Abstract
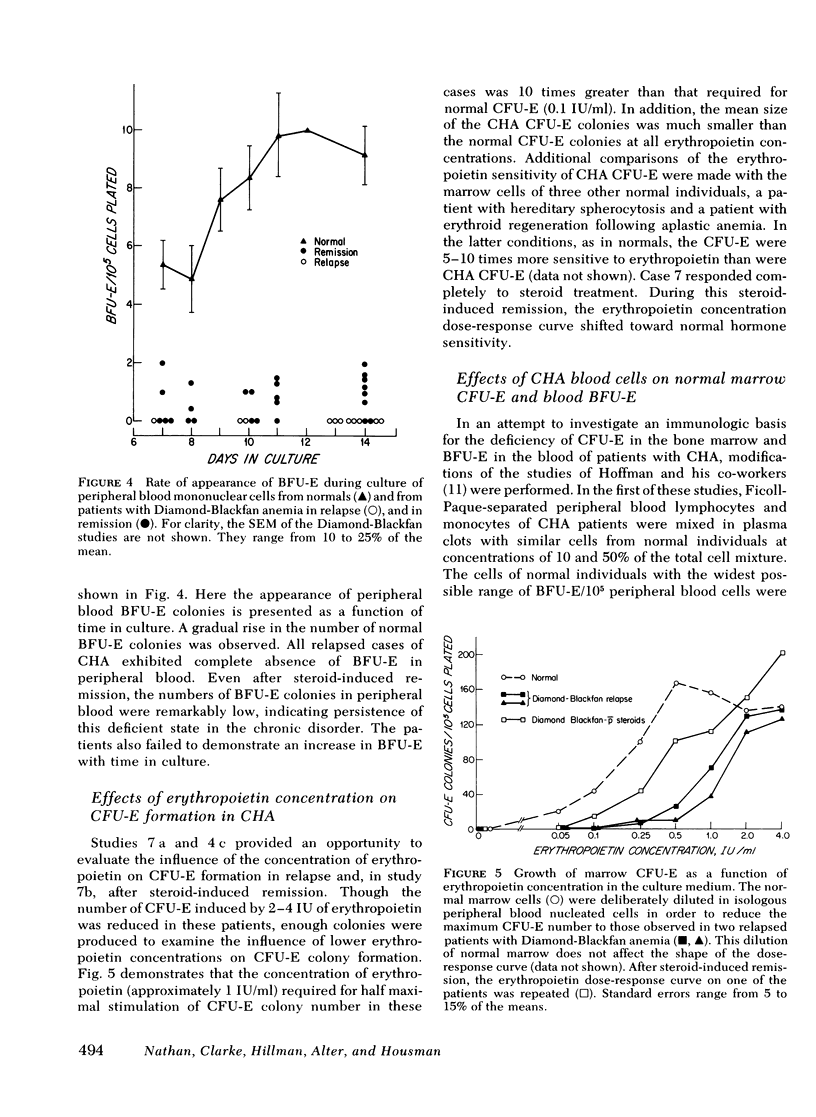
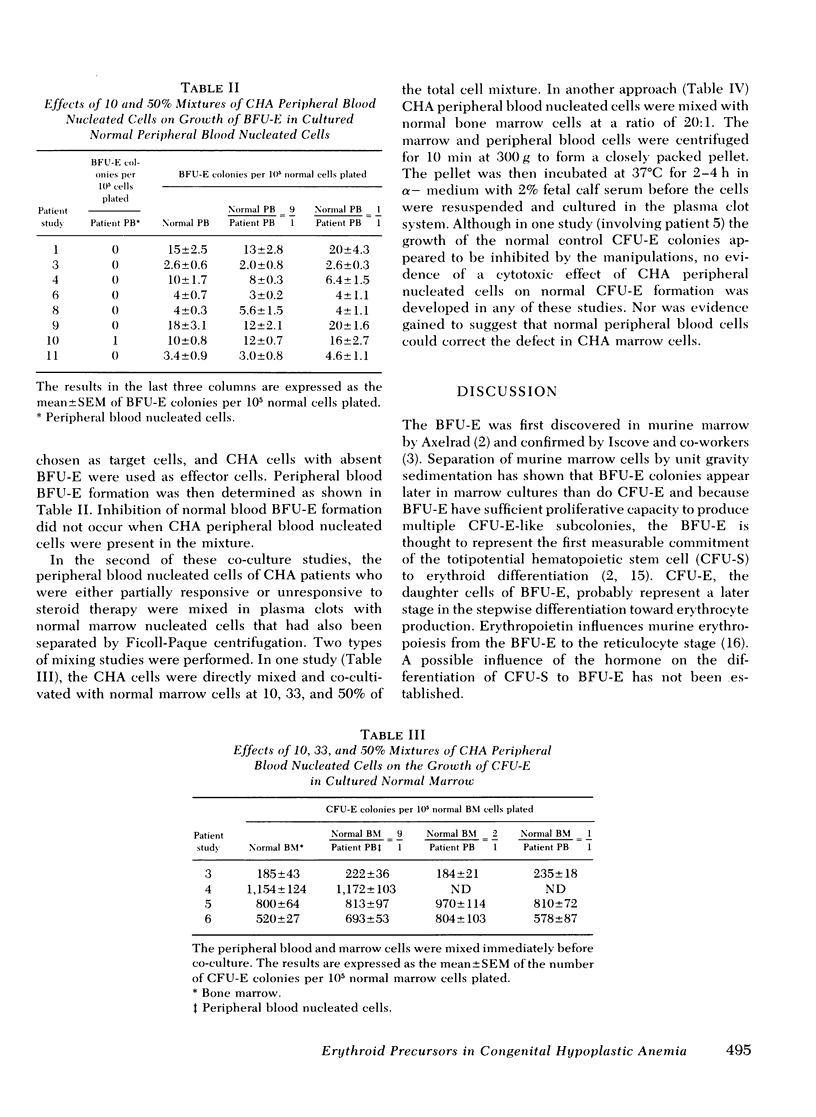
To explore the etiology of congenital hypoplastic anemia (CHA) or the Diamond-Blackfan anemia, erythropoietin responsive committed erythroid precursors were enumerated by the plasma clot method. These included blood and marrow erythroid burst-forming units (BFU-E) and marrow erythroid colony-forming units (CFU-E). The peripheral blood nucleated cells of 11 patients and the marrow cells of seven of these patients were examined. Studies were repeated in several patients during relapse and after induction of remission. BFU-E were undetectable in the marrow and blood of all but one relapsed patient, and the numbers of marrow CFU-E were depressed in all relapsed patients. Blood BFU-E remained low in all of the patients in remission. No evidence was obtained for suppression of normal CFU-E or BFU-E by CHA lymphocytes. Erythropoietin dose-response curves performed in two patients revealed a 10-fold increase in erythropoietin requirement for marrow CFU-E colony growth. This marked unresponsiveness to erythropoietin was strikingly improved by steroid therapy in one patient. We suggest that CHA is the result of a qualitative and/or quantitative deficiency of BFU-E. If BFU-E are produced, they must be relatively unresponsive to erythropoietin. The abnormal BFU-E give rise to erythropoietin unresponsive CFU-E and, thence, to proerythroblasts that are, in turn, trapped in that early stage of development because of their poor erythropoietic response. Hence, red cell production is deficient. Steroids appear to improve the erythropoietin response of CHA erythroid precursors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Adamson J. W. Modulation of in vitro erythropoiesis. The influence of beta-adrenergic agonists on erythroid colony formation. J Clin Invest. 1977 Jul;60(1):70–77. doi: 10.1172/JCI108771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. J., Housman D. Characterization of an erythroid precursor cell of high proliferative capacity in normal human peripheral blood. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1105–1109. doi: 10.1073/pnas.74.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubeleiss K. A., Dancey J. T., Harker L. A., Cheney B., Finch C. A. Marrow erythroid and neutrophil cellularity in the dog. J Clin Invest. 1975 Apr;55(4):825–832. doi: 10.1172/JCI107993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. K., Wang W. C., Alter B. P. Congenital hypoplastic anemia. Adv Pediatr. 1976;22:349–378. [PubMed] [Google Scholar]
