Abstract
The adrenal glands are the primary source of minerocorticoids, glucocorticoids and the so-called adrenal androgens. Under physiological conditions cortisol and adrenal androgen synthesis are controlled primarily by adrenocorticotrophic hormone (ACTH). Although it has been established that ACTH can stimulate steroidogenesis, the effects of ACTH on overall gene expression in human adrenal cells have not been established. In this study, we defined the effects of chronic ACTH treatment on global gene expression in primary cultures of both adult adrenal (AA) and fetal adrenal (FA) cells. Microarray analysis indicated that 48 h of ACTH treatment caused 30 AA genes and 84 AA genes to increase by greater than 4-fold, with 20 genes common in both cell cultures. Among these genes were six encoding enzymes involved in steroid biosynthesis, the ACTH receptor and its accessory protein, MRAP (ACTH receptor accessory protein). These data provide a group of ACTH-regulated genes including many that have not previously been studied with regard to adrenal function. These genes represent candidates for regulation of adrenal differentiation and steroid hormone biosynthesis.
Keywords: adrenal cortex, ACTH, steroidogenesis, microarray
Introduction
Adrenocorticotrophic hormone (ACTH) is a 39 amino acid polypeptide predominantly synthesized and secreted from the anterior lobe of the pituitary gland. The synthesis and secretion of ACTH are tightly controlled by the hypothalamic-pituitary-adrenal axis (HPA axis). Under stress conditions, the paraventricular nucleus of the hypothalamus secretes vasopressin and corticotropin-releasing hormone (CRH). These two peptides regulate the anterior lobe of the pituitary gland and stimulate the secretion of ACTH. ACTH subsequently induces adrenal cortex expansion and corticosteroid production (mainly cortisol in humans). Cortisol once synthesized, in turn acts on the hypothalamus and pituitary (to suppress CRH and ACTH production) causing a negative feedback cycle. In the adrenal glands, ACTH acts by binding to specific cell surface ACTH receptors (MC2R). MC2R is a seven membrane-spanning G-protein coupled receptor that is primarily expressed in adrenocortical cells. Upon ligand binding, the receptor undergoes conformation changes that stimulate adenylyl cyclase, leading to an increase in intracellular cAMP and subsequent activation of protein kinase A.
Although previous studies has identified some ACTH responsive genes that are involved with the steroidogenic and growth related effects of ACTH (Cecim et al. 1996; Gaillard et al. 2000; Le Roy et al. 2000; Markowska et al. 1993; Neri et al. 1991; Phillips et al. 1996; Simmonds et al. 2001), there is a lack of knowledge regarding the global actions of ACTH on gene expression. Given the critical role of ACTH in adrenal development, steroidogenesis and disease, it is appropriate to further define the detailed effects of ACTH on human adrenal cell gene expression. In this study we used oligonucleotide microarray analysis to define the effects of ACTH on gene expression in primary cultures of human adrenocortical cells. The study has defined a series of ACTH responsive genes that greatly expands our understanding of ACTH action in the human.
Materials and methods
Tissue collection
Human adult adrenal glands were obtained from cadaveric kidney donors transplanted at the Medical College of Georgia (Augusta, GA) with the family’s informed consent obtained by LifeLink of Georgia. Human fetal adrenal glands (14–19 wk gestation) were obtained from pathological examination following elective pregnancy terminations from Advanced Bioscience Resources Inc (Alameda, CA) with informed consent. The use of these tissues was approved by the Institutional Review Board of Medical College of Georgia, and the University of Alabama at Birmingham (Birmingham, AL).
Cell culture and treatment
Adult adrenocortical cells were isolated with collogenase-dispase digestion as described previously (Bassett et al. 2004). The adult adrenals were minced and dissociated into single cell suspension by repeated exposure of the tissue fragments to DMEM/F12 medium (Invitrogen, Carlsbad,CA) containing 1 mg/mL of collagenase dispase and 0.25 mg/mL of DNase-1 (F. Hoffmann-La Roche Ltd., Schweiz). Digestion and mechanical dispersion were carried out four times for 1 h each at 37°C. Cells were collected between each digestion and combined before aliquots of AA cells were frozen (2,000,000 cells per vial) and stored at −150 °C.
Fetal adrenals were minced and dissociated into single cell suspensions by repeated exposure of the tissue fragments to 0.4 mg/ml collagenase (Sigma, St. Louis, MO) in phosphate buffered saline (PBS) enriched with 10% bovine serum albumin (Sigma) at 37°C for 15–30 min. After separation of the cells from the collagenase mixture by centrifugation, the cell suspensions were suspended in culture medium [McCoy’s 5A medium (Gibco, Grand Island, NY) that contained 5% fetal bovine serum (Hyclone, Logan, UT) and antibiotics/antimycotics (Gibco)]. Cells were initially cultured in Falcon 75cc flasks (Becton Dickenson & Co, lincoln Park, NJ) for 2–3 days at 37°C in a humidified atmosphere (95% air, 5% CO2) to ensure viability and lack of infection prior to experimental use.
For experiments AA or FA cells were subcultured and plated at a density of 200,000/ well in 24 well dishes and allowed to grow for 5 days in complete growth medium [10% Cosmic calf serum (Hyclone), 1% antibiotic, for adult adrenal cells] (Bassett et al. 2004) [10% Cosmic calf serum, 1% ITS plus (BD Diagnostic Systems, Sparks, MD) and 1% antibiotic for fetal adrenal cells] (Rehman et al. 2007). The day before treatment, the cells were changed to experimental medium (0.1% Cosmic calf serum, 1% antibiotics for adult adrenal cells and 1% cosmic calf serum, 1% ITS plus, 1% antibiotic for fetal adrenal cells) for overnight incubation and then treated for an indicated time either under basal conditions or with ACTH (10 nM) (Organon, Bedford, OH). The cells were lysed for RNA isolation and media were collected for steroid assays as described below.
