Abstract
Drugs absorbed poorly through the skin are commonly delivered via injection with a hypodermic needle, which is painful and increases the risk of transmitting infectious diseases. Microneedles (MNs) selectively and painlessly permeabilize the outermost skin layer, allowing otherwise skin-impermeable drugs to cross the skin through micron-sized pores and reach therapeutic concentrations. However, rapid healing of the micropores prevents further drug delivery, blunting the clinical utility of this unique transdermal technique. We present the first human study demonstrating that micropore lifetime can be extended following MN treatment. Subjects received one-time MN treatment and daily topical application of diclofenac sodium. Micropore closure was measured with impedance spectroscopy, and area under the admittance–time curve (AUC) was calculated. AUC was significantly higher at MN + diclofenac sodium sites vs. placebo, suggesting slower rates of micropore healing. Colorimetry measurements confirmed the absence of local erythema and irritation. This mechanistic human proof-of-concept study demonstrates that micropore lifetime can be prolonged with simple topical administration of a non-specific cyclooxygenase inhibitor, suggesting the involvement of subclinical inflammation in micropore healing. These results will allow for longer patch wear time with MN-enhanced delivery, thus increasing patient compliance and expanding the transdermal field to a wider variety of clinical conditions.
Keywords: Microneedle, Transdermal, Diclofenac, Micropore, Human, Clinical
1. Introduction
Transdermal drug delivery provides significant advantages over other traditional delivery routes by decreasing systemic side effects, avoiding first-pass hepatic metabolism, and increasing ease of application for patients. Despite these advantages, very specific physicochemical properties are required for a drug molecule to passively cross the stratum corneum (SC, the outermost layer of skin), thus limiting this delivery method to a very small number of molecules [1]. Much work has been done towards developing physical methods of disrupting the barrier function of the skin in order to expand the transdermal field to a wider variety of drugs; such methods include iontophoresis, microdermabrasion, and microneedles [2].
Microneedles (MNs) are a minimally invasive means of increasing the permeability of the skin by piercing the SC and creating transient micropores through which a drug can passively diffuse [3]. This novel delivery method allows for a wider variety of molecules to pass the skin's barrier, thus allowing the advantages of transdermal delivery to be applied to a large range of clinical applications including diabetes, severe osteoporosis, and influenza vaccination [3]. MN application generally removes the limitations on the molecular size of the drug moiety, which provides an avenue for the delivery of much larger molecules than what has been previously feasible, including peptides and macromolecules. Perhaps most importantly however, is that treatment with MN arrays is relatively painless and generally well tolerated by most patients, making this a very realistic technique for clinical implementation [3–7]. In fact, the first commercial MN product in the United States was approved in 2011 for influenza vaccination in adults aged 18–64 (Fluzone Intradermal, Sanofi Pasteur) [8].
New advances using MNs have shown promising results towards achievement of therapeutic clinical outcomes, including systemic delivery of naltrexone (an opioid antagonist approved for treatment of alcohol and opioid addiction). A recent study described transdermal delivery of naltrexone in healthy human subjects following pretreatment with solid MNs (a treatment process known as the “poke (press) and patch” method) [9]. In subjects pretreated with MNs, application of a naltrexone patch yielded therapeutic blood levels, while application of the naltrexone patch without MN pretreatment failed to achieve therapeutic levels of naltrexone. This is the only human pharmacokinetic study in the literature describing MN-assisted delivery in humans, and it supports the feasibility of this novel transdermal technique. Other human studies have been completed using MNs to deliver recombinant human parathyroid hormone 1–34, teriparatide (PTH), and positive gains in bone mineral density were seen at the hip and lumbar spine, confirming use of MN delivery techniques for achieving clinical benefit [10].
Several factors affect the efficiency of drug transport following MN treatment, including physical parameters of the MNs, properties of the drug compounds to be delivered, and the lifetime of the micropores created in the skin [11]. There has been a great deal of work examining the MN parameters and properties of drug compounds, but very little is known about the kinetics of micropore closure following MN treatment. This factor is critical to the success of MN-assisted delivery for the “poke (press) and patch” MN technique in which MNs are applied to the skin to create micropores followed by application of a drug patch over the top of the treated area [3,12], and therefore the rates of micropore closure must be optimized for continued forward progress towards clinical implementation. Bal et al. demonstrated that micropores may close as quickly as 15 minutes following MN treatment in healthy human subjects when the treatment sites remain exposed to air, while another study concluded that the micropores may close in a timeframe of hours [13,14]. The pharmacokinetic study previously described suggests that the micropores may close by 48–72 h following MN treatment under occluded conditions, preventing any further transdermal delivery [9]. This severely limits the clinical utility of MN application, and thus it is imperative to develop effective means of extending micropore lifetime to achieve a once weekly dosing schedule (the ideal for transdermal delivery).
The physiological processes underlying micropore closure in humans are not known. One possibility is that there may be mild subclinical local inflammation (at a microscopic level), which would serve as a potential therapeutic target for extending the lifetime of the micropores via topical application of anti-inflammatory agents. Non-steroidal anti-inflammatory drugs (NSAIDs) exert their effects by inhibiting the cyclooxygenase (COX) enzymes that are integral to the body's inflammatory response via the arachidonic acid pathway and prostaglandin production, and several topical NSAID formulations are commercially available. A recent study demonstrated that daily application of 3% diclofenac sodium (a non-specific inhibitor of COX-1 and COX-2) extends micropore lifetime in hairless guinea pigs, allowing transdermal delivery of naltrexone for up to 7 days [15]. This was the first study to demonstrate the feasibility and applicability of extending micropore lifetime with topical NSAIDs.
There are many techniques that can be employed to monitor micropore formation in the skin, both qualitatively and quantitatively. The SC serves as the primary barrier to movement of water and ions, and these properties can be used as a means to evaluate the state of the skin's barrier [16]. Transepidermal water loss (TEWL) measures the movement of water between the skin and the external environment, and an increase in TEWL reflects that the skin's barrier has been compromised [17]. Despite being a widely used technique, however, TEWL measurements are exquisitely sensitive to the hydration status of the skin, presenting a significant challenge for evaluating small changes in SC that has been occluded for hours to days (the typical scenario for a transdermal patch). Impedance spectroscopy is another useful technique that reflects disruption of the SC by measuring the movement of ions. It is well known that human skin presents a large impedance to the movement of electrical current, thus displaying a high electrical resistance that is primarily due to the SC [18–20]. Accordingly, the electrical impedance of the SC provides important information regarding the state of the skin's barrier function (notably, the impedance of intact, healthy skin is quite high, but decreases in response to injury or insult) [18,20–23], and this has been shown to be a reliable method of evaluating barrier function [21,24–26]. Impedance measurements can detect small changes in skin that has been hydrated, and this technique has very recently been described for specifically studying the kinetics of micropore closure under the effects of occlusion, making this an excellent technique for clinical applications and evaluating “real world” transdermal scenarios with microneedle application [19,27]. The inverse of the electrical impedance (admittance) can also be used as a measure of the skin barrier integrity as high admittance values signify compromised barrier integrity, while low baseline values are typical under normal physiological conditions (similar trends to those observed with TEWL).
The objective of the present study was to extend the lifetime of the micropores created from MN insertion in healthy human subjects by targeting the COX enzymes via topical application of diclofenac. Impedance spectroscopy was utilized to monitor micropore closure following one-time MN treatment, and tristimulus colorimetry was employed to assess local erythema and skin irritation. This is the first data to demonstrate that application of small bioactive drugs can effectively delay micropore closure in human subjects.
2. Materials and methods
2.1. Preparation of drug formulations
Solaraze® gel (3% diclofenac sodium, 2.5% hyaluronic acid, PharmaDerm, Melville, NY) and 0.2% sodium hyaluronate gel (Cypress Pharmaceutical, Inc., Madison, MS) were purchased through the University of Kentucky. Sodium hyaluronate powder (Macronan-P) was a gift from American International Chemical, Inc. (Framingham, MA). A 2.5% hyaluronic acid gel served as the placebo vehicle control, and was prepared from the 0.2% sodium hyaluronate gel and the Macronan-P powder.
2.2. Preparation of microneedle arrays and occlusive patches
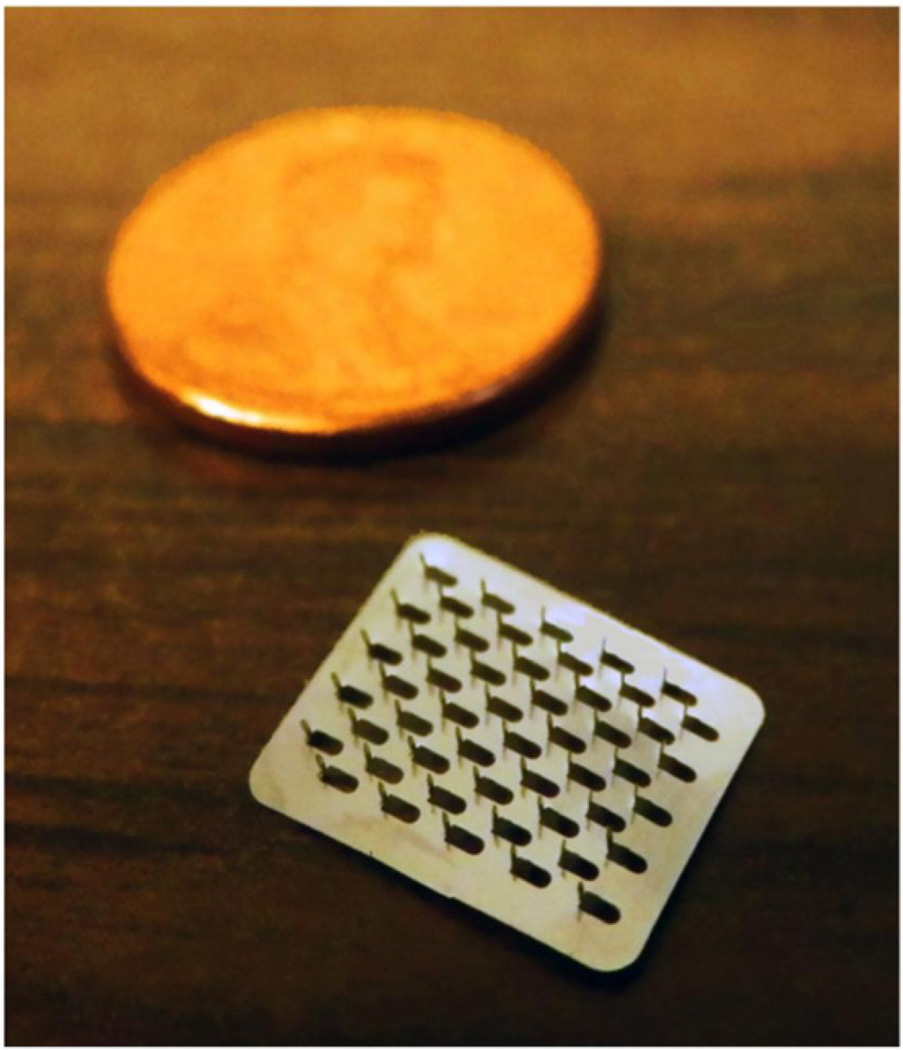
Briefly, fixed MN geometries are cut into 50 µm thick stainless steel sheets and are manually bent perpendicular to the plane of their metal substrate. The arrays contain 50 MNs arranged in a 5 × 10 configuration. The arrays are further assembled into adhesive patches with Arclad (Adhesives Research, Inc., Glen Rock, PA), which allows for close contact between the MNs and the skin during treatment (methods described previously) [9]. This close contact compensates for the mismatch between the flexible skin tissue and the rigid MN substrate. Each MN measures 800 µm in length and 200 µm in width at the base. The MN patches were ethylene oxide sterilized before use. Fig. 1 displays one of the MN arrays.
