Abstract
Large conductance voltage- and Ca2+-activated K+ (BK) channels are key regulators of detrusor smooth muscle (DSM) contraction and relaxation during urine voiding and storage. Here, we explored whether BK channels are regulated by muscarinic receptors (M-Rs) in native freshly isolated rat DSM cells under physiological conditions using the perforated whole cell patch-clamp technique and pharmacological inhibitors. M-R activation with carbachol (1 μM) initially evoked large transient outward BK currents, followed by inhibition of the spontaneous transient outward BK currents (STBKCs) in DSM cells. Carbachol (1 μM) also inhibited the amplitude and frequency of spontaneous transient hyperpolarizations (STHs) and depolarized the DSM cell membrane potential. Selective inhibition of the muscarinic M3 receptors (M3-Rs) with 4-diphenylacetoxy-N-methylpiperidine (4-DAMP; 0.1 μM), but not muscarinic M2 receptors with methoctramine (1 μM), blocked the carbachol inhibitory effects on STBKCs. Furthermore, blocking the inositol 1,4,5-triphosphate (IP3) receptors with xestospongin-C (1 μM) inhibited the carbachol-induced large transient outward BK currents without affecting carbachol inhibitory effects on STBKCs. Upon pharmacological inhibition of all known cellular sources of Ca2+ for BK channel activation, carbachol (1 μM) did not affect the voltage-step-induced steady-state BK currents, suggesting that the muscarinic effects in DSM cells are mediated by mobilization of intracellular Ca2+. In conclusion, our findings provide strong evidence that activation of M3-Rs leads to inhibition of the STBKCs, STHs, and depolarization of DSM cells. Collectively, the data suggest the existence of functional interactions between BK channels and M3-Rs at a cellular level in DSM.
Keywords: 4-DAMP, detrusor, methoctramine, xestospongin-C
coordinated contraction and relaxation of detrusor smooth muscle (DSM), which makes up the wall of the urinary bladder, are responsible for voiding and storing of urine (1). A number of ion channels, receptors, neurotransmitters, and protein kinases regulate DSM function under physiological and pathophysiological conditions. Among the K+ channels, large-conductance voltage- and Ca2+-activated K+ (BK) channels are the key elements in DSM cell excitability and contractility that maintain the resting membrane potential (RMP) and shape the spontaneous action potentials, which trigger DSM phasic contractions (16, 20, 24–26, 31, 32). Rapid and localized cytosolic Ca2+ release via ryanodine receptors (RyRs), known as Ca2+ sparks, activates the BK channels, causing spontaneous transient BK currents (STBKCs) and thus relaxation of DSM (23, 31, 33). In DSM cells, blockade of RyRs with ryanodine inhibits Ca2+ sparks and the associated STBKCs as well as attenuates spontaneous phasic contraction (5, 18, 21, 22, 24, 25, 33). The functional role of the STBKCs, which contribute to setting the RMP, has been well demonstrated in DSM of various animal species (22, 23, 25, 33, 43) and humans (24, 44).
Our group and others have demonstrated that selective pharmacological inhibition of the BK channels with iberiotoxin or paxilline increases DSM excitability and contractility (5, 21, 22, 24, 25, 43, 44). However, whether BK channels are regulated by muscarinic receptors (M-Rs) in DSM cells under physiological conditions remains largely unexplored.
The M-Rs (subtypes M1-M5) belong to the family of heterotrimeric G protein-coupled receptors (7). mRNA and immunoprecipitation studies in rat and human urinary bladders showed predominant expression of muscarinic M2 receptors (M2-Rs) and M3 receptors (M3-Rs) (41, 45). Although expression of M2-Rs is predominant over M3-Rs in mammalian bladders, DSM contraction under physiological conditions is caused largely by activation of M3-Rs (1, 8, 13, 14, 19, 37, 38, 41). The M3-Rs are coupled to the Gq/11 proteins, which activate phospholipase-C inducing inositol 1,4,5-trisphosphate (IP3) production. This subsequently leads to Ca2+ release from the sarcoplasmic reticulum (SR) via IP3 receptors and thus facilitates smooth muscle contraction (27, 42). Unlike M3-Rs, the M2-Rs are coupled with the Gi/0 proteins, which inhibit adenylyl cyclase production and indirectly promote smooth muscle contraction (7). M2-R activation has a modulatory role on airway smooth muscle contraction by suppression of BK channel activity via the cAMP pathway (46). With the use of M3-R and M2-R/M3-R double knockout mice, it has been suggested that M2-Rs enhance the M3-R-mediated DSM contractions (13, 14).
The underlying cellular mechanisms in the M-Rs pathways involved in the regulation of DSM function are not well understood. Here, we tested the hypothesis that under physiological conditions the BK channel activity in DSM is inhibited upon activation of M-Rs. With the use of radiological binding studies, 4-diphenylacetoxy-N-methylpiperidine (4-DAMP) has been shown to have a ∼9 to 10-fold selectivity ratio for M3-Rs over M2-Rs (10, 11). Methoctramine has been demonstrated the most selective M2-Rs inhibitor and has a binding affinity of 33-fold greater than that of M3-Rs (6). Therefore, in the present study, we used 4-DAMP and methoctramine, selective antagonists of the M3-Rs and M2-Rs, respectively, as appropriate pharmacological tools to separate M3-Rs and M2-Rs. Our experimental findings support the concept that activation of M3-Rs suppresses the STBKCs, inhibits spontaneous transient hyperpolarizations (STHs), depolarizes DSM cells, and thus regulates DSM cell excitability. For the first time, we provide strong evidence that under native physiological conditions the BK channels are regulated by M3-Rs in DSM cells.
METHODS
DSM tissue collection and preparation.
Forty-five Sprague-Dawley male rats (Harlan, Frederick, MD) of average weight 342.3 ± 5.8 g were used in this study. All experimental procedures were performed following the ethics of the Animal Use Protocol No. 1747, which was reviewed and approved by the Institutional Animal Care and Use Committee of the University of South Carolina. Rats were euthanized by CO2 inhalation. The urinary bladders were dissected and immediately stored in ice-cold dissection solution. Urinary bladders were cut open longitudinally from the lumen. The urothelium and lamina propria were carefully removed. DSM strips of 5- to 7-mm long and 2- to 3-mm wide were dissected from the bladder wall for single cell isolation.
DSM single cell isolation.
