Abstract
Neuregulin-1 (NRG-1) is a member of a family of neurotrophic factors that is required for the differentiation, migration, and development of neurons. NRG-1 signaling is thought to contribute to both neuronal development and the neuropathology of schizophrenia, which is believed to be a neurodevelopmental disorder. However, few studies have investigated the role of NRG-1 on voltage-gated ion channels. In this study, we report that NRG-1 specifically increases the density of transient outward K+ currents (IA) in rat cerebellar granule neurons (CGNs) in a time-dependent manner without modifying the activation or inactivation properties of IA channels. The increase in IA density is mediated by increased protein expression of Kv4.2, the main α-subunit of the IA channel, most likely by upregulation of translation. The effect of NRG-1 on IA density and Kv4.2 expression was only significant in immature neurons. Mechanistically, both Akt and mammalian target of rapamycin (mTOR) signaling pathways are required for the increased NRG-1-induced IA density and expression of Kv4.2. Moreover, pharmacological blockade of the ErbB4 receptor reduced the effect of NRG-1 on IA density and Kv4.2 induction. Our data reveal, for the first time, that stimulation of ErbB4 signaling by NRG-1 upregulates the expression of K+ channel proteins via activation of the Akt/mTOR signaling pathway and plays an important role in neuronal development and maturation. NRG1 does not acutely change IA and delayed-rectifier outward (IK) of rat CGNs, suggesting that it may not alter excitability of immature neurons by altering potassium channel property.
Keywords: neuregulin, ErbB4, Akt/mTOR, Ia, Kv4.2, CGNs
neuregulin 1 (nrg-1) is a member of a family of neurotrophic factors that contain EGF-like repeats and bind to and activate EGF family receptors (ErbB2–4) (27). NRG-1 is widely expressed throughout development and adulthood, with the highest expression observed in nervous tissue (8). In the central nervous system, NRG-1 is required to induce neuronal differentiation, migration, and development (2, 25). A recent study implicated NRG-1/ErbB4 signaling in the neuropathology of schizophrenia (19), which is believed to be a neurodevelopmental disorder. In vitro studies indicate that NRG-1 can be a neurotrophic or neuroprotective factor for cortical neurons, dopaminergic neurons, cochlear sensory neurons (44), and cereberal neurons following ischemia (36). NRG/ErbB signaling stimulates neurite outgrowth in several populations of primary neurons, such as hippocampal neurons and cerebellar granule cells (14, 31).
Cerebellar granule neurons (CGNs) are small glutamatergic cells and constitute the largest homogeneous neuronal population in the mammalian brain. Due to their postnatal generation and well-defined developmental pathway in vitro, cultures of primary CGNs have been established as a model for studying neuronal maturation, differentiation, and synaptic plasticity (9, 39). Previous studies have indicated that growth and differentiation factors can either stimulate or inhibit CGN development and maturation by regulating multiple signaling pathways (41, 45). In addition, NRG-1 selectively induces the expression of the GABAA receptor β2-subunit in CGNs by binding to the ErbB4 receptor and recruiting PSD-95 (40, 41).
One characteristic of CGN development and maturation is regulation of the expression of K+ channels (28, 35). Primary culture CGNs display several voltage-activated outward K+ currents (26). We previously reported that the enhancement of delayed-rectifier outward (IK) and fast transient outward (IA) potassium currents was associated with CGN apoptosis (17, 20). In addition, IK also plays a role in promoting CGN migration and maturation (24, 45). Recently, our study indicated that neuritin, a new neurotrophic factor that is involved in activity-dependent synaptic plasticity, activates the insulin receptor pathway to upregulate Kv4.2-mediated IA in rat cerebellar granule neurons (43), indicating that neurotrophic factors may alter the status and function of CGNs by modulation of the expression of different potassium channels.
The goal of this study was to examine whether NRG-1 modulates K+ channels as part of its role in synaptic activity and neurodevelopment. To test this hypothesis, we examined the effect of NRG-1 on K+ channels in rat CGNs. We found that NRG-1 increases IA density by regulating the expression of Kv4.2 potassium channel α-subunits and that activation of Akt/mTOR signaling by NRG-1-ErbB4 is required.
EXPERIMENTAL PROCEDURES
Chemicals.
Recombinant human NRG-1 was purchased from Pepro Tech (Rocky Hill, NJ). Triethanolamine (TEA), tetrodotoxin (TTX), 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside (DRB), cycloheximide, AG1478, rapamycin, LY294002, and poly-l-lysine were purchased from Sigma (St. Louis, MO). Fetal calf serum, DMEM, and antibiotic-antimycotic solution were purchased from GIBCO Life Technologies (Grand Island, NY).
Cell culture.
All experimental procedures were carried out in accordance with the European guidelines for the care and use of laboratory animals (Council Directive 86/609/EEC). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Fudan University. Cells were derived from the cerebella of 7-day-old Sprague-Dawley rat pups as described previously (29). Isolated cells were plated at a density of 106 cells/ml onto 35 mm Petri dishes coated with poly-l-lysine (1 μg/ml). Cultured cells were incubated at 37°C in 5% CO2 in DMEM supplemented with 10% fetal calf serum, insulin (5 μg/ml), KCl (25 mM), and 1% antibiotic-antimycotic solution. After culture for 24 h, cytosine β-d-arabinofuranoside (5 μM) was added to the culture medium to inhibit the proliferation of nonneuronal cells. Cells were used for experiments after 2–7 days in culture unless otherwise indicated.
Patch-clamp recordings.
Whole-cell currents of granule neurons were recorded using conventional patch-clamp techniques. Before IA current recording, the culture medium was replaced with a bath solution containing the following (in mM): 125 NaCl, 2.5 KCl, 10 HEPES, 1 MgCl2, 0.001 TTX, 20 TEA, and 10 glucose (pH adjusted to 7.4 with NaOH). Soft-glass recording pipettes were filled with an internal solution containing the following (in mM): 135 potassium gluconate, 10 KCl, 10 HEPES, 1 CaCl2, 1 MgCl2, 10 EGTA, 1 ATP, and 0.1 GTP (with pH adjusted to 7.3 using KOH). The pipette resistance was 4–6 MΩ after filling with an internal solution. The cultured granule cells selected for electrophysiological recording exhibited common morphological characteristics of healthy cells, such as fusiform soma with two main neurites of similar size. In addition, the mean capacitance of the recorded cells in the control group (6.82 ± 0.29 pF) and the neuregulin treatment group (7.13 ± 0.33 pF) showed no significant difference (P = 0.473), indicating that the granule cells used in the experiments were comparable. All recordings were performed at room temperature (23–25°C).
Data acquisition and analysis.
All currents were recorded using an multiclamp 200B amplifier (Axon Instruments, Foster City, CA) operated in voltage-clamp mode. Data acquisition and analysis were performed with pClamp 8.01 software (Axon Instruments) and/or Origin6.1 analysis software (Microcal Software, Northampton, MA). Quantity One software (Version 4.6.2; Bio-Rad Laboratories) was used for background subtraction and for quantification of immunoblotting data. The results were analyzed using one-way ANOVA for comparisons between multiple groups and using Student's t-tests for comparisons between two samples. The values are given as the means ± SE with n as the number of neurons for the electrophysiological recordings and calcium imaging experiments and the number of replicates for the molecular biological experiments. For electrophysiological experiments, data were collected from at least four different batches of neurons, thereby minimizing bias resulting from culture conditions. A P value 0.05 was considered to be statistically significant.
