Abstract
Increased apoptotic death of gastric epithelial cells is a hallmark of H. pylori infection, and altered epithelial cell turnover is an important contributor to gastric carcinogenesis. To address the fate of apoptotic gastric epithelial cells and their role in H. pylori mucosal disease, we investigated phagocyte clearance of apoptotic gastric epithelial cells in H. pylori infection. Human gastric mononuclear phagocytes were analyzed for their ability to take up apoptotic epithelial cells in vivo using immunofluorescence analysis. We then used primary human gastric epithelial cells induced to undergo apoptosis by exposure to live H. pylori to study apoptotic cell uptake by autologous monocyte-derived macrophages. We show that HLA-DR+ mononuclear phagocytes in human gastric mucosa contain cytokeratin-positive and TUNEL-positive apoptotic epithelial cell material, indicating that gastric phagocytes are involved in apoptotic epithelial cell clearance. We further show that H. pylori both increased apoptosis in primary gastric epithelial cells and decreased phagocytosis of the apoptotic epithelial cells by autologous monocyte-derived macrophages. Reduced macrophage clearance of apoptotic cells was mediated in part by H. pylori-induced macrophage TNF-α, which was expressed at higher levels in H. pylori-infected, compared to uninfected, gastric mucosa. Importantly, we show that H. pylori-infected gastric mucosa contained significantly higher numbers of apoptotic epithelial cells and higher levels of non-phagocytosed TUNEL-positive apoptotic material, consistent with a defect in apoptotic cell clearance. Thus, as shown in other autoimmune and chronic inflammatory diseases, insufficient phagocyte clearance may contribute to the chronic and self-perpetuating inflammation in human H. pylori infection.
Introduction
Increased apoptosis of gastric epithelial cells is a hallmark of human H. pylori gastritis (1). Multiple pathways and bacterial virulence factors that cause H. pylori-induced apoptosis have been identified, including enhanced production of reactive oxygen species (2), upregulation of Fas receptor (3), disruption of mitochondrial membranes by H. pylori VacA (4, 5), and cross-linking of major histocompatibility complex class II molecules by H. pylori urease (6). Enhanced apoptosis during prolonged H. pylori infection provides a persistent stimulus for epithelial cell proliferation, a key process in the cascade of carcinogenic events that promote gastric cancer (1, 7). Microbe-stimulated apoptosis also may cause the induction of T helper 17 (Th17) cells (8), crucial cellular contributors to gastric pathology in H. pylori infection (9). However, despite the contribution of H. pylori-induced epithelial cell apoptosis to gastric inflammation and carcinogenesis, the fate of apoptotic epithelial cells in inflamed gastric mucosa is not known.
Macrophage and dendritic cell (DC) removal of dying and dead cells prevents the release of pro-inflammatory signals (10) and is a prerequisite for the maintenance of tissue homeostasis (11). Impaired removal of apoptotic cells allows the dying cell to progress to secondary necrosis, resulting in the loss of membrane integrity and the release of intracellular pro-inflammatory molecules and autoantigens (12). Defective removal of apoptotic cells has been linked to autoimmune syndromes and chronic inflammatory diseases such as systemic lupus erythematosis (SLE) (13). However, in the gastrointestinal tract, apoptotic cell removal occurs not only by phagocytic uptake (14, 15) but also by luminal extrusion independent of phagocyte activity (16), or a combination of both (17). Also, the mechanisms of apoptotic epithelial cell elimination differ between species and between individual regions of the gastrointestinal tract (15, 18). Notably, apoptotic epithelial cell removal in the human gastric mucosa has received little investigative attention.
We previously isolated and characterized HLA-DR+/CD11c+/− mononuclear phagocytes from human gastric mucosa and showed that these cells take up H. pylori bacteria and promote Th1 responses to H. pylori (19, 20). Here we show that normal human gastric mononuclear phagocytes also are involved in the clearance of gastric epithelial cells that have undergone apoptosis. However, prior exposure of phagocytes to H. pylori impairs the cells’ ability, in a TNF-α-dependent manner, to subsequently phagocytose H. pylori-treated apoptotic epithelial cells, resulting in an accumulation of non-phagocytosed apoptotic material in the gastric lamina propria of H. pylori-infected individuals. Thus, H. pylori up-regulates programmed cell death of gastric epithelial cells and down-regulates programmed cell removal of apoptotic epithelial cells by macrophages, features that may provide a potent source of autoimmune stimulatory activity in chronic H. pylori infection.
Materials and Methods
Tissue specimens
Gastric tissue specimens for cell isolation and histological analyses were obtained with Institutional Review Board (IRB) approval and informed consent from adult subjects at the University of Alabama at Birmingham undergoing elective gastric bypass for obesity or diagnostic esophagogastroduodenoscopy. Absence of active and past H. pylori infection was determined by negative serological analysis and/or rapid urease CLO test (Kimberly-Clark, Roswell, GA). Heparinized blood samples were obtained from the same patients. Biopsy specimens for quantitative real-time PCR analysis and tissue microarrays for TUNEL analysis were obtained with IRB approval from adult subjects with abdominal symptoms residing in Santiago, Chile (Supplemental Table I). Exclusion criteria included (a) use of antibiotics, antacid, H2-blocker, proton-pump inhibitor, bismuth compound, non-steroidal anti-inflammatory drug or immunosuppressive agent during the two weeks prior to endoscopy; and (b) stool examination positive for ova or parasites. H. pylori status was determined by rapid urease test and microscopic evaluation, and a study subject was judged colonized with H. pylori if one or both tests were positive for the bacteria.
Cell isolation and culture
Cultures of primary human gastric epithelial cells were prepared as previously described by Smoot et al. (21). Briefly, 10 – 20 gastric biopsies or 1 g of gastric mucosa obtained from gastric bypass donors were minced with a scalpel blade and digested for 1 h at 37°C, 200 rpm, with a digestion solution containing RPMI1640, collagenase (0.5 FALGPA units/mL; Sigma, St. Louis, MO), dispase (1.25 U/mL; Roche, Mannheim, Germany), DNAse (0.2 mg/mL; Sigma) and BSA (0.3%; Fisher, Fair Lawn, NJ). Recovered cells were suspended in F12K medium containing 10% FBS, amphotericin (125 ng/mL), penicillin (100 U/mL), streptomycin (100 µg/mL) and gentamycin (50 µg/mL) and plated on collagen-I-coated plates (Biocoat, Becton Dickinson, San Jose, CA). Non-adherent cells were removed after 18 h of culture. Phenotypic analysis of gastric epithelial cells was performed using anti-ZO-1 (clone 1), anti-cytokeratin (CAM5.2, specific for mucosal epithelial cell associated Moll’s peptides #7 and #8), anti-human CD104 (439-9B), anti-human CD90 (5E10), anti-human CD45 (2D1) and anti-human HLA-DR (L243) (all from Beckton Dickinson), BerEp4 (Dako Cytomation, Carpinteria, CA), and Alexa 488-phalloidin (Molecular Probes, Eugene, OR).
Human gastric lamina propria cells were isolated as previously described (19). Briefly, gastric mucosa was treated with Hank’s BSS containing EDTA (1.25 mM) plus DTT (0.2 mg/mL) (3×30 min) to remove surface epithelial cells and then digested using collagenase solution (0.5 FALGPA units/mL, 3 × 45min). Dead cells and particles were removed by 30 min sedimentation on ice followed by filtration through 40 µm cell strainers.
Monocyte-derived macrophages were differentiated from MACS-isolated CD14+ blood monocytes by culturing 1×106 monocytes per well for 3–4 days in 24-well plates in complete medium (RPMI1640, 10% heat-inactivated human AB serum and antibiotics) supplemented with recombinant human (rh) macrophage colony-stimulating factor (10 ng/mL; R&D Systems, Minneapolis, MN).
H. pylori culture
H. pylori strain 60190 (cagA+, vacA s1/m1) was a kind gift from Dr. G. Perez-Perez, New York University; GFP-labeled H. pylori strain M6 was a kind gift from Dr. John Y. Kao, University of Michigan, Ann Arbor, MI. Bacteria were grown at 36°C under semi-anaerobic conditions on Brucella agar plates, 5% horse blood (Becton Dickinson) for 3 days. Colonies were harvested into warm Brucella broth, and bacterial numbers were determined by spectrophotometry at 600 nm based on a standard curve generated using the LIVE/DEAD BacLight Bacterial Viability and Counting Kit (Molecular Probes, Eugene, OR). Titration experiments with H. pylori revealed 2 × 107 bacteria/mL (a multiplicity of infection (MOI)≈35) as the optimal concentration to induce primary epithelial cell apoptosis, an MOI=10 optimal for suppression of macrophage phagocytosis, and an MOI=50 optimal for the detection of phagocytosed GFP-H. pylori in monocyte-derived macrophages.
