Abstract
The last sixty years of research have provided extraordinary advances of our knowledge of the reward system. Since its initial discovery as a neurotransmitter by Carlsson and colleagues (Carlsson et al., 1957), dopamine (DA) has emerged as an important mediator of reward processing. As a result, a number of electrochemical techniques have been developed to directly measure DA levels in the brain using various preparations. Many of these techniques and preparations differ in the types of questions that they can address. Together, these techniques have begun to elucidate the complex roles of tonic and phasic DA signaling in reward processing and in addiction. In this review, we will first provide a guide for the most commonly used electrochemical methods for DA detection and describe their utility in furthering our knowledge about DA's role in reward and addiction. Second, we will review the value of common in vitro and in vivo preparations and describe their ability to address different types of questions. Last, we will review recent data that has provided new insight of the mechanisms of in vivo phasic DA signaling and its role in reward processing and reward-mediated behavior.
Keywords: Dopamine, Voltammetry, Striatum, Reward-related behavior, Psychostimulant, Reinforcement learning
2. Reward circuitry: a brief historical perspective
The neural system that has come to be known as the “reward pathway” in brain was serendipitously discovered by Olds & Milner when they made the observation that rats would spend more time in the corner of a chamber where they had previously been given non-contingent intracranial electrical stimulation (Olds and Milner, 1954). Additionally, they demonstrated that rats would perform an operant response to receive intracranial self-stimulation (ICSS) only in specific brain regions and would extinguish responding when the stimulation was no longer available, establishing that ICSS is positively reinforcing (Olds and Milner, 1954). Further experiments aimed at mapping the reward pathway indicated that brain regions along the medial forebrain bundle, a fiber tract known to connect the midbrain tegmentum and limbic regions, supported the most robust ICSS (for review see (Olds and Fobes, 1981)). Specifically, several nuclei along the mesocorticolimbic dopaminergic pathway were shown to support ICSS including the ventral tegmental area, dorsal striatum, nucleus accumbens and medial prefrontal cortex (Crow, 1972, Phillips et al., 1975, Mora et al., 1976, Phillips et al., 1976). Alterations in these nuclei have subsequently been shown to play a role in many psychiatric disorders, including drug addiction.
Early research demonstrated that pharmacological manipulations of catecholamine signaling within the mesocorticolimbic pathway altered ICSS such that compounds that increase extracellular catecholamines, like amphetamine and cocaine, facilitate ICSS and those that block catecholamine signaling at the receptor level decrease ICSS (Stein, 1964, Crow, 1970, Ranaldi and Beninger, 1994). While it was initially thought that these findings indicated a significant role for norepinephrine neurotransmission in maintaining ICSS, a more critical role for DA neurotransmission soon emerged (Fibiger, 1978). While the pharmacological manipulations used to characterize the effects of catecholaminergic neurotransmission on ICSS do alter norepinephrine signaling, they also affect the dopaminergic system. Evidence indicating that norepinephrine is not a critical component for ICSS was provided in studies showing that in rats displaying stable ICSS, depletion of norepinephrine behavior had no effect on ICSS nor did it affect amphetamine-induced enhancement of ICSS (Lippa et al., 1973, Cooper et al., 1974). However, selective decreases in DA levels reduce ICSS and decrease amphetamine-induced enhancement of ICSS (Liebman and Butcher, 1973, Lippa et al., 1973, Cooper et al., 1974). Further studies demonstrated that lesions of ascending noradrenergic pathways did not disrupt ICSS, while lesions of ascending dopaminergic pathways reduced ICSS (Clavier and Fibiger, 1977, Phillips and Fibiger, 1978). While other neurotransmitter systems likely contribute to ICSS behavior, these studies indicated a prominent role for DA.
Research indicating that both ICSS and modulation of ICSS behavior by psychostimulants are dependent upon dopaminergic mechanisms provided the basis for the DA hypothesis of drug reward, which posits that drugs of abuse are rewarding because they increase mesolimbic dopaminergic neurotransmission (Wise and Rompre, 1989). As further evidence, self-administration of psychostimulants such as amphetamine, cocaine and nicotine increase extracellular DA in the nucleus accumbens (NAc) (Hurd et al., 1989, Hurd et al., 1990, Di Ciano et al., 1995, Lecca et al., 2006). In addition, studies revealed that animals will self-administer drugs of abuse into specific brain regions of the mesolimbic pathway. For instance, direct infusion of cocaine or amphetamine into the NAc shell and nicotine infusion into the ventral tegmental area (VTA) are sufficient to support self-administration behavior (Hoebel et al., 1983, Carlezon et al., 1995). In light of these findings, particular attention has been given to the dopaminergic neurons of the VTA and their target regions including the NAc and prefrontal cortex and their role in drug reward. The NAc is particularly implicated in drug-seeking behavior as the region where information from limbic regions and the prefrontal cortex is integrated and translated into behavioral output (Mogenson et al., 1980, Goto and Grace, 2008). Thus, changes in tonic (on the order of minutes) or phasic (on the order of seconds) DA have been analyzed under various experimental conditions in order to understand the role of DA dynamics in reward. In the next section, we will review a number of methodologies and preparations that have been used in these research efforts.
3. Methods for analysis of DA dynamics
DA neurons can fire in either a tonic or phasic-like mode. Tonic activity is characterized by 3 to 8 Hz firing frequencies while phasic firing is characterized by bursts of action potentials with a frequency of ∼12 to ∼20 Hz (Grace and Bunney, 1984a, b, Hyland et al., 2002). The firing patterns of DA neurons are minimally modulated during sleep or anesthesia, although different stages of wakefulness can modulate their activity (Miller et al., 1983, Freeman et al., 1985). This neuronal firing leads to the release of DA from presynaptic terminals into the extracellular space, where it binds to receptors. DA receptors are also present in the extrasynaptic space and DA signaling is characterized by “volume transmission” where DA diffuses from the synapse and binds to extrasynaptic receptors to initiate downstream processes (Rice and Cragg, 2008). The levels of extracellular DA in target regions are regulated by four main processes: diffusion, neuronal firing, vesicular release from pre-synaptic terminals and reuptake by the DA transporter (DAT). Further, drugs of abuse typically exert their effects by altering one or more of these processes (Sulzer, 2011). Thus, various methods have been used to measure changes in DA concentration as well as specific changes in DA release and uptake. Among these approaches are microdialysis, electrochemical-based techniques using carbon fiber electrodes (CFE’s) (amperometry, chronoamperometry, fast-scan cyclic voltammetry (FSCV)), rotating disk voltammetry (RDV), and radiolabeling methodologies. Furthermore, a number of additional preparations have been combined with the above techniques. Advantages and disadvantages of using in vitro (synaptosomes, single cell, tissue) and in vivo (anesthetized and awake-behaving animals) techniques will be discussed below, using specific examples from the literature highlighting their advantages, as well as their compatibilities, with DA detection methods.
3.1. Microdialysis
Microdialysis is one of the most commonly used methods to measure neurotransmitter levels in the extracellular space of the brain. The microdialysis technique evolved from the push-pull cannula, an arrangement of two concentric tubes that allowed fluid to be directed into the brain and then removed. First described in the late 1960s and practically implemented in the early 1970s (Delgado et al., 1972, Ungerstedt and Pycock, 1974), over 10,000 papers have been published examining DA levels in the brain using some form of microdialysis (key word search: DA and microdialysis, Web of Knowledge database). Microdialysis itself is a collection method and is not to be confused with methods that are often used in conjunction with microdialysis to detect analytes of interest (i.e. DA). The microdialysis probe consists of a semi-permeable membrane that allows small molecules to pass through (<20 Kd). Typically, a physiological salt solution, such as artificial cerebral spinal fluid (aCSF), is infused through the microdialysis probe. Since most analytes of interest, such as DA, are not in aCSF, they will diffuse down their concentration gradient and across the dialysis probe to be collected and sent to a detector.
Ultimately, the samples collected via microdialysis must be analyzed. Typically the volumes of samples collected are on the order of microliters, therefore, the amount of analyte is very low, often in the femtomole range. Thus, the methods used to analyze dialysate samples must be very sensitive. The most common detection methods used in conjunction with microdialysis are chromotagraphic-based techniques, such as gas (GC) and high-performance liquid chromatography (HPLC). GC is generally too insensitive for measuring neurotransmitters, therefore, HPLC is typically employed. HPLC uses stationary phases that are contained in columns. The mobile phase and sample are pumped into the HPLC column. Each analyte in the sample will interact differently with the stationary phase, which will produce different retention times, or time it takes to emerge from the column. The retention time typically serves as a unique characteristic of an analyte and therefore provides selectivity for this technique. HPLC is usually coupled with a sensitive detection scheme such as electrochemical detection (EC) (Westerink and Van Oene, 1980), florescence (Anderson and Young, 1981), ultraviolet (UV) (Gagnieu et al., 1984), or mass spectrometry (MS) (Bronaugh et al., 1975, Zhang et al., 2007).
Microdialysis with HPLC-EC is one of the most common analytical methods for the detection of DA in vivo, and is best suited to determine long-lasting changes in extracellular DA concentration. To determine absolute DA concentration, the “no-net flux” method is used where several known concentrations of the analyte (such as DA) are perfused through the dialysis probe until equilibrium is reached. When equilibrium is reached, the concentration of analyte at the sampling site equals the concentration of analyte in the perfusate thus allowing estimation of the analyte at the sampling site.
The limits of temporal resolution for microdialysis are usually limited by the detection method employed. Finer temporal resolution requires better mass detection methods. HPLC’s detection limit requires sample times of 5–10 minutes. However, recent advancements in HPLC has allowed temporal resolution to decrease to 1–2 minutes by decreasing the inner diameter of the HPLC column (from 4–5 mm to 0.3–1 mm), resulting in less dilution of the analyte prior to reaching the detector. A more recent advance in detection methods utilized with microdialysis is capillary liquid chromatography (cLC). cLC columns have very small inner diameters (25–150 µm) and, when combined with electrochemical detection methods, can provide temporal resolution as high as 10 seconds (Schultz and Kennedy, 2008).