Western blot analysis.
Cells were lysed in HEPES-NP40 lysis buffer (20 mM HEPES, 150 mM NaCl, 0.5% NP-40, 10% glycerol, 2 mM EDTA, 100 μM Na3VO4, 50 mM NaF, pH 7.5, and 1% proteinase inhibitor cocktail) on ice for 30 min. After centrifugation, the supernatant was mixed with 2× sodium dodecyl sulfate loading buffer and boiled for 5 min. The proteins were separated on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore). The membranes were blocked with 10% nonfat milk and incubated at 4°C overnight with a mouse monoclonal antibody against Kv4.2 (1:2,000; University of California, Davis), a rabbit monoclonal antibody against phosphorylated Akt (1:2,000; Cell Signaling Technology), a rabbit polyclonal antibody against phosphorylated mTOR (1:1,000; Cell Signaling Technology), or a mouse monoclonal antibody against GAPDH (1:10,000; KangChen Bio-Tech). After being washed extensively in TBS-Tween, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (1:10,000; KangChen Bio-Tech) for 2 h at room temperature. Chemiluminescent signals were generated using a SuperSignal West Pico trial kit (Pierce) and detected by exposure to X-ray film or by using the ChemiDoc XRS System (Bio-Rad Laboratories).
Transwell cell migration assays.
Cell culture inserts equipped with an 8-μm pore member of 0.3 cm2 were placed in 24-well culture dishes, forming the upper and lower compartments of the assay, respectively (COSTAR Corning, Cambridge, MA). Most experiments were conducted using granule cells prepared from the cerebellum of 7-day-old Sprague-Dawley rat pups. The bottom wells contained medium alone (DMEM with free serum) as a control. The test agents including different drugs were added to the bottom wells. The top wells were seeded with cell suspension. Then, 106 cells/ml in 100 μl serum-free DMEM were added to the top wells and incubated for 12 h. All incubations were carried out under air/CO2 95%-5%, at 37°C. At the end of experiments, filters were washed twice with PBS, and cells on the upper surface were removed using cotton swabs. Cells on the lower filter surface were fixed in 4% PFA (15–20 min) and stained with crystal violet staining solution and then examined under an Olympus BH-2 microscope. We counted the cells in 10 random high-power fields (×400), and the mean count was determined from duplicate experiments.
RESULTS
NRG-1 increased the IA density of CGNs in a time- and concentration-dependent manner.
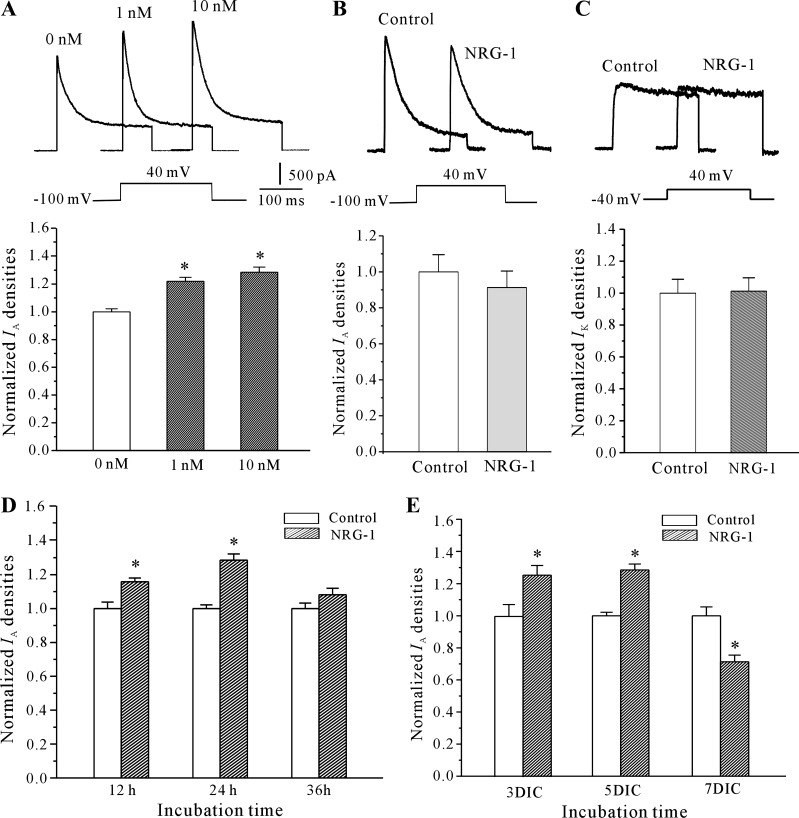
To determine the potential role of NRG-1 on CGNs, we asked whether NRG-1 affected the IA. The IA was evoked by a 200 ms depolarization to +40 mV from a holding potential of −100 mV in the presence of 20 mM TEA, which suppresses the IK and permits better resolution of the IA. Incubation of CGNs with NRG-1 significantly enhanced the IA density. The data obtained from 92 neurons showed that incubation of CGNs with 1 or 10 nM NRG-1 for 24 h increased the current density by 22.5 ± 3.1% (n = 44) or 29.5 ± 3.9% (n = 48), respectively (Fig. 1A). NRG-1, however, did not affect the IA amplitude of CGNs when applied acutely to the bath solution by the perfusion system (Fig. 1B). In the present or absence of 10 nM NRG-1, IA amplitude was 2,017.70 ± 189.30 and 1,897.52 ± 203.41 pA, respectively (n = 17; P > 0.05). Moreover, NRG-1 did not increase the IK current density (Fig. 1C). Incubation of CGNs with 10 nM NRG-1 for 24 h lightly increased the current density by 2.7 ± 5.3% (from 830.47 ± 40.21 to 852.90 ± 40.62 pA; n = 27; P > 0.05). To address whether the NRG-1-induced IA density was time dependent, neurons were incubated with 10 nM NRG-1 for different durations and the IA density was determined. After 12-, 24-, and 36-h incubation, NRG-1 was found to augment IA density by 15.8 ± 2.3% (n = 38), 28.6 ± 3.6% (n = 41), and 8.2 ± 3.8% (n = 37), respectively (Fig. 1D). We also found that the effect of NRG-1 on the induction of the IA was dependent on the number of days that the CGNs were in culture (Fig. 1E). Together, these data indicate that incubating CGNs at 5 different days in culture (DIC) with 10 nM NRG-1 for 24 h produced the most significant increase in IA density. Therefore, we selected 10 nM NRG-1 for 24 h in the subsequent series of study.
Fig. 1.
Neuregulin-1 (NRG-1) enhanced transient outward K+ currents (IA) densities in a concentration- and time-dependent manner in cerebellar granule neurons (CGNs). A: IA obtained from neurons maintained in control medium or medium with different concentrations of NRG-1. IA was elicited by depolarizing pulses to +40 mV from a holding potential of −100 mV in the presence of 20 mM TEA. Top: representative recording samples. Bottom: statistical analysis. B: NRG-1 did not affect the IA amplitude of CGNs when applied acutely to the bath solution by the perfusion system. C: NRG-1 did not affect delayed-rectifier outward (IK) densities in CGNs. IK currents were elicited by depolarizing pulses to +50 mV from a holding potential of −50 mV in the presence of 5 mM 4-aminopyridine (4-AP). D: IA density in CGNs maintained for different incubation times in control medium or in medium containing 10 nM NRG-1. E: IA density in CGNs at different days in culture (DIC) maintained in control medium or in medium containing 10 nM NRG-1 for 24 h. Data are shown as the means ± SE. *P < 0.05, compared with the corresponding control by the unpaired t-test.
