Abstract
Endothelial adhesion molecules are critical effectors of inflammation ensuring coordinated interactions that allow leukocytes to home to sites of injury. These adhesion molecules are often extensively modified posttranslationaly by the addition of N-glycans, but if, or how, these modifications contribute to the protein function remains poorly understood. Herein we show that activated endothelial cells express two distinct N-glycoforms of intercellular adhesion molecule 1 (ICAM-1) that comprise a complex N-glycoform with α-2,6 sialic acid present at relatively high levels and a second, less abundant and previously undescribed high-mannose glycoform (HM-ICAM-1). This novel HM-ICAM-1 glycoform was also detected in human coronary artery specimens and moreover appeared to be the dominant glycoform in vivo. Production of exclusively HM-ICAM-1 in cells by α-mannosidase inhibition increased monocyte rolling and adhesion compared with mature ICAM-1 consistent with high-mannose epitopes providing leukocyte ligands. Cross-linking of ICAM-1 transmits outside-in signals that affect endothelial permeability and survival. Interestingly, cell signaling (assessed using ERK, VE-cadherin, and Akt phosphorylation) was maintained after cross-linking of HM-ICAM-1 compared with mature ICAM-1; however, interactions with the actin cytoskeleton were lost with HM-ICAM-1. These findings suggest that specific ICAM-1 N-glycoforms modulate distinct aspects of the inflammatory response and identify HM-ICAM-1 as a new therapeutic target for controlling leukocyte trafficking and endothelial inflammation.
Keywords: N-glycan, inflammation, adhesion molecule, endothelial cell
during inflammation, leukocytes must migrate across the vessel wall to reach underlying regions of disease (43, 52). This process involves rolling and adhesion steps that facilitate firm contacts between the circulating leukocytes and endothelial cells lining the vessel wall. The protein mediators of this process have been elucidated and based on these findings our current knowledge of the contributions of endothelial adhesion molecules such as ICAM-1, VCAM-1, and E-selectin have been defined (19, 32, 33). Interestingly, these adhesion molecules are all glycoproteins with extensive posttranslational modifications in the form of N-glycans.
Protein N-glycosylation is an enzyme-driven process that covalently attaches a core tetradecasaccharide onto the amide residues of asparagines in an N-X-S/T sequon (X cannot be proline). Subsequent enzymes in the endoplasmic reticulum remove glucose and mannose before enzymes in the Golgi apparatus catalyze the addition of multiple monosaccharide subunits (fucose, galactose, N-acetylglucosamine, and sialic acid) generating a panoply of N-glycan structures (45). N-glycans can be divided into three classes based on their monosaccharide content. These include high-mannose N-glycans, which contain five to nine mannose residues with no additional diversity (beyond the two core N-acetylglucosamines); hybrid N-glycans, which contain three to five mannose residues and only one antennae of saccharides; or complex N-glycans with three mannose residues and two to four antennae of saccharides. As this process is constrained by linearity whereby mannose trimming must occur before addition of other monosaccharides, inhibition of mannose removal can result in increased production of high-mannose and hybrid N-glycan structures, which are considered to be hypo-N-glycosylated.
Under normal physiological conditions, the overwhelming majority of N-glycans are processed to the complex state (12); therefore, production of under processed N-glycans could be viewed as functionally impaired. Indeed, recent studies have reported that inflammation is associated with a decrease in N-glycan complexity and increased expression of high-mannose and hybrid structures (referred to as hypoglycosylated N-glycans; Refs. 21, 23, 31, 36). Moreover, genetic manipulation or pharmacological inhibition of N-glycan processing to limit N-glycan maturation modulates the inflammatory response (1, 4, 21) underscoring the importance of N-glycan branching in immune function. Recently, we demonstrated that proinflammatory stimulation of endothelial cells results in increased hypoglycosylated N-glycans on the cell surface (10, 41), possibly by inhibition of early mannose trimming (α-mannosidase) enzymes (34, 41). However, the proteins carrying these structures remain unknown.
Intercellular adhesion molecule 1 (ICAM-1, CD54) is a member of the Ig-like adhesion molecule superfamily and binds to the cognate leukocyte integrins LFA-1 (CD11a/CD18) and Mac1 (CD11b/CD18). ICAM-1 is known to mediate leukocyte rolling, adhesion, and transmigration (14, 17, 18, 32), and mice lacking functional ICAM-1 display decreased inflammatory disease progression (9, 11, 35). Moreover, ICAM-1 ligation and cross-linking elicit outside-in signaling that regulates endothelial permeability and survival (2, 15, 16, 20, 21, 29, 39, 48–50) highlighting the multiple functions of this protein in the immune response. Previous reports have demonstrated the ICAM-1 N-glycan content depends on the cell type in which the protein is produced (8) and specific ICAM-1 glycoforms are known to have altered function (13, 38). However, whether ICAM-1 N-glycosylation is controlled in endothelial cells during inflammation and whether this modulates the adhesive and signaling functions of ICAM-1 remain unclear.
Herein we identify a novel high-mannose ICAM-1 glycoform with distinct functions We further demonstrate that HM-ICAM-1 is present in human arteries, show that it supports increased monocyte adhesion and maintains normal mitogenic cell signaling, but displays impaired interactions with the actin cytoskeleton.
MATERIALS AND METHODS
Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Rockville, MD), THP-1 cells were purchased from ATCC (Rockville, MD), and Cos-1 cells were a generous gift of Joanne Murphy-Ullrich (University of Alabama, Birmingham). MCDB-131, DMEM (low glucose), HI-FBS, trypsin-EDTA, and l-glutamine were purchased from Invitrogen (Carlsbad, CA). All lectins were from Vector Laboratories (Burlingame, CA), and other reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Cell culture.
HUVECs were propagated on gelatin-coated dishes in MCDB-131 containing 10% FBS, 25 μg/ml endothelial cell growth supplement (BD Biosciences), 1 mM l-glutamine, 30 μg/ml heparin, and penicillin (100 U/ml)/streptomycin (100 μg/ml) and used between passages 3–7. Cos-1 cells were maintained in DMEM containing 10% FBS and penicillin (100 U/ml)/streptomycin (100 μg/ml). THP-1 cells were maintained in RPMI 1640 containing 10% FBS, penicillin (100 U/ml)/streptomycin (100 μg/ml), and 2 mM β-mercaptoethanol. For adhesion experiments, THP-1 monocytes were labeled with Cell-Tracker Green (1 μM) for 15 min at 37°C. To generate hypoglycosylated glycoproteins, HUVECs were treated with kifunensine (40 ng/ml) or swainsonine (1 μM), class I and II α-mannosidase inhibitors respectively, for 2 h before TNF-α stimulation and Cos1 cells were treated at the same concentration at the time of transfections.
Generation of pCMV-ICAM-1 and stable transfections.
pQCXIN-FLAG-ICAM-1 was obtained from Laurent Coscoy (University of California, Berkeley) and subcloned into pCMV-Script using the NotI and EcoRI sites. Lipofectamine 2000 (Invitrogen) was used for transfections according to the manufacturer's protocols. Cos1 cells were selected in media supplemented with 750 μg/ml G418 for 3 wk.
Glycosidase digestion.
Endothelial cells were treated as described and digested with endoglycosidase H (Endo H) or peptide-N-glycosidase F (PNGaseF; both from Prozyme, Hayward, CA) according to the manufacturers protocol.
Coronary artery protein isolation.
Coronary arteries were obtained post mortem, the adventitia was carefully and thoroughly removed, and the vessel (∼2 cm segment) was lysed (50 mM Tris pH 2.4, 150 mM NaCl, 1% Triton X-100, 0.5% NP-40, 0.5% sodium deoxycholate, 1 mM MgCl2, 1 mM CaCl2, and 1 mM PMSF) using 50 strokes of a dounce homogenizer before being cleared by centrifugation at 14,000 g for 10 min. The supernatant was collected and incubated with concanavalin A (ConA; high-mannose binding lectin) agarose (with a small fraction reserved for loading control) for 4 h with gentle rocking before being washed extensively with lysis buffer. Bound proteins were released by boiling in SDS-PAGE sample buffer before being resolved for Western blot analysis.
Adhesion assay.