Epithelial cell death
Apoptosis of primary epithelial cells was induced by 6–8 h treatment with fresh H. pylori (2 × 107 bacteria/mL) or camptothecin (5 µM, Sigma). Apoptosis was determined by Annexin-V FITC binding (Southern Biotechnologies, Birmingham, AL), and necrosis was determined by propidiumiodide uptake and LysotrackerRed™ labeling (both Invitrogen). Cells were analyzed using an LSRII flow cytometer (Becton Dickinson) and FlowJo 7.5.5. software (TreeStar, Ashland, OR).
Apoptotic cell uptake by primary gastric mononuclear phagocytes
Mononuclear phagocytes in cytospins of gastric lamina propria cells or cryosections prepared from healthy gastric tissue were labeled with anti-HLA-DR-PE or -Cy3. Apoptotic DNA was detected by the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling) method using the Fluorescein In situ Cell Death Detection kit (Roche, Mannheim, Germany), and epithelial material was detected with an anti-cytokeratin-FITC antibody (CAM5.2, BD). For immunofluorescence labeling, slides were fixed in ice cold acetone, washed in PBS/0.05% Tween-20, blocked (Dako Protein Block, Dako, Carpinteria, CA) and then incubated with the appropriate antibodies. For quantitative analysis of apoptotic cell uptake in gastric mucosa, formalin-fixed, paraffin-embedded tissue from 9 H. pylori-infected and 7 healthy adults organized on a single slide as a tissue microarray (22) was treated with proteinase K (20 µg/mL; Millipore, Billerica, MA) for 20 min at room temperature, subjected to antigen retrieval with Dako Target Retrieval Solution at 98° C for 20 min, labeled for apoptotic material using the ApopTag® Plus Kit (Millipore) together with an anti Digoxigenin-FITC Fab fragments (Roche), and then labeled for mononuclear phagocytes using anti-HLA-DR (LN-3, Abcam, Cambridge, MA) and a goat anti-mouse-Cy3 secondary antibody (Jackson Immunoresearch, West Grove, PA). In all experiments, cell nuclei were stained with DAPI. Samples were analyzed on a Nikon Eclipse T2000-U fluorescent microscope equipped with a CoolSnap ES digital camera and NIS Elements BR2.30. HLA-DR+ cells, TUNEL+ particles and DAPI+ cell nuclei were counted using the taxonomy feature of the software, with the investigator blinded to the identity of the samples.
Apoptotic epithelial cell uptake in vitro
Gastric epithelial cells treated with H. pylori or camptothecin to induce apoptosis, as described above, were labeled with CellTracker™ Green CMFDA (2 µg/mL; Invitrogen, Eugene, OR) for 1 h at 37°C and harvested using trypsin-EDTA. Simultaneously, macrophages were labeled with CellTracker™ Red CMTPX (2 µg/mL; Invitrogen) for 1 h, washed and then harvested using a cell scraper. Macrophages and epithelial cells (1 × 105 each) then were co-cultured for 2.5 h at 37°C. Control cultures were maintained at 4°C. To inhibit macrophage phagocytosis, macrophages were pre-treated with cytochalasin D (1 µg/mL; Sigma) for 15 min prior to co-culture, with continuing exposure of the cells to cytochalasin D during co-culture. To block binding of apoptotic cells to macrophages, epithelial cells were pre-treated with recombinant RGD-TSR region of the BAI1 ectodomain (residues 202–585), prepared and purified as described previously (23, 24) (10 µg/mL), for 15 min prior to co-culture, with continuing exposure of the cells to BAI1 during co-culture. Macrophage phagocytic activity also was analyzed by feeding the macrophages fluorescein-labeled latex beads (Fluoresbrite YG Microspheres, Polysciences Inc., Warrington, PA) or GFP-labeled H. pylori (MOI=50). After culture, cells were washed, fixed in Cytofix™ (Becton Dickinson) and analyzed on an LSRII flow cytometer (Becton Dickinson). For confocal analysis, cells were imaged on an LSM 710 Laser Confocal Scanning Microscope equipped with Zen 2008 4.7.2 software (Zeiss, Thornwood, NY).
Macrophage treatment
To determine the influence of H. pylori on the ability of macrophages to engulf apoptotic cells, macrophages were treated with H. pylori 60190 at an MOI=10 for 6 h prior to the phagocytosis experiment. Macrophages also were treated with cell-free supernatants from cultures of H. pylori macrophages or rhTNF-α (10 ng/mL; R&D Systems). In addition, macrophages were treated with H. pylori as above plus neutralizing anti-TNF-α antibody (0.02–2 µg/mL, R&D Systems) or an isotype control antibody. The amount of TNF-α in the culture supernatants was determined by ELISA (R&D Systems).
Quantitive RT-PCR
RNA was isolated from snap-frozen gastric biopsy samples using the RNeasy Minikit (Qiagen, Valencia, CA), and cDNA was generated using iScript Reverse Transcriptase (Biorad, Hercules, CA). Target genes were amplified in 25 µL reactions containing TaqMan Universal PCR Master Mix and primer probe sets for TNFα (FAM/MGB, Hs00174128_m1, Ref. Seq. NM_000594.2) and 18s rRNA (VIC/TAMRA, Ref. Seq. X03205.1) or GAPDH (VIC/TAMRA, Ref. Seq. NM_002046.3); all from Applied Biosystems, Foster City, CA. Real-time PCR reactions were run for 40 cycles (15 sec 95°C, 60 sec 60°C) on a Chromo4 PCR system (Biorad), and analysed with Opticon Monitor™ software, version 3.1. Relative expression rates were calculated using the Pfaffl method (25) with gastric tissue from a non-infected donor used as control. PCRs were performed twice with duplicate samples, once with each reference gene, and data are presented as the geometric mean of both reactions.
Study data and statistical analysis
Data were analysed using Microsoft® Excel 2003 and Analyse-it® for Excel, version 1.73. Results are presented as mean ±SEM. Differences between values were analyzed for statistical significance by the two-tailed Student’s t-test, unless stated otherwise. Differences were considered significant at P<0.05.
Results
Mononuclear phagocytes in human gastric mucosa engulf apoptotic gastric epithelial cells
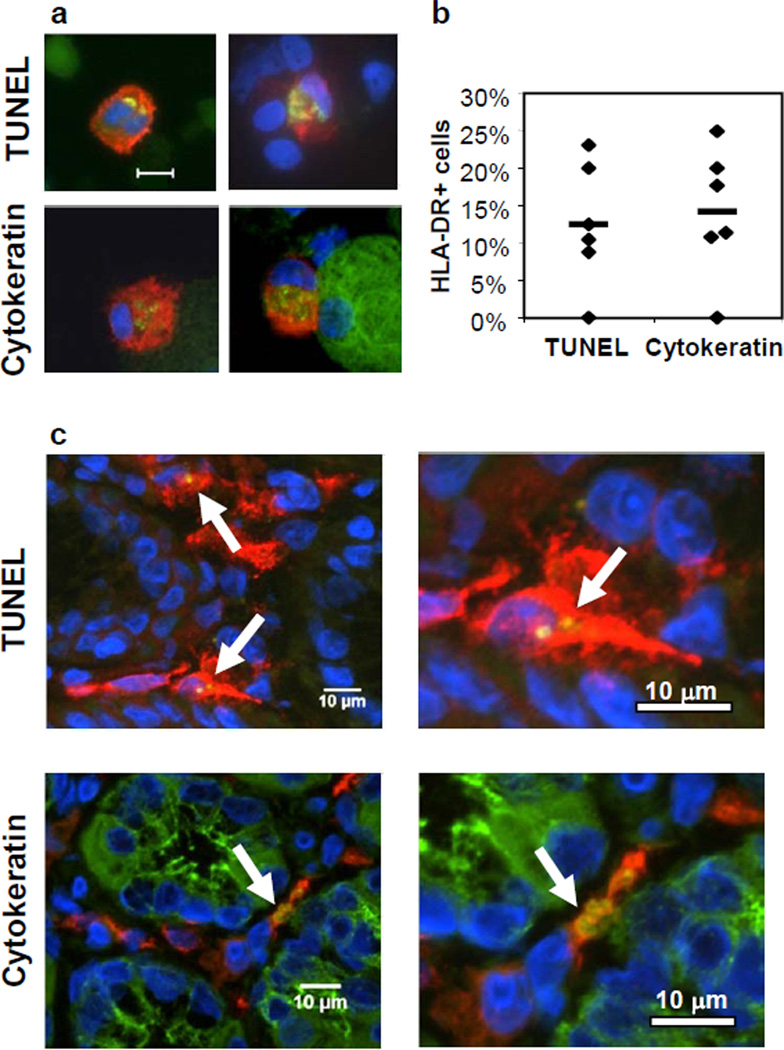
The mechanism by which apoptotic epithelial cells are cleared in human gastric mucosa is largely unknown. Here we analyzed whether HLA-DR+ mononuclear phagocytes are involved in the removal of apoptotic gastric epithelial cells. Cytospins of gastric lamina propria mononuclear cells obtained from H. pylori-negative donors were stained for HLA-DR and, using the TUNEL method, for apoptotic DNA. Microscopic image analysis (Fig. 1a,b) showed that 10–15% of the gastric mononuclear phagocytes contained TUNEL+ inclusions in their cytoplasm, indicating the cells had taken up apoptotic material. A similar proportion of gastric mononuclear phagocytes contained epithelial cell-specific cytokeratin+ inclusions consistent with the epithelial origin of the apoptotic material. The TUNEL+ and cytokeratin+ inclusions were discrete and varied in size, suggesting that fragments of different sizes were ingested. Importantly, TUNEL+ and cytokeratin+ inclusions also were detected in HLA-DR+ mononuclear phagocytes in gastric tissue sections (Fig. 1c), confirming that apoptotic epithelial cell phagocytosis occurs in situ and is not an artifact associated with cell isolation. Thus, under steady state conditions, apoptotic gastric epithelial cells are cleared at least in part by mononuclear phagocytes in human gastric mucosa.