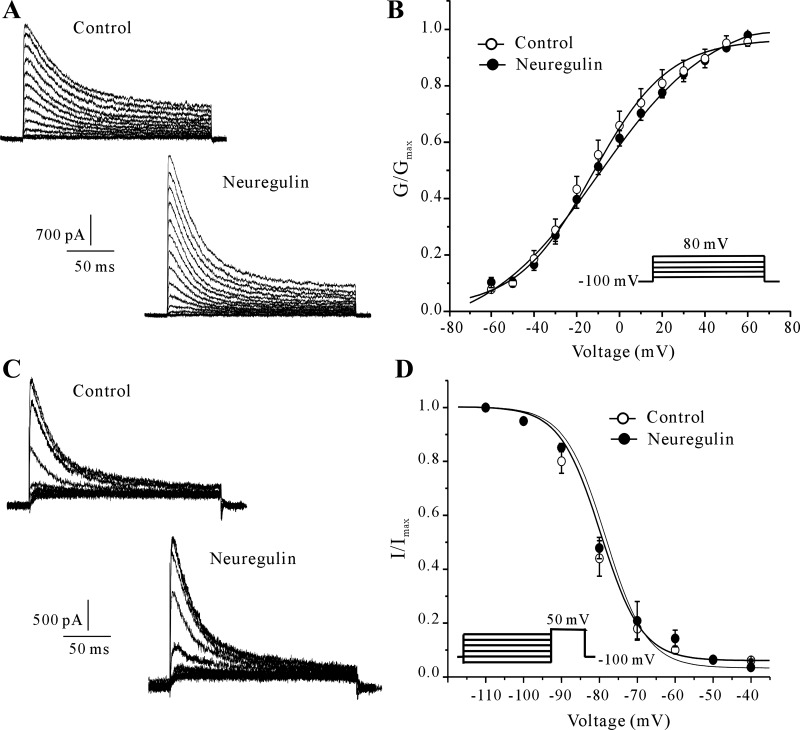
The effects of NRG-1 on the activation and inactivation properties of IA were also explored using corresponding experimental protocols. In steady-state activation experiments, membrane potential was held at −100 mV and the IA was evoked by a 200-ms depolarizing pulse from a first pulse potential of −60 to +60 mV in 10-mV steps in 10-s intervals. Data were analyzed using the equation GK = IK/(Vm − Vrev), where GK is the membrane K+ conductance, Vm is the membrane potential, and Vrev is the reversal potential for K+. The steady-state activation of the IA recorded from neurons maintained in control medium or in medium containing 10 nM NRG-1 is presented in Fig. 2A. The IA activation curve was obtained by plotting normalized conductance as a function of the command potential (Fig. 2B). Incubation with 10 nM NRG-1 for 24 h did not affect the activation curve. The current was half-activated at −8.5 ± 1.3 mV (n = 27) and −7.3 ± 0.8 mV (n = 25) for the control and NRG-1-treated groups, respectively (P > 0.05). These data suggest that NRG-1 treatment does not alter the steady-state activation of the voltage dependence of IA.
Fig. 2.
NRG-1 did not alter the steady-state activation and inactivation properties of IA channels. A: IA recorded using an activation protocol in neurons maintained in control medium or medium containing 10 nM NRG-1. B: steady-state activation curves (G/Gmax) of IA obtained by plotting the normalized conductance as a function of command potential obtained from control and NRG-1-treated groups. Data points were fitted using the Boltzmann function. Data points are shown as the means ± SE (n = 27 for the control group, and n = 25 for the NRG-1-treated group). C: IA recorded using an inactivation protocol in neurons maintained in control medium or medium containing 10 nM NRG-1. D: steady-state inactivation curves (I/Imax) for IA were obtained from the control and NRG-1-treated CGNs using inactivation protocols. Data points were fitted using the Boltzmann function. Data points are shown as the means ± SE (n = 20 for the control group, and n = 22 for the NRG-1-treated group).
To study steady-state inactivation of IA, currents were elicited using 1 s conditioning prepulses from −110 to 0 mV before a 200-ms test pulse of +50 mV. After normalizing each current amplitude to the maximal current, the amplitude obtained from the −110 mV prepulse was used as a function of the conditioning prepulse potential and fitted to the function IA/IA-max = 1/{1 + exp[(Vm1/2 − Vm)/k]}. Hence, we obtained an inactivation curve of the IA and calculated the Vh50 (the voltage at which the current amplitude was half-inactivated). Similarly, the inactivation curve of the IA was not significantly shifted after incubation with 10 nM NRG-1 for 24 h (Fig. 2, C and D). The half-maximal inactivation voltage for the control and NRG-1-treated groups was −73.0 ± 4.7 mV (n = 20) and −76.7 ± 2.4 mV (n = 22), respectively (P > 0.05). These data indicate that NRG-1 does not affect IA inactivation kinetics. Together, the electrophysiological recordings demonstrate that the NRG-1-mediated enhancement of IA amplitude is not associated with the modification of IA activation or inactivation properties.
NRG-1 upregulates the expression of Kv4.2 by promoting translation of Kv4.2 mRNA.
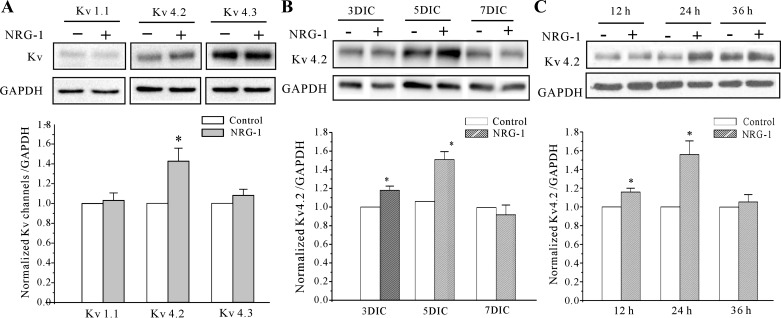
Next, we hypothesized that the NRG-1-induced IA density might be due to upregulation of potassium channel expression levels. The key α-subunits accounting for the IA in CGNs include Kv4.2, Kv4.3, and Kv1.1 (18). Because our previous study indicated that Kv4.2 was the main α-subunit that is sensitive to neurotrophic factors, we focused our investigation on Kv4.2. Antibodies to Kv4.2, Kv4.3, and Kv1.1 α-subunit were used to measure their protein expression levels after incubation of CGNs with NRG-1. Western blotting indicated that protein levels of Kv4.2 were significantly enhanced following incubation of CGNs with NRG-1 for 24 h. There was, however, no significant change in the protein expression levels of Kv4.3 and Kv1.1 (Fig. 3A; n = 5; P > 0.05). Kinetic of Kv4.2 induction was in parallel to the increase in IA amplitudes upon NRG-1 treatment (Fig. 3, B and C). These data indicate that NRG-1 increases IA density by upregulating the expression of Kv4.2.
Fig. 3.
