Abstract
Aims
Abnormal baseline and acutely worsening renal function (WRF) during heart failure (HF) hospitalization are associated with worse outcomes. However, which renal criterion is most predictive of in-hospital and post-discharge mortality is uncertain.
Methods
We analyzed patients hospitalized for HF between January 1, 2000 and June 30, 2008. Preexisting end-stage renal disease was excluded. Blood urea nitrogen (BUN), creatinine (Cr), and MDRD-estimated glomerular filtration rate (eGFR) at admission and during hospitalization were tested for association with in-hospital and 1-year mortality. Logistic regression and conditional receiver operating curves were used to compare criterion in terms of association with mortality.
Results
Among 7,394 patients, 204 died in-hospital, and 1,652 within 1 year. Admission BUN was the strongest correlate for both in-hospital and post-discharge mortality (area under curve [AUC]= 0.724 and 0.656; p<0.001 vs. Cr/eGFR), showing 4.6 and 3.0 fold mortality, respectively. Adjusting for baseline BUN, subsequent changes in Cr and BUN performed similarly for in-hospital death (model AUC 0.812; p<0.001 vs. eGFR) and post-discharge death (all similar, model AUC=0.661). Optimally predictive thresholds of WRF in hospital were dependant on the baseline renal function, and did not always correspond to common definitions.
Conclusions
Among hospitalized HF patients, baseline BUN is the renal index most strongly associated with in-hospital and one year mortality. WRF definitions that use BUN or Cr, have similar discriminative ability overall, but commonly used thresholds are suboptimal for predicting mortality; optimal thresholds varied with baseline renal function and time horizon.
Keywords: Renal function, BUN, Mortality, Heart Failure, Acutely Decompensated Heart Failure
INTRODUCTION
Heart Failure (HF) continues to be a growing epidemic in the Unites States, with a nearly 300,000 deaths and roughly $40 billion cost annually, much of which is attributable to the nearly one million hospitalizations per year.(1) Patients with renal dysfunction during a HF hospitalization have higher in-hospital mortality, longer length of stay, greater resource utilization, and increased long term mortality,(2,3) and thus renal function has become a critical prognostic factor in HF patients. Despite many studies focusing on this topic, there remain several challenges to optimally utilizing common renal function parameters to inform prognosis and clinical decision making.
First is that a wide variety of definitions of worsened renal function (WRF) have been described in the literature as predictive of adverse outcomes. These include increase in serum creatinine (Cr) by more than 0.3 mg/dl from admission baseline, > 25 % increase in Cr, and different cut-offs for the Modification of Diet in Renal Disease (MDRD) estimated glomerular filtration rate (eGFR) values.(4–6) Some definitions included the peak change during hospitalization, while others require a change that persists to discharge or beyond.(7) Besides the inherent confusion on which definition is best, these data have often overlooked another common measure of renal function, blood urea nitrogen (BUN), which some recent studies have suggested may be a better predictor of mortality than Cr-based estimates.(8) An added complexity is that it is also unclear what measure of baseline renal function is most predictive (i.e. Cr, BUN, or eGFR) of outcomes and most existing studies failed to simultaneously account for both baseline renal dysfunction and WRF that occurs during hospitalization. This is critical because WRF only adds value if it is incrementally predictive in addition to baseline renal dysfunction, which clearly impacts outcomes. Moreover, the two factors may interact because baseline renal function modifies the risk of WRF occurring, and could also alter what thresholds or definition for WRF offers the best predictive performance. Finally, a variety of outcomes have been examined in the literature, most often in-hospital mortality, such as that in the ADHERE data.(2) Equally important though is how renal dysfunction may aid in longer term prognostication (e.g. mortality one year after discharge), which has received relatively less attention, and is likely to have differing association with renal dysfunction criteria compared to in-hospital death.
Thus, the optimal criteria for interpreting renal dysfunction in the setting of hospitalized HF have not been established. We undertook this retrospective study to fill this gap by determining the best parameter and definitions for assessing baseline renal dysfunction and WRF in hospitalized HF patients. We compared Cr, BUN, and MDRD eGFR, utilizing baseline and in-hospital changes, in terms of predicting in-hospital mortality and one-year post-discharge mortality.