Figure 1. Human gastric mononuclear phagocytes take up apoptotic epithelial cells in vivo.
(a) Cytospins of freshly isolated human gastric lamina propria cells were analyzed for the presence of fragmented DNA (TUNEL+, upper panels) or epithelial cell cytokeratin (lower panels) in the cytoplasm of HLA-DR+ mononuclear phagocytes. Phagocytes were labeled red with anti-HLA-DR; TUNEL+ apoptotic material and epithelial cell-specific cytokeratin were labeled green (yellow in merge). The bottom right panel shows a large green gastric epithelial cell adjacent to a phagocyte. Bar=20 µm. (b) Quantitative analysis of uptake of apoptotic cells. Samples from 6 donors were analyzed by evaluating an average of 30 randomly selected HLA-DR+ cells for the presence of cytoplasmic apoptotic material. Data points and mean (bar) correspond to the percentage of cells that contain TUNEL+ or cytokeratin+ material. (c) HLA-DR+ (red) mononuclear phagocytes in tissue sections of human gastric lamina propria contain green TUNEL+ (upper panels) and cytokeratin+ (lower panels) epithelial cell-derived material (yellow in merge, arrows).
H. pylori interaction with primary human gastric epithelial cells triggers epithelial cell apoptosis and subsequent clearance by mononuclear phagocytes
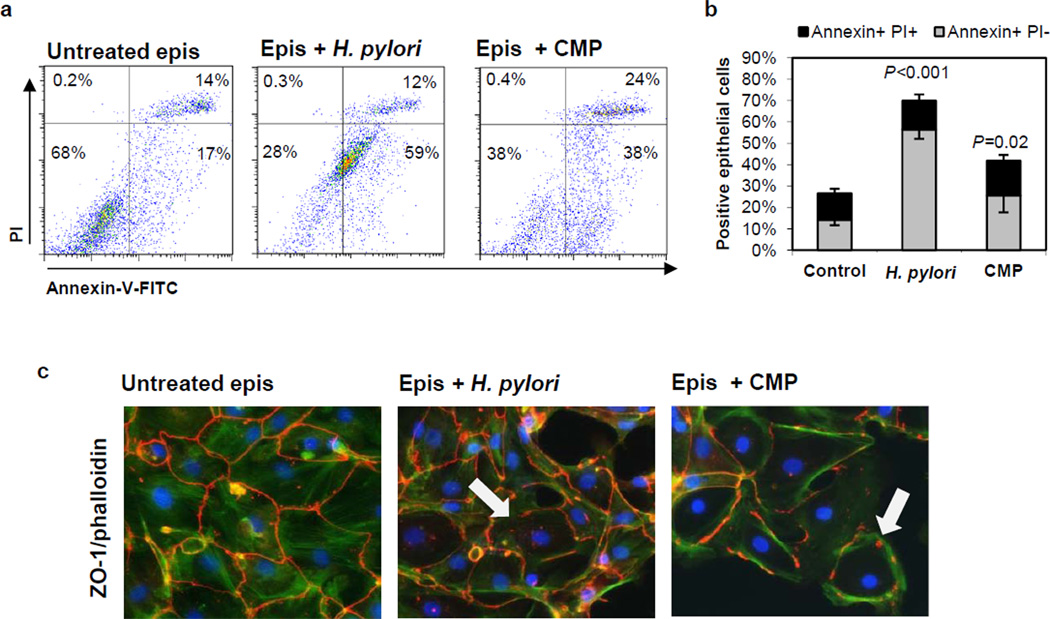
Human gastric H. pylori infection is associated with increased epithelial cell apoptosis (1), and direct induction of apoptotic cell death by H. pylori bacteria has been shown in various gastric epithelial cell lines (2–6). Here, we investigated whether primary human gastric epithelial cells undergo cell death after infection with live H. pylori and are phagocytosed by autologous macrophages in vitro. The cultured gastric epithelial cells expressed epithelial cell-specific markers, including cytokeratin and tight junction protein ZO-1 (Supplemental Fig. 1). As shown in Fig. 2a,b, incubation of epithelial monolayers for 6–8 h with H. pylori (2×107 bacteria/mL; MOI≈35), compared to monolayers incubated in media alone, induced a marked increase in the proportion of apoptotic (Annexin-V+/propidiumiodide−) epithelial cells (56.0 ±4.9% versus 14.7 ±2.0%, n=9; P<0.001) but only a small increase in necrotic (Annexin-V+/propidium iodide+) epithelial cells (16.7 ±3.2% versus 12.5 ±2.1%, n=9; P=0.7). In addition, lysosomal membrane permeabilization, an early event of necrosis, was only detected after 24 and 48 h, but not after 6 and 12 h, of epithelial cell exposure to H. pylori (Supplemental Fig. 2a). Thus, the majority of the epithelial cells exposed to H. pylori for 6 h underwent apoptosis rather than necrotic cell death. Notably, the relatively high background in the propidiumiodide (PI) channel in Fig. 2a was due to binding of bacteria to the cell surface instead of nuclear staining of membrane-permeable necrotic cells (Supplemental Fig. 2c), whereas ethanol-treated necrotic cells were present exclusively in the PI high gate (Supplemental Fig 2d). In agreement with earlier reports (26), H. pylori induction of apoptosis in primary gastric epithelial cells was associated with expression of VacA, as VacA-deficient mutants of the 60190 strain did not induce a significant level of apoptosis (Supplemental Fig. 2d,e). In contrast, CagA- and urease-deficient mutants were each as capable of inducing apoptosis as the wild type strain. Interestingly, primary gastric epithelial cells were significantly more susceptible to H. pylori-induced apoptosis than cells of the AGS gastric cell line (Supplemental Fig. 2a, b), and, surprisingly, camptothecin (CMP) was less efficient than H. pylori at inducing cell death in primary gastric epithelial cells within 6 h (Fig. 2a,b). At 24 h, the epithelial monolayers incubated with both H. pylori and camptothecin, but not the control monolayer, showed prominent architectural distortion and disrupted intercellular ZO-1, consistent with epithelial cell death (Fig. 2c).
Figure 2. H. pylori induces apoptotic cell death in primary human gastric epithelial cells.
(a, b) Gastric epithelial cells were cultured for 3 days on collagen-coated plates and then treated with H. pylori (2×107/mL), camptothecin (CMP, 5 µM) or medium alone. Apoptosis was determined by flow cytometric analysis of Annexin-V-FITC/propidiumiodide (PI)-stained cells after 6–8 h. (a) Representative data and (b) cumulative data from 10 (untreated, H. pylori) and 5 (CMP) experiments; mean ± SEM. (c) Microscopic analysis of ZO-1-Cy3/phalloidin-FITC/DAPI-stained epithelial monolayers after 24 h exposure to H. pylori or camptothecin. Arrows indicate areas with altered ZO-1 immunolocalization. Results are from a representative experiment (n=4).