Rat DSM single cells were freshly isolated as described previously (25). Briefly, one to two DSM isolated strips were incubated for 20–25 min at 37°C in prewarmed 2-ml dissection solution supplemented with 1 mg/ml BSA, 1 mg/ml papain (Worthington, Lakewood, NJ), and 1 mg/ml dl-dithiothreitol. Next, the DSM strips were transferred to prewarmed 2-ml of dissection solution supplemented with 1 mg/ml BSA, 0.5 mg/ml collagenase (type II from Sigma-Aldrich, St. Louis, MO), 0.5 mg/ml trypsin inhibitor, and 100 μM CaCl2 and incubated for 12–15 min at 37°C. The digested DSM tissues were washed several times with dissection solution supplemented with BSA and then were gently triturated with a fire polished Pasteur pipette to release DSM single cells. Freshly isolated DSM single cells were used for patch-clamp experiments within 6 h after isolation.
Patch-clamp recordings.
A few drops of DSM cell suspension were dropped into the glass bottom of a recording chamber, and cells were allowed to adhere to the glass bottom for 20–30 min. DSM cells were washed several times with physiological bath solution to remove cell debris and poorly adhered DSM cells. Morphologically distinct, elongated, and bright DSM cells (when viewed under phase-contrast Axiovert 40CFL microscope) were selected for patch-clamp recordings. Amphotericin-B perforated whole cell patch-clamp technique was applied to record STBKCs, RMP, and voltage-step depolarization-evoked steady-state BK currents from freshly isolated DSM single cells. STBKCs and voltage-step depolarization-induced steady-state BK currents were recorded under voltage-clamp mode, whereas RMP was recorded under current-clamp mode (I = 0) using an Axopatch 200B amplifier, Digidata 1322A, and pCLAMP version 10.2 software (Molecular Devices, Union City, CA). The recorded currents were filtered with an eight-pole Bessel filter 900CT/9L8L (Frequency Devices, Ottawa, IL). Patch-clamp pipettes with a tip resistance of 5–10 MΩ were prepared from borosilicate glass (Sutter Instruments, Novato, CA) using a Narishige PP-830 vertical puller (Narishige Group, Tokyo, Japan) and a Micro Forge MF-830 fire polisher (Narishige Group). Voltage-step-induced whole cell BK currents were recorded by holding the DSM cells at −70 mV and voltage depolarization applied from −40 mV to +80 mV in increments of 20 mV with 200-ms duration, and then cells were repolarized back to −70 mV. Voltages were corrected for the junction potential. A stable continuous recording for 6–10 min (done every 1 min) was used as a control, and another continuous recording for 6–10 min (done every 1 min) was used to examine the effect of 1 or 100 μM carbachol on the whole cell steady-state BK currents. STBKCs were recorded at a holding potential of −20 mV. STBKCs and RMP recorded for at least 8–10 min in the absence of any test compound were used as a control, and another continuous recording for at least 10–15 min was done in the presence of test compounds to examine the effects on STBKCs, STHs, and RMP of DSM cells. Leak currents were not subtracted during the patch-clamp recordings or data analysis. All patch-clamp experiments were conducted at room temperature (22–23°C).
Solutions and drugs.
Dissection solution had a composition of the following (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, 2 MgCl2, and the pH was adjusted to 7.3 with NaOH. Physiological solution used for patch-clamp contained the following (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 10 HEPES, and the pH was adjusted to 7.4 with NaOH. The patch-pipette solution contained the following (in mM): 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, 0.05 EGTA, and the pH was adjusted to 7.2 with NaOH. Freshly dissolved amphotericin-B (200 μg/ml) in DMSO was added to the pipette solution before the experiment and replaced every 1–2 h. Carbachol was dissolved freshly in double-distilled water. 4-DAMP (R&D Systems, McKinley Place NE, MN), methoctramine (Sigma-Aldrich), xestospongin-C (Enzo Life Sciences, Farmingdale, NY), ryanodine (Enzo Life Sciences), nifedipine (Thermo Fisher Scientific, Fair Lawn, NJ), and thapsigargin (Thermo Fisher Scientific) were dissolved in DMSO as stock solutions. The DMSO concentration in the bath solution did not exceed 0.21%.
Data analysis and statistics.
Clampfit 10.2 software (Molecular Device, Union City, CA) was used to analyze patch-clamp data. To evaluate the effect of carbachol on voltage-step depolarization-evoked whole cell steady-state BK currents, the mean value of the last 50 ms of the 200-ms pulse from the average of 6–10 files recorded over 8–10 min (done every 1 min) before and after the application of 1 or 100 μM carbachol was calculated. The last 5 min of at least 8–10 min stable voltage-clamp or current-clamp recordings before the application of test compounds were used as a control and the last 5 min of continuous recordings of at least 10–15 min after the application of test compounds were used to evaluate their effects on STBKCs, STHs, or RMP of DSM cells. Amplitude and frequency of STBKCs or STHs were analyzed using MiniAnalysis software (Synaptosoft, Fort Lee, NJ). Statistical analysis was performed with GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA). The effects of test compounds on the amplitude and frequency of STBKCs or STHs were normalized to control values and were expressed in percentages (%). The data are shown as means ± SE for the n (the number of cells) isolated from N (the number of rats). Corel Draw Graphic Suite X3 software (Corel, Mountain View, CA) and GraphPad Prism 4.03 software were used for data illustration. Statistical analysis was performed using either two-tailed paired Student's t-test or two-way analysis of variance followed by Bonferroni's post hoc test. A P value <0.05 was considered to be statistically significant.
RESULTS
Activation of M-Rs with carbachol suppresses the amplitude and frequency of STBKCs in freshly isolated DSM cells.
