Abstract
Increased levels of 3’–5’-cyclic adenosine monophosphate (cAMP) stimulate cell proliferation and fluid secretion in polycystic kidney disease (PKD). Since hydrolytic capacity of phosphodiesterases (PDE) far exceeds maximum rate of synthesis by adenylyl cyclases (AC), cellular levels of cAMP are more sensitive to PDE inhibition than to AC activity changes. We have used enzymatic, western blot, immunohistochemistry, PCR and biochemical assays to study activity and expression of PDE families and isoforms and expression of downstream effectors of cAMP signaling in wildtype and PKD rat and mouse kidneys. The results indicate: 1) Species specific differences in PDE expression; higher PDE activity in kidneys from mice compared to rats; higher contribution of PDE1, relative to PDE4 and PDE3, to total PDE activity of kidney lysate and lower PDE1, PDE3 and PDE4 activities in murine cystic compared to wildtype kidneys. 2) Reduced levels of several PDE1, PDE3 and PDE4 proteins despite mRNA upregulation, possibly due to increased protein degradation. 3) Increased cGMP levels in polycystic kidneys, suggesting in vivo downregulation of PDE1 activity. 4) Additive stimulatory effect of cAMP and cGMP on cystogenesis in vitro. 5) Upregulation of cAMP-dependent protein kinase (PKA) subunits Iα and IIβ, PKare, CREB-1 mRNA, and CREM, ATF-1 and ICER proteins in cystic compared to wildtype kidneys. In summary, the results of this study suggest that alterations in cyclic nucleotide catabolism may render the cystic epithelium particularly susceptible to factors acting on Gs coupled receptors, account at least in part for the upregulation of cyclic nucleotide signaling in PKD, and contribute substantially to the progression of this disease.
INTRODUCTION
ADPKD and ARPKD are common inherited renal cystic diseases for which there is no proven effective therapy.1–6 ADPKD is caused by mutations in PKD1 encoding polycystin-1 (PC1) or PKD2 encoding polycystin-2 (PC2). ARPKD is caused by mutations in PKHD1 encoding fibrocystin/polyductin (FC). Both diseases display large inter- and intra-familial variability, in part due to the effect of modifier genes which remain largely unidentified.
Recent studies have established that 3’–5’-cyclic adenosine monophosphate (cAMP) stimulates mural epithelial cell proliferation and secretion of fluid into cysts of patients with ADPKD7–11. Increased levels of cAMP are found in the kidney, liver, vascular smooth muscle, and choroid plexus, in various animal models of PKD.12–19 Arginine vasopressin (AVP) V2R antagonists lower the levels of cAMP in the kidneys and inhibit the development and progression of PKD in models orthologous to ARPKD (PCK rat), adolescent nephronophthisis (pcy mouse) and ADPKD type 2 (Pkd2−/WS25 mouse). 13, 14, 20 Genetic elimination of AVP in PCK rats yields animals that remain relative free of cysts unless an exogenous V2R agonist is administered.21 Octreotide also lowers the tissue levels of cAMP and has a protective effect not only in the kidney, but also in the liver.18 The ability to hormonally modulate cAMP in a cell-specific manner minimizes adverse effects, obviously important for the treatment of a chronic disease. Clinical trials of AVP V2R antagonists (NCT00413777, NCT00428948) and long-acting somatostatin analogs (NCT00309283, NCT00565097, NCT00426153) are currently active.
The mechanisms responsible for the accumulation of cAMP in cystic tissues and for its effect on cystogenesis are not well understood. In view of the importance of PKD proteins in the regulation of intracellular calcium and of calcium in the regulation of cAMP metabolism, changes in intracellular calcium may be responsible for the accumulation of cAMP in cystic tissues.13, 14, 22 Since the hydrolytic capacity of phosphodiesterases (PDEs) far exceeds the maximum rate of synthesis by adenylyl cyclases, cellular levels of cAMP are thought to be more sensitive to inhibition of PDEs than to changes in the activity of adenylyl cyclases. Recently a new class of compounds, the 2-(acylamino)-3-thiophenecarboxylates, has been shown to function as nonselective phosphodiesterase activators and inhibit the growth of MDCK cysts.23
Limited information is available on the pattern of expression of the different PDE isoforms and of the downstream effectors thought to mediate the cystogenic effect of cAMP in polycystic kidneys. Therefore we have used enzymatic, western blot, PCR, and biochemical assays to study the expression of different PDE isoforms and downstream effectors of cAMP signaling in wild-type and polycystic rat and mouse kidneys.
RESULTS
PDE1, PDE3 and PDE4 enzymatic activity
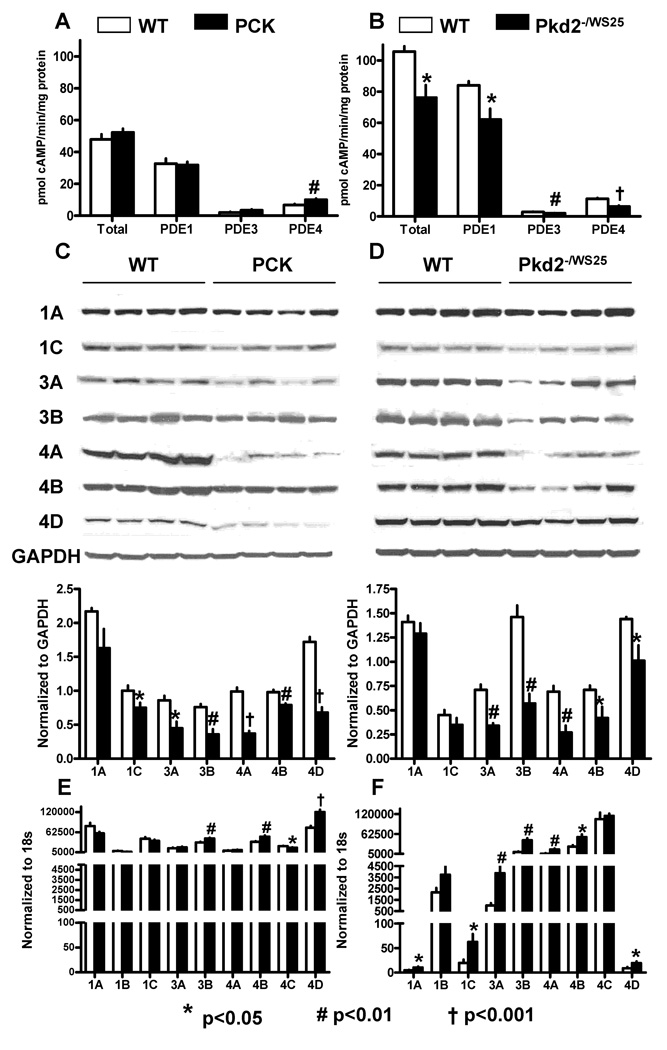
Total PDE activity was significantly higher in kidney lysates from mice than in those from rats (Figure 1A and B). PDE1, PDE3 and PDE4 accounted for 79.6 ± 1.4, 2.8 ± 0.4 and 11.2 ±1.1 % of total PDE activity in lysates from wild-type mice and for 67.6 ± 5.4, 4.5 ± 2.2 and 12.4 ± 3.0 % of total PDE activity in lysates from wild-type rats. PDE1, PDE3 and PDE4 activities were significantly lower in murine cystic compared to wild-type kidneys. However, PDE activities were similar in rat cystic and wild-type kidneys. Since PDE activities were expressed per mg of protein, protein concentrations per wet tissue were significantly lower in the cystic than in wild-type kidneys (12. 4 ± 1.1 vs 17.1 ± 2.7% and 10.5 ± 2.6 vs 17.0 ± 4.6 %, for mice and rats, respectively), and PDE immunoreactivity was confined to cells (see below), the reduction of PDE activities in cystic tissues was likely underestimated. Cystic kidneys were studied at relatively early stages of the disease (10 week old rats with relative cyst volume of 36.9%, fibrosis volume of 1.2% and plasma BUN concentration of 19.0 mg/dl; 16 week old mice with relative cyst volume of 32.6 %, fibrosis volume of 3.3% and plasma BUN concentration of 31.6 mg/dl). Therefore, it is unlikely that the changes observed are secondary to severe azotemia or tubular dropout.
Figure 1.
A) PDE1, PDE3 and PDE4 activities in whole lysates from kidneys of 10 week old male PCK (n=6) and wild-type (n=6) rats. B) PDE1, PDE3 and PDE4 activities in whole lysates from kidneys of 16 week old male Pkd2WS25/− (n=6) and wild-type (n=6) mice. C) Western blots of PDE1, PDE3 and PDE4 isoforms in the kidneys of 10 week old male PCK (n=4) and wild-type (n=4) rats. D) Western blots of PDE1, PDE3 and PDE4 isoforms in the kidneys of 16 week old male Pkd2WS25/− (n=4) and wild-type (n=4) mice. E) PDE1, PDE3 and PDE4 mRNA expression measured by RT-PCR in the kidneys of 10 week old male PCK (n=6) and wild-type (n=6) rats. F) PDE1, PDE3 and PDE4 mRNA expression measured by RT-PCR in the kidneys of 16 week old male Pkd2WS25/− (n=6) and wild-type (n=6) mice.
