Abstract
Fleas are significant ectoparasites of small animals. They can be a severe irritant to animals and serve as a vector for a number of infectious diseases. In this article, we discuss the pharmacological characteristics of four insect nicotinic acetylcholine receptor (nAChR) agonists used as fleacides in dogs and cats, which include three neonicotinoids (imidacloprid, nitenpyram, and dinotefuran) and spinosad. Insect nAChR agonists are one of the most important new classes of insecticides, which are used to control sucking insects both on plants and on companion animals. These new compounds provide a new approach for practitioners to safely and effectively eliminate fleas.
Keywords: Fleas, neonicotinoids, spinosad, fleacides, nicotinic receptors
Introduction
Ectoparasites can cause various levels and types of skin disease and serve as a vector for a number of infectious diseases. In dogs and cats, fleas are the most important ectoparasite world-wide. Among several species that can infest companion animals, the cat flea (Ctenocephalides felis) is most widely found. Repeated flea bites can cause discomfort to animals and sometimes can affect animal owners as well. The bloodsucking of fleas in heavily infested animals can also lead to anemia, especially in young animals. Moreover, fleas are responsible for flea allergy dermatitis in dogs and cats.1,2 They are also vectors of a number of bacteria, e.g., Rickettsia typhi, Rickettsia felis, and Bartonella henselae. In addition, fleas can be an intermediate host for tapeworm Dipylidium caninum, the common intestinal cestode of dogs and cats, which occasionally infects human beings.
The recently introduced insect nicotinic acetylcholine receptor (nAChR) agonists are important novel insecticides that are used to control parasites of companion animals. These compounds are highly selective for insect nAChRs, and thus are effective and safe to eliminate fleas in dogs and cats. To date, there are no records of cross-resistance of insect nAChR agonists with other ectoparasiticies, e.g., carbamates, organophosphates, or pyrethroids, making them important for management of insecticide resistance. Imidacloprid was the first neonicotinoid, the new class of nicotine-like compounds, introduced to the market in 1991 and quickly it became one of the most important insecticides in agriculture and veterinary medicine.3 Subsequently, two other neonicotinoids, nitenpyram and dinotefuran, were also approved for flea control in veterinary practice as well as in agriculture. Their rapid action on fleas is one of the advantages compared with other adult fleacides such as fipronil and selamectin;4 this is an important feature when dealing with ectoparasites that are vectors for zoonotic bacteria.1
Spinosad is a mixture of two natural occurring macrocyclic lactones (spinosyn A and spinosyn D) and acts primarily as a nAChR agonist. In addition, spinosad has a secondary activity in insects as an opener of chloride channels.5 Spinosad was introduced into the market in 1997 for the control of a range of pest insects5 and as an oral fleacide in 2007.6
In this review, we discuss the pharmacology of the neonicotinoids and spinosad on insect nAChRs and their therapeutic uses for flea control in small animals.
Insect nAChRs vs. vertebrate nAChRs
Biochemistry of nAChRs
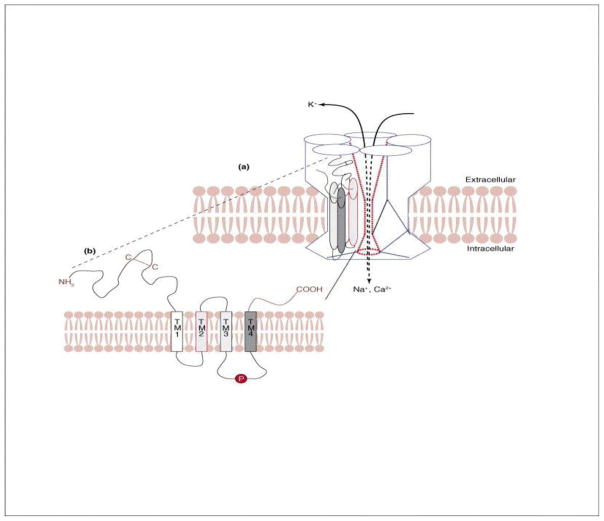
These receptors are prototypical members of the cys-loop ligand-gated ion channel superfamily, consisting of five subunits that are arranged around a central cation permeable pore.7 There are ~500 amino acids in each subunit. Each subunit can be encoded by a separate gene and possesses four transmembrane domains (TM1 TM4), an N-terminal extracellular domain that has six distinct regions (loops A F) involved in ligand binding in which loops A to C are on α-subunits and loops D to F are on non-α-subunits (or β-subunits in insects) (Figure 1).8,9 However, all six loops are located in the homomers of α7- α9 subunits.10
Figure 1.
Structure of nicotinic acetylcholine receptors (nAChRs). (a) nAChRs are pentameric macromolecules composed of five homomeric subunits or heteromeric subunits arranged around a central cation (Na+, K+, and Ca2+) permeable pore. (b) Each subunit includes four transmembrane (TM1-TM4) domains with TM2 lining the ion channel and a potential phosphorylation site between TM3 and TM4 (Modified from Thany et al., Trends Pharmacol Sci 2007;28:14–22, Figure 1 with permission).