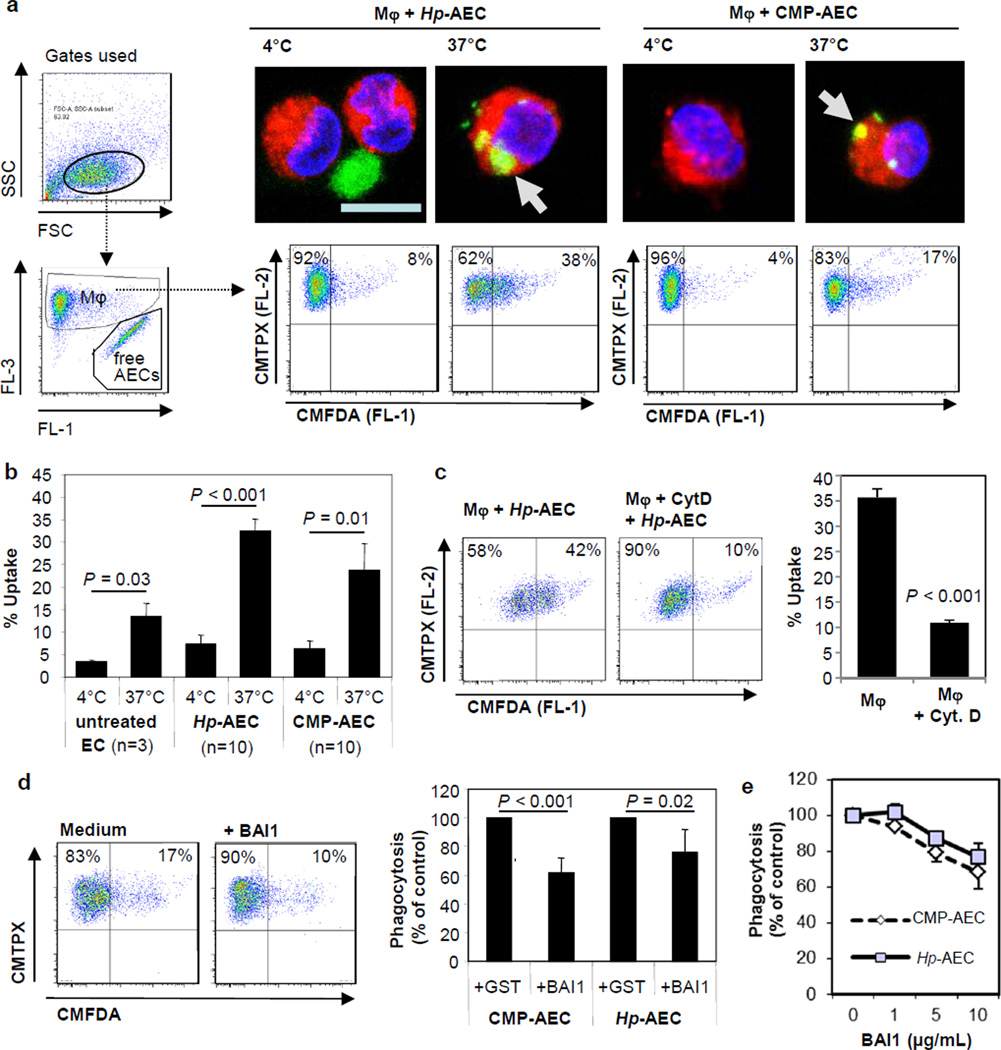
We next generated monocyte-derived macrophages and labeled the macrophages with a red CellTracker™ dye (CMTPX). Epithelial cells obtained from matched donors were cultured with H. pylori (2×107 bacteria/mL) or camptothecin (5 µM) for 6–8 h to induce apoptosis or with media alone and then labeled with a green CellTracker™ dye (CMFDA). After equivalent numbers of red-labeled monocyte-derived macrophages and green-labeled H. pylori-treated apoptotic epithelial cells had been cultured for 2.5 h at 37°C, 32.5 ± 3.2% of the macrophages had acquired green fluorescence, indicating apoptotic cell phagocytosis (Fig. 3a,b). In contrast, flow cytometric analysis of control cultures incubated at 4°C showed only a small population of green fluorescent macrophages (7.3 ± 1.3%, n=10; P<0.001 for 37°C vs. 4°C) after co-culture, likely representing macrophages that had bound epithelial cells on their surface. A slightly lower proportion of macrophages phagocytosed camptothecin-treated apoptotic gastric epithelial cells (23.7 ± 5.5% at 37°C vs. 6.3 ± 1.2% at 4°C, n=10; P=0.01), consistent with the lower rate of apoptotic cell death after this treatment (Fig. 3a,b). The presence of green particles inside red macrophages, indicating phagocytosed apoptotic gastric epithelial cells, was confirmed by confocal microscopy (Fig. 3a, upper panels).
Figure 3. Apoptotic gastric epithelial cells are phagocytosed by monocyte-derived macrophages through a phosphatidylserin-dependent pathway.
(a, b) Gastric epithelial cell cultures were treated with H. pylori (Hp), camptothecin (CMP, 5 µM) or with medium alone for 6–8 h to induce apoptosis and then labeled with CMFDA (green). Monocyte-derived macrophages (MΦ) were stained with CMTPX (red), and equal numbers of stained macrophages and apoptotic epithelial cells (AECs) were co-cultured at 4°C or 37°C for 2.5 h. (a) Macrophages were analyzed by confocal microscopy (right, upper panels) or by flow cytometry (right, lower panels, bar = 10 µm). Representative data; panels on the left show gating strategy. (b) Cumulative data (mean ±SEM) from 3 (untreated) or 10 (Hp, CMP) experiments. (c) To block uptake, macrophages were pre-treated with cytochalasin D (1 µg/mL) for 45 min prior to co-culture with H. pylori-treated AECs (cytochalasin D also present during co-culture). Representative (left) and cumulative data (right), n=3; ***P ≤ 0.001. (d, e) Gastric epithelial cells with apoptosis induced by CMP (CMP-AEC) or H. pylori (Hp-AEC) were treated for 15 min with control GST or recombinant BAI1 RGD-TSR, which neutralizes surface phosphatidylserine, and then cultured with macrophages. (d) Representative (left) and cumulative data (right) from 4 experiments with 10 µg/mL of BAI1 RGD-TSR or control GST. Phagocytosis is expressed as % macrophages that contained BAI1-treated AECs relative to GST-treated AECs (100%). (e) GST and BAI1 used at the indicated concentration, mean ± SEM of 2 (camptothecin) or 3 (H. pylori) experiments.
To further investigate the mechanism of macrophage uptake of apoptotic epithelial cells, we examined the effect of cytochalasin D, an inhibitor of actin polymerization and thus engulfment, on macrophage phagocytosis of apoptotic gastric epithelial cells. Macrophages treated with cytochalasin D (1 µg/mL) before and during the incubation with H. pylori-treated gastric epithelial cells were significantly less capable of phagocytosing the epithelial cells (n=3; P<0.001) (Fig. 3c). Since the expression of phosphatidylserine on the outer leaflet of the plasma membrane is an essential macrophage engulfment signal (12), we assessed macrophage phagocytosis of apoptotic gastric epithelial cells after blockade of surface phosphatidylserine using the recombinant soluble fragment RGD-TSR of brain angiogenesis inhibitor 1 (BAI1). BAI1 is a phospholipid receptor with a phosphtidylserine binding domain that consists of five thrombospondin repeats (TSR motifs) (23, 24). Treatment of apoptotic gastric epithelial cells with the recombinant RGD-TSR region of BAI1 prior to co-culture with the macrophages reduced, but did not completely abrogate, macrophage phagocytosis of the epithelial cells in a dose-dependent manner (Fig. 3d). A similar level of suppression was reported in our earlier study on BAI1-mediated bacterial uptake (23). Notably, higher doses of the RGD-TSR caused significant macrophage cell death, likely due to the glycerol stabilizer present in the peptide preparation. These data support previous reports of phosphatidylserine exposure as an engulfment signal molecule that mediates macrophage phagocytosis of apoptotic gastric epithelial cells.
