Abstract
Objectives
The purpose of this study was to quantify the extent and complexity of residual coronary stenoses following percutaneous coronary intervention (PCI) and to evaluate its impact on adverse ischemic outcomes.
Background
Incomplete revascularization (IR) after PCI is common, and most studies have suggested that IR is associated with a worse prognosis compared with complete revascularization (CR). However, formal quantification of the extent and complexity of residual atherosclerosis after PCI has not been performed.
Methods
The baseline Synergy Between PCI With Taxus and Cardiac Surgery (SYNTAX) score (bSS) from 2,686 angiograms from patients with moderate- and high-risk acute coronary syndrome (ACS) undergoing PCI enrolled in the prospective ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial was determined. The SS after PCI was also assessed, generating the “residual” SS (rSS). Patients with rSS >0 were defined as having IR and were stratified by rSS tertiles, and their outcomes were compared to the CR group.
Results
The bSS was 12.8 ± 6.7, and after PCI the rSS was 5.6 ± 2.2. Following PCI, 1,084 patients (40.4%) had rSS = 0 (CR), 523 (19.5%) had rSS >0 but ≤2, 578 (21.5%) had rSS >2 but ≤8, and 501 patients (18.7%) had rSS >8. Age, insulin-treated diabetes, hypertension, smoking, elevated biomarkers or ST-segment deviation, and lower ejection fraction were more frequent in patients with IR compared with CR. The 30-day and 1-year rates of ischemic events were significantly higher in the IR group compared with the CR group, especially those with high rSS. By multivariable analysis, rSS was a strong independent predictor of all ischemic outcomes at 1 year, including all-cause mortality (hazard ratio: 1.05, 95% confidence interval: 1.02 to 1.09, p = 0.006).
Conclusions
The rSS is useful to quantify and risk-stratify the degree and complexity of residual stenosis after PCI. Specifically, rSS >8.0 after PCI in patients with moderate- and high-risk ACS is associated with a poor 30-day and 1-year prognosis.
Keywords: ACS, incomplete revascularization, PCI, SYNTAX score
Achieving complete revascularization (CR) is intuitively desirable in patients with coronary artery disease undergoing revascularization. However, despite major advances in percutaneous coronary intervention (PCI), CR is often not obtained. The prognostic impact of incomplete revascularization (IR) after PCI has been inconsistent between studies (1–6). One possible reason for these conflicting reports is that there is no universally accepted definition for IR, and the extent, severity, and nature of residual coronary stenoses after PCI may have varying effects on patient outcomes. Indeed, definitions of IR in prior studies have varied according to the degree of coronary stenosis severity (e.g., ≥50% vs. ≥70%) or vessel size diameter (e.g., ≥ 1.5 to ≥2.5 mm) required to be treated (3,4,7,8). Systematic characterization and quantification of residual atherosclerosis after PCI may therefore be important to standardize and improve the prognostic utility of IR. To our knowledge this issue has not been previously addressed.
Since its first description (9), the Synergy Between PCI With Taxus and Cardiac Surgery (SYNTAX) score (SS), a quantitative and reproducible measure of baseline (pre-revascularization) coronary anatomic severity and complexity, has been shown to offer independent prognostic utility in patients undergoing PCI (10–19). We therefore hypothesized that the “residual” SS (rSS) after PCI would provide independent prognostic utility as a quantitative measure of IR. We therefore sought to evaluate the predictive value of IR according to the rSS following PCI in the multicenter, prospective randomized ACUITY (Acute Catheterization and Urgent Intervention Triage StrategY) trial.
Methods
Study protocol
The ACUITY trial design has previously been reported in detail (20). Briefly, the ACUITY trial was a multicenter, prospective, randomized trial of patients with moderate- and high-risk acute coronary syndrome (ACS) who were managed with an early invasive strategy. Patients were randomly assigned before coronary angiography to heparin (unfractionated or low molecular weight) plus a glycoprotein IIb/IIIa inhibitor, bivalirudin plus a glycoprotein IIb/IIIa inhibitor, or bivalirudin monotherapy with provisional glycoprotein IIb/IIIa inhibitor use. Angiography was performed within 72 h of randomization. Depending on coronary anatomy, patients were then treated with PCI, coronary artery bypass graft (CABG) surgery, or medical therapy. In patients undergoing PCI, the choice of either bare-metal or drug-eluting stents was per operator discretion. Dual antiplatelet therapy with aspirin and clopidogrel was strongly recommended for at least 1 year. All major adverse events were adjudicated by an independent clinical events committee blinded to treatment assignment and procedural outcomes.
Objectives, patient population, and angiographic analysis
Our primary objective was to quantify the extent and complexity of residual coronary stenoses following PCI by the rSS, and to evaluate its impact on adverse ischemic outcomes, including the 30-day and 1-year rates of all-cause death, cardiac death, myocardial infarction (MI), and unplanned repeat revascularization for ischemia. We included only the subgroup of PCI patients in whom core laboratory-based quantitative coronary angiography was performed blinded to treatment assignment and clinical outcomes in the formal angiographic substudy of the ACUITY trial (21). As the SS score has been validated only for patients with native coronary artery disease, patients with a history of CABG were excluded.
For the present study, the baseline SS (bSS) was assessed visually by 3 experienced interventional cardiologists, trained for SS assessment, and blinded to treatment assignment and clinical outcomes as previously described (22). Each lesion with >50% diameter stenosis in vessels >1.5 mm in diameter was scored using the SS algorithm fully described elsewhere (9). All data were entered into a dedicated computerized database. Interobserver reproducibility between the 3 readers was determined prior to the beginning of this study (50 angiograms) and was at least substantial (kappa 0.76, 95% confidence interval [CI]: 0.64 to 1.00). Intraobserver reproducibility for the SS was at least moderate for all 3 readers (kappa 0.88, 95% CI: 0.60 to1.00; kappa 0.64, 95% CI: 0.43 to 0.86; kappa 0.66, 95% CI: 0.44 to 0.89, respectively) (22). The rSS was determined as the SS remaining after completion of PCI. In the case of staged PCI procedures, (defined as a second planned PCI procedure after the initial intervention) the final planned procedure was used as the entry point for this study.
Endpoints, definitions, and statistical analysis
CR was defined as a post-PCI rSS = 0. Patients with IR (rSS ≥1) were grouped in tertiles of rSS for analysis and compared to those with CR. Composite of major adverse cardiovascular events (MACE) was defined as death from any cause, MI, or unplanned revascularization for ischemia. The components of the MACE endpoint have been previously described (20).
Continuous data are presented as mean ± SD and were compared using the Student t test or the Mann-Whitney rank sum test, as appropriate. Categorical variables were compared by the chi-square or the Fisher exact test. Thirty-day and 1-year outcomes were determined using Kaplan-Meier methodology and compared using the log-rank test. To assess the association between rSS and 1-year rates of all-cause mortality, cardiac mortality, MI, unplanned revascularization for ischemia, and MACE, stepwise Cox multivariable regression analyses were performed, with variable entry/stay criteria of 0.1/0.1. In addition to the rSS, variables historically known to be associated with these adverse events were included in the models, with the number carefully chosen to avoid overfitting. The proportional hazards assumption was verified for each endpoint using the supremum test. Receiver-operating characteristic (ROC) curves for both bSS and rSS were performed and compared using the nonparametric correlated ROC curves compassion method (23) to assess the relative predictive accuracy for the 1-year ischemic endpoints. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina). A p value <0.05 was considered statistically significant.