Fig. 1.
Image of a microneedle array. The MN arrays are arranged in a configuration of 5×10 needles, with a total of 50 MNs per array. The array is displayed next to a penny in order to demonstrate the relative small size of the whole array.
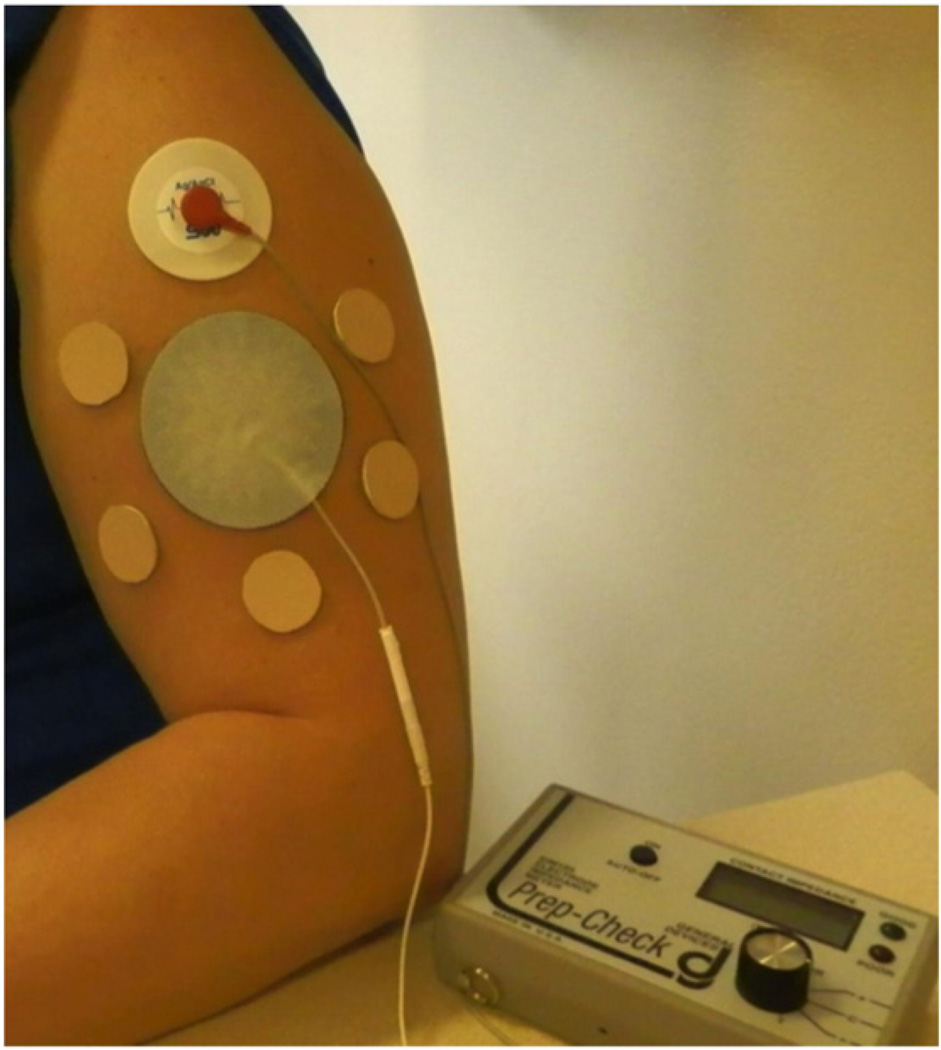
Blank occlusive patches were made by fabricating a rubber-ringed barrier with a drug-impermeable backing membrane on one side (Scotchpak 1109 SPAK 1.34 MIL heat-sealable polyester film; 3M, St. Paul, MN) that was secured to the rubber ring with 3M double-sided medical tape. The patches were held closely to the skin with Bioclusive dressing (Systagenix Wound Management, Quincy, MA). The patches on a subject's arm can be seen in Fig. 2.
Fig. 2.
Treatment patches and electrodes on a subject's upper arm. The reference electrode was placed in the middle of all the treatment sites, which were protected during the study by blank occlusive patches. The top treatment site displays one of the Ag/ AgCl measurement electrodes, which is moved from site to site to make impedance measurements. Both the reference and measurement electrodes are connected by lead wires to the impedance meter.
2.3. Electrodes and impedance measurements
Ag/AgCl measurement electrodes (Thought Technology T-3404; 25 mm × 25 mm total area; 10 mm active electrode diameter; Stens Corporation, San Rafael, CA) were used to measure the impedance at treatment sites. A large electrode with a conductive gel was placed in the middle of the treatment sites and served as the reference electrode (Superior Silver Electrode with PermaGel, 70 mm total and active electrode diameter; Tyco Healthcare Uni-Patch, Wabasha, MN). Lead wires were connected to the measurement and reference electrodes, and the opposite ends of the wires were connected to an impedance meter (EIM-105 Prep-Check Electrode Impedance Meter; General Devices, Ridgefield, NJ). The meter applied a low frequency (30 Hz) alternating current that was modified with a 200 kΩ resistor in parallel (IET labs, Inc., Westbury, NY).
2.4. Clinical study procedures
All study procedures were approved by the University of Kentucky Institutional Review Board and were carried out in accordance with the principles governing clinical research as defined in the World Medical Association Declaration of Helsinki; all subjects provided informed consent prior to beginning any study procedures. Healthy volunteers were examined and interviewed to determine appropriateness for the study. Volunteers were between 18 and 45 years of age and in general good health with no history of dermatological disease. Subjects were excluded if they had any of the following conditions: severe general allergies (indoor, outdoor, or seasonal); allergy to diclofenac sodium, Solaraze® gel, or hyaluronic acid; previous adverse reaction to MN insertion; known allergy or adverse reaction to medical tape or adhesive. Subjects were also excluded if they were pregnant/ nursing or had HIV/AIDS. Immediately prior to and during the study, subjects were asked to refrain from taking any oral anti-inflammatory drugs. At each visit, the subjects sat in the clinic room for 30 minutes prior to any study activities in order to acclimate to normal room temperature of ~25 °C. At the first visit, 6 sites were marked on the arm of each subject, and each site received a different treatment (Table 1).
Table 1.
Description of the various treatments applied to each subject. All treatments consisted of 200 µl total volume. Three subjects in paradigm 1 received treatment on the volar forearm; the remaining two subjects in this treatment paradigm and all subjects in paradigm 2 were treated on the upper arm.
| Site | Treatment paradigm 1 (n=5 subjects)a |
Site(s) | Treatment paradigm 2 (n=8 subjects)b |
|---|---|---|---|
| 1 | MN array + occlusion | 1 and 2 | MN array + diclofenac gel + occlusion |
| 2 | MN array + diclofenac gel + occlusion |
3 and 4 | MN array + placebo gel + occlusion |
| 3 | Diclofenac gel + occlusion | 5 | Diclofenac gel + occlusion |
| 4 | Occlusion of non-treated skin | 6 | Placebo gel + occlusion |
| 5 | MN array, unoccluded | ||
| 6 | MN array + placebo gel + occlusion |
Three subjects were treated on the volar forearm, and two subjects were treated on the upper arm.
All subjects were treated on the upper arm.
The treatments were applied to either the volar forearm or upper arm, and three different treatment schedules were examined (Table 2). Sites were randomly numbered for the subjects treated on the volar forearm. The observed trends in closure kinetics were similar irrespective of the site; thus for the remaining subjects the sites were not randomized, but were kept consistent. Site 1 was marked at the 12 o'clock position (nearest to the shoulder) and Sites 2–6 were numbered consecutively in a clockwise fashion. After the first three subjects, the treatment sites were moved to the upper arm in order to more accurately represent a possible site of patch placement in clinical practice, and the sites were kept consistent to further eliminate any source of inter-subject variability. All gel treatments consisted of 200 µl of total gel, rubbed gently into the skin. Fresh gels and new occlusive patches were re-applied at each study visit.
Table 2.
