Abstract
The fate of iNKT cells following activation remains controversial and unclear. We systemically examined how iNKT cells are regulated following TCR-dependent and -independent activation with alpha-Galactosylceramide (αGC) or IL-18+IL-12 respectively. Our studies reveal activation by αGC or IL-18+IL-12 induced transient depletion of iNKT cells exclusively in the liver that was independent of caspase 3 mediated apoptosis. The loss of iNKT cells was followed by repopulation and expansion of phenotypically distinct cells via different mechanisms. Liver iNKT cell expansion following αGC, but not IL-18+IL-12, treatment required an intact spleen and IFN-γ. Additionally, IL-18+IL-12 induced a more prolonged expansion of liver iNKT cells compared to αGC. iNKT cells that repopulate the liver following αGC had higher levels of suppressive receptors PD-1 and Lag3 while those that repopulate the liver following IL-18+IL-12 had increased levels of TCR and ICOS. In contrast to acute treatment that caused a transient loss of iNKT cells, chronic αGC or IL-18+IL-12 treatment caused long-term systemic loss requiring an intact thymus for repopulation of the liver. This report reveals a previously undefined role for the liver in the depletion of activated iNKT cells. Additionally, TCR-dependent and -independent activation differentially regulate iNKT cell distribution and phenotype. These results provide new insights for understanding how iNKT cells are systemically regulated following activation.
Introduction
Invariant Natural Killer T cells (iNKT cells) express an invariant T-cell receptor and activated/memory markers, but unlike conventional T cells, iNKT cells can rapidly respond to antigenic signals without prior sensitization (1). This functional characteristic permits iNKT cells to rapidly respond in a variety of disease settings, including cancer, autoimmune and infectious diseases (2) and potentially makes them attractive targets for immune intervention. The mechanisms whereby iNKT cells are systemically regulated following activation remain controversial. An improved understanding of iNKT cell regulation may help define their role in particular diseases and/or microenvironments.
iNKT cell activation is primarily mediated through cytokine receptors and the T-cell receptor (TCR). IL-18 and IL-12 activate iNKT cells and are among the first cytokines produced during an immune response (3–5). It has been appreciated that T cells most efficiently produce IFNγ when both IL-18 and IL-12 are present (6). In addition, IL-12 requires TCR engagement for optimal iNKT cell activation (7). In contrast, IL-18+IL-12 activation of iNKT cells and T cells does not require, nor does it enhance activation with TCR engagement (8,9). Thus IL-18+IL-12 can be employed to study TCR-independent activation of iNKT cells. It has been previously reported that IL-18+IL-12 activation of NKT cells in vitro can lead to Fas-dependent NKT cell apoptosis (5). We showed previously that chronic treatment of mice with IL-18+IL-12 results in an inability to detect iNKT cells in the liver (4). However, the in vivo mechanisms underlying the regulation of iNKT cells by this treatment remain unclear. While one report showed IL-12 depleted liver NKT cells with repopulation deriving from the bone marrow (10), another report found that during Listeria monocytogenes infection NK1.1(+) iNKT cells were reduced by internalizing NK1.1(+) receptor in an IL-12 dependent manner (11).
TCR engagement represents another major pathway of iNKT cell activation. In contrast to conventional T cells that recognize a variety of peptide antigens presented by MHC I or II receptors, iNKT cells have a more restricted TCR repertoire (12,13) that recognizes glycolipids presented by CD1d receptor (14,15). Most studies examining antigenic TCR activation of iNKT cells use αGC (1). Studies examining iNKT cell fate following αGC activation report conflicting results. Early reports showed triggering of iNKT cells through the TCR with αGC and anti-CD3 induced a rapid loss of iNKT cells that was attributed to apoptosis (5,10). In contrast, subsequent studies reported that iNKT cells down-modulate TCR following acute αGC activation, rendering them undetectable by standard flow cytometric methods (16–18).
In this report we comprehensively examined iNKT cell regulation in lymphoid tissues following both acute and chronic TCR-dependent and -independent activation with αGC and IL-18+IL-12, respectively. We found a previously undefined role for the liver for depleting activated iNKT cells. Furthermore, we found differential reshaping of the iNKT cell phenotype that depended on the nature of activation. These findings suggest a previously unappreciated adaptability of iNKT cells in response to microenvironmental signals through differential stimulation of their activating and/or regulatory cell surface receptors.
MATERIALS AND METHODS
Mice and treatment approaches
Approval for the animal experimentation presented in this study was received from the Institutional Animal Care and Use Committee at the National Cancer Institute (Office of Laboratory Animal Welfare assurance no. A4159-01). C57BL/6 mice were obtained from the Animal Production Area of the National Cancer Institute-Frederick Cancer Research and Development Center. IFNγ knock-out mice (strain B6.129S7-Ifngtm1Ts/J), mutant FasL mice (strain B6Smn.C3-Tnfsf6gld/J) (gld) and TNFR1 and TNFR2 knock-out mice (strain B6;129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J) were purchased from the Jackson Laboratory (Bar Harbor, Maine) and Trail(−/−) were provided by Mark Smyth. All mice were bred in a dedicated pathogen-free environment at the NCI-Frederick animal facility. All mice were between 8 and 16 weeks of age at the start of the experiment.
Recombinant murine IL-12 was purchased from PeproTech Inc. (Rocky Hill, NJ), recombinant murine IL-18 was kindly provided by GlaxoSmithKline (Upper Merion, PA) and αGC was obtained from Kirin Brewery or purchased from Alexis Biochemicals. Stock aliquots of cytokines were diluted with HBSS containing 0.1% (vol./vol.) sterile-filtered C57/BL6 normal serum. For acute treatment, mice were injected i.p. on day 0 with vehicle control (VC) (HBSS containing 0.1% C57/BL6 normal serum), IL-12 (0.5μg) and IL-18 (0.3μg) or αGC (1μg). For chronic treatment, mice were given VC or IL-18+IL-12 on days 0–4 and 8–10 or α-GC on days 0,2,4,8 and 10 via intraperitoneal (i.p.) injection. General caspase inhibitor Q-Val-Asp(non-omethylated)-OPH (Q-VD-OPh) (Sigma) was dissolved in DMSO at a concentration of 20mM and mice were administered 20mg/kg.
Cell isolation
To isolate liver leukocytes, mice were euthanized. The liver was flushed with 10 cc of cold HBSS through the portal vein. The liver(s) was removed, placed in a stomacher bag (VWR International) containing 25 mls of cold wash buffer (HBSS containing 0.1% BSA (Sigma) and 0.5mM EDTA (Invitrogen)) and disrupted for 30 sec using a stomacher 80 (Seward, West Sussex, UK) set for 30 seconds on medium setting. The disrupted liver was collected and washed with wash buffer by centrifugation at 325 × g for 10 minutes at 4°C. The resulting pellet was re-suspended in 40% Percoll (VWR International, Suwanee, GA) and then under-laid with 80% Percoll. Percoll solutions were made by diluting DPBS-Percoll mixture (1 part 10X DPBS to 9 parts Percoll ) with DMEM medium (Mediatech, Manassas VA) to the proper concentration. The Percoll gradient was centrifuged at room temperature for 25 minutes at 850 xg. The leukocytes were collected at the interface and washed twice in cold wash buffer.
Isolation of lung leukocytes was done by mincing the lung with sharp curved iris scissors and then shaking the minced tissue in 25 mls of complete RPMI 1640 media containing 700u/ml collagenase type I (Worthington) and DNase 1 (0.5mg/ml) (Sigma) for 30 minutes at 37°C. The cell-collagenase mixture was next disrupted using a stomacher 80 and the leukocytes were recovered using a Percoll gradient as described for liver leukocyte isolation.
