Abstract
STriatal-Enriched protein tyrosine Phosphatase (STEP; PTPN5) is expressed in brain regions displaying adult neuroplasticity. STEP modulates neurotransmission by dephosphorylating regulatory tyrosine residues on its substrates. In this way, STEP inactivates extracellular-signal-regulated kinase 1/2 (ERK1/2), limiting the duration and spatial distribution of ERK signaling. Two additional substrates, the tyrosine kinase Fyn and the NR2B subunit of the N-methyl-d-aspartic acid receptor, link STEP to glutamate receptor internalization in the synapse. Thus, STEP may act through parallel pathways to oppose the development of experience-dependent synaptic plasticity. We examined the hypothesis that the absence of STEP facilitates amygdala-dependent behavioral and synaptic plasticity (i.e., fear conditioning and long-term potentiation) using STEP-deficient mice (STEP KO). These mice show no detectable expression of STEP in the brain along with increases in Tyr phosphorylation of STEP substrates. Here we demonstrate that STEP KO mice also display augmented fear conditioning as measured by an enhancement in conditioned suppression of instrumental response when a fear-associated conditioned stimulus was presented. Deletion of STEP also increases long-term potentiation and ERK phosphorylation in the lateral amygdala. The current experiments demonstrate that deletion of STEP can enhance experience-induced neuroplasticity and memory formation and identifies STEP as a target for pharmacological treatment aimed at improving the formation of long-term memories.
Keywords: long-term potentiation, fear conditioning, protein tyrosine phosphatase, ERK
INTRODUCTION
Memory formation involves the coordinated activation of multiple intracellular signaling cascades that underlie experience-dependent neuroplasticity. The critical role for amygdala extracellular-signal-regulated kinase 1/2 (ERK1/2) pathway in consolidation of long-term memories is well established across numerous paradigms, including Pavlovian fear conditioning (Schafe et al., 2000; Sweatt, 2001; Rodrigues et al., 2004). The mechanisms and proteins involved in the regulation of ERK1/2 activity during neuroplasticity remain poorly defined. The STriatal-Enriched protein tyrosine Phosphatase (STEP), present in the lateral amygdala (LA) and other regions displaying adult neuroplasticity (Lombroso et al., 1993; Boulanger et al., 1995; Braithwaite et al., 2006), inactivates ERK1/2 by dephosphorylation of a regulatory Tyr in its activation loop (Paul et al., 2003; Valjent et al., 2005; Choi et al., 2007). Additional experiments have demonstrated STEP-mediated regulation of NMDA and AMPA receptor phosphorylation and endocytosis (Zhang et al., 2008, 2010, 2011; Kurup et al., 2010). By infusing a substrate-trapping TAT-STEP protein into the LA, we showed that blockade of STEP substrates inhibits fear memory consolidation and long-term potentiation (LTP) and that training on Pavlovian fear conditioning increases the expression of STEP in this region in an ERK-dependent manner (Paul et al., 2007). These findings suggest that dynamic co-regulation of STEP and its substrates is involved in amygdala-dependent neuroplasticity.
Several STEP isoforms are produced through alternative splicing and four have been isolated to date (Lombroso et al., 1991; Sharma et al., 1995; Bult et al., 1996, 1997). Of the two most widely expressed, STEP61 is targeted to the postsynaptic density (Oyama et al., 1995), extrasynaptic compartments (Xu et al., 2009), and the endoplasmic reticulum (Boulanger et al., 1995). STEP46 is contained in its entirety within STEP61 (Bult et al., 1996), and is present in the cytosol. Both isoforms contain a kinase interacting motif (KIM) required for the binding of STEP to all known substrates. STEP is activated by calcineurin/PP1-mediated dephosphorylation of a serine residue located in the KIM domain (Paul et al., 2003; Valjent et al., 2005), while protein kinase A (PKA)-phosphorylation of this residue prevents the interaction of STEP with substrates (Paul et al., 2000).
As mentioned above, STEP inactivates ERK but also p38 MAPK, Fyn, and Pyk2 (Nguyen et al., 2002; Pelkey et al., 2002; Xu et al., 2012). Additionally, STEP dephosphorylation promotes internalization of NR1/NR2B receptors (Snyder et al., 2005) and GluR1/GluR2-containing AMPARs (Zhang et al., 2008). These ionotrophic glutamate receptors have been shown to be essential for the induction (i.e., NMDA) and expression (i.e., AMPA) of amygdala LTP and emotionally salient fear memories (Nakazawa et al., 2006; Sotres-Bayon et al., 2007; Zushida et al., 2007).