Vertebrate nAChRs
In vertebrates, nAChRs are located on muscle at the neuromuscular junction and also on neurons expressed in the peripheral and central nervous system.5 The nAChRs consist of two subtypes (skeletal muscular and neuronal subtypes) assembled in pentameric combinations from ten α (α1- α 10), four β (β1- β4); four γ (γ1-γ4); four δ (δ1-δ4), and ε subunits (Figure 2). The skeletal muscular subtype is made up of two α1 subunits and one each of β1, γ, and δ (or ε in adult muscle) subunits and the neuronal nAChR subtypes are assembled in combinations of α2 α10 and β2 β4.11 The pharmacological classification includes two groups based on sensitivity to α-bungarotoxin (a toxin from the elapid snake Bungarus multicinctus, α-BGT). The α7–10 subunits are responsible for α-BGT-sensitive receptors, whereas α2–6 and β2–4 subunits are involved in assembling the α-BGT-insensitive subtypes. The most abundant subtype in vertebrate brain is the α7 subtype (α-BGT-sensitive), which is considered to be a homopentameric structure, and α4β2 (α-BGT-insensitive), which is heteropentameric including two α4 and three β2 subunits. The agonist- or ligand-binding site is localized at the interface region between subunits (Figure 2). The specific subunit combinations confer differences in sensitivity to acetylcholine (ACh) and/or pharmacological profiles among the nAChR subtypes. The ligand-binding site in all subtypes consists of a conserved core of aromatic amino acid residues. Neighboring variable residues are considered to confer individual pharmacological properties to each subtype.8,12
Figure 2.
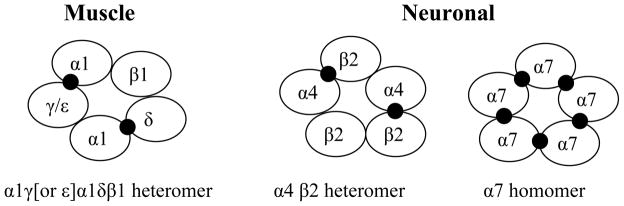
Functional assembly of vertebrate nicotinic receptor subtypes. Homomeric and heteromeric subunit combinations presented in cross-section. The binding sites for cholinergic ligands are at the interface between subunits (Modified from Tomizawa M, Casida JE. Annu Rev Entomol 2003;48:339–364, Figure 4 with permission).
Insect nAChRs
In insects, nAChRs distribute widely and predominantly in the CNS. These receptors are responsible for rapid neurotransmission and are also important targets for insecticides.3 The excitatory synaptic transmission at the neuromuscular junction in insects is glutamatergic, whereas it is cholinergic in vertebrates and in several invertebrates such as nematodes.13 In addition, glutamate is the main excitatory neurotransmitter in mammalian brain, whereas ACh is the main excitatory neurotransmitter in insects.5 These characteristics contribute to the usefulness of insect nAChRs as selective targets for neurotoxic insecticides.5
The subtypes of native insect nAChRs remain unclear, however; studies have shown that insect neurons can express different nAChR subtypes and the pharmacological data have shown that there are at least two different classes of insect nAChRs, α-BGT-sensitive and -insensitive.9
Insect nAChR subunits are less well-understood than those of vertebrates. Ten subunits of insect nAChR gene families have been described in Drosophila melanogaster, including 7 Dα (α1-α7) and 3 Dβ (β1-β3),14 the same number of nAChR subunits is the case for Anopheles gambiae, including 9 Agamα (α1-α9) and 1 Agamβ (β1).15 Eleven subunits have been described in Apis mellifera, including 9 Amelα (α1-9) and 2 Amelβ (β1-2).16 In cat fleas (Ctenocephalides felis), the nAChR family consists of at least seven subunits (Cfα1, Cfα2, Cfα3, Cfα4, Cfα7, Cfα8, and Cfβ1).17 The largest insect nAChR gene family is in silkworm (Bombyx mori), including nine αtype subunits and three β-type subunits.18
Neonicotinoids
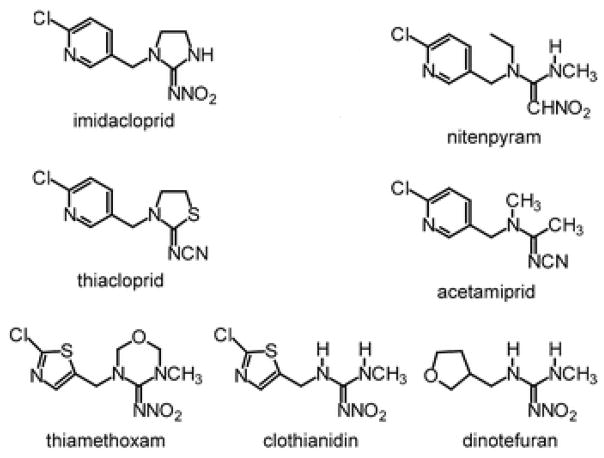
The neonicotinoids, the newest class of insecticides, are very potent for protecting crops against piercing-sucking insects.3 They are also used in veterinary medicine especially for controlling fleas on cats and dogs.1,4 These insecticides include imidacloprid, nitenpyram, acetamiprid, thiacloprid, thiamethoxam, clothianidin and dinotefuran. Base on the differences in chemical structure, these neonicotinoids are divided into three subclasses: “chloronicotinyl” (the first generation neonicotinoids, e.g., imidacloprid, nitenpyram, acetamiprid and thiacloprid), “thianicotinyl” (the second generation neonicotinoids, e.g., thiamethoxam and clothianidin) and “furanicotinyl” (the third generation neonicotinoids, e.g., dinotefuran) (Figure 3).19,20 The classification, first, second or third generation, comes from difference in their chemical structures, which has no relation with their selectivity of binding of insect/vertebrate nAChR. In this review, we focus on three neonicotinoids that are used in veterinary medicine, including imidacloprid, nitenpyram and dinotefuran.
