Abstract
Hearing in laboratory animals is a topic that traditionally has been the domain of the auditory researcher. However, hearing loss and exposure to various environmental sounds can lead to changes in multiple organ systems, making what laboratory animals hear of consequence for researchers beyond those solely interested in hearing. For example, several inbred mouse strains commonly used in biomedical research (e.g., C57BL/6, DBA/2, and BALB/c) experience a genetically determined, progressive hearing loss that can lead to secondary changes in systems ranging from brain neurochemistry to social behavior. Both researchers and laboratory animal facility personnel should be aware of both strain and species differences in hearing in order to minimize potentially confounding variables in their research and to aid in the interpretation of data. Independent of genetic differences, acoustic noise levels in laboratory animal facilities can have considerable effects on the inhabitants. A large body of literature describes the nonauditory impact of noise on the biology and behavior of various strains and species of laboratory animals. The broad systemic effects of noise exposure include changes in endocrine and cardiovascular function, sleep–wake cycle disturbances, seizure susceptibility, and an array of behavioral changes. These changes are determined partly by species and strain; partly by noise intensity level, duration, predictability, and other characteristics of the sound; and partly by animal history and exposure context. This article reviews some of the basic strain and species differences in hearing and outlines how the acoustic environment affects different mammals.
Laboratory animal facilities typically make great efforts to control environmental variables such as lighting, temperature, humidity, airborne particles, and food and water supply (209). The acoustic environment frequently is given less consideration, possibly reflecting an assumption that the sound environment has little impact on the behavior and normal physiology of laboratory animals (127). Nonetheless, data suggest that animal facilities present a more problematic acoustic environment than previously thought (159), due at least in part to the preponderance of high frequency signals not detectable by human ears (10).
Chronic exposure to loud noise causes hearing loss. As early as 1700, Ramazzini noted that men hammering copper ‘have their ears so injured by that perpetual din… that workers of this class became hard of hearing’ (130). Subsequently, more rigorous scientific study has revealed that environmental noise can detrimentally affect humans and other organisms (43). Until recently, researchers focused their efforts on understanding how noise exposure affects the peripheral receptor organ for hearing, the cochlea. This work, often taking place in the context of loud noise exposures in the factory and military settings, has led to public health recommendations regarding the prevention of hearing loss. Animal research conducted in well-controlled environments has clarified many details concerning how the presence or absence of noise affects the cochlea. As a result of human and laboratory animal studies, current recommendations are that chronic exposure to noise levels at ≥ 85 dB sound pressure level (SPL) for any prolonged period of time can cause permanent hearing loss through cochlear damage (131).
Basic guidelines regarding noise in animal care facilities have been published in the Guide for the Care and Use of Laboratory Animals (132). The Guide notes that researchers and animal facility personnel should consider the sound intensity, frequency, rapidity of onset, duration, vibration potential of the noise and the noise exposure history, hearing range, and susceptibility of the exposed species, stock, or strain. The Guide further notes that a) noisy animals should be housed away from quieter animals, b) many species can hear sound at frequencies that are inaudible to humans (e.g., those produced by video display terminals), c) production of unnecessary noise should be minimized, and d) exposure to sound louder than 85 dB can have both auditory and nonauditory effects. The Guide refers to several articles, also found in the present review, that note nonauditory effects of loud sounds (> 85 dB SPL). However, as described later, noteworthy changes in a number of organ systems can occur after exposure even to sounds below the 85-dB standard that the Guide warns about.
Noise is involved in thousands of scientific studies, not as a purposefully manipulated independent variable, but as an unintended environmental variable that can in some cases confound the data. The extra-auditory impact of noise exposure is a largely overlooked factor in animal-based research. A key function of sensory systems input is to maintain an ethologically appropriate level of arousal (123), and noise affects a number of organ systems and can impact nearly every area of biomedical and behavioral research via this mechanism. Whatever the area of study, from immune response to social behavior, the potential impact of environmental noise on the process should be recognized in order to either minimize noise as a confounding variable or, in some cases, to use noise experimentally as a stress manipulation.
The goals of the present article are to review 1) the sources of noise in animal facilities, 2) hearing differences in laboratory animals, 3) the effects of noise on the auditory system, and 4) some of the nonauditory effects of noise on laboratory animals and people. Research on the nonauditory effects of noise has relied much more extensively on human rather than animal subjects. A sample of findings from humans will be presented here because these data may provide insights regarding possible effects in laboratory animals. This overview is not an exhaustive summary of the literature concerning the effects of noise on the auditory system. For more comprehensive reviews, see those by Boettcher and colleagues (13, 14) and Henderson and coworkers (76).
Noise in Animal Facilities
For the purpose of this review, noise can be defined broadly as any sound in the environment. The frequency content and intensity of environmental sounds can be measured or characterized using modern equipment. A basic explanation of the physics of sound and fundamentals of sound measurement can be found at acoustics.org, a Web resource maintained by the Acoustical Society of America. The frequency content of complex sounds, including noise, can be measured as noise energy in different frequency regions by using filters or Fourier fast transformations (FFT). The French mathematician Joseph Fourier showed that any complex sound wave can be represented as a number of sinusoidal waves of different frequencies, amplitudes, and phases (phase refers to the relative timing of two or more sine waves). Spectral analysis refers to the process of specifying the amplitude, frequency, and phase relationships of the sinusoidal components of a complex waveform. The ear generally is insensitive to the effects of phase changes. Therefore, it is common to consider only the amplitude and frequency of the sinusoidal components when specifying the spectrum of a complex waveform. The frequency of a sinusoid refers to the number of repetitions of the sinusoid that take place in 1 sec. The psychological and experiential correlate of frequency is pitch. The unit of measurement for frequency is the Hertz (Hz). Species used in biomedical research differ widely in their sensitivity to the frequency content of a sound.
Sound is the propagation of a disturbance of the normal distribution of particles in a medium. The pressures that produce local movement of air particles and the associated sound waves in air are very small. The amplitude, or intensity of a sound is related to the displacement of the particles. The psychological and experiential correlate of sound amplitude or intensity is loudness. However, it is easier to measure the amplitude of a sound by measuring the pressure changes that move the particles rather than the displacement of the particles themselves. Sound intensity is proportional to the square of the variations in sound pressure. A 10-fold increase in sound pressure reflects a 100-fold increase in intensity. It is convenient to express a sound pressure level as a ratio to some reference sound pressure level. The measurement of amplitude in this manner is expressed in decibels (dB). A number of dB scales and standards have been developed.
The most common way to express the amplitude of a sound in dB is relative to the international sound pressure standard of 20 µPa. This is referred to as dB sound pressure level (SPL). It is also common to see dB expressed with either an A or C weighting scale. Weighting scales are formed by using a set of filters to attenuate sound of certain frequencies. The dB(A) scale is weighted to reflect the sensitivity and frequency range of human hearing, whereas the dB(C) scale is practically linear over a wide frequency range.
Animal facilities tend to be constructed of epoxy-painted ceilings and concrete walls and concrete floors. Such construction, while minimizing surfaces that can collect dust and dander, also compound the problems of environmental noise by providing little noise absorption and increased reverberation times (29). Consequently, considerable environmental noise is virtually unavoidable in animal facilities and generally stems from three basic sources (148):
Figure 1 plots the normal baseline background noise in a standard animal housing room as recorded from the inside of a typical rat plastic shoebox-style cage with a wire-top lid. Notice that the baseline noise level is low (around 42 dB SPL) but that low-frequency building noise (< 1 kHz) and several other noise peaks also occur. Particularly interesting is the elevated background noise in the 30- to 40-kHz ultrasonic range, which could be animal vocalizations or due to some other unidentified source. It should be noted that different caging styles could dramatically alter the noise levels inside an individual cage. For example, sound recordings inside a shoebox-style plastic cage with a wire lid would likely differ from a ventilated isolator caging system with a blower motor pushing air though small diameter tubing into the cage. Further research is needed to compare the acoustic environment as a function of different caging conditions, especially given the increased use of isolator caging.
Figure 1.
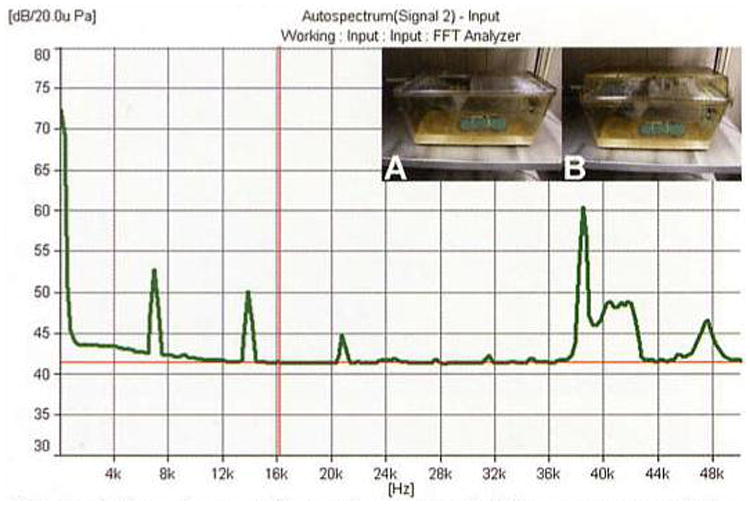
Sample acoustic spectrum recorded from a representative animal housing unit in the Division of Laboratory Animal Medicine at Southern Illinois University School of Medicine. This recording was collected under normal quiet conditions with a Bruel and Kjaer Pulse System using a ½″ free-field microphone (Bruel and Kjaer model 4191-A). The microphone was attached to a model rat at head level in the middle of a plastic shoebox cage with a wire-top lid (A). Measures were also taken with a small particle filter attached to the top of the cage (B). The filter had no noticeable affect on the measurement. Approximately 30 other individually caged rats were present in the 2.5 × 2.5-m room. Baseline levels of noise across the spectrum appear to be around 42 dB SPL. Note the high level of low-frequency noise (≤ 1 kHz) and the harmonics of a 7-kHz signal (i.e., 7, 14, and 21 kHz). These peaks are presumably the result of inherent ventilation and building noise. Particularly interesting is the ultrasonic content ≥ 38 kHz, some of which might be due to rat vocalizations or other unidentified sources.
Pfaff and Stacker (148) recorded noise levels in animal facilities and found approximately 60 episodes/0.5 h at ≥ 90 dB in the morning and > 60 episodes/0.5 h at ≥100 dB in the evening. These intensity measurements were usually associated with short-lasting staff activity in the animal room. More recent studies using better recording of ultrasonic frequencies have shown that much of the noise content in an animal room includes ultrasonic frequencies that are audible to many laboratory animals but not to humans (127, 159). Animal care personnel generated most of the more intense sounds in animal facilities. Many routine duties performed by animal facility personnel and researchers can involve the production of relatively intense sounds. For example, the common practice of simply snapping a wire-top cage lid to a plastic shoebox cage can produce an intense low-frequency noise impulse of nearly 100 dB SPL, with energy of about 80 dB SPL at frequencies up to 40 kHz (Fig. 2).
Figure 2.