Adhesion assays were performed as previously described (41). For static assays, HUVECs or Cos-1 cells were grown in 48-well plates and treated as described for each experiment. Cells were washed with warm PBS and incubated with 6 × 104 Cell-Tracker Green labeled THP-1 monocytes for 30 min at 37°C. Plates were gently washed with PBS and fluorescence was measured on a Victor2 Perkin-Elmer Fluorescent plate reader (Exc = 485 nm and Em = 535 nm). For assays under conditions of flow, Cos1 cells were grown on 35-mm dishes and transfected with pCMV or pCMV-ICAM-1 and 48 h later adhesion was analyzed at 1 dyn/cm2 in RPMI basal media (without serum) containing calcium and magnesium and 2.5 × 105 THP-1 cells/ml. The cells were viewed on a Leica inverted fluorescence microscope equipped with a Hamamatsu Orca ER digital CCD camera (Compix, Cranberry Township, PA). Real-time images were captured for 2 min, and any cell that did not move for 5 s or more was considered to have adhered.
Lectin pull-down.
HUVECs plated in 60-mm dishes were treated as described and at the end of treatment were washed once with ice-cold PBS containing CaCl2 and MgCl2 (1 mM each; PBS + ions) before being incubated in the same buffer at 4°C for 10 min to depolymerize the cytoskeleton and abolish endocytosis. Cells were then incubated with 20 μg of biotinylated lectin (ConA, SNA, MAA, PHA-L, or LCA; see Table 1 for definitions) in 2 ml PBS + ions for 10 min at 4°C. At the end of incubation, cells were washed with PBS + ions and lysed (PBS, 1% Triton X-100, 1 mM CaCl, and 1 mM MgCl2 + protease inhibitors). Lysates were incubated on ice for 10 min and clarified at 14,000 g for 10 min. The resulting supernatant was incubated with streptavidin Dynal beads (Invitrogen) for 2 h at 4°C with gentle rocking (some lysate was reserved for input control). Beads were washed three times with lysis buffer before boiling in SDS-PAGE sample buffer and were resolved for Western blot analysis.
Table 1.
Biotinylated lectins used and their glycan binding specificities
| Lectin Name | Acronym | Sugar Specificity | Glycan Structure |
|---|---|---|---|
| Concanavalin A | ConA | Manα1–6(Manα1–3)Man | High mannose >>> Hybrid N-glycan |
| Lens culinaris agglutinin | LCA | Fuc α1–6GlcNAc, αD-Glc, α-D-Man | Hybrid N-glycan |
| Sambucus nigra lectin | SNA | Siaα2–6Gal/GalNAc | Complex/hybrid, O-glycan |
| Maackia amurensis lectin | MAA | Siaα2,3Gal | Complex N-glycan |
| Phaseolus vulgaris agglutinin | PHA-L | Galβ4GlcNAcβ6 (GlcNAβ2Manα3)Manα3 | Complex N-glycan |
Values are means ± SE.
Analysis of VE-cadherin phosphorylation.
VE-cadherin phosphorylation was determined as previously described (2). Briefly, HUVEC cultured in 35-mm dishes were treated with TNF-α (10 ng/ml, 6 h) and some cells were pretreated with kifunensine as described above. HUVECs were washed with warm PBS and then either incubated in media as before or media containing 1 × 105 THP-1 cells for 15 min. Cells were immediately lysed in boiling SDS-PAGE sample buffer and analyzed by Western blot as described below.
Antibody-mediated ICAM-1 clustering.
HUVECs were treated with TNF-α (10 ng/ml, 6 h) in the presence or absence of kifunensine or swainsonine. At the end of treatment, the media were removed, cells were washed with PBS (37°C), and serum free media containing mouse anti-ICAM-1 (RR1/1; eBiosciences; 1 ug/ml) was added for 15 min. Cells were then washed as before and incubated in media alone or media containing goat anti-mouse (1 ug/ml) for 30 min to cluster ICAM-1.
Immunofluorescence microscopy.
HUVECs were grown on glass coverslips and treated with TNF-α (10 ng/ml, 6 h) in the presence or absence of kifunensine or swainsonine and underwent antibody-mediated ICAM-1 clustering as described above except that an Alexa 488-conjugated goat anti-mouse antibody was used. Cells were fixed with 4% paraformaldehyde for 20 min, washed, and viewed on a Leica DMI600B fluorescent microscope equipped with a Hamamatsu OrcaER digital camera.
Membrane fractionation.
HUVECs grown on 35-mm culture dishes were treated as described for experiments and lysed in 100 μl TST (25 mM Tris-pH 7.4, 150 mM NaCl, 0.02% Triton X-100, and protease inhibitor cocktail) for 5 min on ice. Lysates were cleared at 14,000 g for 3 min, and the supernatant was kept as the soluble fraction. The pellet was washed in TST and cleared at 14,000 g for 3 min. The washed pellet was then lysed in 100 μl of TST containing 60 μM β-octylglucopyranoside for 20 min at 37°C and collected at 20,000 g for 5 min. The resulting supernatant was considered the Triton X-100 insoluble fraction. Equal volumes from each fraction were analyzed by Western blot analysis.
Immunoprecipitation.
For interaction with the Ezrin-Radixin-Moesin (ERM) complex, ICAM-1 immunoprecipitations were performed as previously described (25). Briefly, proteins were collected in lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, and protease inhibitor cocktail), cleared by centrifugation at 14,000 g for 5 min, and incubated with Protein A Dynal beads (Invitrogen) and 1 μg mouse anti -ICAM-1 (Abcam-ab2213) overnight at 4°C with gentle rocking. Beads were washed three times with lysis buffer, and bound proteins were released by boiling in SDS-PAGE sample buffer. A small portion (∼10%) of the original lysate was reserved for input control.
For interaction with caveolin-1, ICAM-1 immunoprecipitations were performed as previously described (25). Briefly, cells were treated and lysed in modified RIPA buffer (50 mM Tris pH 7.4, 150 mM NaCl, 0.1% SDS, 0.5% deoxycholate, 0.5% NP-40, 1 mM MgCl2, 1 mM sodium orthovanadate, and protease inhibitor cocktail) and further processed as above.
Western blotting.
Samples were resolved on 4–15% TGX gels (Bio-Rad, Hercules, CA) and transferred to PVDF membranes. Blots were blocked with 5% milk in TBS + 0.1% Tween-20 (TBST) and incubated overnight at 4°C with antibodies against ICAM-1 (4915) pERK (T202, Y204) (4370), ERK (4695), pAKT (S473) (9271), Akt (9272), and moesin (3146) (all from Cell Signaling Technologies; Danvers, MA); caveolin-1 (sc894) and VE-cadherin (sc28644) (both from Santa Cruz Biotechnology; Santa Cruz, CA); pVE-cadherin (Y658) (441144G) (Invitrogen); and β-actin (ab123020) (Abcam; Cambridge, MA) in 2% heat denatured BSA in TBST. Blots were washed in TBST, incubated with horseradish peroxidase-conjugated secondary antibody raised in the appropriate host, washed again in TBST, and signals were detected using standard enhanced chemiluminescence.
Statistics.
All experiments were conducted a minimum of three times and where appropriate significant differences were calculated as P < 0.05 by one-way ANOVA with Tukey posttest or t-test using GraphPad Prism 5. Western blots were analyzed using ImageJ.
RESULTS
Identification of two ICAM-1 glycoforms in activated endothelium.
