Abstract
Matrix metalloproteinases (MMPs) are enzymes that regulate extracellular matrix composition and contribute to cell migration. Microarray studies in mouse placenta suggested that MMP-9 transcript abundance was dependent on distal-less 3 (Dlx3), a placental-specific transcriptional regulator; however, it was not clear if this was a direct or indirect effect. Here we investigate mechanism(s) for Dlx3-dependent MMP-9 gene transcription and gelatinase activity in placental trophoblasts. Initial studies confirmed that MMP-9 activity was reduced in placental explants from Dlx3−/− mice and that murine MMP-9 promoter activity was induced by Dlx3 overexpression. Two binding sites within a murine MMP-9 promoter fragment bound Dlx3, and mutations in both elements reduced basal MMP-9-luciferase reporter activity and abolished regulation by Dlx3. Chromatin immunoprecipitation studies in JEG3 cells confirmed Dlx3 binding to the endogenous human MMP-9 promoter at three distinct sites and knockdown of human Dlx3 resulted in reduced endogenous MMP-9 transcripts and secreted activity. These studies provide novel evidence that Dlx3 is involved directly in the transcriptional regulation of mouse and human MMP-9 gene expression in placental trophoblasts.
Keywords: distal-less 3, matrix metalloproteinase, placental trophoblast, mouse, human
during pregnancy, the placenta forms the critical fetal-maternal interface to support fetal nutrient supply and exchange of gases and waste products that underlie the coordinated growth and development of the mammalian fetus in utero. In the mouse, the primary site of exchange occurs within the placental labyrinth. The labyrinth layer is formed by distinct interdigitations between fetal blood vessels and maternal blood spaces that are paramount for nutrient exchange to meet the metabolic demands of the growing fetus (43). Accurate morphogenesis and function of the labyrinth compartment depend on a number of dynamic cellular processes such as controlled proliferation, differentiation, cell migration, and (re)organization of the extracellular matrix (ECM) environment. Any genetic or environmental insult(s) that interferes with these dynamic processes can potentially disrupt the morphogenesis of the labyrinth and result in placental insufficiency, intrauterine growth restriction of the fetus, and poor fetal outcomes.
Placental cell migration is critical for normal placental morphogenesis. For example, cytotrophoblasts migrate through the ECM of the maternal decidua and engage in remodeling of the maternal spiral arteries (12, 38). This vascular remodeling leads to enlargement of vessel diameter, decreased vascular resistance, and sustained blood flow into the maternal blood space and ultimately appropriate perfusion of the placental vascular bed (9). Inadequate maternal spiral artery remodeling has been detected in the placentas of women with fetal intrauterine growth restriction and/or symptoms consistent with preeclampsia (PE; Refs. 19, 29, 37). These observations suggest that the dynamic process of maternal spiral artery remodeling is required for successful placental perfusion and a productive pregnancy (33). Matrix metalloproteinases (MMPs) appear to play a key role in ECM remodeling at several levels in the establishment of the feto-placental unit. For example, MMP-1 and 9 have been found in normal human decidual samples collected as biopsies from term pregnancies and MMP-1 specifically was demonstrated to be reduced in deciduas collected from patients with PE (9). Campbell et al. (5) used a bilayer coculture model to examine the effect of decidual endothelial cells on MMP secretion and cytotrophoblast migration from PE pregnancies. These studies provide evidence that cytotrophoblasts from PE pregnancies secreted significantly less MMP-2 and MMP-9 compared with controls. These observations were supported by other studies demonstrating decreased MMP-9 mRNA expression (24, 40) and histological staining (44, 49) in human preeclamptic placentas. Collectively, these results suggest that abnormal trophoblast invasion of the maternal vasculature in at-risk pregnancies may be due in part to misregulation of specific MMPs, including MMP-9; however, these observations remain somewhat controversial since others have been unable to identify a specific role for MMPs during trophoblast invasion (16, 26, 34). While difficult to reconcile, these studies may collectively support the conclusion that the MMP-9 gene profile may be misregulated in at least a subset of patients experiencing at-risk pregnancies potentially reflecting an abnormal trophoblast invasive phenotype. Examination of the regulation of MMPs in the context of the developing placenta and in trophoblasts is clearly warranted.
Dlx3 is homeodomain-containing transcription factor and is necessary for the development of numerous tissues including the mouse placenta (3, 8, 30, 31). Targeted deletion of Dlx3 results in midgestation embryonic lethality due to placental labyrinth defects, suggesting the Dlx3 and Dlx3-dependent gene targets play a critical role in feto-placenta development (30, 43). Microarray analysis of Dlx3 null placentas collected at embryonic day (E) 9.5 linked Dlx3 to aberrant expression of a number of genes critical to placental function, including reduced expression of MMP-9 (11). The present studies examined the hypothesis that Dlx3 may regulate MMP-9 directly at the transcriptional level in placental cells. Data presented here demonstrate that Dlx3 is a key regulator of MMP-9 transcript levels and secreted MMP-9 catalytic activity in mouse placental explants and human choriocarcinoma cells. Dlx3-dependent regulation of the MMP-9 gene promoter occurred through near consensus cis-regulatory elements within the mouse and human MMP-9 gene promoters, suggesting the effects of Dlx3 on the MMP-9 gene promoter were direct. These studies support the conclusion that Dlx3 is a critical transcriptional regulator of mouse and human MMP-9 gene expression in placental trophoblasts.
MATERIALS AND METHODS
Animals.
Dlx3−/− mice were a generous gift from the laboratory of Dr. Maria Morasso at the National Institutes of Health. All mice used in these studies were maintained and used in full compliance with protocols approved by the Cornell University Institutional Animal Care and Use Committee. All animals had free access to food and water; qualified Cornell University laboratory animal veterinarians were responsible for all animal care.
Culture of placental explants, JEG3 cells, and gel zymography.
Mouse placental disk explants were harvested at E9.5 from Dlx3+/− females that were mated with Dlx3+/− males. At the time of dissection, embryos were dissected free of uterine and placental tissues and used for genotyping as described previously (4, 11). Placental explants (essentially the placental disk with minimal maternal decidua; n = 4–6 explants/genotype) were washed three times in Dulbecco's phosphate-buffered saline (DPBS) and then cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with fetal bovine serum (10%) at 37°C in 5% CO2 as previously described (4, 11). Following a 2-h equilibration period, media were removed and replaced with fresh media. Media were collected 4 h later (6 h following the start of culture), and all media samples were snap frozen and stored at −80°C until zymography studies could be completed. In some studies using small interference (si)RNA-mediate Dlx3 knockdown (described below), stably transfected JEG3 cells were split to new culture dishes and cultured to 50–80% confluence. Cells were washed with DPBS and placed in fresh media. Media were collected from these cultures in a manner identical to the placental explants, and media samples were again stored at −80°C until subsequent assays.
Zymography studies were carried out as previously described (13, 14). Briefly, equal volumes of conditioned culture media from the placental explant study and the siRNA knockdown cell lines were resolved in 10% gelatin Zymogram gels (Bio-Rad Laboratories, Hercules, CA). Following electrophoresis, proteins within these gels were allowed to renature in 2.5% Triton X-100 for 30 min at room temperature and incubated in developing buffer (Millipore, Billerica, MA), containing 50m M Tris·HCl, 0.2 M NaCl, 5 mM CaCl2, and 0.02% Brij35, for 30 min at room temperature and then placed in fresh developing buffer overnight at 37°C. The gels were then stained with 0.5% Coomassie Brilliant Blue (Sigma, St. Louis, MO) in methanol/acetic acid/water (40:10:50 vol/vol) for 30 min at room temperature followed by destaining with methanol/acetic acid/water (50:10:40) for 4 h at room temperature. The presence of clear bands in the gels at 92 and 105 kDa reflected the gelatinase activity of human MMP-9 and mouse MMP-9, respectively. Bands were visualized using Bio-Rad Gel Imaging system, and gelatinase activity was quantified using densitometry analysis.
