Abstract
Evidence indicates that the frequency-domain characteristics of surface electromyogram (EMG) signals are modulated according to the contributing sources of neural drive. Modulation of inter-muscular EMG synchrony within the Piper frequency band (30–60Hz) during movement tasks has been linked to drive from the corticospinal tract. However, it is not known whether EMG synchrony is sufficiently sensitive to detect task-dependent differences in the corticospinal contribution to leg muscle activation during walking. We investigated this question in seventeen healthy older men and women. It was hypothesized that, relative to typical steady state walking, Piper band EMG synchrony of the triceps surae muscle group would be reduced for dual-task walking (because of competition for cortical resources), similar for fast walking (because walking speed is directed by an intermediate locomotor pathway rather than by the corticospinal tract), and increased when taking a long step (because voluntary gait pattern modifications are directed by the corticospinal tract). Each of these hypotheses was confirmed. These findings support the use of frequency-domain analysis of EMG in future investigations into the corticospinal contribution to control of healthy and disordered human walking.
Keywords: walking, electromyography, motor control, nervous system, locomotion
Introduction
The corticospinal tract actively contributes to control of walking in humans, and its importance is evident from the severe debilitating effects of corticospinal tract lesions with stroke or spinal cord injury.1 Studying the corticospinal contribution to walking will provide important insight for understanding how walking is controlled under different task conditions and for optimizing function in mobility impaired populations.
Gauging the corticospinal contribution to walking is challenging. Imaging techniques such as fNIRS can reveal motor cortex activity 2,3, but cannot confirm that increased cortical activity reaches the muscle(s) of interest. Transcranial magnetic stimulation (TMS) of the motor cortex (coupled with peripheral electromyography (EMG)) can provide a more direct indication of corticospinal excitation 4,5, but is difficult to perform during walking and requires a perturbing neural stimulus. An alternative approach that avoids some of the drawbacks of imaging and TMS is frequency-domain analysis of surface electrical recordings (e.g., electroencephalography (EEG) and electromyography (EMG)). The premise is that rhythmic firing from the cortical source of excitation will also yield rhythmic firing of populations of motor units in the periphery. The amount of synchronous activity between the sites may indicate the extent to which cortical activation is driving muscular activation.
Studies using frequency-domain analysis of EEG and EMG in humans have demonstrated that submaximal voluntary isometric contractions are characterized by a dominant EEG-EMG synchrony at about 15–30 Hz.6–9 This EEG-EMG synchrony has been observed in a variety of muscles and has been shown to agree with the known somatotopic organization of the motor cortex.7,10 This work in humans is in agreement with earlier invasive studies of cortico-muscular synchrony conducted in monkeys.11,12 During dynamic muscle contractions, the 15–30 Hz EEG-EMG synchrony is diminished.13,14 It is replaced by a dominant synchrony in a higher frequency band10,13, generally in the range of 30–60 Hz which is known as the Piper frequency band.10,15 In the context of walking, a recent study demonstrated that EEG-EMG synchrony (EMG from tibialis anterior) is present in the 24–40 Hz frequency band, which overlaps with the Piper band. This finding strongly supports a direct corticospinal tract contribution to muscle activity during walking.
The same drive that elicits EEG-EMG synchrony may also lead to synchronous activity among paired EMG recordings, particularly within the same muscle or synergistic muscles.11,14,16,17 Support for a link between paired EMG synchrony and corticospinal drive during walking has been provided by a number of studies. Among the first were studies showing that EMG-EMG synchrony is diminished with corticospinal tract damage due to stroke or spinal cord injury 18–21 and that neuro-rehabilitation yields increased synchrony and improved walking function in spinal cord injured individuals 19. Additional support is provided by a recent study showing that Piper band EMG-EMG synchrony within the tibialis anterior muscle is increased during childhood development (ages 4–15 years) and is associated with ankle control, as indicated by reduced step-to-step variability of toe position during swing.22 It was proposed that maturation of corticospinal control of walking is responsible for increased Piper band synchrony and improved ankle control.