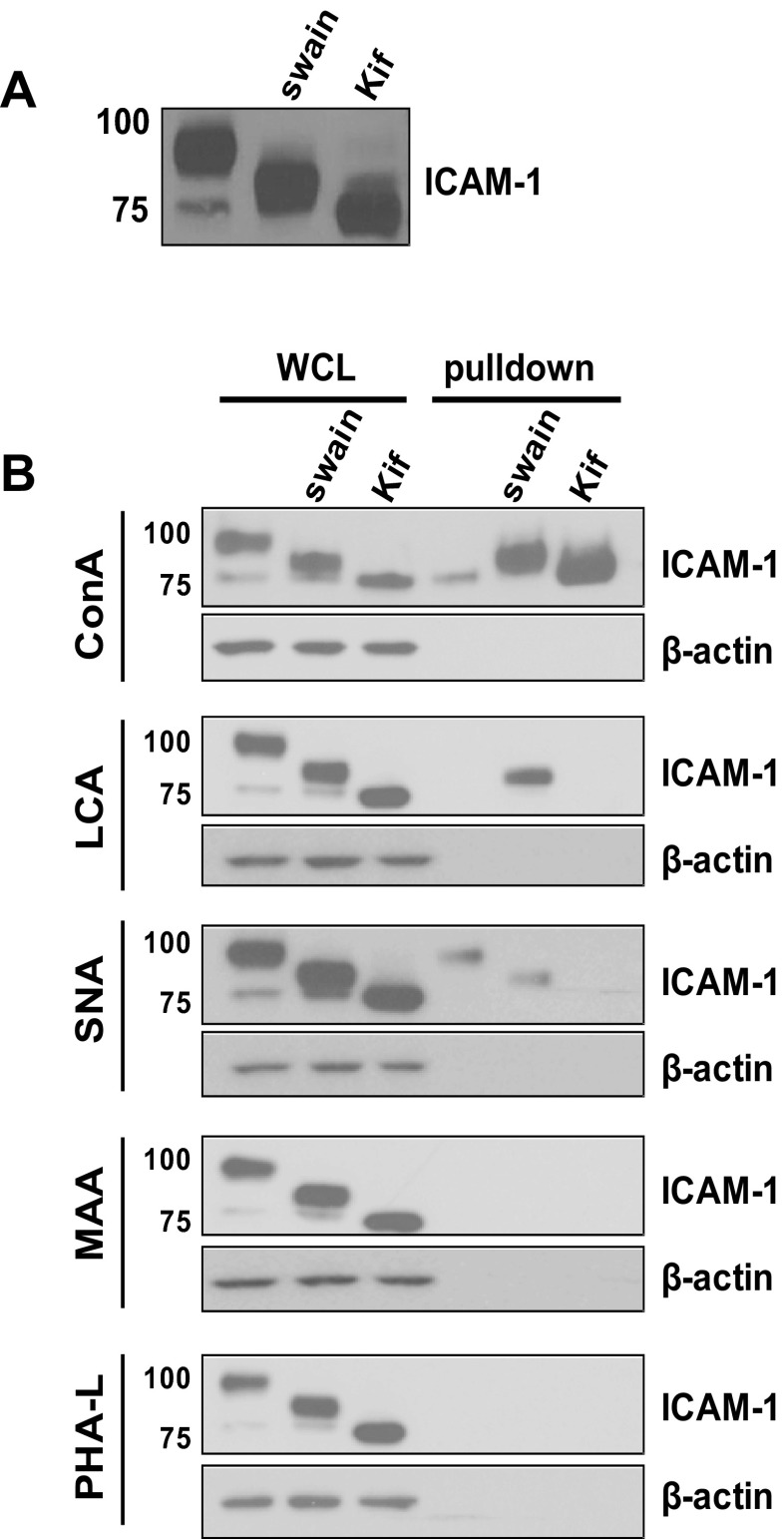
TNF-α stimulation of HUVECs produces two distinct ICAM-1 bands; a major band at ∼95 kDa and a minor and lower molecular mass band at ∼75 kDa (Fig. 1A). The lower band migrates at a similar size to ICAM-1 produced in the presence of the class 1 α-mannosidase inhibitor kifunensine, suggesting that this is a hypoglycosylated ICAM-1 bearing exclusively high-mannose N-glycan structures. To determine the N-glycan structures of ICAM-1 on the surface of activated endothelial cells, HUVECs were stimulated with TNF-α (10 ng/ml, 6 h) and surface labeled with a panel of biotinylated lectins with distinct carbohydrate specificities (Table 1). Immunoprecipitation using streptavadin was then performed followed by blotting for ICAM-1. In parallel whole cell lysates were subject to the same procedure. As seen in Fig. 1B, labeling with the mannose specific lectin ConA selectively pulled down the lower molecular mass glycoform of ICAM-1 from the surface. Enrichment of this ICAM-1 glycoform was not observed with any other lectin tested. In contrast, the higher molecular mass ICAM-1 glycoform interacts with SNA but not ConA, LCA, PHA-L, or MAA. This lectin binding pattern suggests the absence of α-2,3 sialic acid or fully branched structures but supports the presence of α-2,6 sialic acid structures on the higher molecular mass ICAM-1, consistent with previous reports (22). Selectivity of lectins was confirmed by performing pull-downs in cells treated with the α-mannosidase class I inhibitor kifunensine, which produces only high-mannose structures that only reacted with ConA, and with the α-mannosidase class II inhibitor swainsonine, which produces hybrid N-glycan structures that reacted with LCA and SNA but not with ConA or PHA-L.
Fig. 1.
Identification of a concanavalin A (ConA)-reactive intercellular adhesion molecule 1 (ICAM-1) glycoform on activated endothelial cells. A: human umbilical vein endothelial cells (HUVECs) were stimulated with TNF-α (10 ng/ml, 6 h) with or without pretreatment with swainsonine (swain) or kifunensine (kif). Lysates were analyzed by Western blot analysis for ICAM-1 expression. B: HUVECs were stimulated with TNF-α as above and labeled with biotinylated lectins (ConA, SNA, MAA, PHA-L, or LCA; see Table 1 for definitions) and purified against streptavidin. Whole cell lysates (WCL) and streptavidin bound surface proteins were analyzed by Western blot analysis for ICAM-1 and β-actin. Data are representative of at least 4 separate experiments.
Lower molecular mass isoform of ICAM-1 contains high-mannose N-glycans.
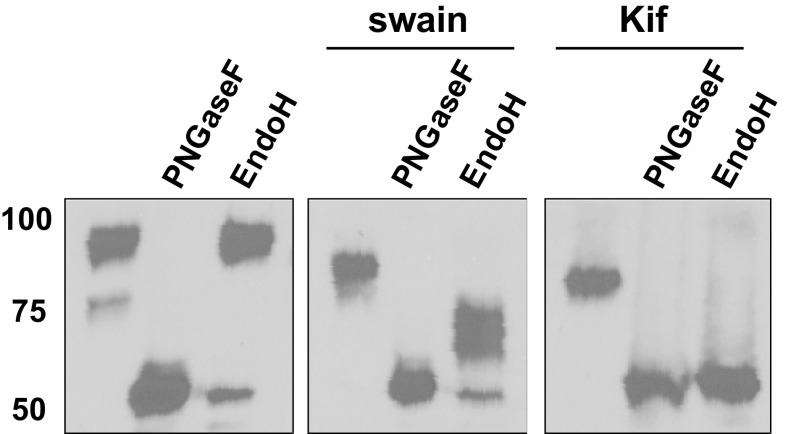
To confirm that the lower molecular mass isoform of ICAM-1 contains high-mannose structures, HUVECs were stimulated with TNF-α as before and lysates were collected and digested with glycosidases. Specifically, lysates were digested with the pan-N-glycosidase PNGaseF or the high-mannose-specific Endo H. As seen in Fig. 2, Endo H treatment selectively removed the lower molecular mass glycoform converting it into a ∼53-KDa species, which equates to the predicted molecular mass of ICAM-1 based on its primary amino acid sequence. Endo H had no effect on the higher molecular mass ICAM-1, confirming the assignment that the ∼75-KDa form is a high-mannose ICAM-1 (HM-ICAM-1) N-glycoform. Enzyme specificity was verified by the ability of Endo H to digest all of ICAM-1 produced in the presence of kifunensine and to partially digest ICAM-1 produced in the presence of swainsonine. Note that ICAM-1 produced in the presence of swainsonine and digested by Endo H appeared as multiple bands by Western blot analysis highlighting the heterogeneity of glycan structures added to the protein even at the hybrid stage.
Fig. 2.
Characterization of high-mannose (HM)-ICAM-1. HUVECs were stimulated with TNF-α (10 ng/ml, 6 h) with or without swainsonine or kifunensine pretreatment. Cells were processed as described in materials and methods and digested with the pan N-glycosidase PNGaseF or the high-mannose N-glycan-specific endoglycosidase H (Endo H). Data are representative of 3 separate experiments.
HM-ICAM-1 is present in vivo.