Figure 3.
Chemical structures of neonicotinoids. The neonicotinoids are nitromethylenes (C=CHNO2), nitroguanidines(C=NNO2), and cyanoamidines (C=NCN). Imidacloprid, nitenpyram, acetamiprid and thiacloprid belong to the first generation and the chloronicotinyl subclass. Thiamethoxam and clothianidin (the second generation neonicotinoids) are the thianicotinyl subclass and dinotefuran (the third generation neonicotinoids) is the furanicotinyl subclass (Modified from Tomizawa M, Casida JE. Annu Rev Pharmacol Toxicol 2005;45:247–268, Figure 1 with permission).
Neonicotinoid-binding sites in insects
Similar to vertebrateAChRs, insect nAChR binding sites are at the interface region between subunits of insect nAChRs and both α and β subunits influence pharmacological properties.21 In fact, the hybrid receptors expressed in Xenopus laevis oocytes between Drosophila (D)-chicken Dα2-β2 and Dα1-β2 show that the neonicotinoid ligands tested activate the Dα2-β2 receptors, but not Dα1-β2 receptors.22 These findings demonstrate that the α-subunit contributes to the selectivity of neonicotinoids for insect nAChRs and those specific residues in the Dα2 subunit could account for the enhancement of neonicotinoid affinity.21,22 In addition, the insect/vertebrate hybrid receptors of Dα3/Rat β2 exhibit higher binding affinity for imidacloprid than those of Dα3/Rat β4, Dα3/Rat γ, and Dα3/Rat δ.23 These findings define the role of β subunits in the pharmacological properties of neonicotinoids.
Why neonicotinoids are so safe in animals?
The high-affinity [3H]imidacloprid-binding site is conserved in neonicotinoid sensitivity and specificity across a broad range of insects.3 The high affinity of neonicotinoids for insect nAChRs and very low affinity for vertebrate nAChRs may explain the high toxicity of neonicotinoids for insects and their low toxicity for vertebrates.24 Neonicotinoids have little or no binding to the vertebrate peripheral (muscular) nAChR α1γα1δβ1 subtype or some neuronal subtypes [α3β2 (and/or β4)α5, α4β2, and α7] and the minor structural modifications of neonicotinoids confer differential subtype selectivity in vertebrate nAChRs.12 The comparison of IC50 of neonicotinoids between insects and vertebrate neuronal nAChRs (α4β2) are shown in Table 1, which strongly suggests that neonicotinoids are very safe for vertebrate species.
Table 1.
Specificity of imidacloprid, nitenpyram and dinotefuran for insect and vertebrate α4β2 nicotinic receptors (Modified from Tomizawa M, Casida JE. Annu Rev Pharmacol Toxicol 2005;45:247–268, Table 4 with permission).
| IC50, nM | |||
|---|---|---|---|
| Compound | Insect | Vertebrate α4β2 | Selectivity ratio |
| Imidacloprid | 4.6 | 2600 | 565 |
| Nitenpyram | 14 | 49,000 | 3500 |
| Dinotefuran | 900 | >100,000 | >111 |
IC50 values for displacing [3H]imidacloprid binding to the fruit fly (Drosophila melanogaster) receptor and [3H]nicotine binding to the vertebrate α4β2 nAChR.12
Mechanism of action of neonicotinoids
Neonicotinoids act selectively by binding to nAChRs, leading to an opening of a nonselective cation channel, promoting the influx of extracellular Na+/Ca2+ and efflux of intracellular K+ to disrupt the equilibrium status of the membrane potential.3 Since the nAChR agonist-induced Na+ influx dominates over K+ efflux, it results in depolarization. The prolonged action of neonicotinoids is due to the fact that they cannot be cleaved by acetylcholinesterase (AChE) in insects, and thus persistently depolarizes neurons, leading to the consequent paralyzing action in insects.1,3
Therapeutic uses of neonicotinoids
Imidacloprid
Imidacloprid,a a first generation neonicotinoid insecticide, was first marketed in 1991 by Bayer Animal Health Division. It has strong insecticidal activity against sucking insects. Imidacloprid is used topically (7.5–10 mg/kg) once a month as a spot-on product in dogs and cats that are ≥4 week-old.25 It rapidly kills adult fleas on dogs and cats and breaks the flea life cycle before eggs being laid.25,26 About 96 % of mature fleas are killed within 8 hours of application and the fleacidal effect is persistent for 34 days, since it is stored in the sebaceous glands of the animals and is not washed out by shampooing.25
Imidacloprid is also available as a mixture with permethrinb to kill ticks in dogs27 and with moxidectinc to prevent heartworms.28,29 and to kill gastrointestinal (GI) nematodes and mites in dogs and cats.