It is not known whether EMG-EMG synchrony is sufficiently sensitive to differentiate the corticospinal demand of different walking tasks. If so, this approach could be used to understand the neural control strategies used by healthy and impaired individuals to accomplish different walking tasks. In the present study, synchronous activity was assessed for a pair of triceps surae muscles using surface EMG during four walking tasks: typical steady state walking, typical walking while performing a distracting cognitive task (i.e., dual-task walking), fast walking, and typical walking plus an intermittent voluntary long step. These specific tasks were chosen because strong evidence from the literature indicates that the corticospinal drive differs across tasks. Relative to typical steady state walking, it was hypothesized that Piper band synchrony would be: 1) reduced for dual-task walking because of competition for cortical resources 23,24, 2) similar to fast walking because walking speed is directed by an intermediate locomotor pathway rather than by the corticospinal tract 25,26, and 3) increased during step lengthening because voluntary gait pattern modifications are directed by the corticospinal tract.27,28
Materials and Methods
Participants
Healthy, high functioning older adults were recruited using the following exclusion criteria: age between 65 and 85 years; body mass index <19 or >32; preferred 10m walking speed <1.0 m/s; Berg Balance Scale score <50 (out of 56); Mini-Mental State Examination score <25 (out of 30), use of assistive device for walking; self-reported difficulty performing mobility tasks; experienced a fall within the previous year; pain, stiffness, numbness or range of motion limitations of the back or legs; involuntary weight gain or loss exceeding 10 pounds within the past six months; resting blood pressure exceeding 160/95; heart attack or symptomatic cardiovascular disease in the past year; fractured or broken bone in the past year; medical condition affecting movement; or terminal illness. All participants provided written informed consent and all study procedures were approved by the University of Florida Institutional Review Board and by the Malcom Randall VA Medical Center Human Research Protection Program.
Procedures
Participants walked on a split-belt treadmill with force plates embedded beneath each belt (Bertec, Columbus OH). Reflective markers were attached to anatomical landmarks on the head, trunk, arms, legs and feet using a modified Helen Hayes marker set and recorded using a 12 camera motion capture system (VICON, Colorado, USA). Disposable 1.5 inch surface gel electrodes (Versa-Trode, Vermed, Bellow Falls VT) were placed on the skin over triceps surae muscles soleus (SO) and medial gastrocnemius (MG). Inter-electrode distance was approximately 2 cm. These muscles were chosen because of the important role of the triceps surae for support and forward propulsion during walking and because EMG synchrony is most readily detected between synergist muscles. The electrode site for SO was distal to the belly of the MG, medial and anterior to the Achilles tendon 29. The site for MG was one hand breadth below the popliteal crease on the medial mass of the calf 29. The distance between these recording sites (generally at least 15 cm) minimizes concern about cross-talk from the same motor units. Each site was shaved and firmly rubbed with a sterile alcohol wipe prior to electrode placement. The electrodes were attached to an EMG preamplifier (Motion Lab Systems MA-420, Baton Rouge LA) and data were recorded using a commercially available EMG system (Motion Lab Systems MA300-28, Baton Rouge LA). Ground reaction force and kinematic data were sample at 200 Hz and EMG were sampled at 1000 Hz using a data acquisition system and software (Vicon Nexus, Los Angeles CA), and saved to disk for offline analysis.
To prevent falls, all participants wore a harness secured to an overhead support. Participants did not hold onto a railing or use any other support devices. The session began with a few minutes of walking on the treadmill at various speeds in order to familiarize the participant with the treadmill. Participants were then tested on the following four treadmill walking tasks: 1) Typical walking was performed at each individual’s own preferred steady state speed; 2) Fast walking was performed at each individual’s own perceived fastest safe walking speed; 3) Dual-task walking involved typical walking plus simultaneously performing a cognitive working memory task (described below); 4) Long step task involved typical walking, but on approximately every sixth gait cycle the participants were instructed to take a left step of maximum possible length. Data were recorded for 60 seconds for each task except fast walking, which was recorded for 30 seconds.