Taken together, STEP appears to participate in the integration of ERK1/2 activation and glutamate receptor functioning and may thus modulate neuroplasticity and memory formation in the LA. While our previous report shows the importance of STEP targets in these processes (Paul et al., 2007), no studies have directly examined the necessity of endogenous STEP in this process. Here we examined if STEP coordinates amygdala-dependent neuroplasticity and long-term memory formation by examining the hypothesis that fear conditioning and amygdala LTP is facilitated in STEP knockout mice.
EXPERIMENTAL PROCEDURES
Animals
Experimentally naïve male transgenic mice, wild-type (STEP+/+; WT), heterozygous (STEP+/−; HET) or homozygous (i.e., STEP−/−; KO) mice were backcrossed for at least nine generations with C57Bl/6WT mice from Charles River Laboratories (Wilmington, MA, USA) and used for all experiments (Venkitaramani et al., 2009). The animals were randomly selected from the breeding colony. All experimental subjects were generated at Yale University. After weaning they were housed in groups of 2–4 mice/cage under controlled temperature and humidity conditions under a 12/12-h light–dark cycle (light on at 7 am and off at 7 pm). Mice had free access to water at all times and limited access to food as detailed below. All animal use was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Animal Care and Use Committee at Yale University.
Conditioned suppression testing
Testing was initiated by training on food-reinforced instrumental responding using standard operant chambers for mice (16 × 14 × 13 cm). The boxes were controlled by MedPC software (Med Associates Inc., St Albans, VT, USA). Each chamber was housed in a sound-attenuating outer chamber equipped with a white noise generator (65 dB) and a fan to reduce the impact of external noise. A house light mounted on the back wall illuminated the chamber. A pellet dispenser delivered food pellets (20 mg; Bio-Serv, USA) as the reinforcer into the magazine. Head entries were detected by a photocell mounted in the reinforcer magazine. In this magazine was also a stimulus light. Two nosepoke apertures were placed on the back wall of the chambers (i.e., opposite to the reinforcer magazine).
Male STEP WT, HET and KO mice (n = 8/group) were used for behavioral testing. During the 5 days immediately prior to the start of training, animals were restricted to 90-min access to food per day and exposed to 20-mg food pellets containing sucrose in their home cages. During the testing period, food pellets were intermittently available in the operant chambers according to the behavioral protocol (see below) as well as in unlimited amounts in the home cage for 90 min, beginning 30 min after the daily testing session. This food access schedule allows for each individual animal to reach their individual satiety point and reduces the variability caused by competition between dominant and subordinate animals. In our hands, this schedule allows for a slow weight gain following the initial weight loss to about 90% of free-feeding weights. Animal weights were monitored throughout the experiment.
All subjects were initially habituated to the testing apparatus for 2 days; during these sessions food pellets were delivered into the reinforcer magazine on a fixed time 15-s (FT-15) schedule. Beginning on the next day, the subjects received daily training sessions for 10 consecutive days. Responding on the correct (i.e., active) nosepoke was reinforced, whereas responding on the other (inactive) nosepoke had no programmed consequences. The position of the active nosepoke (left/right) was balanced for all experimental groups. Completion of the response requirement (see below) resulted in onset of the magazine stimulus light, followed 1 s later by delivery of a single food pellet. Two seconds later the stimulus light was turned off. The first ten (10) reinforcers were obtained after successful completion of responding according to a fixed ratio (FR1) schedule, following which pellets were available after responding on a variable ratio (VR2) schedule. The session lasted for 15 min.
When stable performance on the instrumental response had been acquired, mice were subjected to a single training session where an auditory stimulus was paired with foot shock. Here, mice were placed in a new context, a chamber that was equipped with tone and shock generators. Animals were first allowed to habituate to this environment for 3 min, followed by three pairings of a 30-s auditory stimulus (CS+) at 75 dB coterminating with a 1 s 0.4 mA foot-shock. The training trials were separated by 2 min, and animals were left in the chamber for 1 min after the final shock. All the training procedures were selected based on a pilot experiment using Pavlovian fear conditioning. Moreover, we selected a 75-dB tone intensity for this experiment as pilot experiments showed that it does not significantly disrupt ongoing instrumental performance in well-trained animals when presented in the context of a continuous 65-dB white noise background sound.