Quantitative PCR.
siRNA knockdown cell lines were grown to ∼80% confluence, and RNA was harvested using TRIzol (Invitrogen, Carlsbad, CA). RNA was subjected to quantitative PCR using TaqMan probes (Applied BioSystems, Foster City, CA) for MMP-9 and Dlx3, respectively. Analysis of the quantitative PCR data has been previously described (11).
Western blotting analysis.
Conditioned media from placental explants were suspended in SDS-PAGE loading buffer (100 mM Tris pH 6.8, 4% SDS, 20% glycerol, and 200 mM DTT), boiled for 3 min and incubated on ice for 5 min. Complexes were resolved by SDS-PAGE and transferred to polyvinylidine difluoride membranes by electroblotting. Membranes were blocked in 2× casein (Vector Laboratories, Burlingame, CA) in Tris-buffered saline (10 mM Tris pH 7.6 and 150 mM sodium chloride) containing 0.1% Tween 20 (TBST) for 1 h at room temperature. The following antibodies were used at a 1:1,000 dilution in Western blotting: MMP-9 (Abcam, Cambridge, UK), thrombospondin 2 (BD Transduction Laboratories, San Jose, CA), and Dlx3 antibody (4). Actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:500 dilution. All protein bands were visualized by chemiluminesence reagents. All Western blotting studies were carried out on at least three separate occasions with similar results.
Plasmids and cDNA.
All plasmids used in these studies were purified using two cycles through cesium chloride. A 1.9-kb portion of the mouse MMP-9 promoter was obtained by PCR using mouse genomic DNA and subsequently cloned into a luciferase reporter vector using the following primers: 5′-ggtaccgtcagagcattcattgtagaag-3′and 5′-ggatccgagattttaaagaggcagtaaa-3′. This fragment was selected based on bioinformatics identification of two putative near consensus Dlx3 binding sites. Cloning of this promoter fragment was facilitated by adding restriction sites KpnI and BamHI to the forward and reverse primers, respectively. The fidelity of MMP-9 luciferase reporter was verified by nucleotide sequence analysis. PCR-based site-directed mutagenesis was used to disrupt the two Dlx3 binding sites located at −1,498 and −825 within the mouse MMP-9 promoter luciferase reporter. These mutations substituted a NotI restriction site for the near consensus Dlx3 binding sites. These mutations were confirmed using nucleotide sequence analysis. The pKH3-Dlx3 expression construct has been previously reported (4) and encodes mouse Dlx3 with the addition of three HA epitopes at the amino-terminal end of the protein. Additionally, both the human glycoprotein hormone α-subunit gene promoter luciferase construct and the placental growth factor (PGF) gene promoter luciferase construct have been previously reported (11, 41).
Cell culture and transient transfection studies.
JEG3 cells were cultured in monolayers using DMEM supplemented with fetal bovine serum (10%). Before transfection studies, cells were split into six-well tissue culture dishes and grown to 50–80% confluence. To compare relative gene promoter activity, JEG3 cells were transiently transfected with a luciferase reporter vector that did not contain a promoter fragment (empty), the MMP-9 promoter-luciferase construct, the human −204 α-subunit gene promoter-luciferase construct, or the PGF promoter-luciferase construct, the latter two known to be expressed in human trophoblasts. All transfections were carried out utilizing Genejuice transfection reagent (EMD Biosciences, Gibbstown, NJ) and a constant amount of total DNA by supplementing reactions with the empty expression vector, pKH3. Luciferase activity was determined after 24 h of transfection using reagents from a luciferase reporter assay system (Promega, Madison, WI). Luciferase activity levels were standardized using total cellular protein as determined by a Bradford assay. All transfections were conducted in triplicate on at least three separate occasions. Data are shown as means (n = 3) ± SE for a representative experiment.
Recombinant protein synthesis and EMSA.
Recombinant Dlx3 protein was prepared using a coupled transcription and translation wheat germ extract system (Promega). EMSAs were carried out as described previously (4, 41). Briefly, binding reactions (without radiolabeled probe) containing nonspecific competitor DNA were maintained at room temperature for 20 min followed by the addition of 32P-labeled oligonucleotide Dlx3 binding site probes from the two putative Dlx3 binding sites identified within the mouse MMP-9 promoter. The binding reactions were incubated another 20 min at room temperature and resolved on native polyacrylamide gels. Following electrophoresis, the gels were dried, and DNA-protein complexes were visualized by autoradiography. All DNA binding studies were conducted at least three times with similar results. The nucleotide sequences for the probes were as follows (only the top strand is represented): MMP-9 −1,498 binding site, 5′-ctaaaacaaattaacaaacaa-3′; and MMP-9 −825 binding site, 5′-cagtctcctaatttccaatca-3′.
Preparation of JEG3 cell nuclear extracts.
Near confluent JEG3 cells were washed twice with ice-cold DPBS and collected by scraping in ice-cold DPBS supplemented with a 1:1,000 dilution of protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO), 5 mM benzamidine, and 0.2 mM phenylmethylsulfonyl fluoride. Cells were pelleted by centrifugation and resuspended in a hypotonic buffer consisting of 120 mM potassium chloride, 30 mM sodium chloride, 30 mM HEPES pH 8.0, 0.3 M sucrose, protease inhibitor mix supplemented with 5 mM benzamidine, and 0.2 mM phenylmethylsulfonyl fluoride and allowed to swell for 15 min on ice. Cells were lysed by dounce homogenization, and nuclei were isolated by layering the broken cell lysates over a sucrose cushion (0.9 M sucrose) and then centrifuging at 2,000 g for 30 min at 4°C. The nuclear pellet was resuspended in buffer containing 10 mM Tris pH 7.5, 50 mM NaCl, 5% glycerol, 1 mM EDTA, protease inhibitor mixture, 5 mM benzamidine, and 0.2 mM phenylmethylsulfonyl fluoride. Additional sodium chloride was added to a final concentration of 450 mM, and nuclear proteins were extracted at 4°C for 30 min with constant agitation. Nuclear extracts were clarified by centrifugation (85,000 g for 60 min), and the nuclear extracts were stored in aliquots at −80°C until later use. The protein concentration of each nuclear extract was determined by Bradford assay.
DNA affinity chromatography.
M-280 streptavidin Dynabeads (Invitrogen, Carlsbad, CA) were washed three times in wash buffer, 10 mM Tris pH 7.5, 1 mM EDTA, and 2 M NaCl. The nucleotide sequences for the two putative Dlx3 binding sites on the MMP-9 promoter (−1,498 and −825) were biotinylated on the 5′-end. The biotinylated DNA binding sites were incubated with washed Dynabeads for 15 min at room temperature with constant shaking. Dynabeads-DNA mixture was washed in wash buffer twice followed by one wash in 50 mM Tris pH 7.5, 1 mM EDTA, 100 mM KCl, 5% glycerol, 0.1% Trition X-100, and 1 mM dithiothreitol (incubation buffer) to equilibrate the affinity matrix to incubation conditions. JEG3 cell nuclear extracts (60 ug) were added to the Dynabeads-DNA mixture plus 100 μl incubation buffer and incubated at room temperature 30 min with gentle shaking every 2 min. In some cases, these binding reactions also included 50- or 100-fold molar excess of unbiotinylated competitor oligonucleotides. Following this incubation, the Dynabeads-DNA-protein mixture was washed three times in incubation buffer. Complexes were suspended in 2× SDS loading buffer (100 mM Tris pH 6.8, 4% SDS, 20% glycerol, and 200 mM DTT). Samples were boiled for 3 min and chilled on ice for 5 min, resolved by SDS-PAGE, and immunoblotted using the Dlx3 antibody as described above.
Chromatin immunoprecipitation assay.