The cognitive task performed for dual-task walking was an auditory 2-back test. The examiner read aloud a list of letters throughout the entire walking trial while the participant simultaneously walked and listened. The list was read at a relatively slow pace such that one letter was read at every other heel strike of the left foot (i.e., one letter per two gait cycles). The participant was instructed to listen for a cue in which the same letter was repeated with only one other letter in between, and respond to the cue by saying “yes”. For instance, if part of the sequence of letters was “…R – T – A – P – A – B…” the participant was expected to respond after hearing the second “A”. The list contained four cues during the one-minute walking trial.
Data analysis
Ground reaction force and kinematic data were low-pass filtered (20 Hz and 10 Hz, respectively) with a fourth-order Butterworth filter with zero phase lag. Marker trajectories were fit to an eight-segment musculoskeletal model using Visual 3D (C-Motion, Inc., Germantown MD). The anthropometrics and inertial properties of the model were individualized for each participant and based on the procedures of de Leva.30 Peak ankle power production during walking was calculated from this model for the left and right legs during their respective stance phases. EMG signals were detrended then high-pass filtered at 5 Hz with a fourth-order Butterworth filter. EMG synchrony was calculated using a frequency domain analysis involving Morlet wavelet transform:
Where η is dimensionless time and ω0 is dimensionless frequency (in this study we used ω0 = 6, as suggested by Grinsted and colleagues.31
The Morlet wavelet (with ω0 = 6) is appropriate when performing wavelet and cross-wavelet analysis, because it provides a reasonable balance between time and frequency localization. The wavelet transform applies the wavelet function as a band pass filter to the time-series:
Where s represents the dilation parameter (scale shifting), τ represents the location parameter (time shifting), and the basic function is obtained by dilating and translating the mother wavelet Ψ0(t)0.125
Wavelet and cross-wavelet transforms were calculated using a base algorithm in Matlab (The Mathworks, Natick MA) developed by Torrence and Compo 32 and available at URL: http://paos.colorado.edu/research/wavelets). From the wavelet transform of both EMG signals, the cross-wavelet transform was calculated:
Where W(s,τ)XY is the cross-wavelet transform of signals X(t) and Y(t), W(s,τ)X is the wavelet transform of signal X(t), and W(s,τ)Y* is the complex conjugate of the wavelet transform of signal Y(t).
Upon completing the cross-wavelet analysis, the distribution of cross wavelet power spectra across different frequency bands for each walking task was summarized. For the left long step walking task, the gait cycles containing the long steps were separated from the rest of the walking trial after performing the cross-wavelet analysis. There were generally about 5–7 long step gait cycles per participant, and only data from those gait cycles were used for analysis. Representative data showing cross-wavelet EMG power for one participant are shown in Figure 1. The cross-wavelet results were summarized in two ways: 1) over the entirety of each gait cycle and 2) over just the late stance phase of each gait cycle. Stance phase begins with heel strike and ends with ipsilateral toe off, and late stance phase was defined temporally as the second half of that period. The latter approach specifically isolates triceps surae activity within a period when it is highly active and contributing to the critical biomechanical functions of body support and forward propulsion. The objective of summarizing the data in two different ways was to evaluate the importance of controlling for gait cycle biomechanics when calculating EMG synchrony of the triceps surae.
Figure 1. Representative data from one participant showing EMG-EMG cross-wavelet spectral power calculated from triceps surae muscles.
Three consecutive gait cycles are shown for typical, dual-ask and fast walking tasks, while long step data are presented in separate figures because long steps were not performed consecutively. The 30–60 Hz (Piper) frequency band is located between the dashed lines.
The cross-wavelet spectra were divided into the following six frequency bands: 5–13, 13–30, 30–60, 60–100, 100–200, and 200–300 Hz. Normalized power within each band was then calculated by dividing the power within each band to the total power over the entire 5–300 Hz range. We report normalized power because it considers the relative importance of the commonalities in the variance of the two signals in different frequencies through time.17 We use the interference EMG signal because we 17,33–35 and others 36,37 have shown that the interference EMG more accurately estimates the common activity of two EMG signals compared with rectified EMG.