METHODS
Study Population
Using automated data sources, we identified all patients 18 years of age and older with a primary hospital discharge diagnosis of HF (see appendix for the International Classification of Diseases, 9th Edition/ Revision [ICD] codes used) between January 1, 2000 and June 30, 2008. Only the first (index) hospitalization for each individual during the observation period and meeting the above criteria (a primary diagnosis of HF) were included and analyzed. Patients were required to be members of the system health plan with at least one year of continuous enrollment prior to the index hospitalization, and primarily received their care through health system physicians. Patients with end-stage renal disease requiring renal replacement therapies were excluded from this analysis. Patients were followed until they reached an endpoint (death) or were censored at the earlier of either disenrollment or the final follow up date of December 3, 2008.
Computerized Data Sources
Data for this retrospective study were obtained from the following sources: electronic administrative databases maintained by the health system, vital records for the Michigan Department of Community Health, and the Death Master File (DMF) from the Social Security Administration (SSA). The administrative data captured claims (i.e., coded diagnosis, procedures, and prescription fills) occurring both within and outside the health system. A master patient index contained demographic data (i.e., date of birth, sex and race). Laboratory data was available for all tests performed within the health system. The DMF reported to SSA and was available through the National Technical Information Service. The DMF included the following information on each decedent: social security number, name, date of birth, date of death, state or country of residence, and ZIP codes of last residence and payment. Records of deaths, which occurred in the Michigan, were available from the Division of Vital Records and Health Statistics, Michigan Department of Community Health. Social Security number was used to index both State of Michigan Vital Records and the DMF.
Comorbidities (i.e., hypertension, diabetes mellitus, vascular disease, stroke, atrial fibrillation and coronary artery disease) were identified using ICD9 or CPT codes alone or in combination as described previously. Laboratory data were extracted for admission values and daily changes in serum Cr, BUN, and eGFR. Serum Cr was measured in milligrams/deciliter (mg/dl), BUN measured in mg/dl. eGFR was derived by using the following MDRD formula: eGFR = 186 × serum Cr − 1.154 × age −0.203 × 1.212 (if black) × 0.742 (if female).
End-point Assessment
Primary end points were in-hospital death and 1-year post discharge death. Deaths were ascertained using data from the health system administrative data, as well as vital records from the State of Michigan and the SSA DMF. Deaths within the cohort were obtained by matching patients’ social security numbers with vital records and the DMF. The resulting queries from these two sources were kept separate, and a third query was performed for State vital records by matching on last name, date of birth, and sex. Death records that existed in at least two of the three queries were considered true death. Death records existing in only one file were reviewed individually by study team members and adjudicated as to whether they matched study individuals. Deaths identified by administrative data as having occurred during the index hospitalization were considered true deaths if there were no other subsequent medical claims for the individual following the reported in-hospital event.
Statistical Analysis
Continuous variables were expressed as mean ± SD with categorical variables expressed as percentages. Admission Cr, GFR, and BUN values were each analyzed for their ability to predict mortality using linear logistic regression analysis. Our criterion for choosing the best predictor of mortality was the area under the receiving operator characteristic curve (ROC). A test for paired ROC curves was used to determine if any of the three predictors outperformed the others. We then evaluated the maximum increase observed during hospitalization from the admission value for Cr, eGFR and BUN. A linear logistic regression was again used. Each model was adjusted for the admission BUN (as it was shown to be the best baseline outcome predictor). We again compared the area under the curve (AUC) for the three ROC curves using a test for paired ROC curves. To determine a best cut-point for the admission BUN, we determined the point with the highest sum of the sensitivity and specificity. For models containing two parameters, we generated 4 quartiles of the admission BUN values rather than considering all possible ordered pairs. We then examined the ROC curve within each BUN quartile for the WRF variable (i.e., increase in Cr, eGFR and BUN) and again found the cut-point which generated the maximum sum of sensitivity and specificity. Once determined, we also generated the estimated odds ratio from the resultant two-by-two table. For comparative purposes we also generated the odds ratio resultant from considering the two traditional cut-points of an increase in serum Cr of 0.3 mg/dl and 0.5 mg/dl.
The analysis for the post hospital discharge outcomes was similar to the above method. We restricted this analysis to patients who following index hospitalization discharge had complete follow-up for one year and those who had complete follow-up until their death in that first year. The impact of this restriction along with the exclusion of inhospital deaths restricted our sample size to 6,132 individuals. The remainder of the analysis mimicked the in-hospital analysis. Again admission BUN (i.e., the best predictor variable) was used to adjust all models predicting death at 1-year. All analyses were performed using SAS statistical software, version 9.1.3 (SAS Institute, Cary, North Carolina).
