',5'-Triiodothyronine (reverse T3) and 3,3',5-triiodothyronine (T3) in fetal and adult sheep: studies of metabolic clearance rates, production rates, serum binding, and thyroidal content relative to thyroxine. Endocrinology. 1975 Nov;97(5):1080–1088. doi: 10.1210/endo-97-5-1080. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Sack J., Fisher D. A. Circulating 3,3', 5'-triiodothyronine (reverse T3) in the human newborn. J Clin Invest. 1975 Jun;55(6):1137–1141. doi: 10.1172/JCI108030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J., Williams D. E., Orgiazzi J., Solomon D. H. Opposite effects of dexamethasone on serum concentrations of 3,3',5'-triiodothyronine (reverse T3) and 3,3'5-triiodothyronine (T3). J Clin Endocrinol Metab. 1975 Nov;41(5):911–920. doi: 10.1210/jcem-41-5-911. [DOI] [PubMed] [Google Scholar]
- Conversion of thyroxine into tri-iodothyronine by rat liver homogenate. Biochem J. 1975 Sep;150(3):489–493. doi: 10.1042/bj1500489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duick D. S., Warren D. W., Nicoloff J. T., Otis C. L., Croxson M. S. Effect of single dose dexamethasone on the concentration of serum triiodothyronine in man. J Clin Endocrinol Metab. 1974 Dec;39(6):1151–1154. doi: 10.1210/jcem-39-6-1151. [DOI] [PubMed] [Google Scholar]
- Frumess R. D., Larsen P. R. Correlation of serum triiodothyronine (T3) and thyroxine (T4) with biologic effects of thyroid hormone replacement in propylthiouracil-treated rats. Metabolism. 1975 Apr;24(4):547–554. doi: 10.1016/0026-0495(75)90079-7. [DOI] [PubMed] [Google Scholar]
- Geffner D. L., Azukizawa M., Hershman J. M. Propylthiouracil blocks extrathyroidal conversion of thyroxine to triiodothyronine and augments thyrotropin secretion in man. J Clin Invest. 1975 Feb;55(2):224–229. doi: 10.1172/JCI107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENINGER R. W., LARSON F. C., ALBRIGHT E. C. IODINE-CONTAINING COMPOUNDS OF EXTRATHYROIDAL TISSUES. J Clin Invest. 1963 Nov;42:1761–1768. doi: 10.1172/JCI104861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesch R. D., Brunner G., Söling H. D. Conversion of thyroxine (T4) and triiodothyronine (T3) and the subcellular localisation of the converting enzyme. Clin Chim Acta. 1975 Mar 10;59(2):209–213. doi: 10.1016/0009-8981(75)90031-5. [DOI] [PubMed] [Google Scholar]
- Hillier A. P. Antagonistic effects of dinitrophenol and cyanide on hepatic thyroxine deiodination. Acta Endocrinol (Copenh) 1974 Sep;77(1):122–127. doi: 10.1530/acta.0.0770122. [DOI] [PubMed] [Google Scholar]
- Hillier A. P. Deiodination of thyroid hormones by the perfused rat liver. J Physiol. 1972 Apr;222(2):475–485. doi: 10.1113/jphysiol.1972.sp009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüfner M., Knöpfle M. Pharmacological influences on T4 to T3 conversion in rat liver. Clin Chim Acta. 1976 Nov 1;72(3):337–341. doi: 10.1016/0009-8981(76)90196-0. [DOI] [PubMed] [Google Scholar]
- Ingbar D. H., Galton V. A. The effect of food deprivation of the peripheral metabolism of thyroxine in rats. Endocrinology. 1975 Jun;96(6):1525–1532. doi: 10.1210/endo-96-6-1525. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Schimmel M., Utiger R. D. Changes in serum 3,3',5'-triiodothyronine (reverse T3) concentrations with altered thyroid hormone secretion and metabolism. J Clin Endocrinol Metab. 1977 Sep;45(3):447–456. doi: 10.1210/jcem-45-3-447. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Green W. L. Degradation of thyroid hormones by phagocytosing human leukocytes. J Clin Invest. 1973 Jan;52(1):60–72. doi: 10.1172/JCI107174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Yamada T., Shichijo K. Nonenzymatic deiodination of thyroxine in vitro. Metabolism. 1966 Dec;15(12):1140–1148. doi: 10.1016/0026-0495(66)90104-1. [DOI] [PubMed] [Google Scholar]
- Koch B., Jobin M., Dulac S., Fortier C. Thyrotropin (TSH) response to synthetic TSH-releasing factor following pharmacological blockade of adrenocorticotropin (ACTH) secretion. Can J Physiol Pharmacol. 1972 Apr;50(4):360–363. doi: 10.1139/y72-053. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieblich J., Utiger R. D. Triiodothyronine radioimmunoassay. J Clin Invest. 1972 Jan;51(1):157–166. doi: 10.1172/JCI106786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Ruegamer W. R. Properties of a rat tissue iodothyronine deiodinase and its natural inhibitor. Biochemistry. 1967 May;6(5):1249–1261. doi: 10.1021/bi00857a005. [DOI] [PubMed] [Google Scholar]