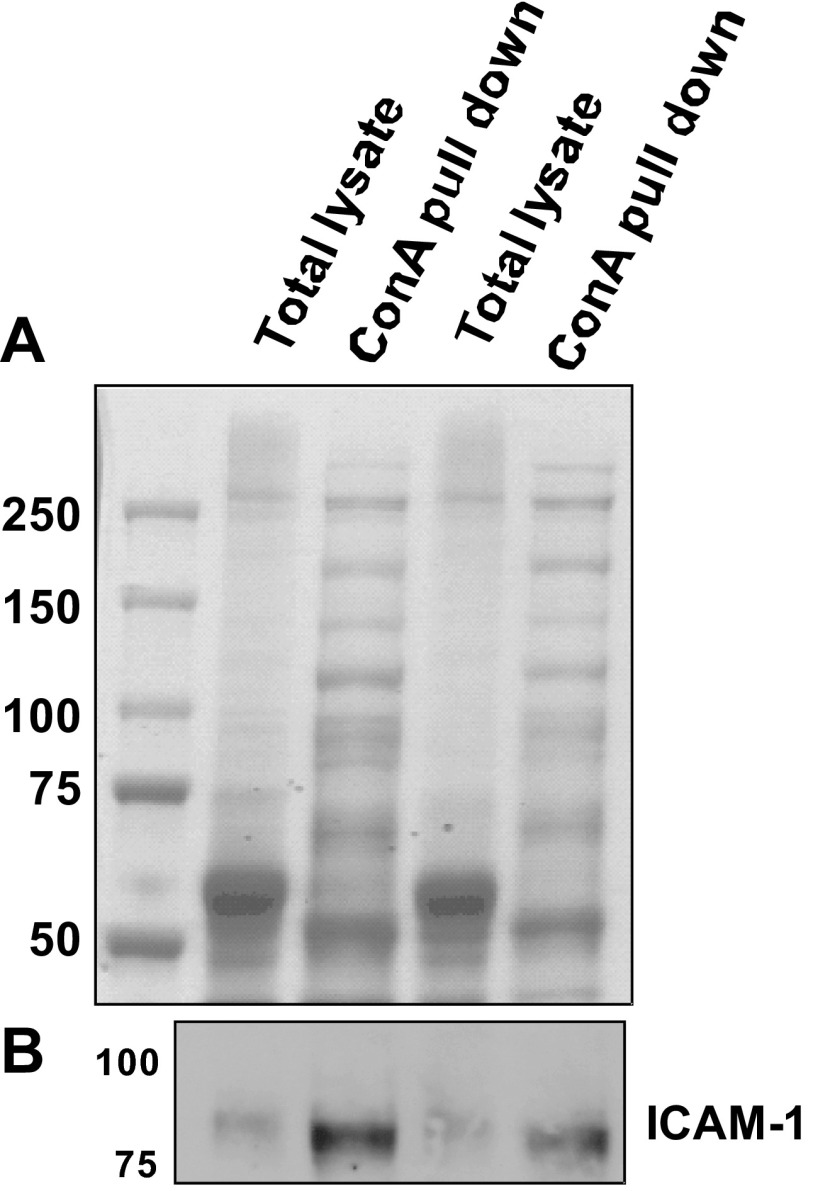
To assess if HM-ICAM-1 was present in vivo and not an artifact of tissue culture, freshly isolated human coronary arteries were lysed, and ConA reactive proteins isolated and processed as described in materials and methods. As seen in Fig. 3A, the protein profile of ConA bound proteins was strikingly different compared with that from the whole cell lysate indicating 1) that ConA enriched proteins, and 2) the presence of a number of high-mannose N-glycan containing proteins. We next performed Western blot analysis on these samples and, as seen in Fig. 3B, were able to detect HM-ICAM-1 in the ConA pull-down samples. The molecular mass of this band was ∼75 kDa, which corresponds to the ConA-reactive band from TNF-α-stimulated endothelial cells and indicates that HM-ICAM-1 is present in vivo.
Fig. 3.
ConA reactive ICAM-1 is present in human atherosclerotic vessels. Coronary arteries were collected postmortem and lysed as described in materials and methods. Lysates were incubated with ConA-Sepharose to enrich for high-mannose N-glycan containing proteins. Total lysate and ConA bound proteins were analyzed by Coomassie staining (A) and Western blot analysis for ICAM-1 (B). Data are representative from 4 independent coronary arteries.
HM-ICAM-1 supports monocyte adhesion with increased adhesion under flow.
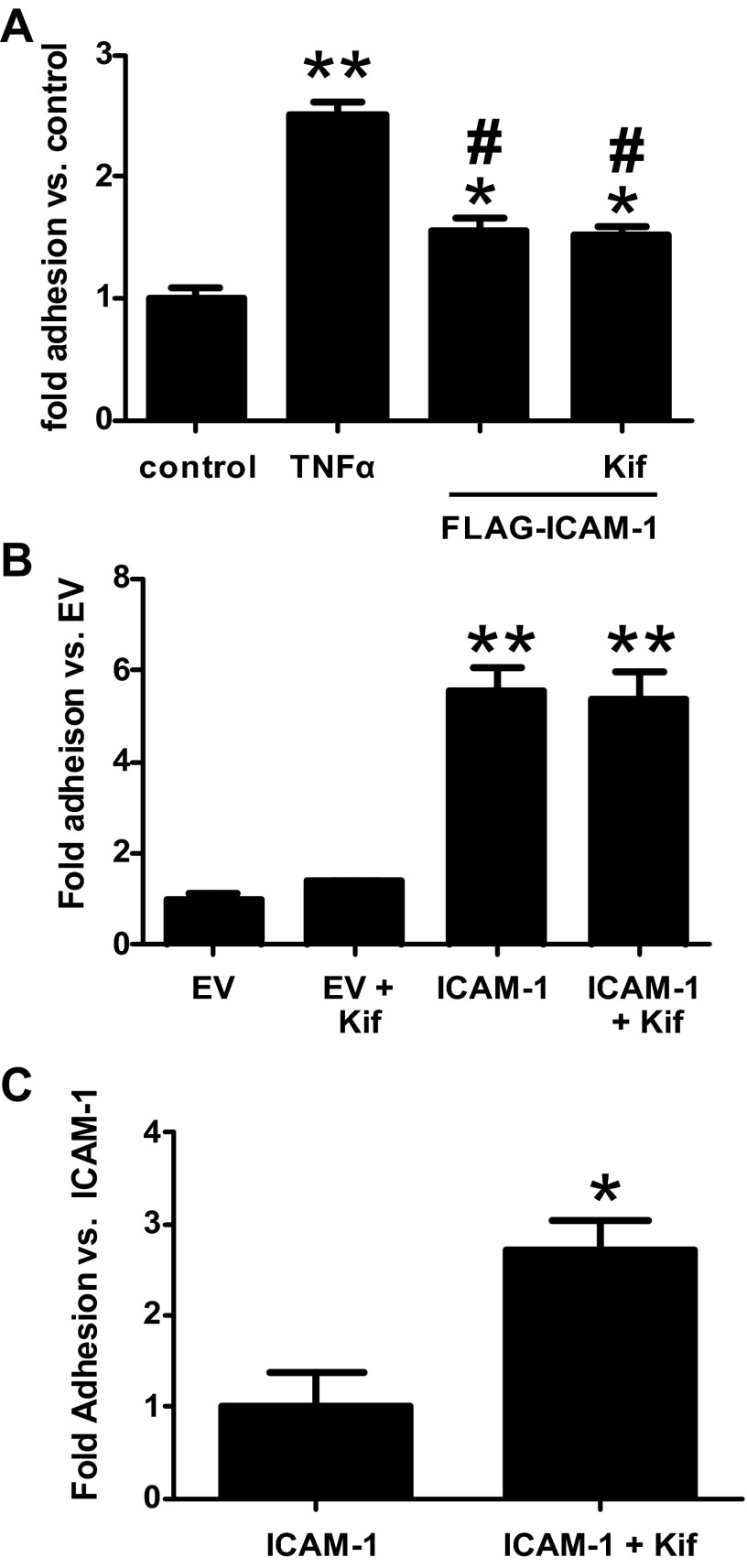
We next determined if HM-ICAM-1 could support monocyte adhesion. For these experiments, HUVECs were transfected with either empty vector (pCMV-script) or pCMV-ICAM-1 to overexpress ICAM-1. Some cells were also treated with kifunensine starting at the time of transfection to generate exclusively HM-ICAM-1. Forty-eight hours after transfection, HUVECs were incubated with THP-1 monocytes and adhesion assessed. As seen in Fig. 4A, kifunensine treatment alone had no effect on monocyte adhesion. However, expression of ICAM-1 in these cells significantly increased THP-1 adhesion, with similar levels seen in the presence or absence of kifunensine but that did not reach the level of adhesion seen with TNF-α treatment (data not shown). Because primary endothelial cells are difficult to transfect (∼50% efficiency), which resulted in lower levels of ICAM-1 expression compared with endogenous induction with TNF-α, these experiments were repeated in stably transfected Cos1 cells. As seen in Fig. 4B, kifunensine treatment alone did not increase THP-1 adhesion to Cos1 cells but overexpression of ICAM-1 or HM-ICAM-1 lead to equally robust monocyte adhesion. Collectively these data demonstrate that HM-ICAM-1 possesses normal function in regards to static monocyte adhesion.