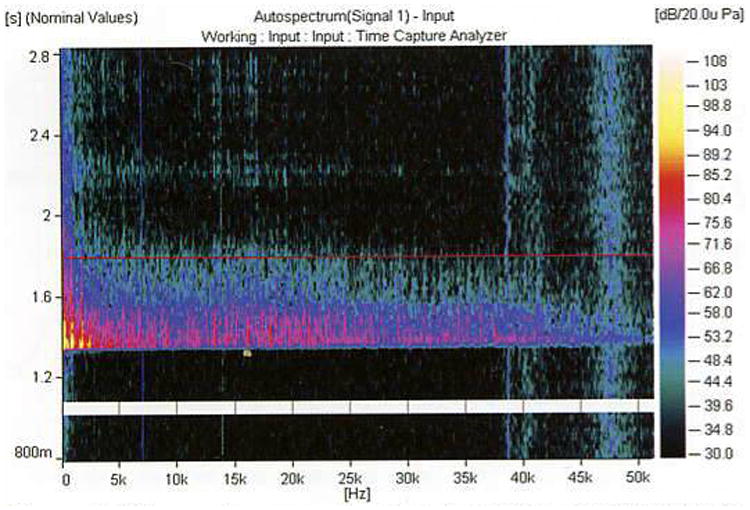
Noise spectrum resulting from the routine activity of attaching a wire-top lid to a typical shoebox-style plastic cage. Sound frequency is on the x-axis, time on the y-axis (response begins at about 1.3 sec), and intensity is color-coded on the z-axis (see legend at right). Measurements were collected as described in Fig. 1. This resulting spectrum produced an intense signal with intense low-frequency content (≤ 5 kHz) of nearly 100 dB SPL and pronounced intensity near 80 dB across the rest of the frequency range.
Recordings conducted on weekends and at other times when workers were not present found that animal facilities are generally quiet in the absence of human activity, suggesting that the noise generated by animals due to activity and communication signals is relatively minor compared with the sounds produced when people are present (127). Exceptions to this depend partly on species and caging type. Marmosets and rabbits produce a remarkably high level of noise regardless of whether humans are present (127). In the case of rabbits, high noise levels were likely due to the animals' thumping on metal cages. Whether high noise levels in marmoset quarters were due to banging on their metal cages or vocalizations was not determined. Rhesus monkeys also generate very high noise levels by rattling and banging on their cages. Peterson (144) showed that such cage rattling/banging generated peak measurements of ≤ 110 dB SPL with sustained levels of > 90 dB. Vocalizations can easily climb to similar levels. For example, barking dogs produced sustained levels > 90 dB and peak levels of 105 to 120 dB SPL (144, 168). These findings suggest that the majority of noise in animal facilities is due to two leading sources: the direct activity of personnel using the facility and the increased activity (vocalization. cage rattling or banging) of animals in response to the presence and actions of people.
Species and Strain Differences in Hearing
The different species and strains of animals commonly used in biomedical research vary in hearing ability. The more commonly used laboratory animals, such as mice, which make up more than 90% of mammals used in research (120), have hearing with frequency sensitivity in the ultrasonic range (≥ 80,000 Hz), far beyond the range of the human ear (20 to 20.000 Hz). Other mammals such as chinchillas, rabbits, cats, and nonhuman primates possess good low-frequency hearing in the same range as that of humans. Figure 3 depicts the audibility curves for humans and some animals commonly used in biomedical research. For a more detailed account of species differences in hearing, see Fay (54).
Figure 3.
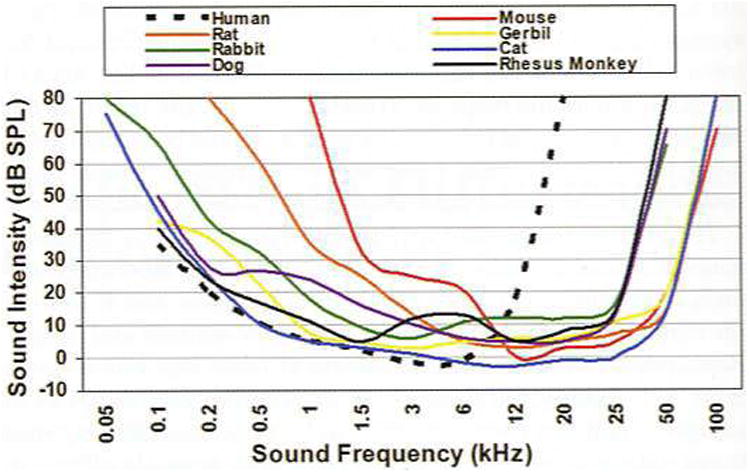
Comparative plot of auditory sensitivity in humans and some animal species commonly used in biomedical research. Adapted from Fay (54); other sources of data include those for human (169), rat (104). mouse (49). gerbil (156), rabbit (73), dog (72), cat (74), and rhesus monkey (149).
To minimize noise as a dangerous and/or confounding variable, researchers and animal care personnel should be aware of the different ranges of hearing sensitivity in the species they work with. In addition to hearing range differences in different species, many breeds and/or strains of commonly used animals differ in hearing function. For example, most of the inbred mouse strains commonly used in biomedical research (including DBA/2J, C57, and BALB) show some degree of genetically determined, progressive hearing loss (204, 206). These same strains show susceptibility to noise-induced seizures induced by sudden sounds such as jingling keys (59, 81). Although noise-induced seizures have been studied most thoroughly in mice, other species (including rats, rabbits, chickens, dogs, goats, cats, hamsters, guinea pigs, and man) also show audiogenic seizures (96). Susceptibility to audiogenic seizures is greatest in the immature auditory system (80). Audiogenic seizures are not just the concern of those investigators using susceptible strains. In a related phenomenon known as acoustic priming, exposure to just a few seconds of loud noise during a critical period early in life can cause seizure-resistant mice to develop a pronounced susceptibility (81). For example, if CBA mice (which hear normally and are not susceptible to audiogenic seizures) are exposed to a 123-dB noise for 2 min when they are 24 to 42 days old, that same exposure repeated 5 days later leads to seizures and death in 75% of the animals (80).
The animals used most often in biomedical research are mice and rats (120). Both genera of rodents provide researchers with a wide variety of strain and stock options. For the hearing researcher, the choice of strain is critically important because of the natural hearing differences present across strains. For example, two commonly used strains of rat are the Fischer 344 (F344), an albino strain developed by M. R. Curtis at Columbia University Institute for Cancer Research in 1920 and the Fl hybrid cross between the F344 and Brown Norway rat (FBN; Fig. 4). These strains have very different patterns of auditory sensitivity. F344s show approximately 20 dB better hearing at low frequencies (4 kHz) whereas FBNs show approximately 20 dB better hearing at higher frequencies (32 kHz; 187).
Figure 4.
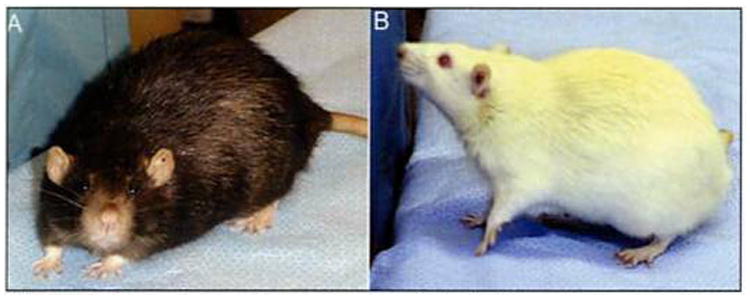
Two commonly used rat models. A. The pigmented FBN is the Fl hybrid cross between F344 females and Brown Norway males. B. F344 is an inbred albino rat. FBNs possess better high-frequency hearing, and F344s better low-frequency hearing.
Variability across mouse strains is potentially even greater. Some commonly used mouse strains develop severe pathologies of the auditory system (201). For example, relative to other strains, DBA/2 mice demonstrate substantial hearing loss beginning at the onset of hearing (around 2 weeks of age) and progressing rapidly until they are profoundly deaf by around 3 to 4 months of age (51, 152, 200). In addition, sudden noise can cause fatal seizures in DBA mice during a critical period between the 3rd and 4th week of life (203). The popular C57BL6 (82, 204) and BALB/c strains (206) commonly used as background strains in genetic studies also show genetically determined, progressive hearing loss that begins relatively early in life. However, the hearing loss in these strains progresses more slowly. Table 1 summarizes the hearing loss found in a number of commonly used mouse strains. For a thorough review of mouse hearing and strain differences, see Willott (201).
Table 1. Hearing loss in some of the most frequently used mouse strains in biomedical research.
| Strain (no. of PubMed hits) | Hearing loss evident at | Reference(s) |
|---|---|---|
| BALB/c (87,266) | 2 months; progresses slowly over next year | 152, 206 |
| C57BL/6 (31,194) | 2 months; progresses slowly over next year | 188,204 |
| A/J (29,424) | 2 months; little further progression | 79 |
| DBA/2 (17,170) | 1 month; progresses rapidly; mice deaf by 8 to 9 months of age | 188, 204 |
| 129 (10,141) | 3 months | 213 |
Frequency of use for different mouse strains was estimated using the National Library of Medicine's PubMed database. Total number of hits for the different strains was determined as of September 2004 by using the keywords “mouse” or “mice” and “BALB/c,” “C57BIV6,” “AJ,” “DBA/2.” and “129.”
Noise and the Auditory System
Intense noise exposure can damage the cochlea and inner ear and lead to a cascade of auditory effects along the entire central auditory pathway. Intense noise exposure is often associated with acute changes in the auditory system, but a completely different set of changes occur as a consequence of the chronic loss of input. Noise overexposure can cause a loss of hearing that is either temporary (temporary threshold shift; TTS) or sometimes permanent (permanent threshold shift; PTS). Partial deafness (loss of hearing input) leads to a reduced sensory input to the brain that has been associated with complex alterations in the balance between excitation and inhibition in the brain (126). These changes can lead to hyperacusis and tinnitus (i.e., ringing in the ears) (24). A number of agents used for other purposes in the animal facility or research laboratory can also impact the auditory system. For example, a number of therapeutic agents used by veterinarians and researchers, including salicylates, loop diuretics, aminoglycoside antibiotics, antithyroid and antitumor drugs, can cause temporary or permanent hearing loss and ringing in the ears (78). Other agents that might be found in animal facilities and laboratories and that can act either alone or interact with noise to produce hearing loss include organic solvents (toluene, styrene, ethylbenzene, xylene, trichloroethylene), metals (mercury, lead, trimethyltin), and asphyxiants (carbon monoxide, hydrogen cyanide) (14, 55).
Intermittent presentation of moderately intense stimuli (85 to 96 dB SPL) can cause TTS but can also ‘toughen’ the auditory system, making it less prone to damage during subsequent intense noise exposure (136). However, noise levels > 100 dB SPL clearly can cause permanent damage in the cochlea. The resulting reduced input to the brain due to hearing loss is associated with hyperacusis and tinnitus, as noted earlier (24). Noise exposure also has been associated with cellular-level changes within the auditory system. Among them are increases in free radical production (210), changes in calcium binding proteins (89), induction of heat-shock proteins (75), c-fos labeling (212), adenosine receptor expression (153), cell loss (202), and changes in inhibitory neurotransmitter activity (126). For a more detailed discussion of the acute and chronic effects of noise exposure on the auditory system, see the reviews by Fay (54), Boettcher and colleagues (13, 14), and Henderson and coworkers (76).
