Abstract
The oxidative metabolism of monocytes and polymorphonuclear leukocytes from human peripheral blood was studied in resting and phagocytosing cells. Monocytes, like neutrophils, showed an increase in oxygen consumption during phagocytosis with a concurrent release of superoxide anions and hydrogen peroxide. Both oxygen products are highly reactive agents with potential bactericidal activity. Neutrophils consumed two and a half times as much oxygen, generated about twice as much superoxide, and released five times as much hydrogen peroxide as monocytes did. Monocytes generated superoxide and hydrogen peroxide at equivalent rates.
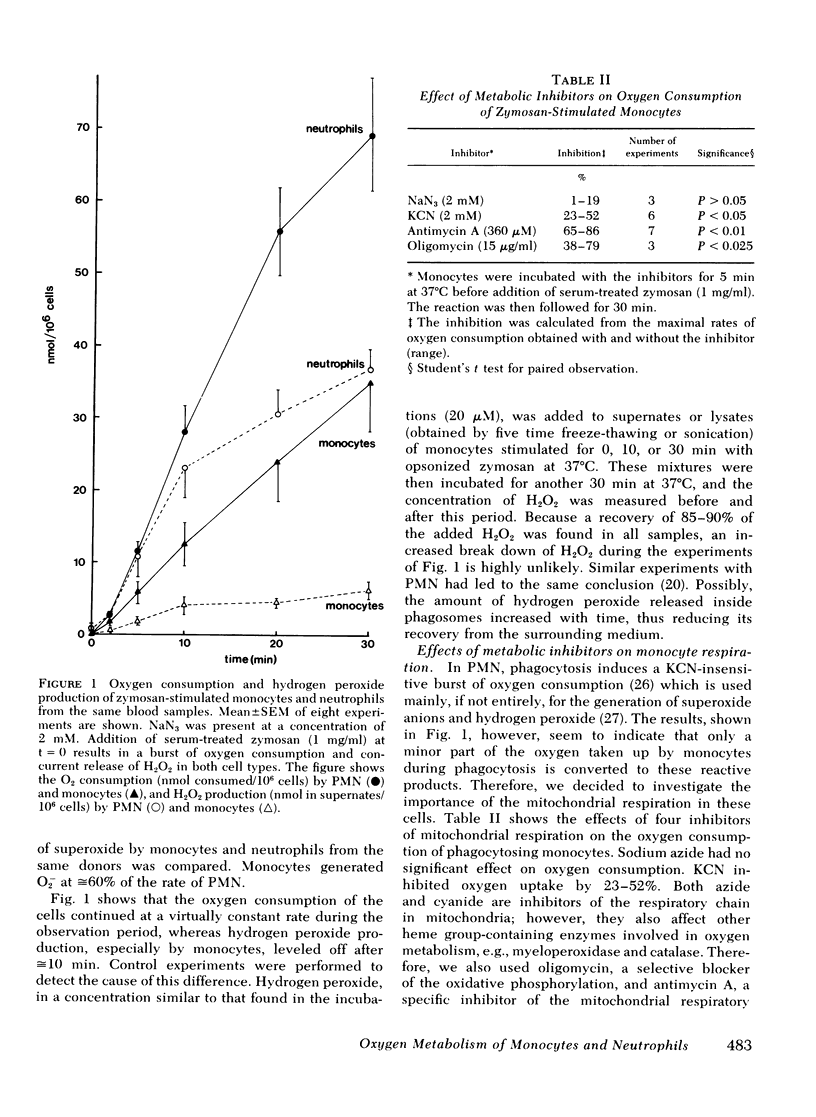
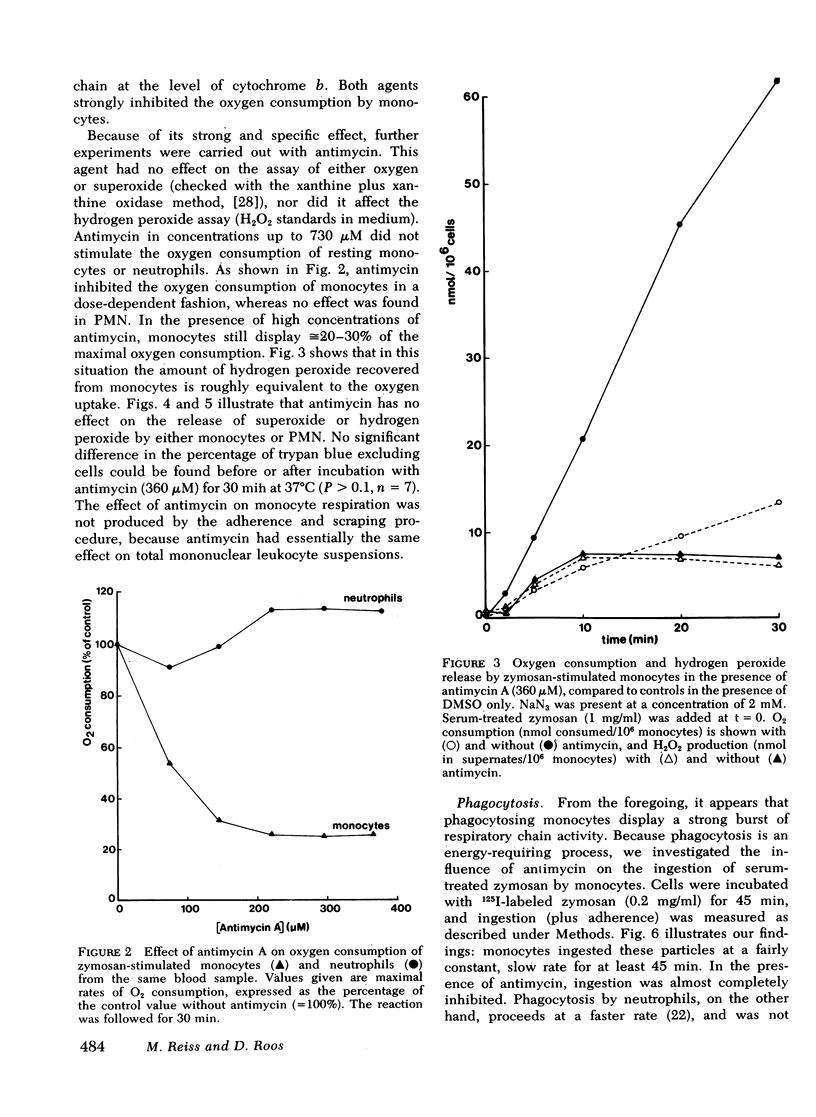
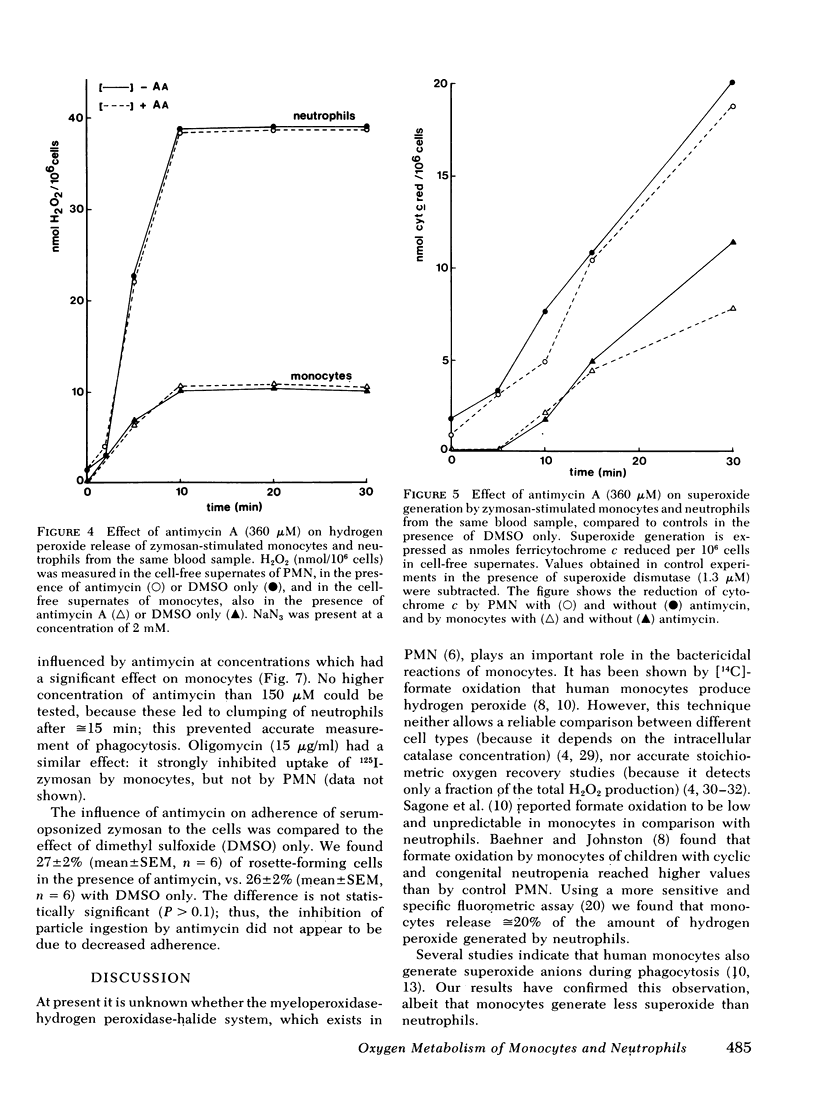
Antimycin A, a specific mitochondrial respiratory chain inhibitor, depressed the oxygen consumption of monocytes by ≅70% but had no effect on neutrophil respiration. Therefore, the oxygen consumed by phagocytosing monocytes appeared to be metabolized in two distinct processes: ≅30% of the oxygen is converted to hydrogen peroxide, whereas the remaining 70% is metabolized via the mitochondrial respiratory chain. The release of superoxide and hydrogen peroxide was unaffected by antimycin in either cell type.