- Nakano M., Tsutsumi Y., Ushijima Y. Degradation of thyroxine by the microsomal particles from rat liver. I. Correlation between thyroxine degradation and lipids peroxides. Biochim Biophys Acta. 1971 Nov 12;252(2):335–347. doi: 10.1016/0304-4165(71)90015-8. [DOI] [PubMed] [Google Scholar]
- Nicod P., Burger A., Staeheli V., Vallotton M. B. Radioimmunoassay for 3,3',5'-triiodo-L-thyronine in unextracted serum: method and clinical results. J Clin Endocrinol Metab. 1976 May;42(5):823–829. doi: 10.1210/jcem-42-5-823. [DOI] [PubMed] [Google Scholar]
- Nicod P., Burger A., Strauch G., Vagenakis A. G., Braverman L. E. The failure of physiologic doses of reverse T3 to effect thyroid-pituitary function in man. J Clin Endocrinol Metab. 1976 Aug;43(2):478–481. doi: 10.1210/jcem-43-2-478. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Shapiro H. C., Bernstein G., Surks M. I. Differences in primary cellular factors influencing the metabolism and distribution of 3,5,3'-L-triiodothyronine and L-thyroxine. J Clin Invest. 1970 May;49(5):1016–1024. doi: 10.1172/JCI106301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnay G. I., O'Brian J. T., Bush J., Vagenakis A. G., Azizi F., Arky R. A., Ingbar S. H., Braverman L. E. The effect of starvation on the concentration and binding of thyroxine and triiodothyronine in serum and on the response to TRH. J Clin Endocrinol Metab. 1974 Jul;39(1):191–194. doi: 10.1210/jcem-39-1-191. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J. L., Hercker E. S. Thyroxine: convesion to triiodothyronine by isolated perfused rat heart. Science. 1971 Sep 24;173(4003):1242–1243. doi: 10.1126/science.173.4003.1242. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Matalon R., Bigazzi M. Metabolism of L-thyroxine (T4) and L-triiodothyronine (T3) by human fibroblasts in tissue culture: evidence for cellular binding proteins and conversion of T4 to T3. Endocrinology. 1972 Oct;91(4):934–947. doi: 10.1210/endo-91-4-934. [DOI] [PubMed] [Google Scholar]
- Saberi M., Sterling F. H., Utiger R. D. Reduction in extrathyroidal triiodothyronine production by propylthiouracil in man. J Clin Invest. 1975 Feb;55(2):218–223. doi: 10.1172/JCI107924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding S. W., Chopra I. J., Sherwin R. S., Lyall S. S. Effect of caloric restriction and dietary composition of serum T3 and reverse T3 in man. J Clin Endocrinol Metab. 1976 Jan;42(1):197–200. doi: 10.1210/jcem-42-1-197. [DOI] [PubMed] [Google Scholar]
- Spector D. A., Davis P. J., Helderman J. H., Bell B., Utiger R. D. Thyroid function and metabolic state in chronic renal failure. Ann Intern Med. 1976 Dec;85(6):724–730. doi: 10.7326/0003-4819-85-6-724. [DOI] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Saldanha V. F. Conversion of thyroxine to triiodothyronine by cultured human cells. Science. 1973 Mar 9;179(4077):1000–1001. doi: 10.1126/science.179.4077.1000. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Metabolism of phenolic- and tyrosyl-ring labeled L-thyroxine in human beings and rats. J Clin Endocrinol Metab. 1971 Oct;33(4):612–618. doi: 10.1210/jcem-33-4-612. [DOI] [PubMed] [Google Scholar]
- TATA J. R. Metabolism of 1-thyroxine and 1-3:5:3'-triiodothyronine by homogenates of rat skeletal muscle. Proc Soc Exp Biol Med. 1957 Jun;95(2):362–364. doi: 10.3181/00379727-95-23222. [DOI] [PubMed] [Google Scholar]
- Taurog A. The mechanism of action of the thioureylene antithyroid drugs. Endocrinology. 1976 Apr;98(4):1031–1046. doi: 10.1210/endo-98-4-1031. [DOI] [PubMed] [Google Scholar]
- Vagenakis A. G., Burger A., Portnary G. I., Rudolph M., O'Brian J. R., Azizi F., Arky R. A., Nicod P., Ingbar S. H., Braverman L. E. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab. 1975 Jul;41(1):191–194. doi: 10.1210/jcem-41-1-191. [DOI] [PubMed] [Google Scholar]
- Wartofsky L., Burman K. D., Dimond R. C., Noel G. L., Frantz A. G., Earll J. M. Studies on the nature of thyroidal suppression during acute falciparum malaria: integrity of pituitary response to TRH and alterations in serum T3 and reverse T3. J Clin Endocrinol Metab. 1977 Jan;44(1):85–90. doi: 10.1210/jcem-44-1-85. [DOI] [PubMed] [Google Scholar]
- Wilber J. F., Utiger R. D. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest. 1969 Nov;48(11):2096–2103. doi: 10.1172/JCI106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeber K. A. A granule-associated L-thyroxine deiodinating system in the human leukocyte. Endocrinology. 1976 Mar;98(3):802–806. doi: 10.1210/endo-98-3-802. [DOI] [PubMed] [Google Scholar]