PDE1, PDE3 and PDE4 protein expression
Figure 1 (C, D) compares the protein expressions of the PDE1, PDE3 and PDE4 isoforms in mouse and rat wild-type and cystic kidneys. Consistent with lower enzymatic activities in murine cystic tissues, protein levels of several PDE1, PDE3, and PDE4 isoforms were significantly reduced in cystic compared to wild-type kidneys. This occurred despite over-expression of the corresponding PDE mRNAs (see below), possibly reflecting increased protein catabolism as suggested in some models of PKD.24
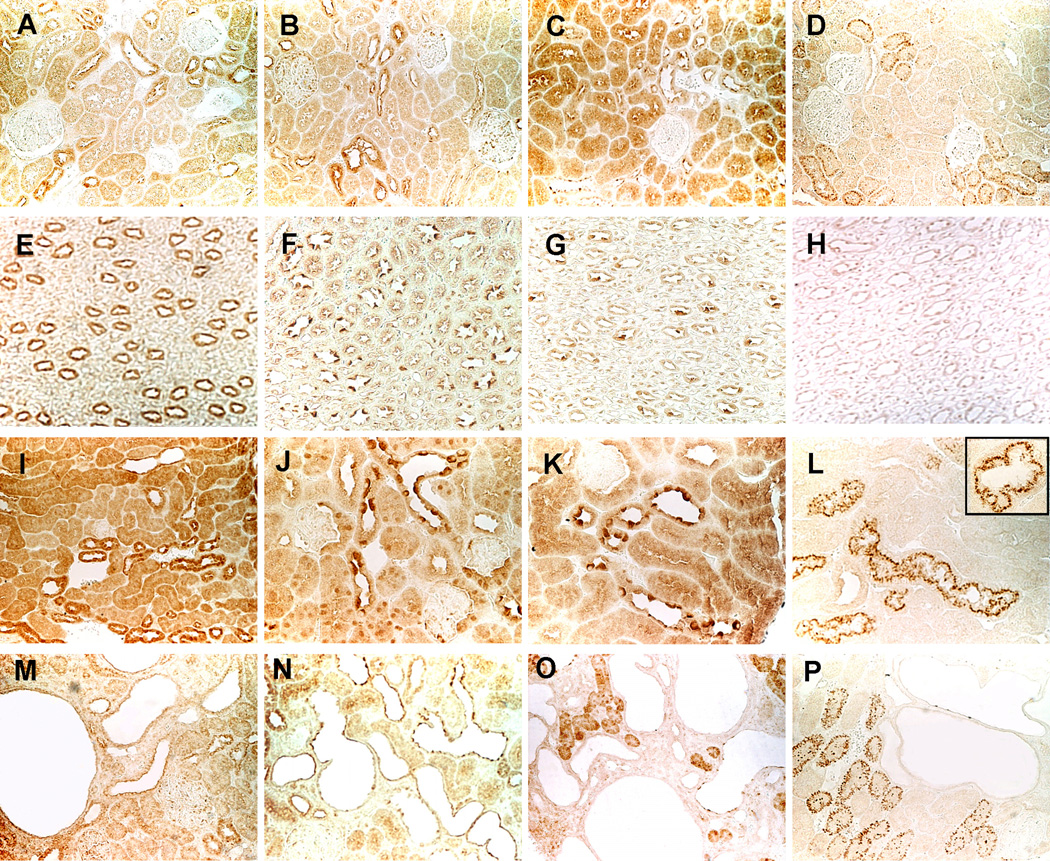
The immunohistochemical localization of PDE1, PDE3 and PDE4 isoforms is shown in Figures 2 and 3. In wild-type animals, PDE1A, PDE1C, and PDE3A were expressed in all tubular segments and in collecting ducts and the highest level of expression was in the distal convoluted tubules (Figure 2). The pattern of expression in the cystic animals was similar, but immunostaining in the cysts was weak or absent. PDE3B was more restricted to the distal tubules and associated with vesicular or granular structures. The immunostaining patterns of PDE4A, PDE4B and PDE4D in the renal cortex were similar to those of PDE1A, PDE1C and PDE3A, but medullary tubules and collecting ducts exhibited only weak immunoreactivity (Figure 3). Moderate immunostaining was detected in the cysts.
Figure 2.
Immunolocalization of PDE1A (A, E, I, M), PDE1C (B, F, J, N), PDE3A (C, G, K, O), and PDE3B (D, H, L, P) in wild-type rat (A-H, X200) and mouse (I-L, X400; insert, X800) and in PCK rat (M-P, X200) kidneys. The immunostaining pattern in Pkd2WS25/− mouse (not shown) was similar to that of PCK rat kidneys.
Figure 3.
Immunolocalization of PDE4A (A, D, G, J), PDE4B (B, E, H, K), and PDE4D (C, F, I, L) in wild-type rat (A-C, X200) and mouse (D-I, X400) and in PCK rat (J-L, X200) kidneys. The immunostaining pattern in Pkd2WS25/− mouse (not shown) was similar to that of PCK rat kidneys.
PDE1, PDE3 and PDE4 mRNA expression
Figure 1 (E, F) compares relative PDE1, PDE3 and PDE4 mRNAs expressions in wild-type and polycystic mouse and rat whole kidney lysates. There were marked species specific differences. The predominant PDE1 mRNAs in the rat kidney were PDE1a and PDE1c, whereas the predominant PDE1 mRNA expressed in the mouse kidney was PDE1b. This was unexpected because previous studies had shown PDE1b expression to be restricted to the brain and not found in the kidney.25, 26 However, the Uniport human kidney EST library (HS.530871-PDE1B) shows expression of 9 transcripts per million in kidney (compared to 94 in the brain). PDE4d mRNA was expressed at a higher level in the rat kidney than in mouse kidney, whereas the reverse was true for PDE4c mRNA. Significant differences were detected between wild-type and cystic kidneys. In the rat, PDE3b, PDE4b, and PDE4d mRNAs were over-expressed in cystic compared to wild-type kidneys. In the mouse, PDE1a, PDE1c, PDE3a, PDE3b, PDE4a, PDE4b, and PDE4d mRNAs were over-expressed in cystic compared to wild-type kidneys.
mRNA expression of other PDE isoforms
Supplementary Figure 1 compares relative expressions of the remaining PDE isoforms in wild-type and cystic kidneys. Of these, PDE2 is known to be localized to the glomeruli and PDE5 predominantly to the vasculature. The specific location of the other PDE isoforms in the kidney has not been characterized. The expressions of PDEA and B and those of PDE8A and B mRNAs are significantly higher in cystic than in wild-type mouse kidneys, whereas no difference between cystic and wild-type kidneys was detected in rats. Both, PDE7 and PDE8, are highly specific for cAMP and may play a role in T-lymphocyte activation.27
Cyclic GMP levels in polycystic kidneys
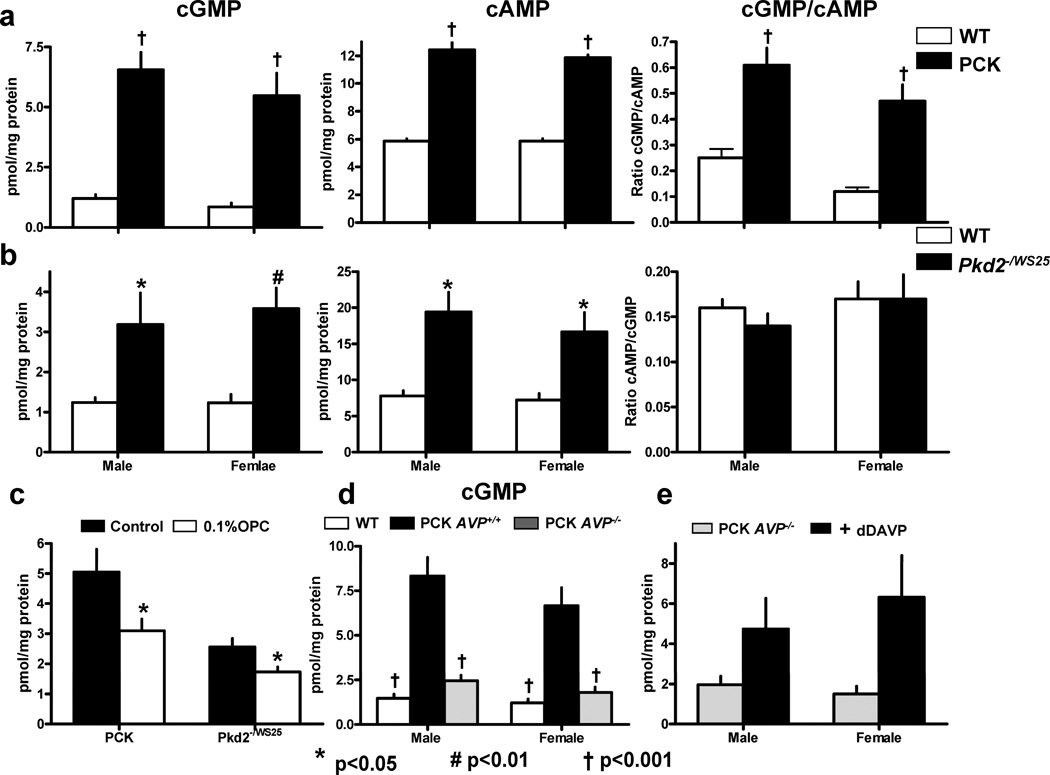
The increased levels of cAMP in cystic tissues may be due to alterations of intracellular calcium and reduced in vivo activity of PDE1, the only PDE regulated by calcium and calmodulin. Since PDE1 isoforms have an affinity for cGMP which is equal or higher than that for cAMP, the levels of cGMP would also be increased if this hypothesis was correct. Indeed, the levels of cGMP were found to be markedly elevated in the kidneys of male and female PCK rats and PKD2−/WS25 mice compared to those in wild-type animals (Figure 4A and B). The ratio of cGMP to cAMP was significantly higher in the kidneys of PCK rats than in those of wild-type rats. Cyclic GMP and cAMP were increased to the same extent in PKD2−/WS25 mice compared to wild-type mice.