Results
Patients and baseline characteristics
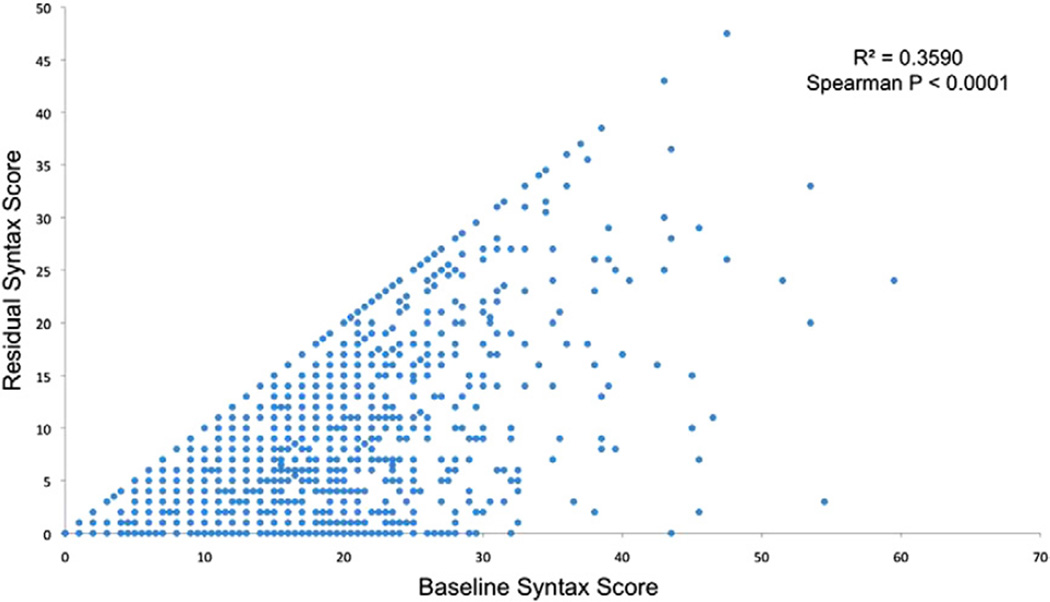
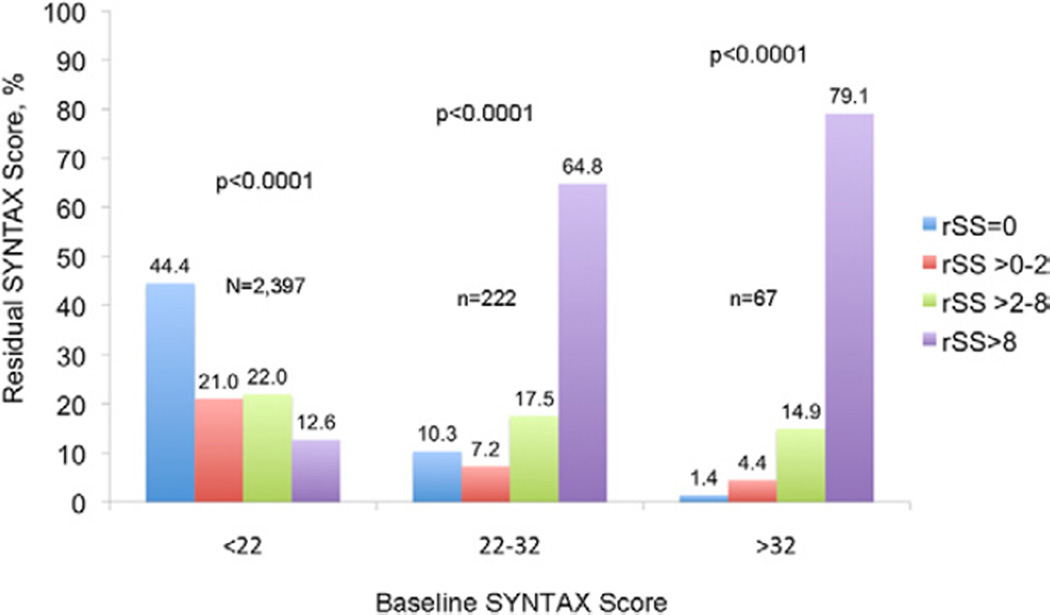
Among the 6,921 patients enrolled in the ACUITY trial angiographic substudy, 3,826 patients underwent PCI. From this group, 1,140 patients were excluded from the present study because of previous CABG surgery (n = 862) or inability to calculate the bSS or rSS for technical reasons (n = 278). Thus, paired bSS and rSS were available in 2,686 patients. The mean bSS was 12.8 ± 6.7 before PCI, ranging from 0 to 59.5. Following PCI, the mean rSS was 5.6 ± 2.2, ranging from 0 to 47.5. CR (rSS = 0) was achieved in 1,084 patients (40.4%). Among patients with IR, by tertile grouping 523 patients (19.5%) had rSS >0 but ≤2 (mean 1.5 ± 0.5), 578 (21.5%) had rSS >2 but ≤8 (mean 5.2 ± 1.6), and 501 patients (18.7%) had rSS >8 (mean 15.8 ± 6.5). A strong correlation was present between bSS and rSS, although for any level of bSS, the range of post-PCI rSS varied considerably (Fig. 1). Figure 2 shows the level of completeness of revascularization stratified by the rSS according to original bSS tertiles.
Figure 1. Correlation Between bSS and rSS.
Relationship between the baseline SYNTAX score (bSS) (x-axis) and the residual SYNTAX score (rSS) (y-axis) after percutaneous coronary intervention in 2,686 patients. Each point may represent more than 1 value. A strong correlation was present between bSS and rSS, although for any level of bSS, the range of post-PCI rSS varied considerably. SYNTAX = Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery.
Figure 2. Completeness of Revascularization Stratified by rSS According to bSS.
Distribution of level of completeness of revascularization stratified by rSS score according to bSS score. Complete revascularization was infrequent in the 2 upper tertiles of the bSS. Abbreviations as in Figure 1.
Clinical and angiographic characteristics of patients stratified by rSS are shown in Table 1. Compared with patients with CR, patients with IR were older, more likely to have insulin-treated diabetes, hypertension, baseline elevated biomarkers or ST-segment deviation, lower left ventricle ejection fraction, and higher Thrombolysis In Myocardial Infarction risk score. Patients with IR were also more likely to have more complex coronary disease, with higher SS at baseline, with more triple-vessel disease, longer lesions, more calcified lesions, and more thrombotic lesions. Thienopyridines were less frequently used in the upper rSS group at discharge and 30-day follow-up, but not at 1 year (Table 2).
Table 1.