Bone marrow cells were recovered by flushing the femurs and tibias. Leukocytes were isolated from thymus, spleen and lymph nodes (pooled inguinal and axillary) by mechanical disruption through a filtra-bag mesh (Fisher Scientific) in 10 mls of QBSF56 containing 0.5 mM EDTA.
Lymphocyte counts were determined from the isolated leukocytes using a Sysmex KX-21 (Roche, Indianapolis, IN) automated cell counter.
Flow cytometric analysis
Data was collected using a LSRII Special Order System equipped with solid state blue (488nm), red (640nm) and violet (405nm) lasers and then analyzed using FCS Express v3 (De Novo software). Appropriately titered monoclonal antibodies against mouse CD4 (FITC or Pacific Blue; clone RM4-5), NK1.1 (PE or PerCP-Cy5.5; clone PK136), Lag3 (PE; clone eBioC9B7W), ICOS (PE; clone 7E.17G9), PD-1 (Fitc; J43), CD28 (PerCP/Cy5.5; clone 37.51) were purchased from eBioscience, CD45 (Pacific Orange; clone 30-F11) was purchased from Invitrogen and CD40L (PE; clone MR1) and anti-active Caspase-3 (Fitc; clone C92-605) was purchased from Pharmingen. CD1d tetramer loaded with PBS-57 was prepared by the NIAID MHC Tetramer Core Facility (Atlanta GA). Cytofix/Cytoperm™ Fixation/Permeablization Kit (Pharmingen) was used for intracellular staining of caspase 3.
Cell proliferation was detected by injecting mice i.p. with BrdU (Sigma) dissolved in PBS at 100ng/μl. BrdU incorporation was detected using a BrdU flow kit (BD Pharmingen™) and analyzed by flow cytometry (FCA).
Assessing relative changes in NKT cell concentration from mouse tissue using real-time PCR
Mice were treated acutely or chronically with VC, αGC or IL-18+IL-12 and leukocytes were isolated from liver, spleen, lung, lymph node, bone marrow and thymus tissues, as described above. Genomic DNA was isolated using a DNeasy kit (Qiagen) according to the manufacturer’s protocol for cultured animal cells. Genomic DNA was probed for Vα14Jα18 TCR gene rearrangement (target gene) (18) and 18s or actin (reference gene) using Taqman primers and universal master mix all purchased from Applied Biosystems and analyzed on an ABI 7300 real time PCR system (Applied Biosystems). The relationship of the iNKT cell concentration (Vα14Jα18) with respect to total cell number (18s or actin) was calculated using the average ΔCt method (ABI system software) from each sample run in triplicate. The equation 2(−ΔCt) was applied to express relative levels of Vα14Jα18 with respect to 18s or actin in linear form. Because treatments can modulate total cell numbers (TCN) in each tissue that may mask absolute changes in the level of Vα14Jα18 signal, we multiplied 2(−ΔCt) by the TCN from each tissue to determine Vα14Jα18 levels in each tissue. The fold change relative to the vehicle control was calculated using the equation . Standard error of the means (SEM) was determined from 6 biological replicates from two separate experiments.
Statistical analysis
P values were determined by the nonparametric Mann-Whitney test using GraphPad Prism 5 software for experiments with 5 or more samples and Student’s t-Tests for samples size of less then 5. The data were considered significant if the two-tailed test was P< 0.05.
Results
IL-18+IL-12 and αGC-induced loss of iNKT cell detection was not dependent on caspase 3 activity
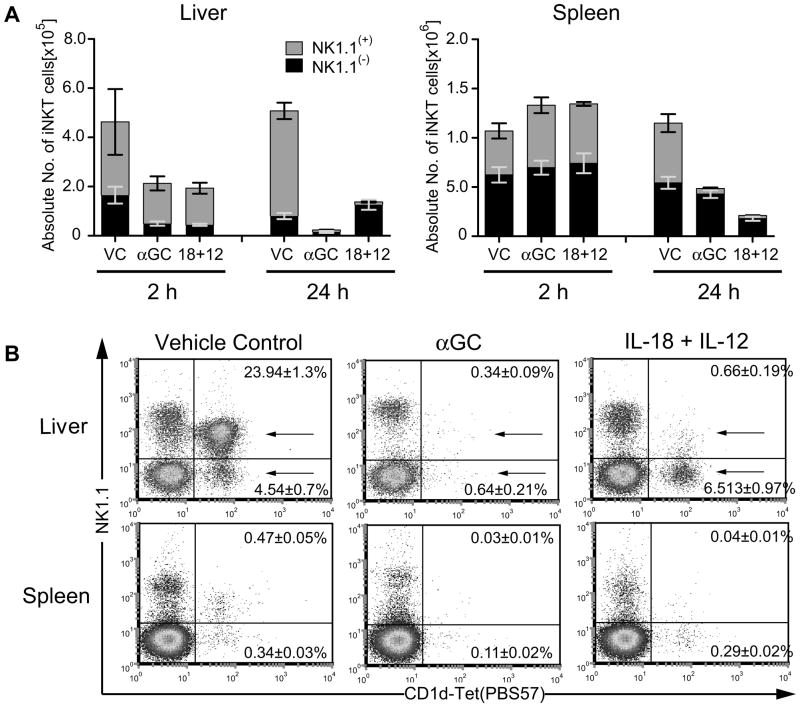
To compare TCR dependent and independent regulation of iNKT cells we treated mice i.p. with αGC and IL-18+IL-12 and examined the modulation of iNKT cells from liver and spleen at 2 and 24h using flow cytometric analysis of cells stained with PBS-57-loaded CD1 tetramer (CD1d-tet). A reduction in detection of absolute iNKT cell numbers was observed at 2h in the liver with both treatments (Figure 1A). In contrast, no loss of iNKT cells was detected at 2h in the spleen. By 24h, iNKT cell detection was reduced in both liver and spleen with both treatments. NK1.1 flow cytometric subset analysis revealed IL-18+IL-12 reduced NK1.1(+) iNKT cell detection in both liver and spleen by 24h similar to IL-12 activation (11) (Figure 1A,B). Consistent with previous reports (5,19) we found αGC reduced the detection of all iNKT cells in the liver. However, in the spleen we found αGC was comparable to IL-18+IL-12 for the reduction of only NK1.1(+) iNKT cell detection.
Figure 1. Disparate regulation of iNKT cells in the spleen vs liver following αGC or IL-18+IL-12 treatment of mice.
(A,B) Leukocytes were isolated from livers and spleens of mice 24h after treatment with vehicle control (VC), αGC (1μg) or IL-18 (0.3μg)+IL-12 (1μg). Cell surface expression of NK1.1+ and NK1.1(−) iNKT cells (CD45+ and CD1d-tetramer/PBS57+) was analyzed on cells from a forward and side scatter lymphocyte gate. (A) Absolute cell number of liver and spleen NK1.1(+) (gray bar) and NK1.1(−) iNKT cells (black bar) were determined as follows: (number of lymphocytes) × (percent of iNKT cell subset determined in A). (B) Percent of NK1.1(−) and NK1.1(+) iNKT cells. The bar graph for 24h and dot plot represents the mean ± SEM of 12 individual animals from 4 independent experiments. The bar graph for 2h represents the mean ± SEM of 6 individual animals from 2 independent experiments.