RESULTS
A total of 8,692 patients were hospitalized for HF. After excluding 547 patients with either pre-existing ESRD or missing data, 7,394 patients remained as the primary analytic dataset. Baseline characteristics are summarized in Table 1. On admission mean Cr was 1.35 mg/dl (SD 0.65), mean BUN was 27.44 mg/dl (SD 17.84), and eGFR was 68.41 ml/min/1.73m2. Overall there were 204 in-hospital deaths (2.8%) among 7,394 patients and 1,652 deaths (26.9%) within one year of discharge among the 6,132 patients with full follow-up information.
Table 1.
Baseline Characteristics
| Variable | In-Hospital Cohort (N=7394) | 1-year Post Discharge Cohort (N=6132) |
|---|---|---|
| Age (years) | 67.4 ±15.5 | 68.0 ±15.3 |
| Length of stay (days) | 4.7 ±6.6 | 5.3 ±6.4 |
| Female | 3750 (50.7%) | 3098 (50.5%) |
| African American | 5038 (68.1%) | 3975 (64.8%) |
| Died | 204 (2.8%) | 1652 (26.9%) |
| Hypertension | 3283 (44.4%) | 2888 (47.1%) |
| Diabetes | 1982 (26.8%) | 1741 (28.4%) |
| Peripheral Vascular Disease | 590 (8.0%) | 503 (8.2%) |
| Atrial Fibrillation | 1398 (18.9%) | 1233 (20.1%) |
| CVA | 846 (11.4%) | 773 (12.6%) |
| Chronic Kidney Disease | 584 (7.9%) | 527 (8.6%) |
| Coronary Artery Disease | 1727 (23.4%) | 1508 (24.6%) |
| Admission Cr (mg/dl) | 1.35 ±0.65 | 1.34 ±0.64 |
| Admission BUN (mg/dl) | 27.4 ±17.8 | 27.44 ±17.1 |
| Admission eGFR (ml/min/1.73M2) | 68.4 ±31.9 | 68.71 ±32.0 |
| Maximum Cr (mg/dl) | 1.66 ±0.9 | 1.63 ±0.86 |
| Maximum BUN (mg/dl) | 75.9 ±37.5 | 76.05 ±37.6 |
| Maximum eGFR (ml/min/1.73M2) | 34.7 ±21.9 | 34.23 ±20.6 |
| Maximum Δ Cr (mg/dl) | 0.31 ±0.54 | 0.29 ±0.49 |
| Maximum Δ BUN (mg/dl) | 7.52 ±20.2 | 7.34 ±20.3 |
| Maximum Δ eGFR (ml/min/1.73M2) | 7.21 ±12.3 | 6.78 ±11.3 |
| Furosemide | 6955 (94.1%) | 5771 (94.1) |
| Angiotensin Converting Enzyme Inhibitor | 5140 (69.5%) | 4223 (70.5%) |
| Beta Blocker | 1265 (17.1%) | 1223 (19.9%) |
| Hemoglobin (g/dl) | 12.1 ±2.1 | 12.0 ±2.1 |
| Left Ventricle Ejection Fraction (%) * | 38.0 ±17.7 | 38.3 ±17.5 |
| Patient Proportion with EF <50% (%) | 60.3 | 59.8 |
Continuous variables are shown as mean ±SD. Categorical variables are shown as count with % in parenthesis. Maximum value means highest absolute value achieved during hospitalization. Maximal change (Δ) = Peak level – Baseline level
n for in-hospital mortality group= 4565, n for 1-year mortality group=3718
Admission renal function parameters and mortality
Admission values of BUN, Cr and eGFR are shown in table 1. Baseline BUN was the strongest correlate for both in-hospital and post-discharge 1-year mortality (AUC = 0.724 and 0.656, respectively; p<0.001 for the comparison with both Cr and eGFR). BUN thresholds of 31mg/dl and 29 mg/dl performed best for predicting in-hospital and post-discharge mortality, and were associated with 4.6 and 3.0 fold increased mortality, respectively. ROC curves for baseline BUN, Cr, and eGFR in predicting in-hospital and 1-year death are shown in Figures 1 and 2, respectively. Since BUN performed best among these parameters, all subsequent models incorporating WRF were adjusted for baseline BUN.
Figure 1.