Fig. 4.
HM-ICAM-1 supports increased monocyte adhesion under conditions of flow. HUVEC (A) or Cos1 (B and C) cells were transfected with empty vector (EV) or pCMV-ICAM-1 and some cells were treated with kifunensine at the time of transfection to produce HM-ICAM-1. Additionally, some HUVECs were treated with TNF-α (10 ng/ml, 4 h). Static adhesion (A and B) or adhesion during flow at 1 dyn/cm2 (C) of THP-1 monocytes was determined as described in materials and methods. For A and B: *P < 0.05 vs. control, **P < 0.05 vs. control, and #P < 0.05 vs. TNF-α by one-way ANOVA. For C, *P < 0.05 vs. ICAM-1 by t-test.
Previously, we demonstrated that high-mannose epitopes on the endothelial surface potentiated TNF-α-induced monocyte adhesion under conditions of flow (41). We next examined THP-1 monocyte adhesion under flow to ICAM-1-expressing Cos1 cells in the presence or absence of kifunensine. As seen in Fig. 4C, addition of kifunensine significantly increased THP-1 monocyte adhesion in Cos1 cells transfected with pCMV-ICAM-1. There was no detectable adhesion in empty vector transfected cells in the presence or absence of kifunensine (data not shown).
HM-ICAM-1 supports cell signaling.
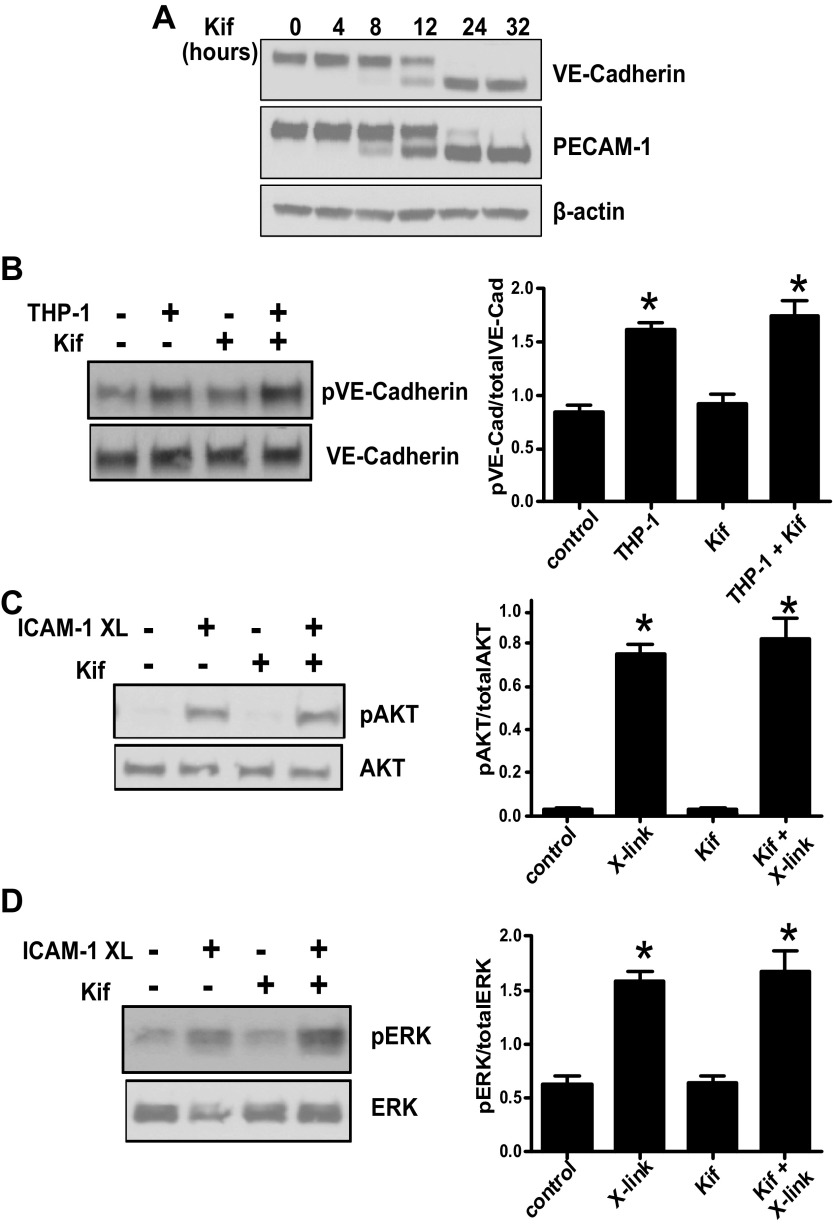
Antibody-mediated clustering or leukocyte engagement to ICAM-1 is known to induce a wide range of cell signaling events as outlined above. To determine if HM-ICAM-1 could support similar signaling we first examined THP-1 monocyte induced VE-cadherin phosphorylation, which was previously shown to require endothelial expression of ICAM-1 (2). However, it was critical to assess these functions before N-glycosylation inhibitors had affected the glycosylation status of constitutively expressed proteins. HUVECs were treated with kifunensine for 0–32 h, and the expression of constitutively expressed VE-cadherin and PECAM-1 was assessed by Western blot analysis. As seen in Fig. 5A, high-mannose glycoforms of these two proteins only become evident between 8–12 h after treatment, and complete expression of the high-mannose forms was not seen until at least 24 h. For this reason, all experiments were conducted before 8 h of kifunensine exposure to only affect newly synthesized proteins. As seen in Fig. 5B, incubation of TNF-α-stimulated endothelial cells, in the presence or absence of kifunensine, with THP-1 monocytes led to an increase in VE-cadherin tyrosine phosphorylation. To further determine if HM-ICAM-1 could support cell signaling we examined Akt and ERK phosphorylation after antibody-mediated clustering as previously described (28, 29). As seen in Fig. 5, C and D, cross-linking of HM-ICAM-1 induced Akt and ERK phosphorylation comparable to fully glycosylated ICAM-1. Combined, these results indicate that HM-ICAM-1 can support cell signaling equally compared with native ICAM-1.
Fig. 5.
HM-ICAM-1 supports cell signaling. HUVECs were treated with kifunensine for 0–32 h, and the effect on VE-cadherin and PECAM-1 N-glycosylation was determined by Western blot analysis (A). HUVECs were treated with TNF-α in the presence of absence of kifunensine (B) and cells then treated with THP-1 monocyte for 15 min and VE-cadherin phosphorylation was detected by Western blot analysis. Shown are representative blots and bar graphs showing means ± SE (n = 3). *P < 0.01, relative to respective control by one-way ANOVA with Tukey posttest. C and D show respectively phosphorylation of Akt and ERK after cross-linking of ICAM-1 as described in materials and methods. Shown are representative blots with bar graphs showing means ± SE (n = 3). *P < 0.01, relative to respective control by one-way ANOVA with Tukey posttest.
HM-ICAM-1 displays reduced interaction with cytoskeletal components.