For the next 3 days, mice received daily training on the instrumental task as above but the schedule of reinforcement was increased such that 1–5 correct responses were required for reinforcement. On the following day the conditioned suppression test was performed. Here, instrumental performance was assessed before, during and after the intermittent presentation of the shock-associated CS+. A total of five CS+ presentations were given, spaced 4 min apart. The response suppression ratio, calculated as responses during the 30-s CS presentation/responses during the 30 s immediately preceding the CS onset, was used as a measure of memory strength.
Western blots of amygdala punches
Immunoblotting of punches from the LA and central nucleus of the amygdala (CeN) was performed as previously described (Paul et al., 2007) with minor modifications. Briefly, STEP WT, HET and KO littermates (n = 15/group) were sacrificed by cervical dislocation, brains rapidly dissected and immediately frozen on dry ice. The brains were stored at −80 °C until sample preparation. Punches from 400-μm frozen sections were taken using 0.5-mm tissue corer and sonicated in buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% SDS, 50 mM NaF and 5 mM Na4P2O7. Each sample consisted of punches pooled from five mice. The sonicated samples were boiled for 10 min, spun down and supernatant stored at −20 °C until use. For immunoblotting, 25 μg of protein was separated on 10% SDS–PAGE, transferred onto nitrocellulose membrane and probed sequentially with pERK1/2 or STEP and ERK2 antibodies. The horseradish peroxidase (HRP) signal from the secondary antibody was detected with Chemi-HR16 gel imaging system (Syngene, Frederick, MD, USA), quantified using ImageJ software and normalized to ERK2 loading control from the same gel.
Electrophysiology data and analysis
This experiment used the experimental procedures described in Paul et al. (2007) for testing amygdala brain slices from STEP WT and KO littermates (n = 3/group). All electrophysiology data were acquired and analyzed using pCLAMP 8.0 (Axon Instruments, Inc., Union City, CA, USA). Field excitatory post-synaptic potentials (fEPSP) amplitude was defined as the maximum DC voltage of a vertical line running tangent to the points of fEPSP onset and offset. In some recordings fEPSP slope (10–60%) was also analyzed. However, the slope measurements were more variable and sensitive to noise, making them difficult to analyze reliably. Therefore, we chose response amplitude for the analysis of extracellular recordings. Responses were averaged and normalized to baseline, which was defined as the mean value obtained in the 10 min prior to tetanus or the 10 min prior to drug application. The potentiation was analyzed by comparing pre-tetanus values (10 min before tetanus) with those collected 50 min after LTP induction. Electrophysiological analyses were conducted using Student’s t-test. The data are presented as mean ± SEM and p < 0.05 was considered statistically significant.
Statistics
The data were analyzed by analysis of variance (ANOVA) using the genotype as the independent factor. Statistically significant main effects were further evaluated using post hoc analysis using the Tukey HSD or Scheffe’s tests that corrects for multiple comparisons. Electrophysiological analyses were conducted using Student’s t-test. The data are as mean ± SEM and p ≤ 0.05 was considered statistically significant.
RESULTS
Deletion of STEP increases amygdala-dependent fear learning
When tested in the conditioned suppression paradigm, HET and KO mice displayed greater conditioned suppression of food-motivated instrumental performance when an auditory CS+ that had previously been associated with foot shock was presented (Fig. 1, right). There were significant main effects of both Genotype (F(2,21) = 4.236; p < 0.05) and CS presentation (F(2,42) = 3.313; p < 0.05) on performance on this task. Post hoc analyses demonstrated that both STEP HET (p < 0.05) and KO (p < 0.05) displayed greater suppression ratios compared to wild-type controls, indicating that the deletion of STEP augmented the strength of the fear-associated memory.
Fig. 1.