RNA isolation
Total RNA was extracted from human adrenal cells using RNeasy mini kit from Qiagen (Valencia, CA) following the manufacturer protocol and the quantity of RNA was checked spectroscopically using a Nanodrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). Deoxyribonuclease I (Ambion Inc, Austin, TX)-treated total RNA (2 µg) was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) and stored at −80°C.
Microarray analysis
Total RNA isolated from human adrenal cells under basal and ACTH-treated conditions was used on genomic expression arrays performed by the Microarray Core Facility at the Medical College of Georgia. Briefly, RNA was hybridized to either Illumina HumanRef-8 (gene targets 24,526) or HumanHT-12 (gene targets 48,804) Expression BeadChips (Illumina, San Diego, CA). Results were analyzed using GeneSpring GX 7.3.1 software (Silicon Genetics, Redwood City, CA) to study global gene expression.
Protein extraction and protein assay
Cells were lysed in 100 µl of Mammalian Protein Extraction Reagent (Pierce Chemical Co., Rockford, IL). The protein content of samples was then determined by the bicinchoninic acid (BCA) protein assay following the micro BCA protocol (Pierce Chemical Co.).
Steroid Immunoassay
The cortisol and dehydroepiandrosterone (DHEA) content of the experimental medium were determined using EIA kits (Diagnostic System Laboratories, Webster, TX). The assays were conducted following the manufacture recommendation, except that standard curves were prepared in the experimental cell culture medium. Results were normalized to the protein per tissue culture well and shown as fold changes compared to basal.
Statistical Analysis
Results are given as mean ± SD. Individual experiments were repeated at least three times, using cells isolated from separate adrenal glands. One way ANOVA were performed using GraphPad Prism 3.0 (GraphPad Software, Inc. San Diego, CA).
Results
ACTH stimulates cortisol production in AA cells
As shown in Fig.1, treatment of AA cells with ACTH (10 nM) resulted in a significant elevation of cortisol biosynthesis. Media content of cortisol increased following ACTH treatment in a time-dependent manner. ACTH caused a significant increase in medium cortisol within 6 hours and by 48 h, ACTH increased cortisol to over 30-fold above that seen in untreated cells.
Fig.1. Time-dependent effects of ACTH on cortisol production in human AA cells.
Primary human AA cells were prepared as described under Methods, and plated at the density of 200,000 cells per well in 24 well dishes. Cells were treated with ACTH (10 nM) for the indicated times and cortisol was quantified in the medium using EIA. Cortisol data was normalized to protein per well and expressed as the fold change over basal. Results represent the mean ±S.E.M of data from at least three independent experiments. Statistics were calculated using one way ANOVA followed by Dunnett test. *, P<0.05, **, P<0.01.
Microarray data of AA 48 h treatment with ACTH
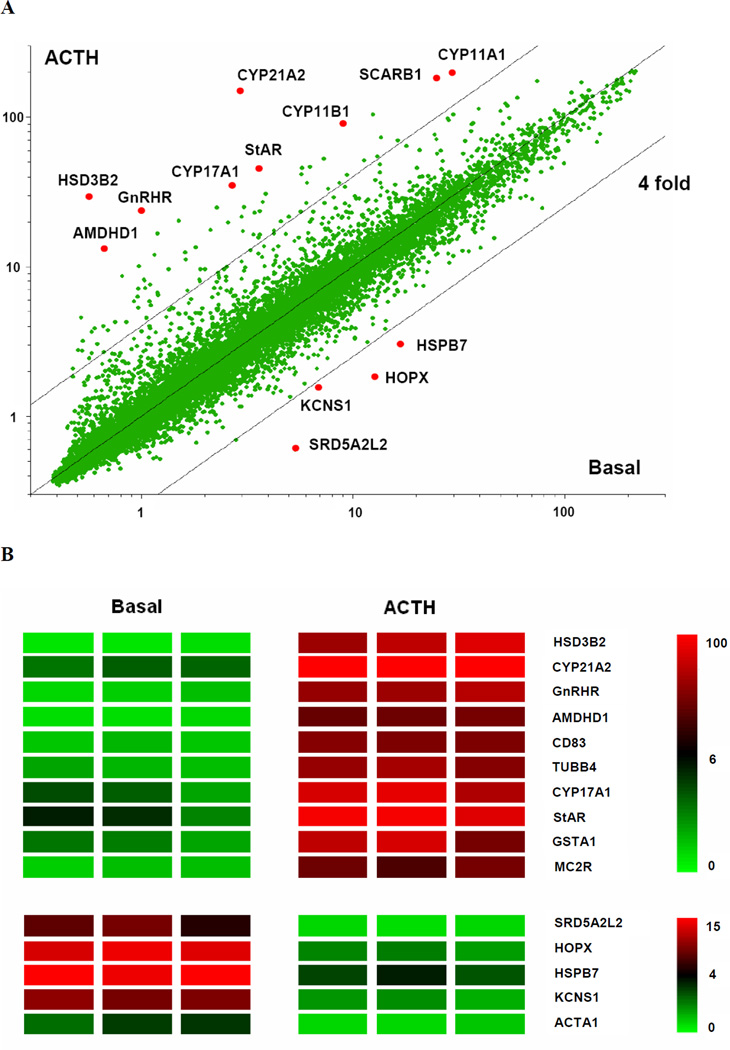
To determine the global gene changes caused by ACTH treatment in AA cells, microarray analysis was performed comparing basal and ACTH (10 nM, 48 h) treated samples (Fig.2A). Cells were isolated from three independent donor adrenal glands and used for independent experiments. Six microarrays were run with three sets of basal and ACTH treated samples. Among the 37,846 genes shown in the scatter plot, 30 genes were upregulated by ACTH over 4-fold, while only one gene (serotonin receptor 2B, HTR2B) was downregulated for more than 4-fold. The top up-regulated/ down-regulated genes are shown in the heatmap (Fig.2B) and listed in Table 1.
