Abstract
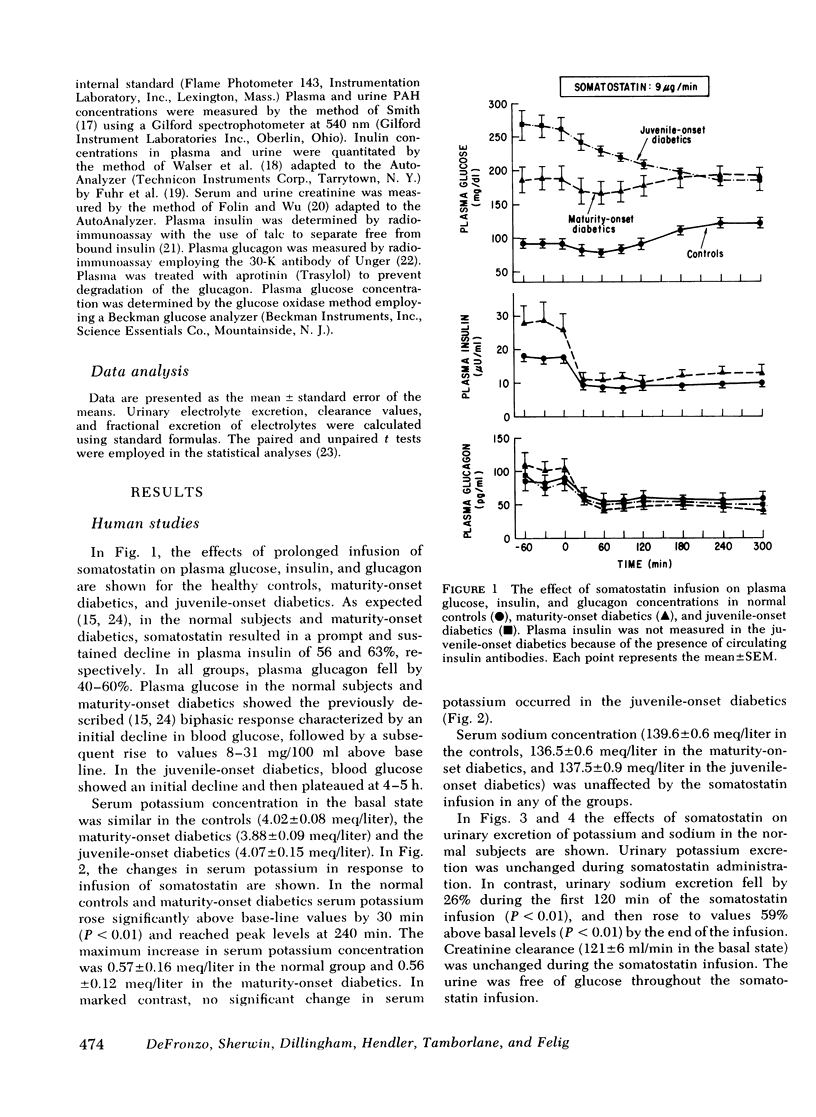
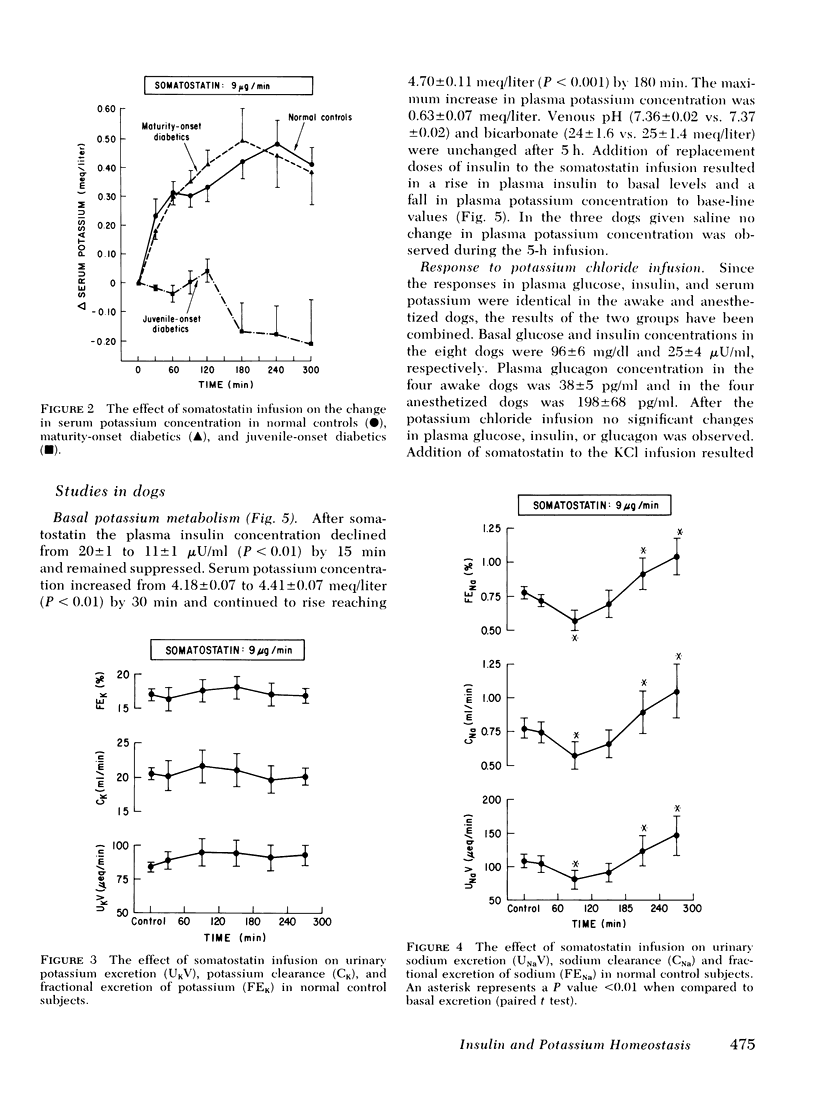
To examine the role of basal insulin and glucagon secretion in potassium and sodium homeostasis, somatostatin, a potent inhibitor of insulin and glucagon secretion, was infused for 5 h into healthy human subjects, maturity-onset diabetes, juvenile-onset diabetics, and normal dogs. Infusion of somatostatin resulted in an increase in serum potassium (0.5-0.6 meq/liter) in normal subjects and maturity-onset diabetics, but not in juvenile-onset diabetics despite equivalent reductions in plasma glucagon in