NRG-1 increased the expression levels of the Kv4.2 α-subunit in CGNs. A: Western blot showing the effect of 10 nM NRG-1 on different Kv channels' subunits expression levels in CGNs. Top: representative Western blots. Bottom: statistical analysis. Data obtained from 5 independent experiments are shown as the means ± SE. B: effect of NRG-1 on Kv4.2 α-subunit protein expression levels in CGNs at different DIC. C: Kv4.2 α-subunit protein expression levels in CGNs maintained for different incubation times in control medium or in medium containing 10 nM NRG-1. Data obtained from 5 independent experiments are shown as the means ± SE. *P < 0.05, compared with corresponding control by the unpaired t-test.
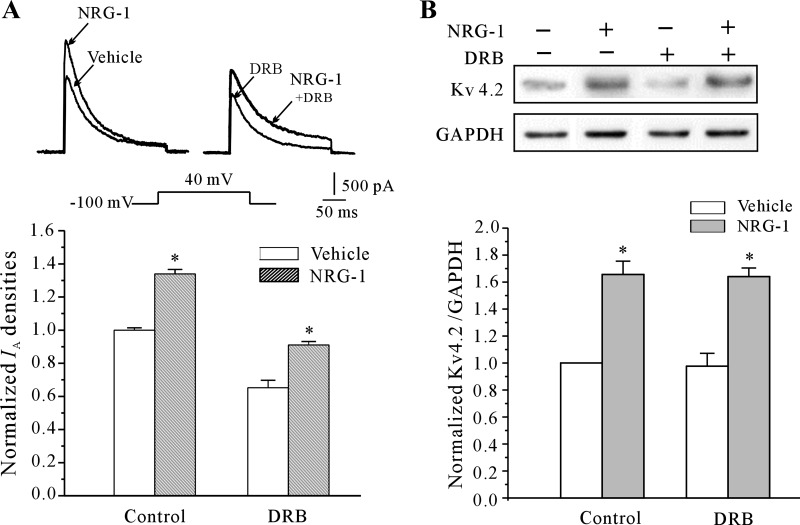
The Kv 4.2 gene expression change in response to neuritin is thought to occur at the transcriptional level (43). Therefore, we used a transcription inhibitor, DRB, to test whether Kv4.2 α-subunit mRNA levels were increased by NRG-1. However, coincubation of CGNs with NRG-1 (10 nM) and DRB (10 μM) failed to abolish the NRG-1-induced increase in IA density and the increased expression of Kv4.2 (Fig. 4, A and B). DRB itself reduced IA density by 34.4 ± 1.7%, but incubation with NRG-1 in the presence of DRB upregulated the IA density by 38.9 ± 0.6% vs. CGNs incubated with DRB alone (P < 0.05), an increase comparable to that observed in cells treated with NRG-1 alone. These results suggested that ongoing transcription is not required for the NRG-1-dependent increase in IA density and upregulation of the expression of Kv4.2.
Fig. 4.
Inhibition of transcription fails to abolish the NRG-1-induced increase in the IA density and the expression of Kv4.2. A: effects of the transcriptional inhibitor 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside (DRB), on the NRG-1-induced increase in IA density. *P < 0.05, compared with the vehicle control (medium without NRG-1) by the unpaired t-test. B: effects of DRB on the NRG-1-induced upregulation of the expression of Kv4.2 measured by Western blot. Data obtained from 5 independent experiments are shown as the means ± SE. *P < 0.05, compared with the vehicle control (medium without NRG-1) by the unpaired t-test.
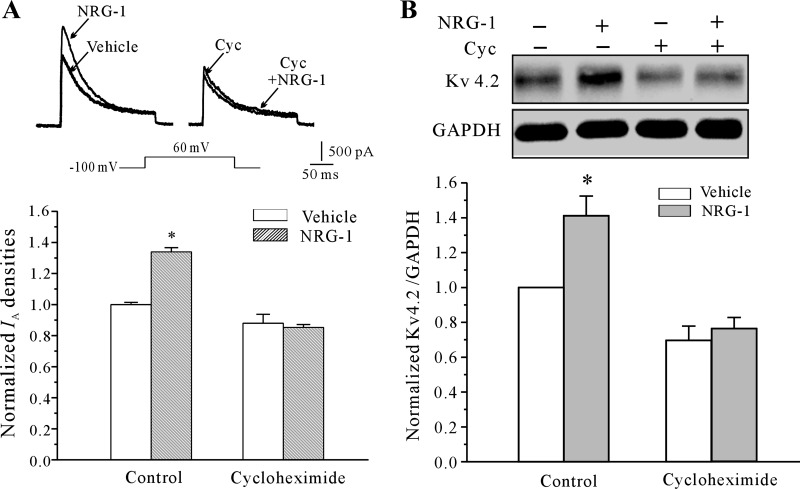
We then addressed whether NRG-1 upregulates the levels of Kv4.2 by promoting the translational efficiency of Kv4.2 mRNA. To test this hypothesis, CGNs were incubated with NRG-1 in the presence or absence of the translation inhibitor cycloheximide (10 μM), and the IA density and Kv4.2 expression levels were determined. As shown in Fig. 5, A and B, incubation with cycloheximide completely abolished the effects of NRG-1 on both the IA density and the expression of Kv4.2. Together these results argue that NRG-1 acts at the translational level to enhance Kv4.2 channel protein expression and IA density.
Fig. 5.
Inhibition of translation abolished the NRG-1-induced increase in the IA density and the expression of Kv4.2. A: effects of the translation inhibitor cycloheximide (Cyc) on the NRG-1-induced increase in IA density. Data are shown as the means ± SE. *P < 0.05, compared with the vehicle control (medium without NRG-1) by the unpaired t-test. B: Western blot analysis of the effect of Cyc on NRG-1-induced upregulation of the expression of Kv4.2. Data obtained from five independent experiments are shown as the means ± SE. *P < 0.05, compared with the vehicle control (medium without NRG-1) by the unpaired t-test.
NRG-1-induced increases in IA density and expression of Kv4.2 are sensitive to inhibitors of the ErbB4 receptor and the AKT/mTOR pathway.