H. pylori interaction with mononuclear phagocytes inhibits engulfment of apoptotic gastric epithelial cells through a TNF-α-dependent mechanism
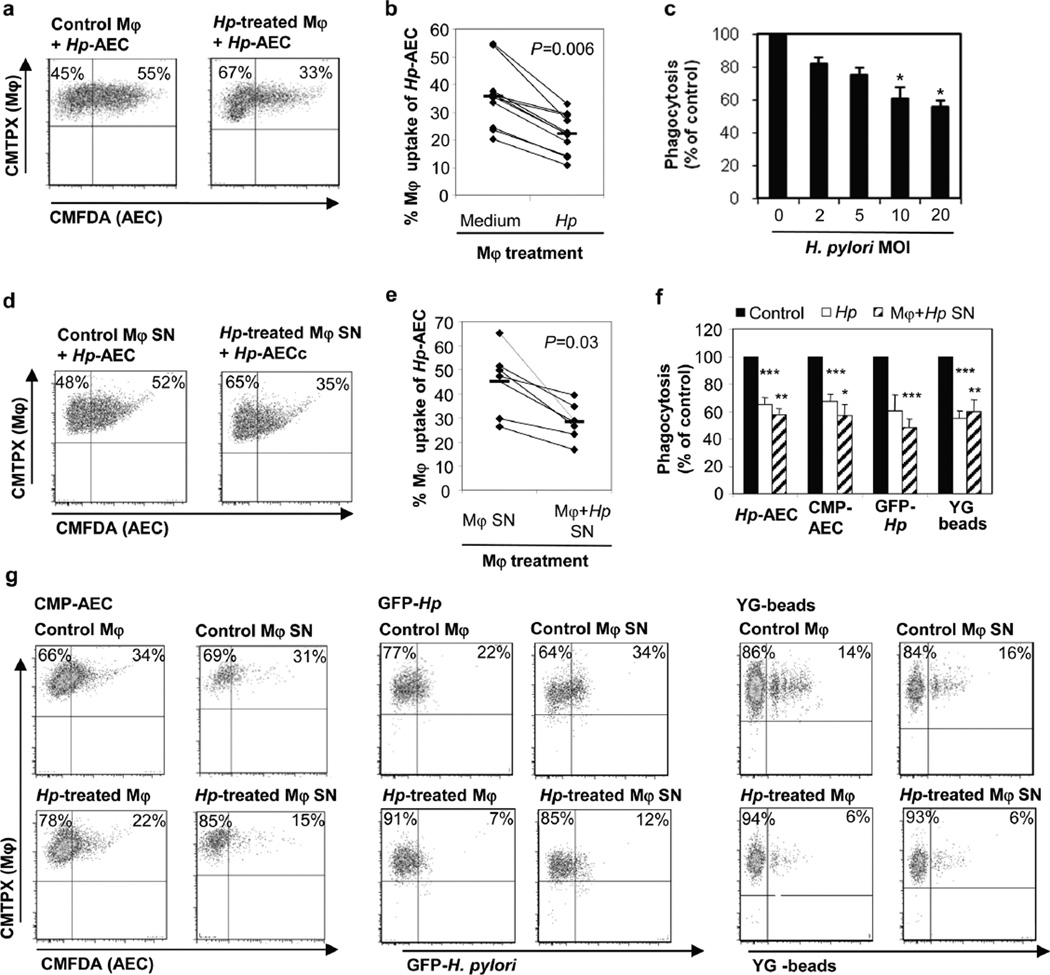
In the H. pylori-infected gastric mucosa, phagocytes likely encounter both H. pylori bacteria and apoptotic epithelial cells. H. pylori have been identified in close contact with lamina propria macrophages (27), and we have shown that human gastric macrophages contain H. pylori surface proteins (28). H. pylori inhibits its own uptake by macrophages (29), raising the possibility that macrophage interaction with H. pylori in the gastric mucosa might interfere with the uptake of apoptotic epithelial cells. Therefore, we investigated the effect of H. pylori on mononuclear phagocyte uptake of apoptotic gastric epithelial cells in vitro. Monocyte-derived macrophages were pre-treated with live H. pylori for 6 h, incubated with H. pylori-treated apoptotic gastric epithelial cells for 2.5 h and then analyzed for engulfed epithelial cells. Exposure of the macrophages to live H. pylori (MOI=10) caused a dose-dependent reduction in subsequent engulfment of the apoptotic epithelial cells with a 38% reduction in phagocytosis at an MOI of 10 (35.6 ±3.5% to 22.1 ±2.2%, n=10; P=0.006) (Fig. 4a,b,c).
Figure 4. H. pylori stimulation inhibits macrophage phagocytic activity for apoptotic gastric epithelial cells.
(a, b) Monocyte-derived macrophages were treated with H. pylori (MOI=10) for 6–8 h or left untreated and then stained with CMTPX (red). Gastric epithelial cells were cultured for 3 days on collagen-coated plates, treated with H. pylori (2×107/mL) or camptothecin (5 µM) for 6–8 h and labeled with CMFDA (green). Macrophages and apoptotic epithelial cells then were harvested and incubated at a ratio of 1:1 at 37°C for 2.5 h to allow macrophage engulfment of epithelial cells. (a) Representative FACS plots and (b) individual values (diamonds) and means (bars), n=10. (c) Phagocytosis of AECs after macrophage pre-treatment with different concentrations of live H. pylori, n=3, mean ± SEM, 1-way ANOVA with Tukey’s post hoc test * P≤0.05. (d, e) Soluble mediators released by H. pylori-treated macrophages inhibit AEC phagocytosis. Cell-free supernatants of macrophages cultured with or without H. pylori for 6–8 h were added to untreated macrophages for 6 h prior to the phagocytosis experiment. (d) Representative FACS plots and (e) individual values (diamonds) and means (bars), n=6. (f, g) H. pylori-induced inhibition of macrophage phagocytosis is not specific to the uptake of AECs. Macrophages were pre-treated with either H. pylori, culture supernatants from H. pylori-treated macrophages or media and then assayed for phagocytosis of Hp-AEC, CMP-AEC, GFP-labeled H. pylori (MOI=50) or YG fluorescent latex beads (40 beads/cell). (f) The relative efficiency of H. pylori-treated macrophages was determined by comparing the phagocytosis of treated macrophages to that of untreated macrophages for the different targets, mean ± SEM, n=4. (g) Representative FACS plots for the data shown in (f).
We next investigated whether the reduced macrophage phagocytosis caused by H. pylori was a direct effect of the bacteria or due to a soluble mediator released from the macrophages in response to H. pylori. Monocyte-derived macrophages were cultured with H. pylori for 6 h and the cell- and bacteria-free supernatants were harvested, added to fresh macrophages for 6 h, and the cells’ phagocytic activity for apoptotic gastric epithelial cells was analyzed as above. As shown in Fig. 4d and e, culture supernatants from H. pylori-treated macrophages (compared to supernatants from macrophages incubated in media alone) significantly suppressed the mean level of macrophage engulfment of the epithelial cells by 35% (from 45.0 ±5.4% to 28.3 ±3.0%, n=6; P=0.03). Culture supernatants from the same concentrations of live H. pylori alone did not impair macrophage phagocytosis (data not shown). These findings implicate a soluble mediator released by the H. pylori-treated macrophages in the reduced phagocytosis of apoptotic gastric epithelial cells. Both H. pylori and culture supernatants from H. pylori-treated macrophages also suppressed macrophage phagocytosis of camptothecin-treated apoptotic gastric epithelial cells, GFP-labeled H. pylori and YG-labeled latex beads (Fig 4f,g). Thus, both H. pylori bacteria and an H. pylori-inducible mediator reduced macrophage clearance of apoptotic epithelial cells, as well as clearance of bacteria and foreign material, suggesting a ‘global’ reduction in macrophage phagocytic activity that could contribute to the accumulation of pro-inflammatory material in the mucosa in H. pylori gastritis.