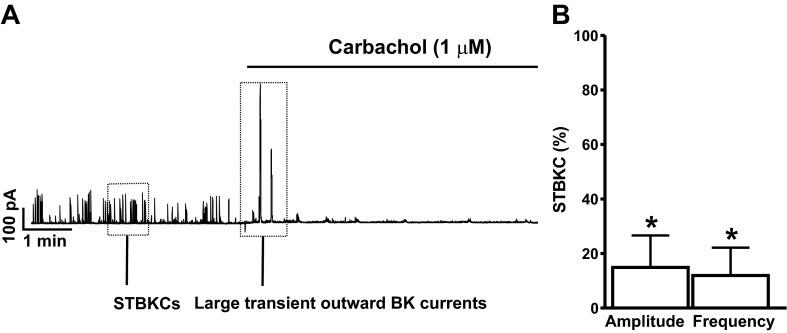
We used the amphotericin-B perforated whole cell patch-clamp technique to preserve the intracellular environment and Ca2+ signaling pathways and study the functional interactions between M-Rs and BK channels in DSM cells under physiological conditions. The average cell capacitance of all DSM cells used in the present study was 21.0 ± 1.2 pF (n = 73, N = 45). We examined the effects of M-Rs activation with carbachol, a nonselective M-R agonist, on STBKCs in DSM cells. Treatment of DSM cells with 1 μM carbachol initially elicited large transient outward BK currents of 985.4 ± 133.7 pA for 20.2 ± 13.8 s (n = 5, N = 4) followed by a significant inhibition of STBKC amplitude and frequency by 85.1 ± 11.7 and 88.1 ± 10.2%, respectively (n = 8, N = 7; P < 0.05; Fig. 1, A and B). These results suggest the existence of a functional link between M-Rs and BK channels in DSM cells. Next, we sought to examine the effect of M-Rs activation on DSM cell RMP.
Fig. 1.
Activation of muscarinic receptors (M-Rs) with 1 μM carbachol induces large transient outward large conductance voltage- and Ca2+-activated K+ (BK) currents followed by suppression of the spontaneous transient outward BK currents (STBKCs) amplitude and frequency in freshly isolated rat detrusor smooth muscle (DSM) cells. A: representative recording illustrating the carbachol-evoked large transient outward BK currents followed by inhibition of the STBKC amplitude and frequency in a freshly isolated DSM cell. B: summarized data illustrating the inhibitory effect of carbachol on the amplitude and frequency of STBKCs (n = 8, N = 7; *P < 0.05). Average amplitude and frequency of STBKCs under control conditions were taken to be 100%, and data were normalized to controls.
Activation of M-Rs with carbachol suppresses the amplitude and frequency of STHs and depolarizes the RMP in freshly isolated DSM cells.
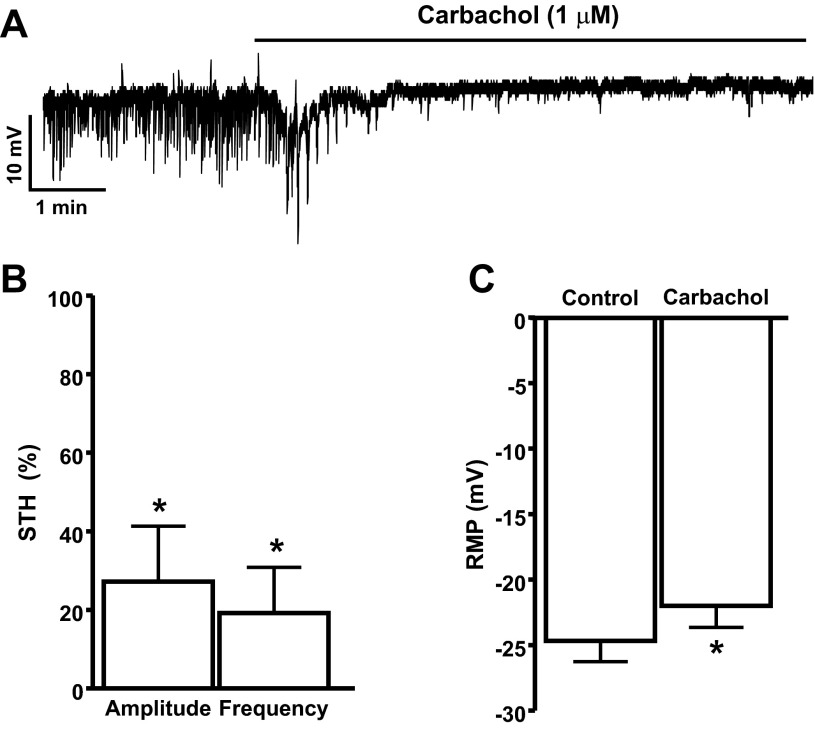
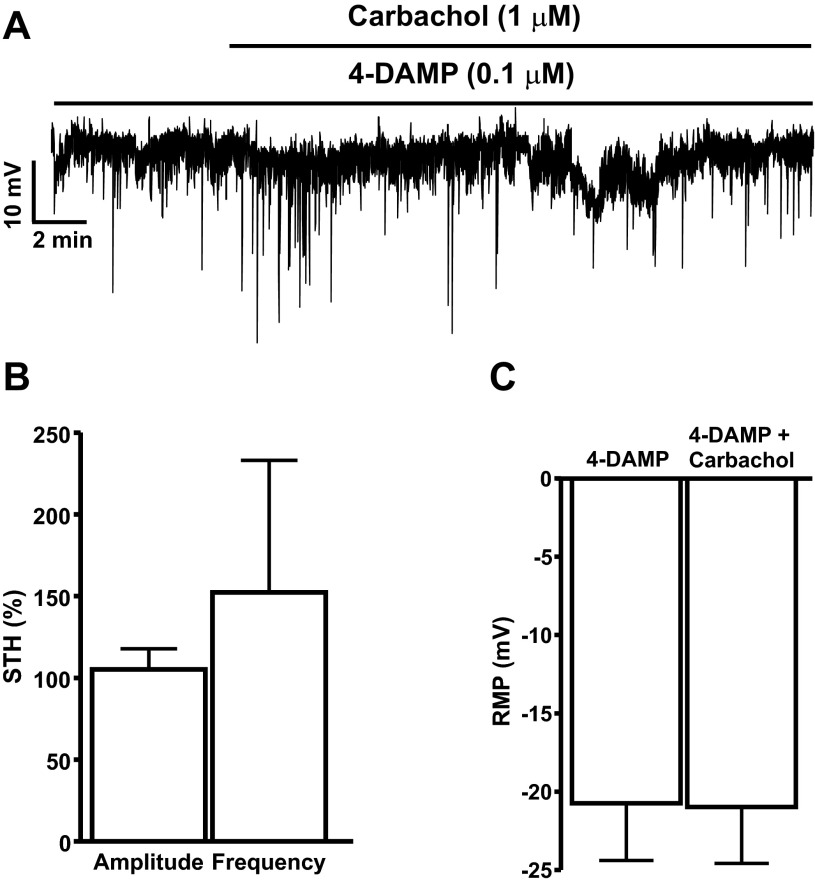
In current-clamp mode, DSM cells exhibit STHs, which are mediated by the BK channels and SR Ca2+ release from RyRs (24). Inhibition of the BK channels with iberiotoxin depolarizes DSM cell RMP and suppresses the STHs (24, 44). In the present study, we recorded RMP from 24 DSM cells (n = 24, N = 21) out of which 15 cells (n = 15, N = 15) exhibited STHs. To explore whether BK channels are functionally coupled to M-Rs at the cellular level, we examined the effects of M-R activation with carbachol on STHs and RMP of DSM cells. As shown in Fig. 2, A and B, 1 μM carbachol significantly inhibited the amplitude and frequency of STHs by 72.8 ± 14.1 and 80.8 ± 11.6%, respectively (n = 10, N = 10; P < 0.05). Furthermore, 1 μM carbachol also caused small but statistically significant depolarization of DSM cell RMP from −24.7 ± 1.6 to −22.0 ± 1.6 mV (n = 19, N = 16; P < 0.05, Fig. 2, A and C).