Baseline Characteristics According to rSS
| rSS = 0 (n = 1,084) |
rSS >0–2 (n = 523) |
rSS >2–8 (n = 578) |
rSS >8 (n = 501) |
p Value All Groups |
|
|---|---|---|---|---|---|
| Age, yrs | 59 (51–68) | 59 (51–69) | 61 (53–69) | 63 (55–72) | <0.001 |
| Male | 66.6% (722/1,084) | 64.6% (338/523) | 70.4% (407/578) | 68.7% (344/501) | 0.17 |
| Weight, kg | 88.0 (75.0–101.9) | 85.0 (75.0–98.0) | 88.0 (77.0–101.0) | 85.0 (75.0–98.0) | 0.13 |
| Diabetes | 25.0% (270/1,079) | 28.9% (151/522) | 31.3% (179/572) | 34.1% (170/499) | <0.01 0.02 |
| Insulin-treated | 6.4% (69/1,079) | 9.4% (49/522) | 7.9% (45/572) | 10.6% (53/499) | <0.01 0.02 |
| Hypertension | 62.0% (669/1079) | 64.0% (333/520) | 70.9% (409/577) | 70.3% (352/501) | <0.001 |
| Hyperlipidemia | 54.0% (576/1,067) | 56.1% (289/515) | 57.9% (329/568) | 60.4% (300/497) | 0.09 |
| Current smoker | 35.8% (387/1,081) | 37.9% (198/522) | 35.0% (201/575) | 31.1% (155/499) | 0.13 |
| Previous MI | 25.7% (273/1,063) | 28.7% (147/513) | 32.1% (181/564) | 34.3% (169/492) | <0.01 |
| Previous PTCA | 44.0% (477/1,083) | 45.5% (237/521) | 46.0% (266/578) | 41.1% (205/499) | 0.37 |
| Renal insufficiency* | 14.0% (143/1,019) | 14.9% (73/491) | 16.1% (87/540) | 18.8% (86/457) | 0.12 |
| Baseline cardiac biomarker elevation | 57.5% (583/1,014) | 57.7% (280/485) | 58.9% (313/531) | 68.5% (315/460) | <0.001 |
| Baseline troponin elevation | 59.8% (553/925) | 57.5% (263/457) | 58.1% (289/497) | 67.5% (295/437) | <0.01 |
| ST-segment deviation >1 mm | 25.3% (274/1,084) | 22.9% (120/523) | 26.1% (151/578) | 27.3% (137/501) | 0.41 |
| Baseline cardiac biomarker elevation or ST-segment deviation | 67.0% (692/1,033) | 66.6% (329/494) | 68.1% (368/540) | 75.9% (356/469) | <0.01 |
| TIMI risk score | |||||
| Low (0–2) | 18.1% (157/866) | 17.8% (77/432) | 14.5% (70/484) | 11.6% (47/404) | 0.01 |
| Intermediate (3–4) | 61.0% (528/866) | 61.3% (265/432) | 56.4% (273/484) | 55.4% (224/404) | 0.12 |
| High (5–7) | 20.9% (181/866) | 20.8% (90/432) | 29.1% (141/484) | 32.9% (133/404) | <0.001 |
| Ejection fraction, % | 66.70 (59.00–73.50) | 67.50 (59.30–73.80) | 66.40 (58.90–72.50) | 62.30 (53.10–71.80) | <0.001 |
| Ejection fraction <35% | 0.6% (4/718) | 0.9% (3/335) | 1.1% (4/356) | 4.0% (13/325) | <0.001 |
| Number with 2-vessel CAD | 40.6% (440/1,084) | 41.9% (219/523) | 36.6% (209/571) | 27.4% (135/493) | <0.001 |
| Number with 3-vessel CAD | 25.2% (273/1,084) | 35.8% (187/523) | 51.5% (294/571) | 64.5% (318/493) | <0.001 |
| Lesions per patient | 3.01 ± 1.79 (1,083) | 3.46 ± 1.83 (523) | 4.22 ± 2.06 (568) | 5.08 ± 2.43 (493) | <0.001 |
| PCI vessel | |||||
| LAD | 41.7% (602/1,444) | 39.2% (291/743) | 36.9% (267/724) | 21.0% (111/529) | <0.001 |
| RCA | 31.7% (458/1,444) | 32.8% (244/743) | 36.2% (262/724) | 40.3% (213/529) | <0.001 |
| LCX | 26.6% (384/1,444) | 28.0% (208/743) | 26.9% (195/724) | 38.8% (205/529) | <0.001 |
| Lesion severity | |||||
| MACC B1 | 31.5% (455/1,444) | 28.3% (210/743) | 30.0% (217/724) | 27.2% (144/529) | 0.20 |
| MACC B2 | 22.0% (318/1,444) | 20.9% (155/743) | 21.8% (158/724) | 18.1% (96/529) | 0.28 |
| MACC C | 34.7% (501/1,444) | 36.2% (269/743) | 38.7% (280/724) | 46.7% (247/529) | <0.001 |
| Lesion length >20 mm | 18.3% (259/1,417) | 19.2% (140/731) | 22.3% (156/701) | 26.8% (134/500) | <0.001 |
| Moderate/severe tortuosity | 2.3% (33/1,442) | 1.9% (14/741) | 3.5% (25/719) | 1.9% (10/528) | 0.16 |
| Severe calcification | 3.1% (44/1,413) | 1.4% (10/726) | 2.9% (20/700) | 5.2% (27/523) | <0.01 |
| Thrombus | 1.4% (20/1,444) | 1.7% (13/751) | 5.1% (40/778) | 9.9% (62/624) | <0.001 |
| Ulceration | 3.4% (49/1,439) | 5.1% (38/740) | 4.9% (35/720) | 5.9% (31/526) | 0.06 |
| Aneurysm | 1.3% (18/1,440) | 0.3% (2/741) | 1.5% (11/720) | 1.0% (5/526) | 0.08 |
| Ectasia | 5.0% (72/1,440) | 4.5% (33/741) | 4.7% (34/720) | 8.4% (44/525) | <0.01 |
| Drug-eluting stent | 86.9% (942/1,084) | 84.6% (439/519) | 86.8% (485/559) | 78.5% (376/479) | <0.001 |
| Bare-metal stent | 12.0% (130/1,084) | 15.0% (78/519) | 15.7% (88/559) | 15.7% (75/479) | 0.08 |
| Baseline SYNTAX score | 7.5 ± 5.6 | 9.3 ± 6.1 | 12.6 ± 6.9 | 21.7 ± 8.6 | <0.001 |
| Residual SYNTAX score | 0 | 1.5 ± 0.5 | 5.2 ± 1.6 | 15.8 ± 6.5 | <0.001 |
| Delta† SYNTAX score | 7.3 ± 5.4 | 7.5 ± 6.1 | 6.9 ± 6.3 | 5.7 ± 6.4 | 0.15 |
Values are median (IQR), % (n/N), or mean ± SD (N).
Renal insufficiency is defined as a calculated creatinine clearance rate <60 ml/min determined by the Cockcroft-Gault equation.
Delta SYNTAX score represents the burden of disease removed by PCI.