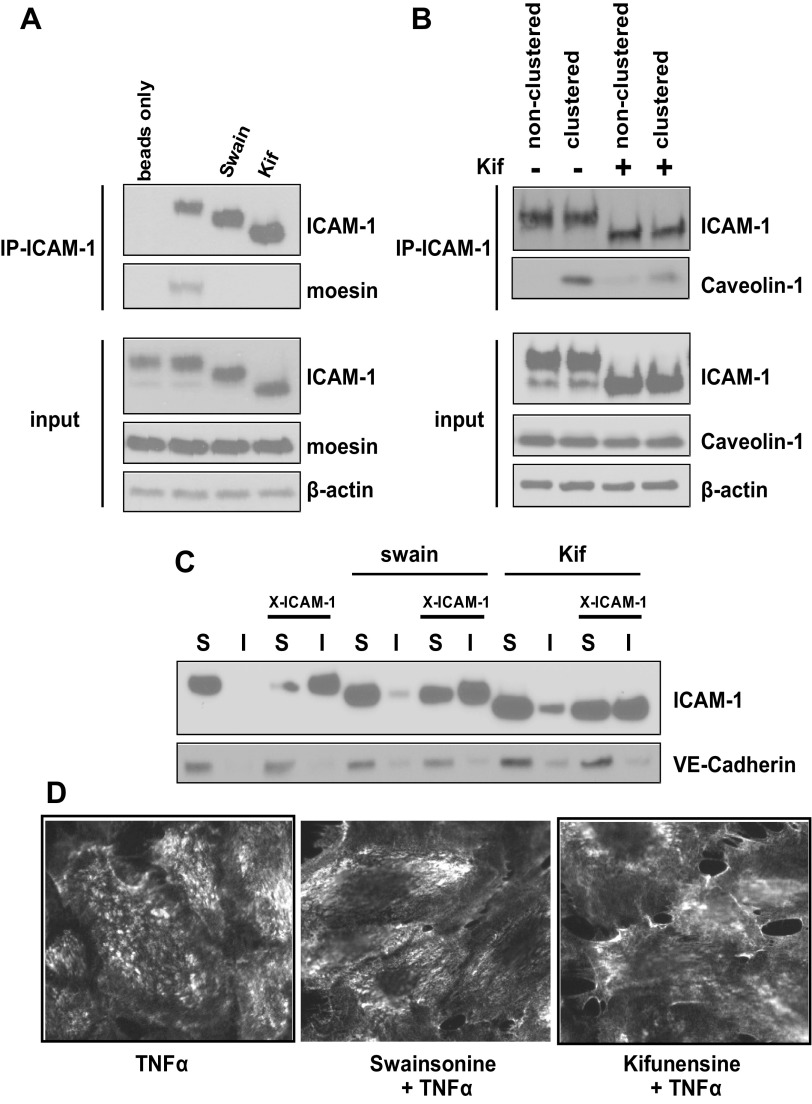
Previous reports indicate that ICAM-1 can interact with the actin cytoskeleton through interactions with the ERM complex (28) or through interactions with filamin A upon clustering which forms a complex with caveolin-1 (32). We next determined if HM-ICAM-1 could interact with moesin or caveolin-1 as previously described. Figure 6A shows that moesin was coimmunoprecipitated from TNF-α-stimulated HUVECs but was not present in samples pretreated with swainsonine or kifunensine. Furthermore, ICAM-1 clustering induced coimmunoprecipitation of caveolin-1 with ICAM-1 but was markedly reduced in cells pretreated with kifunensine (Fig. 6B).
Fig. 6.
HM-ICAM-1 displays impaired interactions with the actin cytoskeleton. HUVECs were treated with TNF-α (10 ng/ml, 6 h) in the presence of absence of α-mannosidase inhibitors swainsonine or kifunensine. A: ICAM-1 was immunoprecipitated as described in experimental procedures for interaction with the Ezrin-Radizin-Moesin (ERM) complex. Whole cell lysates (input) and immunoprecipitated proteins were analyzed by Western blot analysis. B: cells were treated as before and some cells underwent antibody-mediated ICAM-1 clustering. ICAM-1 was immunoprecipitated as described in materials and methods for interaction with caveolin-1. Whole cell lysates (input) and immunoprecipitated proteins were analyzed by Western blot analysis. C: cells were treated and some cells underwent antibody-mediated ICAM-1 clustering (X-ICAM). Lysates were separated into Triton X-100 soluble (S) and insoluble (I) fraction and analyzed by Western blot. Data are representative of at least 3 different experiments. D: cells were treated and underwent antibody-mediated clustering of ICAM-1. After fixation, cells were stained with fluorescently tagged secondary antibody and surface expressed patterns of ICAM-1 were assessed. Data are representative of at least 3 different experiments.
Previous reports have also shown that upon antibody-mediated clustering of cell surface ICAM-1, the latter transitions from Triton X-100 soluble to insoluble membrane fractions (33, 34). Based on our finding that HM-ICAM-1 could not interact with the ERM complex, nor with caveolin-1 when clustered by antibodies, we hypothesized that it would fail to transition to Triton X-100 insoluble membranes after antibody-mediated clustering. Figure 6C shows that both fully processed ICAM-1 and HM-ICAM-1 are found in Triton X-100 soluble fractions before clustering. After clustering, the fully processed ICAM-1 completely enters the Triton X-100 insoluble membrane fraction whereas only a portion of HM-ICAM-1 is able to make this transition. Collectively, these data suggest that despite reaching the membrane, HM-ICAM-1 displays impaired interactions with the actin cytoskeleton and lateral movement on the cell surface. This is further indicated by representative immunofluoresence images of ICAM-1 distribution on the cell surface (Fig. 6D), which show that a punctate focal distribution for mature ICAM-1 but a more diffuse pattern for ICAM-1 produced in the presence of swainsonine or kifunensine.
DISCUSSION
In the current work we have shown that ICAM-1 exists as two N-glycoforms on the surface of activated endothelial cells; a fully processed form containing terminal α-2,6 sialic acid and a minimally processed or hypoglycosylated high-mannose form (Figs. 1 and 2). Further, we have demonstrated that HM-ICAM-1 is detectable in human coronary arteries (Fig. 3), serves as a ligand to support monocyte rolling and adhesion (Fig. 4), can support cell signaling (Fig. 5), but fails to bind the actin cytoskeleton (Fig. 6).
To date several studies have examined the N-glycan structures on ICAM-1, but these studies have relied on plasmid-driven overexpression of the protein rather than examination of endogenous production (8) or examined murine but not human ICAM-1(37). In the latter case, mouse ICAM-1 was overexpressed in three cell lines where the protein plays no relevant function in leukocyte trafficking. Thus, while these data provide a great deal of information, they do not provide insight into ICAM-1 function. Underscoring this perspective is that glycan modifications of a single protein is dependent on the host cell type that in turn imparts unique functions to that protein. As examples, the HIV protein gp120 overexpressed in a panel of immortalized cells retains the N-glycan signature of the host cell type and this host cell glycan signature regulates envelope recognition (40). ICAM-2 produced in platelets contains terminal α-2,3 sialic acid that prevents DC-SIGN and LFA-1 binding (51). Interestingly, ICAM-2 produced in endothelial cells does not contain α-2,3 sialic acid. With respect to ICAM-1, previous studies have shown that murine astrocyte production of macrophage inflammatory protein-2 could be stimulated by murine ICAM-1 when produced in CHO cells, but not when produced in HEK cells, a property dependent on the N-glycan signature (38). The latter study also demonstrated that a high-mannose ICAM-1 was unable to stimulate astrocytes but was able to bind LFA-1 at normal levels. Elegant work by laboratory of Springer (13) has demonstrated that deletion of two consensus N-glycan sequences that flank the Mac-1 binding domain in the IgG like- 3 domain of ICAM-1 (N240 and N269) enhanced Mac-1 binding. Thus glycosylation of ICAM-1 is a posttranslational modification that can regulate interactions with other proteins.
However, while these studies have clearly shown that N-glycosylation can control ICAM-1 function, it is important to note that the N-glycosylation patterns of human and murine ICAM-1 are drastically different. Human ICAM-1 contains 9 while murine ICAM-1 contains 10 N-X-S/T motifs, yet only 4 of the motifs are conserved between the species (42). Of note, the two glycosylation sites in ICAM-1 that are known to regulate Mac1 binding (13) are absent in murine ICAM-1. Additionally, a single mutation at position 20 of human ICAM-1 deletes an N-glycosylation site in the IgG like-1 domain that allows rhinovirus binding (7). Thus extrapolating information from murine ICAM-1 N-glycosylation patterns and applying it to human ICAM-1 is difficult. Many studies that have examined leukocyte binding to ICAM-1 have utilized recombinant protein produced in a variety of cells types but rarely from human (or primate) cells. In many cases, these experiments use recombinant human ICAM-1 produced in murine myeloma cells, which we have observed does not contain HM-ICAM-1 (not shown). Apart from the absence of HM-ICAM-1, these proteins also contain the N-glycan signature of the host cell line (murine myeloma) (8) and may not reflect the action of ICAM-1 produced in an activated human endothelial cell. Figure 3 demonstrates that HM-ICAM-1 can be detected in human coronary arteries, and unlike in cultured endothelial cells where two ICAM-1 glycoforms were present (refer to Figs. 1 and 2), only one glycoform, the HM-ICAM-1 glycoform, was detectable in these samples, even in the whole tissue homogenate. A limitation in this measurement is that we cannot discern whether the HM-ICAM-1 detected is endothelial specific or derived from another cell type. That said, this observation is exciting in that it suggests that the majority of ICAM-1 in vivo is of the high-mannose variety and thus represents the actual therapeutic target rather than the often studied complex (higher molecular mass) N-glycan containing glycoform. This possibility remains to be tested.