Phagocytosis of zymosan particles by monocytes was nearly abolished by antimycin, whereas no effect was noted with neutrophils. Thus, phagocytosis appears to be highly dependent on oxidative phosphorylation in monocytes but not in polymorphonuclear leukocytes. Moreover, in monocytes treated with antimycin, an addition of opsonized zymosan particles induced stimulation of the oxidative metabolism without occurrence of ingestion.
Full text
PDF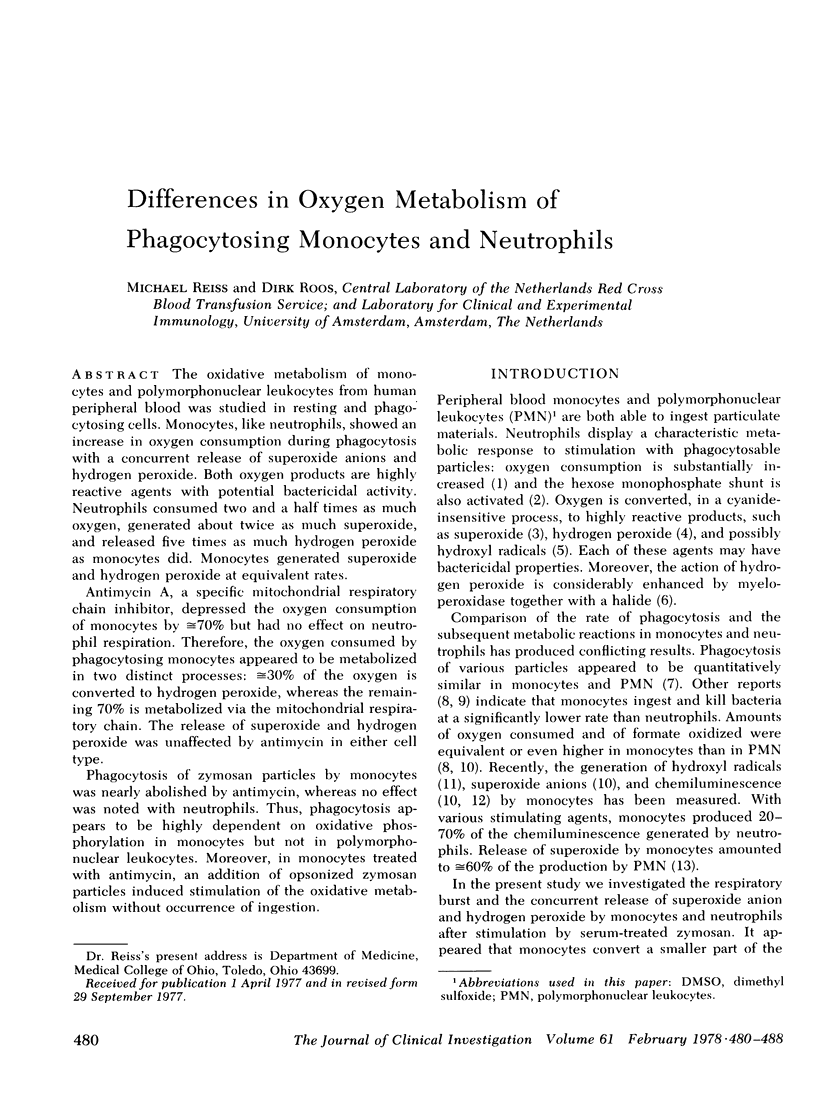




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Monocyte function in children with neutropenia and chronic infections. Blood. 1972 Jul;40(1):31–41. [PubMed] [Google Scholar]
- Briggs R. T., Karnovsky M. L., Karnovsky M. J. Cytochemical demonstration of hydrogen peroxide in polymorphonuclear leukocyte phagosomes. J Cell Biol. 1975 Jan;64(1):254–260. doi: 10.1083/jcb.64.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. T., Karnovsky M. L., Karnovsky M. J. Hydrogen peroxide production in chronic granulomatous disease. A cytochemical study of reduced pyridine nucleotide oxidases. J Clin Invest. 1977 Jun;59(6):1088–1098. doi: 10.1172/JCI108732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. The effect of pH upon the equilibria of catalase compounds. J Biol Chem. 1952 Feb;194(2):483–496. [PubMed] [Google Scholar]
- Cline M. J. Bactericidal Activity of Human Macrophages: Analysis of Factors Influencing the Killing of Listeria monocytogenes. Infect Immun. 1970 Aug;2(2):156–161. doi: 10.1128/iai.2.2.156-161.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Cohen A. B., Cline M. J. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Effects of anaerobiosis and inhibitors on O2-production by human granulocytes. Blood. 1975 Jun;45(6):851–861. [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L. The metabolism of leukocytes. Semin Hematol. 1968 Apr;5(2):156–165. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J., Pincus S. H. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971 Oct;50(10):2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D., Epstein M. B., Norman A. Studies on human monocytes with a multiparameter cell sorter. J Histochem Cytochem. 1976 Jan;24(1):355–362. doi: 10.1177/24.1.56390. [DOI] [PubMed] [Google Scholar]
- Loos H., Blok-Schut B., Kipp B., van Doorn R., Meerhof L. Size distribution, electronic recognition, and counting of human blood monocytes. Blood. 1976 Nov;48(5):743–753. [PubMed] [Google Scholar]