Statistics
Within each of the six frequency bands, one-way ANOVA with main effect of Task was used to determine if cross wavelet relative power differed across walking tasks. A Bonferroni correction was used to account for multiple comparisons (α = .05/6 bands = .008).
Due to having different directional hypotheses for different pairs of walking tasks, post-hoc analysis the of 30–60 Hz band was conducted by comparing typical walking to each other walking task using separate two-way repeated-measures ANOVA models (2 tasks × 2 legs). Of primary interest were task-dependent differences in 30–60 Hz cross wavelet power and whether there were differential responses between the left and right leg (Task x Side interaction). The threshold for statistical significance of post-hoc tests was set to α =0.05. Pearson’s correlation analysis was used to assess associations between continuous outcome variables. Statistical analysis was performed with JMP statistical software (SAS Institute Inc, Cary NC).
Results
Seventeen older adults (8 female, 9 male) participated in the study, but one did not perform the long step task and two did not perform the dual-task condition. The average age of participants was 70.1 ± 3.87 years. The cohort was healthy and high functioning, with body mass index of 26.6 ± 2.0, preferred 10m walking speed of 1.31 ± .15, Berg Balance Scale score of 54.8 ± 1.3 and Mini-Mental State Exam score of 29.2 ± 1.52.
Only the 30–60 Hz (Piper) frequency band revealed significant task-dependent modulation (Figure 2, p<.001). Post-hoc analysis of the Piper band data revealed reduced Piper band synchrony for dual-task walking relative (Figure 2 and Figure 3A, P = 0.015) with no Task x Side interaction (p = 0.267). Unchanged Piper band synchrony was observed for fast walking (Figure 2 and Figure 3B, p = 0.154) with no Task x Side interaction (p=.726). Heightened Piper band synchrony was observed when taking a left long step (Figure 2 and Figure 3C, p < 0.001) and there was a significant Task x Side interaction with the right side increasing more than the left side (p = 0.020).
Figure 2. EMG-EMG synchrony measured by cross wavelet spectral analysis within each frequency band for each walking task.

Data are expressed relative to total cumulative cross wavelet power. Only the 30–60 Hz (Piper) band demonstrated a statistically significant effect of task, as indicated by *. Within the Piper band, significant differences relative to typical walking (black) are indicated by #. Piper band EMG synchrony was lower for dual-task walking (dark gray) and higher for the left long step walking task (light gray). EMG synchrony did not differ for typical and fast walking (white).
Figure 3. Percent change in Piper band synchrony for each task relative to typical walking.
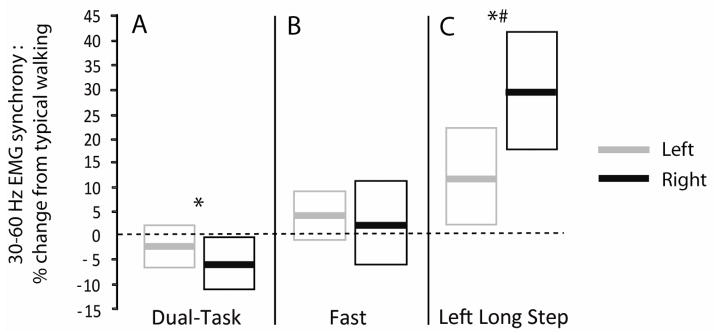
Group means are shown as bold lines for the left (gray) and right (black) legs, and boxes indicate the 95% confidence interval of the group. * indicates a significant main effect of Task and # indicates a significant Task x Side interaction effect (as indicated by the original data prior to transforming to % change values, as presented in the Results section).
Relative to typical walking, peak ankle plantarflexion power increased 69% and 73% in the right and left legs, respectively, during fast walking (p<.0001). Similarly, peak ankle plantarflexion power increased 66% for the right leg when taking a left long step (p<.0001). To assess the extent to which corticospinal drive may have been responsible for altered power production, we examined the association between change in Piper band synchrony and change in power production for these two tasks relative to typical walking. For both tasks, data from left and right legs were pooled when conducting the correlation analysis. There was no association between Piper band synchrony and ankle power in the fast walking condition (p=.95). In contrast, there was a significant association between Piper band synchrony and ankle power during the left long step task (p=.009, r=.42).