Figure 4.
A) Cyclic GMP and cAMP levels and cGMP/cAMP ratios in the kidneys of PCK (male, n=11; female, n=11) and wild-type (male, n=11; female, n=12) rats. B) Cyclic GMP and cAMP levels and cGMP/cAMP ratios in the kidneys of Pkd2WS25/− (male, n=10; female, n=10) and wild-type (male, n=10; female, n=10) mice. C) Cyclic GMP levels in the kidneys of male PCK (n=10) and male and female Pkd2WS25/− (n=10) mice and OPC-31260 treated male PCK (n=7) and and male and female Pkd2WS25/− (n=11) mice. D) Cyclic GMP levels in the kidneys of wild-type (male, n=10; female, n=10), PCK (male, n=10; female, n=10) and PCK/Brattleboro rats (male=10; female=10). E) Cyclic GMP levels in the kidneys of PCK/Brattleboro rats (male, n=4; female, n=4) and their response to the administration of the V2 receptor agonist DDAVP (male, n=4; female, n=4).
Effect of cGMP on cystogenesis in hydrated collagen gels
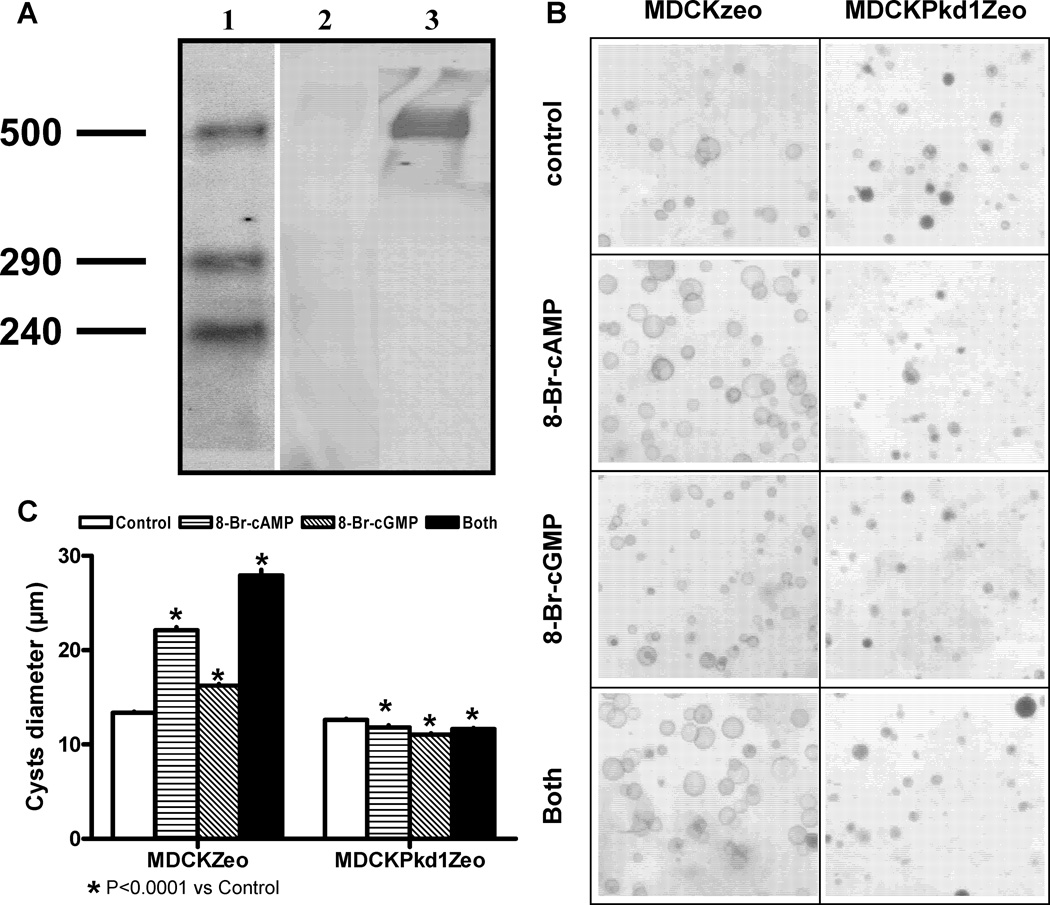
To determine whether the increase in renal cGMP levels may contribute to cystogenesis directly or indirectly, we have studied the effects of 8-Br-cAMP and 8-Br-cGMP on an in vitro system of cystogenesis utilizing a cell line that does not express PC1 at a protein level, MDCKZeo, and its control transfected with full-length human PKD1, MDCKPKD1Zeo (Figure 5A).28 Whereas 8-Br-cAMP and to a lesser extent 8-Br-cGMP stimulated the growth of cysts derived from MDCKZeo cells, they had the opposite effect on cysts derived from MDCKPKD1Zeo (Figure 5B and C). 8-Br-cAMP and 8-Br-cGMP had an additive effect on the growth of MDCKZeo cysts. These results suggest a direct effect of cGMP on cyst growth.
Figure 5.
A) Western analysis of PC1 detected with the mAb 7e12 in membrane fractions from MDCKZeo (lane 2) and MDCKPKD1Zeo (lane 3); lane 1 shows the M.W. standards. B) Development of cysts in collagen gels by “cystic” MDCKZeo and MDCKPKD1Zeo cell lines in medium containing 5% FBS in the absence or presence of 100 µM 8-Br-cAMP, 8-Br-cGMP or both. C) Effect of 100 µM 8-Br-cAMP, 8-Br-cGMP or both on the diameter of MDCKZeo and MDCKPKD1Zeo cysts in collagen gels.
Changes in renal cGMP parallel those in cAMP in response to interventions targeting AVP and the V2R in vivo
AVP V2R antagonists markedly reduce the renal levels of cAMP and inhibit the development of PKD in PCK rats and Pkd2−/WS25 mice13, 14, 20. Genetic elimination of circulating AVP achieved by PCK-Brattleboro crosses has a similar protective effect in the PCK rat. On the other hand, administration of the V2R agonist DDAVP increases the renal levels of cAMP, rescues the cystic phenotype in PCK-Brattleboro rats and aggravates the cystic disease in PCK rats21. To determine whether the renal cGMP levels change in parallel to those of cAMP under these conditions, we measured the cGMP levels in the polycystic kidneys of the animals used in these experiments. The administration of V2R antagonists (Figure 4C) and the elimination of circulating AVP (Figure 4D) significantly reduced the renal levels of cGMP, whereas the administration of DDAVP had the opposite effect (Figure 4E). Although the cGMP levels were lower than those of cAMP, the direction and relative magnitude of the changes were similar.