Microdialysis, coupled with even the most sensitive detection methods, has low temporal resolution (tens of seconds to minutes) compared to the techniques discussed below. However, low temporal resolution is advantageous in some instances. For example, basal, steady-state levels of DA are best assessed using a method with lower temporal resolution. Imperato and Di Chiara used microdialysis to show that ethanol, nicotine, amphetamine and cocaine increase DA levels in the NAc leading the authors to suggest that stimulation of DA transmission in the mesolimbic system may be a pertinent characteristic of drugs of abuse (Imperato and Di Chiara, 1986, Di Chiara and Imperato, 1988). Although the aforementioned techniques have advanced our knowledge about neurotransmitter systems, the temporal resolution limits the questions they can address. Changes in DA levels on the scale of minutes and hours surely contribute to behavior. However, subsecond changes in DA levels correlates with several fundamental aspects of behavior, such as learning, reward and cue processing, saliency, and motivation. Furthermore, measurements of subsecond DA levels allow estimation of DA transporter functioning (DAT), which may play a role in the pathology of addiction. Below, several common techniques to assess subsecond DA levels will be discussed.
3.2. Amperometry
Two types of amperometry have been used to monitor DA. Constant potential amperometry, typically referred to as amperometry, is an electrochemical technique that provides high temporal resolution with limited selectivity. In this method, a constant, continuous potential is applied to the working electrode and the current produced is directly proportional to the number of molecules undergoing oxidation and reduction (Michael and Wightman, 1999a, Willuhn et al., 2010). If maximal temporal resolution is desired, then amperometry is ideal as it can be used to detect individual exocytotic events and provide estimates of quantal release in single cells (Mundroff and Wightman, 2002). The limitation of amperometry is that the current produced by the oxidation or reduction of all molecules at the applied potential will also be detected, limiting specificity. However, the technique can be used for monitoring electrically-stimulated DA in various preparations, by confirming the validity of the signal with anatomical, pharmacological and physiological data (Dugast et al., 1994). Additionally, constant potential amperometry can be combined with enzyme-based detection systems to detect non-electroactive analytes, such as acetylcholine. In chronoamperometry, the potential is periodically pulsed to a value sufficient to oxidize DA (Gulley et al., 2007). Some chemical selectivity is obtained by monitoring the ratio of the current when the potential is returned to its initial value relative to that measured during the potential step. This current ratio is determined by the stability of the electrogenerated product. Additionally, Nafion, a cation exchange membrane, can be coated on the electrode to limit access of anionic species. Coating the carbon fiber with Nafion can also be applied to other voltametric techniques (discussed below). Microdialysis coupled with HPLC, however, allows for enhanced chemical selectivity compared to chronoamperometry. Amperometry may also be used in conjunction with Michaelis-Menten based modeling in order gauge functioning of the DA transporter (DAT).
3.3. Fast-Scan Cyclic Voltammetry
Fast scan cyclic voltammetry (FSCV) provides high chemical selectivity and temporal resolution. During FSCV, the potential at the working electrode is applied as a triangular waveform, and the time between scans is about ten times as long as the time of the scan itself. This allows the cyclic voltammograms to be recorded with high temporal resolution, typically repeated at 10 Hz. While this is very good temporal resolution, it is lower than the resolution possible with constant potential amperometry. In FSCV, current is generated at different potentials and the electrolysis of different species is manifested as distinct peaks. The background current in FSCV is stable over short periods of time, allowing it to be digitally subtracted. The resulting background-subtracted cyclic voltammogram (CV) provides an electrochemical “fingerprint” revealing the identity of the analyte detected (Michael and Wightman, 1999a). FSCV is further advantageous in that the cyclic voltammogram is able to separate the signal of interest from most interferents such as pH through the use of principal component regression to statistically identify DA events (Heien et al., 2004). However, brain regions for DA detection must be carefully selected as the CV for DA is almost indistinguishable from the CV for norepinephrine (NE). Thus, FSCV experiments are typically performed in areas that contain primarily DA or NE. Despite this limitation, FSCV has been used to characterize phasic DA release during reward-seeking behaviors, specifically, showing that the learning of reward-predicting cues correlates to cue-evoked DA release in the NAc (Owesson-White et al., 2008).
FSCV can be used to determine the kinetics of DAT function. Altered DAT functioning can cause three major changes to DA neurotransmission. First, postsynaptic DA receptors may only detect changes in the magnitude of DA release without changes in timing (amplitude-modulated signal). Second, alterations in DAT function can cause a “spread” of the DA signal in time, without changes in magnitude (frequency-modulated signal). Third, both amplitude-modulated and frequency-modulated signal changes can occur (Schenk et al., 1990, Earles et al., 1998, Schenk et al., 2005). These changes in chemical neurotransmission can lead to alterations in the typical physiological functioning. For example, acute and chronic cocaine exposure can cause changes in DAT functioning, which may play a role in cocaine addiction (Addy et al., 2010)
Kinetic modeling has been most useful in slice and anesthetized preparations (Wightman et al., 1988, Jones et al., 1999, Miles et al., 2002, Robinson et al., 2005). The random walk approach, employed in brain slices, uses a Michaelis-Menten based model of uptake coupled to diffusion and release (Schmitz et al., 2001). In both brain slice and in vivo anesthetized preparations, non-linear regression and single curve fitting analyses have been used to determine DA release and uptake kinetics (Garris and Wightman, 1994, Wu et al., 2001). Specifically, the major parameters of interest are [DA]p, the concentration of dopamine released per stimulation pulse, Vmax, the maximal rate of uptake (that reflects the efficiency at which DAT removes DA), and Km, the concentration of DA substrate at which half of Vmax occurs. Different regions of the brain exhibit different Vmax values for DA. However, Km and Vmax are fairly consistent across FSCV in both slice (Jones et al., 1995a, Jones et al., 1995b, Jones et al., 1996) and anesthetized preparations (Garris et al., 1994, Cass and Gerhardt, 1995, Mickelson et al., 1998, Wu et al., 2001, Addy et al., 2010) and are consistent with values obtained using [3H] DA radiolabeling techniques (described below). For example, Km is usually reported in the range of 0.1µM to 0.3µM in the caudate-putamen and nucleus accumbens, with a mean of 0.2µM. (Garris et al., 1994).
3.4. Rotating Disk Voltammetry
Rotating Disk Voltammetry (RDV) provides the most accurate measurements of transport activity. RDE theory is based on the idea of a plane with infinitesimal thickness that is rotating about its axis in solution at a constant rate (Earles et al., 1998). This motion creates drag, which pulls the solution in a direction perpendicular to the electrode. The analyte of interest is brought towards the electrode and then spun radially away via centrifugal forces. If the analyte is electroactive, then RDV can be applied to oxidize or reduce the analyte and produce a current proportional to the analyte concentration. Typically, the applied voltage is fixed at a value sufficient to electrolyze the analyte. Because of the requirement of a liquid sample, RDV is used in synaptosomal preparations, or cell or tissue suspensions.
In the context of DA uptake, diffusion can confound interpretation of results obtained with electrochemical approaches. However, an advantage of RDV is the solution is in constant flux; therefore, the effects of mass transport and diffusion processes are minimized, enhancing the quality of transport measurements with this technique. In RDV experiments, DAT kinetics are determined using non-linear curve fitting that is based on Michaelis-Menten kinetics (Schenk et al., 2005). A drawback of RDV is that it cannot be used in vivo since the methodology is centered around rotation of the solution (Earles et al., 1998).
3.5. [3H] DA radiolabeling and DA radioligand binding
Although not an electrochemical technique per se, radiolabeling techniques are important given their extensive use in the measurements of DA uptake. The general approach is to incubate synaptosomes or tissue slices with [3H] DA, wash off the extracellular fluid, and measure via liquid scintillation counting, the radioactivity accumulated in the tissue (Brown et al., 2002, Fleckenstein et al., 2002). The amount of labeled DA that enters the tissue can provide an estimate of DA uptake. An alternative approach to studying DAT is to use a radioligand that binds to DAT. This approach provides a quantitative estimate of DAT number and location, rather than DA function.
3.6. Optical methods for monitoring presynaptic DA release
Fluorescent styryl dyes, such as FM1–43, are useful tools to monitor the endocytosis and exocytosis of neuronal synaptic vesicles. FM1–43 can be packaged and released with endogenous neurotransmitters. Therefore, neurotransmitter release can be indirectly measured by detecting changes in the intensity of florescence produced by FM1–43, typically detected by two-photon microscopy. This approach is most often used with in vitro preparations such as tissue slices and cell cultures (Angleson et al., 1999, Bamford et al., 2004, Geldwert et al., 2006, Bamford et al., 2008). There are two major stages for optically monitoring neurotransmitter release using FM1–43: staining and destaining. Staining is accomplished by bath application of FM1–43 to the preparation followed by electrical stimulation of cells or terminal fields to provoke endocytosis of the dye followed by a washout period. Destaining is the process caused by a second electrical stimulation, which should release vesicles that contain FM1–43. After stimulation, the intensity of florescence produced by FM1–43 decreases, indicating vesicular release. The change in florescence is a proxy for neurotransmitter release.
Deciding which particular DA detection method can best achieve an experiment’s desired goals is crucial. The utilization of each of the methods described above in the various in vitro and in vivo preparations is detailed below.
4. Experimental Preparations
4.1. Synaptosomal Preparations
Synapotosomes are the simplest preparation to study neurotransmitter release and uptake (Nicholls, 2003). Synaptosomes are isolated nerve terminals or varicosities whose axonal attachments have been removed by shearing the tissue in an isosmotic solution (Nicholls, 2003). In addition, synaptosomes can be used to isolate synaptic vesicles allowing the direct study of release and uptake of DA at the vesicular level. Using synaptosomes, two common techniques have been employed to measure release and uptake kinetics with similar levels of accuracy. These are[3H] DA radiolabeling and RDV, the former being the most popular.