Pharmacokinetics of imidacloprid
By topical application, imidacloprid is localized in the water resistant lipid layer of the skin surface and then spreads over the body surface and onto the hair.30 Within 12 hours of topical application, imidacloprid spreads over the skin surface and throughout the hair coat of dogs and cats.31 When administered orally to rats, 92–99% of imidacloprid is absorbed from the GI tract, rapidly distributed in almost all tissues and organs, and quickly eliminated from the body by degradation to a large number of metabolites formed by multiple pathways, both alternative and sequential. The metabolites are excreted primarily in the urine as glutathione and glycine conjugates of mercaptonicotinic acid and hippuric acid.32 These findings in rats suggest that the systemically absorbed imidacloprid is eliminated from the body rapidly, which would contribute to its safety in animals.
Nitenpyram
Nitenpyram,d another first generation neonicotinoid, was first marketed in 1995 by Takeda Chemical Industries, Ltd. It provides rapid flea relief in dogs and cats and has the highest overall percent kill when compared to fipronil; a blocker of GABA-gated Cl− channels, imidacloprid, selamectin; a macrocyclic lactone, and cythioate; an organophosphate at 3 hours and 8 hours after the treatment4. Nitenpyram is 100% effective in cats and 99% effective in dogs within 3 hours of administration and 100% effective in both cats and dogs within 8 hours of administration.4
Nitenpyram is administered orally (1 mg/kg) for the short-term control of fleas in dogs and cats. Fleas start to fall from the animals 30 minutes post-administration33 and one dose can protect animals for 1–2 days.34
The fast acting of nitenpyram on fleas is particularly important for animals suffering from flea-bite allergy dermatitis, and for the fast control of adult fleas in an integrated flea control strategy.4 However, this drug cannot protect animals for more than 48 hours after the administration. It is normally used in combination with lufenuron,e an insect development inhibitor, to provide continuous flea control.
Pharmacokinetics of nitenpyram
Since nitenpyram is highly lipophilic, it is administered orally after the meal in order to induce bile flow to help dissolve the chemical, thereby increasing GI absorption of the drug. It is rapidly and completely absorbed from the GI tract in less than 90 minutes and is completely excreted in urine within 48 hours after oral administration to dogs and cats.35 Nitenpyram undergoes hydroxylation, followed by conjugation in the liver. The conjugates of nitenpyram are excreted in the urine and nitenpyram is not accumulated in body tissues. The plasma half-life of nitenpyram in dogs and cats are 3 and 8 hours, respectively. It is likely that animals with liver and/or kidney problems may have longer plasma half-life of nitenpyram.
Dinotefuran
Dinotefuran is a third generation neonicotinoid, which was first marketed in 2002 by Mitsui Chemicals Group. It has a characteristic (±)-tetrahydro-3-furylmethyl moiety instead of the pyridine-like moiety of other neonicotinoids.36
Dinotefuranf is used as a spot-on product (7–16 mg/kg) and has slightly faster knockdown action than imidacloprid (killing 96% fleas in 6 hours and 100% within 12 hours). The retaining residual activity can prevent fleas for at least 30 days.37 It is available as a mixture with permethrin (to kill ticks) for use in dogs and pyriproxifen, an insect growth regulator (to kill developing stages of fleas), for use in cats.37,38 The combinations of these compounds effectively break the life cycle of the fleas, including eggs, larvae, and adults, thereby preventing re-infestations.37,38
Pharmacokinetics of dinotefuran
The pharmacokinetics of dinotefuran have been studied in mice and rats. In rats, after oral administration, the absorption is >90% regardless of the dose. The drug is distributed throughout the body. The parent compound is metabolized into numerous minor metabolites by N-demethylation, nitro reduction, tetrahydrofuran hydroxylation, and N-methylene hydroxylation and amine cleavage. Most of dinotefuran is excreted as parent compound in urine and only a small amount is excreted in feces within 24 hours.39,40 These findings in rats suggest that the systemically absorbed dinotefuran is eliminated from the body rapidly, which would contribute to its safety in animals.
Spinosad
Spinosad is not a neonicotinoid. It is a mixture of two natural macrocyclic lactones (spinosyn A and spinosyn D, Figure 4) that is isolated from the soil bacterium Actinomycete - Saccharopolyspora spinosa.5
Figure 4.