Various treatment schedules. Three different schedules were examined, with varying timeframes of pre-hydration (0, 24 or 72 h of pre-hydration). Two subjects completed both Schedules 1 and 2 in a crossover-type design.
| Schedule 1 (n=9a) |
Schedule 2 (n=4b) |
Schedule 3 (n=2c) |
|||
|---|---|---|---|---|---|
| Day of study | Applied treatments | Day of study | Applied treatments | Day of study 0 | Applied treatments |
| 0 | MN treatment, application of gels, occlusion of sites |
0 | Application of blank occlusive patches to intact skin |
0 | Application of blank occlusive patches to intact skin |
| 1–3 | Daily application of fresh gels and occlusive patches |
1 (Following 72 h of occlusion) |
MN treatment, application of gels, occlusion of sites |
1 (Following 24 h of occlusion) | MN treatment, application of gels, occlusion of sites |
| 4 (96 h post-MN) | Removal of gels/patches | 2–4 | Daily application of fresh gels and occlusive patches |
4 (72 h post-MN) | Re-application of gels and occlusive patches |
| 5 (96 h post-MN) | Removal of gels/patches | 8 (7 days post-MN) | Removal of gels/patches | ||
Three subjects in this group completed treatment paradigm 1, applied to the volar forearm. The remaining six subjects completed treatment paradigm 2, applied to the upper arm.
Two of these subjects also completed Schedule 1, in order to compare the effects of pre-hydration on micropore closure kinetics within the same subject. All treatments were applied to the upper arm.
Both subjects were in treatment paradigm 1, applied to the upper arm.
2.5. MN treatments
At each MN-treated site, subjects were treated with 100 MN insertions (50 MN array applied twice). MN insertion was achieved by placing the MN array on the skin and pressing gently for approximately 10–15 seconds; the array was rotated 45 degrees for the second insertion so as not to overlap the same micropores created by the first insertion. All MN applications were performed by the same investigator to eliminate inter-investigator variability.
2.6. Micropore closure kinetics
Micropore closure was assessed via impedance spectroscopy. Prior to any measurements, all excess gel or moisture was gently blotted from the skin with sterile gauze. Each measurement took 30 seconds to obtain. Baseline measurements were obtained, repeated immediately following MN treatment, and then obtained at each clinic visit. Assuming three parallel and independent pathways for electrical current (resistor box (Zbox), intact skin (Zskin) and micropores (Zpores)), the impedance measurements yield a total impedance value (Ztotal) that is a function of the three pathways:
The Zskin was independently estimated from the control sites, and thus the impedance of the micropores could be calculated (employing the assumption that the micropores occupy approximately 2% of the total measurement area) [28]. This approach allows for elimination of confounding variables (influence of the resistor box and hydration state of the skin). The hydration state was further controlled for in Schedules 2 and 3 by the fact that MN treatments were applied to pre-hydrated skin.
The inverse of the impedance (admittance, Y) was calculated from the Zpores. Admittance is also a measure of skin barrier integrity and behaves similarly to transepidermal water loss measurements (e.g. high admittance values signify compromised barrier integrity, while low baseline values are typical under normal physiological conditions). Admittance was normalized to the highest post-MN admittance, and any contribution from the control site was subtracted out (i.e. any effect attributed to the gels or hydration status). For diclofenac and placebo sites, the area under the admittance-time curve (AUC) was calculated (GraphPad Prism® software, version 5.04) to allow for comparison between treatments. At placebo sites it was assumed that the change in admittance values follows approximately first-order kinetics, thus providing an additional means of estimating the kinetics of micropore closure without any active treatment. Micropore closure rate constants (k's) were determined according to the simple model:
Admittance values were logarithmically transformed to fit a loglinear form of the model and obtain apparent first-order rate constants. Each subject served as their own control, and a paired t-test was performed to compare the effects of diclofenac vs. placebo treatment. p<0.05 was considered statistically significant.
2.7. Skin irritation assessments
Tristimulus colorimetry readings were made on the upper arms of six subjects in order to assess the skin irritation potential of the treatments. Erythema was quantified with a Konica Minolta meter (ChromaMeter CR-400, Konica Minolta, Japan) according to previously published guidelines [29]. This technique is non-invasive, measurements are very quick (less than 5 seconds each), and the device is handheld and portable, making it suitable for a clinical environment. The colorimeter was calibrated daily against a white plate provided by Konica Minolta. Measurements were made by placing the head of the instrument gently on the skin area to record the color reflectance. Readings were taken in triplicate at every site at each study visit and the mean a* value was calculated. The change in erythema was reported as a change from the baseline, Δa*, calculated asΔa*=a*t (at time t days after starting the study)–a*0 (at time 0, prior to application of treatment). Statistical analysis was performed using a one-way ANOVA with post-hoc Tukey's analysis (GraphPad Prism®, version 5.04).
3. Results
3.1. Subjects
Thirteen healthy volunteers completed this study: seven males and six females (general demographics described in Table 3); treatment paradigms and schedules are described in Table 4. The various paradigms and schedules allowed for the investigation of different scenarios, including: 1) the effects of pre-hydration on micropore closure; 2) evaluation of micropore closure kinetics under different frequencies of diclofenac application; and 3) comparison of admittance profiles following identical treatment at two independent sites. MN treatments were well tolerated by all of the subjects and no irritation or infection was noted at any of the treatment sites. Some subjects had mild to moderate irritation/allergic reactions to the Bioclusive adhesive tape that was used to secure the blank occlusive patches to the skin (to cover the treatment sites). These local reactions were isolated to the areas of skin covered by the Bioclusive and were determined by the study physicians to be unrelated to the MN or gel treatments themselves. All reactions were treated with brief courses of topical steroids, and all resolved quickly.
Table 3.
Subject demographics (n=13).
| Subject demographics | Count |
|---|---|
| Sex | |
| Male (%) | 7 (54) |
| Female (%) | 6 (46) |
| Mean age, years (SD) | 27.5 (5.8) |
| Minimum age | 22 |
| Maximum age | 45 |
| Race | |
| Caucasian (%) | 11 (85) |
| Asian (%) | 2 (15) |
| Mean body mass index (SD) | 27.4 (5.6) |
| Minimum BMI | 18.7 |
| Maximum BMI | 39.7 |
Table 4.
Description of the combinations of treatment paradigms, schedules, and treatment sites for all subjects. Subjects 6 and 9 completed both Schedules 1 and 2.
| Subjects | Treatment paradigm | Schedule | Treatment site |
|---|---|---|---|
| 1–3 | 1 | 1 | Volar forearm |
| 4–9 | 2 | 1 | Upper arm |
| 6, 9, 10–11 | 2 | 2 | Upper arm |
| 12, 13 | 1 | 3 | Upper arm |