Several important questions about DA uptake and release have been resolved through synaptosomal preparations. Using synaptosomes in conjunction with radiolabeling, the kinetics of DA uptake have been investigated in rat caudate and NAc (Krueger, 1990, McElvain and Schenk, 1992c, a, b, Povlock and Schenk, 1997). Radiolabeling was initially used for uptake characterization and provided evidence for a transporter-mediated uptake system. Subsequent work using RDV extended this work to determine how drugs of abuse modulate DA kinetics (Schenk et al., 1990, McElvain and Schenk, 1992c, a, b, Povlock and Schenk, 1997). More direct measurements of DA uptake were subsequently performed using both RDV and radiolabeling through the isolation of synaptic vesicles (Volz et al., 2006, Volz et al., 2007).
In addition to studying basic mechanisms of DA release and uptake, synaptosomal preparations have also revealed information about specific presynaptic receptors mediating DA release. For example, Grady et. al. demonstrated with [3H] DA radiolabeling that β2 containing nicotinic acetylcholine receptors (nAchR) were required for acetylcholine-induced DA release from the striatal, NAcc, olfactory tubercle, and prefrontal cortex synaptosomes (Grady et al., 2002). These studies highlight uses for synaptosome preparations in studying uptake and release in both intact axon terminals as well as synaptic vesicles (Volz et al., 2006, Volz et al., 2007, Farnsworth et al., 2009).
In comparison to RDV, radiolabeling is more sensitive and can be used to measure DA in sparsely innervated regions. In the experiment by Grady and colleagues (Grady et al., 2002) radiolabeling was most likely used because of the sparse dopaminergic innervations to the prefrontal cortex. However, it would be difficult to detect such low amounts of DA using RDV. On the other hand, radiolabeling techniques can be confounded by metabolic processes which may contaminate the signal measured. For example, DA conversion to DOPAC can give false readouts of DA, since the tritiatum will also be in DOPAC. Therefore, radiolabeling has higher sensitivity, while RDV has better selectivity (Middlemiss and Hutson, 1990).
4.2. Single cell preparations
Single cell experiments using voltammetry provide excellent spatial and temporal resolution for measuring neurotransmitter release mechanisms (Michael and Wightman, 1999b, Travis and Wightman, 1999). This preparation is suitable for use with all electrochemical, electrophysiological, and radiolabelling techniques which are often used in complement. The power of using single cell preparations is that measurements can be made with high spatial accuracy in conjunction with an inverted light microscope. Historically, single cell preparations using electrochemical detection methods have been utilized to investigate catecholamine release from vesicles (Leszczyszyn et al., 1990, Wightman et al., 1991, Chow et al., 1992, Pihel et al., 1994, Travis and Wightman, 1999). Original investigations used bovine adrenal cells, which contain large vesicles with slow release kinetics on the order of milliseconds (Leszczyszyn et al., 1990, Wightman et al., 1991), although neuronal cells such as the giant DA neurons in Planorbus corneus have also been used (Chen et al., 1996, Chen and Ewing, 1997, Anderson et al., 1998, 1999). The contents of these cells can be measured beforehand via high pressure liquid chromatography to verify dopaminergic content, which then allows the use of amperometry for high temporal resolution measurements. However, when the contents of a cell have more than one major electroactive species, FSCV is required (Pihel et al., 1994) for chemical selectivity.
Single cell preparations have been used extensively with electrochemical techniques to study the kinetics of vesicular release in cells (Wightman et al., 1991, Chow et al., 1992, Alvarez de Toledo et al., 1993, Pihel et al., 1994, Albillos et al., 1997, Travis and Wightman, 1999). One notable contribution of single cell preparations coupled with electrochemistry is the finding that catecholamine-containing vesicles may partially fuse with the membrane, releasing a trace amount catecholamine (Alvarez de Toledo et al., 1993, Albillos et al., 1997). This finding was critical since it ruled out an “all or none” mechanism of neurotransmitter release. In addition, extensions of these types of experiments have been performed in neurons (Zhou and Misler, 1995, Staal et al., 2004).
A special consideration for neuronal measurements of single event release is that neuronal release is much faster than non-neuronal release. Therefore, the use of amperometry or FSCV depends largely on the question being asked. Investigations of single event release in neurons must be measured using amperometry to capture its fast release kinetics, 0.5 ms to 2 ms for dense core vesicles in ganglia (Zhou and Misler, 1995) and about 100 µs for small synaptic vesicles (Sulzer and Pothos, 2000, Staal et al., 2004), while FSCV is necessary to identify the released substances (Pihel et al., 1994). Finally, amperometry cannot be used to directly determine absolute concentrations in DA release. As discussed above, concentrations are determined using a flow chamber. Since, in single cell preparations, the electrode is placed on the membrane, the diffusion layer is constrained by the distance from the electrode to the membrane, rather than by the properties of the electrode itself. This altered diffusion layer is difficult to reproduce in the flow chamber, hence concentrations are rarely reported from single cell or tissue (see below) amperometric studies. However, the number of molecules released in a single event can be quantified by integrating the current. The integral has units of charge, which, by Faraday’s law, allows direct calculation of the number of released molecules that were detected. FSCV can be used to measure local concentrations since its diffusion layer is very small. Therefore, there is negligible difference between the diffusion layer near a cell membrane and that in a flow chamber (Michael and Wightman, 1999b).
4.3. Slice preparations
Thin (200–400 µM) slices of brain tissue that are continually perfused with oxygenated artificial cerebrospinal fluid are viable for several hours (Michael and Wightman, 1999a). There are several advantages for using slice preparations. Slices can be placed under a microscope for more precise anatomical placement of the recording electrode, which gives this technique greater spatial resolution than that obtained in vivo. Slices are also suitable for studying the effects of local application of drugs without interference from systemic processes and without surgical placements of cannulae. Therefore, a known concentration of drug can be applied to the slice. Measurements are also easier to make in slices due to enhanced DA release upon electrical stimulation of slices when compared to in vivo stimulation. The reason for higher concentrations of DA is unclear. One potential explanation is that electrical stimulation of the slice itself is more likely to activate the terminals of dopaminergic neurons, whereas in vivo stimulation may activate a smaller population of terminals and a larger population of cell bodies, which do not depolarize easily under most stimulation protocols which utilize as small pulse width (∼4ms) (Yeomans, 1979, Yeomans et al., 1979, Yeomans et al., 1988). An alternative contribution could be due to the demonstrated lack of basal dopamine tone and basal D2 autoreceptor activation in the slice preparation (Phillips et al., 2002), resulting in little inhibitory tone. Therefore, a combination of a lack of inhibition coupled with active excitatory input may explain greater evoked DA release in slices. Additionally, since DA cells are also silent in the slice preparation (Phillips et al., 2002, Perra et al., 2011), there may be a build-up of presynaptic vesicles in the slice, leading to greater evoked DA release.
Due to the larger DA release in slices, it is easier to measure uptake kinetics. Multiple brain regions and multiple slices within a brain region can be used from a single animal, thereby enhancing the utility of each subject and reducing variability, respectively. The use of brain slices precludes the use of anesthetics, which may interfere with DA signaling. Finally, the experimenter has control over the experimental conditions to reduce interference from temperature and pH fluctuations, unlike in vivo preparations (John and Jones, 2007).
FSCV has been the most common electrochemical technique in brain slices, although brain slice preparations do not preclude chronoamperometric techniques. Unlike single cell preparations in which potential analytes can be determined ahead of time, there is no clear way to determine the analyte species present in the slice. Hence, FSCV is the most straightforward approach to studying DA uptake in brain slices because of its ability to identify specific analytes of interest. Caron and coworkers used FSCV in striatal slices to determine changes in DA uptake mice with genetic deletions of the DAT (Giros et al., 1996, Jones et al., 1998). The uptake rate was 300 times faster in striatal tissue from wild type mice than in tissue from the DAT knockout animals whereas the rate of DA uptake in heterozygous animals was approximately one half of the rate in wild type mice. One goal of this experiment was to also verify that DAT was the only transporter responsible in the striatum for DA uptake. Hence, there are three major reasons why Giros et. al chose slice preparations. First, since there is continually flowing fluid over the slice, the authors were able to verify that the removal of DA in knockout slices was attributed to diffusion facilitated by the perfusion fluid. This verification cannot be done easily in vivo. Second, tissue preparations allow application and wash out of drug. Therefore, when NE and 5-HT uptake inhibitors were applied and washed out of the tissue, there was no change in uptake or release in either condition. Ruling out the role of NE and 5-HT transporters is important because they can also transport DA (Duan and Wang, 2010, Larsen et al., 2011). Third, as previously stated, larger DA release in slices allows for easier characterization of the kinetics compared to other preparations. However, some precautions must be taken with slice preparation as the higher DA release in slices versus in vivo are likely due to differences in stimulation areas. Whether this is physiologically relevant is unclear. Overall, however, brain slices with electrochemistry provide a good model for studying release and uptake in complex systems.
4.4.In vivo preparations
In vivo DA measurements serve two major purposes—to verify in vitro measurements in the intact brain, and to allow for simultaneous acquisition of behavioral and electrochemical data. In vivo anesthetized preparations are the simpler choice when deciding on an in vivo preparation because the surgeries are less involved than freely-moving surgeries and because the data acquisition is done in a more controlled environment, providing a larger signal to noise ratio (Gulley et al., 2007, Robinson and Wightman, 2007). Urethane has been reported to not interfere with DA signaling, thereby making it a useful drug for anesthetized preparations (Sabeti et al., 2003). However, awake, freely-moving animals remove any potential effects of anesthesia on DA signaling and provide behavioral data at the time of DA recording.
Both microdialysis and voltammetric techniques can be used in vivo. Microdialysis experiments are conducted most often in awake, behaving animals, but can also be done in anesthetized preparations. Microdialysis is capable of measuring nanomolar amounts of DA in the extracellular environment of the brain. Additionally, microdialysis is ideal for measuring the relatively slow effects of drugs on extracellular concentrations of DA as drug-induced changes in DA receptor function can be correlated with behavioral changes in freely-moving animals (Gulley et al., 2007). For example, Chefer et al. showed that behavioral sensitization to cocaine was correlated with an attenuation of basal DA release and a diminished ability of quinpirole, a D2 receptor agonist, to inhibit DA release (Chefer and Shippenberg, 2002).