The consequences of noise exposure depend greatly on the context and characteristics of the acoustic insult, including stimulus duration, pattern, frequency content, intensity, and predictability. Noise studies frequently have concentrated on the intensity of the sound and often conclude that less-intense sounds (< 85 dB) are relatively safe. More recent work suggests that the effects of noise are much more complex than can be predicted by assessing intensity alone. Ising and Kruppa (94) suggest that even more relevant than sound intensity is the information content of the sound. Meaningful sounds at relatively low intensity levels can have a considerable impact on animal physiology and behavior by engaging limbic structures and higher centers involved in determining context and meaning.
Whereas the effects of excessive auditory stimulation can be damaging, auditory deprivation can have profound effects as well (see review by Moore, 128). Studies have suggested a sensitive period during which the proper structural development in portions of the auditory system may require acoustic stimulation (38). Mice deprived of airborne sound stimulation during postnatal development showed cellular changes similar to that experienced by persons with pure congenital conductive hearing loss (196).
Relatively little is known about the effects of low-level sounds. Chronic exposure to levels ≤ 70 dB SPL are apparently not harmful to the cochlea but rather improve auditory processing of the exposure stimuli. Poon and Chen (150) showed that exposure to trains of tones improved the ability of the exposed animals to process those same signals. Turner and Willott (188) and Willott and Turner (204, 205) showed that exposure to broadband noise improved both brain (inferior colliculus) and behavioral responses to sounds of the same intensity as the exposure signal. However, improved signal processing after low-level sound exposure might come at the expense of processing other acoustic stimuli. Exposure to a broadband noise stimulus (including a wide range of frequencies) reduced the specificity of auditory cortex neurons, causing improved responses to noise at the expense of responses to frequency-specific pure tones (32, 211). The suggestion from this work is that rearing animals in a constant white noise, masking background (which is being recommended in some animal facilities) might have negative consequences for normal development of the auditory system by effectively masking out the normal input to the ear from vocalizations and other sources.
Although sounds < 85 dB may appear to be unimportant because they do not cause hearing loss or trigger severe autonomic stress reactions, chronic exposure to patterns of acoustic input can alter how sounds are processed. Maybe more important, however, is the suggestion that even low-level environmental sounds may mask communication signals between animals (37). Under this scenario, even a relatively quiet acoustic signal might impair an organism's ability to communicate, perhaps explaining some of the adverse effects of noise (37).
Nonauditory Effects of Noise
It is not surprising that environmental and communication sounds are present in animal facilities and that these sound levels can be quite intense. It is also not surprising that such noise can alter the auditory system, as will be described later. However, what is rather surprising is the wide variety of body systems that are affected by such sounds. Noise affects much more than just the auditory system. Because information from sensory systems provides the organism with critical information about the environment, autonomic responses to sounds are highly adaptive. Like somatosensory, visual, olfactory, and gustatory stimulation, sound can induce a wide range of responses that allow the organism to gather information about the source of the stimulus. Such information is essential for determining whether the stimulus is, for example, a territorial signal, a mating signal, or a signal that a predator or prey is near. Stimuli engage a wide variety of neural systems beyond those basic structures involved in detecting stimuli. The stimulus engages central and peripheral systems that help interpret the stimulus information by controlling autonomic motor responses, vigilance, arousal, emotion, learning and memory, and planning and executing, just to name a few. The influential works of Broadbent (20-23), Loeb (114-118), and Kryter (111) outlined some of the diverse nonauditory consequences of noise, with a focus on human noise research. That work helped to establish noise as a regulator of emotion and arousal in humans and described some of the effects of noise on neural systems and behavior. Just as laboratory animal research provides insight into human medical conditions, the rich noise research literature on humans can provide valuable insights into how noise might impact laboratory animals. The first large-scale effort to identify the multiple effects of noise on animals came in an edited book based upon papers presented at an international symposium on the extra-auditory physiological effects of audible sound (198). That book produced a number of influential findings that began to highlight the systemic effects of noise. Many of them are referred to in this article.
Moruzzi and Magoun (129) first demonstrated that electrical stimulation of the brainstem reticular formation elicited arousal in animals. The reticular formation receives a rich array of collaterals from ascending sensory pathways. Ward (195) concluded that acoustic information, transduced at the auditory nerve, stimulates the reticular activating system producing arousal and wakefulness via the cerebral cortex. More recent work suggests that the reticular formation is more involved in controlling specific body movements during arousal, but the adjacent locus coeruleus probably plays a more direct role in mediating arousal and vigilance (28). Aston-Jones and Bloom (4, 5) found that firing of locus coeruleus neurons was related closely to behavioral arousal and that acoustic stimuli increased firing of these neurons. Zhang and colleagues (212) demonstrated that a single 45-min exposure to an 80-dB SPL, 10-kHz tone not only increased c-Fos labeling (a measure of neural activity) in auditory structures in the brain but also dramatically increased labeling in nonauditory structures such as the locus coeruleus, amygdala, and hypothalamus.
Recent work also helps to explain how noise accesses other neural systems (6, 93, 174, 175). The auditory system connects via the amygdala and other circuits to the hypothalamic-pituitary-adrenal axis (HPA axis) and can thereby cause the release of stress-related hormones. Ising and Kruppa (94) indicated that chronic exposure to as little as 65 dB(A) of noise can induce a variety of nonauditory effects. Figure 5 presents a model of how sensory information can affect multiple neural systems to influence an organism's behavior.
Figure 5.
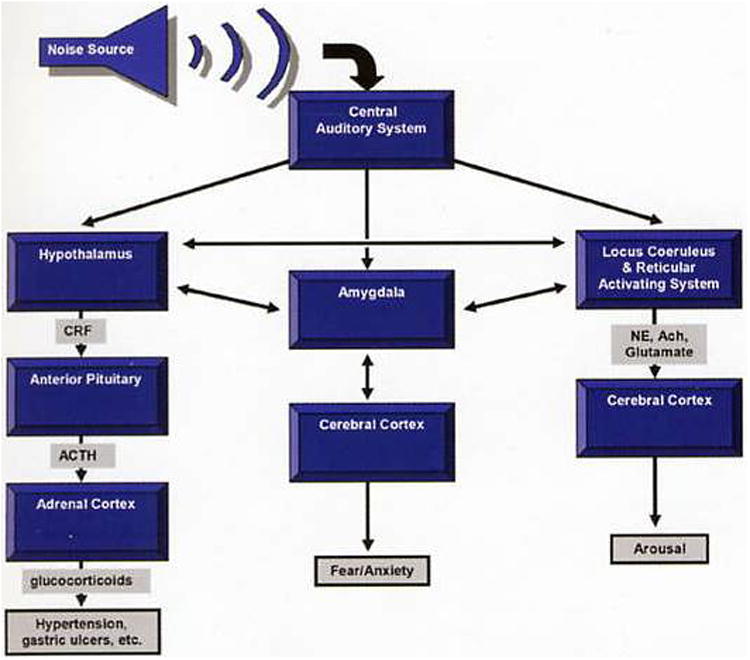
Simplified model of how noise can affect multiple systems. Many additional structures, projections, hormones, and neurotransmitters actually are involved in the process of responding to noise. Ach, acetylcholine; ACTH, adrenocorticotropin hormone; CRF, corticotropin releasing factor; NE, norepinephrine.
Tables 2 and 3 summarize the nonauditory effects of sound in laboratory animals and man. These studies used a variety of different sound stimuli with varied duration of exposure, frequency, and noise intensity as well as different measuring techniques and different species or strains of animal subjects. This summary demonstrates that noise can alter, either directly or indirectly, virtually every major system studied in biomedical research.
Table 2. Summary of the nonauditory effects of sound in laboratory animals.
| System | Results | References |
|---|---|---|
| Cardiovascular | ↑ blood pressure in cat, rat, rhesus monkey, and macaque monkev: ↑ heart rate in desert mule deer and rat | 25, 87, 95,.145, 146, 197 |
| ↑ in vasoconstriction in rat; ↑ respiratory rates and adrenocorticotropin hormone in cat | 15,110 | |
| no change in blood pressure in rat | 16,109 | |
| Hormonal/biochemical | ↑ norepinepherine, Cortisol, cholesterol, and plasma corticosterone in rat; | 9, 42, 46, 77, 193 |
| ↑ noradrenergic and serotonergic activity in dorsal cochlear nucleus, posterior ventral cochlear nucleus, and serotonergic primary auditory cortex in rat; | ||
| ↑ IgM and splenic natural killer cell levels and ↓ splenic lymphatic proliferation and peripheral phagocytic activity in rat | ||
| Reproductive | ↓ in estrus in rat; ↓ fertility rates and ↑ weight of ovaries in both rats and rabbits | 48, 60, 214 |
| ↑ suckling of voung in tree shrew; ↓ milk production in both dairy cattle and tree shrew | 31, 50, 139 | |
| ↑ in fetal mortality and resorption of pups in rat, rabbit, chicken, and pig | 125, 159 | |
| Other | accelerates expression of lupus in a mouse model | 2 |
| can cause audiogenic seizures | 59, 96 | |
| ↑ microvascular permeability and disruption of the intestinal lining in rat | 97 | |
| ↑ in tail flick latency (indication of ↑ analgesia) in rat | 165 | |
| slower wound healing, ↓ in body weight, but no difference in food intake in rat | 208 | |
| ↓ body weight and ↑ in leukocytes and in adrenal gland and liver size in rat and rabbit | 63, 134 | |
| Additional references | 40, 45, 64, 68, 86, 91, 106, 133, 143, 160 |
Table 3. Summary of the nonauditory effects of sound in humans.
| Systems | Results | References |
|---|---|---|
| Cardiovascular | ↑ blood pressure, heart rate, arteriosclerosis, ischemic heart disease, hypertension, coronary artery disease | 36, 47, 52, 70, 90, 99, 140, 155, 176, 192, 207 |
| no change in blood pressure | 112, 121, 122, 178, 191 | |
| Hormonal/biochemical | ↑ levels of norepinepherine. adrenaline, noradrenaline. Cortisol, catecholamines, corticosteriods and hydrocortisone | 6, 17, 93 |
| Reproductive | irregular menstruation, ↓ in birth weight, intrauterine growth retardation, and ↑ in spontaneous abortions | 137 |
| Performance | ↑ risk of overall functional loss notable in aged population; ↓ attention, performance, memory, dual-tasking, cognitive development; affects reading, problem solving, motivation; ↑ irritability and depressed mood | 1, 3, 7, 21-23, 26, 30, 36, 46, 53, 66, 67, 69, 83, 88, 170, 171, 176, 190, 199 |
| Sleep | sleep deprivation, negative affect on immune system and healing, ↑ in adrenaline causing vasoconstriction, ↑ blood pressure and muscle tension, ↓ REM activity and shorter REM duration | 86, 172, 186 |
| Other | migraine headaches, peptic ulcer and irritable bowl syndrome. ↑ neurovascular impairment, ↑ psychological complaints | 11, 71, 155, 173, 177 |
| Additional references | 39, 42, 47, 52, 86, 101, 102, 107, 108, 141, 143, 158, 184, 185, 207 |
REM, rapid eye movement.