To determine the extent to which increased triceps surae activation magnitude might have been responsible for increased 30–60 Hz synchrony, the association between the change in each was calculated for the fast walking task. Fast walking was used for this analysis because it was the only task where we hypothesized considerable change in EMG signal magnitude without corticospinal modulation of Piper band synchrony. The results show no association between triceps surae activation magnitude and Piper band synchrony (p=.92), indicating that normalized Piper band cross wavelet power is not a function of total EMG magnitude.
The results reported above were from cross wavelet analysis conducted over the entire gait cycle. Also calculated for typical walking was the normalized cross wavelet power in each frequency band during only the second half of the stance phase, where SO and MG are most strongly active. The association between cross wavelet power calculated with each approach was determined (full gait cycle versus late stance only). The results show a very strong correlation (Figure 4, R2=.99, p<.0001), indicating that both approaches yield essentially the same results.
Figure 4. Association between EMG synchrony calculated from full gait cycle versus late stance phase only.

For all participants, EMG synchrony values from each of the six frequency bands are plotted from the typical walking task. EMG synchrony values calculated from the full gait cycle and from only late stance are plotted on the abscissa and ordinate, respectively. Both approaches yielded equivalent results (R2=.99, p<.0001), which indicates that controlling for gait cycle biomechanics is not required for calculating triceps surae EMG synchrony.
Discussion
The findings of this study support the hypothesis that EMG-EMG synchrony in the Piper frequency band, as quantified by cross-wavelet spectral analysis, reflects differences in the corticospinal demand of different walking tasks. Dual-task walking resulted in reduced Piper band synchrony relative to typical walking (Figures 2 and 3a). This finding is consistent with earlier studies that have shown that EMG synchrony is reduced when a secondary attention demanding cognitive task is added to a hand motor task.23,24 These findings support the premise that dual-tasking elicits a competition for cortical processing resources between the cognitive and motor tasks 38, which diminishes the cortical drive directed toward the motor task. The difference in Piper band activity between typical and dual-task walking was small, but this is not surprising since the cognitive task was relatively simple and because control of typical steady state walking requires relatively low voluntary/attentional motor control in healthy adults.39–41. We might have found greater dual-task interference had we examined more challenging cognitive and/or motor tasks. Although the Task x Side interaction was not significant for typical and dual-task walking, it is interesting to note that Piper band synchrony during dual-tasking was more consistently reduced for the right leg compared to the left leg (see 95% confidence intervals in Figure 3a). This suggests heightened dual-task interference in the left hemisphere. Such interference is consistent with the verbal comprehension and working memory demands of the auditory 2-back test, which primarily involve processing in the left frontal and parietal cortical regions.42
Piper band synchrony did not differ between typical and fast walking (Figures 2 and 3b), which was consistent with our hypothesis. Although triceps surae muscle activation magnitude increased almost 50% during fast walking compared to typical walking, our findings suggest that this was not due to corticospinal drive. Indeed, we found no association between the change in triceps surae activation magnitude and the change in 30–60 Hz synchrony. Rather, it has previously been proposed that walking speed is regulated by an indirect locomotor pathway that includes the basal ganglia, brainstem locomotor region, and spinal pattern generating circuitry.25,26 This indirect locomotor pathway is likely responsible for increased muscle activation during fast walking, and the present results indicate that it does not elicit modulation of Piper band synchrony.
When taking a longer step during otherwise steady state walking, Piper band synchrony increased considerably relative to typical walking (Figures 2 and 3c). This is consistent with evidence showing that gait pattern modifications are controlled by integrating a corticospinal command with the ongoing locomotor pattern.27,43–45 It is notable that both Piper band synchrony and ankle power in the left and right legs during the left long step differed in a way that is consistent with task demands. As previously described by Varraine and colleagues, increased plantarflexion force in the stance leg is a major contributor to forward propulsion during a contralateral long step.46 Furthermore, the change in ankle power and change in Piper band synchrony (relative to typical walking) were significantly positively correlated. This finding suggests that increased synchrony due to increased corticospinal drive is responsible for the heightened ankle power production. This conclusion is strengthened by the fact that there was no association between the change in Piper band synchrony and ankle power for fast walking relative to typical walking. As discussed earlier, this is consistent with the notion that faster walking speed is not directly mediated by corticospinal drive.