Fig. 2.
Activation of M-Rs with 1 μM carbachol leads to inhibition of the spontaneous transient hyperpolarization (STH) amplitude and frequency in freshly isolated rat DSM cells. A: representative recording depicting the inhibitory effects of carbachol on STH amplitude, frequency, and cell resting membrane potential (RMP) in a freshly isolated DSM cell. B and C: summarized data illustrating the inhibitory effect of carbachol on the amplitude and frequency of STHs (n = 10, N = 10; *P < 0.05) and cell RMP (n = 19, N = 16; *P < 0.05), respectively. Average amplitude and frequency of STHs under control conditions were taken to be 100%, and data were normalized to controls.
Carbachol inhibitory effects on STBKC are mediated by activation of M3-Rs in freshly isolated DSM cells.
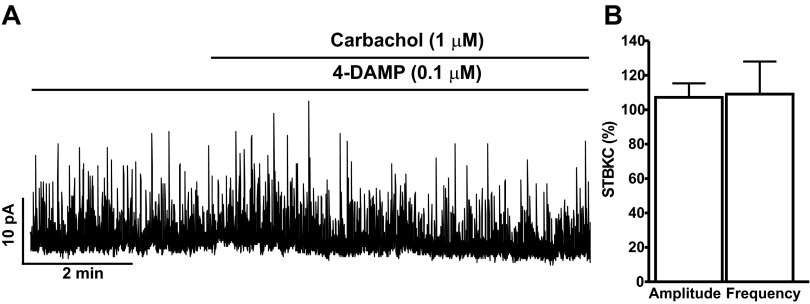
Previous studies on human and rat bladders have revealed that M2-Rs and M3-Rs are the primary M-Rs in DSM cells (45). Here, we examined the relative contribution of M3-Rs and M2-Rs on carbachol-induced inhibitory effects on DSM BK channel activity. To explore whether BK channel activity in DSM is regulated by activation of M3-Rs, we examined the effect of 1 μM carbachol on STBKCs in the presence of 0.1 μM 4-DAMP, a selective antagonist of M3-Rs. Preincubation of DSM cells with 0.1 μM 4-DAMP did not inhibit the STBKCs, which were still observed in the presence of 4-DAMP (n = 5, N = 5). Carbachol-evoked large transient outward BK currents were significantly blocked upon selective inhibition of the M3-Rs (n = 5, N = 5). In the presence of 0.1 μM 4-DAMP, carbachol did not change STBKC amplitude and frequency, and the values were 107.2 ± 8.2 and 109 ± 19.0%, respectively (n = 5, N = 5; P > 0.05; Fig. 3, A and B). These results support the concept that the carbachol-induced inhibitory effects on STBKCs were mediated by activation of M3-Rs.
Fig. 3.
The selective M3-R antagonist 4-diphenylacetoxy-N-methylpiperidine (4-DAMP) suppresses the carbachol inhibitory effects on the STBKC amplitude and frequency in freshly isolated rat DSM cells. A: a representative recording of a freshly isolated DSM cell illustrating the lack of both carbachol-evoked large transient outward BK currents and carbachol inhibitory effects on the STBKC amplitude and frequency in the presence of 0.1 μM 4-DAMP. B: summarized data illustrating the lack of carbachol inhibitory effects on the amplitude and frequency of STBKCs in the presence of 4-DAMP (n = 5, N = 5; P > 0.05). Average amplitude and frequency of STBKCs in the presence of 4-DAMP were taken to be 100% (control), and data were normalized to controls.
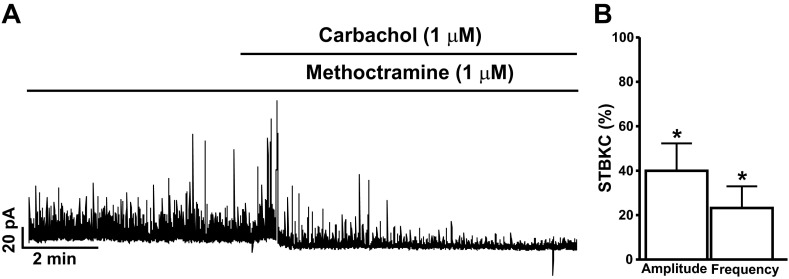
To explore if DSM BK channel activity is also mediated by M2-Rs, we tested the effect of 1 μM carbachol on STBKCs in the presence of 1 μM methoctramine, a selective antagonist of M2-Rs. Preincubation of DSM cells with 1 μM methoctramine did not inhibit the STBKCs, which were still observed in the presence of methoctramine (n = 15, N = 15). As illustrated in Fig. 4, pretreatment of DSM cells with 1 μM methoctramine did not significantly affect carbachol-evoked large transient outward BK currents or the carbachol inhibitory effect on the amplitude and frequency of STBKCs. In the presence of 1 μM methoctramine, 1 μM carbachol still significantly inhibited the amplitude and frequency of STBKCs by 60.1 ± 12.4 and 76.8 ± 9.8%, respectively (n = 15, N = 15; P < 0.05; Fig. 4, A and B). These results indicate that carbachol-induced inhibition of BK channel activity in rat DSM cells was independent of M2-R activation.
Fig. 4.