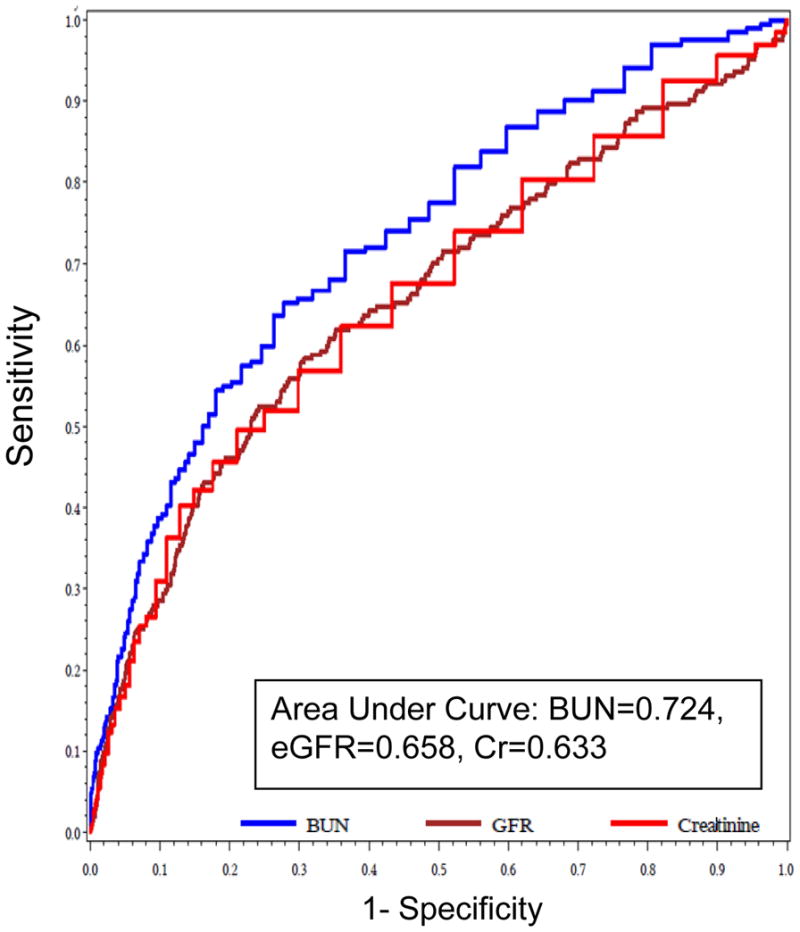
ROC curve of admission BUN, Cr, and eGFR for predicting in-hospital mortality
Figure 2.
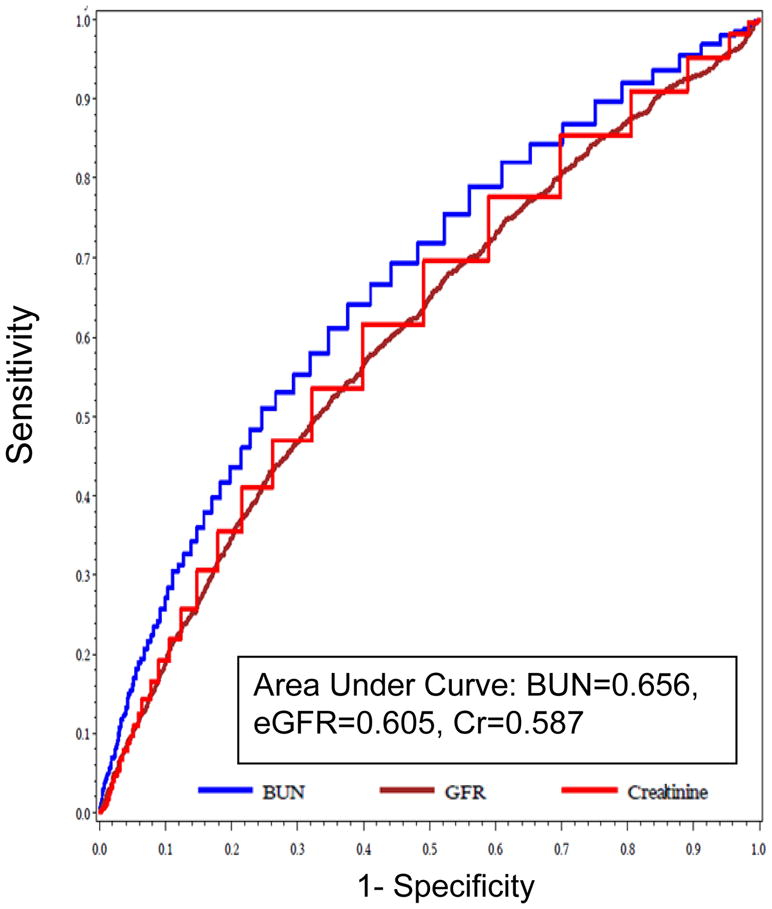
ROC curve of admission BUN, Cr, and eGFR for predicting 1 year post-discharge mortality
Worsening renal function during hospitalization and mortality
After adjustment for baseline BUN, in-hospital change in Cr and BUN performed similarly and superior to eGFR for in-hospital death (model AUC 0.812; p<0.001 vs. eGFR). For post-discharge death, maximal change (i.e. peak value – admission value) performed better than net change (i.e. discharge value - admission value; data not shown). Maximal change in Cr (ΔCr), maximal change in BUN (ΔBUN), and maximal change in eGFR (ΔeGFR), each performed similarly in this setting with model AUC=0.661. Since they were similar, subsequent results are reported in terms of Cr. The sensitivity, specificity, and odds ratio of various WRF definitions (within baseline BUN quartiles) for in-hospital and post-discharge mortality are shown in Table 2. Compared to traditional Cr cut-points (i.e. 0.3 or 0.5 mg/dl) with respective odds-ratio, the optimal values (shown in bold) depended both on the endpoint considered and baseline BUN quartile. For in-hospital death, the optimal cutoffs were often higher (0.6–0.8 mg/dl increase in Cr). When examining post-discharge mortality, baseline BUN was again important but interestingly the optimal threshold was 0.1 mg/dl (i.e. any increase in Cr value was associated with risk) for those with baseline BUN above the median, and 0.3 mg/dl for those patients with baseline BUN below the median.
Table 2.
Cr cutoffs to predict death stratified by baseline BUN quartile
| BUN Strata | ΔCr cutpoint | Sensitivity In-hospital death | Specificity In-hospital death | Sensitivity + Specificity | Odds Ratio |
|---|---|---|---|---|---|
| 0 < BUN ≤ 15 | 0.2 | 94.4 (17/18) | 48.9 (822/1680) | 143.3 | 16.29 |
| 0.3 | 72.2 (13/18) | 67.7 (1137/1680) | 139.9 | 5.44 | |
| 0.5 | 50.0 (9/18) | 88.5 (1487/1680) | 138.5 | 7.70 | |
| 16 ≤ BUN ≤ 22 | 0.3 | 62.5 (20/32) | 63.5 (1279/2014) | 126 | 2.90 |
| 0.5 | 59.4 (19/32) | 82.2 (1656/2014) | 141.6 | 6.76 | |
| 0.6 | 59.4 (19/32) | 88.0 (1772/2014) | 147.4 | 10.70 | |
| 23 ≤ BUN ≤ 32 | 0.3 | 63.9 (23/36) | 60.0 (1039/1731) | 123.9 | 2.66 |