CAD = coronary artery disease; IQR = interquartile range; LAD = left anterior descending artery; LCX = left circumflex artery; MACC = Modified American College of Cardiology/American Heart Association stenosis morphology classification; MI = myocardial infarction; PTCA = percutaneous transluminal coronary angioplasty; RCA = right coronary artery; rSS = residual Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery score; SYNTAX= Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery; TIMI = Thrombolysis In Myocardial Infarction.
Table 2.
Medical Therapies According to the rSS
| rSS = 0 (n = 1,084) |
rSS >0–2 (n = 523) |
rSS >2–8 (n = 578) |
rSS >8 (n = 501) |
p Value All Groups |
|
|---|---|---|---|---|---|
| Antiplatelet medications (pre-PCI) | |||||
| Aspirin | 98.6% (1,067/1,082) | 98.1% (511/521) | 98.6% (563/571) | 98.0% (486/496) | 0.72 |
| Thienopyridine | 57.1% (617/1,081) | 59.1% (306/518) | 56.5% (322/570) | 57.4% (284/495) | 0.84 |
| Antithrombin medications | |||||
| Bivalirudin | 67.8% (735/1,084) | 63.7% (333/523) | 64.9% (375/578) | 62.7% (314/501) | 0.15 |
| Unfractionated heparin | 19.6% (213/1,084) | 17.6% (92/523) | 18.5% (107/578) | 21.8% (109/501) | 0.36 |
| Enoxaparin | 12.0% (130/1,084) | 16.8% (88/523) | 12.8% (74/578) | 13.4% (67/501) | 0.06 |
| Glycoprotein IIb/IIIa inhibitor used | 67.1% (727/1,084) | 68.1% (356/523) | 64.4% (372/578) | 65.3% (327/501) | 0.52 |
| Discharge | |||||
| Aspirin | 89.2% (955/1,071) | 86.1% (445/517) | 89.2% (511/573) | 87.4% (430/492) | 0.25 |
| Thienopyridines | 88.0% (943/1,071) | 82.8% (428/517) | 83.1% (476/573) | 77.2% (380/492) | <0.001 |
| 30 days | |||||
| Aspirin | 97.1% (1,028/1,059) | 98.0% (492/502) | 96.4% (540/560) | 96.3% (463/481) | 0.35 |
| Thienopyridines | 94.1% (996/1,059) | 95.2% (478/502) | 92.1% (516/560) | 89.2% (429/481) | <0.001 |
| Statins | 84.4% (894/1,059) | 82.9% (416/502) | 81.1% (454/560) | 83.5% (401/480) | 0.38 |
| Beta-blockers | 77.3% (819/1,059) | 77.3% (388/502) | 77.5% (434/560) | 79.0% (379/480) | 0.90 |
| ACE inhibitors | 52.3% (554/1,059) | 53.0% (266/502) | 57.4% (321/559) | 61.3% (294/480) | <0.001 |
| 1 year | |||||
| Aspirin | 91.5% (946/1,034) | 90.5% (438/484) | 91.4% (502/549) | 91.1% (418/459) | 0.92 |
| Thienopyridines | 67.5% (697/1,033) | 67.4% (326/484) | 69.0% (379/549) | 66.4% (305/459) | 0.84 |
| Statins | 79.4% (821/1,034) | 77.1% (373/484) | 76.1% (418/549) | 81.5% (374/459) | 0.15 |
| Beta-blockers | 68.6% (709/1,034) | 68.8% (333/484) | 71.0% (390/549) | 70.6% (324/459) | 0.70 |
| ACE inhibitors | 50.6% (523/1,034) | 50.6% (245/484) | 50.1% (275/549) | 55.3% (254/459) | 0.30 |
Values are % (n/N).
ACE = angiotensin-converting enzyme; PCI = percutaneous coronary intervention; rSS = residual Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery score.
SYNTAX score components in the IR groups
Table 3 shows the distribution of the SS variables present in each tertile among patients with IR. The highest rSS tertile was significantly more likely to contain nonrevascularized lesions that were severely calcified, chronic total occlusions; bifurcation/trifurcations; and >20 mm in length. Small vessel and diffuse disease was common in all 3 tertiles of IR patients.
Table 3.
rSS Components in Patients With Incomplete Revascularization According to Tertile
| rSS >0–2 (n = 523) |
rSS >2–8 (n = 578) |
rSS >8 (n = 501) |
p Value All Groups |
|
|---|---|---|---|---|
| Severe calcification | 0 (0%) | 10 (1.7%) | 59 (11.8%) | <0.001 |
| Chronic total occlusion | 1 (0.2%) | 58 (10.0%) | 216 (43.1%) | <0.001 |
| Bifurcation/trifurcation | 0 (0%) | 179 (30.9%) | 287 (57.3%) | <0.001 |
| Aorto-ostial lesion | 1 (0.2%) | 4 (0.7%) | 14 (0.3%) | <0.001 |
| Lesion length >20 mm | 3 (0.6%) | 143 (24.7%) | 351 (70.1%) | <0.001 |
| Small vessel/diffuse disease* | 409 (78.2%) | 303 (52.4%) | 264 (52.7%) | <0.001 |
Values are n (%).
Presence of at least 1 segment in the nonrevascularized vessel described as small vessel/diffuse disease.
rSS = residual Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery score.
Clinical outcomes
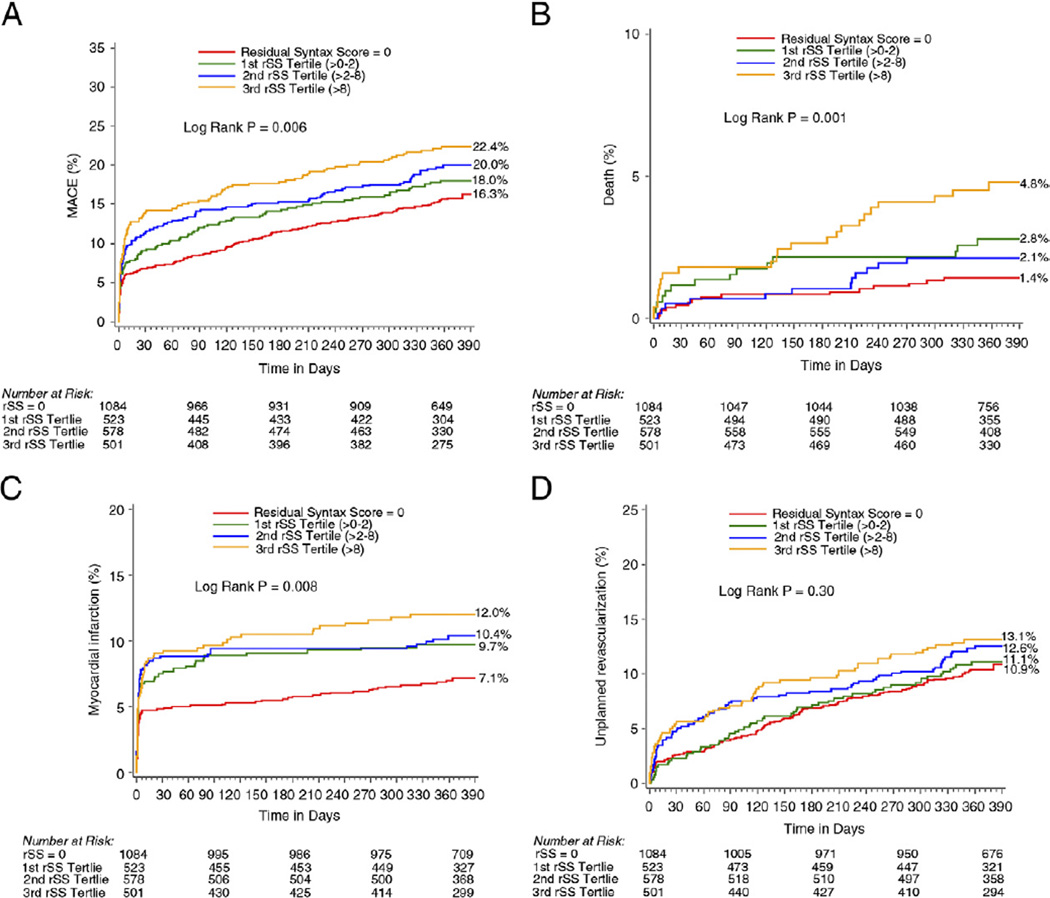
The 30-day and 1-year rates of all ischemic events were significantly higher in the IR compared with CR groups and across all the rSS tertiles (Table 4, Fig. 3). By multivariable analysis, rSS was a strong independent predictor of all ischemic outcomes at 1 year, including all-cause mortality (Table 5).