3.2. Formation of micropores in the stratum corneum
A total of 50 MN treatments were applied in 13 subjects at diclofenac and placebo treated sites (one MN treatment consists of two applications of a 50 MN array, in order to create 100 micropores). Zpores (impedance of the micropores) was calculated as described above in the Materials and methods section. Impedance dropped significantly from baseline immediately following MN treatment in all subjects (p=0.002, paired t-test) indicating formation of micropores in the SC and significant disruption of the skin's barrier function (one subject's measurements were excluded as outliers, n=2).
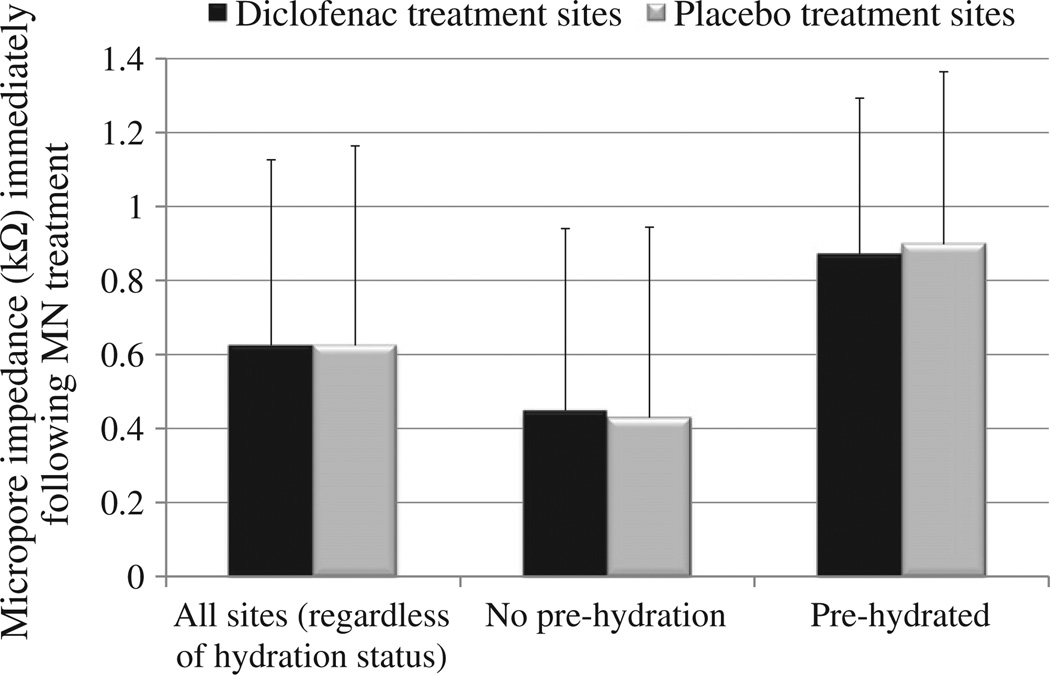
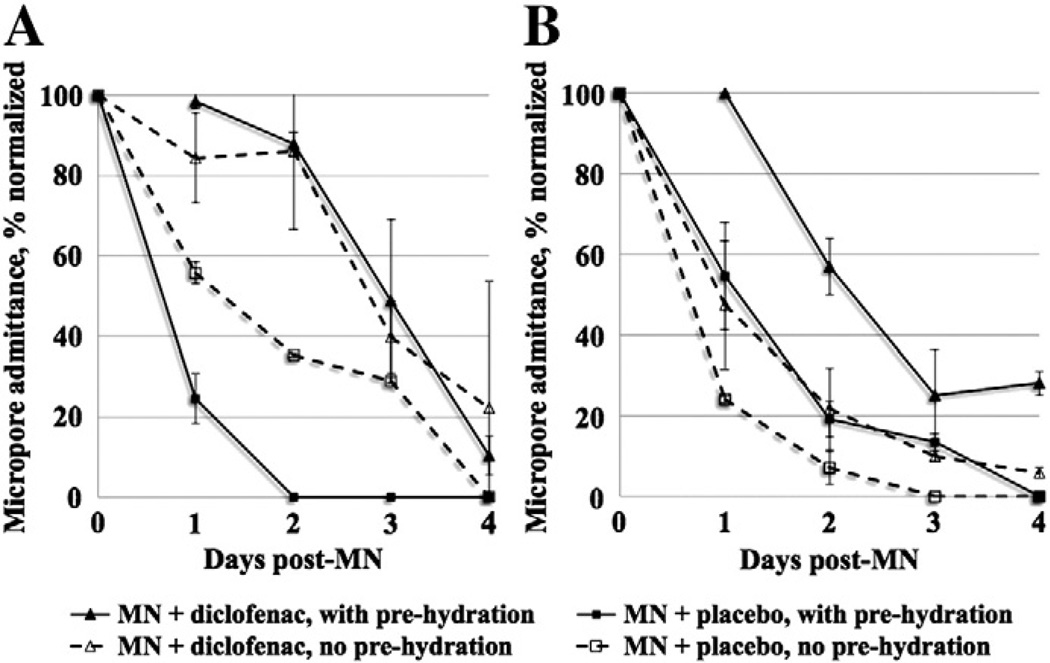
The most relevant clinical scenario for transdermal patches is such that the skin remains occluded beneath a patch for a timeframe of hours to days. Impedance measurements can be made on skin with varied levels of hydration [23], but this does impose an additional factor that could create variability in the measurements. In some subjects the skin was pre-hydrated before MN treatment in order to completely remove any possible effects of hydration on the impedance measurements (n=20 MN treatments). However, the employed basic research method of calculating the Zpores described in the Materials and methods section (using the measurements at corresponding control sites to estimate the value of hydrated intact skin) also allows for removal of hydration effects. Therefore, to more accurately depict the truest clinical scenario, the pre-hydration period was removed from the treatment schedule (n=28 MN treatments). Two subjects completed the study in a crossover design, once with pre-hydration and once without pre-hydration. There was no significant difference (p>0.5, Student's t-test) in the post-MN impedance measurements at diclofenac vs. placebo sites, regardless of hydration status at the time of treatment (Fig. 3); therefore, the hydration of the skin did not appear to introduce additional variability in the measurements. As expected, in all subjects the baseline impedance of intact skin was quite high (always greater than 1500 kΩ, data not shown), which is required for the skin to maintain its effective barrier function. MN treatment breaches this barrier, leading to a substantial decrease in impedance. At pre-hydrated sites (n=20 total; 10 diclofenac sites and 10 placebo sites), micropore impedance (average ± SD) immediately post-MN treatment was 0.87 ± 0.42 kΩ at diclofenac sites vs. 0.90 ± 0.47 kΩ at placebo sites (p=0.9, Student's t-test). At non pre-hydrated sites (n=28 total; 14 diclofenac sites and 14 placebo sites), micropore impedance immediately following MN treatment at diclofenac and placebo sites was 0.45 ± 0.49 kΩ vs. 0.43 ± 0.52 kΩ, respectively (p= 0.9, Student's t-test). All initial post-MN measurements can be seen in Fig. 3. Thus, while there is some variation between subjects in the impedance measurements immediately following MN treatment, in each individual subject the formation of micropores can be easily detected when compared to that subject's high intact skin baseline.
Fig. 3.
Impedance of the micropores immediately following MN treatment. All data are represented as average ± SD. Black bars represent diclofenac treatment sites, and gray bars represent placebo sites. One subject's impedance values were excluded as outliers (n=2 measurements). Regardless of hydration status, no significant difference was found between the impedance measurements at diclofenac vs. placebo sites. Micro-pore impedance (irrespective of hydration status, n=24 in each group) at diclofenac treatment sites was 0.62 ± 0.50 kΩ, compared to 0.62 ± 0.54 kΩ at placebo treatment sites (p=1.0, Student's t-test). At non pre-hydrated sites (n=14 in each group), micropore impedance at diclofenac and placebo treatment sites was 0.45 ± 0.49 kΩ and 0.43 ± 0.52 kΩ, respectively (p=0.9, Student's t-test). Finally, sites that were pre-hydrated (n=10 in each group) at diclofenac and placebo treated sites had an impedance of 0.87 ± 0.42 kΩ vs. 0.90 ± 0.47 kΩ, respectively (p=0.9, Student's t-test).
3.3. Micropore closure kinetics
Micropore closure was assessed via impedance spectroscopy, a method well described for monitoring skin integrity and barrier function under various conditions [19,22,23,30,31]. Admittance values (inverse of the impedance measurements) were calculated and percentage-normalized to the highest post-MN admittance value (in some subjects the admittance increased slightly at 24 h following MN treatment) and plotted vs. time such that the area under the admittance–time curve (AUC, %·days) was calculated. In the first five subjects, additional control sites were applied including occlusion of intact skin, MN-treated skin under occluded conditions (no gels applied), and MN-treated skin exposed to air. In all subjects, measurements at unoccluded MN-treated sites had returned to baseline by the time of the next clinic visit, and the AUC at MN-treated sites under occlusion was not significantly different from placebo (p=0.6, paired t-test). Therefore, the remaining subjects were not treated with these controls, but rather had replicates of MN + diclofenac and MN + placebo treatment sites.
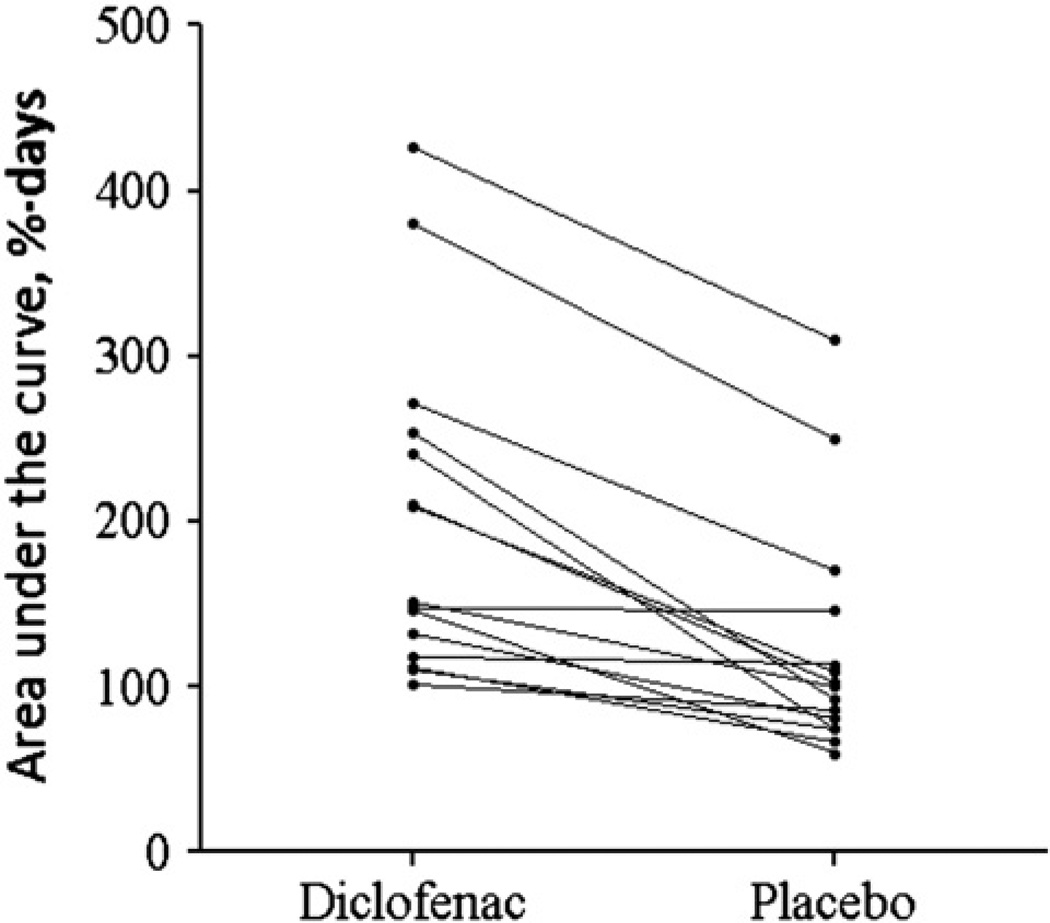
As expected with a biological system, a wide range in the AUC values was observed at both diclofenac and placebo treatment sites (87.3–426.0 %·days vs. 53.5–309.6 %·days, respectively); the overall difference between treatments was significant (p<0.0001, paired t-test). Fig. 4 displays the AUC at diclofenac and placebo sites for each subject. Two subjects had notably higher AUC for both diclofenac and placebo sites, while two subjects did not display any difference between active and placebo AUC. The subjects who received the fewest applications of diclofenac had the highest AUC compared to the other subjects (AUC of 309.6 %·days and 380.2 %·days, vs. values of <300 %·days for all other subjects); the AUC for these subjects was also calculated over a total of 7 days post-MN treatment, which could contribute to the higher values. However, the difference between active and placebo AUC is still significant if these values are removed from the analysis (p<0.0005, paired t-test). Despite these variations, the results are neither surprising nor discouraging, as differences in therapeutic response, outliers, and non-responders are all typical with human clinical data. Representative admittance profiles are seen in Fig. 5. For the subjects who completed the crossover design (Subjects 6 and 9), the overall effect of the pre-hydration period was somewhat inconsistent between the two subjects, although the shapes of the profiles within each subject were consistent (irrespective of the skin's hydration status at the time of MN treatment). The aver-age ± SD AUC values at the pre-hydrated diclofenac treatment sites were 240.5 ± 88.5%·days and 145.8 ± 17.0 %·days (Subjects 6 and 9, re-spectively), vs. the AUC at non-prehydrated diclofenac sites of 271.4 ± 55.6 %·days and 131.9 ± 25.4 %·days. Pre-hydrated placebo site AUC values were 74.6 ± 6.2 %·days (Subject 6) vs. 59.8 ± 8.9 %·days (Subject 9), compared to the non pre-hydrated values of 170.1 ± 3.8 %·days and 81.1 ± 3.4 %·days. Therefore, a positive treatment effect (i.e. higher AUC at diclofenac sites) was seen in both subjects under both treatment schedules (Fig. 6).