| 0.5 | 50.0 (18/36) | 79.4 (1375/1731) | 129.4 | 3.86 | |
| 0.7 | 44.4 (16/36) | 89.3 (1546/1731) | 133.7 | 6.69 | |
| 33 ≤ BUN < 200 | 0.3 | 67.8 (80/118) | 56.9 (1005/1765) | 124.7 | 2.78 |
| 0.5 | 60.2 (71/118) | 74.2 (1310/1765) | 134.4 | 4.35 | |
| 0.8 | 54.9 (65/118) | 85.8 (1514/1765) | 140.7 | 7.40 | |
| BUN Strata | ΔCr cutpoint | Sensitivity Post-discharge death | Specificity Post-discharge death | Sensitivity + Specificity | Odds ratio |
| 0 < BUN ≤ 15 | 0.3 | 83.0 (181/218) | 18.4 (206/1118) | 101.4 | 1.11 |
| 0.5 | 93.6 (204/218) | 7.4 (83/1118) | 101 | 1.17 | |
| 16 ≤ BUN ≤ 22 | 0.3 | 42.6 (142/333) | 65.4 (902/1380) | 108 | 1.40 |
| 0.5 | 22.5 (75/333) | 83.7 (1155/1380) | 106.2 | 1.49 | |
| 23 ≤ BUN ≤ 32 | 0.1 | 73.3 (302/412) | 33.0 (362/1096) | 106.3 | 1.35 |
| 0.3 | 56.6 (233/412) | 46.9 (514/1096) | 103.5 | 1.15 | |
| 0.5 | 20.9 (86/412) | 80.1(878/1096) | 101 | 1.06 | |
| 33 ≤ BUN < 200 | 0.1 | 69.2 (477/689) | 37.0 (331/895) | 106.2 | 1.32 |
| 0.3 | 46.0 (317/689) | 58.4 (523/895) | 104.4 | 1.20 | |
| 0.5 | 28.0 (193/689) | 74.2 (664/895) | 102.2 | 1.19 |
Predictive renal dysfunction criteria
To define a simpler clinically useful classification scheme, we used the baseline BUN cut point of ≤ 30 vs. >30 to determined the optimal Cr change threshold for both mortality outcomes. For in-hospital mortality, when baseline BUN was ≤ 30 the best predictor was a Cr increase of 0.6 mg/dl, and when baseline BUN was >30 the best predictor was a Cr increase of 0.8 mg/dl. For mortality in the first year following discharge, when baseline BUN was ≤ 30 the best predictive inhospital Cr increase was 0.1 mg/dl (i.e. any increase in Cr), and when baseline BUN was >30, the best predictor of 1-year mortality was an in-hospital Cr increase of 0.3 mg/dl. Sensitivity, specificity, and the odds ratio for Cr threshold (within BUN strata) are shown in Table 3a. Mortality rates and risk ratios (compared to lowest risk group) are shown in Table 3b.
Table 3a.
Simplified/optimized renal risk classification
| BUN Strata | ΔCr cutpoint | Sensitivity In-hospital death | Specificity In-hospital death | Odds ratio* |
|---|---|---|---|---|
| BUN ≤ 30 | 0.6 | 54.1 (40/74) | 88.5 (4595/5190) | 9.1 (5.71 –14.5) |