Protein A beads (Millipore, Temecula, CA) were blocked overnight with 1 mg/ml sonicated salmon sperm DNA and 1 mg/ml BSA at 4°C with gentle agitation and then washed in TE buffer twice. JEG3 cells were fixed in 1% formaldehyde in PBS for 10 min at 37°C. Glycine (125 mM) was added to quench the cross-linking, and cells were incubated on ice for 5 min. Cells were rinsed with PBS containing Complete Protease Inhibitors (Roche, Indianapolis, IN). Cells were centrifuged at 4,000 RPM for 5 min and resuspended in 1% SDS, 10 mM EDTA, 50 mM Tris pH7.9, and 1 mM DTT containing Complete Protease Inhibitors (Roche). Cells were incubated on ice for 10 min and sonicated using a Diagenode Bioruptor UCD-200 sonicator at 27% amplitude 15 cycles, 15 s per round with 45 s between sonications. Lysed cells were centrifuged for 10 min at 13,000 rpm at 4°C, and the supernatant was collected. Chromatin was precleared with blocked agarose beads for 1 h at 4°C with gentle agitation. After centrifugation, 10% of each sample was removed for analysis of input controls and 100 μl of each sample were mixed with 900 μl of a buffer containing 0.5% Trition X-100, 2 mM EDTA, and 10 mM Tris pH 7.5, 150 mM NaCl, 1 mM DTT, and Complete Protease Inhibitors (Roche). Complexes were incubated with no antibody, 2 μl of anti-Dlx3 antibody (Abcam, Cambridge, UK), or 2 μl of IgG control antibody overnight at 4°C with gentle agitation. Blocked agarose beads were added to each reaction and incubated at 4°C for an additional 2 h. Complexes were washed four times in washing buffer containing 0.25% Tergitol-type NP-40, 0.5% SDS, 2 mM EDTA, 20 mM Tris pH 8.0, 250 mM NaCl, and Complete Protease Inhibitors, and washed once in TE buffer. Complexes were then resuspended in 100 mM NaHCO3, 1% SDS and incubated at 65°C overnight followed by a phenol-cholorform extraction. Real time PCR was performed using the following primers: MMP-9: −1,812 forward, 5′-gattacaggaatgagccacca-3′ and −1,812 reverse, 5′-ctgtaggttgtaagtccgcaac-3′; MMP-9: −1,268 forward, 5′-tggtggtgtgggaggcttggga-3′ and −1,268 reverse, 5′-gagcagggctggagaactgaaa-3′; MMP-9: −889, forward, 5′-gattacaggaatgagccacca-3′ and −889 reverse, 5′-ctgtaggttgtaagtccgcaac-3′; human glycoprotein hormone α subunit gene (hα JRE): forward. 5′-tgacctaagggttgaaacaagataag-3′ and hα JRE reverse, 5′-ggaaattccatccaatgattga-3′; and distal hα JRE forward, 5′-tggttagactgtggggcatc −3′ and distal hα JRE, reverse 5′-cccaaccccaaagacaaaat-3′. The distal site on the human glycoprotein hormone α subunit gene served as a negative control. A standard curve was generated with the appropriate sample inputs, representing a 1:4, 1:40, and a 1:400 dilution.
Preparation of stable cell lines expressing siRNAs.
The mammalian expression vector pSUPER-retro-neo (OligoEngine, Seattle, WA) was used for preparation and expression of specific siRNAs. Each gene-specific insert targeted a 19-nucleotide sequence within the human Dlx3 mRNA. The Dlx3 siRNA sequence was as follows: 5′-gtagaagactgtagctata-3′. A control siRNA vector was also prepared in pSUPER-retro-neo vector using a nonspecific 19-nuclotide mammalian gene sequence (5′-ttctccgaacgtgtcacgt-3′), which served as a negative control for all studies (Applied Biosystems, Foster City, CA). To create stable cell populations of siRNA expressing cells, JEG3 cells were cultured and transfected as outlined above. Twenty-four hours following transfection, stable cell lines were selected for using 500 μg/ml neomycin in the culture media.
Statistical analysis.
Data were subjected to ANOVA, and differences between treatment groups were analyzed using a Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
Dlx3−/− mouse placentas have reduced levels of MMP-9 protein and gelatinase activity.
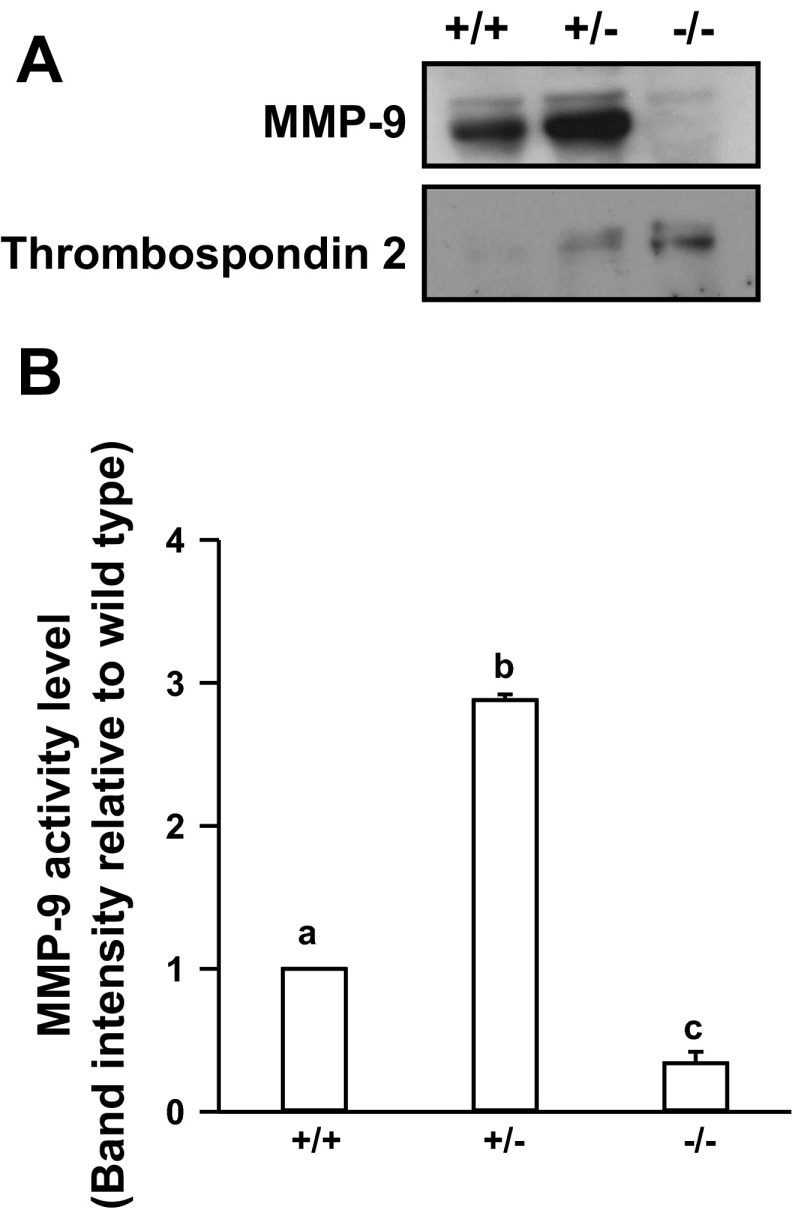
The Dlx3 knockout mouse dies at midgestation (∼E9.5) due to placental dysfunction (4, 8, 30). Further, placental microarray studies revealed that MMP-9 transcript levels were markedly reduced in the placentas of Dlx3−/− mice at E9.5, suggesting that MMP-9 may be an important target of Dlx3-dependent gene regulation (11). Using Dlx3+/+, Dlx+/−, Dlx−/− mouse placental disk explants, we initially examined relative levels of secreted MMP-9 assayed in culture media (Fig. 1A). Western blot analysis revealed that loss of both Dlx3 alleles resulted in a marked reduction in secreted MMP-9 in these cultures. This was corroborated by a marked reduction (P < 0.05) of MMP-9 gelatinase activity using media collected 4 h after culturing the Dlx−/− placental explants (Fig. 1B). Similar observations were made from parallel zymography studies examining media samples collected at the 6-h time point (data not shown). The reduction in secreted MMP-9 activity was not attributable to an overall reduction in secretion from these explants since secretion of the antiangiogenic glycoprotein thrombospondin 2 (7, 21) was increased markedly in a gene dosage-dependent manner where the highest thrombospondin 2 secretion occurred in the Dlx3 null placentas. These observations were consistent with changes in thrombospondin 2 transcript levels in the Dlx3−/− placenta in our previous array studies (11). Interestingly, in the Dlx3+/− placentas, MMP-9 protein levels and gelatinase activity were increased compared with the wild-type controls (P < 0.05; Fig. 1, A and B). On potential interpretation of this observation is that haploinsufficiency at the Dlx3 loci induces an apparent compensatory mechanism associated with elevated MMP-9 protein and gelatinase activity; however, a more complete understanding of this mechanism(s) awaits further investigation.