Importantly, HM-ICAM-1 was equally effective as fully matured ICAM-1 to engaging monocytes under static conditions. During flow, however, HM-ICAM-1 was significantly more potent at mediating monocyte adhesion. Future experiments will be required to determine if these mannose residues are ligands for receptors other than Mac-1 and LFA-1 or if other mannose binding receptors involved in flow-mediated adhesion, such as DC-SIGN, are participating in monocyte adhesion to HM-ICAM-1. Taken together, we speculate that HM-ICAM-1 is the primary mediator of ICAM-1-dependent monocyte rolling and adhesion in vivo and consistent with our previous studies showing that endothelial surface HM-epitopes provide ligands for monocytes (10, 41).
Beyond monocyte adhesion, our data also suggest that the N-glycan complement of ICAM-1 regulates outside in signals. Whereas ICAM-1 ligation-dependent effects on mitogenic signals (indexed by AKT or ERK phosphorylation) or junctional integrity (indexed by VE-cadherin phosporylation) were not different between HM-ICAM-1 or fully mature ICAM-1, basal interactions with the actin cytoskeleton via ERM complexes and lateral movement of ICAM-1 on the cell-surface were dramatically different and lost with HM-ICAM-1. While these results may seem contradictory, that signaling is maintained while lateral mobility is lost, it is important to note that outside-in signaling after ICAM-1 cross-linking is abolished by cytochalasin D while lateral mobility is not (39, 46). In this sense, outside-in signaling requires new stress fiber formation whereas lateral mobility requires preexisting interactions with the cytoskeleton (such as those mediated by interactions with the ERM complex; illustrated in Fig. 7). Data in Fig. 6C demonstrate that HM-ICAM-1 has impaired ability to transition to Triton X-100 insoluble membranes after cross-linking but also show that some HM-ICAM-1 is already in these fractions before cross-linking. This is in direct contrast to mature ICAM-1, which is only found in Triton X-100 soluble membranes before cross-linking. Thus it would appear that surface distribution, at least in the context of membrane domains, is altered in HM-ICAM-1, a conclusion further indicated by Fig. 6D. The functional effects of these differential interactions with cytoskeletal components require further testing as do studies to define which of the eight human N-glycosylation sites are required for these functions. Of particular interest are sites at N358 and N379, which lie in the region where ICAM-1 dimerization is reported to occur (53). N-glycosylation is known to regulate dimer formation of many proteins (6, 24, 26, 27, 44), so it is easy to speculate that ICAM-1 N-glycosylation could be important in this context and could thereby regulate leukocyte adhesion and clustering events.
Fig. 7.
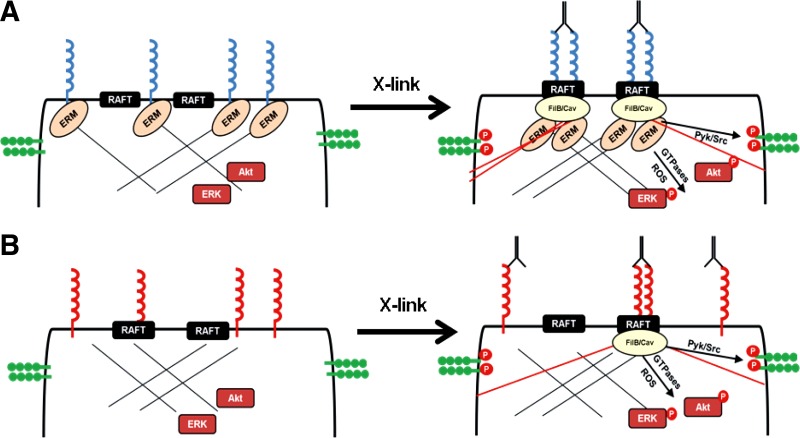
Proposed model for how distinct ICAM-1 glycoforms regulate adhesion and cell signaling. A: complex N-glycan containing ICAM-1 (fully matured) is expressed exclusively in nonraft domains and is bound to the actin cytoskeleton through interactions with the ERM complex. Upon cross-linking, ICAM-1 is transported into raft domains via these cystoskeletal interactions. Additionally, new interactions with filamin A/B and caveolin regulate new stress fiber formation leading to activation of cell signaling (VE-cadherin, Akt, and ERK). B: HM-ICAM-1 resides in both raft and nonraft regions of the plasma membrane during basal conditions and does not interact with the ERM complex. Upon cross-linking, new stress fiber formation is still achievable as some of the protein is already in the raft domains allowing for filamin A/B and caveolin interactions, albeit to a significantly lesser extent compared with complex ICAM-1.
To conclude, the current work demonstrates that ICAM-1 exists in two distinct N-glycoforms on activated endothelial cells in vitro with a major form carrying α-2,6-sialic acid possessing complex N-glycans and a minor form carrying only high-mannose N-glycans. This high-mannose glycoform is abundantly present, however, in human coronary artery samples and when overexpressed in cells is able to support monocyte rolling and adhesion. However, the high-mannose form fails to interact with the ERM complex and displays reduced lateral motility upon clustering despite the ability to support outside-in signaling (Fig. 7). Collectively, these data identify a HM-ICAM-1 as a novel ICAM-1 glycoform that mediates critical functions of this protein and targeting of which offers potentially novel therapeutic strategies to control endothelial inflammation.
GRANTS
This work was funded in part by an American Heart Association Predoctoral Fellowship and Howard Hughes Medical Institute Med-Grad Fellowship (to D. W. Scott) and National Institute of Neurological Disorders and Stroke Grant P30-NS-047466 (to M. Ballestas).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.W.S., M.E.B., and R.P.P. conception and design of research; D.W.S., T.S.D., and M.E.B. performed experiments; D.W.S. and M.E.B. analyzed data; D.W.S., M.E.B., S.H.L., and R.P.P. interpreted results of experiments; D.W.S. and R.P.P. prepared figures; D.W.S. drafted manuscript; D.W.S., T.S.D., M.E.B., S.H.L., and R.P.P. edited and revised manuscript; D.W.S., T.S.D., M.E.B., S.H.L., and R.P.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Joanne Murphy-Ullrich (University of Alabama, Birmingham) for Cos1 cells.