An important finding of this study is that EMG synchrony was essentially the same whether EMG were analyzed from the entire gait cycle or from just the late stance phase of the gait cycle (Figure 4). We made this comparison to determine the extent to which it was important to control for the phase of the gait cycle. Our findings suggest that data analysis of triceps surae Piper band synchrony can be conducted without controlling for the timing of biomechanical gait events. This result is likely aided by the fact that the SO and MG muscles are generally co-active, and primarily during late stance. That is, there is generally less triceps surae activation occurring in other phases of the gait cycle. Controlling for gait timing may be important if studying muscle pairs whose activation timing is less tightly coupled. This finding will considerably simplify our ability to conduct future studies in which triceps surae activation is evaluated during more complex walking tasks or under more diverse environmental conditions where measuring or controlling for biomechanical gait events could be difficult.
There are a number of methodological considerations related to this study. Although we present encouraging evidence of the ability to gauge corticospinal drive during walking, it must be acknowledged that Piper band synchrony is just a small proportion of total EMG power. Indeed, walking is a complex sensorimotor task requiring integration of neural commands from various sources. Some of those sources might contribute to synchrony in the Piper band and/or other frequency bands. For example, certain subcortical outputs might contribute to synchrony in the 5–13 Hz band while certain brainstem outputs might contribute to synchrony in the 60–100 Hz band.15,16 Another consideration is that surface EMG is inherently susceptible to physiological and non-physiological sources of variability and can therefore be challenging to interpret.47,48 One potential factor that is often of concern with surface EMG is the effect of cross talk. However we do not believe that cross talk influenced the results of this study. First, we recorded from two different muscles using recording sites that were separated by about 15–20cm. Given the approximately 2cm inter-electrode distance used for data collection, the detection volume of each set of electrodes should be an approximately 4cm spherical region.49 Accordingly, the electrodes were placed far enough apart to avoid overlapping detection volumes and resultant cross talk. Furthermore, even if crosstalk was present there is no reason to expect that it would preferentially affect activity in the 30–60 Hz over other frequency bands.
In conclusion, EMG synchrony in the Piper frequency band differs across walking tasks in a way that is consistent with the corticospinal demand. This finding will support future studies of Piper band synchrony that will investigate the corticospinal demands of walking in various environments, particularly in the context of understanding compromised mobility function in older adults and individuals with neurological injury.
Acknowledgments
This work was supported by the US Department of Veterans Affairs Rehabilitation Research and Development Service (B7176W to DJC) and by the National Institute on Aging (P30-AG028740-04 to DJC and R01 AG-031769 to EAC).
Footnotes
Conflicts
There are no conflicts of interest.