Table 4.
30-Day and 1-Year Outcomes According to the rSS
| rSS = 0 (a) | rSS >0–2 (b) | rSS >2–8 (c) | rSS >8 (d) | p Value a vs. b |
p Value a vs. c |
p Value a vs. d |
p Value b vs. c |
p Value b vs. d |
p Value c vs. d |
p Value All Groups |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30-day outcomes | |||||||||||
| MACE | 6.9% (75) | 9.2% (48) | 11.8% (68) | 14.2% (71) | 0.11 | <0.001 | <0.001 | 0.16 | 0.01 | 0.24 | <0.001 |
| Death, all-cause | 0.5% (5) | 1.2% (6) | 0.5% (3) | 1.8% (9) | 0.11 | 0.87 | 0.008 | 0.24 | 0.38 | 0.04 | 0.03 |
| Cardiac | 0.3% (3) | 1.2% (6) | 0.5% (3) | 1.6% (8) | 0.03 | 0.43 | 0.003 | 0.24 | 0.53 | 0.07 | 0.02 |
| Myocardial infarction | 4.9% (53) | 7.7% (40) | 8.8% (51) | 9.2% (46) | 0.02 | 0.001 | 0.001 | 0.48 | 0.37 | 0.85 | 0.002 |
| Definite/probable stent thrombosis | 0.8% (8) | 1.2% (6) | 0.8% (3) | 2.0% (9) | 0.42 | 0.93 | 0.03 | 0.59 | 0.29 | 0.05 | 0.18 |
| Death or myocardial infarction | 5.3% (57) | 8.2% (43) | 9.0% (52) | 10.6% (53) | 0.02 | 0.003 | <0.001 | 0.64 | 0.20 | 0.40 | 0.001 |
| Unplanned revascularization for ischemia | 2.7% (29) | 2.3% (12) | 5.2% (30) | 5.7% (28) | 0.65 | 0.008 | 0.003 | 0.01 | 0.006 | 0.73 | 0.002 |
| 1-year outcomes | |||||||||||
| MACE | 16.3% (167) | 18.0% (92) | 20.0% (113) | 22.4% (110) | 0.22 | 0.01 | <0.001 | 0.39 | 0.07 | 0.31 | 0.006 |
| Death, all-cause | 1.4% (15) | 2.8% (14) | 2.1% (12) | 4.8% (23) | 0.06 | 0.28 | <0.001 | 0.49 | 0.10 | 0.02 | 0.001 |
| Cardiac | 0.4% (4) | 2.2% (11) | 1.2% (7) | 2.6% (13) | 0.001 | 0.04 | <0.001 | 0.23 | 0.60 | 0.09 | <0.001 |
| Myocardial infarction | 7.1% (75) | 9.7% (50) | 10.4% (59) | 12.0% (59) | 0.06 | 0.02 | 0.001 | 0.71 | 0.25 | 0.41 | 0.007 |
| Definite/probable stent thrombosis | 1.0% (11) | 1.6% (8) | 1.6% (6) | 2.5% (11) | 0.37 | 0.38 | 0.03 | 0.96 | 0.32 | 0.40 | 0.23 |
| Death or myocardial infarction | 8.1% (86) | 11.1% (57) | 11.8% (67) | 15.0% (74) | 0.04 | <0.001 | <0.001 | 0.71 | 0.07 | 0.13 | <0.001 |
| Unplanned revascularization for ischemia | 10.9% (109) | 11.1% (55) | 12.6% (70) | 13.1% (63) | 0.71 | 0.18 | 0.09 | 0.42 | 0.25 | 0.71 | 0.32 |
Event rates are Kaplan-Meier estimates, % (n).
MACE = major adverse cardiovascular event(s); rSS = residual Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery score.
Figure 3. Kaplan-Meier Curves Showing Cumulative Event Rates Through 1 Year.
(A) MACE, (B) death, (C) myocardial infarction, and (D) unplanned revascularization, stratified by tertiles of rSS score. Adverse ischemic events were significantly higher in incomplete revascularization compared with complete revascularization groups and across all the rSS tertiles. Abbreviations as in Figure 1.
Table 5.
Independent Predictors of 1-Year Ischemic Outcomes
| Hazard Ratio (95% Confidence Interval) |
p Value | |
|---|---|---|
| All-cause death | ||
| rSS* | 1.05 (1.03–1.08) | <0.001 |
| Age (per 10-year increase) | 1.44 (1.10–1.89) | 0.008 |
| Insulin-treated diabetes | 3.66 (2.04–6.57) | <0.0001 |
| Cardiac death | ||
| rSS* | 1.06 (1.03–1.10) | <0.001 |
| Insulin-treated diabetes | 4.89 (2.39–10.00) | <0.0001 |
| Renal dysfunction | 2.23 (1.09–4.55) | 0.03 |
| Myocardial infarction | ||
| rSS* | 1.02 (1.01–1.04) | 0.003 |
| Insulin-treated diabetes | 1.41 (0.94–2.10) | 0.09 |
| Unplanned revascularization for ischemia | ||
| rSS* | 1.04 (1.02–1.05) | <0.001 |
| Age (10-year increase) | 0.86 (0.78–0.96) | 0.005 |
| Insulin-treated diabetes | 1.49 (1.02–2.17) | 0.04 |
| Baseline cardiac biomarker elevation or ST-segment deviation | 0.68 (0.53–0.86) | 0.002 |
The following variables were included in each model: 1) for all-cause death: rSS (continuous variable), age, diabetes, renal dysfunction, baseline troponin elevation, ST-segment deviation, baseline white blood count; 2) for cardiac death: rSS (continuous variable), age, diabetes, and renal dysfunction; 3) for myocardial infarction and unplanned revascularization: rSS (continuous variable), age, male, diabetes, current smoker, renal dysfunction, baseline troponin elevation, ST-segment deviation, prior myocardial infarction, prior percutaneous coronary intervention, baseline white blood count, baseline hemoglobin, and type of stent (drug-eluting stent vs. bare-metal stent).