- Loos J. A., Roos D. Ficoll-isopaque gradients for the determination of density distributions of human blood lymphocytes and other reticulo-endothelial cells. Exp Cell Res. 1974 Jun;86(2):333–341. doi: 10.1016/0014-4827(74)90721-6. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Miller T. E. Metabolic event involved in the bactericidal activity of normal mouse macrophages. Infect Immun. 1971 Mar;3(3):390–397. doi: 10.1128/iai.3.3.390-397.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Mills E. L., Simmons R. L., Quie P. G. Chemiluminescence response of phagocytizing human monocytes. Infect Immun. 1976 Jul;14(1):129–134. doi: 10.1128/iai.14.1.129-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Homan-Müller J. W., Weening R. S. Effect of cytochalasin B on the oxidative metabolism of human peripheral blood granulocytes. Biochem Biophys Res Commun. 1976 Jan 12;68(1):43–50. doi: 10.1016/0006-291x(76)90007-3. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Sagone A. L., Jr, King G. W., Metz E. N. A comparison of the metabolic response to phagocytosis in human granulocytes and monocytes. J Clin Invest. 1976 May;57(5):1352–1358. doi: 10.1172/JCI108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigbigel R. T., Lambert L. H., Jr, Remington J. S. Phagocytic and bacterial properties of normal human monocytes. J Clin Invest. 1974 Jan;53(1):131–142. doi: 10.1172/JCI107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M., Kühner A. V., Glass E. A., David J. R., Karnovsky M. L. Metabolic and functonal studies on activated mouse macrophages. J Exp Med. 1973 Feb 1;137(2):537–542. doi: 10.1084/jem.137.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaka K., O'Brien P. J. Mechanisms of H2O2 formation by leukocytes. Evidence for a plasma membrane location. Arch Biochem Biophys. 1975 Aug;169(2):428–435. doi: 10.1016/0003-9861(75)90184-8. [DOI] [PubMed] [Google Scholar]
- Weening R. S., Roos D., Loos J. A. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974 Apr;83(4):570–577. [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Wong L., Wilson J. D. The identification of Fc and C3 receptors on human neutrophils. J Immunol Methods. 1975 Apr;7(1):69–76. doi: 10.1016/0022-1759(75)90131-3. [DOI] [PubMed] [Google Scholar]
- de Wit J. J., Henrichs H. J., Odink J., Prins H. K. Experiments on the preparation of blood components with the IBM 2991 blood cell processor. Vox Sang. 1975;29(5):352–362. doi: 10.1111/j.1423-0410.1975.tb00519.x. [DOI] [PubMed] [Google Scholar]
- de la Rivière A. B., Verhoef-Karssen P. R., de Wit J. J., Loos J. A., Prins H. K. Large scale preparation of leukocyte concentrates and further purification of lymphocytes and granulocytes with the IBM 2991 cell processor. Transfusion. 1977 Sep-Oct;17(5):509–512. doi: 10.1046/j.1537-2995.1977.17578014593.x. [DOI] [PubMed] [Google Scholar]
- van Furth R. Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):125–141. [PubMed] [Google Scholar]