all three groups. A similar rise in serum potassium was observed in normal conscious dogs given somatostatin and was reversed by insulin replacement. Urinary excretion of potassium was unaffected by somatostatin.
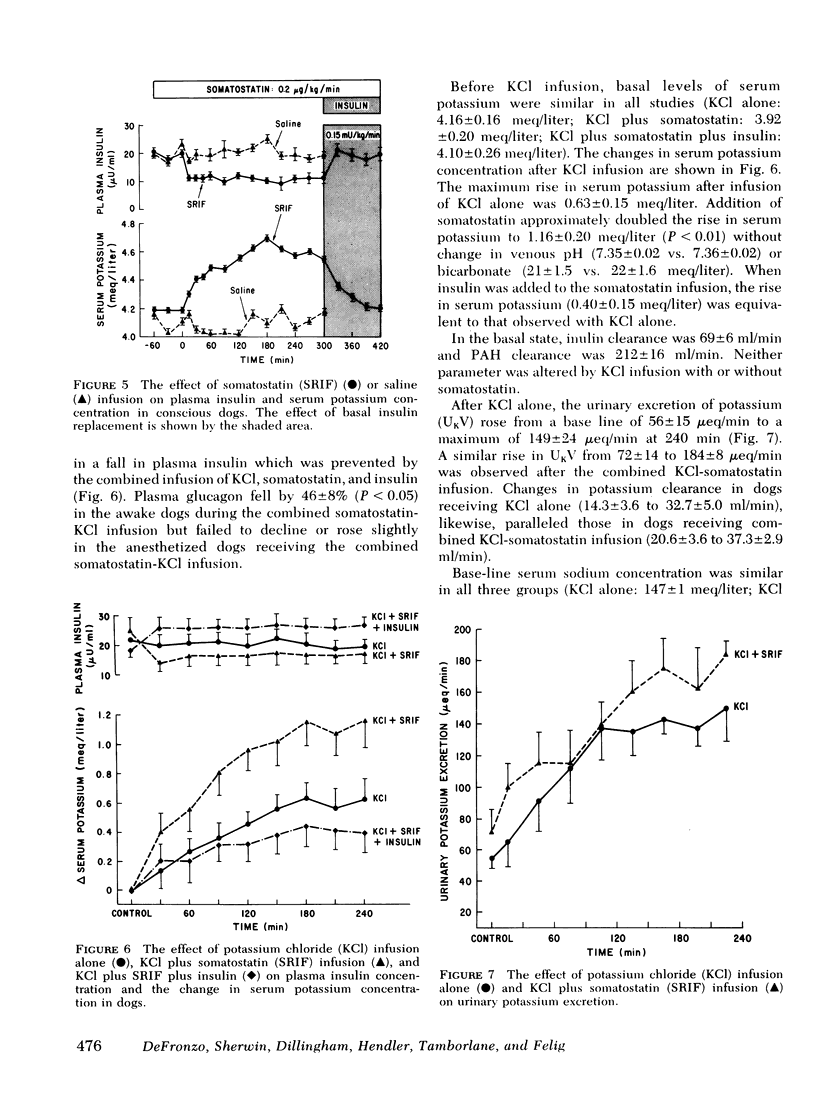
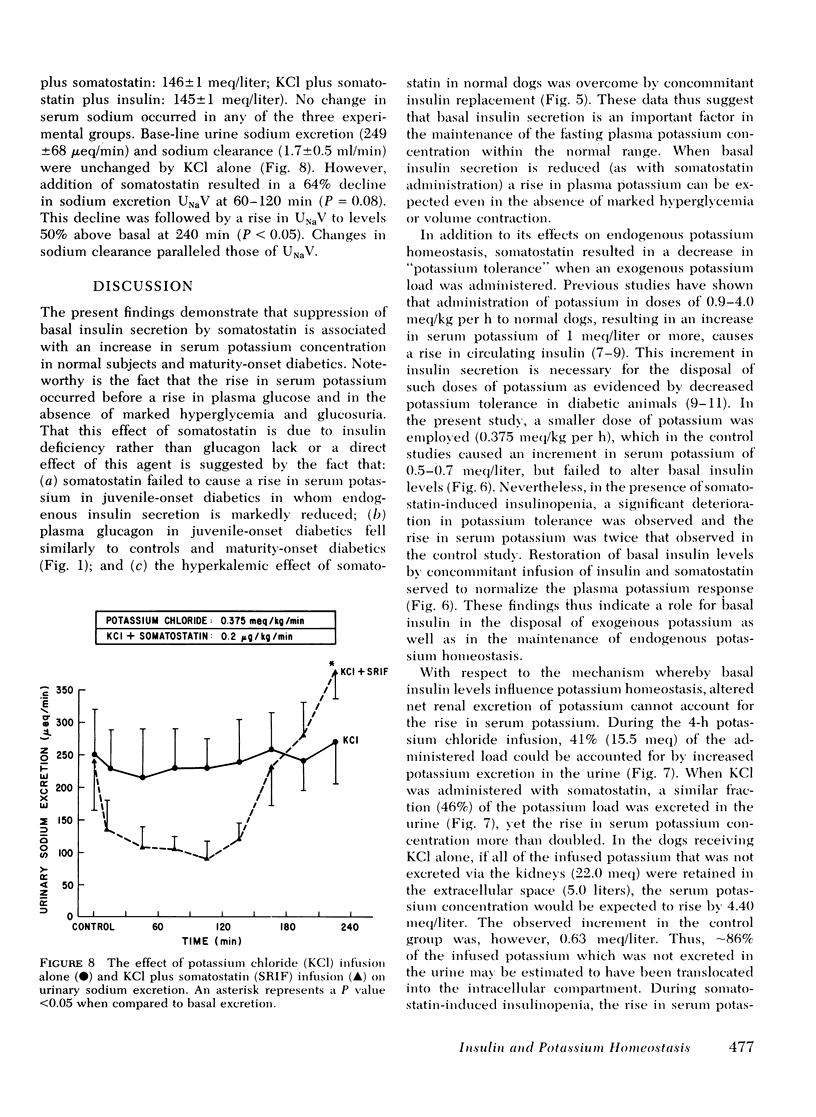
In dogs given intravenous potassium chloride in doses (0.375 meq/kg per h) which do not alter basal insulin levels, the rise in serum potassium (0.6 meq/liter in controls) increased 100% when somatostatin was administered together with the KCl infusion. Addition of replacement doses of insulin to the somatostatin infusion resulted in increments in serum potassium which were comparable to infusion of KCl alone. Urinary potassium excretion rose after KCl administration and was unchanged by the addition of somatostatin.
Serum sodium concentration was unaffected by somatostatin administration in both the human and dog studies. However, urinary sodium excretion displayed a biphasic response falling by 20-60% within the first 2 h of somatostatin administration and then rising to values 50-80% above basal levels at 3-4 h. Inulin and p-aminohippurate clearances were unaffected by somatostatin.