Per SS point.
rSS = residual Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery score.
ROC curves analysis
ROC curve analysis demonstrated a significant association between the rSS and 1-year all-cause mortality, cardiac mortality, MI, unplanned revascularization for ischemia, and MACE. An rSS cutoff of 5 had the best prognostic accuracy for risk prediction of death and cardiac death (Table 6). When compared with the bSS, the rSS had a similar predictive value and discrimination for all-cause mortality (bSS AUC: 0.63, 95% CI: 0.55 to 0.70 vs. rSS AUC: 0.63, 95% CI: 0.56 to 0.70; p = 0.92), unplanned revascularization for ischemia (bSS AUC: 0.56, 95% CI: 0.52 to 0.59 vs. rSS AUC: 0.53, 95% CI: 0.50 to 0.57; p = 0.18), and MACE (bSS AUC: 0.57, 95% CI: 0.54 to 0.60 vs. rSS AUC: 0.55, 95% CI: 0.53 to 0.58; p = 0.16) at 1 year, whereas the bSS was a slightly stronger predictor of MI (bSS AUC: 0.60, 95% CI: 0.57 to 0.64 vs. rSS AUC: 0.57, 95% CI: 0.53 to 0.60; p = 0.03).
Table 6.
Receiver-Operating Characteristic Curve Analysis of rSS and 1-Year Ischemic Outcomes
| AUC | p Value | Optimal Cutoff | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Death, all-cause | 0.63 | <0.001 | 5 | 52 | 69 |
| Death, cardiac | 0.68 | <0.001 | 5 | 57 | 68 |
| Myocardial infarction | 0.57 | <0.001 | 2 | 59 | 52 |
| Unplanned revascularization for ischemia | 0.53 | 0.05 | 4 | 42 | 65 |
| Major adverse cardiovascular events | 0.55 | <0.001 | 2 | 56 | 52 |
AUC = area under the curve; rSS = residual Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery score.
Completeness of revascularization by bSS
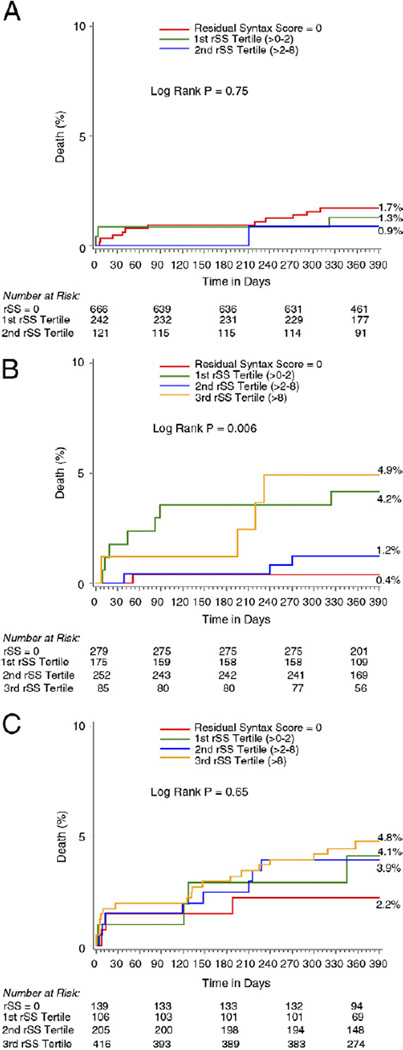
Figure 4 shows the impact of CR versus IR on the 1-year rate of death according to the bSS tertiles of our cohort. In the lower risk tertile (bSS <7), there were no significant differences in the rates of 1-year mortality according to the level of completeness of revascularization (Fig. 4A). However, in the intermediate- (bSS 7 to 13) and high-risk tertile (bSS >13), IR with rSS >8 was associated with greater mortality than CR (Figs. 4B and 4C).
Figure 4. Kaplan-Meier Curves Showing Cumulative Death Rate Through 1 Year.
One-year cumulative death rate for each level of completeness of revascularization stratified by true tertiles of baseline SS. Low risk, 0 to 7 (A); intermediate risk, >7 to 13 (B); and high risk, >13 (C). In the intermediate- and high-risk tertiles, incomplete revascularization with rSS >8 was associated with greater mortality than complete revascularization. Abbreviations as in Figure 1.
Discussion
The current report, drawn from a large cohort of 2,686 patients with moderate- and high-risk ACS, is the first study to quantify the extent and nature of coronary artery disease prior to and following revascularization with PCI. The major results of the present study are as follows: 1) the rSS is a novel instrument to quantify the extent and complexity of atherosclerosis in patients with IR after PCI, and is a strong independent predictor of 1-year mortality, cardiac mortality, MI, unplanned revascularization for ischemia, and MACE; 2) compared with the bSS, rSS had similar prognostic accuracy for risk prediction of 1-year mortality, unplanned revascularization for ischemia, and MACE, but slightly less discrimination for risk prediction of 1-year MI; and 3) despite a strong correlation being present between the bSS and the rSS after PCI, the degree of IR varied greatly for any baseline level of disease, which has important prognostic implications.
Several studies have attempted to compare outcomes between CR versus IR after PCI (1–5,7,24–27). However, the retrospective nature of those studies, the small number of patients enrolled, the lack of consensus on CR definition, numerous statistical and methodological issues, and significant differences in patient populations studied has led to conflicting results as to the prognostic importance of CR. Recently, Aggarwal and colleagues showed in a meta-analysis from 9 studies that CR by PCI was associated with a marginally lower rate of death, MI, and need for CABG at a mean follow-up of 29 months, with no difference in repeat PCI, as compared with IR (28). Conversely, the only randomized controlled trial comparing a strategy of culprit vessel revascularization to CR failed to demonstrate superiority of CR compared with IR regarding 30-day and 1-year rate of major outcomes, including mortality (29). The lack of consensus among previous reports highlights the need for better population characterization, baseline disease stratification, and residual disease quantification.
Surprisingly, few prior studies have been performed to determine whether the extent of IR is meaningful. Leaman et al. (3), in 1981, were the first to attempt to predict prognosis and recurrence of angina after revascularization using a primitive scoring system of coronary artery disease severity. Quantification of extension and severity of coronary artery disease was assessed both at baseline and after CABG (30). To account for revascularization of a given diseased artery, the author decided to subtract from the baseline score the underlying disease associated with the grafted vessel. However, with only 202 patients, both the baseline and “residual” score failed to predict events (30).
Head et al. (7) recently examined the impact of CR versus IR on 3-year patient outcomes in the SYNTAX trial. Consistent with the current study, this report demonstrated that IR occurred more frequently in patients with more complex disease at baseline (total occlusions, greater number of lesions), and in the highest tertile (>32) of the SS. In contrast to the prior report, however, Head et al. (7) did not find a significant difference in mortality and adverse ischemic events according to the extent of revascularization. This difference between these 2 studies may reflect the more precise categorization of IR in the present study, or differences in the patient populations.