Fig. 1.
Matrix metalloproteinase-9 (MMP-9) protein and gelatinase activity are reduced in the distal-less 3-null (Dlx3−/−) mouse placenta. A: wild-type (Dlx3+/+), Dlx3 heterozygous (Dlx3+/−), and Dlx3−/− placental explants (n = 4–6/genotype) were cultured for 4 h and media were collected. Western blot analysis was used to determine secreted MMP-9 and thrombospondin 2 protein abundance in the Dlx3−/− compared with Dlx3+/+. B: zymography studies were performed to determine the amount of secreted MMP-9 gelatinase activity from the explant study. Gels were scanned and densiotometry measurements were obtained to determine the level of gelatinase activity corresponding to a band consistent with the molecular weight of MMP-9. All studies were replicated on at least 3 separate occasions with equivalent results. Bars with varying letters differ, P ≤ 0.05.
An MMP-9 gene promoter fragment is active in placental cells and responsive to Dlx3 overexpression.
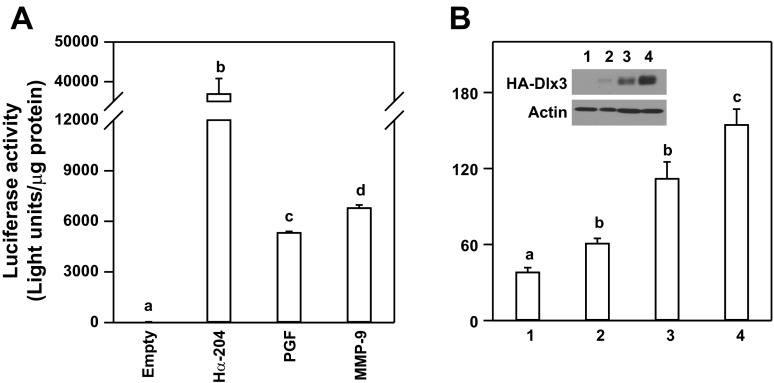
To investigate the mechanisms associated with Dlx3 regulation of the mouse MMP-9 gene, we cloned a 1.9-kb portion of the 5′-flanking region of the mouse MMP-9 promoter and incorporated this promoter fragment into a luciferase reporter gene. Initially, we compared the expression level of the MMP-9 promoter fragment to other promoters known to be expressed in JEG3 choriocarcinoma cells as a model for human placental trophoblasts. In transient transfection studies, MMP-9 promoter activity was observed to be ∼175-fold greater (P < 0.05) than the empty luciferase reporter (Fig. 2A). With the use of equal amounts of reporter plasmid for transfection, MMP-9 promoter activity was modestly higher compared with another placental-specific and Dlx3-dependent gene promoter, 5.2-kb portion of the 5′-flanking region of mouse PGF gene promoter (Fig. 2A) (11); however, both MMP-9 and PGF promoter activities were less (P < 0.05) than the transcriptional activity associated with the human glycoprotein hormone α-subunit gene promoter, another Dlx3-dependent gene in trophoblasts (42).
Fig. 2.
An MMP-9 promoter fragment coupled to a luciferase reporter is active in placental cells and responsive to Dlx3 overexpression. A: transient transfection studies in JEG3 choriocarcinoma cells were used to compare the relative promoter activity of the 1.9-kb portion of the MMP-9 gene promoter-luciferase reporter (MMP-9) to the luciferase reporter construct without a functional promoter (Empty), a 0.2-kb portion of the glycoprotein hormone α-subunit (Hα-204), and 5.2-kb placental growth factor (PGF) gene promoter luciferase reporter. B: Dlx3 overexpression was used to test the inducible activity of the MMP-9 luciferase reporter in JEG3 cells. Cotransfection of the MMP-9 luciferase reporter with a control vector (2.0 μg) or increasing doses of an expression vector encoding hemaglutinin (HA) epitope-tagged Dlx3 (0.5, 1.0, and 2.0 μg) was carried out. Data are reported as relative luciferase activity standardized by total cellular protein content from representative studies (n = 3/treatment). Western blot analysis was used to confirm HA-tagged Dlx3 overexpression (inset). Lane 1, control; lanes 2–4: HA-Dlx3 expression vector doses of 0.5, 1.0, and 2.0 μg, respectively. Actin was used as a lane loading control. All studies were replicated on at least 3 separate occasions with equivalent results. Bars with varying letters differ, P ≤ 0.05.
Transient cotranfection studies with the MMP-9 gene promoter and increasing doses of pKH3-Dlx3 expression vector revealed the MMP-9 gene promoter was responsive to Dlx3 overexpression in a dose-dependent manner (Fig. 2B). Overexpression of Dlx3 was confirmed by Western blot analysis using the HA epitope from the cell lysates used to analyze luciferase activity. These studies provide important evidence that Dlx3 was overexpressed in a dose-dependent manner. These studies support the conclusion that Dlx3 overexpression was sufficient to increase expression of the mouse MMP-9 gene promoter.
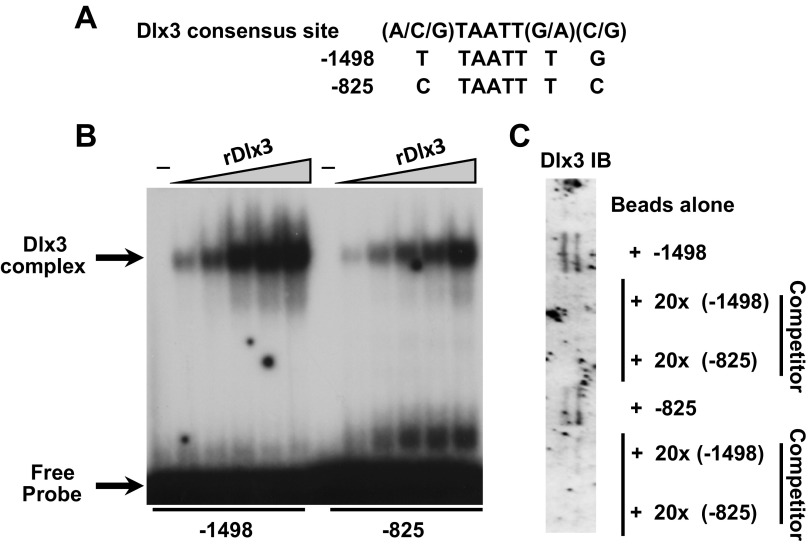
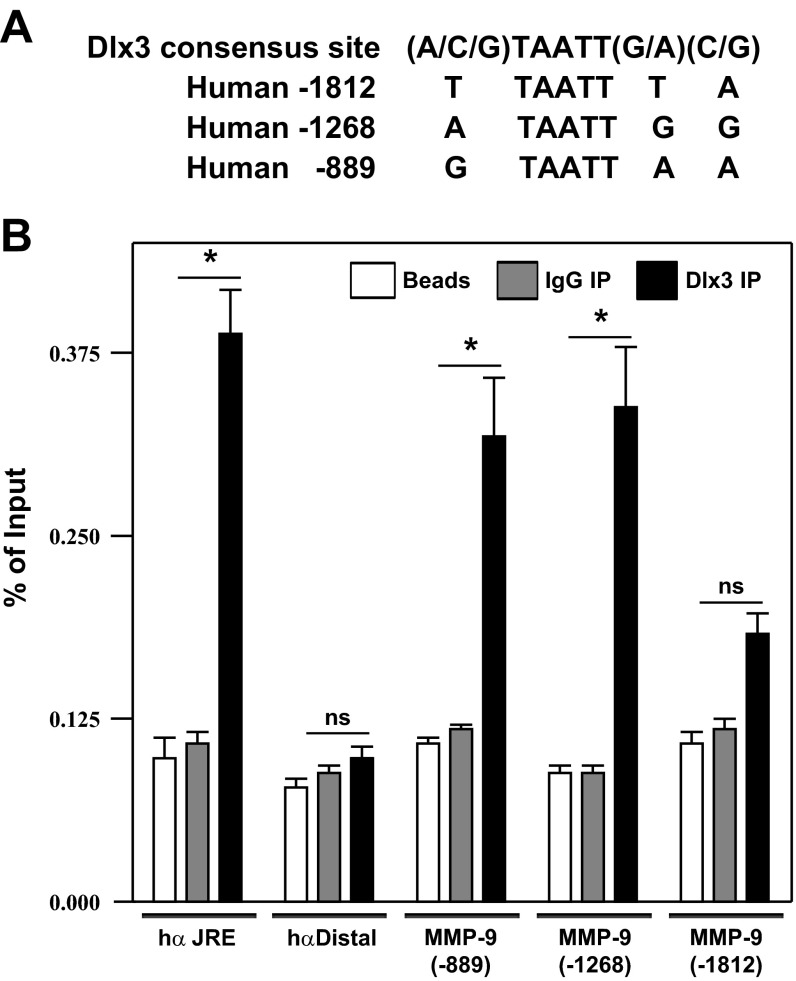