The selective M2-R antagonist methoctramine does not alter the carbachol inhibitory effects on the STBKC amplitude and frequency in freshly isolated rat DSM cells. A: representative recording of a freshly isolated DSM cell illustrating carbachol inhibitory effects on the amplitude and frequency of STBKCs in the presence of 1 μM methoctramine. B: summarized data illustrating the inhibitory effects of carbachol on the amplitude and frequency of STBKCs (n = 15, N = 15; *P < 0.05). Average amplitude and frequency of STBKCs in the presence of methoctramine were taken to be 100% (control), and data were normalized to controls.
Carbachol inhibitory effects on STHs and cell RMP are mediated by activation of M3-Rs in freshly isolated DSM cells.
To further explore whether DSM BK channel activity is mediated by activation of M3-Rs, we examined the effect of 1 μM carbachol on the amplitude and frequency of STHs and cell RMP in the presence of 0.1 μM 4-DAMP. As shown in Fig. 5, A and B, carbachol did not change the amplitude and frequency of STHs in the presence of the M3-R antagonist 4-DAMP (0.1 μM). The carbachol-induced effects on the amplitude and frequency of STHs compared with controls were 105.2 ± 12.6 and 152.3 ± 80.7%, respectively (n = 5, N = 5; P > 0.05; Fig. 5, A and B). Furthermore, the M3-R antagonist 4-DAMP (0.1 μM) also significantly blocked the carbachol-induced RMP depolarization. In the presence of 0.1 μM 4-DAMP, DSM cell RMP was −20.7 ± 3.6 mV, and it was −21.0 ± 3.6 mV in the presence of both 0.1 μM 4-DAMP and 1 μM carbachol (n = 5, N = 5; P < 0.05; Fig. 5, A and C). These results clearly indicate that carbachol-induced inhibition of BK channel activity was mediated by activation of M3-Rs.
Fig. 5.
The selective M3-R antagonist 4-DAMP suppresses the carbachol inhibitory effects on the STH amplitude and frequency in freshly isolated rat DSM cells. A: representative recording illustrating the lack of carbachol inhibitory effects on the amplitude and frequency of STHs and RMP depolarization in a freshly isolated DSM cell. B and C: summarized data illustrating the lack of carbachol inhibitory effects on the amplitude and frequency of STHs and cell RMP, respectively (n = 5, N = 5; P > 0.05). Average amplitude and frequency of STHs in the presence of 4-DAMP were taken to be 100% (control), and data were normalized to controls.
Inhibition of IP3 receptor with xestospongin-C attenuates carbachol-induced large transient outward BK currents without affecting the STBKCs in freshly isolated DSM cells.
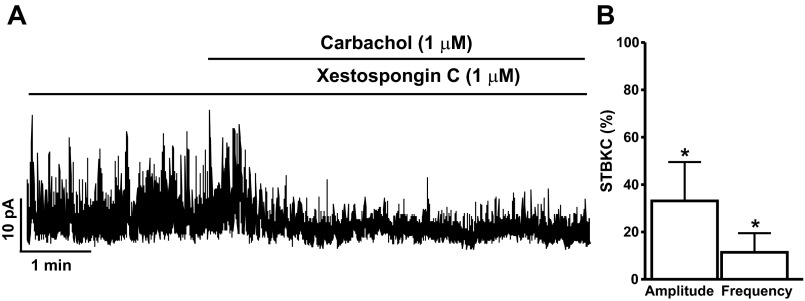
To explore whether carbachol-induced large transient outward BK currents were mediated by Ca2+ release from the IP3 receptors, we examined the effect of the IP3 inhibitor xestospongin-C on DSM BK channel activity. Xestospongin-C (1 μM) did not inhibit the STBKCs, which were still observed in the presence of xestospongin-C (n = 5, N = 4). As illustrated in Fig. 6, in the presence of 1 μM xestospongin-C, 1 μM carbachol did not evoke large transient outward BK currents in DSM cells. Xestospongin-C did not alter the carbachol inhibitory effects on STBKC amplitude and frequency. As shown in Fig. 6, the carbachol inhibitory effects on the amplitude and frequency of STBKCs compared with controls were 33.1 ± 16.4 and 11.4 ± 8.0%, respectively, in the presence of 1 μM xestospongin-C (n = 5, N = 4; P < 0.05). These results suggest the contribution of Ca2+ release from the IP3 receptors in the carbachol-induced large transient outward BK currents and that the IP3 receptors do not regulate STBKCs in DSM cells.
Fig. 6.
The inositol 1,4,5-triphosphate (IP3) receptor blocker xestospongin-C inhibits the carbachol-induced large transient outward BK currents in freshly isolated rat DSM cells. A: representative original recording illustrating the carbachol inhibitory effects on the amplitude and frequency of the STBKCs in the presence of the IP3 receptor blocker xestospongin-C (1 μM). B: summarized data illustrating the effects of carbachol on the STBKC amplitude and frequency in the presence of xestospongin-C (n = 5, N = 4; *P < 0.05). Average amplitude and frequency of STBKCs in the presence of xestospongin-C were taken to be 100% (control), and data were normalized to controls.
Activation of M-Rs with carbachol in the absence of all known cellular sources of Ca2+ for BK channel activation does not change the steady-state BK currents in freshly isolated DSM cells.