It is concluded that (a) potassium homeostasis is influenced by basal insulin levels in the absence of which serum potassium concentration rises and potassium tolerance declines; (b) this effect of insulin is mediated via extrarenal mechanisms of potassium disposal; (c) somatostatin has a biphasic effect on urinary sodium secretion, the mechanism of which remains to be established.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., BALTZAN M. A., CADER G., ZIERLER K. L. Effect of insulin on carbohydrate metabolism and on potassium in the forearm of man. J Clin Invest. 1962 Jan;41:108–115. doi: 10.1172/JCI104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Cooke C. R., Andres R., Faloona G. R., Davis P. J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975 Apr;55(4):845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Goldberg M., Agus Z. S. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976 Jul;58(1):83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Sherwin R. S., Felig P., Bia M. Nonuremic diabetic hyperkalemia. Possible role of insulin deficiency. Arch Intern Med. 1977 Jul;137(7):842–843. doi: 10.1001/archinte.137.7.842. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Sherwin R., Hendler R. Insulin, glucagon, and somatostatin in normal physiology and diabetes mellitus. Diabetes. 1976 Dec;25(12):1091–1099. doi: 10.2337/diab.25.12.1091. [DOI] [PubMed] [Google Scholar]
- Giebisch G. Some reflections on the mechanism of renal tubular potassium transport. Yale J Biol Med. 1975 Sep;48(4):315–336. [PMC free article] [PubMed] [Google Scholar]
- Goldfarb S., Cox M., Singer I., Goldberg M. Acute hyperkalemia induced by hyperglycemia: hormonal mechanisms. Ann Intern Med. 1976 Apr;84(4):426–432. doi: 10.7326/0003-4819-84-4-426. [DOI] [PubMed] [Google Scholar]
- Hiatt N., Davidson M. B., Bonorris G. The effect of potassium chloride infusion on insulin secretion in vivo. Horm Metab Res. 1972 Mar;4(2):64–68. doi: 10.1055/s-0028-1094101. [DOI] [PubMed] [Google Scholar]
- Hiatt N., Morgenstern L., Davidson M. B., Bonorris G., Miller A. Role of insulin in the transfer of infused potassium to tissue. Horm Metab Res. 1973 Mar;5(2):84–88. doi: 10.1055/s-0028-1093988. [DOI] [PubMed] [Google Scholar]
- Himathongkam T., Dluhy R. G., Williams G. H. Potassim-aldosterone-renin interrelationships. J Clin Endocrinol Metab. 1975 Jul;41(1):153–159. doi: 10.1210/jcem-41-1-153. [DOI] [PubMed] [Google Scholar]
- Levy M., Starr N. L. The mechanism of glucagon-induced natriuresis in dogs. Kidney Int. 1972 Aug;2(2):76–84. doi: 10.1038/ki.1972.74. [DOI] [PubMed] [Google Scholar]
- Michelis M. F., Murdaugh H. V. Selective hypoaldosteronism. An editorial revisited after 15 years. Am J Med. 1975 Jul;59(1):1–5. doi: 10.1016/0002-9343(75)90314-9. [DOI] [PubMed] [Google Scholar]
- Perez G. O., Lespier L., Jacobi J., Oster J. R., Katz F. H., Vaamonde C. A., Fishman L. M. Hyporeninemia and hypoaldosteronism in diabetes mellitus. Arch Intern Med. 1977 Jul;137(7):852–855. [PubMed] [Google Scholar]
- Pettit G. W., Vick R. L. Contribution of pancreatic insulin to extrarenal potassium homeostasis: a tow-compartment model. Am J Physiol. 1974 Feb;226(2):319–324. doi: 10.1152/ajplegacy.1974.226.2.319. [DOI] [PubMed] [Google Scholar]
- Pettit G. W., Vick R. L., Swander A. M. Plasma K plus and insulin: changes during KCl infusion in normal and nephrectomized dogs. Am J Physiol. 1975 Jan;228(1):107–109. doi: 10.1152/ajplegacy.1975.228.1.107. [DOI] [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- Santeusanio F., Faloona G. R., Knochel J. P., Unger R. H. Evidence for a role of endogenous insulin and glucagon in the regulation of potassium homeostasis. J Lab Clin Med. 1973 Jun;81(6):809–817. [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R., DeFronzo R., Wahren J., Felic P. Glucose homeostasis during prolonged suppression of glucagon and insulin secretion by somatostatin. Proc Natl Acad Sci U S A. 1977 Jan;74(1):348–352. doi: 10.1073/pnas.74.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M., DAVIDSON D. G., ORLOFF J. The renal clearance of alkali-stable inulin. J Clin Invest. 1955 Oct;34(10):1520–1523. doi: 10.1172/JCI103204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann P., Maxwell M. H., Rowe P., Winer R., Massry S. G. Role of the renin-angiotensin-aldosterone system in the regulation of plasma potassium in chronic renal disease. Nephron. 1975;15(1):35–49. doi: 10.1159/000180491. [DOI] [PubMed] [Google Scholar]
- Wise J. K., Hendler R., Felig P. Influence of glucocorticoids on glucagon secretion and plasma amino acid concentrations in man. J Clin Invest. 1973 Nov;52(11):2774–2782. doi: 10.1172/JCI107473. [DOI] [PMC free article] [PubMed] [Google Scholar]