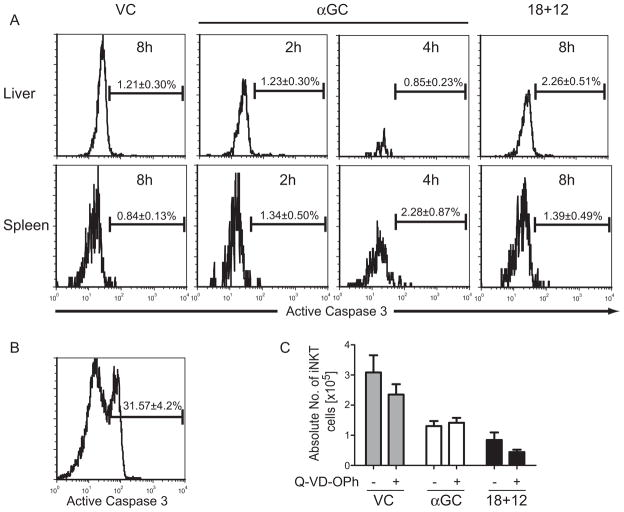
Previous studies found HIV (20,21) and HCV (22) infections caused the loss of iNKT cells in humans. Furthermore, LCMV infection of mice caused the loss of iNKT cells by caspase 3 mediated apoptosis (23,24). To determine if the loss of iNKT cells following αGC or IL-18+IL-12 was due to caspase 3-mediated apoptosis, mice were treated with VC, αGC or IL-18+IL-12 and leukocytes were isolated from the livers and spleens at 2 and 4h for αGC and 4, 6 and 8h for VC and IL-18+IL-12 treatment groups. Isolated leukocytes were surfaced stained with CD1d-tet and intracellularly stained with anti-active caspase 3 (Figure 2A). No change in active caspase 3 was detected in iNKT cells from the liver and spleen of mice treated with αGC for 2 and 4h despite a greater than 50% loss by 2h and an almost complete loss of liver iNKT cells by 4h. In addition, no change in active caspase 3 was detected in iNKT cells from the liver and spleen of mice treated with IL-18+IL-12 for 4, 6 (data not shown) and 8h (Figure 2A). In contrast, control liver leukocytes cultured in the presence of 5μM staurosporine for 4h had significant levels of intracellular active caspase 3, signifying these cells were undergoing apoptosis (25) (Figure 2B). As a second approach to determine whether liver iNKT cells were cleared by apoptosis, mice were pre-treated for 2h with broad-spectrum caspase inhibitor Q-VD-OPh to block caspase mediated apoptosis (26) and then treated with VC, αGC and IL-18+IL-12. iNKT cells from the liver of treated mice were quantitated by staining isolated liver leukocytes with CD1-tet. As shown in Figure 2C, caspase inhibitor Q-VD-OPh was unable to prevent the loss of iNKT cells following αGC treatment at 2h or IL-18+IL-12 treatment at 8h. Thus activation of iNKT cells did not induce detectable levels of active caspase 3 and blocking broad-spectrum capases was unable to prevent the loss of iNKT cells, suggesting this loss was by a mechanism other then caspase 3 mediated apoptosis.
Figure 2. αGC and IL-18+IL-12 induced loss of iNKT cells is independent of caspase mediated apoptosis.
(A) Mice were treated for 2 and 4h with αGC or 8h with VC or IL-18+IL-12 and leukocytes were isolated from the liver and spleens. Levels of active caspase 3 in iNKT cells was determined by surface staining leukocytes with CD1d-tet followed by intracellular staining with active caspase 3 and analyzed by flow cytometry. The histogram represents the mean ± SEM of 6 individual animals from 2 independent experiments for αGC treatment at 2h and IL-18+IL-12 at 8h and mean ± SEM of 3 individual animals for αGC treatment at 4h. (B) Total liver leukocytes were treated in vitro with 5μM staurosporine for 4h. The histogram in represents the mean ± SEM of 3 technical replicates. (C) Liver iNKT cells were quantitated from mice pretreated 2h with DMSO diluent ± Q-VD-OPh and then treated with VC or IL-18+IL-12 for 8h or αGC for 2h. The bar graphs represent the mean ± SEM of 6 individual animals from 2 independent experiments.
IL-18+IL-12 and αGC induced the loss of iNKT cells selectively in the liver
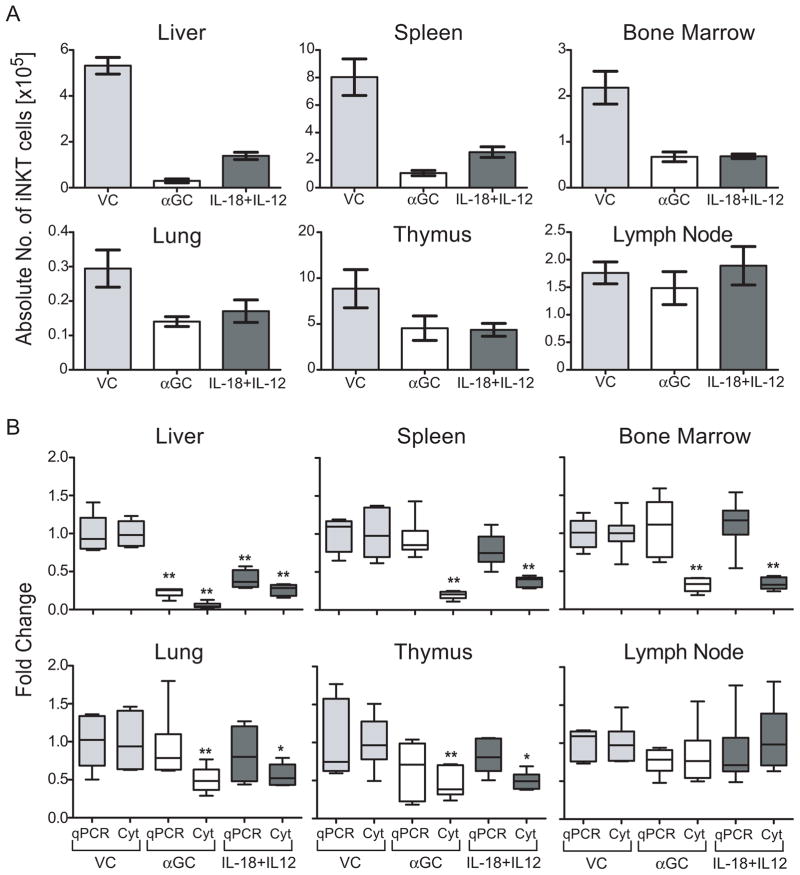
Because iNKT cells were differentially regulated in spleen and liver following αGC or IL-18+IL-12 treatment of mice, we systemically examined their regulation in tissues where iNKT cells are known to reside or accumulate. Mice were treated with VC, αGC or IL-18+IL-12 and leukocytes were isolated from liver, spleen, bone marrow, lung, thymus and lymph nodes. The absolute number of iNKT cells was then determined by flow cytometric analysis of cells that stain with CD1d-tet (Figure 3A). All tissues examined, except lymph nodes, showed a decrease in CD1d-tet visualized iNKT cells following αGC and IL-18+IL-12 treatment. Instead of apoptosis, treatment of mice with αGC has been shown to cause TCR internalization that renders iNKT cells undetectable by flow cytometric techniques (16–18). Thus to rigorously investigate iNKT cell regulation, we compared the fold change of qPCR genomic levels of Vα14 Jα18 TCR gene arrangement (specific to iNKT cells) with fold change in CD1d-tet visualized iNKT cells using flow cytometric analysis. Treatment of mice with αGC reduced, at 24h, the level of CD1d-tet-visualized iNKT cells in lung as well as previously reported tissues, including liver, spleen, bone marrow and thymus, but not lymph nodes (16,17), as determined by flow cytometric analysis (Figure 3B). IL-18+IL-12 also decreased the number of detectable iNKT cells in all of the examined tissues except lymph nodes. However, qPCR analysis revealed the liver was the only tissue that had a significant reduction in the level of iNKT cell-specific Jα14 Vα18 TCR gene arrangement following either IL-18+IL-12 or αGC treatment (Figure 3B). Notably, for both qPCR and flow cytometric analysis, we evaluated the fold change per organ by standardizing the quantitation with the overall cell number in order to avoid treatment-induced changes in total cell numbers that would mask specific iNKT cell changes.