Noise activates the sympathetic division of the autonomic nervous system, producing a stress response with physiological characteristics similar to those triggered by other sensory and psychosocial stimuli (35, 46, 163, 164). Although many early noise studies were poorly designed or qualitative in nature, a relatively consistent finding was the elevation of blood pressure by noise. DeJoy (46) described an intriguing study by Ising et al. (93) that was conducted in a German brewery. In that study, blood pressure and stress hormone levels were compared in the same workers on days when they were either wearing or not wearing hearing protection. Elevated blood pressure and norepinephrine levels were measured on days when workers did not wear hearing protection. In a follow-up study that compared one day of working in 85-dB(A) traffic noise to a quiet condition of < 50 dB(A) on the next day, workers exhibited a similar increase in blood pressure and epinephrine levels, along with increases in cholesterol and Mg and decreases in renin and erythrocyte Na (92). Cantrell (27) also reported elevated serum cholesterol and Cortisol levels after a 30-day exposure to 80- to 90-dB SPL noise bursts. Gitanjali and Ananth (65) found that a single 8-h workday exposure to continuous occupational noise of > 75 dB(A) reduced the normal parasympathetic drop in heart rate during sleep and increased Cortisol levels the next morning.
In laboratory animals, Peterson and colleagues (145-147) reported that chronic (6 or 9 months) exposure of nonhuman primates to moderate noise levels (> 85 dB) resulted in elevated blood pressures that did not return to pre-exposure levels within one month after removal of the noise. Borg and Moller (16) reported no effect of life-long exposure to noise on blood pressure in the rat, but most other studies report noteworthy cardiovascular effects secondary to noise exposure (see Tables 2 and 3). Friedman and coworkers (57) reported elevated cholesterol and increased atherosclerosis in rabbits, whereas Clough (33) suggested that some of the cardiovascular effects of noise in laboratory animals could be explained by activation of the neuro-endocrine stress response system. Consistent with this theory are findings that environmental noise causes a number of changes in laboratory animals: hypertension (25, 154), cardiac hypertrophy (62), changes in electrolyte metabolism (113), reduced body weight (56, 157, 208), increased adrenal weight (63), altered tumor resistance and immune response (98), slower wound healing (208), changes in estrus cycles, increased weight of uterus and ovaries, spontaneous lactation, decreased fertility, termination of pregnancy (214), and embryonic abnormalities (62). Mice stressed by sound during pregnancy also produce offspring with poor learning ability (8).
The almost universal finding that noise increases stress hormone levels suggests that chronic noise-induced activation of the HPA axis might cause a variety of problems because of abnormally high levels of circulating stress hormones. Davis (44) demonstrated that exposure to an 80-dB background noise for just a few minutes led to a state of behavioral sensitization or ‘fear’ in rats. Barrett and Stockham (9) demonstrated that banging of the metal cages in an animal room caused a rapid doubling of rat plasma corticosterone levels that lasted for 2 to 4 h. Henkin and Knigge (77) suggested that noise-induced corticosterone elevations can persist for up to 11.5 h. Sprang (174, 175) argues that long-term activation of the HPA axis, along with associated increase in stress hormones, has been linked to immunosuppression (eosinopenia), insulin resistance (diabetes), cardiovascular disease (hypertension and arteriosclerosis), catabolism (osteoporosis), and gastrointestinal problems (stress ulcers). In addition, abrupt noise and tone bursts (110 and 92 dB, respectively) that startle an animal might activate the endogenous opiate system to produce analgesia in rats (41, 165).
Noise can also interfere with sleep by a) prolonging the time needed to fall asleep, b) causing awakening once asleep, c) interfering with the return to sleep once awakened, and d) inducing shifts from deeper to shallower stages (46). Able (1) reviewed the extra-auditory effects of noise and found that noise caused reliable disturbances in sleep patterns in humans (12, 65, 100, 162, 179-183), rabbits (105), and rats (151, 194). In both rats and humans, noise appeared to decrease both slow-wave sleep (65, 182, 151) and paradoxical (or rapid-eye-movement; REM) sleep (58, 65, 100, 103, 151, 186). Sound intensity as low as 60 to 65 dB(A) was found to consistently disrupt human sleep patterns (182, 183). Women living with a heavy snorer are more likely to report insomnia, daytime sleepiness, and fatigue (189), which is not surprising given that the average snoring male seeking treatment produces nearly 500 snores per hour with a maximum intensity range of 63 to 100 dB (84). Furthermore, an 80-dB(A) intermittent noise has a greater adverse impact on sleep quality, mood, and performance than does an 80-dB(A) continuous noise (138). Lukas and Kryter (119) provided some evidence that older individuals are more likely to be awakened by noise, whereas Thiessen (181) showed no such relationship with age.
Noise also has been associated with more complicated behavioral changes in humans. Participants reported elevated psychological tension and decreased work quality after a 30-day exposure to 80 to 90 dB(A) in their work environment (92). Exposure to background noise also can cause a variety of antisocial behaviors in humans. Sauser and colleagues (161) found that exposure to a relatively low-level [70- to 80-dB(A) ] background noise caused study participants to recommend lower salaries for fictitious employees. Humans in a noisy environment will administer greater levels of shock and noise to another person (22). Introduction of a background noise of 85 dB resulted in fewer individuals helping to pick up something that was dropped (124). The degree to which some of these observations can be generalized to animals remains to be determined.
Noise also affects animal behavior, especially maternal behavior. Among rats, rabbits, chickens, and pigs, noise can cause increased resorption of pups by the mother (62, 125, 159). During suckling, a single fire bell test inhibited milk intake and growth in tree shrews (48). This and similar work spurred the development of the “silent” fire alarm in animal facilities (34, 60). The silent fire alarm was adapted so that much of the energy in the alarm signal was below the optimal hearing range for mice and rats. Unfortunately, many laboratory animals (cats, dogs, rabbits, gerbils, chinchillas, nonhuman primates) also hear low frequency sounds well, making the silent fire alarm less effective in those species. Truly silent alarms using strobe lights would seem a reasonable alternative if it were not for the fact that strobe lights also elevate heart rate and are effective animal stressors (18, 19). Future research will help determine whether using strobe lights as alarms are less stressful for laboratory animals, as some research suggests (135).
Summary and Conclusions
Although much remains to be discovered about the nonauditory effects of noise, researchers and animal care workers should assume that noise exposure could cause any or all of the changes described, potentially impacting a wide variety of biomedical research areas. In a recent review of noise effects in humans, Babisch (6) concludes “Current noise research, in general, does not need to prove any longer the noise–stress hypothesis as such. It is common knowledge that noise is a psychosocial stressor that can affect physiological functioning.” We echo this sentiment as it relates to laboratory animals. As in humans, noise can activate the stress response system in laboratory animals, causing a diverse set of consequences for animal models in a variety of areas of biomedical research.
Several key recommendations emerge from this brief survey of the literature. Researchers and laboratory personnel should:
Monitor the acoustic environment of the animal housing facility with respect to both chronic background noise levels as well as the intensity and frequency components associated with common activities. This can be easily and inexpensively accomplished with very basic laboratory equipment borrowed from a typical university physics, engineering, or speech sciences department.
Minimize noise resulting from daily maintenance. Animal care personnel should minimize loud sounds resulting from handling cages and animal room equipment. For example, carts and other equipment with moving parts should be inspected regularly and lubricated when necessary to minimize high-frequency squeaks. Handling animals also produces vocalizations that often are not audible to humans but that can affect animal physiology and behavior dramatically. Many common husbandry and experimental procedures (handling, injections, decapitation) can increase heart rate when done in the presence of other rats (166, 167). Whether such changes are the result of vocalization, visual, or other cues to the animals is unclear. Nevertheless, such procedures might best be done in a separate room from other animals to minimize such confounds, especially if the measure of interest can be altered by stress. Excessive noise in animal facilities also can be minimized using motor covers on cage-washing machines and sound-absorption panels in hallways and loud animal rooms (29). However, a completely sterile acoustic environment resulting from especially quiet rearing, or the use of a white noise background masker, should not be the goal because deprivation of auditory input can have negative consequences for organisms.
Understand the effects of noise on the biological system being studied. For example, a cardiovascular researcher might require special precautions to maintain chronic noise levels in the animal facility below 65 dB in order to minimize noise stress as a confounding variable (94).
Recognize the hearing range and any unique hearing attributes of the animal species or strain being used. Also be aware of any hearing loss that might be present or develop in an animal model. This issue is critical for the mouse researcher, as many of the commonly used strains exhibit hearing loss. For example, a recent study reported abnormal sound processing in the DBA/2 mouse that was attributed to a sensory filter problem, similar to what occurs in humans with schizophrenia. However, DBA mice have severe hearing loss by 1 month of age, and this attribute also might explain their poor responses to sound (142).
Careful monitoring and control of the acoustic environment in animal facilities could provide valuable insights into the sources of unexplainable variability in behavioral and physiological studies. By understanding some of the diverse consequences of noise in animals, researchers can take actions to minimize this confounding variable. The result could be less-variable data and animal models that can more directly answer the research question at hand. However, before more precise guidelines can be developed, more systematic research on noise affects is necessary.
Acknowledgments
Thanks to Thomas Brozoski (SIU School of Medicine) for his assistance recording noise levels in animal facilities. Supported in part by NM grants AG023910-01 to JGT. DC00151 to DMC, and RR17543 to LAT; by SIU Excellence in Academic Medicine grant to LFH; and by SIU Central Research Committee grant to JGT.