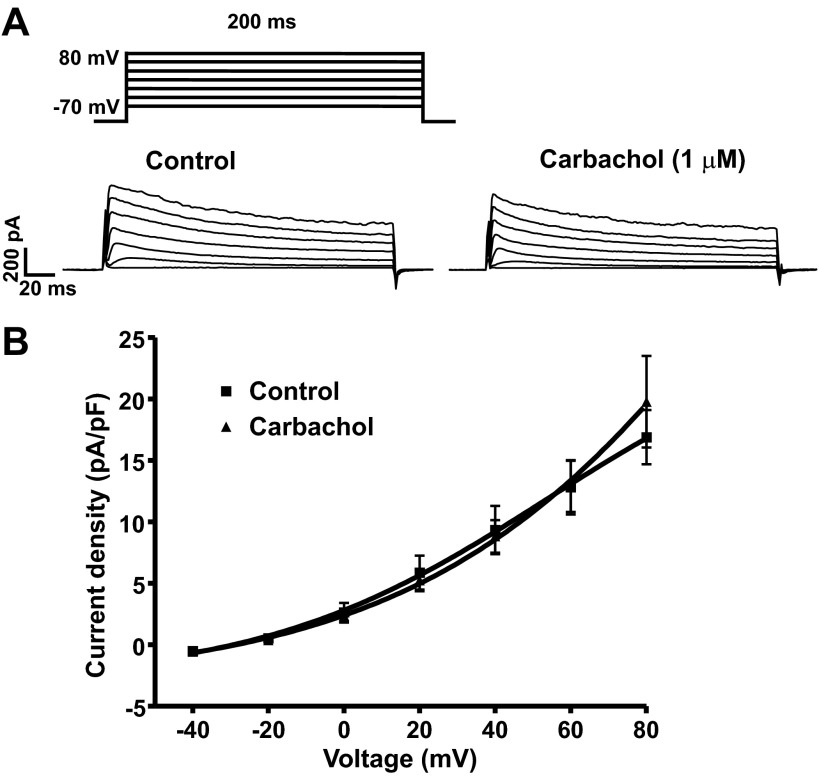
A previous study from our laboratory has shown that in rat DSM cells the voltage-step-induced steady-state outward K+ currents are primarily mediated by BK channel activity (25). To explore whether the activation of M-Rs with carbachol affects the voltage-step-induced steady-state BK currents, we evaluated the effects of 1 or 100 μM carbachol on voltage-step depolarization-induced K+ currents. We performed these experiments in the presence of 30 μM ryanodine (RyR blocker), 1 μM nifedipine (L-type voltage-dependent Ca2+ channel blocker), and 100 nM thapsigargin (SR Ca2+-ATPase blocker) in the bath solution to block all known cellular sources of Ca2+ for BK channel activation. Under these experimental conditions, 1 μM carbachol did not affect the voltage-step depolarization-induced BK currents (n = 7, N = 6; P > 0.05, Fig. 7). A higher concentration of carbachol (100 μM) also failed to affect the steady-state BK currents. At the highest depolarization voltage applied (+80 mV), the steady-state BK currents were 14.7 ± 1.7 and 15.9 ± 2.9 pA/pF in the absence and in the presence of 100 μM carbachol, respectively (n = 9, N = 6; P > 0.05; data not illustrated). These results suggest that in the absence of all known cellular sources of Ca2+ for BK channel activation, M-R activation with carbachol does not directly change the activity of BK channels.
Fig. 7.
Muscarinic receptor agonist carbachol does not affect the voltage-step induced steady-state BK currents upon pharmacological inhibition of all known cellular sources of Ca2+ for BK channel activation in freshly isolated rat DSM single cells. A: representative voltage-clamp recordings illustrating that 1 μM carbachol did not affect the steady-state BK currents at depolarization voltages from −40 to +80 mV. B: current-voltage relationship curve illustrating the lack of carbachol effect on the steady-state BK currents (n = 7, N = 6; P > 0.05). Steady-state BK currents were recorded in the presence of 30 μM ryanodine, 100 nM thapsigargin, and 1 μM nifedipine to block the ryanodine receptors (RyRs), sarcoplasmic reticulum (SR) Ca2+-ATPase, and L-type voltage-dependent Ca2+ channels, respectively.
DISCUSSION
The present study revealed that M3-Rs are functionally coupled with the BK channels in freshly isolated rat DSM cells. Our data demonstrated that activation of M3-Rs in native DSM cells: 1) initially elicits large transient outward BK currents followed by a drastic inhibition of the amplitude and frequency of STBKCs, 2) suppresses the amplitude and frequency of STHs and depolarizes DSM cell RMP, and 3) increases DSM cell excitability.
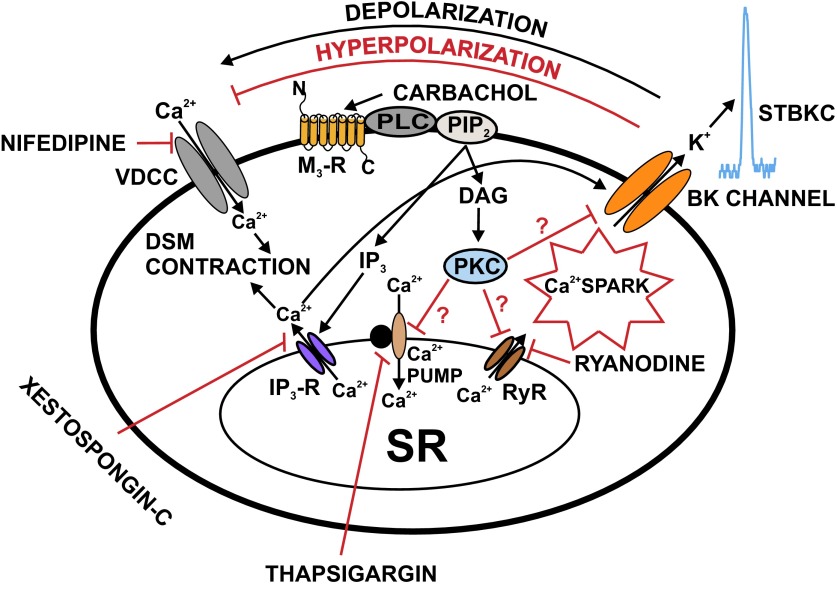
Various studies have shown that M-R agonists transiently increase the BK currents followed by their inhibition in different smooth muscle cell types (3, 28, 30). Following M-R activation in freshly isolated DSM cells, the initial appearance of large transient outward BK currents was due to a rapid IP3-induced release of Ca2+ from the SR and the inhibitory effects on STBKCs were due to either reduced Ca2+ release via RyRs or direct inhibition of the RyRs (Fig. 8). Previous studies have shown that inhibition of RyRs with ryanodine inhibits the STBKCs in DSM of rats (25), guinea pigs (33), and humans (24).
Fig. 8.
Schematic diagram illustrating the mechanisms by which M3-R regulates BK channel function in rat DSM cells. Activation of M3-Rs leads to IP3 and diacylglycerol (DAG) production, via a pathway involving PLC and PIP2. IP3 activates IP3-Rs, which releases Ca2+ from the SR. This IP3-induced Ca2+ release transiently activates the BK channels. Over time, depletion of SR Ca2+ upon activation of M3-Rs reduces Ca2+ spark activity, which leads to inhibition of STBKCs and depolarization of DSM cell RMP causing activation of VDCC, and thus increases DSM contractility. DAG activates PKC, which may lead to direct inhibition of the SR Ca2+ pump and RyRs resulting in suppression of Ca2+ sparks and STBKCs. Pharmacological tools used in this study to inhibit cellular sources of Ca2+ are indicated. DAG, diacylglycerol; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase-C; PLC, phospholipase-C; VDCC, L-type voltage-dependent Ca2+ channel.