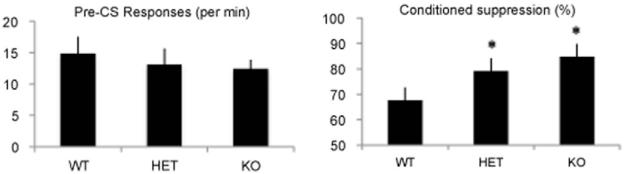
Deletion of STEP levels increases amygdala-dependent fear learning. Deletion of STEP levels increases amygdala-dependent fear learning. Animals hetero- or homozygous for the deletion of the STEP gene displayed greater conditioned suppression when presented with an auditory CS+ that had previously been associated with foot shock. Data shown is the average suppression ratio over the five CS+ presentations. ANOVA analysis identified a main effect of genotype (p ≤ 0.05) on conditioned suppression.
Importantly, there was no group difference in baseline performance prior to the conditioned suppression test and ANOVA analysis found no effects on nosepoke responses (F(2,21) = 0.695; n.s.) or reinforcers (F(2,21) = 0.443; n.s.), demonstrating that the results cannot be explained by changes in motivation or the ability to perform the instrumental response (Fig. 1, left). There was also no difference in performance between the groups prior to presentation of the first CS in the conditioned suppression test session, confirming that the differences in instrumental responding during CS presentations were selectively related to enhancements in Pavlovian fear learning.
Reducing STEP levels results in increased ERK1/2 activation in the amygdala
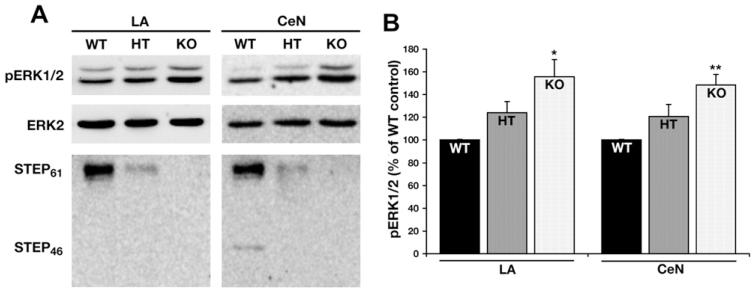
Western blots of punch biopsies from the LA and CeN were probed with STEP antibody. As previously reported (Paul et al., 2007), the only isoform expressed at baseline in the LA is STEP61, while in the CeN both STEP61 and STEP46 are expressed (Fig. 2). The KO mice lacked the expression of all STEP isoforms, while the HET displayed approximately half the amount of STEP proteins.
Fig. 2.
Increased amygdala ERK1/2 activity in STEP−/− mice. (A) Tissue punches from the LA and the CeN were analyzed by SDS–polyacrylamide gel electrophoresis and Western blots probed with anti-ERK1/2, anti-pERK1/2 and anti-STEP antibodies to determine the level of ERK1/2 activity and distribution of STEP isoforms in these regions. (B) Histograms showing an increase in pERK in both the LA and the CeN of STEP−/− mice (*p ≤ 0.05 and **p ≤ 0.01).
We next confirmed the physiological effects of STEP deletion by determining the levels of phosphorylation of the STEP substrate ERK1/2 in the LA and CeN of STEP KO mice. There was a significant difference between the genotypes both in the LA (F(2,6) = 8.91; p < 0.05) and in the CeN (F(2,6) = 7.14; p < 0.05). There were no significant differences between the two regions. STEP KO mice showed significantly higher levels of pERK1/2 in both the LA (155.63 ± 15.14%; p < 0.02) and CeN (148.32 ± 9.34%; p < 0.01), as compared to WT (100%; p < 0.05, both regions). Although the pERK1/2 levels were elevated in both LA (123.89 ± 9.90%) and CeN (120.72 ± 10.51%) of HET mice compared to WT, the differences were not significant. These results were confirmed by immunohistochemical staining (data not shown) and confirmed our previous observations with immunohistochemistry (Venkitaramani et al., 2009).