Fig.2. Microarray analysis of ACTH effects on AA cell gene expression.
Panel A. Scatter plot comparing gene expression between basal and ACTH treated samples. Total RNA from three sets of primary adrenal culture samples treated with/without ACTH were used for six oligonucleotide microarrays and the data were combined to form this plot. The graph represents 37,846 transcripts that were found to have a signal which indicated that it was present in at least one tissue sample. The transcripts with the highest variation between the basal and ACTH are labeled. Panel B. Heatmap of the fifteen most differentially expressed transcripts between three basal and three ACTH treated samples. The top ten upregulated and top five downregulated genes are given here. Colors represent the expression level from the median of all the samples for each probe set. Results represent data from three experiments using cells isolated from three independent donor adrenal glands.
Table.1. Genes most regulated by ACTH treatment in AA cells.
Primary human AA cell culture were treated with/without ACTH (10 nM) for 48 h and RNA was isolated for microarray analysis. Ten genes most upregulated and five genes downregulated by ACTH treatment in human AA are shown. The gene symbol and full name of each gene is given in the first and last columns, followed by the fold change between basal and ACTH and gene accession number. Results represent data from three experiments using cells isolated from three independent donor adrenal glands.
| Gene symbol |
Fold change |
Gene bank access number |
Gene name |
|---|---|---|---|
| CYP17A1 | 31.60 | NM_000102.3 | Cytochrome P450, family 17, subfamily A, polypeptide 1 |
| CYP11B1 | 29.19 | NM_001026213.1 | Cytochrome P450, family 11, subfamily B, polypeptide 1, nuclear gene encoding mitochondrial protein, transcript variant 2 |
| MRAP | 15.85 | NM_178817.3 | Melanocortin 2 receptor accessory protein, transcript variant 1 |
| INHA | 14.73 | NM_002191.2 | Inhibin, alpha |
| STAR | 13.86 | NM_001007243.1 | Steroidogenic acute regulatory protein, nuclear gene encoding mitochondrial protein, transcript variant 2 |
| CYP21A2 | 13.45 | NM_000500.5 | Cytochrome P450, family 21, subfamily A, polypeptide 2 |
| MC2R | 12.22 | NM_000529.2 | Melanocortin 2 receptor (ACTH receptor) |
| HSD3B2 | 10.02 | NM_000198.2 | Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 |
| RDH12 | 8.28 | NM_152443.1 | Retinol dehydrogenase 12 (all-trans/9-cis/11-cis) |
| AMDHD1 | 7.84 | NM_152435.1 | Amidohydrolase domain containing 1 |
| Gene symbol |
Fold change |
Gene bank access number |
Gene name |
|---|---|---|---|
| HTR2B | 0.24 | NM_000867.3 | 5-hydroxytryptamine (serotonin) receptor 2B |
| HOPX | 0.27 | NM_032495.4 | HOP homeobox (HOPX), transcript variant 1 |
| CPB1 | 0.37 | NM_001871.2 | Carboxypeptidase B1 |
| C7 | 0.38 | NM_000587.2 | Complement component 7 |
| WISP2 | 0.38 | NM_003881.2 | WNT1 inducible signaling pathway protein 2 |
As shown in the scatter plot and table, all steroidogenic enzymes involved in adrenal cortisol production were increased following treatment with ACTH for 48 h. CYP17 showed the largest increase of 32-fold. This confirms that ACTH is a very potent steroidogenic activator that chronically increases the capacity of adrenal cells to produce steroid hormones by increasing steroidogenic enzyme/protein expression. In addition, ACTH treatment increased the expression of its own receptor—MC2R (12-fold). Interestingly, a previously reported important component and effector of the MC2R system—the MC2R accessory protein (MRAP) was also upreguted by ACTH (16-fold). Another interesting finding is the significantly increased expression of the scavenger receptor type B class 1 (SCARB1) transcript with ACTH treatment. As the major HDL receptor in the adrenal gland, SCARB1 is shown to be regulated by cAMP and PKC pathway. Although it is established that SCARB1 mediated selective uptake is not the major course of cholesterol supply in the human adrenal, this change of SCARB1 expression may suggest that HDL plays other important roles in adrenal function.
ACTH stimulates cortisol and DHEA-S production in FA
Like AA cells, human FA cells are dependent on ACTH to increase steroidogenesis. To test the effects of ACTH on human FA, we isolated cells from three separate adrenals and ran three independent experiments. As shown in Fig.3, both cortisol and DHEA-S levels were significantly stimulated by ACTH treatment and these effects were time-dependent, continuing to magnify with time. By 48 h, ACTH was able to increase DHEA-S to about 8-fold and cortisol to over 300-fold when compared to basal steroid production. Six microarrays were performed on the 48 h ACTH treatment groups (Fig.4A) and the genes that were most upregulated and downregulated are summarized in Fig.4B and Table 2. Among the 18,391 genes shown in the scatter plot, 84 of them were upregulated more than 4-fold by 48 h ACTH treatment and 5 genes were downregulated for more than 4-fold. Four genes of steroidogenic enzymes/protein including HSD3B2, CYP21A2, CYP17A1 and StAR were found in the 10 most ACTH upregulated gene list, while the mRNA encoding HSD3B2 increased the most, at 53-fold above that seen in untreated cells.
Fig.3. Time-dependent effects of ACTH on cortisol and DHEA-S production in FA primary cultures.
Primary human FA cell were prepared as described in Method section, and plated at a density of 300,000 cells per well in 24-well dishes. The day before experiments, cells were changed to 1% low serum medium overnight. Cells were treated with ACTH (10 nM) for the indicated times followed by quantification of medium cortisol and DHEA-S using EIA. Steroid data was normalized to protein per well and expressed as fold change over basal. Results represent the mean ±S.E.M of data from at least three independent experiments. Statistics were calculated using one way ANOVA followed by Dunnett test. *, P<0.05, **, P<0.01.