Upregulation of PKA regulatory subunits in polycystic kidneys
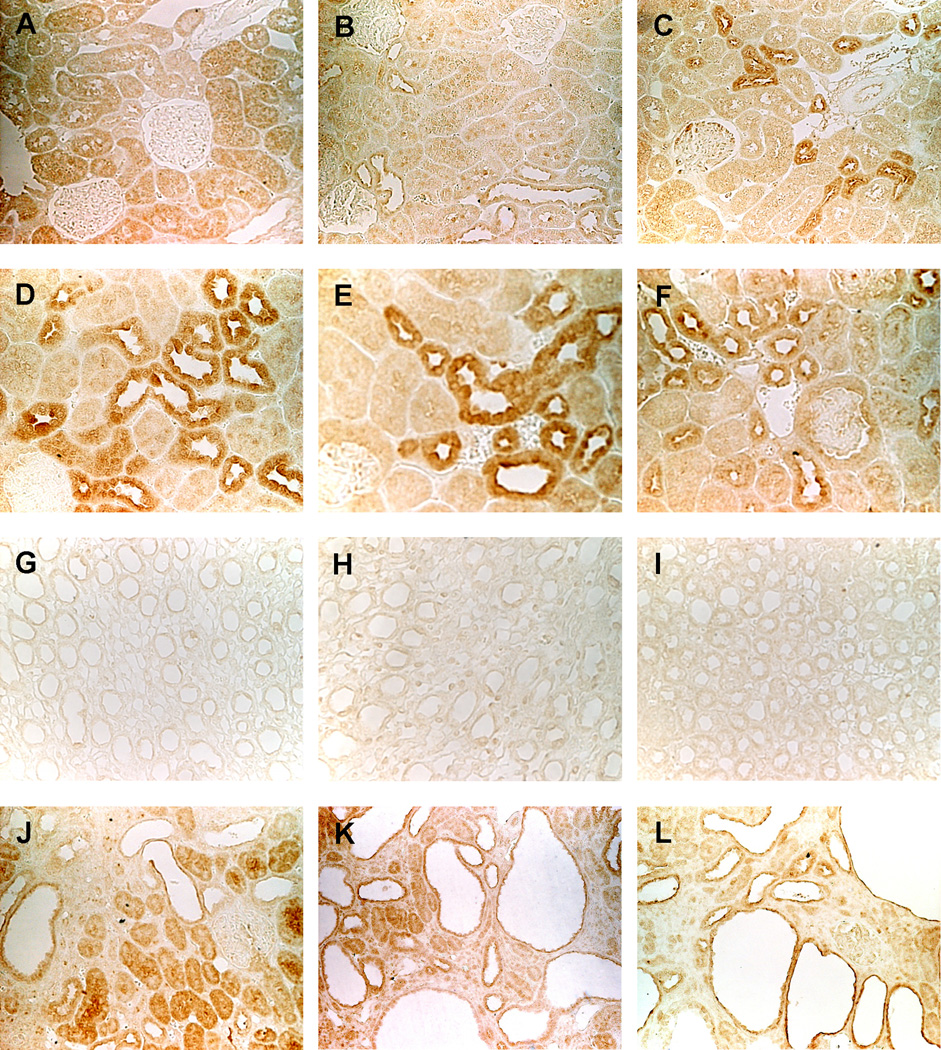
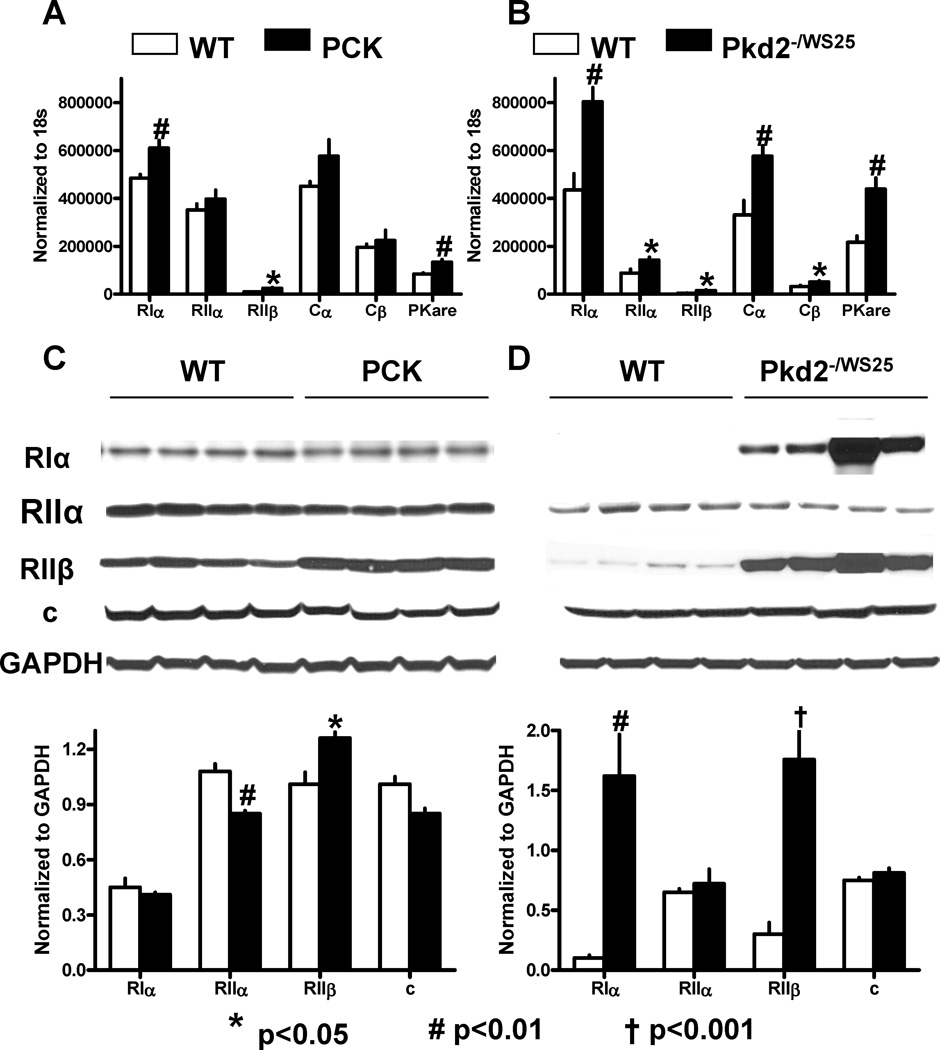
The proliferative response of the cystic epithelium to cAMP in the kidney is mediated by PKA, since it can be completely abrogated by a PKA inhibitor. To examine whether alterations in PKA accompany the development of PKD, we studied the renal expression of PKA regulatory (R) and catalytic (C) subunits in wild-type and polycystic kidneys. RIα and RIIβ mRNAs were significantly upregulated in the PCK rats and Pkd2−/WS25 mice compared to wild-type animals (Figure 6A and B). At the protein level, RIIβ was upregulated in the cystic kidneys of both, rats and mice, whereas RIα was markedly upregulated in mouse, but not ostensibly in rat cystic kidneys (Figure 6C and D). Strong RIα and RIIα immunoreactivities were observed in all tubular segments (particularly in distal convoluted tubules) and collecting ducts (Figure 7). Contrary to the reduced expression of PDE1A, PDE1C, and PDE3A, RIα and RIIα were strongly expressed in cysts, RIIβ immunoreactivity was restricted to stromal structures and vasa recta, and to tubular epithelial cells in the outer medulla, and appeared to be more prominent in cystic than in wild-type animals. PKare mRNA was significantly upregulated in the cystic kidneys of both rats and mice, whereas Cα and Cβ subunits tended to be increased at the mRNA but not at the protein level.
Figure 6.
A) PKA regulatory- and catalytic-subunit mRNA expression measured by RT-PCR in the kidneys of 10 week old male PCK (n=6) and wild-type (n=6) rats. B) PKA regulatory- and catalytic-subunit mRNA expression measured by RT-PCR in the kidneys of 16 week old male Pkd2WS25/− (n=6) and wild-type (n=6) mice. C) Western blots of PKA regulatory and catalytic subunits in the kidneys of 10 week old male PCK (n=4) and wild-type (n=4) rats. D) Western blots of PKA regulatory and catalytic subunits in the kidneys of 16 week old male Pkd2WS25/− (n=4) and wild-type (n=4) mice.
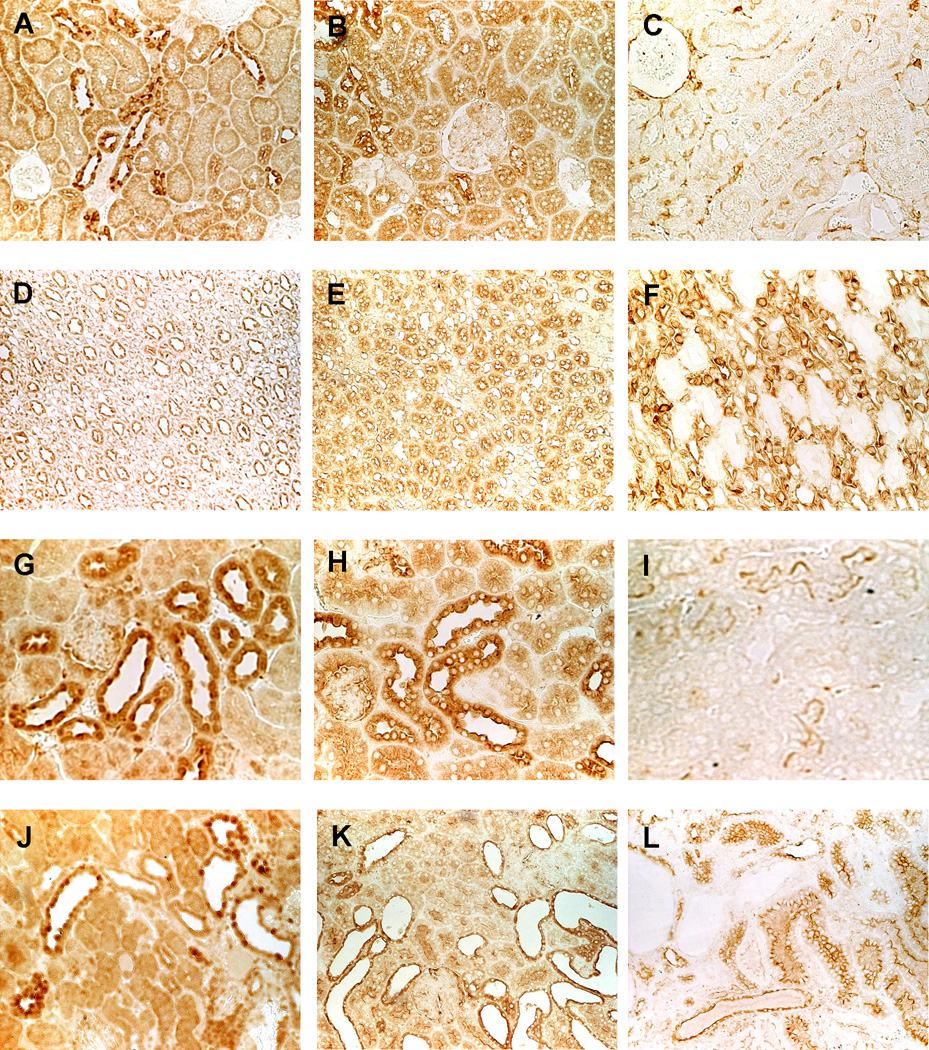
Figure 7.