References
- 1.Nielsen JB. How we walk: central control of muscle activity during human walking. Neuroscientist. 2003;9:195–204. doi: 10.1177/1073858403009003012. [DOI] [PubMed] [Google Scholar]
- 2.Belda-Lois JM, Mena-del Horno S, Bermejo-Bosch I, Moreno JC, Pons JL, Farina D, Iosa M, Molinari M, Tamburella F, Ramos A, Caria A, Solis-Escalante T, Brunner C, Rea M. Rehabilitation of gait after stroke: a review towards a top-down approach. J Neuroeng Rehabil. 2011;8:66. doi: 10.1186/1743-0003-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki M, Miyai I, Ono T, Kubota K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. Neuroimage. 2008;39:600–7. doi: 10.1016/j.neuroimage.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 4.Schubert M, Curt A, Jensen L, Dietz V. Corticospinal input in human gait: modulation of magnetically evoked motor responses. Exp Brain Res. 1997;115:234–46. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]
- 5.Petersen NT, Butler JE, Marchand-Pauvert V, Fisher R, Ledebt A, Pyndt HS, Hansen NL, Nielsen JB. Suppression of EMG activity by transcranial magnetic stimulation in human subjects during walking. J Physiol. 2001;537:651–6. doi: 10.1111/j.1469-7793.2001.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol. 1995;489(Pt 3):917–24. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77:3401–5. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- 8.Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20:8838–45. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- 10.Brown P, Salenius S, Rothwell JC, Hari R. Cortical correlate of the Piper rhythm in humans. J Neurophysiol. 1998;80:2911–7. doi: 10.1152/jn.1998.80.6.2911. [DOI] [PubMed] [Google Scholar]
- 11.Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol. 1997;501(Pt 1):225–41. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A. 1992;89:5670–4. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omlor W, Patino L, Hepp-Reymond MC, Kristeva R. Gamma-range corticomuscular coherence during dynamic force output. Neuroimage. 2007;34:1191–8. doi: 10.1016/j.neuroimage.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol. 1999;516(Pt 2):559–70. doi: 10.1111/j.1469-7793.1999.0559v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol. 2000;60:97–108. doi: 10.1016/s0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 16.Grosse P, Cassidy MJ, Brown P. EEG-EMG, MEG-EMG and EMG-EMG frequency analysis: physiological principles and clinical applications. Clin Neurophysiol. 2002;113:1523–31. doi: 10.1016/s1388-2457(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 17.Neto OP, Baweja HS, Christou EA. Increased voluntary drive is associated with changes in common oscillations from 13 to 60 Hz of interference but not rectified electromyography. Muscle Nerve. 2010;42:348–54. doi: 10.1002/mus.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen JB, Brittain JS, Halliday DM, Marchand-Pauvert V, Mazevet D, Conway BA. Reduction of common motoneuronal drive on the affected side during walking in hemiplegic stroke patients. Clin Neurophysiol. 2008;119:2813–8. doi: 10.1016/j.clinph.2008.07.283. [DOI] [PubMed] [Google Scholar]
- 19.Norton JA, Gorassini MA. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J Neurophysiol. 2006;95:2580–9. doi: 10.1152/jn.01289.2005. [DOI] [PubMed] [Google Scholar]
- 20.Hansen NL, Conway BA, Halliday DM, Hansen S, Pyndt HS, Biering-Sorensen F, Nielsen JB. Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J Neurophysiol. 2005;94:934–42. doi: 10.1152/jn.00082.2005. [DOI] [PubMed] [Google Scholar]
- 21.Barthelemy D, Willerslev-Olsen M, Lundell H, Conway BA, Knudsen H, Biering-Sorensen F, Nielsen JB. Impaired transmission in the corticospinal tract and gait disability in spinal cord injured persons. J Neurophysiol. 2010;104:1167–76. doi: 10.1152/jn.00382.2010. [DOI] [PubMed] [Google Scholar]
- 22.Petersen TH, Kliim-Due M, Farmer SF, Nielsen JB. Childhood development of common drive to a human leg muscle during ankle dorsiflexion and gait. J Physiol. 2010;588:4387–400. doi: 10.1113/jphysiol.2010.195735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson AN, Wheaton LA, Shinohara M. Attenuation of corticomuscular coherence with additional motor or non-motor task. Clin Neurophysiol. 2011;122:356–63. doi: 10.1016/j.clinph.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Kristeva-Feige R, Fritsch C, Timmer J, Lucking CH. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol. 2002;113:124–31. doi: 10.1016/s1388-2457(01)00722-2. [DOI] [PubMed] [Google Scholar]
- 25.Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Shigemi M. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Elsevier; Amsterdam: 2004. pp. 239–249. [DOI] [PubMed] [Google Scholar]