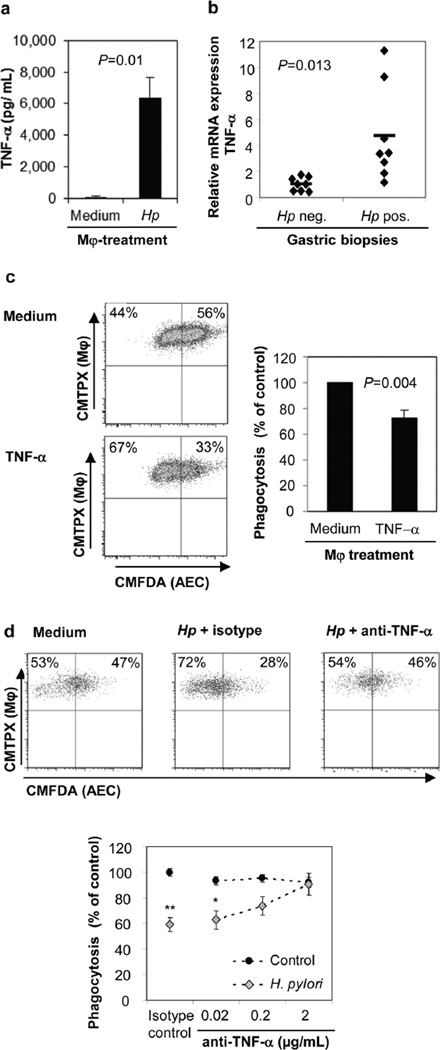
Since TNF-α has been shown to inhibit macrophage clearance of apoptotic cells (30), we measured TNF-α secretion of monocyte-derived macrophages incubated with H. pylori (MOI=10) for 6 h and tested whether the released TNF-α could inhibit macrophage phagocytosis of apoptotic gastric epithelial cells. Indeed, H. pylori-stimulated macrophages released significant amounts of TNF-α (>6,000 pg/mL, n=4; P=0.01) (Fig. 5a). The in vivo relevance of this finding is reflected in the >4-fold increase in TNF-α mRNA expression in the gastric tissue of H. pylori-infected subjects compared to that of uninfected subjects (Fig. 5b, n=8; P=0.013), confirming our previous observation (31). Moreover, pre-treatment of macrophages with rhTNF-α (10 ng/mL) caused a significant reduction in macrophage engulfment of apoptotic epithelial cells (26.8 ±5.2%, n=4; P=0.004) (Fig. 5c), and, conversely, incubation of H. pylori-treated macrophages with a neutralizing anti-TNF-α, but not an isotype control, antibody completely restored the ability of the macrophages to phagocytose apoptotic epithelial cells (Fig. 5d). In summary, these results implicate TNF-α release by macrophages in response to H. pylori as a potent inhibitor of the cells’ capacity to phagocytose apoptotic gastric epithelial cells.
Figure 5. TNF-α mediates reduced apoptotic cell clearance by H. pylori treated macrophages.
(a) TNF-α concentrations in culture supernatants of macrophages treated with live H. pylori (MOI=10) or medium for 6 h were determined by ELISA, n=4. (b) Human H. pylori infection increases gastric expression of TNF-α. Gastric biopsies obtained from non-infected or H. pylori-infected human subjects were analyzed for TNF-α gene expression by quantitative real-time PCR, n=8. (c) Macrophage pre-treatment with rhTNF-α (10 ng/mL) inhibits phagocytosis of AECs. Representative FACS plots (left panels) and cumulative data from 4 experiments, mean ± SEM (right panel). (d) Neutralization of TNF-α in H. pylori-treated macrophage cultures reverses the suppressive effect of H. pylori on macrophage clearance of AECs. Macrophages were pre-treated for 6–8 h with both H. pylori and anti-TNF-α antibodies (or isotype control) prior to co-culture with Hp-AECs Representative FACS plots (top panels) and cumulative data from 3 experiments, mean ± SEM (bottom panel). *P≤0.05 and **P≤0.01 compared to untreated control; Student’s t test.
Gastric mucosa from H. pylori-infected subjects contains increased amounts of non-phagocytosed apoptotic material
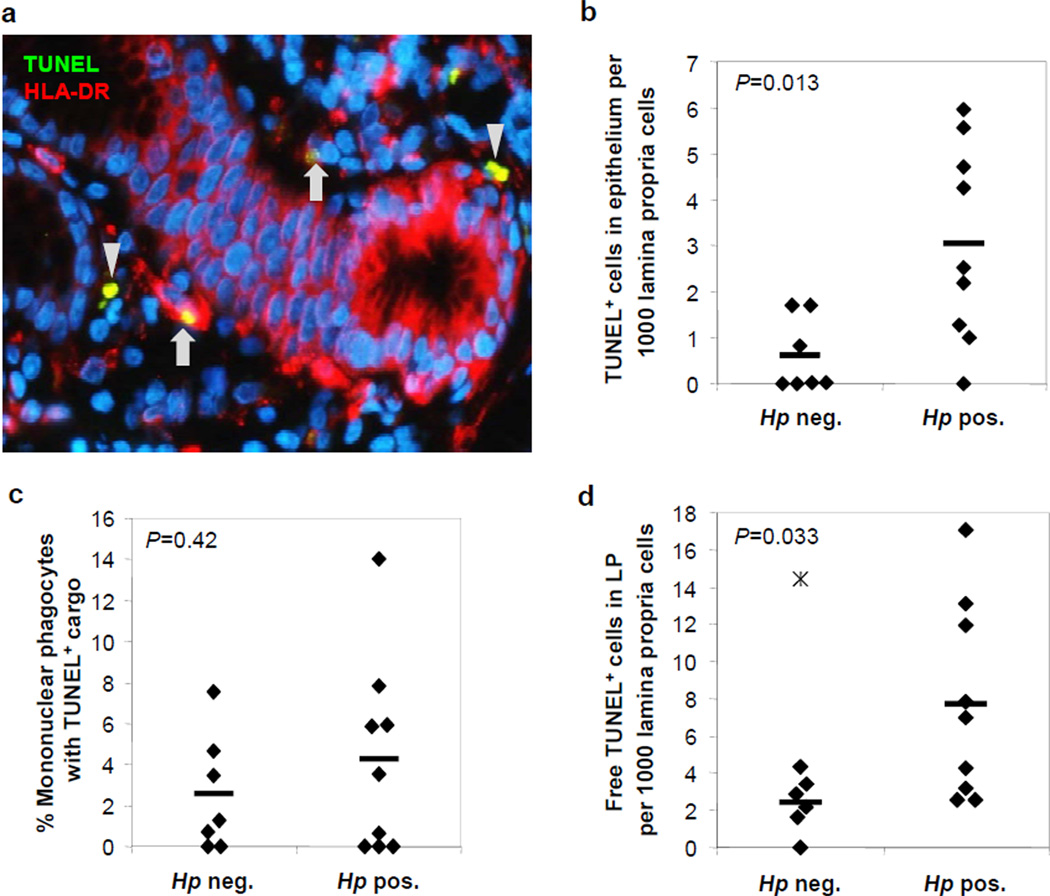
In order to determine whether phagocytosis of apoptotic cells is impaired in human H. pylori gastritis, we analyzed gastric mucosa from H. pylori-infected (n=9) and non-infected (n=7) subjects for the presence of HLA-DR+ mononuclear phagocytes and TUNEL+ material, with an average of 954 ± 157 cells analyzed per sample (Fig. 6a–d). As anticipated, H. pylori-infected gastric mucosa contained significantly higher numbers of TUNEL+ cells in the gastric epithelial layer, consistent with H. pylori-induction of epithelial cell apoptosis (Fig. 6b). Surprisingly, in contrast to our published digital image analysis study (19) , we did not find an increased number of HLA-DR+ mononuclear phagocytes in the gastric mucosa of H. pylori-infected versus uninfected subjects (data not shown). This discrepancy be due to the exclusion of tertiary lymphoid follicle areas, which are rich in DCs but do not allow accurate cell counts, from the present study. The percentage of mononuclear phagocytes containing apoptotic material did not differ significantly between non-infected and H. pylori-infected mucosa, with 2.6 ± 1.4% and 4.7 ± 1.5% of positive cells, respectively (Fig. 6c). Notably, the percentage of mononuclear phagocytes containing TUNEL inclusions in gastric tissue was lower than that seen in isolated cells on cytospins (Fig. 1b), possibly reflecting additional apoptotic cell uptake by the mononuclear phagocytes during the collagenase digestion procedure. Importantly, the amount of TUNEL+ apoptotic material not associated with HLA-DR+ mononuclear phagocytes was significantly increased in H. pylori-infected compared to non-infected tissue. (Fig. 6d). Thus, results of the HLA-DR/TUNEL analysis of gastric tissue support the hypothesis that chronic H. pylori gastritis is associated with insufficient apoptotic cell clearance in the human gastric mucosa.
Figure 6. Increased epithelial cell apoptosis and decreased apoptotic cell clearance in H. pylori infected human gastric mucosa.
Paraffin-embedded gastric tissue from seven non-infected and nine H. pylori-infected human subjects was quantitatively analyzed for HLA-DR+ mononuclear phagocytes, TUNEL+ apoptotic cells/cell fragments and DAPI+ nuclei. (a) Gastric mucosa of an H. pylori-infected subject. Arrows: HLA-DR-Cy3+ (red) mononuclear phagocytes containing TUNEL-FITC+ (green) apoptotic material; arrowheads: non-phagocytosed TUNEL+ apoptotic material. Note that some glandular epithelial cells express HLA-DR. Original magnification 400x. (b) Frequency of TUNEL+ cells in gastric epithelial layer relative to total lamina propria cells. (c) Percentage of HLA-DR+ mononuclear phagocytes containing TUNEL+ material. (d) Free TUNEL+ material not associated with HLA-DR+ mononuclear phagocytes in gastric lamina propria relative to total lamina propria cells. Mean (bars) and individual values (diamonds) are shown; star – patient #3, outlier, excluded from analysis (value – 3 SD from mean); Student’s t test.