Fig.4. Microarray analysis of ACTH effects on FA cell gene expression.
Panel A. Scatter plot comparing gene expression between basal and ACTH treated samples in FA cells. Total RNA from three sets of primary adrenal samples treated with/without ACTH were used for oligonucleotide microarray analysis and the data were combined. The graph represents 18,391 transcripts that were found to have a signal which indicated that it was present in at least one cell sample. The transcripts with the highest variation between the basal and ACTH are labeled. Results represent data from three experiments using cells isolated from three independent adrenal glands. Panel B. Heatmap of the fifteen most differentially expressed transcripts between basal and ACTH treatment. The top ten upregulated and top five downregulated genes are shown. Colors represent the expression level from the median of all the samples for each probe set. Results represent data from three experiments using cells isolated from three independent FA glands.
Table.2. Genes most regulated by ACTH treatment in FA cells.
Primary human FA cell cultures were treated with/without ACTH (10 nM) for 48 h and RNA was isolated for microarray analysis. Ten genes most upregulated and five genes downregulated by ACTH treatment in human FA are shown. The gene symbol and full name of each gene is given in the first and last columns, followed by the fold change between basal and ACTH and gene accession number. Result represent data from three experiments using cells isolated from three independent donor fetal adrenal glands.
| Gene symbol |
Fold change |
Gene bank access number |
Gene name |
|---|---|---|---|
| HSD3B2 | 53.05 | NM_000198.2 | Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 |
| CYP21A2 | 52.00 | NM_000500.5 | Cytochrome P450, family 21, subfamily A, polypeptide 2 |
| GNRHR | 23.60 | NM_001012763.1 | Gonadotropin-releasing hormone receptor, transcript variant 2 |
| AMDHD1 | 19.92 | NM_152435.1 | Amidohydrolase domain containing 1 |
| CD83 | 14.16 | NM_004233.3 | CD83 molecule, transcript variant 1 |
| TUBB4 | 13.78 | NM_006087.2 | Homo sapiens tubulin, beta 4 (TUBB4), mRNA. |
| CYP17A1 | 13.22 | NM_000102.3 | Cytochrome P450, family 17, subfamily A, polypeptide 1 |
| STAR | 12.90 | NM_001007243.1 | Steroidogenic acute regulatory protein, nuclear gene encoding mitochondrial protein |
| GSTA1 | 11.40 | NM_145740.2 | Glutathione S-transferase A1 |
| MC2R | 11.01 | NM_000529.2 | Melanocortin 2 receptor (ACTH receptor) |
| Gene symbol |
Fold change |
Gene bank access number |
Gene name |
|---|---|---|---|
| SRD5A2L2 | 0.11 | NM_001010874.3 | Steroid 5 alpha-reductase 2-like 2 |
| HOPX | 0.15 | NM_032495.4 | HOP homeobox (HOPX), transcript variant 1 |
| HSPB7 | 0.18 | NM_014424.3 | Heat shock 27kDa protein family, member 7 (cardiovascular) |
| KCNS1 | 0.23 | NM_002251.3 | Potassium voltage-gated channel, delayed-rectifier, subfamily S, member 1 |
| ACTA1 | 0.25 | NM_001100.3 | Actin, alpha 1, skeletal muscle |
Common genes shared by AA and FA
Comparing the pan-genomic effects of ACTH on AA and FA cells, we found a series of common genes that were increased in both cell types. When setting the threshold at a 4-fold increase, there were 30 genes regulated by ACTH in AA, with 84 genes in FA (Fig.5A). Among them, 20 genes were common; suggesting that those genes may represent a universal set of human adrenal ACTH targets and perform the critical functions associated with ACTH action. The heatmap for those 20 genes was shown (Fig.5B), followed by the detailed fold changes in both cell types (Table 3). Among the 20 common genes, six were steroidogenic enzyme/protein, namely StAR, CYP11A1, CYP17A1, HSD3B2, CYP21A2 and CYP11B1. The ACTH receptor (MC2R) and MC2R accessory protein (MRAP) were also upregulated by ACTH in both cell cultures.
Fig.5. Common genes that are increased by ACTH treatment in AA and FA cells.
Panel A. Venn diagram of genes upregulated by ACTH by at least 4 fold in AA and FA cells. Grey: AA cell. White: FA cells. Panel B. Heatmap of the twenty commonly regulated genes between AA and FA after ACTH treatment. Colors represent the expression level from the median of all the samples for each probe set. Results represent data from three experiments using cells isolated from three independent donor adrenal glands.
Table.3. Genes with increased expression in both human AA and FA cells.
The genes upregulated more than 4-fold in AA and FA cells after 48 h ACTH treatment were compared and common genes are listed below. The gene symbol and full name of each gene are given in the first and last columns, followed by the fold change between basal and ACTH and gene accession number.