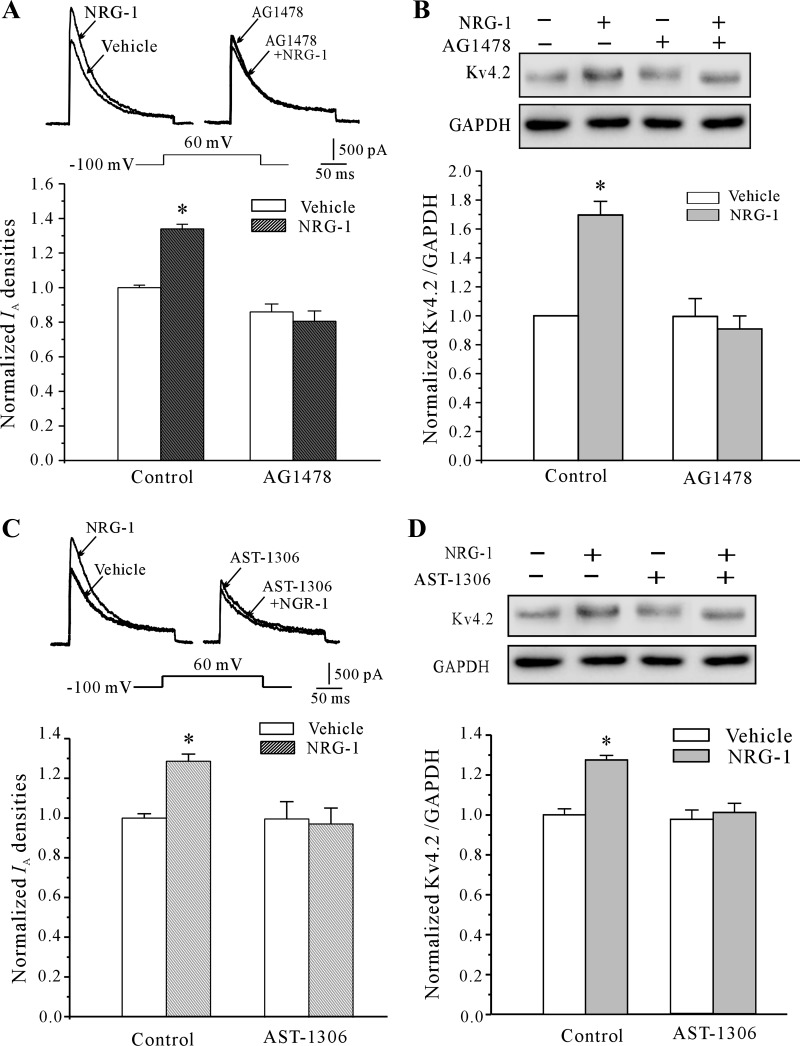
Previous observations suggested that NRG-1 signals through the ErbB4 receptor (27). Next, we asked whether blockade of the ErbB4 pathway would affect the NRG-1-induced increase in IA density and Kv4.2 protein level. To address this question, we used AG 1478, the EGF receptor tyrosine kinase inhibitor that also inhibits the ErbB4 receptor Tyr kinase. Blockade of ErbB4 activity by AG 1478 reduced NRG-1-mediated increases in both the IA density and the Kv4.2 protein level (Fig. 6, A and B). In the presence of 5 μM AG 1478, the IA density increased by NRG-1 was significantly reduced from 28.6 ± 3.6 to −6.3 ± 1.7% (n = 25, P < 0.05). Similarly, the induction of the Kv4.2 α-subunit was decreased to −9 ± 0.1%. We also tested the effect of AST-1306, a novel irreversible inhibitor of the epidermal growth factor receptor 1 and 2, which has a much lower IC50 for the ErbB4 than ErbB2 receptor (42). Our study of its effects on neuregulin-induced IA density and Kv4.2 expression, presented in Fig. 6, C and D, indicated that in the presence of 2 nM AST1306, the IA density and Kv4.2 α-subunit expression increased by NRG-1 were significantly reduced to −3.56 ± 8.0% (n = 24; P < 0.05) and −5.6 ± 7.9% (n = 5; P < 0.05), respectively. These data indicate that NRG-1 may activate ErbB4 receptor pathway to increase the IA density and Kv4.2 protein level.
Fig. 6.
Activation of the ErbB4 receptor is needed for the NRG-1-induced increase in IA density and increased Kv4.2 α-subunit expression in CGNs. A, AG 1478, the blocker of the ErbB4 receptor, reduced the NRG-1-induced increase in IA density. Data are shown as the means ± SE. *P < 0.05, compared with the vehicle control (medium without NRG-1) by unpaired t-test. B: Western blot analysis of the effect of 5 μM AG 1478 on NRG-1-induced upregulation of expression of Kv4.2. Data obtained from 5 independent experiments are shown as the means ± SE. *P < 0.05, compared with the vehicle control (medium without NRG-1) by the unpaired t-test. C: AST-1306, another blocker of the ErbB4 receptor, reduced the NRG-1-induced increase in IA density. Data are shown as the means ± SE. *P < 0.05, compared with the vehicle control (medium without NRG-1) by unpaired t-test. D: Western blot analysis of the effect of 2 nM AST-1306 on NRG-1-induced upregulation of expression of Kv4.2. Data obtained from 5 independent experiments are shown as the means ± SE. *P < 0.05, compared with the vehicle control (medium without NRG-1) with an unpaired t-test.
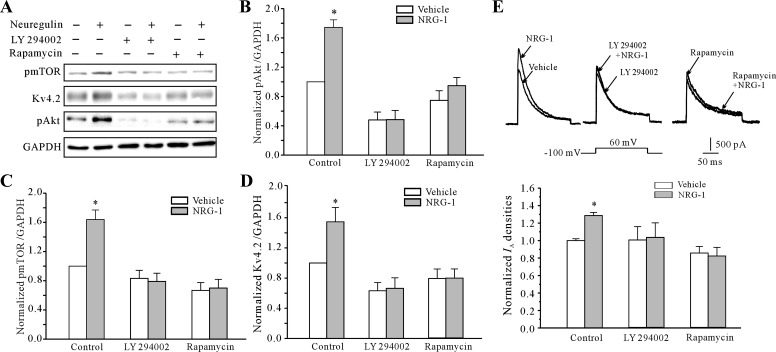
Phosphatidylinositol 3-kinase (PI3K)-Akt-S6K pathways are frequently activated by NRG-1-induced stimulation of ErbB receptor homo- or heterodimers (27). In addition to ErbB4 activity, we also determined whether the Akt/mTOR pathway, which was reported recently to affect protein synthesis and is relevant to synaptic plasticity (15), is activated by NRG-1. After NRG-1 treatment, the phosphorylation levels of Akt (pAkt) and mTOR (pmTOR) were significantly increased to 74.4 ± 10.3 and 63.8 ± 13.2%, respectively (n = 5) at 24 h (Fig. 7, B and C). Activation of Akt/mTOR by NRG-1 was confirmed using pharmacological inhibitors. Blockade of Akt activity by LY294002 or mTOR activity by rapamycin prevented the NRG-1-mediated increase in pAkt and pmTOR levels, respectively (Fig. 7, A–C). Moreover, in the presence of 20 μM LY294002, the increase in the pmTOR level induced by NRG-1 was significantly inhibited. These data indicate that the Akt/mTOR pathway is activated by NRG-1 and NRG-1 activates mTOR via the Akt kinase.
Fig. 7.
NRG-1-induced increase in IA density and the increased expression of the Kv4.2 α-subunit are dependent the Akt/mammalian target of rapamycin (mTOR) signaling pathway. A: Western blot shows the levels of activated Akt (pAkt), activated mTOR (p-mTOR) and Kv4.2 expression induced by 10 nM NRG-1 in the presence or absence of the mTOR inhibitor, rapamycin and the Akt inhibitor, LY294002. B–D: statistical analysis shows the effect of 20 μM LY294002 and 50 nM rapamycin on the levels of pAkt, p-mTOR, and Kv4.2 expression. Data obtained from 5 independent experiments are shown as the means ± SE. *P < 0.05, compared with the vehicle control (medium without NRG-1) by the unpaired t-test. E: effect of the mTOR inhibitor rapamycin and the Akt inhibitor LY294002 on the NRG-1-induced increase in IA density. Data are shown as the means ± SE. *P < 0.05, compared with the vehicle control (medium without NRG-1) by the unpaired t-test.