Discussion
Chronic H. pylori gastritis is associated with self-perpetuating inflammation that contributes to the development of autoimmune atrophic gastritis and gastric adenocarcinoma and may persist even after clearance of the bacteria in advanced disease (32, 33). The mechanism for this chronic and persistent inflammation is not fully understood. Here, we present findings that support inefficient clearance of apoptotic epithelial cells by gastric mononuclear phagocytes as a pathogenic mechanism in chronic H. pylori gastritis.
Increased gastric epithelial cell apoptosis is well-established in H. pylori infection and is thought to contribute to gastric carcinogenesis (1). In this regard, our results show that primary human gastric epithelial cells cultured in vitro are highly susceptible to H. pylori-induced cell death. Epithelial cell apoptosis was significantly associated with H. pylori expression of the virulence factor VacA, which causes loss of mitochondrial membrane potential through formation of an anion channel in the inner mitochondrial membrane, thereby inducing apoptosis through the intrinsic pathway (26).
The increased epithelial cell death in H. pylori-infected gastric mucosa results in a higher demand for apoptotic epithelial cell removal. In addition, chronic active H. pylori gastritis also involves infiltration of the gastric mucosa with short-lived neutrophils and lymphocytes (32), further increasing the need for apoptotic cell clearance. Here we show that apoptotic epithelial cell-derived material was present in a proportion of HLA-DRhigh cells in human gastric mucosa, indicating that gastric mononuclear phagocytes are routinely involved in the clearance of apoptotic gastric epithelial cells. In our previous studies, we identified mononuclear phagocytes with high expression of HLA-DR in human gastric mucosa and showed that these cells express variable levels of CD11c, DC-SIGN, CD206, CD14 and CD86 (19, 20). Based on their high HLA-DR expression and ability to drive Th1 T cells in response to H. pylori, we classified those cells as DCs, although some cells may have more macrophage-like characteristics. Within the gastric mucosa, the HLA-DRhigh mononuclear phagocytes are present in the epithelial layer and in the lamina propria, where they are ideally positioned for apoptotic epithelial cell clearance (19). In agreement with previous reports, we show that the number of apoptotic epithelial cells was higher in H. pylori infected samples (1). However, the percentage of MNPs that had phagocytosed apoptotic material was not significantly changed, consistent with a reduced capacity by mononuclear phagocytes in H. pylori-infected mucosa to clear apoptotic cells. Importantly, the amount of apoptotic material not phagocytosed by mononuclear phagocytes was increased in H. pylori infection, supporting our hypothesis of an apoptotic cell clearance defect in chronic H. pylori gastritis.
Under steady-state conditions, the uptake of apoptotic epithelial cells by mononuclear phagocytes contributes to tissue homeostasis by preventing the release of pro-inflammatory signals that occurs when the dying cell undergoes secondary necrosis (12) and by releasing the anti-inflammatory mediators TGF-β and PGE2 (10). Our data indicate that H. pylori infection suppresses mononuclear phagocyte clearance of apoptotic gastric epithelial cells through a TNF-α-dependent mechanism. H. pylori reduced the ability of mononuclear phagocytes to clear apoptotic cells by approximately 40%, similar to the level of reduced macrophage clearance of apoptotic cells in SLE (34, 35). Insufficient apoptotic cell clearance is widely recognized as a key disease mechanism in SLE (13) and chronic inflammatory diseases such as atherosclerosis (36), chronic obstructive pulmonary disease (37) and cystic fibrosis (38).
Reduced clearance of apoptotic epithelial cells in the H. pylori-infected human gastric mucosa could exacerbate chronic gastritis by three putative mechanisms. First, non-phagocytosed apoptotic cells that undergo secondary necrosis in the gastric mucosa could release pro-inflammatory intracellular molecules including nucleic acids, which promote innate immune cell activation (39). This likely results in additional release of pro-inflammatory cytokines, including TNF-α, by mononuclear phagocytes, which may further inhibit apoptotic cell clearance. Second, epithelial cell autoantigens released from necrotic cells may induce gastric autoimmune responses. In this connection, gastric autoimmunity has been closely associated with H. pylori infection, and approximately 50% of H. pylori-infected subjects possess autoantibodies to gastric epithelial cell antigens (40). Conversely, most persons diagnosed with autoimmune gastritis show signs of active or past H. pylori infection (41). A third potential consequence of reduced phagocytosis of apoptotic epithelial cells in H. pylori infection is decreased tolerization due to reduced antigen presenting cell engulfment of apoptotic cells that contain H. pylori antigens, leading to enhanced inflammatory responses.
Our results indicate that H. pylori-induced macrophage release of TNF-α suppressed apoptotic gastric epithelial cell clearance in an auto- and paracrine manner. Thus, our results extend previous reports that TNF-α inhibits macrophage clearance of apoptotic neutrophils (30, 42). TNF-α levels in gastric tissue of H. pylori-infected subjects were increased compared to non-infected subjects, confirming our previous finding (31), and macrophages treated with H. pylori released significant amounts of TNF-α. A number of different pathways for the induction TNF-α by H. pylori have been described to date. H. pylori TNF-α-inducing protein (TIP-α) (43) and protein HP986 (44), both of which signal through NF-κB, have been shown to induce TNF-α secretion directly. In addition, bacterial danger signals that trigger MyD88 signaling through activation of TLRs are involved in the induction of TNF-α and other pro-inflammatory cytokines in response to H. pylori stimulation (45). Notably, phagocytosis of H. pylori is required for maximal stimulation of TNF-α secretion (46). Although H. pylori bacteria have been identified in the gastric lamina propria, often in close contact with mononuclear phagocytes (27), direct contact between these cells and H. pylori, resulting in autocrine TNF-α signals, is likely infrequent. However, the majority of gastric mononuclear phagocytes in the H. pylori-infected gastric mucosa will be exposed to paracrine TNF-α signals, which may derive from both mononuclear phagocytes and H. pylori-reactive Th1 cells (47). Notably, the suppressed phagocytosis by H. pylori-treated macrophages was not specific to the uptake of apoptotic epithelial cells, since macrophage uptake of latex beads and of H. pylori bacteria also was reduced, corroborating previous observations that H. pylori can inhibit its own uptake by macrophages (29).
In conclusion, we propose that an increased apoptotic cell load in the H pylori-infected human gastric mucosa exacerbates the persistent inflammatory state that leads to gastric atrophy and adenocarcinoma through a mechanism in which H. pylori-induced mononuclear phagocyte secretion of TNF-α suppresses apoptotic cell clearance. The observed insufficient apoptotic cell clearance in the gastric mucosa may then result in a release of inflammatory components and autoantigens from the dying cells, which further enhances gastric inflammation, independent of H. pylori bacteria.
Supplementary Material
Acknowledgements
We would like to thank Donna Crabb and Amy Ratliff for preparation of H. pylori cultures. We also thank the Analytic and Preparative Cytometry Facility (P30 AR48311), the DDRDC Human Cell/Tissue Core (DK-64400) and the UAB High Resolution Imaging Facility for supporting this study.
Grant Support: Funding was provided by the National Institutes of Health grants: DK-54495 (PDS); AI-079145 and AI-07047 (PBE); DK-084063 (PBE, PDS); AI-083539 (LES); DK-097144 (DB); RR-20136; DK-064400, Mucosal HIV and Immunobiology Center (PDS, LES); the UAB Autoimmunity, Immunology and Transplantation Steering Committee Pilot Program (DB); the UAB CCTS Pilot Program UL1 TR000165 (DB); FONDECYT 1130387 (PRH); and the Research Service of the Veterans Administration (PDS).
Abbreviations
- AEC
apoptotic epithelial cell
- ANOVA
analysis of variance
- BAI1
brain angiogenesis inhibitor 1
- CMP
camptothecin
- DC
dendritic cell
- DTT
dithiothreitol
- EC
epithelial cell
- ELISA
enzyme-linked immunosorbent assay
- HLA
human leukocyte antigen
- IRB
institutional review board
- MOI
multiplicity of infection
- Mφ
macrophage
- rh
recombinant human
- SLE
systemic lupus erythematosus
- TNF-α
tumor necrosis factor alpha
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labelling
Footnotes
The authors have no conflicting financial interests.