Fig. 4.
Comparison of AUC values at diclofenac vs. placebo treatment sites. The AUC (%·days) at MN + diclofenac and MN + placebo treated sites was calculated from percentage-normalized admittance measurements and compared within each subject over the entire treatment period (n=15 treatment periods in 13 subjects, because 2 subjects completed a crossover design). For those subjects who had two independent sites each for diclofenac and placebo treatments, the average was calculated and used to determine the AUC. The overall difference in AUC was statistically significant (p<0.0001, paired t-test).
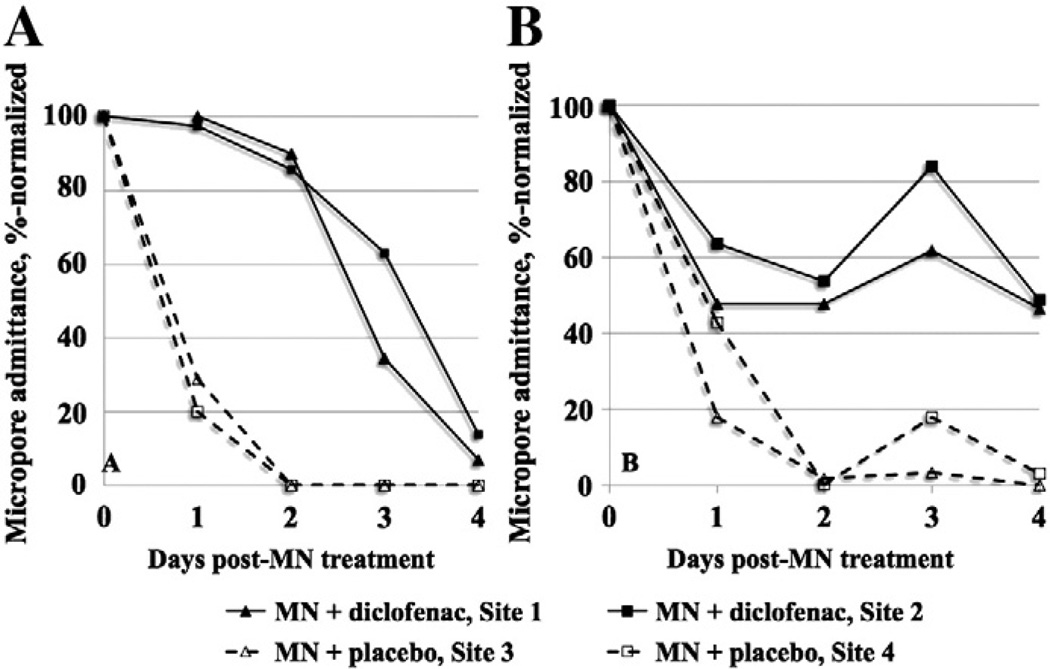
Fig. 5.
Representative profiles of micropore admittance from two subjects. These profiles demonstrate inter-subject differences in the change in micropore admittance over a 5 day period (MN treatment occurring on Day 0). Values were normalized to the highest post-MN admittance value. Two independent sites were treated with MN + diclofenac (solid lines with solid shapes), and two additional sites were treated with MN + placebo (dashed lines with open shapes), and the area under the admittance–time curve, %·days (AUC) was calculated from the normalized admittance values. Subject A completed treatment Schedule 2, and Subject B completed treatment Schedule 1. Subject A: AUC for diclofenac and placebo was 240.5 ± 88.5 %·days vs. 74.6 ± 6.2 %·days, respectively. Subject B: Average (± SD) AUC for diclofenac sites was 253.2 ± 32.1 %·days vs. 92.9 ± 28.0 %·days at placebo sites. Despite differences in the shape of the profiles at diclofenac sites, all placebo treatment sites follow an approximately exponential decay. Under this model, the calculated t1/2 of the micropores at placebo sites for Subject A is 11.7 ± 2.1 h and 18.3 ± 4.5 h for Subject B.
Fig. 6.
Comparison of admittance profiles in two subjects who completed a crossover design. Subject 6 (graph A) and Subject 9 (graph B) completed both Schedules 1 and 2 in order to examine the effect of pre-hydration on the AUC. Triangles represent the diclofenac treatment sites, and squares represent the placebo sites. Solid lines with solid shapes display the sites with pre-hydration, and dashed lines with open shapes represent non pre-hydrated sites. As seen in the profiles, the effect of pre-hydration was not consistent between the two subjects, though the shape of the profiles (regardless of hydration status) was consistent within each individual subject. The average ± SD AUC at the pre-hydrated diclofenac treatment sites was 240.5 ± 88.5 %·days and 145.8 ± 17.0 %·days (Subject 6 and 9, respectively), vs. the AUC at non-prehydrated diclofenac sites of 271.4 ± 55.6 %·days and 131.9 ± 25.4 %·days. Pre-hydrated placebo site AUC values were 74.6 ± 6.2 %·days (Subject 6) vs. 59.8 ± 8.9 %·days (Subject 9), compared to the non pre-hydrated values of 170.1 ± 3.8 %·days and 81.1 ± 3.4 %·days.
For the majority of subjects, admittance of the micropores exhibited approximately exponential decay at the MN + placebo treatment sites, and logarithmically transforming this data allowed for determination of apparent first-order rate constants (k's). The average ± SD rate constant was 0.92 ± 0.32 days−1 (range 0.41–1.60 days−1). Based on the rate constants, the average first-order t1/2 of micropore closure (without any active drug moiety to prolong micropore lifetime) was approximately 0.76 ± 0.35 days (range 0.43–1.67 days). In contrast, the kinetics at sites treated with MN + diclofenac did not generally follow an exponential decay process, and there was more inter-subject variability in the shape of the profiles. In subjects who had duplicate treatments (i.e. the same treatment applied to two independent sites), the shapes of the profiles were markedly similar, for both diclofenac and placebo treated sites, demonstrating the reproducibility of this surrogate marker technique for monitoring micropore closure (Fig. 5).
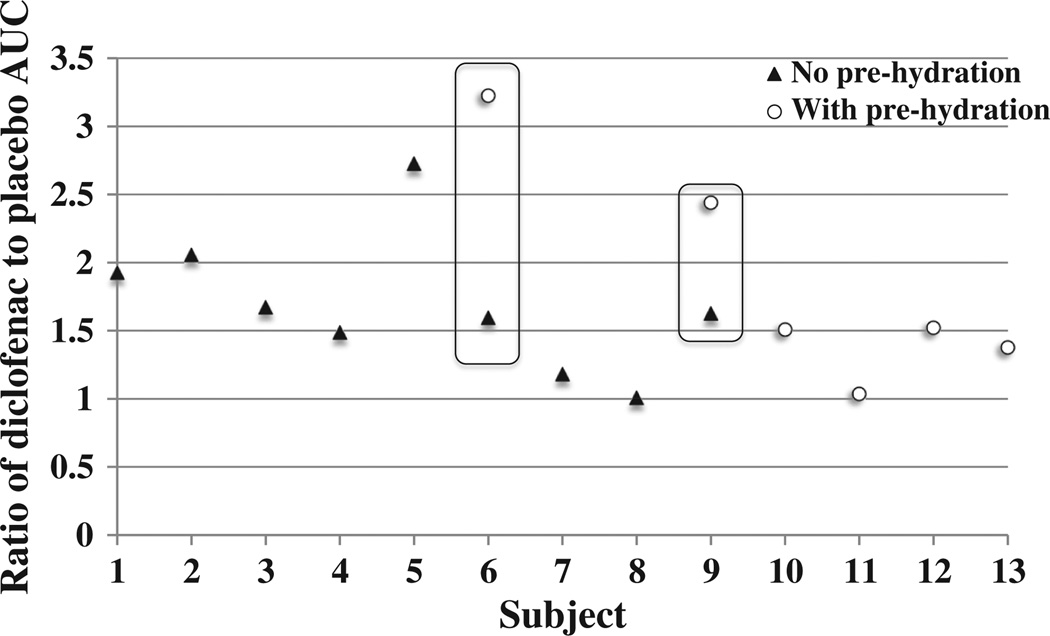
To more consistently compare treatment effects between subjects, the ratio of diclofenac to placebo AUC was calculated (Fig. 7). By comparing the active to placebo treatment sites within the same subject, a better comparison of the magnitude of treatment effect can be made between subjects. All ratios were >1.0 (range 1.01–3.23), demonstrating a positive treatment effect (slower micropore closure) attributed to diclofenac. The hydration status of the skin did not appear to have any consistent effect on the magnitude of the treatment effect, which was especially notable in Subjects 6 and 9, who completed the crossover design (Fig. 6).
Fig. 7.
Ratios of diclofenac to placebo AUC. A simple ratio of the AUC values of active treatment (diclofenac) to placebo was calculated for each subject in order to demonstrate the magnitude of a treatment effect, thus allowing for a more direct comparison between subjects (n=13). Solid triangles represent subjects with no pre-hydration, open circles represent subjects with pre-hydration. For subjects who had 2 treatment sites each for diclofenac or placebo, the average AUC for each treatment type was calculated and used to determine the ratio. The 2 subjects who completed the treatments with and without pre-hydration are outlined by the open boxes. Any ratio >1 demonstrates a favorable treatment effect for diclofenac. The average ± SD ratio was 1.76 ± 0.62 (range 1.01–3.23). The skin's hydration state at the time of MN treatment did not have a consistent impact on the treatment effect, which is particularly evident for the 2 subjects who completed the crossover schedule.