| Gene symbol |
Fold change in FA |
Fold change in AA |
Gene bank access number |
Gene name |
|---|---|---|---|---|
| MRAP | 8.54 | 16.20 | NM_178817.3 | Melanocortin 2 receptor accessory protein (MRAP), transcript variant 1 |
| CYP11B1 | 10.73 | 43.31 | NM_001026213.1 | Cytochrome P450, family 11, subfamily B, polypeptide 1 (CYP11B1), nuclear gene encoding mitochondrial protein, transcript variant 2 |
| HSD3B2 | 53.05 | 10.58 | NM_000198.2 | Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 |
| CYP21A2 | 52.00 | 20.64 | NM_000500.5 | Cytochrome P450, family 21, subfamily A, polypeptide 2 |
| MVD | 7.57 | 5.81 | NM_002461.1 | Mevalonate (diphospho) decarboxylase |
| SCARB1 | 7.38 | 5.94 | NM_005505.3 | Scavenger receptor class B, member 1 |
| MT3 | 8.04 | 6.72 | NM_005954.2 | Metallothionein 3 |
| IDI1 | 4.77 | 5.76 | NM_004508.2 | Isopentenyl-diphosphate delta isomerase 1 |
| MC2R | 11.01 | 12.42 | NM_000529.2 | Melanocortin 2 receptor (ACTH receptor) |
| AMDHD1 | 19.92 | 8.19 | NM_152435.1 | Amidohydrolase domain containing 1 |
| ACSS2 | 5.61 | 5.75 | NM_018677.2 | Acyl-CoA synthetase short-chain family member 2 |
| TMEM97 | 8.68 | 5.43 | NM_014573.2 | Transmembrane protein 97 |
| GSTA4 | 4.27 | 4.87 | NM_001512.2 | Glutathione S-transferase A4 |
| STAR | 12.90 | 14.30 | NM_001007243.1 | Steroidogenic acute regulatory protein, nuclear gene encoding mitochondrial protein, transcript variant 2 |
| CYP11A1 | 6.83 | 5.55 | NM_000781.2 | Cytochrome P450, family 11, subfamily A, polypeptide 1, nuclear gene encoding mitochondrial protein, transcript variant 1 |
| EBP | 7.82 | 6.94 | NM_006579.1 | Emopamil binding protein (sterol isomerase) |
| FDX1 | 7.65 | 6.04 | NM_004109.3 | Ferredoxin 1, nuclear gene encoding mitochondrial protein |
| CYP17A1 | 13.22 | 31.64 | NM_000102.3 | Cytochrome P450, family 17, subfamily A, polypeptide 1 |
| EPB41L1 | 8.32 | 4.08 | NM_177996.1 | Erythrocyte membrane protein band 4.1-like 1, transcript variant 2 |
| INHA | 6.15 | 14.75 | NM_002191.2 | Inhibin, alpha |
Discussion
ACTH is the major regulator of adrenal steroidogenesis and has been shown to increase aldosterone, cortisol and DHEA production. Aside from steroidogenic enzymes/proteins, several other gene targets of ACTH have been identified. Schimmer et al has identified a series of ACTH response genes using microarry analysis in the Y-1 mouse adrenal tumor cell model (Schimmer et al. 2006). However, there is a lack of knowledge on ACTH global actions on human adrenal cells. Herein, we used primary cultures of human adrenocortical cells as models and described the genomic effects of ACTH.
The steroidogenic action of ACTH in the adrenal cortex has been well studied and is believed to be mediated by cAMP and protein kinase A (PKA) via two temporal responses (Cote & Yasumura 1975; Simpson et al. 1987; Durand et al. 1987; Sewer & Waterman 2003). The acute response is mediated by the activity of the StAR protein, which leads to mobilization of cholesterol from cellular stores to the inner mitochondrial membrane where cholesterol side-chain cleavage (CYP11A1) is located (Fleury et al. 1998; Ariyoshi et al. 1998; Fleury et al. 1996; Clark & Combs 1999; Lehoux et al. 2003). Studies in fetal and adult adrenals across several species have shown that the chronic response of ACTH includes the activation of genes encoding the steroidogenic enzymes, HSD3B2, CYP21, CYP17, CYP11B1 (Neri et al. 1991; Phillips et al. 1996; Simmonds et al. 2001; Bassett et al. 2004; Schimmer et al. 2006; Carey et al. 2006; Coulter & Jaffe 1998; Di Blasio et al. 1990; Galtier et al. 1996; Lebrethon et al. 1994; Mason et al. 1993; Su et al. 2005; Su & Rose 2008; Tangalakis et al. 1990; Zhang et al. 1999). These gene targets were confirmed in the current study in AA and FA cells using microarray analysis. All the steroidogenic enzyme/proteins needed for cortisol synthesis were upregulated by ACTH treatment.
In agreement with previous studies (Le Roy et al. 2000; Rehman et al. 2007; Carey et al. 2006; Su et al. 2005; Johnston et al. 2007; Mountjoy et al. 1994), we found that the ACTH receptor-MC2R was significantly stimulated by ACTH incubation (12-fold). We also noticed a significant increase in MRAP mRNA level. As an essential component for MC2R function, MRAP was first identified by Clark and colleagues in 2004 (Metherell et al. 2004). Its mutation has been shown to be responsible for some cases of familial glucocorticoid deficiency type 2, in which there is elevated ACTH but low or absent circulating cortisol (Metherell et al. 2005; Rumie et al. 2007). By stimulating expression of both MC2R and MRAP concomitantly, ACTH forms a feed forward loop leading to amplification of responsiveness.
As mentioned above, the only other microarray study about ACTH genomic effects on adrenal function was performed using the Y-1 mouse adrenal tumor cells (Schimmer et al. 2006; Schimmer et al. 2007). In this study, Y-1 adrenal cells were treated with ACTH (20 nM) for 24 h and 588 genes were found to be significantly increased. Compared to their results, we also confirmed upregulation of steroidogenic enzyme/proteins, as well as two other transcripts, SCARB1 and INHA. When treating Y-1 mouse adrenal cells with ACTH for 24 h, Schimmer et al was able to detect a 4-fold change in SCARB1 transcript (Schimmer et al. 2006), suggesting this multifunction HDL receptor is a common ACTH target shared between humans and mice. INHA was also stimulated in FA cells and shown to increase in Y-1 cells (Schimmer et al. 2006). Although normally associated with gonadal function, INHA has been shown to influence adrenal activity. Its expression correlates with CYP17 expression in adrenocortical tissues (Hofland et al. 2006; Wang et al. 2003) and can antagonize the inhibitory effects of activin and bone morphogenetic protein on cyp17 mRNA in a mouse adrenocortical cell line (Farnworth et al. 2006).