We then determined whether the Akt/mTOR pathway is required for the NRG-1-induced upregulation of the IA density and Kv4.2 α-subunit expression. Figure 7, D and E, shows that blockade of Akt/mTOR activity by 20 μM LY294002 or 50 nM rapamycin prevented the NRG-1-mediated increase in Kv4.2 protein expression and the increased IA density. In the presence of LY294002 or rapamycin, the increase in IA density induced by NRG-1 was reduced (Fig. 7E) from 28.6 ± 3.6 to 12.5 ± 18.3 and −4.1 ± 8.8% (n = 4 and 5; P > 0.05). Similarly, the expression levels of the Kv4.2 α-subunit protein (Fig. 7D) were decreased to 5.2 ± 13.9% (n = 5) and 0.6 ± 12.1% (n = 5), respectively. These data indicate that the mTOR pathway is also required for the NRG-1-stimulated upregulation of the IA density and increase in Kv4.2 α-subunit expression.
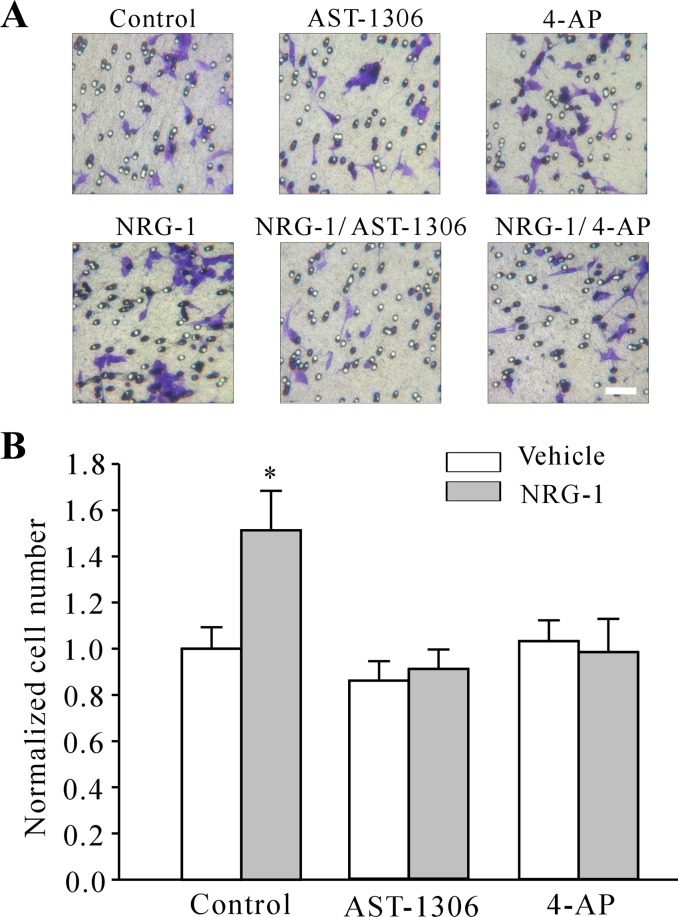
Because our previous experiments revealed the existence of K+ channel-dependent migration in granule cells (22), the effects of neuregulin on the migration of cerebellar granule cells were then tested using the Transwell migration assay, which is an in vitro model for invasive migration. In this assay, purified granule cells are plated on one side of a porous membrane and migration through the membrane into a second compartment is quantified. Control medium, neuregulin, AST-1306, and 4-aminopyridine (4-AP), which is a specific blocker of IA channels, were placed in the lower compartment, and granule cells were seeded into the top wells and allowed to migrate toward the lower surface of the separating membrane for 12 h. Figure 8A shows that a directional granule cell movement across the Transwell filter under vehicle control (medium without NRG-1) and addition of 10 nM neuregulin significantly increased granule cell migration by 51.3 ± 17.04% (P < 0.05). These neuregulin-induced migration effects could be modified by the addition of AST-1306, which decreased the number of granule cells migrating through the filter by 40.0 ± 8.35%. Moreover, this increase in migration effects induced by neuregulin was eliminated by cotreatment with 4-AP. Preincubation of cells with 4-AP, followed by treatment with neuregulin, only increased cell migration by 7.07 ± 6.32 or 8.09 ± 12.41% (Fig. 8B).
Fig. 8.
NRG-1 stimulates the migration of cerebellar granule cells across the Transwell membrane. A: representative microscopic fields showing cerebellar granule cells that have migrated across an 8-μm pore size (*). Transwell membrane in the absence and presence of NRG-1, AST-1306, and 4-AP. Scale bar = 20 μm. B: percentage of migration neuron induced by the corresponding treatment and control media. Results are expressed as the means ± SE of 3–5 separate experiments performed in duplicate. *P < 0.05, compared with the corresponding controls.
DISCUSSION
CGNs contain two major voltage-dependent outward K+ currents: a delayed rectifier potassium current (IK) and a fast transient potassium current (IA). NRG-1 treatment for 24 h failed to modulate IK in CGNs. We thus examined the effect of neuregulin on IA in this study. The IA has been described in neurons from many regions of the central nervous system (16, 37). Short-term modulation of the IA density could arise from a rapid mechanism due to changes in voltage-gating properties or intracellular trafficking of the channel proteins (23, 45). Alternatively, a long-term mechanism by upregulation of channel mRNA and protein expression could be involved (30). We observed that NRG-1 applied acutely to the bath solution does not affect the IA amplitude. NRG-1, however, significantly increased the IA density without modification of IA activation and inactivation properties, indicating that the mechanism of action of NRG-1 involves long-term effects.
Recent studies have suggested that the Kv4 shal family forms the major component of the IA in the central nervous system (32). Expression of the Kv4 family was found in CGNs, pyramidal neurons of the hippocampus and cortical neurons (30, 34). We have shown previously that Kv4.2, Kv4.3, and Kv1.1 are the main α-subunits expressed in rat CGNs (18). Upon NRG-1 treatment, only Kv4.2 α-subunit mRNA and protein levels were significantly increased. More importantly, the kinetics of Kv4.2 induction paralleled the increase in IA upon NRG-1 treatment. These data indicate that NRG-1 specifically increases the expression of Kv4.2. Interestingly, our recent studies indicated that neuritin, an important neurotrophin that plays multiple roles in the process of neural development, synaptic plasticity, and synaptic maturation, could activate the IR and ERK/mTOR signaling pathways to increase the IA amplitude and the expression of Kv4.2 in CGNs (43). Together, the results suggested that Kv4.2 might be the main target of neurotrophins in developing neurons.
Previous studies using cultured CGNs showed that the subcellular redistribution of Kv4.2 from the soma to the dendrites and synapses is induced by synapse formation. The regulation of Kv4.2 can be further influenced by synaptic activity (33). Indeed, the expression of Kv4.2 in CGNs was increased with the duration of the micro explant culture period (35) and CGNs in culture (43), in agreement with our findings showing a concomitant increase in the IA and Kv4.2 from 3 to 5 DIC. Notably, after NRG-1 treatment, the levels of IA and Kv4.2 in 3 DIC CGNs were similar to those in 5 DIC CGNs. Moreover, the NRG-1-induced increase in IA density and Kv4.2 expression only occurs in 3 DIC and 5 DIC CGNs. These observations suggest that the effect of NRG-1 on Kv4.2 expression may be developmentally regulated and associated with neuronal maturation. Our result is consistent with the findings of Ting et al. (38), which demonstrated that NRG-1 increases the number of excitatory synapses in GABAergic interneurons, and this effect only occurs in developing but not mature neurons. Meanwhile, our primary data also showed that NRG-1 promoted migrations of CGNs as measured by Transwell analysis and that incubation of CGNs with 4-AP (a blocker of IA) suppressed the effect of NRG-1 on CGNs migration, suggesting that the 4-AP-sensitive IA current was connected to neuronal migration of granule cells. Although neuronal migration is the one of the signs of differentiation and mutation of CGNs, the mechanisms involved in the NRG-1-induced increase of IA on CGNs migration remain to be tested.