Figure 3. αGC or IL-18+IL-12 activated iNKT cells are cleared specifically in the liver.
Leukocytes were isolated from indicated tissues 24h after treatment with VC, αGC or IL-18+IL-12 and divided to analyze iNKT cell levels by flow cytometric analysis (Cyt) of the absolute number of iNKT cells (A) and fold change (B) as described in figure 1 and (B) qPCR genomic analysis of the Vα14Jα18 TCR arrangement levels. The fold calculation is described in the materials and methods section. For flow cytometric analysis of iNKT cell fold change, absolute number of iNKT cells were determined as described in figure 1A. The boxplot graph depicts the five-number summaries (sample minimum, lower quartile, median, upper quartile, and sample maximum) of 6 individual animals from two independent experiments. *, P<0.05 and **, P<0.01
Taken together these results reveal that the liver microenvironment is distinct from other organ sites for the depletion of activated iNKT cells. While acute activation with αGC or IL-18+IL-12 caused iNKT cell detection to be lost by TCR internalization in most organs, iNKT cells in the liver were almost entirely depleted with αGC and substantially depleted with IL18+IL12 treatments.
Expansion of iNKT cells in the liver following αGC but not IL-18+IL-12 treatment requires an intact spleen and IFNγ
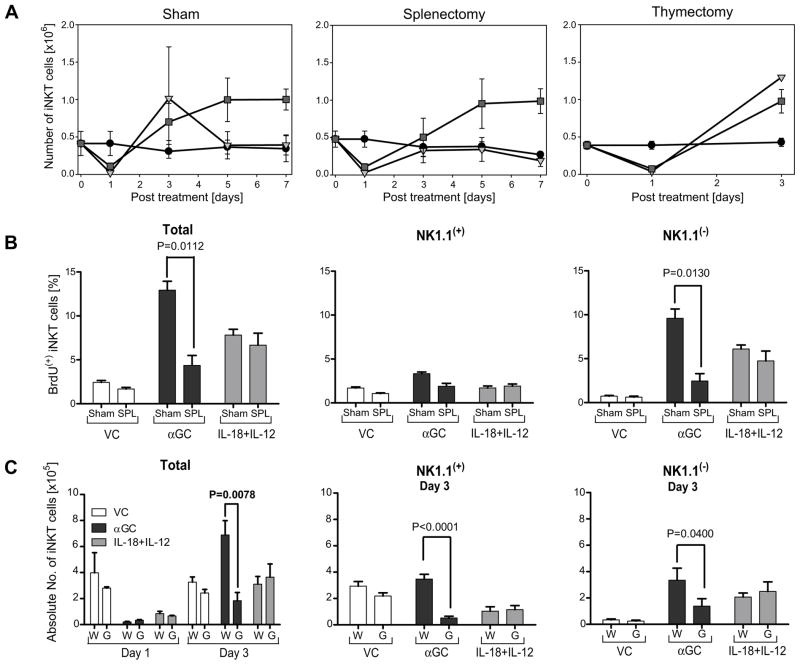
Treatment of mice with αGC initiates a well-defined sequence of iNKT modulation, beginning with rapid disappearance at day 1, followed by expansion by day 3 and finally a Bim-dependent contraction by about day 5 (27). To determine whether IL-18+IL-12 treatment induces expansion and contraction of liver iNKT cells similar to αGC, mice were treated with VC, αGC or IL-18+IL-12 and then leukocytes were isolated from the liver at days 0, 1, 3, 5 and 7. Both IL-18+IL-12 and αGC treatment decreased iNKT cells at day 1, followed by expansion by day 3 as compared to VC. However, liver iNKT cell levels from αGC-treated mice returned to baseline by day 5, while IL-18+IL-12 treated mice maintained peak levels of liver iNKT cells that persisted at least through day 7 (Figure 4A; left panel).
Figure 4. iNKT cell expansion in the liver caused by αGC or IL-18+IL-12 treatment utilized different mechanisms.
(A) Liver leukocytes from sham control, splenectomized and thymectomized mice treated with VC, αGC or IL-18+IL-12 were analyzed for the number of iNKT cells by FCA at indicated times as described in figure 1. Line graph of sham and splenectomy experiments for days 0, 1 and 3 represent 9 individual animals from 3 independent experiments and days 5 and 7 represent 3 individual animals. Line graph for thymectomy experiment represent 4 animals from 2 individual experiments. The symbol and error bar depict mean ± standard error. (B) Proliferating iNKT cell NK1.1(+/−) subset analysis. Sham control (Sham) and splenectomized mice (SPL) were treated with VC, αGC or IL-18+IL-12 on day 0 and BrdU on days 1 and 2. On day 3 the proliferating NK1.1(+) and NK1.1(−) iNKT cells was determined using FCA of the percent of iNKT cells that incorporated BrdU. Bars depict mean ± standard error representing 6 individual animals from 2 independent experiments. (C) Wild type (W) and IFNγ(−/−) mice (G) were treated with VC, αGC or IL-18+IL-12 and then on days 1 and 3 leukocytes were isolated from the liver and total iNKT cell numbers or on day 3 NK1.1(+) and NK1.1(−) iNKT cell numbers were determined as described in figure 1. Bars depict mean ± SEM representing 9 individual animals from 3 independent experiments.
Previous reports have proposed that αGC causes iNKT cells to simultaneously internalize TCR receptors and proliferate, rendering their expansion undetectable to conventional staining of cell surface molecules. Not until day 3 is iNKT cell expansion revealed by TCR re-expression (16–18). However, results presented here (Figure 3) demonstrate that iNKT cells are depleted in the liver following αGC and IL-18+IL-12 treatment, suggesting proliferating resident liver iNKT cells are not likely the major source of expansion, but instead, more likely the result of recruitment from other sites. Two of the possible sites from which iNKT cells may be recruited are the spleen, which hosts a large number of iNKT cells, and the thymus, which is the site of iNKT cell development. To test these hypotheses, we analyzed iNKT cell expansion in αGC and IL-18+IL-12 treated mice that had been previously thymectomized (thx) or splenectomized (splx). We found expansion of iNKT cells at day 3 was not compromised in thx mice treated with αGC, consistent with a previous report (17), or with IL-18+IL-12 (Figure 4A; right panel). In contrast, splx mice did not expand iNKT cells following αGC treatment and only partially increased iNKT cell numbers with IL-18+IL-12 treatment as compared to respectively treated sham control mice (Figure 4A; center panel). This finding is consistent with recruitment either from the spleen or via the spleen as the source of iNKT cell expansion in the liver following αGC treatment. Because IL-18+IL-12-induced expansion was only partially blocked in splx mice this suggests at least some of the increase in response to this type of activation could be due to proliferating resident iNKT cells (Figure 4A; center panel). Additionally, the bone marrow could contribute to the expansion of liver iNKT cells as this site has been previously reported to be a source for IL-12-induced expansion of liver NKT cells (defined by CD3(+) NK1.1(+) cells) (10).