Upon examination of 1.9-kb portion of the mouse MMP-9 gene promoter, two Dlx3 near-consensus binding sites were identified; one near the proximal end of the MMP-9 gene promoter at −825 and one near the distal portion of the promoter at −1,498 relative to the transcription start site (1). Although both sites contain the core TAATT motif, the flanking nucleotides from each site varied from the Dlx3 consensus site (Fig. 3A). EMSA compared relative DNA binding of both the distal (−1,498) and proximal (−825) sites using recombinant Dlx3 (rDlx3). Addition of rDlx3 to these binding reactions resulted in dose-dependent DNA binding (Fig. 3B). These studies were corroborated with studies using DNA affinity chromatography (Fig. 3C). With the use of JEG3 nuclear extracts as a source of endogenous Dlx3, the distal (−1,498) and proximal (−825) Dlx3 binding sites were able to bind Dlx3 in this assay and Dlx3 binding was competed for using 20-fold molar excess of unbiotinylated oligonucleotides for the −1,498 and −825 binding sites. These studies support the conclusion that recombinant and endogenous Dlx3 can bind these sites in vitro.
Fig. 3.
Dlx3 binds to 2 cis-elements within the murine MMP-9 promoter fragment. A: bioinformatic investigation of the MMP-9 gene promoter identified 2 near-consensus Dlx3 binding sites based upon homology to a consensus Dlx3 site. Positions of these sites (designated as probes) were at −1,498 and −825 relative to the start site of transcription. B: with the use of EMSA, both binding sites were found to bind recombinant (r)Dlx3 with differing relative affinity. Dlx3 complexes as well as free probe are identified by arrows at left. C: with the use of JEG3 nuclear extracts as a source of Dlx3 binding activity, DNA affinity chromatography followed by Western blot studies revealed Dlx3 bound the −1,498 and −825 sites consistent with EMSA. Competition studies using the −1,498 and −825 binding sites at 20-fold molar excess of unbiotinylated oligonucleotides were used to demonstrate specificity of binding. All studies were replicated on at least 3 separate occasions with equivalent results.
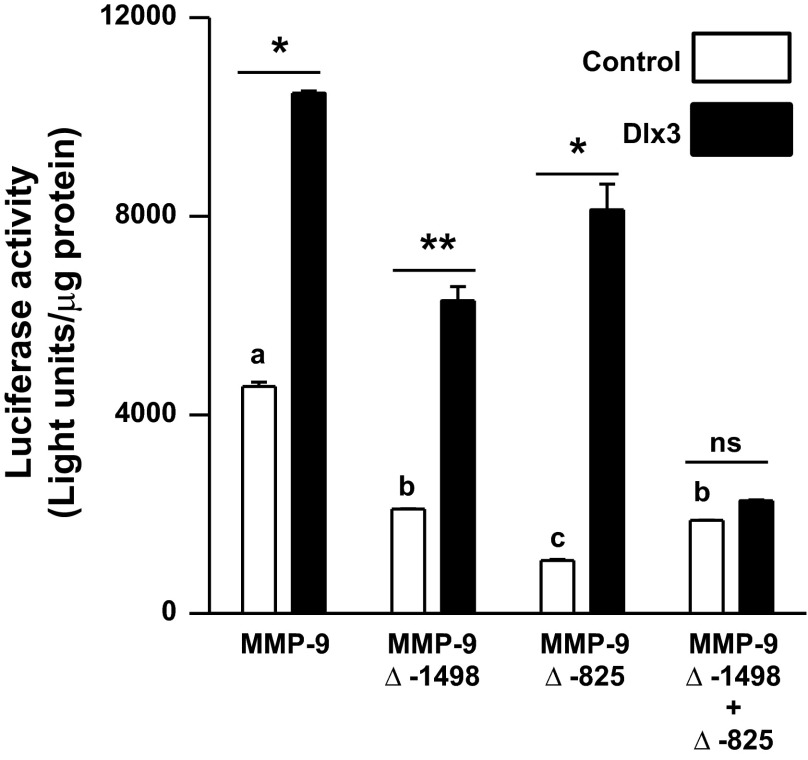
Dlx3 binding sites within the mouse MMP-9 gene promoter are required for basal and Dlx3-induced transactivation.
To evaluate the importance of the Dlx3 binding sites within the mouse MMP-9 gene promoter, both the distal (MMP-9 Δ−1,498) and proximal (MMP-9 Δ−825) sites were mutated alone and in combination in the context of the luciferase reporter. Each construct was transiently cotransfected into JEG3 cells with control vector or pKH3-Dlx3 to facilitate Dlx3 overexpression. Both of the individual mutants resulted in a reduction (P < 0.05) in basal expression of the MMP-9 luciferase reporter; however, neither mutation alone blunted the response to Dlx3 overexpression (Fig. 4). Only the combined mutations (MMP-9 Δ−1,498 + Δ−825) abolished (P < 0.05) responsiveness of this promoter to Dlx3 overexpression (Fig. 4). These findings indicate the interaction between Dlx3 and the cis-elements within the MMP-9 gene promoter are necessary for full transcriptional activation in placental trophoblasts.
Fig. 4.
Mutations within the 2 Dlx3 binding sites reduce basal murine MMP-9 promoter activity and abolish response to overexpression of Dlx3. Dlx3 binding sites within the MMP-9 gene promoter were mutated using a PCR-based site-directed mutagenesis. The 1.9-kb portion of the MMP-9 gene promoter (wild type), the mutation at the distal −1,498 site (MMP-9 Δ−1,498), proximal −825 site (MMP-9 Δ−825), and the combined mutations (MMP-9 Δ−1,498 + 825) were cloned into a luciferase reporter construct. Transient transfection studies were performed in JEG3 cells with MMP-9 gene promoter-luciferase reporter constructs cotransfected with a control pKH3 vector (2.0 μg) or 2.0 μg of pKH3-Dlx3 expression vector. Data are reported as relative luciferase activity standardized by total cellular protein content from representative studies (n = 3/treatment). All studies were replicated on at least 3 separate occasions with equivalent results. *P ≤ 0.05, increased MMP9 promoter activity with Dlx3 overexpression; **P ≤ 0.1, indicates trend; ns, nonsignificant difference. Bars with varying letters differ, P ≤ 0.05.
Dlx3 binds to the human MMP-9 gene promoter and is required for full MMP-9 expression in JEG3 choriocarcinoma cells.