It has been reported that stimulation of NRG-1/ErbB signaling upregulates the expression of NMDA and GABAA receptors in cultured cortical GABAergic interneurons and rat CGNs in vivo (1, 41), although Gajendran et al. (13) recently found that the developmental expression patterns of the mRNAs encoding the NMDA and GABAA-R is normal in mice lacking the NRG receptors ErbB2 and ErbB4. In addition to ligand-gated ion channels, reports have shown that the expression of voltage-gated ion channels is also induced by NRG-1. In developing chick ciliary ganglion neurons, NRG-1 regulates the functional expression of large-conductance Ca2+-activated K+ channels (BK) in an acute and sustained manner by the trafficking of channel proteins (4, 6, 10). Recently, the laboratory of Li et al.(22) reported that NRG-1-ErbB4 signaling increased the excitability of parvalbumin interneurons through downregulation of Kv1.1 which is as a risk factor for epilepsy. However, their data demonstrated that there was no significant change in the expression level of Kv1.1 after NRG-1 treatment but the level of tyrosine-phosphorylation of the Kv1.1 channel protein in the membrane was regulated by NRG-1 signaling (22), which is a mechanism different from NRG-1-induced upregulation of Kv4.2. This difference may be caused by neuregulin activation of a different signal transduction pathway involving different neuron types, different developmental statue, and the different animal used.
Although NRG-1 is known to contain EGF-like repeats and mediates its effects by binding to members of the ErbB receptor family including ErbB2, ErbB3, and ErbB4, (5, 11), ErbB4 homodimers bind NRG-1 and are activated in the CNS. NRG-1 may function as the ligand for ErbB4 forward signaling (27). Recently, evidence obtained from cultured postnatal hippocampal slices showed that NRG-1-ErbB4 signaling is essential for synapse maturation and plasticity (21). Coincidentally, previous studies in CGNs demonstrated that both ErbB3 and ErbB4 are present in cultured CGNs, but ErbB2 was virtually undetectable and that ErbB4 receptor activation is required for NRG-1-induced GABAA receptor β2/3-subunit expression (41). Considering the fact that ErbB3 must heterodimerize with ErbB2 to be activated and that ErbB4 receptors can function as homodimers (5), we focused on NRG-1-induced activation of ErbB4. Our studies indicate that selective blockade of ErbB4 receptor activation inhibits the effects of NRG-1 on the expression of Kv4.2. This is consistent with the earlier findings of Xie and colleagues (40, 41) in CGNs and demonstrates that the ErbB4 receptor is responsible for regulating Kv4.2 expression and the IA density in cultured CGNs.
In canonical forward signaling, NRG-1-induced ErbB dimerization activates the ErbB kinase domain, resulting in auto- and trans-phosphorylation of the intracellular domains. The phosphorylated tyrosine residues serve as docking sites for phosphopeptide-binding adaptor proteins or enzymes (27). The Raf-MEK-ERK and PI3K-Akt-S6K pathways are frequently activated by NRG-1-induced stimulation of ErbB receptor homo- or heterodimers (22, 27). Other downstream kinases such as CDK5 and Fyn have been reported (3, 12). In our study, we show that the Akt and mTOR pathways are required for the upregulation of IA density and induction of Kv4.2 gene translation by NRG-1, because administration of LY294002 or rapamycin completely eliminated the effect of NRG-1. Moreover, application of LY294002 blocks NRG-1-induced phosphorylation of mTOR, suggesting that NRG-1 activates mTOR via the Akt kinase. These data indicate that NRG-1-ErbB4 signaling activates the PI3K/Akt pathway and subsequently mTOR-dependent protein synthesis. However, it is interesting to note in our study that rapamycin also significantly reduced the phosphorylated Akt level when added to the culture medium. This result is in agreement with previous studies from Chen et al. (7) in which rapamycin was found to regulate Akt phosphorylation through the mTORC1 and mTORC2 signaling pathways.
However, we noted that NRG-1-induced regulation of GABAA receptor β2/3-subunit expression in CGNs reported by Xie et al. (41) requires at least three signaling pathways, including MAPK, PI3K, and CDK5, and their data suggest that signals from these three pathways converge, possibly at the level of GABAA receptor β2/3-subunit gene transcription. In this study, we showed that both LY294002 and rapamycin completely reduced the NRG-1-induced upregulation of Kv4.2 and IA, and in studies with nuclear factor of activated T cells transcription factor knockout mice or with a calcineurrin caciunulin inhibitor, there was no effect on the actions of NRG-1 (data not shown). Moreover, NRG-1-induced upregulation of Kv4.2 subunit expression was at level of Kv4.2 gene translation rather than gene transcription. All together, these data suggest that the NRG-1-induced upregulation of Kv4.2 subunit expression in CGNs occurs mainly through the Akt/mTOR pathway. Interestingly, neuritin also modulated Kv4.2 expression and the IA density in CGNs by upregulation of Kv4.2 subunit expression at the transcriptional and translational levels by activating both ERK and mTOR signaling (43). Together, these studies indicated that the same NRG-1-ErbB4 signaling pathway may regulate the expression of different proteins by activating different downstream signaling pathways; alternatively, the same gene expression could be regulated by different neurotrophins through different mechanisms.
In summary, our studies provide insight into the mechanism used by NRG-1 to regulate Kv4.2 subunit expression in CGNs. These studies suggest that proteins known to play important roles in neuronal development and maturation also participate in NRG-induced signaling. How these signaling pathways interact and activate downstream signaling pathways leading to translation remains to be determined.
GRANTS
This work was supported by the National Natural Science Foundation of China (NSFC 31070745) and Shanghai Leading Academic Discipline Project (B111). Q.-R. Zhao was supported by National Talent Training Fund in Basic Research of China (J1030627). The monoclonal antibody Kv4.2 was obtained from the University of California, Davis/National Institute of Neurological Disorders and Stroke/National Institute of Mental Health NeuroMab Facility.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.-J.Y. and Y.-A.M. conception and design of research; J.-J.Y., J.S., and Q.-R.Z. performed experiments; J.-J.Y., J.S., C.-Y.W., and Y.-A.M. analyzed data; J.-J.Y., J.S., and C.-Y.W. interpreted results of experiments; J.-J.Y., J.S., and Q.-R.Z. prepared figures; Y.-A.M. drafted manuscript; Y.-A.M. edited and revised manuscript; Y.-A.M. approved final version of manuscript.