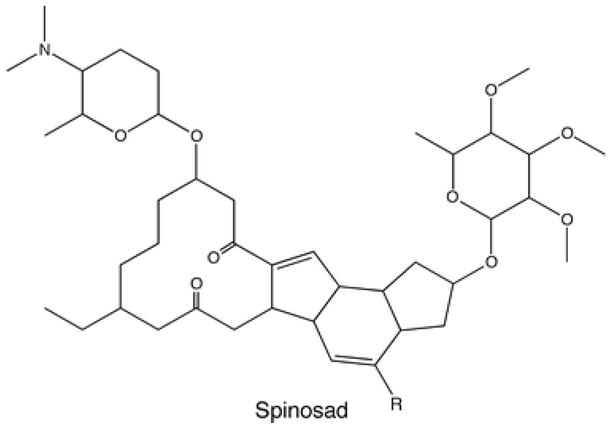
Structure of spinosad. The mixture of two natural macrocyclic lactones (spinosyn A (R=H) and spinosyn D (R=CH3)) that is isolated from the soil bacterium Actinomycete Saccharopolyspora.
Spinosad-binding sites in insect neurons
The binding sites of spinosad in insect neurons are still not well-understood. However, the mutagenesis studies have identified that the Drosophila nAChR Dα6 subunit is the target site for spinosad.5 A null mutation of the nAChR subunit Dα6 in Drosophila melanogaster causes the resistance to spinosad.41 Radioligand binding studies have shown that neonicotinoid insecticides do not bind to Dα6-containing nAChRs.42 These findings suggest that spinosad and neonicotinoids act upon different α-subunits of nAChRs43 since Dα2 subunit seems to be the binding site for neonicotinoids.21,22
Mechanism of action of spinosad
The spinosad acts primarily by binding to nAChRs, leading to hyperexcitation, and disruption of the insect CNS. This effect causes involuntary muscle contractions and tremors resulting from the widespread excitation of neurons in the CNS.44,45 The prolonged spinosad-induced hyperexcitation results in paralysis that is due to neuromuscular fatigue.44 This activity of spinosad is similar to that of neonicotinoids. Moreover, spinosad has been shown to increase the Cl− channel activity in the neurons of the insect CNS.46,47 This effect might modulate the effect of spinosad on insect nChRs. In addition, spinosad can modulate γ-aminobutyric acid (GABA) responses from small-diameter cockroach neurons, suggesting that it has potent effects on the function of GABA-gated Cl− in insect neurons.46 There are at least two types of Cl− channels in arthropods, namely glutamate-gated Cl− channels and GABA-gated Cl− channels;48 it is important to explore the effects of spinosad on these two types of channels in insects. As indicated before, spinosad opens non-selective cation channels, leading to depolarization of the neuronal membrane,44,45 while its opening of Cl− channel can lead to hyperpolarization of the membrane,46 further research is warranted to determine how spinosad’s effect on Cl− channels would modulate its effect on neuronal depolarization.
Pharmacokinetics of spinosad
The pharmacokinetics of spinosad have been studied in rats, in which spinosad is readily absorbed from the GI tract following oral administration. It is rapidly metabolized and eliminated. Within 48 hours of dosing in rats, 60–80% of spinosad and their metabolites are eliminated via the urine and feces.48,49 The depletion of spinosad residues from rat tissues occurs rapidly following cessation of administration.49,50 The conjugation with glutathione, either directly or after O or N demethylation, is the major path of metabolism.50 In contrast, after oral administration of spinosad in dogs, the plasma half-life of this insecticide in this species is ~10 days.51 This information accounts for the prolonged fleacidal effect of spinosad in dogs and suggests a species difference, at least between dogs and rats, in the pharmacokinetics of spinosad.
Therapeutic uses of spinosad
Spinosad was introduced by Dow AgroSiences LLC for controlling the lepidopterous pests in cotton in 1997. In veterinary medicine, spinosadg is an FDA approved oral drug for use as a flea adulticide.51 It is highly effective (97–100% 30 days after the treatment) and safe in dogs. The repeated monthly oral administrations with spinosad at 30 mg/kg provide sustained control of Ctenocephalide felis in dogs.6 It is available as chewable tablets for dogs, administered orally once a month.51 However, spinosad has not been approved or recommended for use in cats.
Adverse effects of insect nAChR agonists
The three approved neonicotinoids and spinosad for use as fleacides in dogs and cats are very effective and safe since they have high affinity for insect nAChRs and extremely low affinity for mammalian nAChRs.24 However, overdose of the nAChR agonist can cause vomiting in dogs and cats. The scratching behavior after the administration/application of an insect nAChR agonist is due to the reaction to the fleas when they begin to die. There are no noticeable chronic adverse effects associated with these insect nAChR agonists at the recommended dose regimen.
According to the FDA Adverse Drug Experiences report, concurrent use of spinosad and high doses of ivermectin (0.4–0.6 mg/kg/day) in the treatment of canine demodicosis has caused increased frequency of ivermectin toxicity than without spinosad.52 Therefore; one should avoid concurrent administration of spinosad and high doses of ivermectin. Since spinosad is a macrocyclic lactone, it may cause additive adverse effect when it is used concurrently with a large dose of a macrocyclic lactone. Further research is needed to investigate the drug interaction between spinosad and other macrocyclic lactones.