| BUN > 30 | 0.8 | 52.3 (68/130) | 86.1 (1722/2000) | 6.8 (4.71 – 9.8) |
| BUN Strata | ΔCr cutpoint | Sensitivity Post-discharge death | Specificity Post-discharge death | Odds ratio |
| BUN ≤ 30 | 0.1 | 75.5 (673/891) | 31.2 (1080/3462) | 1.4 (1.18 – 1.66) |
| BUN > 30 | 0.3 | 44.5 (339/761) | 59.7 (608/1018) | 1.2 (0.99 – 1.44) |
Odds ratio of Cr above threshold vs. below, within given BUN category
Table 3b.
Simplified/optimized renal risk classification
| BUN Strata | ΔCr | In-hospital death, % (n) | Risk Ratio (95% CI)* |
|---|---|---|---|
| BUN ≤ 30 | <0.6 | 0.8% (34/4629) | 1.0 |
| BUN ≤ 30 | ≥0.6 | 6.3% (40/635) | 9.09 (5.71 –14.5) |
| BUN > 30 | <0.8 | 3.5% (62/1784) | 4.87 (3.19 –7.42) |
| BUN > 30 | 0.8 | 19.7% (68/346) | 33.06 (21.5 –50.8) |
| BUN Strata | ΔCr cutpoint | Post-discharge death % (n) | Risk Ratio* |
| BUN ≤ 30 | <0.1 | 16.8% (218/1298) | 1.0 |
| BUN ≤ 30 | ≥0.1 | 22.0% (673/3055) | 1.4 (1.18 –1.66) |
| BUN > 30 | <0.3 | 41.0% (422/1030) | 3.44 (2.84 –4.16) |
| BUN > 30 | ≥0.3 | 53.3% (339/749) | 4.1 (3.34 –5.03) |
Compared to lowest risk group (i.e. BUN 30 and ΔCr below threshold)
DISCUSSION
This retrospective analysis reaffirms the prognostic importance of renal dysfunction among hospitalized heart failure patients, but extends existing knowledge in several important ways. First, we found that the baseline measure of renal dysfunction is more important than subsequent in-hospital fluctuations in terms of predicting short and long term risk, as judged by AUC (i.e. while subsequent changes are statistically significant the incremental AUC increase was modest). Second, a baseline measure of BUN outperforms Cr in predicting subsequent death. In addition, although future risk of death can be further refined with information about subsequent, in-hospital changes in renal dysfunction, these cut-points vary substantially depending on the baseline renal function and the outcome horizon (i.e., in-hospital death vs. death within 1 year). In fact the optimal thresholds were often not consistent with those in common use today (e.g. increased Cr by 0.3 mg/dl). Finally, we tried to define the optimized yet practical risk assessment thresholds. This was BUN >30 mg/dl at admission, and dynamic Cr rise of 0.1 – 0.8 mg/dl depending on baseline BUN and time frame of interest.