3.4. Effects of diclofenac on human skin
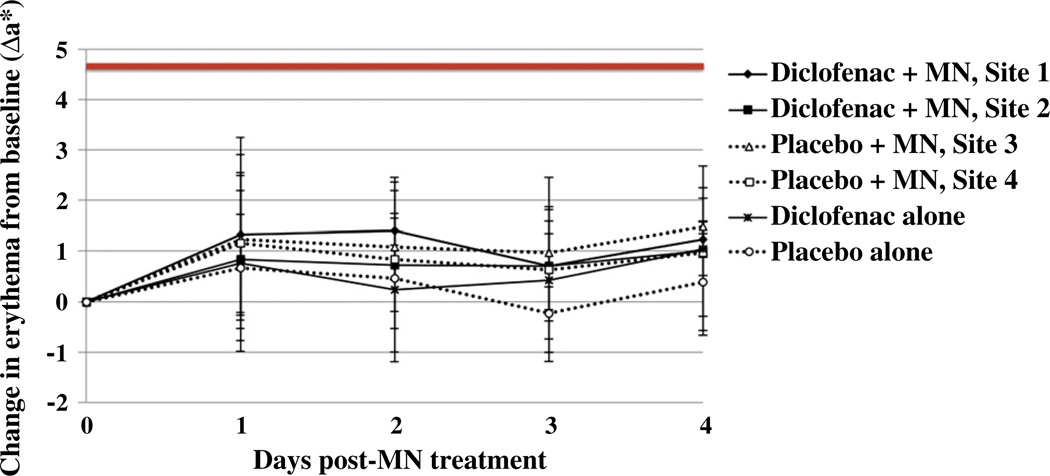
Colorimetry measurements in six subjects (treatment paradigm 2, Schedule 1) were taken to confirm that the observed differences between diclofenac and placebo sites were in fact a result of the diclofenac sodium and were not due to nonspecific irritation. In all subjects, a* and Δa* values over the entire treatment course were lower than values expected for positive controls following treatment with mild skin irritants in humans or guinea pigs [32,33] (Fig. 8). There was no significant difference in erythema between any of the treatment sites (p=0.2, one-way ANOVA) and no redness or irritation was observable by the naked eye. These combined factors indicate that no clinically significant skin irritation contributed to the enhanced admittance observed at the diclofenac treatment sites, and also confirms other findings that microneedle treatment in humans is not irritating [4,5,7,9].
Fig. 8.
Assessment of skin irritation. Erythema was quantified via daily colorimetry readings. Change in erythema from baseline at each site was assessed by calculating the Δa* value according to the equation: Δa* = a*t (at time t days after starting the study) –a*0 (at time 0, prior to application of treatment); data are displayed as the mean ± SD at each timepoint (n=6 subjects). The bold line at 4.7 depicts a typical value expected after treating humans with sodium lauryl sulfate 33. Sites treated with diclofenac are depicted by solid lines, whereas dashed lines represent placebo treated sites. Overall, no significant skin erythema was noted between diclofenac and placebo treatment sites (p=0.2, one-way ANOVA).
3.5. Benefits of examining multiple treatment schedules
Examining the effects of diclofenac under the 3 different treatment schedules provided some unique insight about the measurement techniques and treatment effects. In the crossover subjects, completing Schedules 1 and 2 helped confirm that the impedance measurements were reproducible regardless of the skin's hydration status, demonstrating the consistency of this measurement technique under various clinical conditions. Second, a positive treatment effect from the diclofenac was seen in both subjects for both schedules, confirming that the skin's healing response after MN treatment (and attenuation of that response with an anti-inflammatory) is not altered based on the skin's hydration status. For all subjects who completed either Schedule 1 or 2, it was valuable from a safety perspective for the investigators to remove the gels and patches daily to visually inspect the skin to ensure that no infection or irritation was present. This also provided an opportunity to measure subclinical erythema with the colorimetry methods. Finally, Schedule 3 demonstrated that daily application of the diclofenac gel was not necessary in order to observe a positive effect from the diclofenac. This suggests that the unique gel base (containing hyaluronate sodium) created a depot in the skin providing a local anti-inflammatory effect, rather than a systemic effect. Lastly, this schedule further confirmed that the skin follows the same healing response with or without antiinflammatory treatment under short (24 h) vs. longer (72 h) prehydration periods, suggesting that the hydration under a transdermal patch applied for a full week would not negatively affect the treatment response from diclofenac.
4. Discussion
The benefits of transdermal drug delivery are well established, but the strict physicochemical parameters required for a drug to cross the skin barrier limit the number of molecules that can be passively delivered. Physical enhancement methods have greatly expanded the potential number of drug molecules that can be transdermally delivered, but despite these advances none of the methods are yet suitable for delivering a drug over a week-long time frame, (the ideal for transdermal patches). This is the first human study to demonstrate that the lifetime of micropores following one-time application of MN arrays can be enhanced via simple topical application of a nonspecific COX inhibitor. The commercial development of this technology would allow for transdermal treatment of a variety of indications with less frequent patch application, which would likely increase patient compliance and satisfaction with therapy.
Previous studies have demonstrated that daily application of diclofenac prolongs micropore lifetime in hairless guinea pigs [15], and the present work demonstrates that micropore closure kinetics are also prolonged in human subjects via evaluation of the AUC over several days following MN treatment. The AUC is typically reported for drug concentration-time data to demonstrate total drug exposure, allowing for a comparison of exposure between different treatments. In this case, we used AUC to compare the change in the admittance values between diclofenac and placebo treatment sites. While this does not explicitly describe the kinetics of micropore closure, per se, it can be extrapolated that a higher AUC would correlate with slower rates of closure, as a higher AUC would be a result of higher admittance values over the entire treatment period. Therefore, this model incorporates the general assumption that slower kinetics of micropore closure are described by higher AUC values.
The diclofenac formulation used in these studies is unique in that the vehicle contains 2.5% hyaluronic acid, a naturally occurring polysaccharide found in the skin. Studies have demonstrated that hyaluronic acid aids the partitioning of diclofenac into the skin and promotes its retention within the epidermis, ultimately creating a local depot of drug [34]. For the purposes of enhancing micropore lifetime this property is particularly appealing, as it ensures that the effects of the diclofenac are a result of local concentrations at the micropores, rather than any effects from systemic delivery. The subjects with the highest AUC were those that completed the treatment schedule with the fewest applications of diclofenac, suggesting that daily application of the diclofenac is not necessary (which is likely partly attributable to the depot of diclofenac formed in the skin). The AUC values for the subjects in Schedule 3 were calculated over a total of 7 days post-MN treatment, which could also contribute to the higher values. It was not feasible to accurately determine the AUC over a 4 day post-MN period in these subjects because this would have generated an AUC from a much smaller number of data points, which is likely to greatly overestimate the values and produce the appearance of a more pronounced treatment effect. It is also possible that the increased exposure to air (created by daily impedance measurements) at the MN-treated sites in the other subjects may have contributed to faster micropore closure, as MN-treated skin heals markedly faster when unoccluded. Any systemic absorption of diclofenac from the topical dose (200 µl of a 3% w/w gel, 6 mg total) would be considered negligible, as this is far below the lowest oral dose of diclofenac given for systemic indications (100 mg/day with 50% oral bioavailability equating to a 50 mg systemic dose) [35].
4.1. Effect of formulation pH on micropore closure kinetics
The experimentally measured pH values of the diclofenac and placebo gels were 7.3 and 4.7, respectively. The effect of pH on skin wound healing has been investigated in the literature and it has been shown that pH does not play a role in wound healing for lesser insults [36]. However, in the case of acetone disruption when the “acid mantle” of the skin is disturbed, the rate of healing is slower at pH 7.4 compared to pH 5.5. This can be attributed to the acidic pH optimum of β-glucocerebrosidase, an enzyme responsible for the post-secretion modifications of polar to non-polar ceramides [36]. Since the pH of the diclofenac gel was higher compared to the placebo in this study, we examined the role of formulation pH in the healing of the micropores. Micropore closure kinetics were evaluated in vivo under 3 different pH conditions in a Yucatan miniature pig: pH 5.5, 6.5 and 7.4 (gels made in 100 mM citrate buffer and gelled with 3% hydroxyethylcellulose). Three MN treated sites and 1 untreated control site were used for each pH condition; all sites were under occlusion after one time MN application. There was no significant difference in admittance values among the 3 conditions after the first 24 h (p>0.05). The difference in admittance up to 24 h is consistent with previous reports demonstrating significant differences in TEWL (following acetone treatment) between pH 5.5 and pH 7.4 at 2 h and 4 h (p<0.01) and 24 h (p<0.05) [36]. After the early time points, however, the rates of recovery normalize in spite of the pH difference. Thus, as the skin begins to heal itself over time, the effect of formulation pH becomes less evident in the repair mechanism. Hence it can be concluded that the effect of diclofenac seen in this study is independent of the formulation pH of the gels, beyond the 24 h time point. Based on the pH data and all of the above mentioned factors it can be concluded that the differences in micropore closure (between diclofenac and placebo) that were observed in this study are related to the local concentration of diclofenac, not from a systemic anti-inflammatory effect, or from the effects of the gel vehicles.
4.2. Potential factors contributing to inter-subject variability
The ability of drugs to permeate intact human skin is related both to the individual characteristics of the subject's skin as well as the structural and physicochemical properties of the drug compound (molecular weight, octanol/water partition coefficient, and hydrogen bonding) [37]. For in vitro human skin experimental permeability data alone, it is not unusual to observe as much as 30% variation [38,39]. Responses to topical treatments can sometimes be quite unpredictable, and there are currently no methods to accurately predict whether or not a subject will be an outlier in the typical response to a topically applied drug. In the present study there are various reasons why some subjects had higher or lower levels of response. First, previous work has demonstrated that drug-metabolizing enzymes (DMEs) are expressed in human skin [40], and inter-subject differences in the expression of these enzymes could be related to the magnitude of treatment effect observed from a variety of topical treatments. Diclofenac metabolism in the skin is thought to be similar to the metabolism seen following oral administration (conversion to glucuronide conjugates) [41], and varied expression of the enzymes involved in this metabolism could impact the treatment effect observed following topical diclofenac application. It is noteworthy here, however, that the amount of drug applied per unit area for typical topical drug applications would likely saturate the enzyme systems, and therefore differences in metabolism of diclofenac in the skin would likely have a minimal effect in this regard. Second, expression of the COX enzymes in normal skin can vary [42], and it is possible that a subject with lower COX enzyme expression may not display as pronounced an effect to the diclofenac treatment (the opposite would be true for subjects with greater COX expression in the skin). Third, expression of DMEs in the skin can be increased or decreased to varying degrees in response to topical treatments used in clinical practice, which could further contribute to the observed variation [40]. Despite these possible inter-subject variations, the overall trend demonstrated a significant difference between active and placebo treatments in a relatively small sample size.