The physiological contractions of DSM, which are required for voiding, are under control of the parasympathetic nervous system (1). Parasympathetic nerve fibers releasing acetylcholine (ACh) and ATP contribute to DSM contraction. ACh, which is the primary contractile neurotransmitter in DSM cells, activates M-Rs. M3-Rs are the main pathway for the different phases of the cholinergic response in both adult and newborn DSM tissue whereas the M2-Rs are of lesser importance (15). The carbachol-induced smooth muscle contraction has been reported to be due to M3-R activation leading to IP3-induced Ca2+ release and then membrane potential depolarization (39). Earlier studies by our group have showed that pharmacological inhibition of the BK channels with iberiotoxin or paxilline suppressed the STBKCs, STHs, and depolarized the RMP in freshly isolated DSM cells of humans (24, 44), guinea pigs (22, 43), and rats (25). The present data demonstrated that activation of M-Rs with carbachol inhibited the STBKCs, STHs, and depolarized cell RMP, and these inhibitory effects on the BK channel activity were eliminated by the selective M3-R antagonist 4-DAMP (Figs. 1–3, and 5). 4-DAMP has been shown to inhibit M3-Rs with a higher affinity (pA2) value of 8.5 to 8.9 (∼1 nM) in rat DSM (4, 19). Our data are consistent with those studies because carbachol-induced inhibitory effects on BK channel activity were significantly blocked by 0.1 μM 4-DAMP (Fig. 5). This suggests that M3-Rs are functionally coupled with the BK channels. Activation of M3-R causes BK channel inhibition, thus increasing DSM cell excitability (Fig. 8).
The functional role of M2-Rs in DSM excitability and contractility is not clear, although its contribution has been demonstrated under some pathological conditions such as neurogenic bladder dysfunction and bladder hypertrophy (12, 34, 36). In other smooth muscle types such as tracheal and gastrointestinal, activation of M2-Rs has been shown to cause a direct inhibition of the BK channels (9, 40). In rat DSM-isolated strips, 4-DAMP and methoctramine have the potency (pA2) of 8.5 and 6.5 for M3-Rs and M2-Rs, respectively (4). Methoctramine, which discriminates between M2-Rs and M3-Rs, has a lower potency (pA2) value of 5.9 for M2-Rs in rat DSM (19). Our results showed that selective inhibition of M2-Rs with 1 μM methoctramine did not alter carbachol inhibitory effect on the BK channel activity (Fig. 4). The functional role of M2-Rs in carbachol-induced inhibition of the BK channel activity has been demonstrated in rat DSM cells using elevated intracellular Ca2+ concentrations and conventional whole cell patch-clamp technique (30). Unlike this study, our study using the perforated patch-clamp technique, which preserves the native intracellular environment including Ca2+ signaling pathways, showed that M2-Rs were not involved in BK channel regulation in rat DSM cells under physiological conditions. Our data clearly indicate that carbachol inhibitory effects on STBKCs were not mediated by M2-Rs, but only by M3-Rs.
Previous studies on DSM of experimental animals and humans showed that M-R agonist-induced DSM contractions were either due to Ca2+ entry through L-type voltage-dependent Ca2+ channels or Ca2+ release from the SR (17, 35, 37, 42). The contribution of the SR Ca2+ release via RyRs for activation of STBKCs has been shown in DSM cells isolated from guinea pigs (22, 33). Simultaneous recordings of Ca2+ sparks and whole cell BK currents directly demonstrated the requirement of the SR Ca2+ release via RyRs for activation of STBKC in DSM of guinea pigs (22). Our data showed that in the presence of all known cellular sources of Ca2+ for BK channel activation, M3-R activation with carbachol inhibited the STBKCs, STHs, and depolarized cell RMP (Figs. 1 and 2). Moreover, M-R activation with carbachol did not affect the depolarization-evoked steady-state BK currents in the presence of thapsigargin, ryanodine, and nifedipine-pharmacological inhibitors of Ca2+ sources for BK channel activation (Fig. 7). These results indicate that mobilization of intracellular Ca2+ contributes to interaction between BK channels and M-Rs in DSM cells.
It has been demonstrated that M-R activation with carbachol enhanced the IP3 formation in DSM cells (29, 37, 42). An involvement of IP3 receptors has also been reported in ACh-induced suppression of the BK channel activity in murine colonic myocytes (2). Our data showed that pretreatment of DSM cells with IP3 receptor inhibitor xestospongin-C completely blocked carbachol-induced large transient outward BK currents. These data suggest that carbachol-induced large transient outward BK currents were attributed to the IP3-induced Ca2+ release from intracellular stores (Figs. 1, 6, and 8). This IP3-induced Ca2+ release transiently activates the BK channels and eventually causes a depletion of SR Ca2+. Depletion of SR Ca2+ upon activation of M3-Rs reduces Ca2+ spark activity, which leads to inhibition of STBKCs and depolarization of DSM cell RMP causing enhancement of voltage-dependent Ca2+ channels and DSM contractility (Figs. 1, 2, and 8). Ca2+ influx or releases associated with PKC pathways have been suggested to be involved in regulating DSM function (1, 7). As illustrated in Fig. 8, in rat DSM cells, M3-Rs activation with carbachol leads to diacylglycerol production, which activates PKC. Thus activated PKC may lead to direct inhibition of the SR Ca2+ pumps and RyRs resulting in suppression of Ca2+ sparks and STBKCs. However, our data cannot rule out a possible inhibition of the BK channel by PKC via direct channel phosphorylation in rat DSM cells (Fig. 8).