To place our findings in context regarding different renal function measures, our data is consistent with several recent reports indicating that BUN has the best ability to discriminate risk (8–11), contrasting with another recent study.(12) In addition, at least one study in the setting of acute coronary syndrome patients (13), as well as two retrospective analyses of large randomized controlled trials in HF patients (10,14) have also shown that BUN is superior to Creatinine based measures. Given these data, the preponderance of pre-existing evidence and our own data clearly suggest BUN is the best renal predictor of mortality in HF patients. Why this is the case continues to be an intriguing question. Unfortunately, our data are not able to address why BUN appears to be superior to other markers, though a brief review of existing studies can suggest some clues. BUN concentration is determined by the balance between urea generation versus urea reabsorption and excretion in the kidneys.(19) Thus, BUN can rise independent of changes in GFR (or creatinine), due in part to proximal tubular reabsorption. This in turn is affected by both the sympathetic nervous system and the renin-angiotensin-aldosterone system.(13) Also, vasopressin levels are increased in the setting of acutely decompensated heart failure which is known to enhance water reabsorption via up-regulation of aquaporin-2 expression thereby raising urea concentration resulting in back diffusion of urea into the renal medulla. (20) Therefore, BUN elevations may reflect adrenergic activation and excess vascular tone in addition to renal function which may explain it greater ability to gauge overall risk in HF (since adrenergic and RAAS activation are clearly associated with worse disease and higher risk independent of kidney function). In addition, it has been hypothesized that BUN levels can also denote hypoperfusion from hypovolemia, existing reno-vascular disease, or even reduced cardiac output.(13) Given ours and the other results noted above, it is likely that one or more of these factors are at play. Thus our results support the idea that BUN provides a more comprehensive assessment of cardiorenal function and status by encompassing both renal function as well as directly reflecting sympathetic, vascular and cardiac status.
Our findings extend existing knowledge in several ways. First, we found that the timeline for assessing outcomes was quite influential. Whereas most previous studies have focused on inhospital or short-term risk, ours is one of only a few documenting longer-term risk, (3,15) and the only that we know of that compares these parameters demonstrating that the marker cutoffs are quite different depending on what endpoint is of most interest. Equally important, our data show that admission renal function must be accounted for when interpreting the predictive importance of in-hospital changes in renal function. In contrast, none of the aforementioned studies assessed which combination of baseline and worsening renal function was prognostically most important. This is a key question because having simple and informative markers of longer term outcomes is necessary in order to be helpful in planning medical care subsequent to hospitalization. It is interesting that smaller creatinine changes were required when baseline renal function was better (i.e. in the lower BUN strata). It may be that a certain magnitude of renal decrement carries risk, and if that’s the case it would be expected to be associated with smaller changes in creatinine when the baseline function is normal; where as when baseline function is impaired, a relatively larger change in creatinine is required in order to correspond to the same absolute change in renal function. Our data should be clinically useful to clinicians to help them identify patients at higher risk after HF hospitalization and who may benefit from closer follow up.
LIMITATIONS
Several limitations of this work should be noted. First, while utilizing claims data allows large numbers of patients to be analyzed they can be imperfect representations of clinical diagnoses. However a primary discharge diagnosis for HF (our inclusion criteria) has been shown to have approximately 95% specificity for patients meeting the Framingham definition of HF.(16) Furthermore, we have previously shown that these criteria are nearly 100% specific in our patient population.(17) Second, exposure to non-steroidal anti-inflammatory drugs or intravenous contrast usage is not included in this analysis. These medications may influence measure of renal function and obscure the relationship between renal function and mortality. Finally, information on left ventricular ejection fraction was not available for this study. However, we feel this is unlikely to affect our findings as previous data indicates that renal insufficiency is an independent predictor of mortality among HF patients with both systolic and diastolic dysfunction.(18)
CONCLUSION
Among hospitalized HF patients, baseline (i.e., admission) BUN was the renal index most strongly associated with mortality. Common WRF definitions are suboptimal for predicting mortality and the optimal thresholds must account for baseline renal function. BUN is a simple clinical marker that may better reflect the cumulative effects of hemodynamic and neurohormonal effects, thereby providing useful prognostic information in HF patients. Thus it may be preferential to use BUN over other measures of renal function in daily clinical practice and clinical trials. Further studies are needed to elucidate the mechanism for BUN being a better prognosticator than other available renal measures, and to assess whether renal-based risk stratification can prospectively identify patients that benefit from closer monitoring at discharge.