4.3. Drug delivery window in relation to micropore lifetime and transdermal systems
It is important to note that extending micropore lifetime is not the only factor that will contribute to enhanced delivery of a drug molecule to therapeutically relevant systemic concentrations. The nature of transdermal patches dictates that a treatment site is occluded for the duration of patch application, which leads to a local increase in skin hydration. This natural byproduct of the treatment system can lead to enhanced drug delivery for many compounds [43] and helps the micropores to remain open for days (as seen by the drug delivery window observed in the human pharmacokinetic study) vs. approximately 15 minutes (observed when the micropores remain unoccluded) [9,13]. The enhanced micropore lifetime seen in this study combined with the increased drug delivery related to the local hydration represents the truest clinical scenario and would be expected to produce an additive effect on drug delivery.
In MN-assisted drug delivery, the concept of micropore lifetime is only useful in the context of a window during which a drug can be transdermally delivered to a therapeutic plasma concentration. The first MN-assisted pharmacokinetic study demonstrated that naltrexone can be transdermally delivered through micropores for 2–3 days under occluded conditions in the absence of any active drug moiety to prolong micropore lifetime [9]. An average micropore closure half-life of 0.76 days was observed in the current study, corresponding with the pharmacokinetic drug delivery window of approximately 2–3 days (or 3–5 half-lives) when ~87.5–97% of the micropores would be closed (according to the impedance measurements). While the notion of first-order rate constants cannot be extrapolated directly to the diclofenac sites because of the non-exponential decay of the admittance profiles, it does illustrate that the true drug delivery window is significantly longer than what is predicted based on the kinetics of micropore closure alone. It is probable that the impedance measurements overestimate the micropore closure rate, and this method is not as sensitive as evaluating drug diffusion in a pharmacokinetic study. A proof-of-concept pharmacokinetic study will be necessary, but impedance is a useful surrogate marker to conduct micropore closure formulation study screening.
In addition to providing a better understanding of the drug delivery window, the consistency of rate constants at the placebo treatment sites also provides a novel method of evaluating other treatments for enhancing the lifetime of micropores. The ratio of active treatment to placebo treatment effects (in this case, AUC) offers a means to understand the magnitude of a treatment effect, allowing for more direct comparison between subjects. Without drug plasma concentrations to measure the amount of drug delivery through the micropores, this ratio solely describes the difference between AUC values; thus, a ratio of 2.0 would correlate to 2-fold slower rates of micropore closure at diclofenac sites vs. placebo sites (based on the assumption that higher AUC indirectly describes slower kinetics of micropore closure). This ratio of 2.0 would then also loosely predict that the maximum amount of drug that could be delivered through the micropores at diclofenac treatment sites would be approximately twice that seen at placebo sites. This analysis was not only useful in the current work, but will likely also prove to be beneficial for screening additional compounds for similar effects. This ratio allows for a direct comparison of the utility of various treatments within the same subject by allowing the subject to serve as their own control, while also allowing for comparison between subjects. Furthermore, these techniques could be expanded to measure pore closure in other physical enhancement techniques that create pores in the skin (e.g. microdermabrasion or electroporation).
4.4. Tolerability of microneedles and topical treatments
Skin erythema after MN treatment and topical gel applications was quantified using a tristimulus colorimeter. This technique allows for analysis of blue, red, and green light reflected from the skin, providing a quantitative means of assessing skin color that mimics the perception of the human eye. The a* measurement represents the red–green axis, and this value becomes more positive as erythema appears on the skin (i.e. less green light is reflected) [29]. The a* values obtained with a colorimeter correlate well with erythema and can be used to quantify skin irritation; the Δa* demonstrates change in local erythema from a pre-treatment baseline at that site [29]. It has been reported that Δa* values can reach up to 4.73 in humans following treatment with sodium lauryl sulfate (a known skin irritant) and as high as 8.9 in hairless guinea pigs [33,44]. Hairless guinea pigs are a well accepted model for studying skin irritation as they are more sensitive than humans to mild irritation, allowing for amore conservative estimation of skin irritation potential [45]. In this study, Δa* values were well below those of typical positive controls, confirming the lack of erythema and providing additional support that the observed changes in micropore lifetime are not related to nonspecific local irritation.
The concern often arises that prolonging micropore lifetime may increase the risk of local infection at MN treated sites. From a practical point of view, however, this is not a prominent concern. Prior to application of MN arrays, the skin is treated with 70% isopropyl alcohol and the arrays are sterile and only used once (similar precautions to those used in routine clinical care for inserting a hypodermic needle into the skin). All topical treatments applied to the MN treated skin would be in a preparation suitable for human use, i.e. the formulation would contain bacteriostatic/cidal preservatives designed to prevent local bacterial infection [9]. Finally, despite all of the research performed on MN-assisted delivery, no reports have described any kind of infection (local or systemic), and in vitro work has demonstrated that microbial penetration is significantly less following treatment with a MN array vs. a 21G hypodermic needle [46].
4.5. Limitations
There are some limitations to this work. Due to the rapid closure of the micropores in unoccluded conditions, repeated impedance measurements could not be made at each time point because of the prolonged exposure to air that this would allow. Despite this limitation, the profiles were similar in shape in those subjects who had multiple sites for identical treatments, and placebo rate constants were similar between subjects, demonstrating the reliability and reproducibility of the results. Secondly, MN insertion is a somewhat imprecise process and there are currently no non-proprietary standardized means of applying MN arrays to the skin. This was controlled for as much as possible by having the same investigator apply all of the MN treatments, to avoid inter-investigator variability. There was no significant difference in the post-MN measurements between diclofenac and placebo treatment sites, and therefore the imprecise nature of MN application would not be expected to substantially affect the results. Finally, in this study diclofenac was applied daily to the skin for the majority of subjects. This does not represent the ideal clinical situation, as it would be cumbersome for a patient to apply the gel daily. Additionally, in a regulatory sense the concept of using diclofenac to enhance micropore lifetime might seem impractical because of the frequent applications and off-label use of a commercial product. However, the application schedule was a necessary component of this work given the proof-of-concept nature of the study and the need to remove the gels in order to make impedance measurements; furthermore, the diclofenac gel represented the safest formulation due to the lack of systemic delivery from the gel vehicle. Current work in our lab is focused on developing codrugs for integrating diclofenac into a patch system that would allow for continued local delivery of the diclofenac. A codrug consists of two drug moieties joined by a chemical linker (in this case diclofenac sodium linked to another drug), in order to improve the delivery of one or both drugs. Our efforts are aimed at developing a codrug system with diclofenac that will dissociate within the skin, thus separating the two independent drugs and allowing for local delivery of diclofenac sodium while allowing the other drug moiety to passively diffuse through the micropores into the systemic circulation. This would thereby eliminate the need for daily application of the diclofenac moiety and would be a product designed specifically for enhancing micropore lifetime to allow for a longer drug delivery window [47]. Incorporation of diclofenac into a patch formulation could also be done in other ways, and specific drug development studies would need to be done.
In summary, this is the first study in human volunteers to demonstrate that topical application of a nonspecific COX inhibitor can prolong micropore lifetime. Future directions of this work will include a pharmacokinetic proof-of-concept study to demonstrate the clinical utility of extending micropore lifetime, as well as continued development of diclofenac codrugs. This work indicates that MN-assisted trans-dermal delivery has immense potential to continue expanding to allow for delivery of a vast array of drug compounds for a variety of clinical uses.
Acknowledgments
We would like to acknowledge Dr. Mark Prausnitz and Dr. Vladimir Zarnitsyn (Georgia Institute of Technology) for their expert advice and assistance with the MN arrays, and Dr. Bruce Walcott (University of Kentucky) for his contributions to the impedance setup. Additionally, thank you to Priyanka Ghosh (doctoral candidate, University of Kentucky College of Pharmacy) for completing the preliminary animal pH studies. This work was funded by the following NIH grants: CTSA 1UL1RR033173-01, R01DA13425, 1F31DA029374, and the Clinical Loan Repayment Program; additional funding sources included the University of Kentucky Center for Clinical and Translational Science Pilot Research Program and the University of Kentucky Center for Clinical and Translational Science Seed Grant.
Footnotes
Conflict of interest
Audra L. Stinchcomb is a significant shareholder in and Chief Scientific Officer of AllTranz Inc., a Lexington, KY specialty pharmaceutical company involved in the development of transdermal formulations for microneedle delivery.
References
- 1.Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin's barrier function. Pharm. Sci. Technol. 2000;3(9):318–326. doi: 10.1016/s1461-5347(00)00295-9. Today. [DOI] [PubMed] [Google Scholar]
- 2.Prausnitz MR, Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008 Nov;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prausnitz MR. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004;56(5):581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, Morrissey A, Birchall JC. Clinical administration of microneedles: skin puncturepain and sensation. Biomed. Microdevices. 2009 Feb;11(1):35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 5.Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR. Lack of pain associated with microfabricated microneedles. Anesth. Analg. 2001 Feb;92(2):502–504. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 6.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002 Apr;8(4):415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 7.Smart WH, Subramanian K. The use of silicon microfabrication technology in painless blood glucose monitoring. Diabetes Technol. Ther. 2000 Winter;2(4):549–559. doi: 10.1089/15209150050501961. [DOI] [PubMed] [Google Scholar]
- 8.Vaccine Shoppe. [Accessed March 7, 2012]; http://www.vaccineshoppe.com/static/FluID2/fluid-video-desktop.html.