Because NK1.1(−) iNKT cells persist in the liver following IL-18+IL-12 treatment, and their expansion only partially requires an intact spleen, we hypothesized that a portion of iNKT cell expansion could arise from NK1.1(−) iNKT proliferation. To test this hypothesis and determine whether αGC induces the recruitment of proliferating NK1.1(−) iNKT cells, we treated splx and sham control mice with αGC or IL-18+IL-12 on day 0 and BrdU on days 1 and 2. BrdU incorporation by NK1.1(+) or (−) iNKT cells was then examined on day 3. Approximately 75% of the proliferating liver iNKT in αGC and IL-18+IL-12 treated mice were found to be NK1.1(−) (Figure 4B; left panel). Splenectomized mice treated with αGC had a significantly lower proportion of proliferating liver NK1.1(−) iNKT cells than sham control mice, again suggesting that αGC-induced expansion of liver iNKT cells is due to proliferating iNKT cells recruited to the liver via the spleen. However, splx mice treated with IL-18+IL-12 were virtually identical to sham control mice in the proportion of proliferating iNKT cells (Figure 4B; right panel), suggesting IL-18+IL-12-induced expansion is at least partially due to proliferating resident iNKT cells.
Both αGC and IL-18+IL-12 induce IFNγ that in turn can upregulate adhesion molecules, chemokine and chemokine receptors necessary for recruitment (28,29). Thus we investigated whether IFNγ was a possible effector cytokine for αGC-induced hepatic recruitment of iNKT cells. WT and IFNγ−/− mice were treated with αGC and IL-18+IL-12 on day 0 and the total number of liver iNKT cells was determined on days 1 and 3, and NK1.1(+) and NK1.1(−) iNKT cells were determined on day 3. Neither NK1.1(+) nor NK1.1(−) iNKT cells expanded in IFNγ−/− mice following αGC treatment (Figure 4C). However, no difference in iNKT cell expansion at day 3 was observed between WT and IFNγ−/− mice treated with IL-18+IL-12. Collectively, these data indicate qualitatively different mechanisms exist between TCR and cytokine activation-induced expansion of liver iNKT cells.
αGC and IL-18+IL-12 activation causes divergent phenotypic reshaping of liver iNKT cells
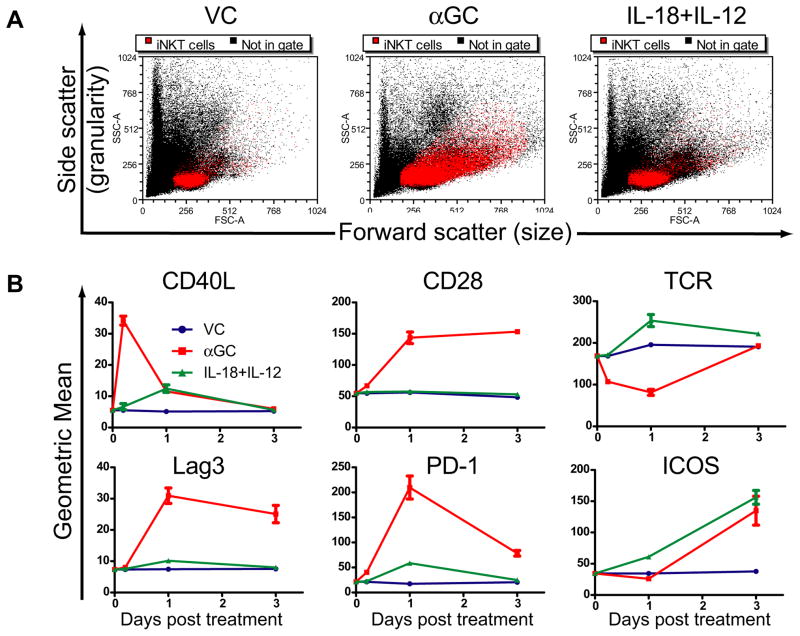
Because αGC and IL-18+IL-12 treatments induced the proliferation of NK1.1(−) iNKT cells (Figure 4B), we examined whether these treatments induced other phenotypic changes in liver iNKT cells. Liver iNKT cell size and granularity was evaluated three days after treatment with VC, αGC or IL-18+IL-12. αGC caused a substantial increase in iNKT cell size and granularity compared to VC and IL-18+IL-12 (Figure 5A). We next investigated activation-induced changes in cell surface markers known to regulate T cell functions at 2, 24 and 72h. IL-18+IL-12 treatment caused a pronounced increase in iNKT cell TCR and ICOS levels by day 1, demonstrating this treatment induces iNKT cells with an effector phenotype (Figure 5B). As previously reported (30) and we report here αGC caused upregulation of costimulatory receptors ICOS and CD28. However, the increase in these molecules was in conjunction with an immediate decrease in TCR levels, an increase in the suppressive surface molecule Lag3 and, as previously reported, PD-1 expression (31). We also analyzed CD40L expression by iNKT cells, since this signaling pathway is a major effector mechanism by which αGC induces a proinflammatory cytokine cascade (32). Interestingly, IL-18+IL-12 did not induce significant levels of CD40L, while αGC transiently induced CD40L expression that was lost as PD-1 and Lag3 molecules increased. Collectively, these data demonstrate αGC or IL-18+IL-12 treatments differentially regulate activating and inhibitory surface molecules on iNKT cells in the liver microenvironment, causing a shift in phenotype.
Figure 5. Phenotype of iNKT cells from the liver are differentially reshaped by αGC vs IL-18+IL-12 treatment of mice.
(A) Leukocytes were isolated from the liver of mice three days after treatment with vc, αGC or IL-18+IL-12. Size and granularity of iNKT cells (red dots) was visualized by side and forward flow cytometry scatter, respectively, on back gated lymphocytes that stained double positive for CD1d-tet and CD45 markers. The dot plots shown are representative of 9 animals from three independent experiments. (B) Expression levels iNKT cell effector and regulatory surface markers. Leukocytes from the liver of mice treated with vc, αGC or IL-18+IL-12 were isolated at 2h, day 1 and day 3 and cell were stained with mAbs for indicated surface markers and iNKT cell markers as described in A. The line graph represents the mean ± SEM of 6 individual animals from 2 independent experiments.