Although microdialysis offers these advantages and excellent chemical selectivity, it cannot offer the high spatial or temporal resolution of electrochemical recording techniques. Carbon fiber electrodes (CFEs) CFEs can be made more than 20-fold smaller than microdialysis probes (∼7 µm diameter CFEs vs. ∼200 to ∼500 µm diameter microdialysis probes). Their small size allows for minimal damage when they are implanted into living brain tissue. The small size also allows for the recording of electrical stimulation events of DA cell bodies with less diffusional distortion (Borland and Michael, 2007).
Voltammetric methods with CFEs are used in both anesthetized and freely-moving animals. For example, Gerhardt et al. coupled a microinjection cannula to a CFE, allowing them to locally apply DA in the mammalian brain. They employed chronoamperometry in freely-moving animals to compare uptake rates in anesthetized and freely moving animals (Gerhardt et al., 1999). Chronoamperometry can also be used in anesthetized preparations to analyze baseline differences in DA function and also the effects of drugs that alter DA activity. This is less technically challenging than recording in freely-moving animals, however, data cannot be correlated to behavior (Gulley et al., 2007).
An even more powerful benefit of using FSCV in vivo is that discrete, naturally-occurring DA signals can be detected in awake rats during behavior (Robinson and Wightman, 2007). This was first done by Rebec et al. in 1997 in the NAc shell (Rebec et al., 1997). These naturally-occuring DA signals, or transients, are not time-locked to external stimuli such as cues and rewards and therefore are likely to reflect either the motivational state of the animal or spontaneous DA release. In the dorsal and ventral striatum, DA transients occur at baseline frequencies, an average of 0.24 per minute (Robinson et al., 2002) and vary from 10 nM to over 1 µM in concentration. They increase in frequency at the presentation of salient stimuli to the animal (Rebec et al., 1997), social interaction (Robinson et al., 2002), pharmacological application (Cheer et al., 2004), and self-administration of drug reinforcers (Phillips et al., 2003a). Over the last 10 years, studies employing in vivo FSCV in freely moving animals have continued to provide new insights of the complex role of phasic DA signaling in reinforcement learning and reward seeking-behavior.
5. The role of in vivo DA signaling: recent findings
5.1. Tonic and phasic DA signaling
DA neurons fire in two distinct modes, tonic and burst firing, which leads to different types of DA release at terminals in target regions. Tonic DA firing occurs at a frequency of 3–8 Hz and leads to DA release that results in steady-state DA levels. In contrast, burst firing occurs with intra-burst frequencies of ∼12 to ∼20 Hz and leads to transient, phasic increases in DA concentration ranging from 10 nM to 1 µM (Grace and Bunney, 1983, 1984a, Freeman et al., 1985, Wightman and Zimmerman, 1990, Hyland et al., 2002, Aragona et al., 2008). Different regulatory systems are capable of selective modulation of tonic and phasic DA signaling. For example, glutamatergic/cholinergic afferents from the pedunculopontine tegmental nucleus (PPT) to the VTA contribute to phasic DA firing/release, whereas GABAergic input from the ventral pallidum (VP) to the VTA modulates tonic DA/release (Floresco et al., 2003). Distinct characteristics of phasic and tonic DA firing/release suggest that these differential temporal dynamics of DA release might have distinct roles in physiology and behavior. Therefore, distinguishing tonic and phasic contributions of DA release may shed light on DA-related central nervous disorders such as Parkinson's, schizophrenia, and addiction.
5.2. Physiological consequences of phasic DA firing
DA neurons in the VTA and substantia nigra (SN) project to the nucleus accumbens (NAc), dorsal striatum, prefrontal cortex, amygdala and hippocampus. Phasic firing of DA cells activates excitatory, low affinity DA D1-like receptors, leading to activation of the direct pathway of the basal ganglia and facilitation of long term potentiation (LTP) at excitatory cortico-striatal synapses (Goto and Grace, 2005, Grace et al., 2007). In contrast, tonic DA activity is proposed to activate DA D2-like receptors, producing long term depression (LTD) of the medium spiny neurons (MNS) within NAc and dorsal striatum and leading to suppressed activity of indirect pathway of the basal ganglia (Goto and Grace, 2005, Shen et al., 2008). It has been proposed that coordination of D1 and D2 activation, by controlling striatal plasticity, modulates motor and cognitive function and facilitates behavioral flexibility (Shen et al., 2008). In line with these electrophysiological results is the recent electrochemical data demonstrating that phasic DA signaling in NAc selectively modulates excitatory but not inhibitory responses of NAc neurons during reward-seeking behavior (Cacciapaglia et al., 2011). These observations suggest that phasic DA selectively activates discrete NAc microcircuits that influence goal-directed behaviors (Cacciapaglia et al., 2011).
5.3. Phasic DA signaling is modulated by excitatory afferents
Burst activity of midbrain DA neurons resulting in transient increase in synaptic DA (Grace et al., 2007) is mediated by large amplitude, slow inactivating excitatory postsynaptic currents (EPSCs). Burst firing of DA neurons and subsequent DA transients are modulated by excitatory afferents from the midbrain pedunculopontine tegmental (PPTg) and laterodorsal tegmental (LDT) nuclei, which process cue-related sensory information to DA neurons (Lodge and Grace, 2006, Lester et al., 2008, Zweifel et al., 2009). These afferents have been shown to be both glutamatergic and cholinergic, implicating the involvement of glutamatergic and/or cholinergic signaling in the modulation of DA neuronal activity. Indeed, activation of the PPTg results in burst firing in SNc DA cells and striatal phasic DA release (Floresco et al., 2003) - an effect blocked by intra-SNc infusions of nonselective nicotinic ACh receptors (nAChRs) antagonists (Forster and Blaha, 2003). In addition, LDT projections to the VTA have been demonstrated to evoke burst firing of VTA DA cells (Imperato et al., 1991, Oakman et al., 1995). Specifically, pharmacological activation of the VTA muscarinic ACh receptors (mAChRs) and nAChRs produces increased DA release in DA terminals, whereas blockade of VTA mAChRs and nAChRs attenuates phasic DA signaling in the NAc (Lacey et al., 1990, Blaha et al., 1996, Floresco et al., 2003, Lester et al., 2008, Ishibashi et al., 2009). Furthermore, inhibition of nicotinic or muscarinic receptors in the VTA attenuates LDT stimulated phasic DA signaling in the nucleus accumbens (Lester et al., 2008).
Glutamatergic mechanisms in the VTA have also been demonstrated to influence phasic DA mechanisms. For instance, inhibition of NMDA receptors (NMDAR) in the VTA reduces the frequency of naturally occurring DA transients, decreases cocaine-evoked DA release and attenuates phasic DA encoding of reward-predictive cues during ICSS behavior (Sombers et al., 2009). More specifically, genetic inactivation of the essential NR1 subunit of the NMDAR selectively on midbrain DA neurons attenuates both burst firing of DA neurons in the VTA/SN and phasic DA transients in the striatum (Zweifel et al., 2009). Importantly, disruption of burst firing of DA neurons and phasic DA signaling observed in those transgenic mice selectively attenuated learning of many tasks in which cues predicted rewarding or aversive events (Zweifel et al., 2009, Zweifel et al., 2011). However, NR1 deletion on DA neurons does not impact the learning of a conditioned approach in response to cue associated with reward (Parker et al., 2010). Together, the data from these studies suggests that the NMDAR-dependent DA cell burst firing and phasic DA signaling might play an important role in learning contingencies and encoding valence and/or salience of important environmental cues, but is not essential for stimulus-response (S-R) associations (Parker et al., 2010).
5.4. Phasic DA signaling and reward
A growing body of literature examining DA signaling in freely moving rats continues to delineate the complex role of DA in reward mechanisms. For instance, the frequency of naturally occurring phasic DA in rat dorsal and ventral striatum has been shown to increase in response to rewarding events such as novelty, social interaction, and experimenter delivered or self-administered drugs of abuse (Rebec et al., 1997, Robinson et al., 2001, Robinson et al., 2002, Phillips et al., 2003b, Cheer et al., 2004, Robinson and Wightman, 2004, Stuber et al., 2005b). Interestingly, cues associated with both natural and pharmacological rewards produce time-locked DA transients in the NAc (Phillips et al., 2003b, Roitman et al., 2004, Day et al., 2007, Owesson-White et al., 2008). Similarly, DA transients also occur in the NAc shell during intracranial self-stimulation (ICSS) in response to the intracranial stimulus as well as to the conditioned stimuli that predict reward availability (Cheer et al., 2007). Furthermore, phasic DA contributes to the maintenance of self-administration behavior, as DA transients in the NAc core precede lever depression in rats trained for cocaine self-administration and electrically evoked phasic DA release is sufficient to promote cocaine self-administration behavior (Phillips et al., 2003b, Stuber et al., 2005b). These observations suggest that phasic DA may play a crucial role in incentive learning and goal-directed behaviors and are consistent with the idea that DA modulates reward-seeking behaviors (Goto and Grace, 2005, Nicola et al., 2005, Morris et al., 2006). Interestingly, recent evidence points to a selective role of phasic DA in NAc in encoding anticipated benefits but not costs to obtain food reward in rats trained on a decision-making task (Gan et al., 2010). Similarly, the magnitude of the cue-elicited phasic DA firing and release has been shown to depend on the size of the reward, the time to reward delivery and the probability of receiving a reward (Fiorillo et al., 2003, Beyene et al., 2010). In contrast, aversive stimuli such as quinine taste, stimuli conditioned with electric shock, or delayed cocaine availability modulates phasic DA signaling by producing a decrease in phasic DA levels detected with FSCV (Wheeler et al., 2011, Zweifel et al., 2011). These observations suggest that phasic DA might encode the attribution of salience and/or valence to the environmentally salient stimuli. Taken together, the data suggest that phasic DA signaling plays an important role in reinforcement learning.