REFERENCES
- 1. Akama TO, Nakagawa H, Wong NK, Sutton-Smith M, Dell A, Morris HR, Nakayama J, Nishimura S, Pai A, Moremen KW, Marth JD, Fukuda MN. Essential and mutually compensatory roles of α-mannosidase II and α-mannosidase IIx in N-glycan processing in vivo in mice. Proc Natl Acad Sci USA 103: 8983–8988, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 179: 4053–4064, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Amos C, Romero IA, Schultze C, Rousell J, Pearson JD, Greenwood J, Adamson P. Cross-linking of brain endothelial intercellular adhesion molecule (ICAM)-1 induces association of ICAM-1 with detergent-insoluble cytoskeletal fraction. Arterioscler Thromb Vasc Biol 21: 810–816, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Bahaie NS, Kang BN, Frenzel EM, Hosseinkhani MR, Ge XN, Greenberg Y, Ha SG, Demetriou M, Rao SP, Sriramarao P. N-glycans differentially regulate eosinophil and neutrophil recruitment during allergic airway inflammation. J Biol Chem 286: 38231–38241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 157: 1233–1245, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bell SL, Xu G, Khatri IA, Wang R, Rahman S, Forstner JF. N-linked oligosaccharides play a role in disulphide-dependent dimerization of intestinal mucin Muc2. Biochem J 373: 893–900, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bella J, Kolatkar PR, Marlor CW, Greve JM, Rossmann MG. The structure of the two amino-terminal domains of human ICAM-1 suggests how it functions as a rhinovirus receptor and as an LFA-1 integrin ligand. Proc Natl Acad Sci USA 95: 4140–4145, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bloom JW, Madanat MS, Ray MK. Cell line and site specific comparative analysis of the N-linked oligosaccharides on human ICAM-1des454-532 by electrospray ionization mass spectrometry. Biochemistry 35: 1856–1864, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Bullard DC, Hu X, Schoeb TR, Collins RG, Beaudet AL, Barnum SR. Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J Immunol 178: 851–857, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Chacko BK, Scott DW, Chandler RT, Patel RP. Endothelial surface N-glycans mediate monocyte adhesion and are targets for anti-inflammatory effects of peroxisome proliferator-activated receptor gamma ligands. J Biol Chem 286: 38738–38747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med 191: 189–194, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell 139: 1229–1241, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell 65: 961–971, 1991 [DOI] [PubMed] [Google Scholar]
- 14. Eniola AO, Krasik EF, Smith LA, Song G, Hammer DA. I-domain of lymphocyte function-associated antigen-1 mediates rolling of polystyrene particles on ICAM-1 under flow. Biophys J 89: 3577–3588, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol 165: 3375–3383, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol 161: 5755–5761, 1998 [PubMed] [Google Scholar]
- 17. Forlow SB, Ley K. Selectin-independent leukocyte rolling and adhesion in mice deficient in E-, P-, and L-selectin and ICAM-1. Am J Physiol Heart Circ Physiol 280: H634–H641, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Gaboury JP, Kubes P. Reductions in physiologic shear rates lead to CD11/CD18-dependent, selectin-independent leukocyte rolling in vivo. Blood 83: 345–350, 1994 [PubMed] [Google Scholar]
- 19. Galkina E, Ley K. Leukocyte influx in atherosclerosis. Curr Drug Targets 8: 1239–1248, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Gardiner EE, D'Souza SE. A mitogenic action for fibrinogen mediated through intercellular adhesion molecule-1. J Biol Chem 272: 15474–15480, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Green RS, Stone EL, Tenno M, Lehtonen E, Farquhar MG, Marth JD. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity 27: 308–320, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Hanasaki K, Varki A, Stamenkovic I, Bevilacqua MP. Cytokine-induced beta-galactoside alpha-2,6-sialyltransferase in human endothelial cells mediates alpha 2,6-sialylation of adhesion molecules and CD22 ligands. J Biol Chem 269: 10637–10643, 1994 [PubMed] [Google Scholar]
- 23. Hashii N, Kawasaki N, Itoh S, Nakajima Y, Kawanishi T, Yamaguchi T. Alteration of N-glycosylation in the kidney in a mouse model of systemic lupus erythematosus: relative quantification of N-glycans using an isotope-tagging method. Immunology 126: 336–345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He J, Xu J, Castleberry AM, Lau AG, Hall RA. Glycosylation of beta(1)-adrenergic receptors regulates receptor surface expression and dimerization. Biochem Biophys Res Commun 297: 565–572, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Kanters E, van Rijssel J, Hensbergen PJ, Hondius D, Mul FP, Deelder AM, Sonnenberg A, van Buul JD, Hordijk PL. Filamin B mediates ICAM-1-driven leukocyte transendothelial migration. J Biol Chem 283: 31830–31839, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Kitazume S, Imamaki R, Ogawa K, Komi Y, Futakawa S, Kojima S, Hashimoto Y, Marth JD, Paulson JC, Taniguchi N. Alpha2,6-sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J Biol Chem 285: 6515–6521, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langer MD, Guo H, Shashikanth N, Pierce JM, Leckband DE. N-glycosylation alters cadherin-mediated intercellular binding kinetics. J Cell Sci 125: 2478–2485, 2012 [DOI] [PubMed] [Google Scholar]
- 28. Lawson C, Ainsworth M, Yacoub M, Rose M. Ligation of ICAM-1 on endothelial cells leads to expression of VCAM-1 via a nuclear factor-kappaB-independent mechanism. J Immunol 162: 2990–2996, 1999 [PubMed] [Google Scholar]
- 29. Liu G, Place AT, Chen Z, Brovkovych VM, Vogel SM, Muller WA, Skidgel RA, Malik AB, Minshall RD. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood 120: 1942–1952, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol 8: 113–123, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Mkhikian H, Grigorian A, Li CF, Chen HL, Newton B, Zhou RW, Beeton C, Torossian S, Tatarian GG, Lee SU, Lau K, Walker E, Siminovitch KA, Chandy KG, Yu Z, Dennis JW, Demetriou M. Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat Commun 2: 334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 6: 323–344, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res 105: 223–230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mun GI, Lee SJ, An SM, Kim IK, Boo YC. Differential gene expression in young and senescent endothelial cells under static and laminar shear stress conditions. Free Radic Biol Med 47: 291–299, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Nageh MF, Sandberg ET, Marotti KR, Lin AH, Melchior EP, Bullard DC, Beaudet AL. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler Thromb Vasc Biol 17: 1517–1520, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Ohtsubo K, Chen MZ, Olefsky JM, Marth JD. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat Med 17: 1067–1075, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Otto VI, Damoc E, Cueni LN, Schurpf T, Frei R, Ali S, Callewaert N, Moise A, Leary JA, Folkers G, Przybylski M. N-glycan structures and N-glycosylation sites of mouse soluble intercellular adhesion molecule-1 revealed by MALDI-TOF and FTICR mass spectrometry. Glycobiology 16: 1033–1044, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Otto VI, Schurpf T, Folkers G, Cummings RD. Sialylated complex-type N-glycans enhance the signaling activity of soluble intercellular adhesion molecule-1 in mouse astrocytes. J Biol Chem 279: 35201–35209, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Pluskota E, D'Souza SE. Fibrinogen interactions with ICAM-1 (CD54) regulate endothelial cell survival. Eur J Biochem 267: 4693–4704, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Raska M, Takahashi K, Czernekova L, Zachova K, Hall S, Moldoveanu Z, Elliott MC, Wilson L, Brown R, Jancova D, Barnes S, Vrbkova J, Tomana M, Smith PD, Mestecky J, Renfrow MB, Novak J. Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J Biol Chem 285: 20860–20869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scott DW, Chen J, Chacko BK, Traylor JG, Jr, Orr AW, Patel RP. Role of endothelial N-glycan mannose residues in monocyte recruitment during atherogenesis. Arterioscler Thromb Vasc Biol 32: e51–59, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scott DW, Patel RP. Endothelial heterogeneity and adhesion molecules N-glycosylation: implications in leukocyte trafficking in inflammation. Glycobiology 23: 622–633, 2013 [DOI] [PubMed] [Google Scholar]
- 43. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11: 762–774, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sohail A, Marco M, Zhao H, Shi Q, Merriman S, Mobashery S, Fridman R. Characterization of the dimerization interface of membrane type 4 (MT4)-matrix metalloproteinase. J Biol Chem 286: 33178–33189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stanley P, Schachter H, Taniguchi N. N glycans. In: Essentials of Glycobiology, edited by Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, and Etzler ME. New York: Cold Spring Harbor, 2009 [Google Scholar]
- 46. Tilghman RW, Hoover RL. E-selectin and ICAM-1 are incorporated into detergent-insoluble membrane domains following clustering in endothelial cells. FEBS Lett 525: 83–87, 2002 [DOI] [PubMed] [Google Scholar]
- 47. van Buul JD, van Rijssel J, van Alphen FP, Hoogenboezem M, Tol S, Hoeben KA, van Marle J, Mul EP, Hordijk PL. Inside-out regulation of ICAM-1 dynamics in TNF-alpha-activated endothelium. PLoS One 5: e11336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Q, Doerschuk CM. The p38 mitogen-activated protein kinase mediates cytoskeletal remodeling in pulmonary microvascular endothelial cells upon intracellular adhesion molecule-1 ligation. J Immunol 166: 6877–6884, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil-endothelial cell adhesion. Antioxid Redox Signal 4: 39–47, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Wang Q, Pfeiffer GR, 2nd, Stevens T, Doerschuk CM. Lung microvascular and arterial endothelial cells differ in their responses to intercellular adhesion molecule-1 ligation. Am J Respir Crit Care Med 166: 872–877, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Weber KS, Alon R, Klickstein LB. Sialylation of ICAM-2 on platelets impairs adhesion of leukocytes via LFA-1 and DC-SIGN. Inflammation 28: 177–188, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol 32: 461–469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang Y, Jun CD, Liu JH, Zhang R, Joachimiak A, Springer TA, Wang JH. Structural basis for dimerization of ICAM-1 on the cell surface. Mol Cell 14: 269–276, 2004 [DOI] [PubMed] [Google Scholar]