The present study demonstrates for the first time that quantification of the extent and complexity of residual coronary stenoses remaining after PCI carries substantial prognostic information. In this regard, since its first publication (9), the predictive value of the bSS (prior to PCI) has been validated in several subsets of coronary artery disease patients (10–19). However, the role of the SS after PCI has not previously been described. The present study demonstrates that the rSS effectively risk-stratifies patients with ACS undergoing an early invasive strategy with PCI. The rSS had similar accuracy to predict and discriminate all ischemic events compared with bSS, except for MI, for which bSS was slightly superior. This finding is not surprising because most MIs were periprocedural. Baseline angiographic characteristics therefore have more discriminatory power to predict MI than residual untreated disease.
Predictably, a strong correlation was observed between coronary artery disease severity at baseline and completeness of revascularization. CR was infrequent in the 2 upper tertiles of the bSS (Fig. 2). Because the rSS was a strong predictor of adverse ischemic outcomes after PCI, the knowledge that >90% of the patients in the 2 highest baseline SYNTAX tertiles will have IR suggests that alternative strategies of revascularization (such as CABG or “hybrid” PCI + CABG) should be considered unless the operator is confident that the level of ICR after PCI will be low (i.e., rSS <2). Capodanno et al. (11) also reported similar results, finding that only 12% of patients with an SS >34 have CR after PCI. In this study, CR was also found to be an independent predictor of survival (hazard ratio: 0.55, 95% CI: 0.31 to 0.98, p = 0.04), similar to our results. Thus, quantification and risk stratification of the extent and complexity of both baseline and residual CAD is essential for full prognostic characterization.
Of interest, the “delta SS” from baseline to post-PCI, representing the amount of disease “removed” by PCI, did not significantly vary according to the rSS (Table 1). This suggests in general that the net burden reduction in coronary artery disease by PCI is comparable, regardless of initial disease extent and complexity. Moreover, the proportion of chronic total occlusions, severe calcification, and complex bifurcations/trifurcations were disproportionately represented in the higher rSS tertiles, lesions not favorable to PCI. Small vessel and diffuse disease was also common in all patients with IR. These findings suggest that IR was most likely not by “choice,” but was rather consequent to unfavorable anatomy for PCI. Aggressive secondary prevention is therefore indicated in patients with substantial IR after PCI.
Of note, CR (rSS = 0) was associated with an improved 1-year prognosis in the intermediate- and high-risk bSS tertiles of our cohort compared with “extensive” IR (rSS >8). Conversely, in the low-risk bSS tertile, completeness of revascularization did not significantly influence the 1-year mortality rate. This finding suggests that baseline severity of the coronary artery disease is an important determinant of prognosis, but also that CR, when possible, may be particularly important to improve patient survival in those with high bSS. Conversely, relatively high survival rates may be seen in patients with low bSS whether or not CR is achieved. This finding is in line with the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, which enrolled patients at the lower end of the “patient-risk spectrum” (31).
In the present report, a relatively low proportion (40.4%) of patients obtained CR. In the pivotal SYNTAX trial, CR (defined by intent, not by quantitative measurement) was achieved in 56.7% of patients in the PCI group and in 63.2% in the CABG group (p = 0.005) (32). As the relative results of PCI compared with CABG in the SYNTAX trial varied according to the bSS (7), knowledge of the rSS might provide further insights into which high-risk patients may have an acceptable prognosis after PCI.
Study limitations
Several important limitations of the present analysis should be discussed. First, ACUITY was a trial performed in patients with moderate- and high-risk ACS undergoing an early invasive strategy. Therefore, although the SS has been validated in this patient population (13), our conclusions apply to patients with similar presentation and treatments. Second, the SS (and all its components) was visually assessed by 3 interventional cardiologists in whom good reproducibility for bSS evaluation has been demonstrated (22). Nonetheless, the SS is based on angiographic interpretation that has inherent limitations (33), and the results may have varied if the SS was assessed by less-trained readers, core lab technicians, by QCA analysis (22), or derived from fractional flow reserve (34). Third, the bSS and rSS are anatomic measures of revascularization, and theoretically may be improved upon by knowledge of ischemia or viability. However, such studies were not routinely available in patients with non-ST-segment elevation ACS in the ACUITY trial in whom the median time from emergency room presentation to catheterization was <24 h. Fourth, both the bSS and rSS often equally weight narrowings in small versus large vessels, and with moderate versus severe stenoses; despite this shortcoming, both scores have substantial prognostic utility. Additional studies are required to determine if the predictive ability of these scores can be improved by a different weighting algorithm. Finally, although we adjusted for measured imbalances, potential unmeasured cofounders may still be present. The results of this report should therefore be considered hypothesis generating. Prospective randomized trials are required to further evaluate the clinical importance of achieving CR after PCI.
Conclusions
In moderate- and high-risk ACS patients with IR following PCI, the newly described rSS is an independent predictor of 1-year mortality, cardiac mortality, MI, and unplanned revascularization. The rSS has a good discriminatory power for risk prediction of 1-year ischemic outcomes, is a useful tool to stratify the extent and complexity of residual coronary stenoses after PCI, and may identify patients who could benefit from further revascularization. Specifically, in our population of moderate- and high-risk ACS, an rSS >8 was associated with an increased risk of 1-year mortality, MI, and MACE.
Acknowledgments
Dr. Mehran has received research grant from sanofi-aventis, The Medicines Company, Abbott Vascular, Bristol-Myers Squibb, AstraZeneca; and has served as consultant/advisory board for Eli Lilly, AstraZeneca, Johnson & Johnson, Regado, and Daiichi Sankyo. Dr. Stone has served as consultant for Abbott Vascular, Boston Scientific, Medtronic, and The Medicines Company.
Abbreviations and Acronyms
- ACS
acute coronary syndrome(s)
- bSS
baseline SYNTAX score
- CABG
coronary artery bypass graft
- CI
confidence interval
- CR
complete revascularization
- IR
incomplete revascularization
- MACE
major adverse cardiovascular event(s)
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- ROC
receiver-operating characteristic
- rSS
residual SYNTAX score
- SS
SYNTAX score
- SYNTAX
Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery
Footnotes
All other authors have reported that they have no relationships relevant to the contents to this paper to disclose.