5.5. Phasic DA and reinforcement learning
Several lines of evidence point to a role for phasic DA in reinforcement learning. During Pavlovian training for food reward, both burst firing of midbrain DA neurons and phasic DA transients in NAc shift from primary reward to the reward-predictive stimulus (Schultz, 1998, Day et al., 2007). This phasic DA plasticity is also apparent during ICSS training with a conditional stimulus (CS). Initially, DA transients are only seen at ICSS delivery. However, with increasing number of repeated parings with the CS and ICSS delivery, DA transients begin to emerge and increase in response to CS – a process proposed to underlie S-R (stimulus-response) associative learning. Furthermore, ICSS extinction leads to the decrease and eventual elimination of CS-evoked DA transients across trials – an effect accompanied by significant decline in goal-directed behaviors. Furthermore, reinstatement of ICSS behavior is accompanied by the return of DA transients to pre-extinction amplitudes in response to the CS (Owesson-White et al., 2008). Such results, showing that phasic DA release is associated with a CS that predicts reward, are in line with theories of reward-prediction error, in which DA neurons responsiveness encodes the predictive value of stimuli during associative learning (Schultz et al., 1997). More recently, increased phasic DA encoding during S-R learning has also been demonstrated to reflect the incentive salience of the CS (Flagel et al., 2011).
Additional evidence also points to the role of phasic DA in reinforcement learning as pharmacological and genetic disruption of DA D1R signaling – which is thought to arise from phasic DA - impairs acquisition of S-R associations (Lisman and Grace, 2005, Grace et al., 2007). Interestingly, DA-deficient mice are capable of learning the S-R association but lack the motivation to work for food reward. In addition, hyperdopaminergic mice can learn an instrumental task as fast as controls but exhibit increased motivation to work for food reward (Hnasko et al., 2005, Cagniard et al., 2006). Such results suggest the possibility that DA is not necessary for attributing salience and/or valence to environmentally-relevant cues but is instead more important for encoding motivation to obtain the reward. Importantly not all forms of S-R learning require phasic DA signaling. Sign-tracking behavior, where an animal will first approach and seek the CS cue, (the “sign” that is associated with reward) is dependent on phasic DA signaling. In contrast, goal-tracking behavior, where an animal approaches the reward or reward location (the “goal”) after CS presentation and not the CS itself, is not dependent on phasic DA release. In both cases, the CS is a strong predictor of the reward delivery and evokes a conditional approach behavior. However, a shift in phasic DA signaling to the CS is only present in sign-trackers and is absent in goal-trackers. In addition, the systemic blockade of DA transmission suppresses sign-tracking but not goal-tracking behaviors (Flagel et al., 2011). These results indicate that DA signaling may underlie a form of an S-R learning in which incentive salience is assigned to a CS, as is observed in sign-trackers, rather than encoding a predictive error signal.
5.6. Phasic DA signaling, drugs of abuse, and drug-mediated behavior
While microdialysis studies demonstrate that alcohol, nicotine, opiates, psychostimulants, and cannabinoids, increase DA levels (Wise and Bozarth, 1987, Di Chiara and Imperato, 1988), recent in vivo FSCV studies have highlighted the importance of phasic DA signaling physiological responses to cocaine and in cocaine-mediated behavior. While cocaine self-administration behavior is mediated by cocaine inhibition of the DAT (Ritz et al., 1988), cocaine also increases phasic DA release, in freely moving rats and this is likely a consequence of phasic firing of the VTA DA neurons (Wightman et al., 2007). Interestingly, cocaine-induced increases in phasic DA release are more pronounced in the shell of NAc in comparison to the NAc core (Aragona et al., 2008). This subregion difference is due to DA autoreceptor function, since it is abolished by DA D2 autoreceptor blockade before cocaine administration. These observations implicate that cocaine directly increases DA release in a regionally specific manner and demonstrates the significance of autoregulation in cocaine-evoked DA transmission (Aragona et al., 2008, Aragona et al., 2009).
FSCV studies in freely moving rats have also shown that i.v. cocaine produces increases in DA concentration in the NAc as fast as 20–40s after the end of cocaine infusion (Mateo et al., 2004, Heien et al., 2005, Stuber et al., 2005a). This DA rise in concentration is readily observed in the shell but not core of the NAc (Aragona et al., 2008). This delay is thought to reflect the time-course for cocaine to cross the blood-brain barrier. However, it has been demonstrated that DA transients occur faster in animals which are well trained in cocaine self-administration (Stuber et al., 2005a). These DA transients are observed as early as 0–2 s after cocaine i.v. infusion, followed by a second DA rise occurring 2–8 s later (Stuber et al., 2005a, Aragona et al., 2008, Wise and Kiyatkin, 2011). The first DA peak after cocaine i.v. infusion reflects DA response to external cues such as the sound of the lever press or the onset of audiovisual cues (such tone or cue light), whereas the second DA peak is thought to be associated with interoceptive cocaine effects in periphery (Aragona et al., 2008, Wise and Kiyatkin, 2011). Consistent with the evidence for phasic DA in reinforcement learning, these observations indicate that, after conditioning, both external and internal cues can serve as predictive cues of the later unconditioned cocaine effect on the DA neurotransmission. In contrast, if the CS predicts a delay in cocaine availability, it produces a decrease in DA concentration associated with aversive state, whereas DA concentration rapidly increase in response to cues signaling imminent cocaine delivery in the subsequent self-administration session (Wheeler et al., 2011). These findings show rapid, bivalent contextual control over brain reward processing.
During cocaine self-administration behavior, DA transients occur in the NAc core both pre- and post-response (ie lever depression resulting in i.v. cocaine administration and cue presentation). The pre-response DA transients are associated with the lever approach, whereas post-response DA peaks are time-locked to cocaine predictive cues, as discussed above. Furthermore, pre-response DA appears to contributes to the maintenance of self-administration behavior, as electrically evoked phasic DA release is sufficient to promote cocaine self-administration behavior (Phillips et al., 2003b, Stuber et al., 2005a). Interestingly, only the post-response DA release is subject to change in response to extinction or reinstatement procedures (Stuber et al., 2005b). Therefore, changes in phasic DA signaling appear to reflect associative processes linking cues with drug rewards. These observations suggest that phasic DA may play a crucial role in incentive learning and goal-directed behaviors and are consistent with the idea that DA modulates reward-seeking behaviors (Goto and Grace, 2005, Nicola et al., 2005, Morris et al., 2006).
5.7. Other mechanisms for phasic DA modulation
Several lines of evidence suggest that norepinephrine (NE) signaling in VTA modulates DA neuronal activity and DA release at terminals. It has been demonstrated that (i) the locus ceruleus – a major source of NE in the CNS - projects to the VTA (Jones et al., 1977, Geisler and Zahm, 2005); (ii) NE terminals make synaptic contacts onto midbrain DA neurons (Liprando et al., 2004); (iii) α-1 and α-2 adrenoceptors are present within the VTA (Jones et al., 1985, Lee et al., 1998) and (iv) blockade of α-1 adrenoceptors as well as activation of α-2 adrenoceptors reduces burst firing in DA cells (Grenhoff and Svensson, 1988, 1989, Grenhoff et al., 1993). These observations suggest that the NE system may modulate phasic DA signaling. The orexin/hypocretin system is also capable of modulating phasic DA release. Afferent projections from the lateral hypothalamus provide the source of orexin in the VTA (Harris et al., 2005, Narita et al., 2006, Zheng et al., 2007). In addition, orexin release onto VTA neurons activates DA cells and produces DA release at the DA cell terminal fields (Narita et al., 2006, Vittoz and Berridge, 2006, Muschamp et al., 2007, Vittoz et al., 2008). Furthermore, orexin signaling facilitates cocaine-induced glutamate-dependent LTP in the VTA (Borgland et al., 2009). Several lines of evidence suggest a role for orexin in reward-seeking behavior (Harris et al., 2005, Narita et al., 2006, Zheng et al., 2007). Blockade of VTA orexin signaling decreases both basal and cocaine-evoked phasic DA release in NAc core as well as motivation to self-administer cocaine (Espana et al., 2010). In addition, activation of the orexin system by hypocretin-1 infusion into the VTA increases the effects of cocaine on tonic and phasic DA signaling as well as the motivation to self-administer cocaine (Espana et al., 2011).
Phasic DA release can also be modulated by facilitation of activity at DA terminals. Glutamatergic afferents originating from basolateral amygdala (BLA) and forming synapses with NAc DA terminals are capable of modulating phasic DA release (Floresco et al., 1998). Such BLA inputs are crucial for processing motivationally salient stimuli during reward-seeking (Jones et al., 2010). Another system that can modulate phasic DA is the opiod system via the kappa opioid receptors (KOR) located presynaptically within DA terminals or on DA midbrain cells bodies (Mansour et al., 1995, Svingos et al., 2001). Activation of the KOR by systemic administration of the KOR agonist, salvinorin A, decreased phasic DA release in both NAc core and shell and resulted in attenuated motivation to obtain reward (Ebner et al., 2010). The role of the cannabinoid system has also been investigated as the CB1 receptor agonist, WIN55,212-2, increases both the number of spikes in a burst and the frequency of burst firing of VTA DA cells (Cheer et al., 2003). Similarly, intravenous administration of the CB1 agonist increases both the rate of DA transients as well as DA concentration detected with FSCV in the NAc, an effect blocked by a CB1 antagonist (Cheer et al., 2004). These observations suggest that phasic DA signaling is controlled and modulated by multiple neurotransmitter systems at the level of DA cell bodies and the DA terminals. Such a complex network is suited to control sub-second DA release in response to salient environmental stimuli, and therefore, is a crucial component of the reward system of the brain. Phasic DA signaling leads to goal-directed behaviors which favor survival in the changing environment. On the other hand, it may also underlie pathological conditions which may lead to psychiatric disorders such as schizophrenia and addiction.