Immunolocalization of PKA RIα (A, D, G, J), RIIα (B, E, H, K), RIIβ (C, F, I, L) in wild-type rat (A-F, X200) and mouse (G-I, X400) and in PCK rat (J-L, X200) kidneys. The immunostaining pattern in Pkd2WS25/− mouse (not shown) was similar to that of PCK rat kidneys.
Upregulation of CREM and ICER in polycystic kidneys
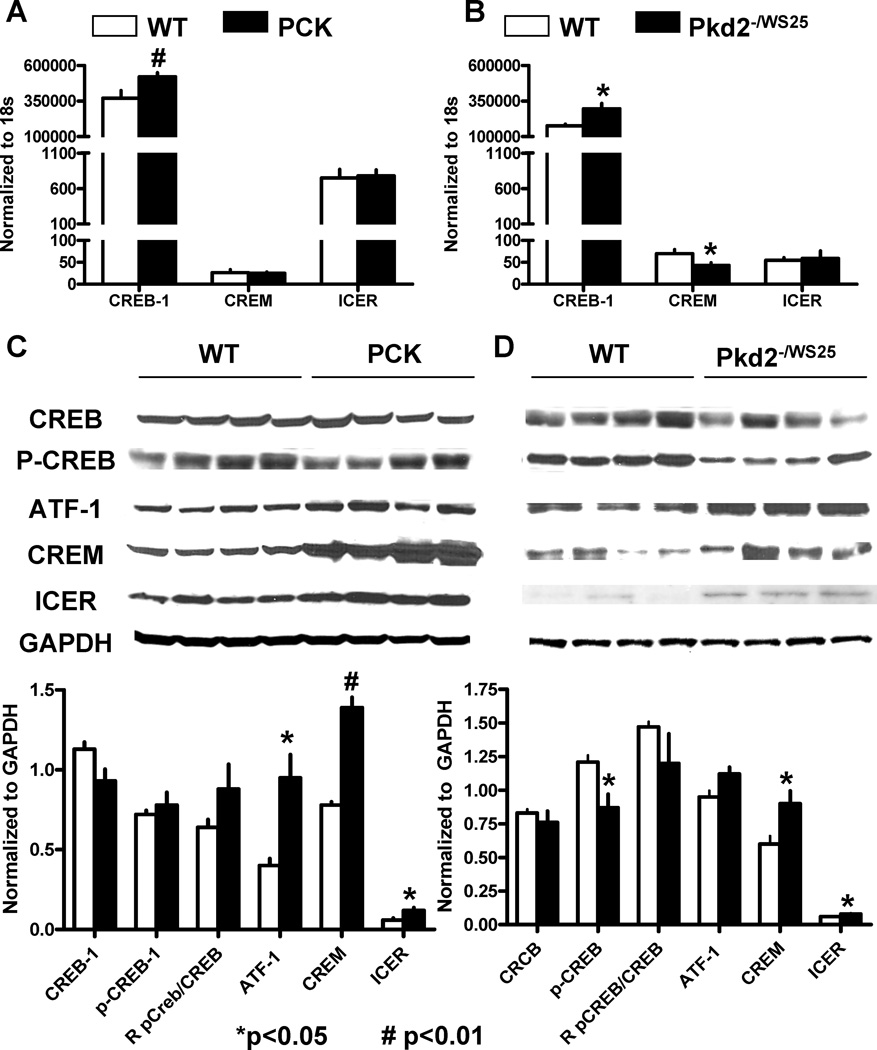
The effects of cAMP on cell proliferation are likely mediated, at least in part, by PKA mediated phosphorylation of transcription factors that bind to cAMP responsive promoter elements (CREs). We examined the expression of CRE binding protein (CREB) and CRE modulator proteins (CREMs) in wild-type and polycystic kidneys. CREB-1 mRNA was significantly increased in the cystic tissues while no consistent difference was detected in the renal level of expression of CREM-1 and ICER mRNAs between wild-type and cystic rats or mice (Figure 8A and B). At the protein level, no consistent differences were detected in the levels of CREB or P-CREB between cystic and wild-type kidneys, while CREM-1, ATF-1, and ICER were significantly increased in the nuclear extracts of cystic compared to wild-type animals (Figure 8C and D). The increased protein expression, without a change in mRNA expression, is consistent with protein stabilization associated with PKA activation, as it has been shown in for ICER in cardiac myocytes.29
Figure 8.
A) CREB-1, CREM-1 and ICER mRNA expression measured by RT-PCR in the kidneys of 10 week old male PCK (n=6) rats and wild-type (n=6) rats. B) CREB-1, CREM-1 and ICER mRNA expression measured by RT-PCR in the kidneys of 16 week old male Pkd2WS25/− (n=6) and wild-type (n=6) mice. C) Western blots of CREB, P-CREB, CREM-1, ATF, and ICER in the kidneys of 10 week old male PCK (n=4) rats and wild-type (n=4) rats. D) Western blots of CREB, P-CREB, CREM-1, ATF, and ICER in the kidneys of 16 week old male Pkd2WS25/− (n=4) and wild-type (n=4) mice.
DISCUSSION
The main observations of this study include: 1) Species specific differences in the expression of cyclic nucleotide PDEs; higher PDE activity in whole kidney lysates from mice compared to those from rats; higher contribution of PDE1, relative to PDE4 and PDE3, to total PDE activity of whole kidney lysate, and lower PDE1, PDE3 and PDE4 activities in murine cystic compared to wild-type kidneys. 2) Reduced levels of several PDE1, PDE3 and PDE4 protein isoforms despite upregulation at mRNA level, possibly due to increased protein catabolism. 3) Increased cGMP levels in polycystic kidneys, suggesting functional in vivo downregulation of phosphodiesterase PDE1, the only calcium dependent PDE active against cGMP and cAMP. 4) Additive stimulatory effect of cAMP and cGMP on cystogenesis in vitro. 5) Upregulation of cAMP-dependent protein kinase (PKA) subunits Iα and IIβ, PKA catalytic subunit PKare, CREB-1 mRNA, and CREM, ATF-1 and ICER proteins in cystic compared to wild-type kidneys.
The superfamily of mammalian PDEs consists of eleven families (PDE1-PDE11) with twenty-one genes and more than 50 isoenzymes.27, 30–32 PDE family members hydrolyze exclusively cAMP (PDE4, PDE7, and PDE8), exclusively cGMP (PDE5, PDE6, and PDE9), or both (PDE1, PDE2, PDE3, PDE10, and PDE11). They are regulated at the genetic level, as well as by phosphorylation, cGMP or cAMP binding, -calmodulin binding, and protein-protein interactions.
Information on PDEs in renal tubules is limited.33, 34, 35 PDE1, PDE3, and PDE4 account for most of the cAMP PDE activity, while PDE1 is responsible for most of the cGMP PDE activity. In mixed cortical tubules and microdissected tubular segments, 50–70% of cAMP PDE activity was found to be inhibitable by an inhibitor of the calcium/calmodulin sensitive PDE1 and PDE activity was higher in renal tubules from mice than in those from rats and rabbits.36 Our results are consistent with both observations. This, along with species specific differences noted in this study, raises the possibility that variation in cyclic nucleotide metabolism could result in variable susceptibility to renal cystic disease.