Acknowledgments
FUNDING
Dr. Lanfear is supported by National Heart, Lung, and Blood Institute Career Development Award (K23HL085124). Dr. Williams receives funding from the Fund for Henry Ford Hospital, the American Asthma Foundation, and the National Institute of Allergy and Infectious Diseases (AI79139, AI61774), the National Heart Lung and Blood Institute (HL79055), and the National Institute of Diabetes and Digestive and Kidney Diseases (DK64695), National Institutes of Health.
Footnotes
Conflicts of interest: The authors have no conflicts to disclose.
References
- 1.Association AH. Heart Disease and Stroke Statistics - 2009 Update. Dallas, Texas: American Heart Association; 2009. [Google Scholar]
- 2.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. Jama. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 3.Lanfear DE, Peterson EL, Campbell J, et al. Relation of worsened renal function during hospitalization for heart failure to long-term outcomes and rehospitalization. Am J Cardiol. 2011;107:74–8. doi: 10.1016/j.amjcard.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–7. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb SS, Abraham W, Butler J, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–41. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 6.Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–74. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 7.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) Eur J Heart Fail. 2009;11:847–54. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 8.Smith GL, Shlipak MG, Havranek EP, et al. Serum urea nitrogen, creatinine, and estimators of renal function: mortality in older patients with cardiovascular disease. Arch Intern Med. 2006;166:1134–42. doi: 10.1001/archinte.166.10.1134. [DOI] [PubMed] [Google Scholar]
- 9.Aronson D, Mittleman MA, Burger AJ. Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am J Med. 2004;116:466–73. doi: 10.1016/j.amjmed.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Klein L, Massie BM, Leimberger JD, et al. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 11.Cauthen CA, Lipinski MJ, Abbate A, et al. Relation of blood urea nitrogen to long-term mortality in patients with heart failure. Am J Cardiol. 2008;101:1643–7. doi: 10.1016/j.amjcard.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 12.Scrutinio D, Passantino A, Lagioia R, Santoro D, Cacciapaglia E. Detection and prognostic impact of renal dysfunction in patients with chronic heart failure and normal serum creatinine. Int J Cardiol. 2011;147:228–33. doi: 10.1016/j.ijcard.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Kirtane AJ, Leder DM, Waikar SS, et al. Serum blood urea nitrogen as an independent marker of subsequent mortality among patients with acute coronary syndromes and normal to mildly reduced glomerular filtration rates. J Am Coll Cardiol. 2005;45:1781–6. doi: 10.1016/j.jacc.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 14.Filippatos G, Rossi J, Lloyd-Jones DM, et al. Prognostic value of blood urea nitrogen in patients hospitalized with worsening heart failure: insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) study. J Card Fail. 2007;13:360–4. doi: 10.1016/j.cardfail.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Belziti CA, Bagnati R, Ledesma P, Vulcano N, Fernandez S. Worsening renal function in patients admitted with acute decompensated heart failure: incidence, risk factors and prognostic implications. Rev Esp Cardiol. 2010;63:294–302. doi: 10.1016/s1885-5857(10)70062-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee WY, Capra AM, Jensvold NG, Gurwitz JH, Go AS. Gender and risk of adverse outcomes in heart failure. Am J Cardiol. 2004;94:1147–52. doi: 10.1016/j.amjcard.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 17.Alqaisi F, Williams LK, Peterson EL, Lanfear DE. Comparing methods for identifying patients with heart failure using electronic data sources. BMC Health Services Research. 2009;9:237. doi: 10.1186/1472-6963-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–8. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 19.Conte G, Dal Canton A, Terribile M, et al. Renal handling of urea in subjects with persistent azotemia and normal renal function. Kidney Int. 1987;32:721–7. doi: 10.1038/ki.1987.266. [DOI] [PubMed] [Google Scholar]
- 20.Schrier RW. Vasopressin and aquaporin 2 in clinical disorders of water homeostasis. Semin Nephrol. 2008;28:289–96. doi: 10.1016/j.semnephrol.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