6. Conclusions
The brain DA system and its relation to reward and addiction have been studied extensively since initial findings showing its role for ICSS and reward circuitry. The mesocorticolimbic DA system, consisting of VTA/SN DA neurons which send projections to limbic (ventral and dorsal striatum, amygdala, hippocampus) and cortical sites (medial prefrontal cortex, orbitofrontal cortex, cingulate cortex), has been implicated in processing salient stimuli and influencing goal-directed behaviors. DA signaling has been shown to be involved in both reinforcement learning and habit formation (Schultz et al., 1997). The use of electrochemical techniques (microdialysis, amperometry, chronoamperometry, FSCV and RDV) and as well as radiolabeling techniques, both in the in vitro and in vivo preparations has established a broad foundation for DA system physiology. Improvements in the electrochemical detection sensitivity have allowed for analysis of 10 nM DA fluctuations with very high spatial and temporal resolution (Robinson et al., 2003), providing an excellent tool to study DA release dynamics. Thus, combined results from electrophysiological and electrochemical studies have allowed for the distinction of tonic and phasic DA activity. Results obtained with electrochemical techniques such as FSCV, strengthened many electrophysiological findings which suggested a role for DA neurons burst firing in the processing of reward and reward associated stimuli. In addition, FSCV allows for the distinction and examination of in vivo DA release and uptake kinetics providing insight into the changes of DA signaling caused by presynaptic plasticity (e.g. evoked by psychostimulant treatment) (Greco and Garris, 2003, Addy et al., 2010). Finally, the use of FSCV in freely moving animals has allowed for functional determination of in vivo, real time DA release. These studies point to the crucial role of phasic DA signaling in the NAc – the brain motivation-action interface in the process of reinforcement learning. While phasic DA signaling has been postulated to encode prediction error signals, recent findings also suggested its role in encoding salience of environmental stimuli as well as motivation to work for reward. Importantly, changes in phasic DA signaling in NAc during drug self-administration, extinction and reinstatement may underlie the mechanisms of how drug-associated cues influence different phases of addiction including drug-seeking behavior during drug-taking and abstinence.
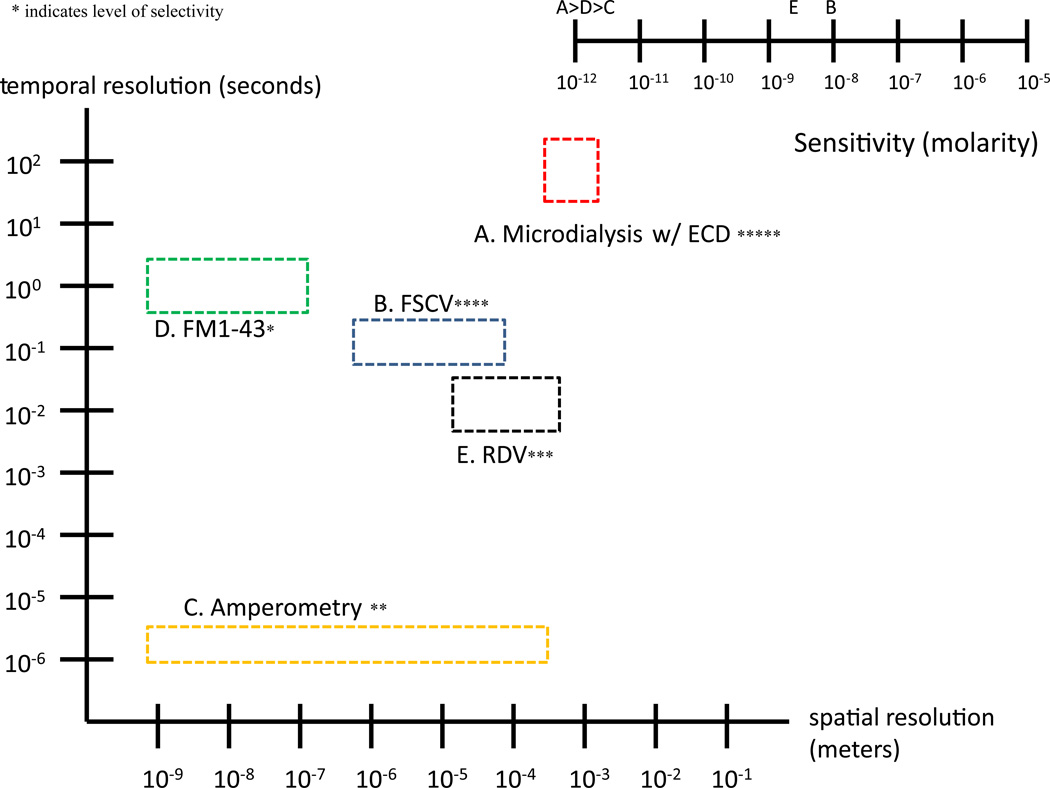
Figure 1.
Temporal versus spatial resolution plot of five commonly used techniques to monitor DA levels. Values plotted are typical and not theoretical ranges. Inset: Ranking of sensitivity of each of these five techniques. A greater number of asterisks indicate greater sensitivity.
Acknowledgments
Research in this area was supported by NIH (DA 10900 to RMW and DA 026698 to NAA) and by the Carolina Postdoctoral Program for Faculty Diversity (to NAA).
Footnotes
This is an un-copyedited author manuscript that has been accepted for publication in the Frontiers in Bioscience. Cite this article as it appears in the Journal of Frontiers in Bioscience. Full citation can be found by searching the Frontiers in Bioscience (Search for articles) following publication and at PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=pubmed) following indexing. This article may not be duplicated or reproduced, other than for personal use or within the rule of "Fair Use of Copyrighted Materials" (section 107, Title 17, U.S. Code) without permission of the copyright holder, the Frontiers in Bioscience. From the time of acceptance following peer review, the full final copy edited article of this manuscript will be made available at http://www.bioscience.org. The Frontiers in Bioscience disclaims any responsibility or liability for errors or omissions in this version of the un-copyedited manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM. Sensitization of Rapid Dopamine Signaling in the Nucleus Accumbens Core and Shell After Repeated Cocaine in Rats. Journal of Neurophysiology. 2010;104:922–931. doi: 10.1152/jn.00413.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Anderson BB, Chen GY, Gutman DA, Ewing AG. Dopamine levels of two classes of vesicles are differentially depleted by amphetamine. Brain Research. 1998;788:294–301. doi: 10.1016/s0006-8993(98)00040-7. [DOI] [PubMed] [Google Scholar]
- Anderson BB, Chen GY, Gutman DA, Ewing AG. Demonstration of two distributions of vesicle radius in the dopamine neuron of Planorbis corneus from electrochemical data. Journal of Neuroscience Methods. 1999;88:153–161. doi: 10.1016/s0165-0270(99)00024-2. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Young JG. Applications of liquid chromatographic-fluorometric systems in neurochemistry. Life Sci. 1981;28:507–517. doi: 10.1016/0024-3205(81)90144-2. [DOI] [PubMed] [Google Scholar]
- Angleson JK, Cochilla AJ, Kilic G, Nussinovitch I, Betz WJ. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci. 1999;2:440–446. doi: 10.1038/8107. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Joyce JA, Scarlis CA, Hanan W, Wu NP, Andre VM, Cohen R, Cepeda C, Levine MS, Harleton E, Sulzer D. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron. 2008;58:89–103. doi: 10.1016/j.neuron.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Beyene M, Carelli RM, Wightman RM. Cue-evoked dopamine release in the nucleus accumbens shell tracks reinforcer magnitude during intracranial self-stimulation. Neuroscience. 2010;169:1682–1688. doi: 10.1016/j.neuroscience.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–722. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland LM, Michael AC. An Introduction to Electrochemical Methods in Neuroscience. 2007 [PubMed] [Google Scholar]
- Bronaugh RL, Hattox SE, Hoehn MM, Murphy RC, Rutledge CO. The separation and identification of dopamine 3-O-sulfate and dopamine 4-O-sulfate in urine of Parkinsonian patients. J Pharmacol Exp Ther. 1975;195:441–452. [PubMed] [Google Scholar]
- Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, Ugarte YV, Gibb JW, Hanson GR, Fleckenstein AE. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. Journal of Pharmacology and Experimental Therapeutics. 2002;302:497–501. doi: 10.1124/jpet.302.2.497. [DOI] [PubMed] [Google Scholar]
- Cacciapaglia F, Wightman RM, Carelli RM. Rapid Dopamine Signaling Differentially Modulates Distinct Microcircuits within the Nucleus Accumbens during Sucrose-Directed Behavior. J Neurosci. 2011;31:13860–13869. doi: 10.1523/JNEUROSCI.1340-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology. 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA. In vivo assessment of dopamine uptake in rat medial prefrontal cortex: comparison with dorsal striatum and nucleus accumbens. J Neurochem. 1995;65:201–207. doi: 10.1046/j.1471-4159.1995.65010201.x. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54:237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Mason R, Marsden CA. Differential cannabinoid-induced electrophysiological effects in rat ventral tegmentum. Neuropharmacology. 2003;44:633–641. doi: 10.1016/s0028-3908(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Changes in basal and cocaine-evoked extracellular dopamine uptake and release in the rat nucleus accumbens during early abstinence from cocaine: quantitative determination under transient conditions. Neuroscience. 2002;112:907–919. doi: 10.1016/s0306-4522(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Chen GY, Ewing AG. Chemical analysis of single cells and exocytosis. Critical Reviews in Neurobiology. 1997;11:59–90. doi: 10.1615/critrevneurobiol.v11.i1.40. [DOI] [PubMed] [Google Scholar]
- Chen GY, Gutman DA, Zerby SE, Ewing AG. Electrochemical monitoring of bursting exocytotic events from the giant dopamine neuron of Planorbis corneus. Brain Research. 1996;733:119–124. doi: 10.1016/0006-8993(96)00754-8. [DOI] [PubMed] [Google Scholar]
- Chow RH, Vonruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Clavier RM, Fibiger HC. On the role of ascending catecholaminergic projections in intracranial self-stimulation of the substantia nigra. Brain research. 1977;131:271–286. doi: 10.1016/0006-8993(77)90520-0. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Cott JM, Breese GR. Effects of catecholamine-depleting drugs and amphetamine on self-stimulation of brain following various 6-hydroxydopamine treatments. Psychopharmacologia. 1974;37:235–248. doi: 10.1007/BF00421537. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Enhancement of coaine of intra-cranial self-stimulation in the rat. Life sciences. 1970;9:375–381. doi: 10.1016/0024-3205(70)90190-6. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Catecholamine-containing neurones and electrical self-stimulation. 1. A review of some data. Psychological medicine. 1972;2:414–421. doi: 10.1017/s0033291700045232. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Delgado JM, DeFeudis FV, Roth RH, Ryugo DK, Mitruka BM. Dialytrode for long term intracerebral perfusion in awake monkeys. Arch Int Pharmacodyn Ther. 1972;198:9–21. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Coury A, Depoortere RY, Egilmez Y, Lane JD, Emmett-Oglesby MW, Lepiane FG, Phillips AG, Blaha CD. Comparison of changes in extracellular dopamine concentrations in the nucleus accumbens during intravenous self-administration of cocaine or d-amphetamine. Behavioural pharmacology. 1995;6:311–322. [PubMed] [Google Scholar]
- Duan HC, Wang J. Selective Transport of Monoamine Neurotransmitters by Human Plasma Membrane Monoamine Transporter and Organic Cation Transporter 3. Journal of Pharmacology and Experimental Therapeutics. 2010;335:743–753. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugast C, Suaud-Chagny MF, Gonon F. Continuous in vivo monitoring of evoked dopamine release in the rat nucleus accumbens by amperometry. Neuroscience. 1994;62:647–654. doi: 10.1016/0306-4522(94)90466-9. [DOI] [PubMed] [Google Scholar]