References
- 1.Able SM. The extra-auditory effects of noise and annoyance: an overview of research. J Otolaryngol. 1990;19(1):1–13. [PubMed] [Google Scholar]
- 2.Aguas AP, Esguay N, Grande NR, Castro AP, Castclo Branco NAA. Acceleration of lupus erythematosus-like processes by low frequency noise in the hybrid NZB/VV mouse model. Aviat Space Environ Med. 1999;70(3 part 2):A132–A136. [PubMed] [Google Scholar]
- 3.Anders K. Subjective, behavorial and psychophysiological effects of noise. Scand J Work Environ Health. 1990;16(1):29–38. doi: 10.5271/sjweh.1825. [DOI] [PubMed] [Google Scholar]
- 4.Aston-Jones G, Bloom FE. Nonrepinephrine-containing locus coerulcus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1(8):887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aston-Jones G, Bloom FE. Activity of norepinepherine-containing locus eoeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babisch W. Stress hormones in the research on cardiovascular effects of noise. Noise Health. 2003;18(5):1–11. [PubMed] [Google Scholar]
- 7.Balfour JL, Kaplan GA. Neighborhood environment and loss of physical function in older adults: evidence from the Alameda county study. Am J Epidemiol. 2002;155(6):507–515. doi: 10.1093/aje/155.6.507. [DOI] [PubMed] [Google Scholar]
- 8.Barlow SM. Ph D thesis. University of London; 1972. Teratogenic effects of restraint, cold and audiogenic stress in mice and rats. [Google Scholar]
- 9.Barrett AM, Stockham MA. The effect of housing conditions and simple experimental procedures upon the corticos-terone level in the plasma of rats. J Endocrinol. 1963;26:97–105. doi: 10.1677/joe.0.0260097. [DOI] [PubMed] [Google Scholar]
- 10.Bell RW. Ultrasounds in small rodents: arousal-produced andarousal-producing. Dev Psychobiol. 1974;7(1):39–42. doi: 10.1002/dev.420070107. [DOI] [PubMed] [Google Scholar]
- 11.Berglund B, Lindvall T, Nordin S. Adverse effects of aircraft noise. Environ Int. 1990;16:315–338. [Google Scholar]
- 12.Berry B, Thiessen GJ. The effects of impulsive noise on sleep. National Research Council of Canada, Division of Physics; Ottawa: 1970. [Google Scholar]
- 13.Boettcher FA, Gratton MA, Schmiedt RA. Effects of noise and age on the auditory system. Occup Med. 1995;10(3):577–591. [PubMed] [Google Scholar]
- 14.Boettcher FA, Henderson D, Gratton MA, Danielson RW, Byrne CD. Synergistic interactions of noise and other ototraumatic agents. Ear Hear. 1987;8(4):192–212. doi: 10.1097/00003446-198708000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Borg E. Peripheral vasoconstriction in the rat in response to sound: dependence on stimulus duration. Acta Otolaryngol. 1978;85:153–157. doi: 10.3109/00016487809111921. [DOI] [PubMed] [Google Scholar]
- 16.Borg E, Moller AR. Noise and blood pressure: effect of lifelong exposure in the rat. Acta Physiol Scand. 1978;103(3):340–342. doi: 10.1111/j.1748-1716.1978.tb06223.x. [DOI] [PubMed] [Google Scholar]
- 17.Brandenberger G, Follenius M, Wittersheim G, Salame P. Plasma catecholamines and pituitary adrenal hormones related to mental task demand under quiet and noise conditions. Biol Psychol. 1980;10:239–252. doi: 10.1016/0301-0511(80)90037-x. [DOI] [PubMed] [Google Scholar]
- 18.Briski KP, Sylvester PW. Effect of specific acute stressors on luteinizing hormone release in ovariectomized and ovariectomized estrogen-treated female rats. Neuroendocrinology. 1988;47:194–202. doi: 10.1159/000124913. [DOI] [PubMed] [Google Scholar]
- 19.Briski KP, Sylvester PW. Comparative effects of various stressors on immunoreactive versus bioactive prolactin release in old and young male rats. Neuroendocrinology. 1990;51:625–31. doi: 10.1159/000125402. [DOI] [PubMed] [Google Scholar]
- 20.Broadbent DE. The role of auditory localization in attention and memory span. J Exp Psychol. 1954;47(3):191–196. doi: 10.1037/h0054182. [DOI] [PubMed] [Google Scholar]
- 21.Broadbent DE. Noise and behavior. Proc R Soc Med. 1957;50(4):225–228. [PubMed] [Google Scholar]
- 22.Broadbent DE. Human performance and noise. In: Harris CM, editor. Handbook of noise control. Vol. 17. McGraw-Hill; New York: 1979. pp. 1–17.pp. 20 [Google Scholar]
- 23.Broadbent DE. Recent advances in understanding performance in noise. In: Rossi G, editor. Proceedings of the IVth International Congress on Noise as a Public Health Problem. Vol. 2. Edizioni Techniche a cura del Centro Ricerche e Studi Amplifon; Milan: 1983. [Google Scholar]
- 24.Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22(6):2283–2290. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckley JP, Smookler HH. Cardiovascular and biochemical effects of chronic intermittent neurogenic stimulation. In: Welch BL, Welch AS, editors. Physiological effects of noise. Plenum Press; New York: 1970. pp. 75–84. [Google Scholar]
- 26.Bullinger M, Hygge S, Evans GW. A prospective study of some effects of aircraft noise on cognitive performance in schoolchildren. Psychol Sci. 2002;13(5):469–474. doi: 10.1111/1467-9280.00483. [DOI] [PubMed] [Google Scholar]
- 27.Cantrell RW. Prolonged exposure to intermittent noise: audiometric, biochemical, motor, psychological and sleep effects. Laryngoscope. 1974;84:4–55. [PubMed] [Google Scholar]
- 28.Carlson DE, Gann DS. Alpha-adrenergic input in the locus coeruleus modulates plasma adrenocorticotropin in cats. Endocrinology. 1992;130(5):2795–2803. doi: 10.1210/endo.130.5.1349279. [DOI] [PubMed] [Google Scholar]
- 29.Carlton DL, Richards W. Affordable noise control in a laboratory animal facility. Lab Anim. 2002;31(1):47–48. doi: 10.1038/5000117. [DOI] [PubMed] [Google Scholar]
- 30.Carter NL. Transportation noise, sleep and possible after-affects. Environ Int. 1996;22:105–116. [Google Scholar]
- 31.Casady RB, Lehmann RP. Response of farm animals to sonic booms: studies at Edwards Air Force Base, June 6-30, 1966. U.S. Department of Agriculture Research Division; Beltsville, Md: 1967. [Google Scholar]
- 32.Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- 33.Clough G. Environmental effects on animals used in biomedical research. Biol Rev. 1982;57:487–523. doi: 10.1111/j.1469-185x.1982.tb00705.x. [DOI] [PubMed] [Google Scholar]
- 34.Clough G, Fasham JA. A ‘silent’ fire alarm. Lab Anim. 1975;9(3):193–196. doi: 10.1258/002367775780994538. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S. Extra-auditory effects of acoustic stimulation. In: Lee DH, editor. Handbook of physiology: reactions to environmental agents. American Physiology Society; Bethesda, Md: 1977. [Google Scholar]
- 36.Cohen S, Evans GW, Krantz DS, Stokols D. Physiological, motivational and cognitive effects of aircraft noise on children: moving from the laboratory to the field. Am Psychol. 1980;35:231–243. doi: 10.1037//0003-066x.35.3.231. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Weinstein N. Nonauditory effects of noise on behavior and health. J Soc Issues. 1981;37(1):36–70. [Google Scholar]
- 38.Coleman J, Blatchley BJ, Willimans JE. Development of the dorsal and ventral cochlear nuclei in rat and the effects of acoustic deprivation. Brain Res. 1982;256(1):119–123. doi: 10.1016/0165-3806(82)90104-3. [DOI] [PubMed] [Google Scholar]
- 39.Colle HA, Welsh A. Acoustic masking in primary memory. J Verbal Learn Verbal Behav. 1976;15:17–32. [Google Scholar]
- 40.Cook RO, Nawrot PS, Hamm CW. Effects of high-frequency noise on prenatal development and maternal plasma and uterine catacholamine concentrations in the CD-I mouse. Toxicol Appl Pharmacol. 1982;66:338–348. doi: 10.1016/0041-008x(82)90300-3. [DOI] [PubMed] [Google Scholar]
- 41.Cranney J. Analgesia following startle-eliciting stimuli. Psychobiology. 1988;16:67–69. [Google Scholar]
- 42.Cransac H, Cottet-Emard JM, Hellström S, Peyrin L. Specific sound-induced noradrenergic and serotonergic activation in central auditory structures. Hear Res. 1998;118:151–156. doi: 10.1016/s0378-5955(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 43.Davis H. Survey of the problem BENOX Report: An exploratory study of the biological effects of noise. The University of Chicago; Chicago: 1953. [Google Scholar]
- 44.Davis M. Sensitization of the rat startle response by noise. J Comp Physiol. 1974;87:571–581. doi: 10.1037/h0036985. [DOI] [PubMed] [Google Scholar]
- 45.Davis RR, Kozel P, Erway LC. Genetic influences in individual susceptibility to noise: a review. Noise Health. 2003;20(5):19–28. [PubMed] [Google Scholar]
- 46.Dejoy DM. The nonauditory effects of noise: review and perspective for research. J Aud Res. 1984;24:123–150. [PubMed] [Google Scholar]
- 47.Demeter I, Drasoveanu C, Cherestes I, Kertesz I, Demeter E. The inter-relationship between sonic trauma and arteriosclerosis. Arch Otorhinolaryngol. 1979;24(3):197–203. [Google Scholar]
- 48.D'Souza F, Martin RD. Maternal behavior and the effects of stress in tree shrews. Nature. 1974;251:309–311. doi: 10.1038/251309a0. [DOI] [PubMed] [Google Scholar]
- 49.Ehret G. Naturwissenchaften. Vol. 11. German: 1974. Age-dependent hearing loss in normal hearing mice; p. 506. [DOI] [PubMed] [Google Scholar]
- 50.Ely F, Peterson WE. Factors involved in the ejection of milk. J Dairy Sci. 1941;14:211–222. [Google Scholar]
- 51.Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice. I. Inbred and Fl hybrid strains. Hear Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- 52.Evans GW, Bullinger M, Hygge S. Chronic noise exposure and physiological response: a prospective study of children living under environmental stress. Psychol Sci. 1998;9:75–77. [Google Scholar]
- 53.Evans GW, Maxwell L. Chronic noise exposure and reading deficits. Environ Behav. 1997;29:638–656. [Google Scholar]
- 54.Fay RR. Hearing in vertebrates: a psychophysics data book. Hill Fay Associates; Winnetka, Ill: 1988. [Google Scholar]
- 55.Fechter LD. Promotion of noise-induced hearing loss by chemical contaminants. J Toxicol Environ Health. 2004;67:727–740. doi: 10.1080/15287390490428206. [DOI] [PubMed] [Google Scholar]
- 56.Fink GB, Itturian WB. Influence of age, auditory conditioning and environmental noise on sound-induced seizure and seizure threshold in mice. In: Welch BL, Welch AS, editors. Physiological effects of noise. Plenum Press; New York: 1970. pp. 211–226. [Google Scholar]
- 57.Friedman M, Byers SO, Brown AE. Plasma lipid responses of rats and rabbits to an auditory stimulus. Am J Physiol. 1967;212(5):1174–1178. doi: 10.1152/ajplegacy.1967.212.5.1174. [DOI] [PubMed] [Google Scholar]