αGC, but not IL-18+IL-12, treatment requires an intact spleen for maximal induction of inhibitory and activating co-receptors
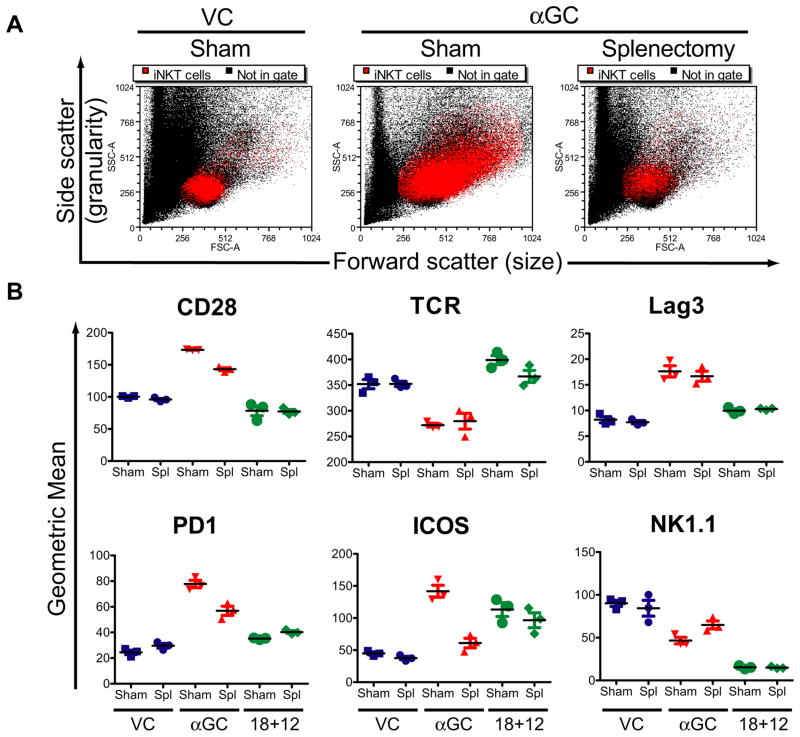
We next compared the activation phenotype of resident vs recruited liver iNKT cells following αGC or IL-18+IL-12 treatment of sham control and splenectomized mice. Sham control and splx mice were treated with αGC and on day 3 the size and granularity of liver iNKT cells were analyzed by flow cytometry. αGC treatment of sham control mice increased iNKT cell size and granularity in sham control mice but not in splx mice (Figure 6A), suggesting iNKT cells recruited to the liver are activated differentially compared to iNKT cells resident to the liver. We therefore compared cell surface marker expression on resident and recruited iNKT cells from the liver 3 days after treatment with αGC and IL-18+IL-12 (Figure 6B). Surface expression levels of TCR, ICOS and NK1.1 were comparable on liver iNKT cells from IL-18+IL-12 treated splx and sham control mice (Figure 6B). In contrast, iNKT cells from splx mice treated with αGC had reduced expression levels of CD28, PD-1 and ICOS and slightly more NK1.1 expression compared to sham control mice treated with αGC. Thus, recruitment via the spleen following αGC, but not IL-18+IL-12, treatment is needed to repopulate the liver with iNKT cells expressing maximal levels of activating and inhibitory receptors.
Figure 6. αGC modulation of cell surface markers are differentially regulated on resident vs recruited liver iNKT cells.
Sham control or splenectomized mice were treated with VC, αGC or IL-18+IL-12 and 3 days latter leukocytes were isolated from the liver and (A) size and granularity of iNKT cells was determined as described in figure 5A and (B) CD28, TCR, Lag3, PD-1, ICOS and NK1.1 expression levels was determined as described in 5B. The dot plots shown are a representative experiment of 3 animals that was repeated with similar results. The dot plot represents the mean ± SEM of 3 individual animals.
Chronic treatment with αGC or IL-18+IL-12 induces a profound and systemic iNKT cell depletion that requires the thymus for repopulation of the liver
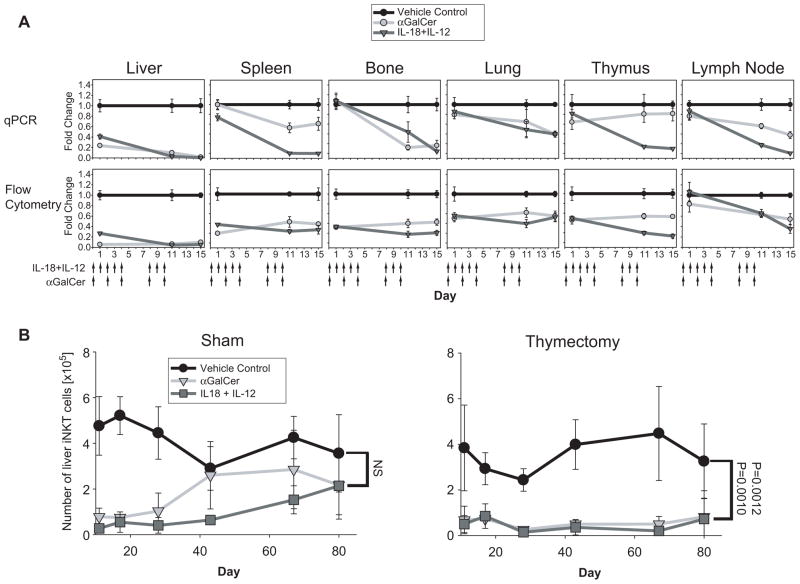
Because αGC and IL-18+IL-12 treatment causes depletion of liver iNKT cells, we tested whether continual removal of iNKT cells in the liver induced by chronic αGC or IL-18+IL-12 treatment would exhaust systemic iNKT cells. Using qPCR and flow cytometric analysis (FCA), we examined changes in iNKT cells in the liver, spleen, bone marrow, lung, thymus and lymph nodes of mice treated chronically with αGC on days 0,2,4,8 and 10 or IL-18+IL-12 on days 0–4 and 8–10. In all tissues examined, chronic IL-18+IL-12 treatment profoundly reduced iNKT cell number by the end of treatment as evaluated by both FCA and qPCR analysis, (Figure 7A). Similarly, chronic αGC treatment reduced iNKT cells in all tissues examined, but only a minor reduction was observed in the thymus using qPCR analysis. After cessation of treatment with either agent at day 10, the liver was slowly repopulated with iNKT cells and levels comparable to those of untreated mice were reached between day 40 and 80 (Figure 7B). However, the rate by which iNKT cells repopulated the liver varied with treatment. While liver iNKT cell numbers recovered from chronic αGC treatment by day 43, it took until day 80 before these cells recovered from chronic IL-18+IL-12 treatment, suggesting that the signaling mechanisms induced by IL-18+IL-12 and αGC differentially impact iNKT cell renewal/development (Figure 7B).
Figure 7. Chronic treatment of mice with αGC or IL-18+IL-12 ablates peripheral iNKT cells followed by a protracted repopulation of the liver that requires the thymus.
(A) Mice were treated chronically with αGC on days 0, 2, 4, 8, 10, and VC and IL-18+IL-12 on days 0–4 and 8–10 (chronic treatment regimen). Fold change in iNKT cells relative to VC at indicated time points was determined by qPCR and FCA as in figure 1 using 6 individual animals from 2 independent experiments ± SEM. (B) Liver repopulation of iNKT cell kinetics in sham and thymectomized mice treated chronically, as in A, with VC, αGC and IL-18+IL-12. Liver leukocytes were isolated from the liver at indicated days and the number of iNKT cells was determined by FCA as described in figure 1. Day 80 represents 9 individual animals from 3 independent experiments ± standard error. All other days represent 6–9 animals from 2–3 independent experiments ± SEM.
Early studies using surrogate markers presented evidence that NKT cells could undergo extrathymic development (33–35). With the advent of CD1d tetramers loaded with iNKT cell antigens it was reported that development of iNKT cells occurs in the thymus (36–38). To test whether the iNKT cells that repopulate the liver after the systemic depletion induced by chronic treatment with either agent are newly developed cells in the thymus (36), we compared the efficiency of the repopulation in thx and sham control mice. Unlike sham treated mice, iNKT cells from thx mice treated chronically with αGC or IL-18+IL-12 did not repopulate the liver by day 80 (Figure 7B), demonstrating that thymic development is required to repopulate the liver with iNKT cells following the depletion induced by either agent.
Discussion
In this study, we identify a previously undefined and unique role for the liver in the elimination of iNKT cells following acute activation with αGC or IL-18+IL-12. Following αGC-induced depletion of iNKT cells in the liver our data demonstrated subsequent iNKT cell expansion was likely through the recruitment of iNKT cells via the spleen, while IL-18+IL-12 expansion was only partially dependent on the spleen. Furthermore we show cells that expand in the liver following αGC or IL-18+IL-12 treatment of mice had divergent phenotypes. Finally we found while acute treatment of mice induced transient depletion of liver iNKT cells, chronic αGC or IL-18+IL-12 treatment caused a systemic and persistent depletion of iNKT cells that required an intact thymus for subsequent repopulation of the liver.