Studies thus far focused on the regulation of the mouse MMP-9 gene promoter consistent with our previous array studies of the Dlx3−/− mouse placenta (11). We next sought to examine the putative binding of Dlx3 on the human MMP-9 gene promoter using a chromatin immunoprecipitation assay (ChIP). Bioinformatic analysis of the human MMP-9 gene promoter revealed three near consensus Dlx3 binding sites containing the core TAATT motif within 2-kb portion of the 5′-flanking region located at −1,812, −1,268 and −889 relative to the transcriptional start site (Fig. 5A). Dlx3 ChIP revealed specific Dlx3 binding on the MMP-9 gene promoter at or near the −889 and −1,268 elements (P < 0.05; Fig. 5B). Dlx3 binding as position −1,812 approached statistical significance (P = 0.081); however, putative association of Dlx3 and the −1,912 site was clearly weaker than the other two sites examined. Negative and positive controls for the ChIP study included examining an IP control (Beads), an IgG control (IgG IP) and Dlx3 ChIP of the human glycoprotein hormone α-subunit gene promoter at the junctional regulatory element (hα JRE) and a ChIP target ∼2 kb upstream from the JRE on the same segment of DNA (hα Distal). Dlx3 engaged the JRE robustly in this assay (P < 0.05), while the distal portion of the α-subunit gene promoter served as a negative control. Lastly, all three targets were identified by ChIP using an acetylated-histone H3 (Ac-H3 IP) immunoprecipitation (data not shown). These ChIP studies provide strong evidence that the human MMP-9 gene promoter binds Dlx3 in placental trophoblasts in vitro.
Fig. 5.
Dlx3 occupies the human MMP-9 gene promoter endogenously. A: analyses of the human MMP-9 gene promoter revealed 3 near consensus Dlx3 binding sites containing the core TAATT motif within a 2-kb portion of the 5′-flanking region specifically located at −1,812, −1,268 and −889 relative to the transcriptional start site of this gene. B: chromatin immunoprecipitation (ChIP) studies were carried out in JEG3 cells expressing Dlx3 endogenously. Several separate targets were assayed by PCR following ChIP. As a positive control, the junctional regulatory element from the human α-subunit gene promoter (hα JRE) was used; a target ∼2 kb downstream of the JRE within the α-subunit gene was used as a negative control (hα Distal); the putative Dlx3 binding at position −889, −1,268, and −1,812 were selected within the human MMP-9 gene promoter. For each condition, a ChIP reaction was carried out with protein A beads alone or with an IgG control antibody to serve as controls. This ChIP study was carried out on at least 3 separate occasions, each in triplicate, with similar results. *P ≤ 0.05, increased MMP9 promoter occupancy with Dlx3 overexpression.
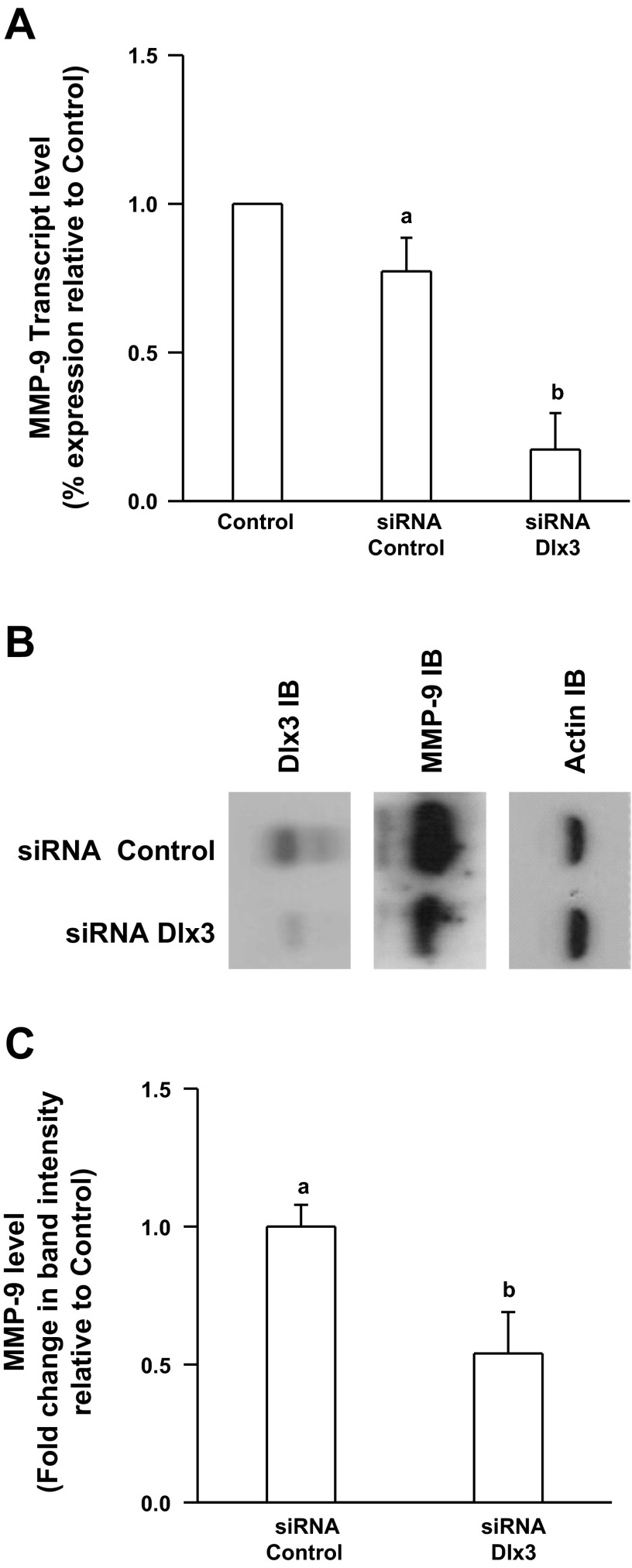
siRNA-mediated knockdown of human Dlx3 resulted in decreased endogenous MMP-9 transcript levels and gelatinase activity.
To examine a requirement for Dlx3 on the human MMP-9 gene promoter, we sought to investigate endogenous MMP-9 transcript and protein abundance in an environment where Dlx3 was reduced in JEG3 cells. To create such an environment, we developed JEG3 cell lines stably expressing a specific Dlx3 siRNA hairpin. The siRNA Dlx3 cell line was characterized by reduction of ∼60–70% of MMP-9 transcript abundance relative to the control siRNA cell line (Fig. 6A). Consistent with the transcript data, Dlx3 protein levels were markedly reduced and this correlated well with reduced MMP-9 protein levels as measured by Western blot analysis (Fig. 6B). To evaluate a relationship between reduced Dlx3 protein levels and MMP-9 gelatinase activity, media from siRNA knockout cell lines were collected and subjected to zymography to determine gelatinase activity associated with MMP-9. Results indicated ∼50% reduction (P < 0.05) in secreted MMP-9 activity in the siRNA Dlx3 cell line compared with the siRNA control cell line (Fig. 6C). Combined these data support our working hypothesis that in human trophoblasts, Dlx3 engages the MMP-9 gene promoter and contributes to full MMP-9 expression and MMP-9-specific gelatinase activity.
Fig. 6.
Knockdown of human Dlx3 using small interference (si)RNA results in reduced expression of human MMP-9 in JEG3 cells. A: knockdown of Dlx3 was accomplished in stably transfected JEG3 cells using specific siRNAs (siRNA control or Dlx3). In the siRNA Dlx3 cell lines, mRNA levels of endogenous MMP-9 were decreased ∼60% compared with control cells or the control siRNA cell line as measured by quantitative PCR. B: Dlx3 protein levels were reduced by ∼70% in the siRNA Dlx3 cell line as detected by immunoblot. This result was correlated with reductions in MMP-9 protein levels in the same cell line. Actin was used as a lane loading control. C: endogenously secreted MMP-9 gelatinase activity was reduced by ∼50% in JEG3 cells expressing Dlx3 siRNA. These studies were carried out on 3 separate occasions with similar results. Bars with varying letters differ, P ≤ 0.05.