- Earles C, Wayment H, Green M, Schenk JO. Resolution of biogenic amine transporter kinetics by rotating disk electrode voltammetry: Methodology and mechanistic interpretations. Neurotransmitter Transporters. 1998;vol. 296:660–675. doi: 10.1016/s0076-6879(98)96047-5. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth SJ, Volz TJ, Hanson GR, Fleckenstein AE. Cocaine Alters Vesicular Dopamine Sequestration and Potassium-Stimulated Dopamine Release: The Role of D2 Receptor Activation. Journal of Pharmacology and Experimental Therapeutics. 2009;328:807–812. doi: 10.1124/jpet.108.146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger HC. Drugs and reinforcement mechanisms: a critical review of the catecholamine theory. Annual review of pharmacology and toxicology. 1978;18:37–56. doi: 10.1146/annurev.pa.18.040178.000345. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Brown JM, Sandoval V, Riddle EL, Hansen JP, Ugarte YV, Gibb JW, Hanson GR. D-2 receptor-mediated regulation of vesicular dopamine uptake. Catecholamine Research: From Molecular Insights to Clinical Medicine. 2002;53:39–42. [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci. 2003;17:751–762. doi: 10.1046/j.1460-9568.2003.02511.x. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Meltzer LT, Bunney BS. Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci. 1985;36:1983–1994. doi: 10.1016/0024-3205(85)90448-5. [DOI] [PubMed] [Google Scholar]
- Gagnieu MC, Menouni-Foray V, Guardiola P, Quincy C, Renaud B. Liquid chromatographic determination of homovanillic acid, 5-hydroxyindoleacetic acid and probenecid levels in human cerebrospinal fluid during probenecid test. Clin Chim Acta. 1984;139:1–12. doi: 10.1016/0009-8981(84)90186-4. [DOI] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PE. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nat Neurosci. 2010;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Geldwert D, Norris JM, Feldman IG, Schulman JJ, Joyce MP, Rayport S. Dopamine presynaptically and heterogeneously modulates nucleus accumbens medium-spiny neuron GABA synapses in vitro. BMC Neurosci. 2006;7:53. doi: 10.1186/1471-2202-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt GA, Ksir C, Pivik C, Dickinson SD, Sabeti J, Zahniser NR. Methodology for coupling local application of dopamine and other chemicals with rapid in vivo electrochemical recordings in freely-moving rats. J Neurosci Methods. 1999;87:67–76. doi: 10.1016/s0165-0270(98)00158-7. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends in neurosciences. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984a;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984b;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced H-3 dopamine release from synaptosomes prepared from four mouse brain regions. Journal of Pharmacology and Experimental Therapeutics. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Greco PG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur J Pharmacol. 2003;479:117–125. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Clonidine regularizes substantia nigra dopamine cell firing. Life Sci. 1988;42:2003–2009. doi: 10.1016/0024-3205(88)90500-0. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Clonidine modulates dopamine cell firing in rat ventral tegmental area. Eur J Pharmacol. 1989;165:11–18. doi: 10.1016/0014-2999(89)90765-6. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Larson GA, Zahniser NR. Using High-Speed Chronoamperometry with Local Dopamine Application to Assess Dopamine Transporter Function. 2007 [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Monaco AP, Hernandez L, Aulisi EF, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacology. 1983;81:158–163. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob G, Ungerstedt U. The influence of cocaine self-administration on in vivo dopamine and acetylcholine neurotransmission in rat caudate-putamen. Neuroscience letters. 1990;109:227–233. doi: 10.1016/0304-3940(90)90568-t. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain research. 1989;498:199–203. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Angelucci L. Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res. 1991;538:111–117. doi: 10.1016/0006-8993(91)90384-8. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Leonard CS, Kohlmeier KA. Nicotinic activation of laterodorsal tegmental neurons: implications for addiction to nicotine. Neuropsychopharmacology. 2009;34:2529–2547. doi: 10.1038/npp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CE, Jones SR. Fast Scan Cyclic Voltammetry of Dopamine and Serotonin in Mouse Brain Slices. 2007 [PubMed] [Google Scholar]
- Jones BE, Halaris AE, McIlhany M, Moore RY. Ascending projections of the locus coeruleus in the rat. I. Axonal transport in central noradrenaline neurons. Brain Res. 1977;127:1–21. doi: 10.1016/0006-8993(77)90377-8. [DOI] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biol Psychiatry. 2010;67:737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LS, Gauger LL, Davis JN. Anatomy of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J Comp Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- Jones SR, Bowman BP, Kuhn CM, Wightman RM. Development of dopamine neurotransmission and uptake inhibition in the caudate nucleus as measured by fast-cyclic voltammetry. Synapse. 1996;24:305–307. doi: 10.1002/(SICI)1098-2396(199611)24:3<305::AID-SYN13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Kilts CD, Wightman RM. Comparison of dopamine uptake in the basolateral amygdaloid nucleus, caudate-putamen, and nucleus accumbens of the rat. J Neurochem. 1995a;64:2581–2589. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J Pharmacol Exp Ther. 1995b;274:396–403. [PubMed] [Google Scholar]
- Jones SR, Joseph JD, Barak LS, Caron MG, Wightman RM. Dopamine neuronal transport kinetics and effects of amphetamine. Journal of Neurochemistry. 1999;73:2406–2414. doi: 10.1046/j.1471-4159.1999.0732406.x. [DOI] [PubMed] [Google Scholar]
- Krueger BK. Kinetics and block of dopamine uptake in synaptosomes from rat caudate-nucleus. Journal of Neurochemistry. 1990;55:260–267. doi: 10.1111/j.1471-4159.1990.tb08847.x. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Calabresi P, North RA. Muscarine depolarizes rat substantia nigra zona compacta and ventral tegmental neurons in vitro through M1-like receptors. J Pharmacol Exp Ther. 1990;253:395–400. [PubMed] [Google Scholar]
- Larsen MB, Sonders MS, Mortensen OV, Larson GA, Zahniser NR, Amara SG. Dopamine Transport by the Serotonin Transporter: A Mechanistically Distinct Mode of Substrate Translocation. Journal of Neuroscience. 2011;31:6605–6615. doi: 10.1523/JNEUROSCI.0576-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Gronli J, Spiga S, Di Chiara G. Preferential increase of extracellular dopamine in the rat nucleus accumbens shell as compared to that in the core during acquisition and maintenance of intravenous nicotine self-administration. Psychopharmacology. 2006;184:435–446. doi: 10.1007/s00213-005-0280-4. [DOI] [PubMed] [Google Scholar]
- Lee A, Wissekerke AE, Rosin DL, Lynch KR. Localization of alpha2C-adrenergic receptor immunoreactivity in catecholaminergic neurons in the rat central nervous system. Neuroscience. 1998;84:1085–1096. doi: 10.1016/s0306-4522(97)00578-2. [DOI] [PubMed] [Google Scholar]
- Lester DB, Miller AD, Pate TD, Blaha CD. Midbrain acetylcholine and glutamate receptors modulate accumbal dopamine release. Neuroreport. 2008;19:991–995. doi: 10.1097/WNR.0b013e3283036e5e. [DOI] [PubMed] [Google Scholar]
- Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ, Near JA, Wightman RM. Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells -chemical evidence for exocytosis. Journal of Biological Chemistry. 1990;265:14736–14737. [PubMed] [Google Scholar]
- Liebman JM, Butcher LL. Effects on self-stimulation behavior of drugs influencing dopaminergic neurotransmission mechanisms. Naunyn-Schmiedeberg's archives of pharmacology. 1973;277:305–318. doi: 10.1007/BF00505669. [DOI] [PubMed] [Google Scholar]
- Lippa AS, Antelman SM, Fisher AE, Canfield DR. Neurochemical mediation of reward: a significant role for dopamine? Pharmacology, biochemistry, and behavior. 1973;1:23–28. doi: 10.1016/0091-3057(73)90050-6. [DOI] [PubMed] [Google Scholar]
- Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR. Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat and monkey ventral tegmental area. Synapse. 2004;52:233–244. doi: 10.1002/syn.20023. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, Morgan D, Roberts DC, Jones SR. Fast onset of dopamine uptake inhibition by intravenous cocaine. Eur J Neurosci. 2004;20:2838–2842. doi: 10.1111/j.1460-9568.2004.03736.x. [DOI] [PubMed] [Google Scholar]
- McElvain JS, Schenk JO. Blockade of dopamine autoreceptors by haloperidol and the apparent dynamics of potassium-stimulated endogenous release of dopamine from and reuptake into striatal suspensions in the rat. Neuropharmacology. 1992a;31:649–659. doi: 10.1016/0028-3908(92)90143-d. [DOI] [PubMed] [Google Scholar]
- McElvain JS, Schenk JO. A multisubstrate mechanism of striatal dopamine uptake and its inhibition by cocaine. Biochemical Pharmacology. 1992b;43:2189–2199. doi: 10.1016/0006-2952(92)90178-l. [DOI] [PubMed] [Google Scholar]
- McElvain JS, Schenk JO. Studies of the mechanism of inhibition of the dopamine uptake carrier by cocaine in vitro using rotating-disk electrode voltammetry. In: Kalivas PW, Samson HH, editors. Neurobiology of Drug and Alcohol Addiction. vol. 654. 1992c. pp. 480–482. [DOI] [PubMed] [Google Scholar]
- Michael DJ, Wightman RM. Electrochemical monitoring of biogenic amine neurotransmission in real time. J Pharm Biomed Anal. 1999a;19:33–46. doi: 10.1016/s0731-7085(98)00145-9. [DOI] [PubMed] [Google Scholar]
- Michael DJ, Wightman RM. Electrochemical monitoring of biogenic amine neurotransmission in real time. Journal of Pharmaceutical and Biomedical Analysis. 1999b;19:33–46. doi: 10.1016/s0731-7085(98)00145-9. [DOI] [PubMed] [Google Scholar]