References
- 1.Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am. J. Gastroenterol. 2001;96:16–26. doi: 10.1111/j.1572-0241.2001.03447.x. [DOI] [PubMed] [Google Scholar]
- 2.Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes VE, Patel J, Dirden-Kramer B, Boldogh I, Ernst PB, Crowe SE. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect. Immun. 2007;75:4030–4039. doi: 10.1128/IAI.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones NL, Day AS, Jennings HA, Sherman PM. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect. Immun. 1999;67:4237–4242. doi: 10.1128/iai.67.8.4237-4242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cover TL, Krishna US, Israel DA, Peek RM., Jr Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951–957. [PubMed] [Google Scholar]
- 5.Jain P, Luo ZQ, Blanke SR. Helicobacter pylori vacuolating cytotoxin A (VacA) engages the mitochondrial fission machinery to induce host cell death. Proc. Natl. Acad. Sc.i U.S.A. 2011;108:16032–16037. doi: 10.1073/pnas.1105175108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan X, Gunasena H, Cheng Z, Espejo R, Crowe SE, Ernst PB, Reyes VE. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J. Immunol. 2000;165:1918–1924. doi: 10.4049/jimmunol.165.4.1918. [DOI] [PubMed] [Google Scholar]
- 7.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat. Rev. Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 8.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Liu XF, Zhuang Y, Zhang JY, Liu T, Yin Z, Wu C, Mao XH, Jia KR, Wang FJ, Guo H, Flavell RA, Zhao Z, Liu KY, Xiao B, Guo Y, Zhang WJ, Zhou WY, Guo G, Zou QM. Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J. Immunol. 2010;184:5121–5129. doi: 10.4049/jimmunol.0901115. [DOI] [PubMed] [Google Scholar]
- 10.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokines production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat. Rev. Cancer. 2012;12:58–67. doi: 10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- 12.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao WH, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res. Ther. 2011;13:202. doi: 10.1186/ar3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang F-P, Platt N, Wykes M, Major JR, Powell J, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 2000;191:435–443. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkla DH, Gibson PR. The fate of epithelial cells in the human large intestine. Pathology. 1999;31:230–238. doi: 10.1080/003130299105043. [DOI] [PubMed] [Google Scholar]
- 16.Mayhew TM, Myklebust R, Whybrow A, Jenkins R. Epithelial integrity, cell death and cell loss in mammalian small intestine. Histol. Histopathol. 1999;14:257–267. doi: 10.14670/HH-14.257. [DOI] [PubMed] [Google Scholar]
- 17.Iwanaga T, Han H, Adachi K, Fujita T. A novel mechanism for disposing of effete epithelial cells in the small intestine of guinea pigs. Gastroenterology. 1993;105:1089–1097. doi: 10.1016/0016-5085(93)90953-a. [DOI] [PubMed] [Google Scholar]
- 18.Han H, Iwanaga T, Fujita T. Species-differences in the process of apoptosis in epithelial cells of the small intestine: an ultrastructural and cytochemical study of luminal cell elements. Arch. Histol. Cytol. 1993;56:83–90. doi: 10.1679/aohc.56.83. [DOI] [PubMed] [Google Scholar]
- 19.Bimczok D, Clements RH, Waites KB, Novak L, Eckhoff DE, Mannon PJ, Smith PD, Smythies LE. Human primary gastric dendritic cells induce a Th1 response to H. pylori. Muc. Immunol. 2010;3:260–269. doi: 10.1038/mi.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bimczok D, Grams JM, Stahl RD, Waites KB, Smythies LE, Smith PD. Stromal regulation of human gastric dendritic cells restricts the Th1 response to Helicobacter pylori . Gastroenterology. 2011;141:929–938. doi: 10.1053/j.gastro.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smoot DT, Sewchand J, Young K, Desbordes BC, Allen CR, Naab T. A method for establishing primary cultures of human gastric epithelial cells. Methods Cell Science. 2000;22:133–136. doi: 10.1023/a:1009846624044. [DOI] [PubMed] [Google Scholar]
- 22.Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J. Clin. Oncol. 2008;26:5630–5637. doi: 10.1200/JCO.2008.17.3567. [DOI] [PubMed] [Google Scholar]
- 23.Das S, Owen KA, Ly KT, Park D, Black SG, Wilson JM, Sifri CD, Ravichandran KS, Ernst PB, Casanova JE. Brain angiogenesis inhibitor 1 (BAI1) is a pattern recognition receptor that mediates macrophage binding and engulfment of Gram-negative bacteria. Proc. Natl. Acad. Sci., U.S.A. 2011;108:2136–2141. doi: 10.1073/pnas.1014775108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rassow J, Meinecke M. Helicobacter pylori VacA: a new perspective on an invasive chloride channel. Microbes Infect. 2012;14:1026–1033. doi: 10.1016/j.micinf.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, Ricci V, Solcia E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori . Gastroenterology. 2007;132:1009–1023. doi: 10.1053/j.gastro.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 28.Mai UE, Perez-Perez GI, Allen JB, Wahl SM, Blaser MJ, Smith PD. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramarao N, Meyer TF. Helicobacter pylori resists phagocytosis by macrophages: quantitative assessment by confocal microscopy and fluorescence-activated cell sorting. Infect. Immun. 2001;69:2604–2611. doi: 10.1128/IAI.69.4.2604-2611.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPhillips K, Janssen WJ, Ghosh M, Byrne A, Gardai S, Remigio L, Bratton DL, Kang JL, Henson P. TNF-α inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J. Immunol. 2007;178:8117–8126. doi: 10.4049/jimmunol.178.12.8117. [DOI] [PubMed] [Google Scholar]
- 31.Harris PR, Weber HC, Wilcox CM, Jensen RT, Smith PD. Cytokine gene profile in gastric mucosa in Helicobacter pylori infection and Zollinger-Ellison syndrome. Am. J. Gastroenterol. 2002;97:312–318. doi: 10.1111/j.1572-0241.2002.05463.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Weck MN, Gao L, Brenner H. Helicobacter pylori infection and chronic atrophic gastritis: associations according to severity of disease. Epidemiology. 2009;20:569–574. doi: 10.1097/EDE.0b013e3181a3d5f4. [DOI] [PubMed] [Google Scholar]
- 34.Tas SW, Quartier P, Botto M, Fossati-Jimack L. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann. Rheum. Dis. 2006;65:216–221. doi: 10.1136/ard.2005.037143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 37.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 2003;81:289–296. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 38.Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J. Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faller G, Steininger H, Kranzlein J, Maul H, Kerkau T, Hensen J, Hahn EG, Kirchner T. Antigastric autoantibodies in Helicobacter pylori infection: implications of histological and clinical parameters of gastritis. Gut. 1997;41:619–623. doi: 10.1136/gut.41.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amedei A, Bergman MP, Appelmelk BJ, Azzurri A, Benagiano M, Tamburini C, van der Zee R, Telford JL, Vandenbroucke-Grauls CM, D'Elios MM, Del Prete G. Molecular mimicry between Helicobacter pylori antigens and H+, K+ --adenosine triphosphatase in human gastric autoimmunity. J. Exper. Med. 2003;198:1147–1156. doi: 10.1084/jem.20030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng X, Deng T, Zhang Y, Su S, Wei C, Han D. Lipopolysaccharide inhibits macrophage phagocytosis of apoptotic neutrophils by regulating the production of tumour necrosis factor-α and growth arrest-specific gene 6. Immunology. 2011;132:287–295. doi: 10.1111/j.1365-2567.2010.03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang CL, Hao B, Zhang GX, Shi RH, Cheng WF. Helicobacter pylori tumor necrosis factor-α inducing protein promotes cytokine expression via nuclear factor-κB. World Journal of Gastroenterology. 2013;19:399–403. doi: 10.3748/wjg.v19.i3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvi A, Ansari SA, Ehtesham NZ, Rizwan M, Devi S, Sechi LA, Qureshi IA, Hasnain SE, Ahmed N. Concurrent proinflammatory and apoptotic activity of a Helicobacter pylori protein (HP986) points to its role in chronic persistence. PloS one. 2011;6:e22530. doi: 10.1371/journal.pone.0022530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rad R, Brenner L, Krug A, Voland P, Mages J, Lang R, Schwendy S, Reindl W, Dossumbekova A, Ballhorn W, Wagner H, Schmid RM, Bauer S, Prinz C. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori . Gastroenterology. 2007;133:150–163. doi: 10.1053/j.gastro.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 46.Kranzer K, Sollner L, Aigner M, Lehn N, Deml L, Rehli M, Schneider-Brachert W. Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells. Infect. Immun. 2005;73:4180–4189. doi: 10.1128/IAI.73.7.4180-4189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alam MS, Kurtz CC, Wilson JM, Burnette BR, Wiznerowicz EB, Ross WG, Rieger JM, Figler RA, Linden J, Crowe SE, Ernst PB. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunology. 2009;2:232–242. doi: 10.1038/mi.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.