In addition to the genes previously mentioned, RDH12 was also among the top genes upregulated by ACTH in AA cells. RDH12 is an efficient NADPH-dependent retinal reductase, displaying high activity toward 9-cis and all-trans-retinol (Haeseleer et al. 2002; Janecke et al. 2004; Maeda et al. 2006). The ability of human RDH12 to convert dihydrotestosterone to androstanediol was found in HEK293 cells with overexpression of RDH12 (Keller & Adamski 2007), however its ability to produce androgens has not been validated in adrenocortical cells. The function of these candidates in ACTH regulation of the adrenal gland needs further elucidation.
Of all the genes upregulated in human FA, one interesting finding is the GnRHR gene. In the previous study, we have shown that GnRHR is the most differentially expressed g-protein coupled receptor between AA and FA (Xing et al. 2009). In the current study, GnRHR transcript was also greatly stimulated in FA after ACTH treatment, suggesting its regulation by the ACTH, cAMP and PKA pathways. The role of GnRHR in the human FA, however, remains to be determined.
The comparison of AA and FA microarray data gave 20 common genes that are upregulated by ACTH in both cell models. Comparison of these unique models provides a series of broad ACTH target genes. Other published targets of ACTH in adrenal glands include a series of transcription factors (Sewer & Waterman 2003), bTREK-1 potassium channel (Liu et al. 2008) and genes involved in cell proliferation (Rocha et al. 2003; Lotfi et al. 2000). However, due to the chronic treatment period (48 h) used in the current study; we were not able to detect increases of these rapid response genes in our microarray analysis results.
In summary, by applying microarray approaches, we defined the genomic effects of ACTH in human adult and fetal primary cultures. The newly defined adrenal ACTH response genes can provide clues to the mechanism of ACTH regulated steroidogenesis and cell growth and may lead to further understanding of the global functions of ACTH in the adrenal gland. This knowledge may be helpful in solving and developing treatments for adrenal diseases including hyperplasia and tumors.
Acknowledgment
We would like to thank Medical College of Georgia, Adrenal Center for helping to collect adrenal samples. This work was supported by National Institute of Health grant DK069950 and DK43140 to WER, R01 HD11149 to WER and CRP and an MCG Diabetes and Obesity Discovery Institute pilot grant to ME.
Footnotes
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Reference
- 1.Cecim M, Alvarez-Sanz M, Van de Kar L, Milton S, Bartke A. Increased plasma corticosterone levels in bovine growth hormone (bGH) transgenic mice: effects of ACTH, GH and IGF-I on in vitro adrenal corticosterone production. Transgenic Res. 1996;5:187–192. doi: 10.1007/BF01969708. [DOI] [PubMed] [Google Scholar]
- 2.Gaillard I, Keramidas M, Liakos P, Vilgrain I, Feige JJ, Vittet D. ACTH-regulated expression of vascular endothelial growth factor in the adult bovine adrenal cortex: a possible role in the maintenance of the microvasculature. J Cell Physiol. 2000;185:226–234. doi: 10.1002/1097-4652(200011)185:2<226::AID-JCP7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 3.Le Roy C, Li JY, Stocco DM, Langlois D, Saez JM. Regulation by adrenocorticotropin (ACTH), angiotensin II, transforming growth factor-beta, and insulin-like growth factor I of bovine adrenal cell steroidogenic capacity and expression of ACTH receptor, steroidogenic acute regulatory protein, cytochrome P450c17, and 3beta-hydroxysteroid dehydrogenase. Endocrinology. 2000;141:1599–1607. doi: 10.1210/endo.141.5.7457. [DOI] [PubMed] [Google Scholar]
- 4.Markowska A, Rebuffat P, Rocco S, Gottardo G, Mazzocchi G, Nussdorfer GG. Evidence that an extrahypothalamic pituitary corticotropin-releasing hormone (CRH)/adrenocorticotropin (ACTH) system controls adrenal growth and secretion in rats. Cell Tissue Res. 1993;272:439–445. doi: 10.1007/BF00318550. [DOI] [PubMed] [Google Scholar]
- 5.Neri G, Andreis PG, Nussdorfer GG. Comparison of ACTH and corticotropin-releasing hormone effects on rat adrenal steroidogenesis in vitro. Res Exp Med (Berl) 1991;191:291–295. doi: 10.1007/BF02576685. [DOI] [PubMed] [Google Scholar]
- 6.Phillips ID, Ross JT, Owens JA, Young IR, McMillen IC. The peptide ACTH(1–39), adrenal growth and steroidogenesis in the sheep fetus after disconnection of the hypothalamus and pituitary. J Physiol. 1996;491(Pt 3):871–879. doi: 10.1113/jphysiol.1996.sp021264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmonds PJ, Phillips ID, Poore KR, Coghill ID, Young IR, Canny BJ. The role of the pituitary gland and ACTH in the regulation of mRNAs encoding proteins essential for adrenal steroidogenesis in the late-gestation ovine fetus. J Endocrinol. 2001;168:475–485. doi: 10.1677/joe.0.1680475. [DOI] [PubMed] [Google Scholar]
- 8.Bassett MH, Suzuki T, Sasano H, De Vries CJ, Jimenez PT, Carr BR, Rainey WE. The orphan nuclear receptor NGFIB regulates transcription of 3beta-hydroxysteroid dehydrogenase. implications for the control of adrenal functional zonation. J Biol Chem. 2004;279:37622–37630. doi: 10.1074/jbc.M405431200. [DOI] [PubMed] [Google Scholar]