In conclusion, we revealed that in rat DSM cells the M3-Rs are functionally coupled to the BK channels. Our findings provide strong evidence that BK channel activity in DSM cells is inhibited upon M3-R activation. Activation of M3-Rs leads to inhibition of the STBKCs and STHs and depolarizes DSM cell RMP. M3-R dysfunction or its uncoupling from BK channels may contribute to some urinary bladder disorders such as overactive bladder. Because the antimuscarinic agents are the mainstay in treating overactive bladder, the present findings provide important mechanistic details of muscarinic signaling pathways in DSM.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-084284 (to G. V. Petkov) and a fellowship from Urological Care Foundation Research Scholars Program and Allergan Foundation (to S. P. Parajuli).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.P.P. and G.V.P. performed experiments; S.P.P. and G.V.P. analyzed data; S.P.P. and G.V.P. interpreted results of experiments; S.P.P. and G.V.P. prepared figures; S.P.P. and G.V.P. drafted manuscript; S.P.P. and G.V.P. edited and revised manuscript; S.P.P. and G.V.P. approved final version of manuscript; G.V.P. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Kiril Hristov, Dr. John Malysz, Dr. Wenkuan Xin, Dr. Ning Li, and Amy Smith for the critical evaluation of the manuscript.
REFERENCES
- 1. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bayguinov O, Hagen B, Sanders KM. Muscarinic stimulation increases basal Ca2+ and inhibits spontaneous Ca2+ transients in murine colonic myocytes. Am J Physiol Cell Physiol 280: C689–C700, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bolton TB, Lim SP. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol 409: 385–401, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braverman A, Legos J, Young W, Luthin G, Ruggieri M. M2 receptors in genito-urinary smooth muscle pathology. Life Sci 64: 429–436, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckley NJ, Bonner TI, Buckley CM, Brann MR. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol 35: 469–476, 1989 [PubMed] [Google Scholar]
- 7. Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50: 279–290, 1998 [PubMed] [Google Scholar]
- 8. Chess-Williams R, Chapple CR, Yamanishi T, Yasuda K, Sellers DJ. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J Auton Pharmacol 21: 243–248, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Cole WC, Sanders KM. G proteins mediate suppression of Ca2+-activated K+ current by acetylcholine in smooth muscle cells. Am J Physiol Cell Physiol 257: C596–C600, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Doods HN, Mathy MJ, Davidesko D, van Charldorp KJ, de Jonge A, van Zwieten PA. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther 242: 257–262, 1987 [PubMed] [Google Scholar]
- 11. Dorje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther 256: 727–733, 1991 [PubMed] [Google Scholar]
- 12. Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev 48: 531–565, 1996 [PubMed] [Google Scholar]
- 13. Ehlert FJ. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci 74: 355–366, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Ehlert FJ, Griffin MT, Abe DM, Vo TH, Taketo MM, Manabe T, Matsui M. The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J Pharmacol Exp Ther 313: 368–378, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Ekman M, Andersson KE, Arner A. Signal transduction pathways of muscarinic receptor mediated activation in the newborn and adult mouse urinary bladder. BJU Int 103: 90–97, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hashitani H, Bramich NJ, Hirst GD. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol 524: 565–579, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hashitani H, Yanai Y, Shirasawa N, Soji T, Tomita A, Kohri K, Suzuki H. Interaction between spontaneous and neurally mediated regulation of smooth muscle tone in the rabbit corpus cavernosum. J Physiol 569: 723–735, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol 120: 1409–1418, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol 541: 483–492, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 302: C1632–C1641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hulme EC, Birdsall NJ, Buckley NJ. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol 30: 633–673, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Hurley BR, Preiksaitis HG, Sims SM. Characterization and regulation of Ca2+-dependent K+ channels in human esophageal smooth muscle. Am J Physiol Gastrointest Liver Physiol 276: G843–G852, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Ikeda K, Kobayashi S, Suzuki M, Miyata K, Takeuchi M, Yamada T, Honda K. M3 receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Naunyn Schmiedebergs Arch Pharmacol 366: 97–103, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Nakamura T, Kimura J, Yamaguchi O. Muscarinic M2 receptors inhibit Ca2+-activated K+ channels in rat bladder smooth muscle. Int J Urol 9: 689–696, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Pontari MA, Braverman AS, Ruggieri MR, Sr The M2 muscarinic receptor mediates in vitro bladder contractions from patients with neurogenic bladder dysfunction. Am J Physiol Regul Integr Comp Physiol 286: R874–R880, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rivera L, Brading AF. The role of Ca2+ influx and intracellular Ca2+ release in the muscarinic-mediated contraction of mammalian urinary bladder smooth muscle. BJU Int 98: 868–875, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Ruggieri MR, Sr, Braverman AS. Regulation of bladder muscarinic receptor subtypes by experimental pathologies. Auton Autacoid Pharmacol 26: 311–325, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schneider T, Fetscher C, Krege S, Michel MC. Signal transduction underlying carbachol-induced contraction of human urinary bladder. J Pharmacol Exp Ther 309: 1148–1153, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Schneider T, Hein P, Michel MC. Signal transduction underlying carbachol-induced contraction of rat urinary bladder. I. Phospholipases and Ca2+ sources. J Pharmacol Exp Ther 308: 47–53, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Wade GR, Sims SM. Muscarinic stimulation of tracheal smooth muscle cells activates large-conductance Ca2+-dependent K+ channel. Am J Physiol Cell Physiol 265: C658–C665, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther 273: 959–966, 1995 [PMC free article] [PubMed] [Google Scholar]
- 42. Wuest M, Hiller N, Braeter M, Hakenberg OW, Wirth MP, Ravens U. Contribution of Ca2+ influx to carbachol-induced detrusor contraction is different in human urinary bladder compared to pig and mouse. Eur J Pharmacol 565: 180–189, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Xin W, Cheng Q, Soder RP, Petkov GV. Inhibition of phosphodiesterases relaxes detrusor smooth muscle via activation of the large-conductance voltage- and Ca2+-activated K+ channel. Am J Physiol Cell Physiol 302: C1361–C1370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xin W, Cheng Q, Soder RP, Rovner ES, Petkov GV. Constitutively active phosphodiesterase activity regulates urinary bladder smooth muscle function: critical role of KCa1.1 channel. Am J Physiol Renal Physiol 303: F1300–F1306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamaguchi O, Shishido K, Tamura K, Ogawa T, Fujimura T, Ohtsuka M. Evaluation of mRNAs encoding muscarinic receptor subtypes in human detrusor muscle. J Urol 156: 1208–1213, 1996 [PubMed] [Google Scholar]
- 46. Zhou XB, Wulfsen I, Lutz S, Utku E, Sausbier U, Ruth P, Wieland T, Korth M. M2 muscarinic receptors induce airway smooth muscle activation via a dual, Gbetagamma-mediated inhibition of large conductance Ca2+-activated K+ channel activity. J Biol Chem 283: 21036–21044, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]