Initially it was reported that iNKT cells undergo apoptosis following activation with αGC, anti-CD3 or IL-12 (5,10). More recently, several elegant studies proposed that upon activation with αGC, iNKT internalize their TCR instead of undergoing apoptosis and rapidly expand (16–18). Our data provides evidence that iNKT cells are differentially regulated following activation depending on the microenvironment in which they reside. Our data shows that treatment of mice with αGC induces an almost complete loss of iNKT cells while IL-18+IL-12 induce a partial loss of iNKT cells specifically in the liver. However, TCR internalization by iNKT cells occurs in all other tissues tested except the lymph node as determined by FCA and qPCR analysis. Two of the studies that reported TCR internalization by activated iNKT cells focused on iNKT cells from the spleen, which is in agreement with our findings. Similar to our results, using CD1d-tet staining Crowe et al. found liver iNKT cells disappeared following αGC activation and expanded 3 days later (16). However, in an apparent contrast to our results, they proposed the loss of liver iNKT cells following αGC treatment of mice was due to receptor internalization (16). This discrepancy is likely due to the use of intracellular staining with NK1.1 and αβTCR markers used in that study to detect NKT cells with internalized receptors following αGC activation, while our study used qPCR analysis of genomic levels of Vα14 Jα18 TCR gene arrangement. NK1.1 and αβTCR, markers are not always synonymous with iNKT cells and can be expressed by activated T cells and non-invariant NKT cells (16,28,37,39,40). The use of these markers can be further complicated by the fact that αGC activation of iNKT cells by dendritic cells can cause bystander activation and proliferation of T cells (41,42). Thus the proliferation of NK1.1(+) αβTCR(+) cells, and the IFNγ production by cells that have their αβTCR internalized, could be an αGC-induced bystander effect on T cells or non-invariant NKT cells. However, it is likely that liver iNKT cells are internalizing their TCR following αGC activation but as a precursor to elimination.
From data presented in this report, we propose a more complex model in which the liver can be an important site for the elimination of activated iNKT cells. Consistent with our model, it has been shown that the liver also eliminates activated CD8 T cells (43). In this process, activated CD8 T cells are trapped in the liver primarily due to ICAM interactions and subsequently cleared by apoptosis. Similar to this process, iNKT cells were also shown to undergo migratory arrest in the liver following αGC and IL-18+IL-12 treatment of mice (44). These results suggest unique mechanisms exist in the liver that immobilizes activated iNKT cells with subsequent eradication. Our model, in which activated iNKT cells are eliminated by liver-specific mechanisms, would explain why studies examining iNKT cells in vitro found no apoptosis in response to αGC activation. Consistent with reports that find NKT cells are relatively resistant to apoptosis (45), we found the loss of iNKT cells appears to be independent of caspase 3 mediated apoptosis.
Re-interpretation of the current paradigm of iNKT cell regulation is further supported by data obtained with αGC-induced expansion of iNKT cells. Treatment of mice with αGC induces an expansion of NK1.1(−) iNKT cells which becomes apparent at day 3. While previous studies proposed that αGC-induced expansion was from local proliferation of iNKT cells that had internalized TCR, we clearly show that full expansion of liver NK1.1(−) iNKT cells requires an intact spleen and IFNγ, suggesting recruitment is the mechanism for iNKT cell expansion. Recruitment of iNKT cells to the liver has been previously shown in a study that found sulfatide activation of type II NKT cells caused the recruitment of iNKT cells to the liver in an IL-12 and MIP-2-dependent manner (46). In contrast to αGC treatment, we found acute IL-18+IL-12 treatment induced expansion of iNKT cells was independent of IFNγ and only partially dependent on an intact spleen demonstrating a mechanism distinct from that induced by αGC. A possible explanation for this difference is that IL-18+IL-12 removes only a subset of iNKT cells, unlike αGC treatment that removes all subsets of liver iNKT cells. IL-18+IL-12 causes the loss of the NK1.1(+) subset of iNKT cells either through receptor internalization as previously reported (11,16) or through targeted deletion of this subset. Therefore expansion of iNKT by IL-18+IL-12 could be due to proliferation of the remaining iNKT cells.
Differences between αGC and IL-18+IL-12 activation of iNKT cells are further demonstrated at the contraction phase. Following αGC-induced expansion, iNKT cell numbers contract to baseline around day 5 (27). In contrast, IL-18+IL-12-induced expansion of iNKT cells remained at maximal levels out to at least day 7. Taken together, these results indicate that TCR-dependent and -independent activation induce disparate programmed responses in iNKT cells and illustrate the potential to reshape effector or regulatory functions of these cells. This difference was further highlighted by the shift in iNKT cell phenotype. IL-18+IL-12 treatment induced the expansion of liver iNKT cells that have higher levels of TCR and ICOS both of which can enhance effector functions (47,48), while αGC-induced TCR reduction and increased suppressive molecules PD-1 and Lag3 shown to inhibit iNKT cell proliferation and function (31,49). Differences between αGC and IL-18+IL-12 for the induction of various inhibitory and activating receptors could be due to feed back of specific cytokines these stimuli may differentially induce. For example IL-18+IL-12 preferentially induces iNKT cells to produce IFNγ (50), while αGC induces iNKT cells to produce numerous other cytokines such as IL-4, IL-17 and IL-13 (51–54). Thus iNKT cells phenotype can be reshaped with type of activation.
While acute activation by αGC and IL-18+IL-12 caused a transient loss in liver iNKT cells, we found that chronic stimulation with these agents caused a systemic loss iNKT cells that required an intact thymus for repopulation of the liver. Repopulation of the liver following chronic αGC treatment of mice occurred with faster kinetics than did IL-18+IL-12, which is probably due to αGC and IL-18+IL-12 targeting iNKT cells at different stages of development. While IL-18+IL-12 was shown to disrupt developing cells at an early CD4(−)/CD8(−) double negative stage (3,55), αGC targets iNKT cells that have acquired the properly rearranged TCR, after differentiating from a CD4(−) CD8(−) cell precursor. Furthermore our data shows iNKT cell reduction of thymic iNKT cells by chronic αGC was less then by chronic IL-18+IL-12 treatment.
The data presented in this study show acute or chronic activation of iNKT cells have distinctly different effects on the fate of iNKT cells that is determined by the liver microenvironment. Additionally, chronic TCR-dependent and -independent inflammatory signals can reshape iNKT cell phenotype and frequency.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI/NIH).
We thank Drs. Steve Anderson and Giorgio Trinchieri for their critical editing of the manuscript; Loretta Smith for her assistance with animal experiments.
Abbreviations
- αGC
α-galactosylceramide
- VC
vehicle control
- iNKT cell
invariant NKT cell
- TCR
T-cell receptor
- TNC
total number of cells
- intraperitoneal
i.p
- flow cytometry
FCA
- thymectomized
thx
- splenectomized
splx
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. government.