- 58.Fruhstorfer B, Pritsch MG, Fruhstorfer H. Effects of daytime noise load on the sleep-wake cycle and endocrine patterns in man. I. 24 hours neurophysiological data. Int J Neurosci. 1988;39:197–209. doi: 10.3109/00207458808985704. [DOI] [PubMed] [Google Scholar]
- 59.Fuller JL, Easier C, Smith ME. Inheritance of audiogenic seizures susceptibility in the mouse. Genetics. 1950;35:622–632. doi: 10.1093/genetics/35.6.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gamble MR. Fire alarms and oestrus in rats. Lab Anim. 1976;10:161–163. doi: 10.1258/002367776781071468. [DOI] [PubMed] [Google Scholar]
- 61.Gamble MR. Sound and its significance for laboratory animals. A review Biol Rev Camb Philos Soc. 1982;57(3):395–421. doi: 10.1111/j.1469-185x.1982.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 62.Geber WF. Cardiovascular and teratogenic effects of chronic intermittent noise stress. In: Welch BL, Welch AS, editors. Physiological effects of noise. Plenum Press; New York: 1970. pp. 85–90. [Google Scholar]
- 63.Geber WF, Anderson TA, Van Dyne B. Physiologic responses in the albino rat to chronic noise stress. Arch Environ Health. 1966;12:751–754. doi: 10.1080/00039896.1966.10664476. [DOI] [PubMed] [Google Scholar]
- 64.Gesi M, Fornai F, Lenzi P, Natale G, Soldani P, Paparelli A. Time-dependent changes in adrenal cortex ultra-structure and corticosterone levels after noise exposure in male rats. Eur J Morphol. 2001;39(3):129–135. doi: 10.1076/ejom.39.3.129.4673. [DOI] [PubMed] [Google Scholar]
- 65.Gitanjali B, Ananth R. Effect of acute exposure to loud occupational noise during daytime on the nocturnal sleep architecture, heart rate, and Cortisol secretion in healthy volunteers. J Occup Health. 2003;45:146–152. doi: 10.1539/joh.45.146. [DOI] [PubMed] [Google Scholar]
- 66.Glass DC, Singer JE. Urban stress: experiments on noise and social stressors. Academic Press; New York: 1972. [Google Scholar]
- 67.Gomes LMP, Martinho Pimenta AJF, Castelo Branco NAA. Effects of occupational exposure to low-frequency noise on cognition. Aviat Space Environ Med. 1999;70:A115–A118. [PubMed] [Google Scholar]
- 68.Gunther T, Ising H, Mohr-Nawroth F, Chahoud I, Merker HJ. Embryotoxic effects of magnesium deficiency and stress on rats and mice. Teratology. 1981;24:225–33. doi: 10.1002/tera.1420240213. [DOI] [PubMed] [Google Scholar]
- 69.Haines MM, Stansfeld SA, Job RFS, Berglund B. Chronic aircraft noise exposure and child cognitive performance and stress. In: Carter NL, Job RFS, editors. Proceedings of noise as a public health problem. 1-2. University of Sydney; Australia: 1998. pp. 329–35. [Google Scholar]
- 70.Hannunkari I, Jarvinen E, Partanen T. Work conditions and health of locomotive engineers. II. Questionnaire study, mortality and disability. Scand J Work Environ Health. 1978;4(3):15–38. doi: 10.5271/sjweh.2763. [DOI] [PubMed] [Google Scholar]
- 71.Hattori H. A field study of health effects of noise in adults around Komatsu Air Base. Nippon Koshu Eisei Zasshi. 2000;47(1):20–31. In Japanese with English summary. [PubMed] [Google Scholar]
- 72.Heffner HE. Hearing in large and small dogs: absolute thresholds and size of the tympanic membrane. Behav Neurosci. 1983;97:310–318. [Google Scholar]
- 73.Heffner HE, Masterton B. Hearing in glires: domestic rabbit, cotton rabbit, house mouse, and kangaroo rat. J Acoust Soc Am. 1980;68:1584–1599. [Google Scholar]
- 74.Heffner RS, Heffner HE. Hearing range of the domestic cat. Hear Res. 1985;19:85–88. doi: 10.1016/0378-5955(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 75.Helfert RH, Glatz FR, III, Wilson TS, Ramkumar V, Hughes LF. Hsp70 in the inferior colliculus of Fischer-344 rats: effects of age and noise exposure. Hear Res. 2002;170(1-2):155–165. doi: 10.1016/s0378-5955(02)00487-2. [DOI] [PubMed] [Google Scholar]
- 76.Henderson D, Prasher D, Kopke RD, Salvi RJ, Hamernik RP. Noise induced hearing loss: basic mechanisms, prevention and control. NRN Publications; London: 2001. [Google Scholar]
- 77.Henkin RI, Knigge KM. Effect of sound on the hypothalamic-pituitary-adrenal axis. Am J Physiol. 1963;204:701–704. doi: 10.1152/ajplegacy.1963.204.4.710. [DOI] [PubMed] [Google Scholar]
- 78.Henley CM, Rybak LP. Ototoxicity in developing mammals. Brain Res Brain Res Rev. 1995;20(1):68–90. doi: 10.1016/0165-0173(94)00006-b. [DOI] [PubMed] [Google Scholar]
- 79.Henry KR. Abnormal auditory development resulting from exposure to ototoxic chemicals, noise, and auditory restriction. In: Romarrd R, editor. Development of auditory and vestigle systems. Academic Press; New York: 1983. [Google Scholar]
- 80.Henry KR. Noise and the young mouse: genotype modifies the sensitive period for effects on cochlear physiology and audiogenic seizures. Behav Neurosci. 1984;98(6):1073–1082. doi: 10.1037//0735-7044.98.6.1073. [DOI] [PubMed] [Google Scholar]
- 81.Henry KR, Bowman RE. Acoustic priming of audiogenic seizures in mice. In: Welch BE, Welch AS, editors. Physiological effects of noise. Plenum Press; New York: 1970. pp. 185–201. [Google Scholar]
- 82.Henry KR, Lepkowski CM. Evoked potential correlates of genetic progressive hearing loss. Age-related changes from the ear to the inferior colliculus of C57BL/6 and CBA/J mice. Acta Otolaryngol. 1978;86(5-6):366–374. doi: 10.3109/00016487809107515. [DOI] [PubMed] [Google Scholar]
- 83.Hockey GRJ. Stress and the cognitive components of skilled performance. In: Hamilton V, Warburton DM, editors. Human stress and cognition. John Wiley and Sons; New York: 1979. pp. 141–177. [Google Scholar]
- 84.Hoffstein V, Haight J, Cole P, Zamel N. Does snoring contribute to presbycusis? Am J Respir Crit Care Med. 1999;159:1351–1354. doi: 10.1164/ajrccm.159.4.9808147. [DOI] [PubMed] [Google Scholar]
- 85.Honkus VI. Sleep deprivation in critical care units. Crit Care Nurs Q. 2003;26(3):179–189. doi: 10.1097/00002727-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Horner KC. The emotional ear in stress. Neurosci Biobehav Rev. 2003;27:437–446. doi: 10.1016/s0149-7634(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 87.Hudak WJ, Buckley JP. Production of hypertensive rats by experimental stress. J Pharmaceut Sci. 1961;50:263–264. doi: 10.1002/jps.2600500321. [DOI] [PubMed] [Google Scholar]
- 88.Hygge S, Evans G, Bullinger M. Proceedings of Inter-Noise 96. Institute of Acoustics; Liverpool, United Kingdom: 1996. The Munich airport noise study: cognitive effects on children from before to after the change over of airports. [Google Scholar]
- 89.Idrizbegovic E, Bogdanovic N, Canlon B. Modulating calbindin and parvalbumin immunoreactivity in the cochlear nucleus by moderate noise exposure in mice: a quantitative study on the dorsal and posteroventral cochlear nucleus. Brain Res. 1998;800(1):86–96. doi: 10.1016/s0006-8993(98)00504-6. [DOI] [PubMed] [Google Scholar]
- 90.Idzior-Walus B. Coronary risk factors in men occupationally exposed to vibration and noise. Eur Heart J. 1987;8:1040–1046. doi: 10.1093/oxfordjournals.eurheartj.a062168. [DOI] [PubMed] [Google Scholar]
- 91.Ising H. Extraaural effects of chronic noise exposure in animals: a review. Schriftenr Ver Wasser Boden Lufthyg. 1993;88:48–64. [PubMed] [Google Scholar]
- 92.Ising H, Dienel D, Gunther T, Marker B. Health effects of traffic noise. Int Arch Occup Environ Health. 1980;47:179–80. doi: 10.1007/BF00716376. [DOI] [PubMed] [Google Scholar]
- 93.Ising H, Günther T, Haverstadt C, Krause Ch, Market B, Melchert HU, Schoknecht G, Thefeld W, Tietze KW. EPA translation TR-79-0857. Office of Noise Abatement and Control; Washington, D.C.: 1979. Study on the quantification of risk for the heart and circulatory system associated with noise on workers. [Google Scholar]
- 94.Ising H, Kruppa B. Health effects caused by noise: evidence in the literature from the past 25 years. Noise Health. 2004;22(6):5–13. [PubMed] [Google Scholar]
- 95.Ising H, Melchert HU. Endocrine and cardiovascular effects of noise. In: Tobias JV, Jansen G, Ward WD, editors. Proceedings of the Third International Congress on Noise as a Public Health Problem (ASHA Reports 10) American Speech, Language, and Hearing Association; Rockville, Md: 1980. [Google Scholar]
- 96.Iturrian WB. Cartworth letter, no 92. Cartworth, New York: 1973. The effect of noise on immature rodents. [Google Scholar]
- 97.Jain M, Baldwin AL. Are laboratory animals stressed by their housing environment and are investigators aware that this stress can affect physiological data? Med Hypotheses. 2003;60(2):284–289. doi: 10.1016/s0306-9877(02)00387-0. [DOI] [PubMed] [Google Scholar]
- 98.Jensen MM, Ramussen SF., Jr . Audiogenic stress and susceptibility to infection. In: Welch BL, Welch AS, editors. Physiological effects of noise. Plenum Press; New York: 1970. pp. 7–19. [Google Scholar]
- 99.Jonsson A, Hansson L. Prolonged exposure to a stressful stimulus (noise) as a cause of raised blood pressure in man. Lancet. 1977;1:86–87. doi: 10.1016/s0140-6736(77)91093-5. [DOI] [PubMed] [Google Scholar]
- 100.Jurriens AA, Griefhan B, Kumar A, Vallet M, Wilkinson RT. An essay in European research collaborations: common results from the project on traffic noise and sleep in the home. In: Rossi G, editor. Proceedings of the 4th International Congress on Noise as a Public Health Problem. Vol. 2. Edizioni Techniche a cura del Centro Ricerche e Studi Amplifon; Milan: 1983. pp. 929–937. [Google Scholar]
- 101.Kawada T. Effects of traffic noise on sleep: a review. Jpn J Hyg. 1995;50:932–938. doi: 10.1265/jjh.50.932. [DOI] [PubMed] [Google Scholar]
- 102.Kawada T. The effect of noise on the health of children. J Nippon Med Sch. 2004;71(1):5–10. doi: 10.1272/jnms.71.5. [DOI] [PubMed] [Google Scholar]
- 103.Kawada T, Suzuki S. Change in rapid eye movement (REM) sleep in response to exposure to all-night noise and transient noise. Arch Environ Health. 1999;54:336–340. doi: 10.1080/00039899909602497. [DOI] [PubMed] [Google Scholar]
- 104.Kelly JB, Masterton B. Auditory sensitivity of the albino rat. J Comp Physiol Psychol. 1977;91:930–936. doi: 10.1037/h0077356. [DOI] [PubMed] [Google Scholar]
- 105.Khazan N, Sawyer CH. Rebound recovery from deprivation of paradoxical sleep in the rabbit. Proc Soc Exp Biol Med. 1963;114:536–539. doi: 10.3181/00379727-114-28725. [DOI] [PubMed] [Google Scholar]
- 106.Kimmel CA, Cook RO, Staples RE. Teratogenic potential of noise in mice and rats. Toxicol Appl Pharmacol. 1976;36:239–245. doi: 10.1016/0041-008x(76)90003-x. [DOI] [PubMed] [Google Scholar]
- 107.King RP, Davis JR. Community noise: health effects and management. Int J Hyg, Environ Health. 2003;206:123–131. doi: 10.1078/1438-4639-00202. [DOI] [PubMed] [Google Scholar]