The PDE1 family consists of three members (PDE1A, PDE1B and PDE1C).37, 38 They are the only PDEs activated by calcium and integrate cyclic nucleotide and calcium signaling pathways. Phosphorylation of PDE1A and PDE1C by PKA and of PDE1B by calcium-calmodulin kinase II reduces their affinity for calmodulin-calcium. PDE1 isoforms are dephosphorylated and reactivated by calcium-calmodulin dependent protein phosphatase (calcineurin; protein phosphatase 2B). Activation of Gs coupled receptors increases the concentration of cAMP (via stimulation of adenylyl cyclase, but also via PKA-mediated inhibition of PDE1 and possibly cGMP mediated inhibition of PDE3, see below) and evokes a physiological response. A subsequent increase in intracellular calcium through calcium influx activates calcineurin, overrides the inhibition of PDE1 by PKA, and reduces intracellular cAMP levels. A reduction in intracellular calcium in PKD would be expected to result in increased cellular levels of cGMP due to PDE1 inhibition and of cAMP due to inhibition of PDE1 and cGMP-induced inhibition of PDE3 (see below). This may account for the increased levels of cAMP and cGMP in polycystic kidneys. Interestingly, administration of the calcineurin inhibitor cyclosporine has been associated with acquired renal cystic disease following renal transplantation and pediatric liver transplantation.39–41
The PDE3 family consists of two members (PDE3A and PDE3B).29–31 PDE3 enzymes are mostly cAMP-specific PDEs, are inhibited by cGMP, and mediate cross talk between cAMP and cGMP pathways. Thus, PDE3 inhibition by increased cGMP levels may contribute to the accumulation of cAMP in polycystic kidneys. In turn, increased accumulation of cAMP may be responsible for the upregulation of PDE3 mRNA expression observed in this study.42
The PDE4 family consists of four subfamilies (PDE4A through D) with approximately 20 isoforms.43 These have tissue and cell type-specific expression. Their subcellular targeting and physiological function are regulated by A-kinase anchoring proteins (AKAPs).44 For example, PDE4d 3/9 isoforms interact with AKAP18 in aquaporin-2 bearing vesicles and regulate PKA activity, aquaporin-2 phosphorylation and shuttling and water permeability in the collecting duct principal cells.45, 46 Despite its importance in the regulation of urinary concentration, the cAMP pool controlled by PDE4 does not appear to be important in the regulation of tubular cell proliferation (see below). Increased expression of certain PDE4 mRNAs in cystic kidneys in this study is consistent with cAMP-driven upregulation of PDE4 isoforms observed in other tissues in other studies.47–49
The subcellular localization of the different PDEs determines the functional compartmentalization of cAMP-mediated responses. Inhibitory and stimulatory effects of cAMP on cell proliferation have been linked to a cAMP pool regulated by PDE3. In mesangial cells, PDE3 and PDE4 inhibitors increase cAMP levels and activate PKA to a similar extent, but only PDE3 inhibitors block phosphorylation of Raf-1 on serine 338 and suppress Raf-1 kinase activity and ERK activation.50, 51 PDE3, but not PDE4 inhibitors, inhibit the proliferation of vascular smooth muscle and endothelial cells.52 Conversely, only PDE3 inhibitors suppress the proliferation of renal tubular epithelial cells following the administration of folic acid, despite the fact that PDE4 is three times more active than PDE3 in suspensions of renal cortical tubules.53 PDE4 inhibitors are more effective than PDE3 inhibitors in elevating intracellular cAMP levels in MDCK cells, but only PDE3 inhibitors stimulate MDCK cell proliferation.54
The proliferative response of the cystic epithelium to cAMP can be completely abrogated by a PKA inhibitor. Other cAMP effectors such as Epac (exchanging protein directly activated by cAMP) and cAMP gated ion channels do not play a significant role in the renal cystic epithelium, although Epac-specific stimulation may contribute to the proliferation of the biliary epithelium in polycystic liver disease.55 PKA is a tetrameric holoenzyme with two R subunits and two C subunits.56–59 There are two major forms of PKA, PKA-I and PKA-II, determined by their different R-subunits (RI and RII). R-subunits are classified into α- and β-forms, resulting in four distinct R-subunits, RIα, RIβ, RIIα, and RIIβ, encoded by separate genes. Three C subunit isoforms have been described (Cα, Cβ, and Cγ). In addition, a human X chromosome-encoded protein kinase X (PRKX)60–62 and its rodent homolog PKare63 have been identified as a novel C-subunit of PKA-I. Binding of two cAMP molecules to each R-subunit releases the C-subunits which are then able to phosphorylate target proteins.
PKA R subunits have different patterns of expression and biological functions. RIα and RIIα are ubiquitously expressed. RIβ is expressed in neuronal tissues. RIIβ is expressed in brain, adipose, and some endocrine tissues. RI subunits are more readily dissociated by cAMP, are involved in the transition from G1 to S phase of the cell cycle and the control of cell proliferation 64,65,63, and are overexpressed in cancer cell lines.66, 67 Early embryonic lethality of RIα null mice68 and lack of a severe phenotype of RIIα, RIIβ, and RIβ null mice point to the importance of the RIα in maintaining the catalytic subunit under cAMP control.56 Heterozygous Prkar1a mice develop tumors arising from cAMP-responsive cells such as Schwann, adrenal and thyroid cells,69, 70 and tissue-specific ablation of Prkar1a from cranial neural crest cells, pituitary cells and cardiac myocytes leads to development of schwannomas, pituitary adenomas71and cardiac myxomas.72
Very few studies have focused on the expression of various PKA subunits in polycystic kidneys. Higher expression of RII and C have been described in epithelial cells cultured from cpk mouse cystic kidneys compared to wild-type kidneys.73 PRKX (human X chromosome-encoded protein kinase), a novel C subunit of PKA-I, is overexpressed in ADPKD kidneys and has been reported to interact with PC1.60–62 Our results show upregulation of RIα mRNA expression in cystic kidneys of rats and mice, although at a protein level RIα expression was increased only in the kidneys of Pkd2−/WS25 mice. Cα, Cβ and PKare (the rodent homolog of PRKX) mRNA were also overexpressed in cystic kidneys.
Cyclic AMP effects on cell proliferation may be due, at least in part, to PKA mediated phosphorylation of transcription factors that bind to cAMP responsive promoter elements (CREs) in the regulatory region of numerous genes.74, 75 These include CREB, CRE modulator proteins (CREMs), and activating transcription factor-1 (ATF-1). The increased expression of CREB-1 mRNA in cystic tissues and the significant elevations of CREM-1, ATF-1, and ICER proteins in the nuclear extracts of cystic compared to wild-type animals in this study is consistent with enhanced PKA activity.
In summary, the results of this study suggest that alterations in cyclic nucleotide catabolism may render the cystic epithelium particularly susceptible to factors acting on Gs coupled receptors, account at least in part for the upregulation of cyclic nucleotide signaling in PKD, and contribute substantially to the progression of this disease.
METHODS
Tissue samples
Tissue samples from PCK and wild-type rats and from Pkd2WS25/− and wild type mice and Pkd2WS25/WS25 mice were obtained as part of previously published studies13, 14, 20,21. At sacrifice, the left kidney was removed under ketamine and xylazine anesthesia, immediately frozen in liquid nitrogen, and ground to fine powder under liquid nitrogen in a stainless steel mortar for determination of cyclic nucleotides and for PCR and western analysis.
Cell lines
MDCKZeo and MDCKPKD1Zeo cell lines have been previously described.28 The MDCKZeo cell line was derived by transfection with pCIZeo of a parental MDCK that has no detectable PC1 expression by western blot analysis. The MDCKPKD1Zeo cell line was derived from the same parental MDCK cell line and stably expresses full-length human PKD1 cDNA.
PDE activity assay
Kidneys were homogenized in ice-cold homogenization buffer containing 50 mM Tris pH 7.5, 0.25M sucrose, 5mM MgCl2, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT, and supplemented with a protease inhibitor tablets (Roche). PDE activities were measured using 1µM cAMP as substrate in buffer containing 50 mM Tris (pH 7.5), 5 mM MgCl2, 4mM 2-mercaptoethanol and 0.1% BSA.76 3H-cAMP was included as a tracer for quantitation. Assays were initiated by the addition of substrate and incubated for 10 min at 30°C. The reaction was stopped by incubation for 3 min in a boiling-water. Crotalus atrox snake venom was then added and after 15-min incubation at 30°C, hydrolyzed nucleotide was separated using high capacity pre-activated ion exchange resin (FabGennix, Frisco, TX). Slurries were mixed thoroughly and left to stand for 15 min on ice before centrifugation at 12,000g for 3min. The radioactivity in 150ul aliquots of the resulting supernatants was determined by liquid scintillation counting. To determine the activity in a sample due to a specific PDE, various activators or inhibitors were included in the assay. The PDE activity in aliquots incubated without calcium was determined as a basal activity. Calmodulin-stimulated PDE1 activity was determined by subtracting the basal activity from the activity in the presence of 2.01 mM CaCl2 and 10µg/ml calmodulin. PDE3 and PDE4 activities were determined as cAMP-PDE inhibitable by 10uM cilostamide or rolipram, respectively. Hydrolysis of cAMP was linearly proportional to incubation time and enzyme protein. Specific activities were defined as pmoles of cAMP hydrolyzed/minute/mg protein.
Quantitative Immunoblotting and Immunohistochemistry
Whole tissue lysates were heated in a sample buffer, electrophoresed on SDS-polyacrylamide gel, and transferred to PVDF membrane. After blocking at room temperature for 1h and incubating with primary antibody overnight at 4°C, membranes were washed and incubated with secondary antibody for 1h at room temperature. Detection was performed using enhanced ECL (Pierce Chemical Co). Four micron transverse, paraffin embedded tissue sections, including cortex, medulla and papilla were used for immunohistochemical staining as described.14 PDE antibodies used were PDE1A(ab14599, Abcam,Cambrige,MA); PDE1C(ab14602, Abcam); PDE3A(sc20792, Santa Cruz, CA); PDE3B(sc20793, Santa CRUZ); PDE4A(ab14607, Abcam); PDE4B(ab14611, Abcam); PDE4D(ab14613, Abcam). PKA antibodies used were: PKARIα(610609, BD Biosciences, San Jose, CA); PKARIIα(612242, BD Biosciences); PKARIIβ(610625, BD Biosciences); PKAC(610980, BD Biosciences). CREB and phospho-CREB were purchased from Cell Signaling (9190, cell signaling, Beverly MA). CREB, ICER and ATF-1 were detected by polyclonal antibody CREM-1(sc440, Santa Cruz). PC1 was detected with the monoclonal antibody 7e12.
Real-time polymerase chain reaction
PDE isoforms, PKA R and C subunits, CREB-1, CREM-1 and ICER mRNAs were quantified using real-time PCR. Total RNA was extracted from kidneys using an RNeasy plus mini kit (Qiagen, Valencia, CA). First-strand cDNA synthesis was performed using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA). Accumulation of PCR products was measured in real-time by using SYBR Green ER qPCR Supermix Universal Kit (Invitrogen). The sequences of primers are listed in Supplementary Table 1. Reaction was performed in ABI7900 (Applied Biosystems), starting with 10 min of preincubation at 95°C followed by 40 amplification cycles. 18S was used as housekeeping gene for arbitrary unit calculation for every tested gene. All PCR products were confirmed by sequencing.