Reference List
- 1.Kronenberg M. TOWARD AN UNDERSTANDING OF NKT CELL BIOLOGY: Progress and Paradoxes. Annual Review of Immunology. 2005;23:877. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 4.Subleski JJ, V, Hall L, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 2006;66:11005. doi: 10.1158/0008-5472.CAN-06-0811. [DOI] [PubMed] [Google Scholar]
- 5.Leite-de-Moraes MC, Herbelin A, Gouarin C, Koezuka Y, Schneider E, Dy M. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J Immunol. 2000;165:4367. doi: 10.4049/jimmunol.165.8.4367. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA, Fantuzzi G. Interleukin-18 and host defense against infection. J Infect Dis. 2003;187(Suppl 2):S370–S384. doi: 10.1086/374751. [DOI] [PubMed] [Google Scholar]
- 7.Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Both CD1d antigen presentation and interleukin-12 are required to activate natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73:1890. doi: 10.1128/IAI.73.3.1890-1894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leite-de-Moraes MC, Hameg A, Arnould A, Machavoine F, Koezuka Y, Schneider E, Herbelin A, Dy M. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement 1. J Immunol. 1999;163:5871. [PubMed] [Google Scholar]
- 9.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 10.Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3epsilon- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 11.Emoto M, Yoshizawa I, Emoto Y, Takahashi Y, Hurwitz R, Miamoto M, Kaufmann SH. Reversible NK1.1 surface expression on invariant liver natural killer T cells during Listeria monocytogenes infection. Microbes Infect. 2007;9:1511. doi: 10.1016/j.micinf.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Budd RC, Miescher GC, Howe RC, Lees RK, Bron C, MacDonald HR. Developmentally regulated expression of T cell receptor beta chain variable domains in immature thymocytes 1. J Exp Med. 1987;166:577. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai K, Kanno M, Kimoto H, Shigemoto K, Yamamoto S, Taniguchi M. Sequence and expression of transcripts of the T-cell antigen receptor alpha-chain gene in a functional, antigen-specific suppressor-T-cell hybridoma 1. Proc Natl Acad Sci U S A. 1986;83:8708. doi: 10.1073/pnas.83.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules 1. Science. 1994;263:1774. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 15.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells 1. J Exp Med. 1997;186:109. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, Keating R, Kronenberg M, Smyth MJ, Godfrey DI. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171:4020. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 17.Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- 18.Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, Van KL. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A. 2003;100:10913. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motsinger A, Haas DW, Stanic AK, Van KL, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection 1. J Exp Med. 2002;195:869. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandberg JK, Fast NM, Palacios EH, Fennelly G, Dobroszycki J, Palumbo P, Wiznia A, Grant RM, Bhardwaj N, Rosenberg MG, Nixon DF. Selective loss of innate CD4(+) V alpha 24 natural killer T cells in human immunodeficiency virus infection 1. J Virol. 2002;76:7528. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deignan T, Curry MP, Doherty DG, Golden-Mason L, Volkov Y, Norris S, Nolan N, Traynor O, McEntee G, Hegarty JE, O’Farrelly C. Decrease in hepatic CD56(+) T cells and V alpha 24(+) natural killer T cells in chronic hepatitis C viral infection 1. J Hepatol. 2002;37:101. doi: 10.1016/s0168-8278(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 23.Hobbs JA, Cho S, Roberts TJ, Sriram V, Zhang J, Xu M, Brutkiewicz RR. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus 1. J Virol. 2001;75:10746. doi: 10.1128/JVI.75.22.10746-10754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Roberts TJ, Wang CR, Cho S, Brutkiewicz RR. Long-term loss of canonical NKT cells following an acute virus infection. Eur J Immunol. 2005;35:879. doi: 10.1002/eji.200425495. [DOI] [PubMed] [Google Scholar]
- 25.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment 1. Annu Rev Immunol. 1999;17:221. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 26.Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E. Broad-spectrum caspase inhibitors: from myth to reality? 1. Cell Death Differ. 2007;14:387. doi: 10.1038/sj.cdd.4402044. [DOI] [PubMed] [Google Scholar]
- 27.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 29.Subleski JJ, Wiltrout RH, Weiss JM. Application of tissue-specific NK and NKT cell activity for tumor immunotherapy 4. J Autoimmun. 2009;33:275. doi: 10.1016/j.jaut.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneda H, Takeda K, Ota T, Kaduka Y, Akiba H, Ikarashi Y, Wakasugi H, Kronenberg M, Kinoshita K, Yagita H, Okumura K. ICOS costimulates invariant NKT cell activation 2. Biochem Biophys Res Commun. 2005;327:201. doi: 10.1016/j.bbrc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, Kumar V, Wu L, Kaer LV. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells 1. J Immunol. 2009;182:2816. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van KL, Kawano T, Taniguchi M, Nishimura T. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells 4. J Exp Med. 1999;189:1121. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikly K, Dennert G. Evidence for extrathymic development of TNK cells. NK1+ CD3+ cells responsible for acute marrow graft rejection are present in thymus-deficient mice 1. J Immunol. 1992;149:403. [PubMed] [Google Scholar]
- 34.Makino Y, Yamagata N, Sasho T, Adachi Y, Kanno R, Koseki H, Kanno M, Taniguchi M. Extrathymic development of V alpha 14-positive T cells 2. J Exp Med. 1993;177:1399. doi: 10.1084/jem.177.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation 3. J Exp Med. 1995;182:759. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 37.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage 1. J Exp Med. 2002;195:835. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development 1. J Exp Med. 2005;202:485. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNab FW, Pellicci DG, Field K, Besra G, Smyth MJ, Godfrey DI, Berzins SP. Peripheral NK1.1 NKT cells are mature and functionally distinct from their thymic counterparts. J Immunol. 2007;179:6630. doi: 10.4049/jimmunol.179.10.6630. [DOI] [PubMed] [Google Scholar]
- 40.Pellicci DG, Hammond KJ, Coquet J, Kyparissoudis K, Brooks AG, Kedzierska K, Keating R, Turner S, Berzins S, Smyth MJ, Godfrey DI. DX5/CD49b-positive T cells are not synonymous with CD1d-dependent NKT cells 1. J Immunol. 2005;175:4416. doi: 10.4049/jimmunol.175.7.4416. [DOI] [PubMed] [Google Scholar]
- 41.Eberl G, Brawand P, MacDonald HR. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J Immunol. 2000;165:4305. doi: 10.4049/jimmunol.165.8.4305. [DOI] [PubMed] [Google Scholar]
- 42.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 44.Velazquez P, Cameron TO, Kinjo Y, Nagarajan N, Kronenberg M, Dustin ML. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J Immunol. 2008;180:2024. doi: 10.4049/jimmunol.180.4.2024. [DOI] [PubMed] [Google Scholar]
- 45.Seino K, Harada M, Taniguchi M. NKT cells are relatively resistant to apoptosis 1. Trends Immunol. 2004;25:219. doi: 10.1016/j.it.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease 1. J Clin Invest. 2007;117:2302. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akbari O, Stock P, Meyer EH, Freeman GJ, Sharpe AH, Umetsu DT, DeKruyff RH. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival 1. J Immunol. 2008;180:5448. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wasserman HA, Evavold BD. Induction of anergy by antibody blockade of TCR in myelin oligodendrocyte glycoprotein-specific cells 1. J Immunol. 2008;180:7259. doi: 10.4049/jimmunol.180.11.7259. [DOI] [PubMed] [Google Scholar]
- 49.Byun HJ, Jung WW, Lee DS, Kim S, Kim SJ, Park CG, Chung HY, Chun T. Proliferation of activated CD1d-restricted NKT cells is down-modulated by lymphocyte activation gene-3 signaling via cell cycle arrest in S phase 2. Cell Biol Int. 2007;31:257. doi: 10.1016/j.cellbi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18 1. J Immunol. 2000;165:1847. doi: 10.4049/jimmunol.165.4.1847. [DOI] [PubMed] [Google Scholar]
- 51.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 52.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production 1. Science. 1995;270:1845. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 53.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion 1. J Immunol. 2008;180:5167. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Galan MC, Bream JH, Farr A, Young HA. Synergistic effect of IL-2, IL-12, and IL-18 on thymocyte apoptosis and Th1/Th2 cytokine expression. J Immunol. 2005;174:2796. doi: 10.4049/jimmunol.174.5.2796. [DOI] [PubMed] [Google Scholar]