DISCUSSION
MMPs are a complex family of proteolytic enzymes linked to various developmental processes including cell invasion, migration, and ECM/tissue remodeling. This complexity is underscored by multiple levels of regulation of MMPs including changes in gene transcription and transcript abundance; requirements for zinc for MMP endopeptidase activity; the necessity, in some cases, for posttranslational cleavage of some of these enzymes for activity; subcellular localization (membrane associated vs. secreted); and control of MMP activity by tissue inhibitors of MMP or tissue inhibitors of metalloproteinase (45). The current studies provide important direct evidence for the role of Dlx3 in the regulation of MMP-9 gene transcription and activity in placental trophoblasts. In mice, the loss of Dlx3 has been linked to placental insufficiency and midgestation lethality (30). These findings suggest a crucial role for Dlx3 and presumably the Dlx3 gene network in facilitating proper placentation and support the hypothesis that misregulation of Dlx3-dependent MMP-9 activity during placental morphogenesis may be linked to inappropriate ECM remodeling/gelatinase activity in the establishment of the feto-placental interface. Interestingly, the relationship between Dlx3 and MMP-9 is not restricted to placental trophoblasts. In skin, Park and Morasso (35) demonstrated that Dlx3 is upregulated during keratinocyte differentiation induced by calcium (35). Elevations in cell calcium during keratinocyte differentiation also result in increased MMP-9 transcript levels (20). In those studies, responsiveness of the MMP-9 gene promoter was linked to regulatory elements within the proximal ∼100 nucleotides of the MMP-9 transcription start site and more distal elements (including the Dlx3 binding sites identified herein) were not included in the analysis. Thus the coincident upregulation of Dlx3 and MMP-9 in keratinocytes with increase calcium stimuli may indeed reflect a direct effect of Dlx3 on MMP-9 promoter activity via the combined actions of these proximal elements with more distal Dlx3 binding sites on the MMP-9 promoter.
MMP-9 proteolytic activity has been shown to degrade denatured collagens and basement membrane components (15), organize collagen fibrils (22), cleave fibrin (23), serpin α1-proteinase inhibitor (25), vascular endothelial growth factor (2), interleukin-1β (17), and transforming growth factor-β (48) revealing the involvement of MMP-9 in many processes including tissue remodeling, inflammatory responses, and metastasis in the progression of cancer. As there are currently many recognized MMP family members in humans, it is difficult at present to ascribe specific functions to discrete members of this family without important consideration of potential redundancies. For instance in the placenta, it would appear that MMP-2 and MMP-9 may be playing central roles for proper placental development based on several recent studies linked to at-risk pregnancies (5, 24, 44); including recent evidence of MMP-9 misregulation in the placentas of women (6) and rats (28) with maternal diabetes. One of the primary steps for proper placentation includes invasion of extravillous trophoblasts into the maternal decidua. In the normal placenta, this trophoblast invasion allows for extensive maternal spiral artery remodeling by trophoblasts to facilitate appropriate perfusion of the placental vascular bed ultimately resulting in suitable placental development (38). Aberrant trophoblast invasion in either a spatially and temporally restricted manner may result in placental dysfunction (36, 47). For example in placental tissues examined from patients experiencing PE, shallow trophoblast invasion impairs spiral artery and arteriole remodeling causing reduced uteroplacental blood flow and compromised fetal fitness (37, 39). Interestingly, the phenotype of cytotrophoblasts originating from human embryonic stem cell differentiation includes increased invasiveness properties coincident with increased MMP-2 and -9 expression and activity (46), reinforcing the potential importance of these MMPs on trophoblast invasive behavior.
An intriguing relationship emerging from the present studies is the secretion pattern of thrombospondin 2 relative to MMP-9 in the Dlx3−/− placenta (Fig. 1). These observations were consistent with predictions made from our previous array data comparing Dlx3+/+ and Dlx−/− placentas (11) and was the rationale for the selection and use of thrombospondin 2 as a control in these secretion studies. Thrombospondin 2 is a secreted glycoprotein known to inhibit angiogenesis by slowing proliferation and potentially inducing apoptosis in endothelial cells; however, perhaps more relevant to the current studies is the role of thrombospondin 2 in the regulation of extracellular matrix remodeling (21). In thrombospondin 2 null mice, MMP-9 and VEGF are elevated associated with wounds in the skin (27). Conversely, when thrombospondin 2 is overexpressed in a colon cancer (18) or pancreatic cancer cell lines (32), MMP-2 and -9 levels are specifically downregulated, which has important implication for development of tumor vasculature. It is presently unclear if Dlx3 may be playing a direct role in transcriptional repression of the thrombospondin 2 gene promoter or if the apparent elevation of thrombospondin 2 transcript levels and secretion observed in the current studies reflects a more complex mechanism(s). Although entirely speculative, the reciprocal relationship between loss of MMP-9 secretion concurrent with elevated thrombospondin 2 secretion from the E9.5 Dlx3−/− mouse placenta has raised the intriguing possibility that Dlx3 may regulate both the invasive properties of placental trophoblasts to establish fetal/maternal vascular relationships through regulation of MMP-9 (and potentially other ECM remodeling factors) and modulate angiogenic tone within the developing placenta through control of thrombospondin 2 secretion. What is conclusive from these findings is that aberrant expression of these two secreted proteins, MMP-9 and thrombospondin 2, likely contributes to placental failure of the Dlx3−/− mouse at multiple levels. Additional studies are necessary to ascertain the exact relationship between MMP-9 and thrombospondin 2 in compromised placentation in the Dlx3−/− animal.
The present studies highlight the important interplay between Dlx3 and MMP-9 in the placenta suggesting a role for both of these genes in proper placental morphogenesis. The MMP-9 gene promoter appears to be a direct target of Dlx3 transcriptional regulation in both mouse and human trophoblasts. The finding that thrombospondin 2 expression is also coordinately regulated within this gene network provides compelling evidence that Dlx3 may be controlling placental labyrinth development at multiple levels; controlling both an invasive trophoblast phenotype and modulating the angiogenic environment. While not directly related to the present studies, it is notable that two separate Dlx3 target genes, MMP-9 and placental growth factor (11), have been used as targets of overexpression to restore vascular networks and reduce ECM deposition as a cell therapy in aging dystrophic myocytes in mice (10). Thus the role of the Dlx3 gene network may be more far reaching than originally appreciated.
GRANTS
This research was supported by National Institute of Child Health and Human Development Grant R21-HD-056414 (to M. S. Roberson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.A.C., S.A.C., and M.S.R. conception and design of research; P.A.C., J.X., S.L., and X.Z. performed experiments; P.A.C., J.X., S.L., and X.Z. analyzed data; P.A.C., S.L., X.Z., S.A.C., and M.S.R. interpreted results of experiments; P.A.C. and M.S.R. drafted manuscript; P.A.C. and M.S.R. edited and revised manuscript; P.A.C., J.X., S.L., X.Z., S.A.C., and M.S.R. approved final version of manuscript; J.X. and M.S.R. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. Maria I. Morasso for generously providing mice for these studies.