Cyclic AMP and GMP content of whole kidneys
Tissues were homogenized in 10 volumes of cold 5% TCA in a glass-Teflon tissue grinder. After centrifugation at 600g for 10 min, the supernatants were extracted with 3 volumes of water-saturated ether. After drying the aqueous extracts, reconstituted samples were processed without acetylation using enzyme immunoassay kits (Sigma-Aldrich, Inc., St. Louis MO). The results were expressed as pmol/mg of protein.
3D Culture in Collagen Gel
Cell lines were maintained as monolayer cultures in medium containing a 1:1 mixture of Dulbecco’s Modified Eagle’s Medium/Ham’s F12 nutrient medium (DMEM/F12) containing 10% (vol/vol) fetal bovine serum (FBS; Invitrogen). After trypsinization and removal of trypsin, 4,000 MDCKPKD1Zeo cells or 2,000 MDCKZeo cells were suspended in ice-cold Minimum Essential Medium (MEM) containing 2.9mg/ml collagen type I (BD Biosciences), 10mM HEPES, 27mM NaHCO3 and deposited in 24-well plates (0.4 ml per well). The temperature was raised to 37°C to cause gelation and trapping of the cells within the matrix that attaches to the bottom of the well. DME/F12 medium (1.5 mL) supplemented with 5% FBS, 100 U/ml penicillin G and 0.1 mg/ml streptomycin was added above the gelled medium. The plates were kept in a cell incubator in a humidified atmosphere of 5% CO2 in room air at 37°C. The medium was replaced three times a week and the cells are inspected by light microscopy and photographed at regular intervals. The number and diameters of the cysts in each well were measured using MetaMorph software (Universal Imaging, West Chester, PA). Results were expressed as absolute number of cysts and average cyst volumes.
Statistical Methods
Data were expressed as means ± SD. Comparisons between the groups were performed by t-test.
Supplementary Material
PDE2 and PDE5 through PDE11 mRNA expression in the kidneys of PCK rats, Pkd2WS25/− mice and wild-type rats and mice.
ACKNOWLEDGEMENTS
This work was supported by grants from the PKD Foundation and by the National Institutes of Health grant DK44863 (V.E.T.). We thank Dr. Gregory Germino, Johns Hopkins University School of Medicine, Baltimore, Maryland, for the generous gift of MDCKZeo and MDCKPKD1Zeo cell lines.
Footnotes
DISCLOSURE
The authors have no competing interests.
REFERENCES
- 1.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Grantham JJ. Cystic Diseases of the Kidney IN 8th Edition, Chapter 41. Bermedica: Brenner & Rector; 2007. [Google Scholar]
- 3.Somlo S, Torres VE, Caplan MJ. Autosomal Dominant Polycystic Kidney Disease and Inherited Cystic Disease. In: Alpern RJ, Hebert SC, editors. The Kidney. Fourth Edition edn. 2007. pp. 2283–2313. Chapter 81. [Google Scholar]
- 4.Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics. 2003;111:1072–1080. doi: 10.1542/peds.111.5.1072. [DOI] [PubMed] [Google Scholar]
- 5.Adeva M, El-Youssef M, Rossetti S, et al. Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD) Medicine (Baltimore) 2006;85:1–21. doi: 10.1097/01.md.0000200165.90373.9a. [DOI] [PubMed] [Google Scholar]
- 6.Gunay-Aygun M, Avner ED, Bacallao RL, et al. Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis: Summary statement of a First National Institutes of Health/Office of Rare Diseases conference. J Pediatr. 2006;149:159–164. doi: 10.1016/j.jpeds.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grantham JJ. Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: a systems biology approach. Kidney Int. 2003;64:1157–1162. doi: 10.1046/j.1523-1755.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi T, Nagao S, Wallace DP, et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanaoka K, Guggino W. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11:1179–1187. doi: 10.1681/ASN.V1171179. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi T, Wallace DP, Magenheimer BS, et al. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem. 2004:40419–40430. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Hempson SJ, Reif GA, et al. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006;17:178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi T, Nagao S, Kasahara M, et al. Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am J Kidney Dis. 1997;30:703–709. doi: 10.1016/s0272-6386(97)90496-0. [DOI] [PubMed] [Google Scholar]
- 13.Gattone VH, Wang X, Harris PC, et al. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nature Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 14.Torres VE, Wang X, Qian Q, et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nature Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 15.Kip SN, Hunter LW, Ren Q, et al. [Ca2+]i reduction increases cellular proliferation and apoptosis in vascular smooth muscle cells: Relevance to the ADPKD phenotype. Circ Res. 2005;96:873–880. doi: 10.1161/01.RES.0000163278.68142.8a. [DOI] [PubMed] [Google Scholar]
- 16.Smith LA, Bukanov NO, Husson H, et al. Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol. 2006;17:2821–2831. doi: 10.1681/ASN.2006020136. [DOI] [PubMed] [Google Scholar]
- 17.Banizs B, Pike MM, Millican CL, et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. doi: 10.1242/dev.02153. [DOI] [PubMed] [Google Scholar]
- 18.Masyuk TV, Masyuk AI, Torres VE, et al. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5'-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Starremans PG, Li X, Finnerty PE, et al. A mouse model for polycystic kidney disease through a somatic in-frame deletion in the 5' end of Pkd1. Kidney Int. 2008;73:1394–1405. doi: 10.1038/ki.2008.111. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Gattone VH, Somlo S, et al. Effectiveness of OPC-41061 on polycystic kidney disease development in Pkd2WS25/−. J Am Soc Nephrol. 2005;16:361A. doi: 10.1681/ASN.2004121090. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Wu Y, Ward CJ, et al. Vasopressin directly regulates cyst growth in the PCK rat. J Am Soc Nephrol. 2008;19:102–108. doi: 10.1681/ASN.2007060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nature Clin Prac Nephro. 2006;2:40–54. doi: 10.1038/ncpneph0070. [DOI] [PubMed] [Google Scholar]
- 23.Tradtrantip L, Yangthara B, Padmawar P, et al. Thiophenecarboxylate suppressor of cyclic nucleotides discovered in a small-molecule screen blocks toxin-induced intestinal fluid secretion. Mol Pharmacol. 2009;75:134–142. doi: 10.1124/mol.108.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada S, Misaka T, Tanaka Y, et al. Aquaporin-11 knockout mice and polycystic kidney disease animals share a common mechanism of cyst formation. FASEB J. 2008;22:3672–3684. doi: 10.1096/fj.08-111872. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Wolda SL, Frazier AL, et al. Identification and characterisation of a human calmodulin-stimulated phosphodiesterase PDE1B1. Cell Signal. 1997;9:519–529. doi: 10.1016/s0898-6568(97)00046-6. [DOI] [PubMed] [Google Scholar]
- 26.Polli JW, Kincaid RL. Molecular cloning of DNA encoding a calmodulin-dependent phosphodiesterase enriched in striatum. Proc Natl Acad Sci U S A. 1992;89:11079–11083. doi: 10.1073/pnas.89.22.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 28.Boletta A, Qian F, Onuchic LF, et al. Polycystin-1, the gene product of PKD1, induces resistance to apoptosis and spontaneous tubulogenesis in MDCK cells. Molecular cell. 2000;6:1267–1273. doi: 10.1016/s1097-2765(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 29.Ding B, Abe J, Wei H, et al. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 32.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 33.Dousa TP. Cyclic-3',5'-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 1999;55:29–62. doi: 10.1046/j.1523-1755.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamaki M, McIntyre S, Rassier ME, et al. Cyclic 3',5'-nucleotide diesterases in dynamics of cAMP and cGMP in rat collecting duct cells. Am J Physiol. 1992;262:F957–F964. doi: 10.1152/ajprenal.1992.262.6.F957. [DOI] [PubMed] [Google Scholar]
- 35.Torres VE, Northrup TE, Edwards RM, et al. Modulation of cyclic nucleotides in islated rat glomeruli: role of histamine, carbamylcholine, parathyroid hormone, and angiotensin-II. J Clin Invest. 1978;62:1334–1343. doi: 10.1172/JCI109254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusano E, Yoshida I, Takeda S, et al. Nephron distribution of total low Km cyclic AMP phosphodiesterase in mouse, rat and rabbit kidney. Tohoku J Exp Med. 2001;193:207–220. doi: 10.1620/tjem.193.207. [DOI] [PubMed] [Google Scholar]