- 9.Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, Stinchcomb AL. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc. Natl. Acad. Sci. U. S. A. 2008;105(6):2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daddona PE, Matriano JA, Mandema J, Maa YF. Parathyroid hormone (1– 34)-coated microneedle patch system: clinical pharmacokinetics and pharma-codynamics for treatment of osteoporosis. Pharm. Res. 2011 Jan;28(1):159–165. doi: 10.1007/s11095-010-0192-9. [DOI] [PubMed] [Google Scholar]
- 11.Milewski M, Brogden NK, Stinchcomb AL. Current aspects of formulation efforts and pore lifetime related to microneedle treatment of skin. Expert Opin. Drug Deliv. 2010 May;7(5):617–629. doi: 10.1517/17425241003663228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.3M Microneedle Technology. [Accessed March 27, 2011]; http://solutions.3m.com/wps/portal/3M/en_WW/DrugDeliverySystems/DDSD/technology-solutions/transdermal-technologies/microstructured-transdermal-systems/
- 13.Bal S, Kruithof AC, Liebl H, Tomerius M, Bouwstra J, Lademann J, Meinke M. In vivo visualization of microneedle conduits in human skin using laser scanning microscopy. Laser Phys. Lett. 2010;7(3):242–246. [Google Scholar]
- 14.Enfield J, O'Connell ML, Lawlor K, Jonathan E, O'Mahony C, Leahy M. In-vivo dynamic characterization of microneedle skin penetration using optical coherence tomography. J. Biomed. Opt. 2010 Jul-Aug;15(4):046001. doi: 10.1117/1.3463002. [DOI] [PubMed] [Google Scholar]
- 15.Banks SL, Paudel KS, Brogden NK, Loftin CD, Stinchcomb AL. Diclofenac enables prolonged delivery of naltrexone through microneedle-treated skin. Pharm. Res. 2011 May;28(5):1211–1219. doi: 10.1007/s11095-011-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn GL. Cutaneous and transdermal delivery: processes and systems of delivery. In: Banker GS, Rhodes CT, editors. Modern Pharmaceutics. 2nd ed. New York: Marcel Dekker; 1990. pp. 239–298. [Google Scholar]
- 17.Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. J. Lipid Res. 1989 Mar;30(3):323–333. [PubMed] [Google Scholar]
- 18.Lackermeier AH, McAdams ET, Moss GP, Woolfson AD. In vivo ac impedance spectroscopy of human skin. Theory and problems in monitoring of passive percutaneous drug delivery. Ann. N. Y. Acad. Sci. 1999;873:197–213. doi: 10.1111/j.1749-6632.1999.tb09468.x. [DOI] [PubMed] [Google Scholar]
- 19.Tagami H, Ohi M, Iwatsuki K, Kanamaru Y, Yamada M, Ichijo B. Evaluation of the skin surface hydration in vivo by electrical measurement. J. Invest. Dermatol. 1980 Dec;75(6):500–507. doi: 10.1111/1523-1747.ep12524316. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Yamamoto Y. Electrical properties of the epidermal stratum corneum. Med. Biol. Eng. 1976 Mar;14(2):151–158. doi: 10.1007/BF02478741. [DOI] [PubMed] [Google Scholar]
- 21.Kalia YN, Guy RH. The electrical characteristics of human skin in vivo. Pharm. Res. 1995 Nov;12(11):1605–1613. doi: 10.1023/a:1016228730522. [DOI] [PubMed] [Google Scholar]
- 22.Karande P, Jain A, Mitragotri S. Relationships between skin's electrical impedance and permeability in the presence of chemical enhancers. J. Control. Release. 2006;110(2):307–313. doi: 10.1016/j.jconrel.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Kohli R, Archer WI, Roberts JMC, Cochran AJ, Li Wan Po A. Impedance measurements for the non-invasive monitoring of skin hydration: a reassessment. Int. J. Pharm. 1985;26:275–287. [Google Scholar]
- 24.Curdy C, Naik A, Kalia YN, Alberti I, Guy RH. Non-invasive assessment of the effect of formulation excipients on stratum corneum barrier function in vivo. Int. J. Pharm. 2004;271(1–2):251–256. doi: 10.1016/j.ijpharm.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Kawai E, Nakanishi J, Kumazawa N, Ozawa K, Denda M. Skin surface electric potential as an indicator of skin condition: a new, non-invasive method to evaluate epidermal condition. Exp. Dermatol. 2008 Aug;17(8):688–692. doi: 10.1111/j.1600-0625.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 26.White E, Bunge A. Reporting Skin Resistance and Impedance, in: Paper presented at: Barrier Function of Mammalian Skin Gordon Research Conference. Waterville Valley, NH: 2009. [Google Scholar]
- 27.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release. 2011 Sept 5;154(2):148–155. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milewski M. Microneedle-assisted transdermal delivery of naltrexone species: in vitro permeation and in vivo pharmacokientic studies. Lexington, KY: University of Kentucky Doctoral; 2011. [Google Scholar]
- 29.Fullerton A, Fischer T, Lahti A, Wilhelm KP, Takiwaki H, Serup J. Guidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1996 Jul;35(1):1–10. doi: 10.1111/j.1600-0536.1996.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 30.Curdy C, Kalia YN, Guy RH. Post-iontophoresis recovery of human skin impedance in vivo. Eur. J. Pharm. Biopharm. 2002 Jan;53(1):15–21. doi: 10.1016/s0939-6411(01)00216-8. [DOI] [PubMed] [Google Scholar]
- 31.Gupta J, Prausnitz MR. Recovery of skin barrier properties after sonication in human subjects. Ultrasound Med. Biol. 2009 Aug;35(8):1405–1408. doi: 10.1016/j.ultrasmedbio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiptoo PK, Paudel KS, Hammell DC, Hamad MO, Crooks PA, Stinchcomb AL. In vivo evaluation of a transdermal codrug of 6-beta-naltrexol linked to hydroxybupropion in hairless guinea pigs. Eur. J. Pharm. Sci. 2008;33(4–5):371–379. doi: 10.1016/j.ejps.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnetz E, Diepgen TL, Elsner P, Frosch PJ, Klotz AJ, Kresken J, Kuss O, Merk H, Schwanitz HJ, Wigger-Alberti W, Fartasch M. Multicentre study for the development of an in vivo model to evaluate the influence of topical formulations on irritation. Contact Dermatitis. 2000 Jun;42(6):336–343. doi: 10.1034/j.1600-0536.2000.042006336.x. [DOI] [PubMed] [Google Scholar]
- 34.Brown MB, Jones SA. Hyaluronic acid: a unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005 May;19(3):308–318. doi: 10.1111/j.1468-3083.2004.01180.x. [DOI] [PubMed] [Google Scholar]
- 35.Micromedex® 2.0. [Accessed March 7, 2011]; http://www.thomsonhc.com/micromedex2/librarian/2011.
- 36.Mauro T, Holleran WM, Grayson S, Gao WN, Man MQ, Kriehuber E, Behne M, Feingold KR, Elias PM. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch. Dermatol. Res. 1998 Apr;290(4):215–222. doi: 10.1007/s004030050293. [DOI] [PubMed] [Google Scholar]
- 37.Potts RO, Guy RH. A predictive algorithm for skin permeability: the effects of molecular size and hydrogen bond activity. Pharm. Res. 1995 Nov;12(11):1628–1633. doi: 10.1023/a:1016236932339. [DOI] [PubMed] [Google Scholar]
- 38.Guy RH, Hadgraft J. Physicochemical aspects of percutaneous penetration and its enhancement. Pharm. Res. 1988 Dec;5(12):753–758. doi: 10.1023/a:1015980516564. [DOI] [PubMed] [Google Scholar]
- 39.Potts RO, Guy RH. Predicting skin permeability. Pharm. Res. 1992 May;9(5):663–669. doi: 10.1023/a:1015810312465. [DOI] [PubMed] [Google Scholar]
- 40.Smith G, Ibbotson SH, Comrie MM, Dawe RS, Bryden A, Ferguson J, Wolf Ref CR. Regulation of cutaneous drug-metabolizing enzymes and cytoprotective gene expression by topical drugs in human skin in vivo. Br. J. Dermatol. 2006 Aug;155(2):275–281. doi: 10.1111/j.1365-2133.2006.07317.x. [DOI] [PubMed] [Google Scholar]
- 41.PharmaDerm. Solaraze® gel package insert. 2009 [Google Scholar]
- 42.Muller-Decker K, Reinerth G, Krieg P, Zimmermann R, Heise H, Bayer C, Marks F, Fürstenberger G. Prostaglandin-H-synthase isozyme expression in normal and neoplastic human skin. Int. J. Cancer. 1999;82(5):648–656. doi: 10.1002/(sici)1097-0215(19990827)82:5<648::aid-ijc6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 43.Bucks D, Maibach H. Percutaneous Absorption. New York: Marcel Dekker, Inc.; 1999. [Google Scholar]
- 44.Swadley CL. Investigation of Endocannabinoid System Modulators for Percutaneous Drug Delivery. Lexington, KY: University of Kentucky Doctoral; 2011. [Google Scholar]
- 45.Andersen F, Hedegaard K, Fullerton A, Bindslev-Jensen C, Andersen KE. Comparison of the response to topical irritants in hairless guinea pigs and human volunteers. J. Toxicol.-Cutan. Ocul. Toxicol. 2005;24:31–43. [Google Scholar]
- 46.Donnelly RF, Singh TR, Tunney MM, Morrow DI, McCarron PA, O'Mahony C, Woolfson AD. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm. Res. 2009 Nov;26(11):2513–2522. doi: 10.1007/s11095-009-9967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh P, Pinninti RR, Lee DM, Hammell DC, Paudel KS, Kim KB, Stinchcomb AL. Microneedle enhanced transdermal delivery using a codrug approach; Abstract presented at: American Association of Pharmaceutical Scientists, Annual meeting; Washington D.C. 2011. [Google Scholar]