REFERENCES
- 1. Backstrom JR, Tokes ZA. The 84-kDa form of human matrix metalloproteinase-9 degrades substance P and gelatin. J Neurochem 64: 1312–1318, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res 63: 5224–5229, 2003 [PubMed] [Google Scholar]
- 3. Berghorn KA, Clark PA, Encarnacion B, Deregis CJ, Folger JK, Morasso MI, Soares MJ, Wolfe MW, Roberson MS. Developmental expression of the homeobox protein Distal-less 3 and its relationship to progesterone production in mouse placenta. J Endocrinol 186: 315–323, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Berghorn KA, Clark-Campbell PA, Han L, McGrattan M, Weiss RS, Roberson MS. Smad6 represses Dlx3 transcriptional activity through inhibition of DNA binding. J Biol Chem 281: 20357–20367, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Campbell S, Rowe J, Jackson CJ, Gallery ED. Interaction of cocultured decidual endothelial cells and cytotrophoblasts in preeclampsia. Biol Reprod 71: 244–252, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Capobianco E, White V, Sosa M, Di M, I, Basualdo MN, Faingold MC, Jawerbaum A. Regulation of matrix metalloproteinases 2 and 9 activities by peroxynitrites in term placentas from type 2 diabetic patients. Reprod Sci 19: 814–822, 2012 [DOI] [PubMed] [Google Scholar]
- 7. De SD, Nicolaus G, Maiuri MC, Cipolletta D, Galluzzi L, Cinelli MP, Tajana G, Iuvone T, Carnuccio R. NF-kappaB blockade upregulates Bax, TSP-1, and TSP-2 expression in rat granulation tissue. J Mol Med 87: 481–492, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Depew MJ, Simpson CA, Morasso M, Rubenstein JL. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat 207: 501–561, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gallery ED, Campbell S, Arkell J, Nguyen M, Jackson CJ. Preeclamptic decidual microvascular endothelial cells express lower levels of matrix metalloproteinase-1 than normals. Microvasc Res 57: 340–346, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Gargioli C, Coletta M, De GF, Cannata SM, Cossu G. PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med 14: 973–978, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Han L, Dias FM, Berghorn KA, Iwata TN, Clark-Campbell PA, Welsh IC, Wang W, O'Brien TP, Lin DM, Roberson MS. Analysis of the gene regulatory program induced by the homeobox transcription factor distal-less 3 in mouse placenta. Endocrinology 148: 1246–1254, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Harris LK, Keogh RJ, Wareing M, Baker PN, Cartwright JE, Whitley GS, Aplin JD. BeWo cells stimulate smooth muscle cell apoptosis and elastin breakdown in a model of spiral artery transformation. Hum Reprod 22: 2834–2841, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Herron GS, Banda MJ, Clark EJ, Gavrilovic J, Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem 261: 2814–2818, 1986 [PubMed] [Google Scholar]
- 14. Herron GS, Werb Z, Dwyer K, Banda MJ. Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenase and prostromelysin exceeds expression of proteolytic activity. J Biol Chem 261: 2810–2813, 1986 [PubMed] [Google Scholar]
- 15. Hibbs MS, Hoidal JR, Kang AH. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest 80: 1644–1650, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huisman MA, Timmer A, Zeinstra M, Serlier EK, Hanemaaijer R, Goor H, Erwich JJ. Matrix-metalloproteinase activity in first trimester placental bed biopsies in further complicated and uncomplicated pregnancies. Placenta 25: 253–258, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Ito A, Mukaiyama A, Itoh Y, Nagase H, Thogersen IB, Enghild JJ, Sasaguri Y, Mori Y. Degradation of interleukin 1beta by matrix metalloproteinases. J Biol Chem 271: 14657–14660, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Kamochi J, Tokunaga T, Tomii Y, Abe Y, Hatanaka H, Kijima H, Yamazaki H, Watanabe N, Matsuzaki S, Ueyama Y, Nakamura M. Overexpression of the thrombospondin 2 (TSP2) gene modulated by the matrix metalloproteinase family expression and production in human colon carcinoma cell line. Oncol Rep 10: 881–884, 2003 [PubMed] [Google Scholar]
- 19. Khong TY, Lane EB, Robertson WB. An immunocytochemical study of fetal cells at the maternal-placental interface using monoclonal antibodies to keratins, vimentin and desmin. Cell Tissue Res 246: 189–195, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi T, Kishimoto J, Ge Y, Jin W, Hudson DL, Ouahes N, Ehama R, Shinkai H, Burgeson RE. A novel mechanism of matrix metalloproteinase-9 gene expression implies a role for keratinization. EMBO Rep 2: 604–608, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krady MM, Zeng J, Yu J, MacLauchlan S, Skokos EA, Tian W, Bornstein P, Sessa WC, Kyriakides TR. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am J Pathol 173: 879–891, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, Senior RM, Bornstein P. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol 28: 65–73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, Ronco PM. Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J Exp Med 193: 793–802, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li JK, Xiong Q, Zhou S, Yang PF. [Differences between the expression of matrix metalloproteinase-2, 9 in preeclampsia and normal placental tissues]. Zhonghua Fu Chan Ke Za Zhi 42: 73–75, 2007 [PubMed] [Google Scholar]
- 25. Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell 102: 647–655, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Lockwood CJ, Oner C, Uz YH, Kayisli UA, Huang SJ, Buchwalder LF, Murk W, Funai EF, Schatz F. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod 78: 1064–1072, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacLauchlan S, Skokos EA, Agah A, Zeng J, Tian W, Davidson JM, Bornstein P, Kyriakides TR. Enhanced angiogenesis and reduced contraction in thrombospondin-2-null wounds is associated with increased levels of matrix metalloproteinases-2 and -9, and soluble VEGF. J Histochem Cytochem 57: 301–313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martinez N, Sosa M, Higa R, Fornes D, Capobianco E, Jawerbaum A. Dietary treatments enriched in olive and safflower oils regulate seric and placental matrix metalloproteinases in maternal diabetes. Placenta 33: 8–16, 2012 [DOI] [PubMed] [Google Scholar]
- 29. McFadyen IR, Price AB, Geirsson RT. The relation of birthweight to histological appearances in vessels of the placental bed. Br J Obstet Gynaecol 93: 476–481, 1986 [PubMed] [Google Scholar]
- 30. Morasso MI, Grinberg A, Robinson G, Sargent TD, Mahon KA. Placental failure in mice lacking the homeobox gene Dlx3. Proc Natl Acad Sci USA 96: 162–167, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morasso MI, Markova NG, Sargent TD. Regulation of epidermal differentiation by a Distal-less homeodomain gene. J Cell Biol 135: 1879–1887, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakamura M, Oida Y, Abe Y, Yamazaki H, Mukai M, Matsuyama M, Chijiwa T, Matsumoto H, Ueyama Y. Thrombospondin-2 inhibits tumor cell invasion through the modulation of MMP-9 and uPA in pancreatic cancer cells. Mol Med Rep 1: 423–427, 2008 [PubMed] [Google Scholar]
- 33. Paiva P, Salamonsen LA, Manuelpillai U, Dimitriadis E. Interleukin 11 inhibits human trophoblast invasion indicating a likely role in the decidual restraint of trophoblast invasion during placentation. Biol Reprod 80: 302–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palei AC, Sandrim VC, Cavalli RC, Tanus-Santos JE. Comparative assessment of matrix metalloproteinase (MMP)-2 and MMP-9, and their inhibitors, tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in preeclampsia and gestational hypertension. Clin Biochem 41: 875–880, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Park GT, Morasso MI. Regulation of the Dlx3 homeobox gene upon differentiation of mouse keratinocytes. J Biol Chem 274: 26599–26608, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech 5: 9–18, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van AA. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 98: 648–655, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4: 397–413, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27: 939–958, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Qiao C, Wang CH, Shang T, Lin QD. [Clinical significance of KiSS-1 and matrix metalloproteinase-9 expression in trophoblasts of women with preeclampsia and their relation to perinatal outcome of neonates]. Zhonghua Fu Chan Ke Za Zhi 40: 585–590, 2005 [PubMed] [Google Scholar]
- 41. Roberson MS, Meermann S, Morasso MI, Mulvaney-Musa JM, Zhang T. A role for the homeobox protein Distal-less 3 in the activation of the glycoprotein hormone alpha subunit gene in choriocarcinoma cells. J Biol Chem 276: 10016–10024, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Roberson MS, Misra-Press A, Laurance ME, Stork PJ, Maurer RA. A role for mitogen-activated protein kinase in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol Cell Biol 15: 3531–3539, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet 2: 538–548, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Shokry M, Omran OM, Hassan HI, Elsedfy GO, Hussein MR. Expression of matrix metalloproteinases 2 and 9 in human trophoblasts of normal and preeclamptic placentas: preliminary findings. Exp Mol Pathol 87: 219–225, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev 27: 57–66, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Udayashankar R, Baker D, Tuckerman E, Laird S, Li TC, Moore HD. Characterization of invasive trophoblasts generated from human embryonic stem cells. Hum Reprod 26: 398–406, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 24: 147–158, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176, 2000 [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang H, Lin QD, Qiao C. [Expression of trophoblast invasion related genes mRNA and protein in human placenta in preeclampsia]. Zhonghua Fu Chan Ke Za Zhi 41: 509–513, 2006 [PubMed] [Google Scholar]