- 26.Grillner S, Wallen P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates--an overview. Brain Res Rev. 2008;57:2–12. doi: 10.1016/j.brainresrev.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Schubert M, Curt A, Colombo G, Berger W, Dietz V. Voluntary control of human gait: conditioning of magnetically evoked motor responses in a precision stepping task. Exp Brain Res. 1999;126:583–8. doi: 10.1007/s002210050767. [DOI] [PubMed] [Google Scholar]
- 28.Bonnard M, Camus M, Coyle T, Pailhous J. Task-induced modulation of motor evoked potentials in upper-leg muscles during human gait: a TMS study. Eur J Neurosci. 2002;16:2225–30. doi: 10.1046/j.1460-9568.2002.02295.x. [DOI] [PubMed] [Google Scholar]
- 29.Perroto AO. Anatomical Guide for the Electromyographer. 3. Springfield, IL: Charles C. Thomas; 1994. [Google Scholar]
- 30.de Leva P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J Biomech. 1996;29:1223–30. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]
- 31.Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet tranform and wavelet coherence to geophysical time series. Nonlinear Proc Geophys. 2004;11:561–556. [Google Scholar]
- 32.Torrence C, Compo GP. A practical guide to wavelet analysis. Bull Am Meteorol Soc. 1998;79 [Google Scholar]
- 33.Neto OP, Christou EA. Rectification of the EMG signal impairs the identification of oscillatory input to the muscle. J Neurophysiol. 2010;103:1093–103. doi: 10.1152/jn.00792.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christou EA, Neto OP. Identification of oscillations in muscle activity from surface EMG: reply to Halliday and Farmer. J Neurophysiol. 2010;103:3548–3549. [Google Scholar]
- 35.Christou EA, Neto OP. Reply to Boonstra: the nature of periodic input to the muscle. J Neurophysiol. 2010;104:577. doi: 10.1152/jn.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–95. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 37.McClelland VM, Cvetkovic Z, Mills KR. Rectification of the EMG is an unnecessary and inappropriate step in the calculation of Corticomuscular coherence. J Neurosci Methods. 2012;205:190–201. doi: 10.1016/j.jneumeth.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–33. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regnaux JP, David D, Daniel O, Smail DB, Combeaud M, Bussel B. Evidence for cognitive processes involved in the control of steady state of walking in healthy subjects and after cerebral damage. Neurorehabil Neural Repair. 2005;19:125–32. doi: 10.1177/1545968305275612. [DOI] [PubMed] [Google Scholar]
- 40.Bock O. Dual-task costs while walking increase in old age for some, but not for other tasks: an experimental study of healthy young and elderly persons. J Neuroeng Rehabil. 2008;5:27. doi: 10.1186/1743-0003-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gage WH, Sleik RJ, Polych MA, McKenzie NC, Brown LA. The allocation of attention during locomotion is altered by anxiety. Exp Brain Res. 2003;150:385–94. doi: 10.1007/s00221-003-1468-7. [DOI] [PubMed] [Google Scholar]
- 42.Glascher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Grabowski T, Damasio H, Adolphs R. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–91. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beloozerova IN, Farrell BJ, Sirota MG, Prilutsky BI. Differences in movement mechanics, electromyographic, and motor cortex activity between accurate and non-accurate stepping. J Neurophysiol. 2010 doi: 10.1152/jn.00360.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drew T. Motor cortical cell discharge during voluntary gait modification. Brain Res. 1988;457:181–7. doi: 10.1016/0006-8993(88)90073-x. [DOI] [PubMed] [Google Scholar]
- 45.Amos A, Armstrong DM, Marple-Horvat DE. Changes in the discharge patterns of motor cortical neurones associated with volitional changes in stepping in the cat. Neurosci Lett. 1990;109:107–12. doi: 10.1016/0304-3940(90)90546-l. [DOI] [PubMed] [Google Scholar]
- 46.Varraine E, Bonnard M, Pailhous J. Intentional on-line adaptation of stride length in human walking. Exp Brain Res. 2000;130:248–57. doi: 10.1007/s002219900234. [DOI] [PubMed] [Google Scholar]
- 47.De Luca CJ. The use of surface electromyography in biomechanics. Journal of Applied Biomechanics. 1997;13:135–163. [Google Scholar]
- 48.Farina D. Interpretation of the surface electromyogram in dynamic contractions. Exerc Sport Sci Rev. 2006;34:121–7. doi: 10.1249/00003677-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Kamen G, Gabriel DA. Essentials of electromyography. Champaigne, IL: Human Kinetics; 2010. [Google Scholar]