REFERENCES
- 1.Rogers WJ, Bourassa MG, Andrews TC, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study: outcome at 1 year for patients with asymptomatic cardiac ischemia randomized to medical therapy or revascularization. The ACIP investigators. J Am Coll Cardiol. 1995;26:594–605. doi: 10.1016/0735-1097(95)00228-v. [DOI] [PubMed] [Google Scholar]
- 2.Hannan EL, Racz M, Holmes DR, et al. Impact of completeness of percutaneous coronary intervention revascularization on long-term outcomes in the stent era. Circulation. 2006;113:2406–2412. doi: 10.1161/CIRCULATIONAHA.106.612267. [DOI] [PubMed] [Google Scholar]
- 3.Hannan EL, Wu C, Walford G, et al. Incomplete revascularization in the era of drug-eluting stents: impact on adverse outcomes. J Am Coll Cardiol Intv. 2009;2:17–25. doi: 10.1016/j.jcin.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Kim YH, Park DW, Lee JY, et al. Impact of angiographic complete revascularization after drug-eluting stent implantation or coronary artery bypass graft surgery for multivessel coronary artery disease. Circulation. 2011;123:2373–2381. doi: 10.1161/CIRCULATIONAHA.110.005041. [DOI] [PubMed] [Google Scholar]
- 5.van den Brand MJ, Rensing BJ, Morel MA, et al. The effect of completeness of revascularization on event-free survival at one year in the ARTS trial. J Am Coll Cardiol. 2002;39:559–564. doi: 10.1016/s0735-1097(01)01785-5. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 7.Head SJ, Mack MJ, Holmes DR, Jr, et al. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3-year SYNTAX data. Eur J Cardiothorac Surg. 2012;41:535–541. doi: 10.1093/ejcts/ezr105. [DOI] [PubMed] [Google Scholar]
- 8.Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Lancet. 1999;354:708–715. [PubMed] [Google Scholar]
- 9.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 10.Valgimigli M, Serruys PW, Tsuchida K, et al. Cyphering the complexity of coronary artery disease using the SYNTAX score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am J Cardiol. 2007;99:1072–1081. doi: 10.1016/j.amjcard.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 11.Capodanno D, Capranzano P, Di Salvo ME, et al. Usefulness of SYNTAX score to select patients with left main coronary artery disease to be treated with coronary artery bypass graft. J Am Coll Cardiol Intv. 2009;2:731–738. doi: 10.1016/j.jcin.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Capodanno D, Di Salvo ME, Cincotta G, Miano M, Tamburino C. Usefulness of the SYNTAX score for predicting clinical outcome after percutaneous coronary intervention of unprotected left main coronary artery disease. Circ Cardiovasc Interv. 2009;2:302–308. doi: 10.1161/CIRCINTERVENTIONS.108.847137. [DOI] [PubMed] [Google Scholar]
- 13.Palmerini T, Genereux P, Caixeta A, et al. Prognostic value of the SYNTAX score in patients with acute coronary syndromes undergoing percutaneous coronary intervention: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage StrategY) trial. J Am Coll Cardiol. 2011;57:2389–2397. doi: 10.1016/j.jacc.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Park DW, Kim WJ, et al. Validation of SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) score for prediction of outcomes after unprotected left main coronary revascularization. J Am Coll Cardiol Intv. 2010;3:612–623. doi: 10.1016/j.jcin.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Wykrzykowska JJ, Garg S, Girasis C, et al. Value of the SYNTAX score for risk assessment in the all-comers population of the randomized multicenter LEADERS (Limus Eluted from A Durable versus ERodable Stent coating) trial. J Am Coll Cardiol. 2010;56:272–277. doi: 10.1016/j.jacc.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Girasis C, Garg S, Raber L, et al. SYNTAX score and clinical syntax score as predictors of very long-term clinical outcomes in patients undergoing percutaneous coronary interventions: a substudy of SIRolimus-eluting stent compared with pacliTAXel-eluting stent for coronary revascularization (SIRTAX) trial. Eur Heart J. 2011;32:3115–3127. doi: 10.1093/eurheartj/ehr369. [DOI] [PubMed] [Google Scholar]
- 17.Garg S, Sarno G, Girasis C, et al. A patient-level pooled analysis assessing the impact of the SYNTAX (synergy between percutaneous coronary intervention with taxus and cardiac surgery) score on 1-year clinical outcomes in 6,508 patients enrolled in contemporary coronary stent trials. J Am Coll Cardiol Intv. 2011;4:645–653. doi: 10.1016/j.jcin.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Garg S, Serruys PW, Silber S, et al. The prognostic utility of the SYNTAX score on 1-year outcomes after revascularization with zotarolimus- and everolimus-eluting stents: a substudy of the RESOLUTE All Comers Trial. J Am Coll Cardiol. 2011;4:432–441. doi: 10.1016/j.jcin.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Magro M, Nauta S, Simsek C, et al. Value of the SYNTAX score in patients treated by primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: the MI SYNTAXscore study. Am Heart J. 2011;161:771–781. doi: 10.1016/j.ahj.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, McLaurin BT, Cox DA, et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 21.Stone GW, White HD, Ohman EM, et al. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Lancet. 2007;369:907–919. doi: 10.1016/S0140-6736(07)60450-4. [DOI] [PubMed] [Google Scholar]
- 22.Genereux P, Palmerini T, Caixeta A, et al. SYNTAX score reproducibility and variability between interventional cardiologists, core laboratory technicians, and quantitative coronary measurements. Circ Cardiovasc Interv. 2011;4:553–561. doi: 10.1161/CIRCINTERVENTIONS.111.961862. [DOI] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 24.Srinivas VS, Selzer F, Wilensky RL, et al. Completeness of revascularization for multivessel coronary artery disease and its effect on one-year outcome: a report from the NHLBI dynamic registry. J Interv Cardiol. 2007;20:373–380. doi: 10.1111/j.1540-8183.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 25.Kloeter UC, Jander NG, Buser PT, Osswald S, Mueller-Brand J, Pfisterer ME. Long-term outcome of angioplasty for multivessel coronary disease: importance and price of complete revascularization. Int J Cardiol. 2001;79:197–205. doi: 10.1016/s0167-5273(01)00421-1. [DOI] [PubMed] [Google Scholar]
- 26.Nikolsky E, Gruberg L, Patil CV, et al. Percutaneous coronary interventions in diabetic patients: is complete revascularization important? J Invasive Cardiol. 2004;16:102–106. [PubMed] [Google Scholar]
- 27.Tamburino C, Angiolillo DJ, Capranzano P, et al. Complete versus incomplete revascularization in patients with multivessel disease undergoing percutaneous coronary intervention with drug-eluting stents. Catheter Cardiovasc Interv. 2008;72:448–456. doi: 10.1002/ccd.21666. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal V, Rajpathak S, Singh M, Romick B, Srinivas VS. Clinical outcomes based on completeness of revascularisation in patients undergoing percutaneous coronary intervention: a meta-analysis of multivessel coronary artery disease studies. EuroIntervention. 2012;7:1095–1102. doi: 10.4244/EIJV7I9A174. [DOI] [PubMed] [Google Scholar]
- 29.Ijsselmuiden AJ, Ezechiels J, Westendorp IC, et al. Complete versus culprit vessel percutaneous coronary intervention in multivessel disease: a randomized comparison. Am Heart J. 2004;148:467–474. doi: 10.1016/j.ahj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Leaman DM, Brower RW, Meester GT, Serruys P, van den Brand M. Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation. 1981;63:285–299. doi: 10.1161/01.cir.63.2.285. [DOI] [PubMed] [Google Scholar]
- 31.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 32.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 33.Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the fame study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 34.Nam CW, Mangiacapra F, Entjes R, et al. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol. 2011;58:1211–1218. doi: 10.1016/j.jacc.2011.06.020. [DOI] [PubMed] [Google Scholar]