REFERENCES
- 1. Abe Y, Namba H, Kato T, Iwakura Y, Nawa H. Neuregulin-1 signals from the periphery regulate AMPA receptor sensitivity and expression in GABAergic interneurons in developing neocortex. J Neurosci 31: 5699–5709, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci 7: 1319–1328, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, Novak TJ, Stefansson K, Gurney ME, Andresson T. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci 27: 4519–4529, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cameron JS, Dryer L, Dryer SE. beta -Neuregulin-1 is required for the in vivo development of functional Ca2+-activated K+ channels in parasympathetic neurons. Proc Natl Acad Sci USA 98: 2832–2836, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carpenter G. ErbB-4: mechanism of action and biology. Exp Cell Res 284: 66–77, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chae KS, Martin-Caraballo M, Anderson M, Dryer SE. Akt activation is necessary for growth factor-induced trafficking of functional K(Ca) channels in developing parasympathetic neurons. J Neurophysiol 93: 1174–1182, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Chen XG, Liu F, Song XF, Wang ZH, Dong ZQ, Hu ZQ, Lan RZ, Guan W, Zhou TG, Xu XM, Lei H, Ye ZQ, Peng EJ, Du LH, Zhuang QY. Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol Carcinog 49: 603–610, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron 14: 103–115, 1995 [DOI] [PubMed] [Google Scholar]
- 9. D'Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci 32: 30–40, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Dryer SE, Lhuillier L, Cameron JS, Martin-Caraballo M. Expression of K(Ca) channels in identified populations of developing vertebrate neurons: role of neurotrophic factors and activity. J Physiol (Paris) 97: 49–58, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 284: 14–30, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Fu AK, Fu WY, Cheung J, Tsim KW, Ip FC, Wang JH, Ip NY. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat Neurosci 4: 374–381, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Gajendran N, Kapfhammer JP, Lain E, Canepari M, Vogt K, Wisden W, Brenner HR. Neuregulin signaling is dispensable for NMDA- and GABA(A)-receptor expression in the cerebellum in vivo. J Neurosci 29: 2404–2413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Mol Cell Neurosci 27: 379–393, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci 18: 3521–3528, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu CL, Liu Z, Gao ZY, Zhang ZH, Mei YA. 2-Iodomelatonin prevents apoptosis of cerebellar granule neurons via inhibition of A-type transient outward K+ currents. J Pineal Res 38: 53–61, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hu CL, Zeng XM, Zhou MH, Shi YT, Cao H, Mei YA. Kv1.1 is associated with neuronal apoptosis and modulated by protein kinase C in the rat cerebellar granule cell. J Neurochem 106: 1125–1137, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci 32: 485–495, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiao S, Wu MM, Hu CL, Zhang ZH, Mei YA. Melatonin receptor agonist 2-iodomelatonin prevents apoptosis of cerebellar granule neurons via K(+) current inhibition. J Pineal Res 36: 109–116, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron 54: 583–597, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li KX, Lu YM, Xu ZH, Zhang J, Zhu JM, Zhang JM, Cao SX, Chen XJ, Chen Z, Luo JH, Duan S, Li XM. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci 15: 267–273, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Liu LY, Fei XW, Li ZM, Zhang ZH, Mei YA. Diclofenac, a nonsteroidal anti-inflammatory drug, activates the transient outward K+ current in rat cerebellar granule cells. Neuropharmacology 48: 918–926, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Liu LY, Hoffman GE, Fei XW, Li Z, Zhang ZH, Mei YA. Delayed rectifier outward K+ current mediates the migration of rat cerebellar granule cells stimulated by melatonin. J Neurochem 102: 333–344, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Ford BD, Mann MA, Fischbach GD. Neuregulin-1 increases the proliferation of neuronal progenitors from embryonic neural stem cells. Dev Biol 283: 437–445, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Mathie A, Clarke CE, Ranatunga KM, Veale EL. What are the roles of the many different types of potassium channel expressed in cerebellar granule cells? Cerebellum 2: 11–25, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 9: 437–452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mei YA, Vaudry D, Basille M, Castel H, Fournier A, Vaudry H, Gonzalez BJ. PACAP inhibits delayed rectifier potassium current via a cAMP/PKA transduction pathway: evidence for the involvement of I k in the anti-apoptotic action of PACAP. Eur J Neurosci 19: 1446–1458, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Mei YA, Wu MM, Huan CL, Sun JT, Zhou HQ, Zhang ZH. 4-aminopyridine, a specific blocker of K(+) channels, inhibited inward Na(+) current in rat cerebellar granule cells. Brain Res 873: 46–53, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Plant LD, Webster NJ, Boyle JP, Ramsden M, Freir DB, Peers C, Pearson HA. Amyloid beta peptide as a physiological modulator of neuronal “A”-type K+ current. Neurobiol Aging 27: 1673–1683, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Rieff HI, Raetzman LT, Sapp DW, Yeh HH, Siegel RE, Corfas G. Neuregulin induces GABA(A) receptor subunit expression and neurite outgrowth in cerebellar granule cells. J Neurosci 19: 10757–10766, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol 79: 1081–1091, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Shibasaki K, Nakahira K, Trimmer JS, Shibata R, Akita M, Watanabe S, Ikenaka K. Mossy fibre contact triggers the targeting of Kv4.2 potassium channels to dendrites and synapses in developing cerebellar granule neurons. J Neurochem 89: 897–907, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J Neurosci 20: 4145–4155, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shibata R, Wakazono Y, Nakahira K, Trimmer JS, Ikenaka Expression of Kv3 K.1 Kv4.2 genes in developing cerebellar granule cells. Dev Neurosci 21: 87–93, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Shyu WC, Lin SZ, Chiang MF, Yang HI, Thajeb P, Li H. Neuregulin-1 reduces ischemia-induced brain damage in rats. Neurobiol Aging 25: 935–944, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv41 subunits. J Neurosci 18: 3124–3137, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, Liu X, Xiong WC, Mei L. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci 31: 15–25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vaudry D, Falluel-Morel A, Leuillet S, Vaudry H, Gonzalez BJ. Regulators of cerebellar granule cell development act through specific signaling pathways. Science 300: 1532–1534, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Xie F, Padival M, Siegel RE. Association of PSD-95 with ErbB4 facilitates neuregulin signaling in cerebellar granule neurons in culture. J Neurochem 100: 62–72, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Xie F, Raetzman LT, Siegel RE. Neuregulin induces GABAA receptor beta2 subunit expression in cultured rat cerebellar granule neurons by activating multiple signaling pathways. J Neurochem 90: 1521–1529, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Xie H, Lin L, Tong L, Jiang Y, Zheng M, Chen Z, Jiang X, Zhang X, Ren X, Qu W, Yang Y, Wan H, Chen Y, Zuo J, Jiang H, Geng M, Ding J. AST1306, a novel irreversible inhibitor of the epidermal growth factor receptor 1 and 2, exhibits antitumor activity both in vitro and in vivo. PLoS One 6: e21487, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yao JJ, Gao XF, Chow CW, Zhan XQ, Hu CL, Mei YA. Neuritin activates insulin receptor pathway to up-regulate Kv4.2-mediated transient outward K+ current in rat cerebellar granule neurons. J Biol Chem 287: 41534–41545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang L, Fletcher-Turner A, Marchionni MA, Apparsundaram S, Lundgren KH, Yurek DM, Seroogy KB. Neurotrophic and neuroprotective effects of the neuregulin glial growth factor-2 on dopaminergic neurons in rat primary midbrain cultures. J Neurochem 91: 1358–1368, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Zhuang JL, Wang CY, Zhou MH, Duan KZ, Mei YA. TGF-beta1 enhances Kv2.1 potassium channel protein expression and promotes maturation of cerebellar granule neurons. J Cell Physiol 227: 297–307, 2012 [DOI] [PubMed] [Google Scholar]