Environmental impact of insect nAChR agonists
There is a controversy over the role of insect nAChR agonists in relation to their toxicity in bees. Neonicotinoids have been strictly limited in France since 1990s, when they were implicated in a massive destruction of the bee population.54,55 The nAChR agonists may cause worker bees to neglect providing food for larvae, and they may evoke a breakdown of the navigational abilities of bees, thereby possibly leading to what has become generally known as the Colony Collapse Disorder, which is usually associated with the mite pest Varroa destructor.54,55
Germany has banned the treatment of seeds with neonicotinoids in May 2008, due to negative effects on bee colonies. Bee keepers suffered a severe decline linked to the use of a neonicotinoid clothianidin in the Baden-Württemberg region of Germany,54 allegedly connected to a manufacturer’s failure to apply a ‘glue’ agent that affixes the compound to the coats of seeds. Without the fixative agent, the compound could have drifted into the environment from sown rapeseed and sweet corn and then affected the honeybees. Fortunately, the use of insect nAChR agonists in veterinary medicine has not caused significant environmental problems.
Conclusions
This article reviews the characteristics of nAChRs in both vertebrates and insects and the use of nAChR agonists as fleacides in dogs and cats. These receptors are the important targets for neonicotinoids and spinosad. Since they have high affinity for insect nAChRs and extremely low affinity for vertebrate nAChRs, they have become very important insecticides in veterinary medicine. Neonicotinoids (imidacloprid, nitenpyram, and dinotefuran) and spinosad have been used widely as fleacides. These compounds are very effective and safe for flea control in dogs and cats and their rapid flea killing action is highly beneficial to the control of flea-bite allergy dermatitis and flea-transmitted zoonosis.
Acknowledgments
Dr. Dai Tan Vo was supported by a grant from Project 322, the Ministry of Education and Training, Vietnamese Government, Vietnam
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- α-BGT
α-bungarotoxin
- EPA
U.S. Environmental Protection Agency
- FDA
Food and Drug Administration
- GABA
gamma-aminobutyric acid
- NADA
New Animal Drug Application
- nAChR
nicotinic acetylcholine receptor
Footnotes
Advantage®
K9 Advantix®
Advantage Multi®
Capstar®
Program®
Vectra 3D®
Comfortis®
References
- 1.Mencke N, Jeschke P. Therapy and prevention of parasitic insects in veterinary medicine using imidacloprid. Curr Top Med Chem. 2002;2:701–715. doi: 10.2174/1568026023393598. [DOI] [PubMed] [Google Scholar]
- 2.Rust MK. Advances in the control of Ctenocephalides felis (cat flea) on cats and dogs. Trends Parasitol. 2005;21:232–236. doi: 10.1016/j.pt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol. 2003;48:339–364. doi: 10.1146/annurev.ento.48.091801.112731. [DOI] [PubMed] [Google Scholar]
- 4.Schenker R, Tinembart O, Humbert-Droz E, et al. Comparative speed of kill between nitenpyram, fipronil, imidacloprid, selamectin and cythioate against adult Ctenocephalides felis (Bouché) on cats and dogs. Vet Parasitol. 2003;112:249–254. doi: 10.1016/s0304-4017(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 5.Millar NS, Denholm I. Nicotinic acetylcholine receptors: targets for commercially important insecticides. Invert Neurosci. 2007;7:53–66. doi: 10.1007/s10158-006-0040-0. [DOI] [PubMed] [Google Scholar]
- 6.Snyder DE, Meyer J, Zimmermann AG, et al. Preliminary studies on the effectiveness of the novel pulicide, spinosad, for the treatment and control of fleas on dogs. Vet Parasitol. 2007;150:345–351. doi: 10.1016/j.vetpar.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- 8.Corringer PJ, Le Novère N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 9.Thany SH, Lenaers G, Raymond-Delpech V, et al. Exploring the pharmacological properties of insect nicotinic acetylcholine receptors. Trends Pharmacol Sci. 2007;28:14–22. doi: 10.1016/j.tips.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda K, Shimomura M, Ihara M, et al. Neonicotinoids show selective and diverse actions on their nicotinic receptor targets: electrophysiology, molecular biology, and receptor modeling studies. Biosci Biotechnol Biochem. 2005;69:1442–1452. doi: 10.1271/bbb.69.1442. [DOI] [PubMed] [Google Scholar]
- 11.Tomizawa M, Casida JE. Structure and diversity of insect nicotinic acetylcholine receptors. Pest Manag Sci. 2001;57:914–922. doi: 10.1002/ps.349. [DOI] [PubMed] [Google Scholar]
- 12.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45:247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- 13.Usherwood PNR. Insect glutamate receptors. Adv Insect Physiol. 1994;24:309–341. [Google Scholar]
- 14.Sattelle DB, Jones AK, Sattelle BM, et al. Edit, cut and paste in the nicotinic acetylcholine receptor gene family of Drosophila melanogaster. Bioessays. 2005;27:366–376. doi: 10.1002/bies.20207. [DOI] [PubMed] [Google Scholar]