- 9.Rehman KS, Sirianni R, Parker CR, Jr, Rainey WE, Carr BR. The regulation of adrenocorticotrophic hormone receptor by corticotropin-releasing hormone in human fetal adrenal definitive/transitional zone cells. Reprod Sci. 2007;14:578–587. doi: 10.1177/1933719107307908. [DOI] [PubMed] [Google Scholar]
- 10.Schimmer BP, Cordova M, Cheng H, Tsao A, Goryachev AB, Schimmer AD, Morris Q. Global profiles of gene expression induced by adrenocorticotropin in Y1 mouse adrenal cells. Endocrinology. 2006;147:2357–2367. doi: 10.1210/en.2005-1526. [DOI] [PubMed] [Google Scholar]
- 11.Cote TE, Yasumura S. Effect of ACTH and histamine stress on serum corticosterone and adrenal cyclic AMP levels in immature rats. Endocrinology. 1975;96:1044–1047. doi: 10.1210/endo-96-4-1044. [DOI] [PubMed] [Google Scholar]
- 12.Simpson ER, Mason JI, John ME, Zuber MX, Rodgers RJ, Waterman MR. Regulation of the biosynthesis of steroidogenic enzymes. J Steroid Biochem. 1987;27:801–805. doi: 10.1016/0022-4731(87)90152-x. [DOI] [PubMed] [Google Scholar]
- 13.Durand P, Cathiard AM, Naaman E, Saez JM. Adrenal adenylate cyclase and steroidogenic activities of 63 day old ovine fetuses: in vitro effects of ACTH1–24 and forskolin. Biochimie. 1987;69:629–638. doi: 10.1016/0300-9084(87)90182-9. [DOI] [PubMed] [Google Scholar]
- 14.Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech. 2003;61:300–307. doi: 10.1002/jemt.10339. [DOI] [PubMed] [Google Scholar]
- 15.Fleury A, Ducharme L, LeHoux JG. In vivo effects of adrenocorticotrophin on the expression of the hamster steroidogenic acute regulatory protein. J Mol Endocrinol. 1998;21:131–139. doi: 10.1677/jme.0.0210131. [DOI] [PubMed] [Google Scholar]
- 16.Ariyoshi N, Kim YC, Artemenko I, Bhattacharyya KK, Jefcoate CR. Characterization of the rat Star gene that encodes the predominant 3.5-kilobase pair mRNA. ACTH stimulation of adrenal steroids in vivo precedes elevation of Star mRNA and protein. J Biol Chem. 1998;273:7610–7619. doi: 10.1074/jbc.273.13.7610. [DOI] [PubMed] [Google Scholar]
- 17.Fleury A, Cloutier M, Ducharme L, Lefebvre A, LeHoux J, LeHoux JG. Adrenocorticotropin regulates the level of the steroidogenic acute regulatory (StAR) protein mRNA in hamster adrenals. Endocr Res. 1996;22:515–520. doi: 10.1080/07435809609043740. [DOI] [PubMed] [Google Scholar]
- 18.Clark BJ, Combs R. Angiotensin II and cyclic adenosine 3',5'-monophosphate induce human steroidogenic acute regulatory protein transcription through a common steroidogenic factor-1 element. Endocrinology. 1999;140:4390–4398. doi: 10.1210/endo.140.10.7085. [DOI] [PubMed] [Google Scholar]
- 19.Lehoux JG, Mathieu A, Lavigne P, Fleury A. Adrenocorticotropin regulation of steroidogenic acute regulatory protein. Microsc Res Tech. 2003;61:288–299. doi: 10.1002/jemt.10338. [DOI] [PubMed] [Google Scholar]
- 20.Carey LC, Su Y, Valego NK, Rose JC. Infusion of ACTH stimulates expression of adrenal ACTH receptor and steroidogenic acute regulatory protein mRNA in fetal sheep. Am J Physiol Endocrinol Metab. 2006;291:E214–E220. doi: 10.1152/ajpendo.00578.2005. [DOI] [PubMed] [Google Scholar]
- 21.Coulter CL, Jaffe RB. Functional maturation of the primate fetal adrenal in vivo: 3. Specific zonal localization and developmental regulation of CYP21A2 (P450c21) and CYP11B1/CYP11B2 (P450c11/aldosterone synthase) lead to integrated concept of zonal and temporal steroid biosynthesis. Endocrinology. 1998;139:5144–5150. doi: 10.1210/endo.139.12.6333. [DOI] [PubMed] [Google Scholar]
- 22.Di Blasio AM, Fujii DK, Yamamoto M, Martin MC, Jaffe RB. Maintenance of cell proliferation and steroidogenesis in cultured human fetal adrenal cells chronically exposed to adrenocorticotropic hormone: rationalization of in vitro and in vivo findings. Biol Reprod. 1990;42:683–691. doi: 10.1095/biolreprod42.4.683. [DOI] [PubMed] [Google Scholar]
- 23.Galtier A, Liakos P, Keramidas M, Feige JJ, Chambaz EM, Defaye G. ACTH angiotensin II and TGF beta participate in the regulation of steroidogenesis in bovine adrenal glomerulosa cells. Endocr Res. 1996;22:607–612. doi: 10.1080/07435809609043754. [DOI] [PubMed] [Google Scholar]
- 24.Lebrethon MC, Jaillard C, Naville D, Begeot M, Saez JM. Regulation of corticotropin and steroidogenic enzyme mRNAs in human fetal adrenal cells by corticotropin, angiotensin-II and transforming growth factor beta 1. Mol Cell Endocrinol. 1994;106:137–143. doi: 10.1016/0303-7207(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 25.Mason JI, Ushijima K, Doody KM, Nagai K, Naville D, Head JR, Milewich L, Rainey WE, Ralph MM. Regulation of expression of the 3 beta-hydroxysteroid dehydrogenases of human placenta and fetal adrenal. J Steroid Biochem Mol Biol. 1993;47:151–159. doi: 10.1016/0960-0760(93)90069-9. [DOI] [PubMed] [Google Scholar]