- 108.Kjellberg A. Subjective, behavioral and psychophysiological effects of noise. Scand J Work Environ Health. 1990;161(1):29–38. doi: 10.5271/sjweh.1825. [DOI] [PubMed] [Google Scholar]
- 109.Kraft-Schreyer N, Angelanos ET. Efforts of sound stress on norepinepherine responsiveness and blood pressure. Fed Proc. 1979;38:883. [Google Scholar]
- 110.Kristensen MP, Rector DM, Poe GR, Harper RM. Activity changes of the cat paraventricular hypothalamus during stressor exposure. Neuroenclocrinology. 2004;15(1):43–48. doi: 10.1097/00001756-200401190-00010. [DOI] [PubMed] [Google Scholar]
- 111.Kryter KD. Evaluation of exposures to impulse noise. Arch Environ Health. 1970;20(5):624–635. doi: 10.1080/00039896.1970.10665675. [DOI] [PubMed] [Google Scholar]
- 112.Lees REM, Roberts JH. Noise-induced hearing loss and blood pressure. Can Med Assoc J. 1979;120:1082–1084. [PMC free article] [PubMed] [Google Scholar]
- 113.Lockett MF. Effects of sound on endocrine function and electrolyte excretion. In: Welch BL, Welch AS, editors. Physiological effects of noise. Plenum Press; New York: 1970. pp. 21–42. [Google Scholar]
- 114.Loeb M. The present state of research on effects of noise: are we asking the right questions? J Aud Res. 1981;21:93–104. [PubMed] [Google Scholar]
- 115.Loeb M. Noise and human efficiency. Wiley; Chichester, United Kingdom: 1986. [Google Scholar]
- 116.Loeb M, Holding DH, Baker MA. Noise stress and circadian arousal in self-paced computation. Motiv Emotion. 1982;6:43–48. [Google Scholar]
- 117.Loeb M, Jones PD. Noise exposure, monitoring and tracking performance as a function of signal bias and task priority. Ergonomics. 1978;21(4):265–272. doi: 10.1080/00140137808931723. [DOI] [PubMed] [Google Scholar]
- 118.Loeb M, Percival L. Influence of noise characteristics on behavioral aftereffects. Hum Factors. 1980;22(3):341–352. doi: 10.1177/001872088002200308. [DOI] [PubMed] [Google Scholar]
- 119.Lukas JS, Kryter KD. Awakening effects of simulated sonic booms and subsonic aircraft noise. In: Welch BL, Welch AS, editors. Physiological effects of noise. Plenum Press; New York: 1970. pp. 283–293. [Google Scholar]
- 120.Malakoff D. The rise of the mouse: biomedicine's model mammal. Science. 2000;288(5464):248–253. doi: 10.1126/science.288.5464.248. [DOI] [PubMed] [Google Scholar]
- 121.Malchaire JB, Mullier M. Occupational exposure to noise and hypertension: a retrospective study. Am Occup Hyg. 1979;22:63–66. doi: 10.1093/annhyg/22.1.63. [DOI] [PubMed] [Google Scholar]
- 122.Manninen O, Aro S. Noise-induced hearing loss and blood pressure. Int Arch Occup Environ Health. 1979;42:251–256. doi: 10.1007/BF00377779. [DOI] [PubMed] [Google Scholar]
- 123.Martin JH. Coding and processing of sensory information. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of neural science. 3rd. Appleton & Lange; East Norwalk, Conn: 1991. pp. 329–339. [Google Scholar]
- 124.Matthews KE, Jr, Canon LK. Environmental noise level as a determinant of helping behavior. J Pers Soc Psychol. 1975;32:571–577. [Google Scholar]
- 125.Meyer RE, Aldrich TE, Eastley CE. Effects of noise and electromagnetic fields on reproductive outcomes. Environ Health Perspect. 1989;81:193–200. doi: 10.1289/ehp.8981193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147:251–260. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 127.Milligan SR, Sales GD, Khirnykh K. Sound levels in rooms housing laboratory animals: an uncontrolled daily variable. Phys Behav. 1993;53:1067–1076. doi: 10.1016/0031-9384(93)90361-i. [DOI] [PubMed] [Google Scholar]
- 128.Moore DR. Postnatal development of the mammalian central auditory system and the neural consequences of auditory deprivation. Acta Otolaryngol Suppl. 1985;421:19–30. doi: 10.3109/00016488509121753. [DOI] [PubMed] [Google Scholar]
- 129.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEC Electroencephalogr. Clin Neurol. 1949;1:455–473. [PubMed] [Google Scholar]
- 130.National Institute for Occupational Safety and Health. Education and Information Division and Division of Biomedical and Behavioral Science. United States Department of Health and Human Services; Washington, D.C.: 1996. Criteria for a recommended standard occupational noise exposure revised criteria 1996; pp. 1–77. [Google Scholar]
- 131.National Institute of Health Consensus Report. Noise and hearing loss: consensus conference. JAMA. 1990;263(23):3185–3190. [PubMed] [Google Scholar]
- 132.National Research Council. Guide for the care and use of animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- 133.Nawrot PS, Cook RO, Staples RE. Embryotoxicity of various noise stimuli in the mouse. Teratology. 1980;22:279–289. doi: 10.1002/tera.1420220304. [DOI] [PubMed] [Google Scholar]
- 134.Nayfield KC, Besch EL. Comparative responses of rabbits and rats to elevated noise. Lab Anim Sci. 1981;31(4):386–390. [PubMed] [Google Scholar]
- 135.Nephew BC, Kahn SA, Romero LM. Heart rate and behavior are regulated independently of corticosterone following diverse acute stressors. Gen Comp Endocrinol. 2003;133:173–180. doi: 10.1016/s0016-6480(03)00165-5. [DOI] [PubMed] [Google Scholar]
- 136.Niu X, Canlon B. Protective mechanisms of sound conditioning. Adv Otorhinolaryngol. 2002;59:96–105. doi: 10.1159/000059246. [DOI] [PubMed] [Google Scholar]
- 137.Nurminen T. Female noise exposure, shift work, and reproduction. J Occup Environ Med. 1995;37(8):945–950. doi: 10.1097/00043764-199508000-00010. [DOI] [PubMed] [Google Scholar]
- 138.Öhrström E, Rylander R, Björkman M. Effects of night time road traffic noise: an overview of laboratory and field studies on noise dose and subjective noise sensitivity. J Sound Vib. 1988;122:277–290. [Google Scholar]
- 139.Parker JB, Bayley ND. Investigation of effects of aircraft sound on milk production of dairy cattle 1957-1958. United States Department of Agriculture; Washington, D.C.: 1960. p. 22. [Google Scholar]
- 140.Parvizpoor D. Noise exposure and prevalence of high blood pressure. J Occup Med. 1976;18:730–731. doi: 10.1097/00043764-197611000-00007. [DOI] [PubMed] [Google Scholar]
- 141.Passchier-Vermeer W, Passchier WF. Noise exposure and public health. Environ Health Perspect. 2000;108(1):123–131. doi: 10.1289/ehp.00108s1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology. 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 143.Pepper CB, Nascarella MA, Kendall RJ. A review of the effects of aircraft noise on wildlife and humans, current control mechanisms, and the need for further study. Environ Manag. 2003;32(4):418–432. doi: 10.1007/s00267-003-3024-4. [DOI] [PubMed] [Google Scholar]
- 144.Peterson EA. Noise and laboratory animal. In: Melby EC, editor. Defining the laboratory animal and its environment Lab Anim Sci. 2. Vol. 30. 1980. pp. 422–439. [PubMed] [Google Scholar]
- 145.Peterson EA, Augenstein JS, Haselton CL, Tanis DC, Augenstein DG. Noise raises blood pressure without impairing auditory sensitivity. Science. 1981;211:1450–1452. doi: 10.1126/science.7466404. [DOI] [PubMed] [Google Scholar]
- 146.Peterson EA, Augenstein JS, Haselton CL, Tanis DC. Some cardiovascular effects of noise. J Aud Res. 1984;24(1):35–62. [PubMed] [Google Scholar]
- 147.Peterson EA, Haselton CL, Augenstein JS. Daily noise duration influences cardiovascular responses. J Aud Res. 1984;24(2):69–86. [PubMed] [Google Scholar]
- 148.Pfaff J, Stecker M. Loudness level and frequency content of noise in the animal house. Lab Anim. 1976;10:111–117. doi: 10.1258/002367776781071521. [DOI] [PubMed] [Google Scholar]
- 149.Pfingst BJ, Laycock J, Flammino F, Lonsbury-Martin B, Martin G. Pure tone thresholds for the rhesus monkey. Hear Res. 1978;1:43–47. doi: 10.1016/0378-5955(78)90008-4. [DOI] [PubMed] [Google Scholar]
- 150.Poon PW, Chen X. Postnatal exposure to tones alters the tuning characteristics of inferior follicular neurons in the rat. Brain Res. 1992;585:391–394. doi: 10.1016/0006-8993(92)91243-8. [DOI] [PubMed] [Google Scholar]
- 151.Rabat A, Bouyer JJ, Aran JM, Courtiere A, Mayo W, Le Moal M. Deleterious effects of an environmental noise on sleep and contribution of its physical components in a rat model. Brain Res. 2004;1009:88–97. doi: 10.1016/j.brainres.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 152.Ralls K. Auditory sensitivity in mice, Peromyscus and Mus musculus. Anim Behav. 1967;15:123–128. doi: 10.1016/s0003-3472(67)80022-8. [DOI] [PubMed] [Google Scholar]
- 153.Ramkumar V, Whitworth CA, Pingle SC, Hughes LF, Rybak LP. Noise induces A(1) adenosine receptor expression in the chinchilla cochlea. Hear Res. 2004;88(1-2):47–56. doi: 10.1016/S0378-5955(03)00344-7. [DOI] [PubMed] [Google Scholar]
- 154.Rosecrans JS, Watzman N, Buckley JP. The production of hypertension in male albino rats subjected to experimental stress. Biochem Pharmacol. 1966;15:1707–1718. [Google Scholar]
- 155.Rosenlund M, Berglind N, Pershagen G, Jarup L, Bluhm G. Increased prevalence of hypertension in a population exposed to aircraft noise. Occup Environ Med. 2001;58:769–773. doi: 10.1136/oem.58.12.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ryan A. Hearing sensitivity of the Mongolian gerbil, Meriones unguiculatus. J Acoust Soc Amer. 1976;59:1222–1226. doi: 10.1121/1.380961. [DOI] [PubMed] [Google Scholar]
- 157.Sackler AM, Weltman AS, Bradshaw M, Jurtshuck P., Jr . Acta Endocrinol. Vol. 31. Copenhagen: 1959. Endocrine changes due to auditory stress; pp. 405–418. [DOI] [PubMed] [Google Scholar]
- 158.Salamé P, Baddeley A. Differential effects of noise and speech on short-term memory. In: Rossi G, editor. Proceedings of the 4th International Congress on Noise as a Public Health Problem. Vol. 1. Edizioni Techniche a cura del Centro Ricerche e Studi Amplifon; Milan: 1983. pp. 751–758. [Google Scholar]
- 159.Sales GD, Wilson KJ, Spencer KE, Milligan SR. Environmental ultrasound in laboratories and animal houses: a possible cause for concern in the welfare and use of laboratory animals. Lab Anim. 1988;22(4):369–375. doi: 10.1258/002367788780746188. [DOI] [PubMed] [Google Scholar]
- 160.Sanz P, Rodriguez-Vicente MC, Villar P, Repetto M. Uncontrollable atmospheric conditions which can affect animal experimentation. Vet Hum Toxicol. 1988;30(5):452–454. [PubMed] [Google Scholar]
- 161.Sauser WI, Jr, Arauz CG, Chambers RM. Exploring the relationship between level of office noise and salary recommendations: a preliminary research note. J Manag. 1978;4:57–63. [Google Scholar]