- 15.Jones AK, Grauso M, Sattelle DB. The nicotinic acetylcholine receptor gene family of the malaria mosquito, Anopheles gambiae. Genomics. 2005;85:176–187. doi: 10.1016/j.ygeno.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Jones AK, Raymond-Delpech V, Thany SH, et al. The nicotinic acetylcholine receptor gene family of the honey bee, Apis mellifera. Genome Res. 2006;16:1422–1430. doi: 10.1101/gr.4549206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bass C, Lansdell SJ, Millar NS, et al. Molecular characterisation of nicotinic acetylcholine receptor subunits from the cat flea, Ctenocephalides felis (Siphonaptera: Pulicidae) Insect Biochem Mol Biol. 2006;36:86–96. doi: 10.1016/j.ibmb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Shao YM, Dong K, Zhang CX. The nicotinic acetylcholine receptor gene family of the silkworm, Bombyx mori. BMC Genomics. 2007;8:324. doi: 10.1186/1471-2164-8-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maienfisch P, Huerlimann H, Rindlisbacher A, et al. The discovery of thiamethoxam: a second generation neonicotinoid. Pest Manag Sci. 2001;57:165–176. doi: 10.1002/1526-4998(200102)57:2<165::AID-PS289>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Wakita T, Kinoshita K, Yamada E, et al. The discovery of dinotefuran: a novel neonicotinoid. Pest Manag Sci. 2003;59:1016–1022. doi: 10.1002/ps.727. [DOI] [PubMed] [Google Scholar]
- 21.Tomizawa M, Millar NS, Casida JE. Pharmacological profiles of recombinant and native insect nicotinic acetylcholine receptors. Insect Biochem Mol Biol. 2005b;35:1347–1355. doi: 10.1016/j.ibmb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Ihara M, Matsuda K, Otake M, et al. Diverse actions of neonicotinoids on chicken α7, α4β2 and Drosophila-chicken SADβ2 and ALSβ2 hybrid nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Neuropharmacology. 2003;45:133–144. doi: 10.1016/s0028-3908(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 23.Lansdell SJ, Millar NS. The influence of nicotinic receptor subunit composition upon agonist, α bungarotoxin and insecticide (imidacloprid) binding affinity. Neuropharmacology. 2000;39:671–679. doi: 10.1016/s0028-3908(99)00170-7. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda K, Buckingham SD, Kleier D, et al. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci. 2001;22:573–580. doi: 10.1016/s0165-6147(00)01820-4. [DOI] [PubMed] [Google Scholar]
- 25.Arther RG, Cunningham J, Dorn H, et al. Efficacy of imidacloprid for removal and control of fleas (Ctenocephalides felis) on dogs. Am J Vet Res. 1997;58:848–850. [PubMed] [Google Scholar]
- 26.Jacobs DE, Hutchinson MJ, Fox MT, et al. Comparison of flea control strategies using imidacloprid or lufenuron on cats in a controlled simulated home environment. Am J Vet Res. 1997;58:1260–1262. [PubMed] [Google Scholar]
- 27.Epe C, Coati N, Stanneck D. Efficacy of the compound preparation imidacloprid 10%/permethrin 50% spot-on against ticks (I. ricinus, R. sanguineus) and fleas (Ct. felis) on dogs. Parasitol Res. 2003;90:122–124. doi: 10.1007/s00436-003-0911-9. [DOI] [PubMed] [Google Scholar]
- 28.Arther RG, Bowmann DD, McCall JW, et al. Feline Advantage Heart (imidacloprid and moxidectin) topical solution as monthly treatment for prevention of heartworm infection (Dirofilaria immitis) and control of fleas (Ctenocephalides felis) on cats. Parasitol Res. 2003;90:137–139. doi: 10.1007/s00436-003-0917-3. [DOI] [PubMed] [Google Scholar]
- 29.Arther RG, Bowman DD, Slone RL, et al. Imidacloprid plus moxidectin topical solution for the prevention of heartworm disease (Dirofiloria immitis) in dogs. Parasitol Res. 2005;97:76–80. doi: 10.1007/s00436-005-1448-x. [DOI] [PubMed] [Google Scholar]
- 30.Mehlhorn H, Mencke N, Hansen O. Effects of imidacloprid on adult and larval stages of the flea Ctenocephalides felis after in vivo and in vitro application: a light- and electron-microscopy study. Parasitol Res. 1999;85:625–637. doi: 10.1007/s004360050607. [DOI] [PubMed] [Google Scholar]
- 31.Fichtel M. Dissertation. Free University of Berlin, School of Veterinary Medicine; Berlin, Germany: 1998. Investigations of the effect of the adulticide imidacloprid against the cat flea Ctenocephalides felis on cats and dogs. [Google Scholar]
- 32.FDA-Federal Register. Imidacloprid (Admire, Provado, Gaucho) - Pesticide Petition Filing. 2000;65:7010. [Google Scholar]
- 33.Dobson P, Tinembart O, Fisch RD, et al. Efficacy of nitenpyram as a systemic flea adulticide in dogs and cats. Vet Rec. 2000;147:709–713. [PubMed] [Google Scholar]
- 34.Rust MK, Waggoner MM, Hinkle NC, et al. Efficacy and longevity of nitenpyram against adult cat fleas (Siphonaptera: Pulicidae) J Med Entomol. 2003;40:678–681. doi: 10.1603/0022-2585-40.5.678. [DOI] [PubMed] [Google Scholar]
- 35.Schenker R, Tinembart O, Barnett SH, et al. A brief introduction to nitenpyram: a new systemic flea adulticide for cats and dogs. Compend Contin Educ Pract Vet. 2001;23:4–6. [Google Scholar]
- 36.Wakita T, Kinoshita K, Yamada E, et al. The discovery of dinotefuran: a novel neonicotinoid. Pest Manag Sci. 2003;59:1016–1022. doi: 10.1002/ps.727. [DOI] [PubMed] [Google Scholar]
- 37.Summit VetPharm LLC. VECTRA 3D™ Technical Monograph. 2008 Available at: http://www.summitvetpharm.com/File/Vectra%203D%20Technical%20Monograph.pdf.