- 37.Goraya TA, Cooper DM. Ca2+-calmodulin-dependent phosphodiesterase (PDE1): current perspectives. Cell Signal. 2005;17:789–797. doi: 10.1016/j.cellsig.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Kakkar R, Raju RV, Sharma RK. Calmodulin-dependent cyclic nucleotide phosphodiesterase (PDE1) Cell Mol Life Sci. 1999;55:1164–1186. doi: 10.1007/s000180050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calvo-Garcia MA, Campbell KM, O'Hara SM, et al. Acquired renal cysts after pediatric liver transplantation: association with cyclosporine and renal dysfunction. Pediatr Transplant. 2008;12:666–671. doi: 10.1111/j.1399-3046.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- 40.Franchi-Abella S, Mourier O, Pariente D, et al. Acquired renal cystic disease after liver transplantation in children. Transplant Proc. 2007;39:2601–2602. doi: 10.1016/j.transproceed.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Lien YH, Hunt KR, Siskind MS, et al. Association of cyclosporin A with acquired cystic kidney disease of the native kidneys in renal transplant recipients. Kidney Int. 1993;44:613–616. doi: 10.1038/ki.1993.288. [DOI] [PubMed] [Google Scholar]
- 42.Liu H, Maurice DH. Expression of cyclic GMP-inhibited phosphodiesterases 3A and 3B (PDE3A and PDE3B) in rat tissues: differential subcellular localization and regulated expression by cyclic AMP. Br J Pharmacol. 1998;125:1501–1510. doi: 10.1038/sj.bjp.0702227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100:950–966. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 44.Baillie GS, Scott JD, Houslay MD. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett. 2005;579:3264–3270. doi: 10.1016/j.febslet.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 45.McSorley T, Stefan E, Henn V, et al. Spatial organisation of AKAP18 and PDE4 isoforms in renal collecting duct principal cells. Eur J Cell Biol. 2006;85:673–678. doi: 10.1016/j.ejcb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Stefan E, Wiesner B, Baillie GS, et al. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol. 2007;18:199–212. doi: 10.1681/ASN.2006020132. [DOI] [PubMed] [Google Scholar]
- 47.Le Jeune IR, Shepherd M, Van Heeke G, et al. Cyclic AMP-dependent transcriptional up-regulation of phosphodiesterase 4D5 in human airway smooth muscle cells. Identification and characterization of a novel PDE4D5 promoter. J Biol Chem. 2002;277:35980–35989. doi: 10.1074/jbc.M204832200. [DOI] [PubMed] [Google Scholar]
- 48.Rena G, Begg F, Ross A, et al. Molecular cloning, genomic positioning, promoter identification, and characterization of the novel cyclic amp-specific phosphodiesterase PDE4A10. Mol Pharmacol. 2001;59:996–1011. doi: 10.1124/mol.59.5.996. [DOI] [PubMed] [Google Scholar]
- 49.Vicini E, Conti M. Characterization of an intronic promoter of a cyclic adenosine 3',5'-monophosphate (cAMP)-specific phosphodiesterase gene that confers hormone and cAMP inducibility. Mol Endocrinol. 1997;11:839–850. doi: 10.1210/mend.11.7.9941. [DOI] [PubMed] [Google Scholar]
- 50.Cheng J, Thompson MA, Walker HJ, et al. Differential regulation of mesangial cell mitogenesis by cAMP phosphodiesterase isozymes 3 and 4. Am J Physiol Renal Physiol. 2004;287:F940–F953. doi: 10.1152/ajprenal.00079.2004. [DOI] [PubMed] [Google Scholar]
- 51.Chini CC, Grande JP, Chini EN, et al. Compartmentalization of cAMP signaling in mesangial cells by phosphodiesterase isozymes PDE3 and PDE4. Regulation of superoxidation and mitogenesis. J Biol Chem. 1997;272:9854–9859. doi: 10.1074/jbc.272.15.9854. [DOI] [PubMed] [Google Scholar]
- 52.Osinski MT, Schror K. Inhibition of platelet-derived growth factor-induced mitogenesis by phosphodiesterase 3 inhibitors: role of protein kinase A in vascular smooth muscle cell mitogenesis. Biochem Pharmacol. 2000;60:381–387. doi: 10.1016/s0006-2952(00)00328-2. [DOI] [PubMed] [Google Scholar]
- 53.Matousovic K, Tsuboi Y, Walker H, et al. Inhibitors of cyclic nucleotide phosphodiesterase isozymes block renal tubular cell proliferation induced by folic acid. J Lab Clin Med. 1997;130:487–495. doi: 10.1016/s0022-2143(97)90125-6. [DOI] [PubMed] [Google Scholar]
- 54.Cheng J, Thompson MA, Walker HJ, et al. Lixazinone stimulates mitogenesis of Madin-Darby canine kidney cells. Exp Biol Med (Maywood) 2006;231:288–295. doi: 10.1177/153537020623100308. [DOI] [PubMed] [Google Scholar]
- 55.Banales JM, Masyuk TV, Gradilone SA, et al. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bossis I, Stratakis CA. Minireview: PRKAR1A: normal and abnormal functions. Endocrinology. 2004;145:5452–5458. doi: 10.1210/en.2004-0900. [DOI] [PubMed] [Google Scholar]
- 57.Griffioen G, Thevelein JM. Molecular mechanisms controlling the localisation of protein kinase A. Curr Genet. 2002;41:199–207. doi: 10.1007/s00294-002-0308-9. [DOI] [PubMed] [Google Scholar]
- 58.Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression,regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–D693. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- 59.Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Burrow CR, Polgar K, et al. Protein kinase X (PRKX) can rescue the effects of polycystic kidney disease-1 gene (PKD1) deficiency. Biochim Biophys Acta. 2008;1782:1–9. doi: 10.1016/j.bbadis.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Li X, Li HP, Amsler K, et al. PRKX, a phylogenetically and functionally distinct cAMP-dependent protein kinase, activates renal epithelial cell migration and morphogenesis. Proc Natl Acad Sci U S A. 2002;99:9260–9265. doi: 10.1073/pnas.132051799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimmermann B, Chiorini JA, Ma Y, et al. PrKX is a novel catalytic subunit of the cAMP-dependent protein kinase regulated by the regulatory subunit type I. J Biol Chem. 1999;274:5370–5378. doi: 10.1074/jbc.274.9.5370. [DOI] [PubMed] [Google Scholar]
- 63.Blaschke RJ, Monaghan AP, Bock D, et al. A novel murine PKA-related protein kinase involved in neuronal differentiation. Genomics. 2000;64:187–194. doi: 10.1006/geno.2000.6116. [DOI] [PubMed] [Google Scholar]
- 64.Burton KA, Treash-Osio B, Muller CH, et al. Deletion of type IIalpha regulatory subunit delocalizes protein kinase A in mouse sperm without affecting motility or fertilization. J Biol Chem. 1999;274:24131–24136. doi: 10.1074/jbc.274.34.24131. [DOI] [PubMed] [Google Scholar]
- 65.Malmberg AB, Brandon EP, Idzerda RL, et al. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. J Neurosci. 1997;17:7462–7470. doi: 10.1523/JNEUROSCI.17-19-07462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho-Chung YS, Nesterova MV. Tumor reversion: protein kinase A isozyme switching. Ann N Y Acad Sci. 2005;1058:76–86. doi: 10.1196/annals.1359.014. [DOI] [PubMed] [Google Scholar]
- 67.Vincent-Dejean C, Cazabat L, Groussin L, et al. Identification of a clinically homogenous subgroup of benign cortisol-secreting adrenocortical tumors characterized by alterations of the protein kinase A (PKA) subunits and high PKA activity. Eur J Endocrinol. 2008;158:829–839. doi: 10.1530/EJE-07-0819. [DOI] [PubMed] [Google Scholar]
- 68.Amieux PS, Cummings DE, Motamed K, et al. Compensatory regulation of RIalpha protein levels in protein kinase A mutant mice. J Biol Chem. 1997;272:3993–3998. doi: 10.1074/jbc.272.7.3993. [DOI] [PubMed] [Google Scholar]
- 69.Kirschner LS, Kusewitt DF, Matyakhina L, et al. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–4514. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- 70.Pavel E, Nadella K, Towns WH, 2nd, et al. Mutation of Prkar1a causes osteoblast neoplasia driven by dysregulation of protein kinase A. Mol Endocrinol. 2008;22:430–440. doi: 10.1210/me.2007-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yin Z, Williams-Simons L, Parlow AF, et al. Pituitary-specific knockout of the Carney complex gene Prkar1a leads to pituitary tumorigenesis. Mol Endocrinol. 2008;22:380–387. doi: 10.1210/me.2006-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin Z, Jones GN, Towns WH, 2nd, et al. Heart-specific ablation of Prkar1a causes failure of heart development and myxomagenesis. Circulation. 2008;117:1414–1422. doi: 10.1161/CIRCULATIONAHA.107.759233. [DOI] [PubMed] [Google Scholar]
- 73.Marfella-Scivittaro C, Quinones A, Orellana SA. cAMP-dependent protein kinase and proliferation differ in normal and polycystic kidney epithelia. Am J Physiol Cell Physiol. 2002;282:C693–C707. doi: 10.1152/ajpcell.00122.2001. [DOI] [PubMed] [Google Scholar]
- 74.Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20:460–466. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Zambon AC, Zhang L, Minovitsky S, et al. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc Natl Acad Sci U S A. 2005;102:8561–8566. doi: 10.1073/pnas.0503363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deng C, Wang D, Bugaj-Gaweda B, et al. Assays for cyclic nucleotide-specific phosphodiesterases (PDEs) in the central nervous system (PDE1, PDE2, PDE4, and PDE10) Current Protocols in Neuroscience. 2007:21. doi: 10.1002/0471142301.ns0721s38. Chapter 7: Unit 7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDE2 and PDE5 through PDE11 mRNA expression in the kidneys of PCK rats, Pkd2WS25/− mice and wild-type rats and mice.