- 162.Scott TD. The effects of continuous, high intensity, white noise on the human sleep cycle. Psychophysiology. 1972;9:227–232. doi: 10.1111/j.1469-8986.1972.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 163.Selye H. Further thoughts on “stress without distress”. Med Times. 1976;104(11):124–144. [PubMed] [Google Scholar]
- 164.Selye H. Forty years of stress research: principal remaining problems and misconceptions. Can Med Assoc J. 1976;115(1):53–56. [PMC free article] [PubMed] [Google Scholar]
- 165.Shankar N, Awasthy N, Mago H, Tandon OP. Analgesic effect of environmental noise: a possible stress response in rats. Ind J Phys Pharmacol. 1999;43(3):337–346. [PubMed] [Google Scholar]
- 166.Sharp J, Zammit T, Azar T, Lawson D. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci. 2003;42(1):9–18. [PubMed] [Google Scholar]
- 167.Sharp J, Zammit T, Azar T, Lawson D. Are “bystander” female Sprague-Dawley rats affected by experimental procedures? Contemp Top Lab Anim Sci. 2003;42(1):19–27. [PubMed] [Google Scholar]
- 168.Sierens SE, Ingley HA, Besch EL. Ninth Conference on Space Simulation. National Aeronautics and Space Administration; Washington, D.C.: 1977. A methodology for estimating dog noise in an animal housing facility; pp. 167–177. [Google Scholar]
- 169.Sivian LJ, White SD. On minimum audible fields. J Acoustic Soc Am. 1933;4:288–321. [Google Scholar]
- 170.Smith A. A review of the effects of noise on human performance. Scand J Psychol. 1989;30:185–206. doi: 10.1111/j.1467-9450.1989.tb01082.x. [DOI] [PubMed] [Google Scholar]
- 171.Smith A, Miles C. The combined effects of noise and nightwork on human function. In: Oborne DJ, editor. Contemporary ergonomics: proceedings of the Ergonomics Society's annual conference 1985 in Nottingham. Taylor & Francis; London: 1985. pp. 33–41. [Google Scholar]
- 172.Snyder-Halpern R. The effect of critical care unit noise on patient sleep cycles. Crit Care Q. 1985;7:41–51. [PubMed] [Google Scholar]
- 173.Spitzer M, Neumann M. Noise in models of neurological and psychiatric disorders: review. Int J Neural Syst. 1996;7(4):355–361. doi: 10.1142/s0129065796000312. [DOI] [PubMed] [Google Scholar]
- 174.Spreng M. Central nervous system activation by noise. Noise Health. 2000;2(7):49–58. [PubMed] [Google Scholar]
- 175.Spreng M. Possible health effects of noise induced Cortisol increase. Noise Health. 2000;2(7):59–64. [PubMed] [Google Scholar]
- 176.Standsfeld SA, Matheson MP. Noise pollution: non-auditory effects on health. Br Med Bull. 2003;68:243–257. doi: 10.1093/bmb/ldg033. [DOI] [PubMed] [Google Scholar]
- 177.Suvarov GA, Denisov EI, Ovakimov VG, Tavtin IUK. Gigiena Truda i Professional'nye Zabolevaniia. Vol. 7. Russian: 1979. Correlations between hearing losses and neurovascular impairments in workers in relation to noise levels; pp. 18–22. [PubMed] [Google Scholar]
- 178.Talbott E, Helmkamp J, Matthews K, Kuller L, Cottington E, Redmond G. Occupational noise exposure, noise-induced hearing loss, and the epidemiology of high blood pressure. Am J Epidemiol. 1985;121(4):501–514. doi: 10.1093/oxfordjournals.aje.a114028. [DOI] [PubMed] [Google Scholar]
- 179.Terzano MG, Parrino L, Fioriti G, Orofiamma B, Depoortere H. Modifications of sleep structure induced by increasing levels of acoustic perturbation in normal subjects. Electroencephalogr Clin Neurophysiol. 1990;76:29–38. doi: 10.1016/0013-4694(90)90055-o. [DOI] [PubMed] [Google Scholar]
- 180.Thiessen GJ. Effects of noise during sleep. In: Welch BL, Welch AS, editors. Psychological effects of noise. Plenum Press; New York: 1970. pp. 271–275. [Google Scholar]
- 181.Thiessen GJ. Disturbance of sleep by noise. J Acoust Soc Am. 1978;64:216–222. doi: 10.1121/1.381964. [DOI] [PubMed] [Google Scholar]
- 182.Thiessen GJ, Lapointe AC. Effect of intermittent truck noise on percentage of deep sleep. J Acoust Soc Am. 1978;64:1078–1080. doi: 10.1121/1.382066. [DOI] [PubMed] [Google Scholar]
- 183.Thiessen GJ, Lapointe AC. Effect of continuous traffic noise on percentage of deep sleep, waking and sleep latency. J Acoust Soc Am. 1983;73:225–229. doi: 10.1121/1.388853. [DOI] [PubMed] [Google Scholar]
- 184.Thompson SJ. Extraaural health effects of chronic noise exposure in humans: a review. Schriftenr Ver Wasser Boden Lufthyg. 1993;88:91–117. [PubMed] [Google Scholar]
- 185.Topf M. Hospital noise pollution: an environmental stress model to guide research and clinical interventions. Adv Nursing. 2000;31(3):520–528. doi: 10.1046/j.1365-2648.2000.01307.x. [DOI] [PubMed] [Google Scholar]
- 186.Topf M, Davis J. Critical care unit noise and rapid eye movement (REM) sleep. Heart Lung. 1993;22:252–58. [PubMed] [Google Scholar]
- 187.Turner JG, Caspary DM. Comparison of two rat models of aging: Peripheral pathology and GABA changes in the inferior colliculus. In: Syka J, Merzenich M, editors. Plasticity of the central auditory system and processing of Complex acoustic signals. Kluwer-Plenum; London: in press. [Google Scholar]
- 188.Turner JG, Willott JF. Exposure to an augmented acoustic environment alters auditory function in hearing-impaired DBA/2J mice. Hear Res. 1998;118:101–113. doi: 10.1016/s0378-5955(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 189.Ulfberg J, Carter N, Talback M, Edling C. Adverse health effects among women living with heavy snorers. Health Care Women Int. 2000;21(2):81–90. doi: 10.1080/073993300245311. [DOI] [PubMed] [Google Scholar]
- 190.United States Environmental Protection Agency. Office of the Scientific Assistant, Office of Noise Abatement and Control. National Association of Noise Control Officials; Fort Walton Beach, Fla: 1981. Noise effects handbook. [Google Scholar]
- 191.Van Dijk FJ, Ettema JH, Zielhuis RL. Non-auditory effects of noise in industry. VII: Evaluation, conclusions and recommendations. Int Arch Occup Environ Health. 1987;59:133–145. doi: 10.1007/BF00378492. [DOI] [PubMed] [Google Scholar]
- 192.Van Kempen E, Kruize H, Boshuizen HC, Ameling CB, Staatsen B, de Hollander A. The association between noise exposure and blood pressure and ischemic heart disease: a meta-analysis. Environ Health Perspect. 2002;110(3):307–317. doi: 10.1289/ehp.02110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Van Raaij MTM, Oortgiesen M, Timmerman HH, Dobbe CJG, Van Loveren H. Time-dependent differential changes of immune function in rats exposed to chronic intermittent noise. Physiol Behav. 1996;60:1527–1533. doi: 10.1016/s0031-9384(96)00327-7. [DOI] [PubMed] [Google Scholar]
- 194.Van Twyver H, Levitt R, Dunn R. The effects of high intensity white noise on the sleep pattern of the rat. Psychon Sci. 1966;6:355–356. [Google Scholar]
- 195.Ward AA. BENOX report: an exploratory study of the biological effects of noise. The University of Chicago; Chicago: 1953. Central nervous system effects. [Google Scholar]
- 196.Webster DB, Webster M. Neonatal sound deprivation affects brain stem auditory nuclei. Arch Otolaryngol. 1977;103(7):392–396. doi: 10.1001/archotol.1977.00780240050006. [DOI] [PubMed] [Google Scholar]
- 197.Weisenberger ME, Krausman PR, Wallace MC, De Young DW, Maughan OE. Effects of simulated jet aircraft noise on heart rate and behavior of desert ungulates. J Wildlife Manag. 1996;60:52–61. [Google Scholar]
- 198.Welch BL, Welch AS. Physiological effects of noise. Plenum Press; New York: 1970. [Google Scholar]
- 199.Wilkinson RT. Interaction of noise with knowledge of results and sleep deprivation. J Exp Psychol. 1963;66:332–337. doi: 10.1037/h0044161. [DOI] [PubMed] [Google Scholar]
- 200.Willott JF. Comparison of response properties of inferior colliculus neurons of two inbred mouse strains differing in susceptibility to audiogenic seizures. J Neurophysiol. 1981;45:35–47. doi: 10.1152/jn.1981.45.1.35. [DOI] [PubMed] [Google Scholar]
- 201.Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; New York: 2001. [Google Scholar]
- 202.Willott JF, Bross L. Effects of prolonged exposure to an augmented acoustic environment on the auditory system of middle-aged C57BI7J6 mice: cochlear and central histology and sex differences. J Camp Neurol. 2004;472(3):358–370. doi: 10.1002/cne.20065. [DOI] [PubMed] [Google Scholar]
- 203.Willot JF, Henry KR. Roles of anoxia and noise-induced hearing loss in the postictal refractory period for audiogenic seizures in mice. J Comp Physiol Psychol. 1976;90(4):373–381. doi: 10.1037/h0077204. [DOI] [PubMed] [Google Scholar]
- 204.Willott JF, Turner JG. Prolonged exposure to an augmented acoustic environment ameliorates age-related auditory-changes in C57BL/6J and DBA/2J mice. Hear Res. 1999;135(1-2):78–88. doi: 10.1016/s0378-5955(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 205.Willott JF, Turner JG. Neural plasticity in the mouse inferior colliculus: relationship to hearing loss, augmented acoustic stimulation, and pre-pulse inhibition. Hear Res. 2000;147(1-2):275–281. doi: 10.1016/s0378-5955(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 206.Willott JF, Turner JG, Carlson S, Ding D, Seegers-Bross L, Falls WA. The BALB/c mouse as an animal model for progressive sensorineural hearing loss. Hear Res. 1998;115(1-2):162–174. doi: 10.1016/s0378-5955(97)00189-5. [DOI] [PubMed] [Google Scholar]
- 207.Wu TN, Ko YC, Chang PY. Study of noise exposure and high blood pressure in shipyard workers. Am J Ind Med. 1987;12:431–438. doi: 10.1002/ajim.4700120408. [DOI] [PubMed] [Google Scholar]
- 208.Wysocki AB. The effect of intermittent noise exposure on wound healing. Adv Wound Care. 1996;9(1):35–39. [PubMed] [Google Scholar]
- 209.Yamaguchi C. Studies on the environmental control of laboratory animals. Exp Anim. 1995;44(1):9–21. doi: 10.1538/expanim.44.9. [DOI] [PubMed] [Google Scholar]
- 210.Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019(1-2):201–209. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- 211.Zhang JS, Kaltenbach JA, Wang J, Kim SA. Kos-like iinmunoreactivity in auditory and nonauditory brain structures of hamsters previously exposed to intense sound. Exp Brain Res. 2003;153:655–660. doi: 10.1007/s00221-003-1612-4. [DOI] [PubMed] [Google Scholar]
- 212.Zhang LI, Bao S, Merzenich MM. Disruption of primary auditory cortex by synchronous auditorv inputs during a critical period. Proa Natl Acad Sci USA. 2002;99(4):2309–2314. doi: 10.1073/pnas.261707398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zondek B, Tamari I. Effects of auditory stimuli on reproduction. In: Wolstenholme GEW, O'Connor M, editors. Effects of external stimuli on reproduction. Ciba Foundation Study No. 26; A. Churchill, London: 1967. pp. 4–19. [Google Scholar]