- Mickelson GE, Garris PA, Bunin M, Wightman RM. In vivo and in vitro assessment of dopamine uptake and release. Adv Pharmacol. 1998;42:144–147. doi: 10.1016/s1054-3589(08)60716-4. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Hutson PH. Measurement of the in vitro release of endogenous monoamine neurotransmitters as a means of identification of prejunctional receptors. Journal of Neuroscience Methods. 1990;34:23–28. doi: 10.1016/0165-0270(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Miles PR, Mundorf ML, Wightman RM. Release and uptake of catecholamines in the bed nucleus of the stria terminalis measured in the mouse brain slice. Synapse. 2002;44:188–197. doi: 10.1002/syn.10069. [DOI] [PubMed] [Google Scholar]
- Miller JD, Farber J, Gatz P, Roffwarg H, German DC. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273:133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Progress in neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Mora F, Phillips AG, Koolhaas JM, Rolls ET. Prefrontal cortex and neostriatum self-stimulation in the rat: differential effects produced by apomorphine. Brain research bulletin. 1976;1:421–424. doi: 10.1016/0361-9230(76)90110-6. [DOI] [PubMed] [Google Scholar]
- Morris G, Nevet A, Arkadir D, Vaadia E, Bergman H. Midbrain dopamine neurons encode decisions for future action. Nat Neurosci. 2006;9:1057–1063. doi: 10.1038/nn1743. [DOI] [PubMed] [Google Scholar]
- Mundroff ML, Wightman RM. Amperometry and cyclic voltammetry with carbon fiber microelectrodes at single cells. Curr Protoc Neurosci Chapter. 2002;6 doi: 10.1002/0471142301.ns0614s18. Unit 6 14. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG. Bioenergetics and transmitter release in the isolated nerve terminal. Neurochemical Research. 2003;28:1433–1441. doi: 10.1023/a:1025653805029. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of comparative and physiological psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olds ME, Fobes JL. The central basis of motivation: intracranial self-stimulation studies. Annual review of psychology. 1981;32:523–574. doi: 10.1146/annurev.ps.32.020181.002515. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci U S A. 2008;105:11957–11962. doi: 10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Zweifel LS, Clark JJ, Evans SB, Phillips PE, Palmiter RD. Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proc Natl Acad Sci U S A. 2010;107:13491–13496. doi: 10.1073/pnas.1007827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Clements MA, Bernier BE, Morikawa H. In vivo ethanol experience increases D(2) autoinhibition in the ventral tegmental area. Neuropsychopharmacology. 2011;36:993–1002. doi: 10.1038/npp.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Brooke SM, Fibiger HC. Effects of amphetamine isomers and neuroleptics on self-stimulation from the nucleus accumbens and dorsal noradrenergic bundle. Brain research. 1975;85:13–22. doi: 10.1016/0006-8993(75)90998-1. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Carter DA, Fibiger HC. Dopaminergic substrates of intracranial self-stimulation in the caudate-putamen. Brain research. 1976;104:221–232. doi: 10.1016/0006-8993(76)90615-6. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. The role of dopamine in maintaining intracranial self-stimulation in the ventral tegmentum, nucleus accumbens, and medial prefrontal cortex. Canadian journal of psychology. 1978;32:58–66. doi: 10.1037/h0081676. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Hancock PJ, Stamford JA. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse. 2002;44:15–22. doi: 10.1002/syn.10049. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med. 2003a;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003b;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pihel K, Schroeder TJ, Wightman RM. Rapid and selective cyclic voltammetric measurements of epinephrine and norepinephrine as a method to measure secretion from single bovine adrenal-medullary cells. Analytical Chemistry. 1994;66:4532–4537. [Google Scholar]
- Povlock SL, Schenk JO. A multisubstrate kinetic mechanism of dopamine transport in the nucleus accumbens and its inhibition by cocaine. Journal of Neurochemistry. 1997;69:1093–1105. doi: 10.1046/j.1471-4159.1997.69031093.x. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Beninger RJ. Rostral-caudal differences in effects of nucleus accumbens amphetamine on VTA ICSS. Brain research. 1994;642:251–258. doi: 10.1016/0006-8993(94)90929-6. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz M, Lamb R, Goldberg S, Kuhar M. Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:233–239. doi: 10.1016/0278-5846(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien ML, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci. 2002;22:10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Phillips PE, Budygin EA, Trafton BJ, Garris PA, Wightman RM. Sub-second changes in accumbal dopamine during sexual behavior in male rats. Neuroreport. 2001;12:2549–2552. doi: 10.1097/00001756-200108080-00051. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Volz TJ, Schenk JO, Wightman RM. Acute ethanol decreases dopamine transporter velocity in rat striatum: In vivo and in vitro electrochemical measurements. Alcoholism-Clinical and Experimental Research. 2005;29:746–755. doi: 10.1097/01.alc.0000164362.21484.14. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. J Neurochem. 2004;90:894–903. doi: 10.1111/j.1471-4159.2004.02559.x. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. Rapid Dopamine Release in Freely Moving Rats. 2007 [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Chloral hydrate and ethanol, but not urethane, alter the clearance of exogenous dopamine recorded by chronoamperometry in striatum of unrestrained rats. Neurosci Lett. 2003;343:9–12. doi: 10.1016/s0304-3940(03)00301-x. [DOI] [PubMed] [Google Scholar]
- Schenk JO, Patterson TA, McElvain JS. Rotating-disk voltammetric measurements in neurobiology and neuropharmacology. Trac-Trends in Analytical Chemistry. 1990;9:325–330. [Google Scholar]
- Schenk JO, Wright C, Bjorklund N. Unraveling neuronal dopamine transporter mechanisms with rotating disk electrode voltammetry. Journal of Neuroscience Methods. 2005;143:41–47. doi: 10.1016/j.jneumeth.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci. 2001;21:5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KN, Kennedy RT. Time-resolved microdialysis for in vivo neurochemical measurements and other applications. Annu Rev Anal Chem (Palo Alto Calif) 2008;1:627–661. doi: 10.1146/annurev.anchem.1.031207.113047. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal RGW, Mosharov EV, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nature Neuroscience. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- Stein L. Self-Stimulation of the Brain and the Central Stimulant Action of Amphetamine. Federation proceedings. 1964;23:836–850. [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005a;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005b;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Pothos EN. Regulation of quantal size by presynaptic mechanisms. Rev Neurosci. 2000;11:159–212. doi: 10.1515/revneuro.2000.11.2-3.159. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Chavkin C, Colago EE, Pickel VM. Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse. 2001;42:185–192. doi: 10.1002/syn.10005. [DOI] [PubMed] [Google Scholar]
- Travis ER, Wightman RM. Spatio-temporal resolution of exocytosis from individual cells. Annual Review of Biophysics and Biomolecular Structure. 1999;28:VII–VIII. doi: 10.1146/annurev.biophys.27.1.77. (vol 27, pg 102, 1998) [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bull Schweiz Akad Med Wiss. 1974;30:44–55. [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, King JL, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate administration alters vesicular monoamine transporter-2 function in cytoplasmic and membrane-associated vesicles. Journal of Pharmacology and Experimental Therapeutics. 2007;323:738–745. doi: 10.1124/jpet.107.126888. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Hanson GR, Fleckenstein AE. Measurement of kinetically resolved vesicular dopamine uptake and efflux using rotating disk electrode voltammetry. Journal of Neuroscience Methods. 2006;155:109–115. doi: 10.1016/j.jneumeth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Van Oene JC. Evaluation of the effect of drugs on dopamine metabolism in the rat superior cervical ganglion by HPLC with electrochemical detection. Eur J Pharmacol. 1980;65:71–79. doi: 10.1016/0014-2999(80)90210-1. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from indivudal chromaffin cells. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wise RA, Kiyatkin EA. Differentiating the rapid actions of cocaine. Nat Rev Neurosci. 2011;12:479–484. doi: 10.1038/nrn3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annual review of psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Yeomans JS. The absolute refractory periods of self-stimulation neurons. Physiol Behav. 1979;22:911–919. doi: 10.1016/0031-9384(79)90336-6. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Maidment NT, Bunney BS. Excitability properties of medial forebrain bundle axons of A9 and A10 dopamine cells. Brain Res. 1988;450:86–93. doi: 10.1016/0006-8993(88)91547-8. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Matthews GG, Hawkins RD, Bellman K, Doppelt H. Characterization of self-stimulation neurons by their local potential summation properties. Physiol Behav. 1979;22:921–929. doi: 10.1016/0031-9384(79)90337-8. [DOI] [PubMed] [Google Scholar]
- Zhang MY, Hughes ZA, Kerns EH, Lin Q, Beyer CE. Development of a liquid chromatography/tandem mass spectrometry method for the quantitation of acetylcholine and related neurotransmitters in brain microdialysis samples. J Pharm Biomed Anal. 2007;44:586–593. doi: 10.1016/j.jpba.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Misler S. Amperometric detection of stimulus-induced quantal release of catecholamines from cultures superior cervical-ganglion neurons. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:6938–6942. doi: 10.1073/pnas.92.15.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]