- Freedman M. H., Amato D., Saunders E. F. Erythroid colony growth in congenital hypoplastic anemia. J Clin Invest. 1976 Mar;57(3):673–677. doi: 10.1172/JCI108323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. J., Eaves A. C. Human marrow cells capable of erythropoietic differentiation in vitro: definition of three erythroid colony responses. Blood. 1977 Jun;49(6):855–864. [PubMed] [Google Scholar]
- Gregory C. J., Tepperman A. D., McCulloch E. A., Till J. E. Erythropoietic progenitors capable of colony formation in culture: response of normal and genetically anemic W-W-V mice to manipulations of the erythron. J Cell Physiol. 1974 Aug;84(1):1–12. doi: 10.1002/jcp.1040840102. [DOI] [PubMed] [Google Scholar]
- Heath D. S., Axelrad A. A., McLeod D. L., Shreeve M. M. Separation of the erythropoietin-responsive progenitors BFU-E and CFU-E in mouse bone marrow by unit gravity sedimentation. Blood. 1976 May;47(5):777–792. [PubMed] [Google Scholar]
- Hoffman R., Zanjani E. D., Vila J., Zalusky R., Lutton J. D., Wasserman L. R. Diamond-Blackfan syndrome: lymphocyte-mediated suppression of erythropoiesis. Science. 1976 Sep 3;193(4256):899–900. doi: 10.1126/science.986086. [DOI] [PubMed] [Google Scholar]
- Hunter R. E., Hakami N. The occurrence of congenital hypoplastic anemia in half brothers. J Pediatr. 1972 Aug;81(2):346–348. doi: 10.1016/s0022-3476(72)80309-3. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Minagi H., Steinbach H. L. Roentgen appearance of anomalies associated with hypoplastic anemias of childhood: Fanconi's anemia and congenital hypoplastic anemia (erythrogenesis imperfecta). Am J Roentgenol Radium Ther Nucl Med. 1966 May;97(1):100–109. doi: 10.2214/ajr.97.1.100. [DOI] [PubMed] [Google Scholar]
- Miyake T., Kung C. K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977 Aug 10;252(15):5558–5564. [PubMed] [Google Scholar]
- Mott M. G., Apley J., Raper A. B. Congenital (erythroid) hypoplastic anaemia: modified expression in males. Arch Dis Child. 1969 Dec;44(238):757–760. doi: 10.1136/adc.44.238.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. G., Chess L., Hillman D. G., Clarke B., Breard J., Merler E., Housman D. E. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978 Feb 1;147(2):324–339. doi: 10.1084/jem.147.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L., Shreeve M. M. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman A. D., Curtis J. E., McCulloch E. A. Erythropietic colonies in cultures of human marrow. Blood. 1974 Nov;44(5):659–669. [PubMed] [Google Scholar]