- 26.Su Y, Carey LC, Valego NK, Rose JC. Developmental changes in adrenocorticotrophin (ACTH)-induced expression of ACTH receptor and steroid acute regulatory protein mRNA in ovine fetal adrenal cells. J Soc Gynecol Investig. 2005;12:416–420. doi: 10.1016/j.jsgi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Su Y, Rose JC. The impact of ACTH receptor knockdown on fetal and adult ovine adrenocortical cell function. Reprod Sci. 2008;15:253–262. doi: 10.1177/1933719107310991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tangalakis K, Coghlan JP, Crawford R, Hammond VE, Wintour EM. Steroid hydroxylase gene expression in the ovine fetal adrenal gland following ACTH infusion. Acta Endocrinol (Copenh) 1990;123:371–377. doi: 10.1530/acta.0.1230371. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Hatta T, Ma L, Hashimoto R, Kihara I, Otani H. Acute in vivo effects of ACTH by exo utero microinjection on differentiation, steroidogenesis and proliferation of fetal mouse adrenocytes. Endocr Res. 1999;25:51–66. doi: 10.1080/07435809909066129. [DOI] [PubMed] [Google Scholar]
- 30.Johnston H, King PJ, O'Shaughnessy PJ. Effects of ACTH and expression of the melanocortin-2 receptor in the neonatal mouse testis. Reproduction. 2007;133:1181–1187. doi: 10.1530/REP-06-0359. [DOI] [PubMed] [Google Scholar]
- 31.Mountjoy KG, Bird IM, Rainey WE, Cone RD. ACTH induces up-regulation of ACTH receptor mRNA in mouse and human adrenocortical cell lines. Mol Cell Endocrinol. 1994;99:R17–R20. doi: 10.1016/0303-7207(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 32.Metherell LA, Cooray S, Huebner A, Ruschendorf F, Naville D, Begeot M, Clark AJ. Mutations in a novel gene, encoding a single transmembrane domain protein are associated with familial glucocorticoid deficiency type 2. Endocr Res. 2004;30:889–890. doi: 10.1081/erc-200044136. [DOI] [PubMed] [Google Scholar]
- 33.Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, Huebner A, Cheetham ME, Clark AJ. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 34.Rumie H, Metherell LA, Clark AJ, Beauloye V, Maes M. Clinical and biological phenotype of a patient with familial glucocorticoid deficiency type 2 caused by a mutation of melanocortin 2 receptor accessory protein. Eur J Endocrinol. 2007;157:539–542. doi: 10.1530/EJE-07-0242. [DOI] [PubMed] [Google Scholar]
- 35.Schimmer BP, Cordova M, Cheng H, Tsao A, Morris Q. A genome-wide assessment of adrenocorticotropin action in the Y1 mouse adrenal tumor cell line. Mol Cell Endocrinol. 2007;265–266:102–107. doi: 10.1016/j.mce.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Hofland J, Timmerman MA, de Herder WW, van Schaik RH, de Krijger RR, de Jong FH. Expression of activin and inhibin subunits, receptors and binding proteins in human adrenocortical neoplasms. Clin Endocrinol (Oxf) 2006;65:792–799. doi: 10.1111/j.1365-2265.2006.02668.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang EY, Ma EY, Woodruff TK. Activin signal transduction in the fetal rat adrenal gland and in human H295R cells. J Endocrinol. 2003;178:137–148. doi: 10.1677/joe.0.1780137. [DOI] [PubMed] [Google Scholar]
- 38.Farnworth PG, Stanton PG, Wang Y, Escalona R, Findlay JK, Ooi GT. Inhibins differentially antagonize activin and bone morphogenetic protein action in a mouse adrenocortical cell line. Endocrinology. 2006;147:3462–3471. doi: 10.1210/en.2006-0023. [DOI] [PubMed] [Google Scholar]
- 39.Haeseleer F, Jang GF, Imanishi Y, Driessen CA, Matsumura M, Nelson PS, Palczewski K. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem. 2002;277:45537–45546. doi: 10.1074/jbc.M208882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janecke AR, Thompson DA, Utermann G, Becker C, Hubner CA, Schmid E, McHenry CL, Nair AR, Ruschendorf F, Heckenlively J, Wissinger B, Nurnberg P, Gal A. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36:850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- 41.Maeda A, Maeda T, Imanishi Y, Sun W, Jastrzebska B, Hatala DA, Winkens HJ, Hofmann KP, Janssen JJ, Baehr W, Driessen CA, Palczewski K. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller B, Adamski J. RDH12, a retinol dehydrogenase causing Leber's congenital amaurosis, is also involved in steroid metabolism. J Steroid Biochem Mol Biol. 2007;104:190–194. doi: 10.1016/j.jsbmb.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Xing Y, Nakamura Y, Rainey WE. G protein-coupled receptor expression in the adult and fetal adrenal glands. Mol Cell Endocrinol. 2009;300:43–50. doi: 10.1016/j.mce.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Enyeart JA, Enyeart JJ. ACTH inhibits bTREK-1 K+ channels through multiple cAMP-dependent signaling pathways. J Gen Physiol. 2008;132:279–294. doi: 10.1085/jgp.200810003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocha KM, Forti FL, Lepique AP, Armelin HA. Deconstructing the molecular mechanisms of cell cycle control in a mouse adrenocortical cell line: roles of ACTH. Microsc Res Tech. 2003;61:268–274. doi: 10.1002/jemt.10336. [DOI] [PubMed] [Google Scholar]
- 46.Lotfi CF, Lepique AP, Forti FL, Schwindt TT, Eichler CB, Santos MO, Rebustini IT, Hajj GN, Juliano L, Armelin HA. Proliferative signaling initiated in ACTH receptors. Braz J Med Biol Res. 2000;33:1133–1140. doi: 10.1590/s0100-879x2000001000002. [DOI] [PubMed] [Google Scholar]