- 38.Bowman DD, Ball CA. Introducing a brand new flea technology. Summit VetPharm Vol. 2007;1(4) Available at: www.summitvetpharm.com. [Google Scholar]
- 39.Ford KA, Casida JE. Unique and common metabolites of tiamethoxam, cothianidin, and dinotefuran in mce. Chem Res Toxicol. 2006;19:1549–1556. doi: 10.1021/tx0601859. [DOI] [PubMed] [Google Scholar]
- 40.EPA. Dinotefuran: PesticideFactSheet. 2004 Available at: http://www.epa.gov/opprd001/factsheets/dunotefuran.pdf.
- 41.Perry T, McKenzie JA, Batterham P. A Dα6 knockout strain of Drosophila melanogaster confers a high level of resistance to spinosad. Insect Biochem Mol Biol. 2007;37:184–188. doi: 10.1016/j.ibmb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Lansdell SJ, Millar NS. Molecular characterisation of Dα6 and Dα7 nicotinic acetylcholine receptor subunits from Drosophila: formation of a high-affinity α-bungarotoxin binding site revealed by expression of subunit chimeras. J Neurochem. 2004;90:479–489. doi: 10.1111/j.1471-4159.2004.02499.x. [DOI] [PubMed] [Google Scholar]
- 43.Salgado VL, Saar R. Desensitizing and non-desensitizing subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in cockroach neurons. J Insect Physiol. 2004;50:867–879. doi: 10.1016/j.jinsphys.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Salgado VL. Studies on the mode of action of spinosad: insect symptoms and physiological correlates. Pestic Biochem Physiol. 1998;60:91–102. [Google Scholar]
- 45.Salgado VL, Sheets JJ, Watson GB, et al. Studies on the mode of action of spinosad: the internal effective concentration and the concentration dependence of neural excitation. Pestic Biochem Physiol. 1998;60:103–110. [Google Scholar]
- 46.Watson GB. Actions of insecticidal spinosyns on γ-aminobutyric acid responses from small-diameter cockroach neurons. Pestic Biochem Physiol. 2001;71:20–28. [Google Scholar]
- 47.Sparks TC, Crouse GD, Durst G. Natural products as insecticides: the biology, biochemistry and quantitative structure activity relationships of spinosyns and spinosoids. Pest Manag Sci. 2001;57:896–905. doi: 10.1002/ps.358. [DOI] [PubMed] [Google Scholar]
- 48.Janssen D, Derst C, Buckinx R, et al. Dorsal unpaired median neurons of Locusta migratoria express ivermectin- and fipronil-sensitive glutamate-gated chloride channels. J Neurophysiol. 2007;97:2642–2650. doi: 10.1152/jn.01234.2006. [DOI] [PubMed] [Google Scholar]
- 49.EPA. Spinosad Pesticide Fact Sheet No. HJ 501C. EPA, Office of Pesticides and Toxic Substances; 1997. www.epa.gov. [Google Scholar]
- 50.Dow AgroSciences. Spinosad Technical Bulletin. 1997. p. 15. [Google Scholar]
- 51.FDA website. NADA; 2007. pp. 141–277. Available at. http://www.fda.gov/cvm/FOI/141-277o092507.pdf. [Google Scholar]
- 52. [Accessed June 24, 2008];FDAwebsite.CVM update. Available at: http://www.fda.gov/cvm/CVM_Updates/ComfortisSafety.htm.
- 53.LiveScience®. [Accessed June 15, 2007];Mysterious Bee Deaths Linked to Pesticides. Available at: http://www.livescience.com/animals/070615_ap_bee_trouble.html.
- 54.Copping Jasper. [Accessed Mar 31, 2007];Flowers and fruit crops facing disaster as disease kills off bees. Available at: http://www.telegraph.co.uk/news/main.jhtml?xml=/news/2007/04/01/nbees01.xml.