Eliminating STEP facilitates LTP in the LA
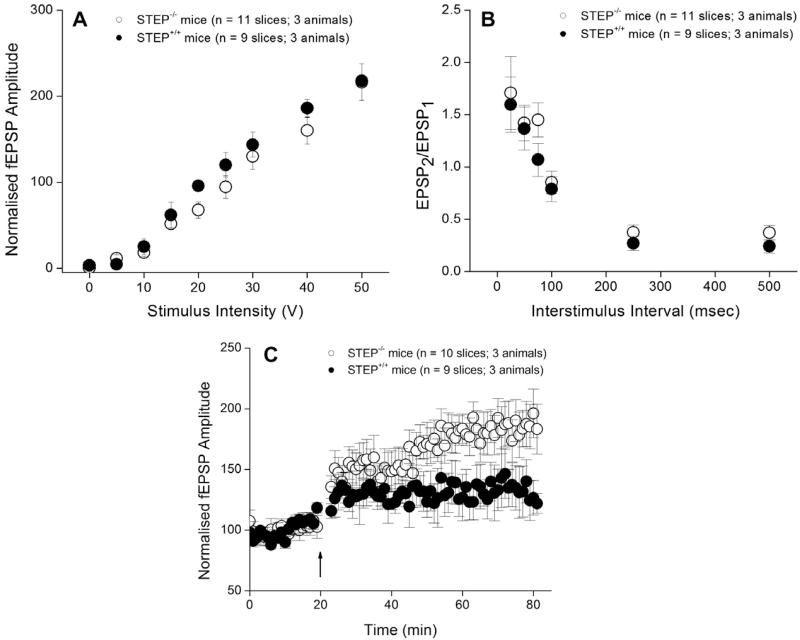
We next determined the effect of STEP deletion on LTP in the LA (Fig. 3). First we examined basal synaptic transmission in amygdala slices from WT and STEP−/− mice. The fEPSP amplitude recorded as a function of stimulus intensity in slices from WT mice (n = 9 slices, 3 mice) was not different in slices from STEP−/− mice (n = 11 slices, 3 mice; Fig. 3A). Moreover, paired synaptic responses from WT and STEP−/− slices at different interstimulus intervals did not differ in WT (n = 9 slices, 3 mice, black circles) versus STEP−/− mice (n = 11 slices, 3 mice, open circles; (Fig. 3 B). Together, these measures indicate that deletion of STEP does not alter baseline synaptic transmission or short-term facilitation in the LA.
Fig. 3.
Deletion of STEP facilitates induction of LTP in LA without affecting basal synaptic transmission. (A) Basal synaptic transmission and LTP in STEP−/− mice. Synaptic responses to increasing stimulus intensity (upper left panel). The fEPSP amplitude is plotted as a function of stimulus intensity in slices from WT mice (n = 9 slices, 3 mice, black circles) or from STEP−/− (KO) mice (n = 11 slices, 3 mice, open circles). (B) Paired synaptic responses from WT and STEP−/− slices at different interstimulus intervals do not differ (upper right panel). Ratio of the second fEPSP the first fEPSP (mean ± SEM) is plotted for WT (n = 9 slices, 3 mice, black circles) and STEP−/− mice (n = 11 slices, 3 mice, open circles). (C) Pooled fEPSP amplitude (lower panel) (mean ± SEM) is plotted for WT slices (n = 9 slices, 3 mice, black circles) and STEP−/− slices (n = 10 slices, 3 mice, open circles circles). Tetanic stimulation was delivered at t = 20 min as indicated by the arrow.
To examine whether STEP deletion altered persistent synaptic plasticity in the LA, we investigated the induction of LTP. Tetanic stimulation (three 1 s, 100 Hz trains, 90 s inter-train interval) to the internal capsule in control slices produced a sustained increase in fEPSP amplitude in both WT (n = 9 slices; p < 0.0001; Fig. 3C) and STEP−/− slices (n = 10 slices; p < 0.0001). In slices from WT mice, the fEPSP amplitude was 124 ± 16% (mean ± SEM) of the baseline level 60 min after tetanus, whereas in slices STEP−/− mice fEPSP amplitude was 185 ± 18% (p < 0.05 compared with WT slices). These results demonstrate that deletion of STEP increased the magnitude of LTP in the LA but did not affect baseline synaptic transmission or short-term facilitation.
DISCUSSION
The current research demonstrates that the protein tyrosine phosphatase STEP exerts an inhibitory influence on neuroplasticity in the LA, and that elimination of STEP in the brain of genetically modified mice results in facilitated Pavlovian fear conditioning, increased ERK1/2 phosphorylation and enhanced LTP in the LA.
STEP, as a regulator of tyrosine phosphorylation in the brain, has been shown to temporally and spatially modulate ERK1/2 activity (Paul et al., 2003; Venkitaramani et al., 2009) and synaptic glutamate receptor function (Pelkey et al., 2002). The activity or levels of STEP protein may thus play a central role in coordinating several key elements of experience-induced neuroplasticity. To further understand the physiological roles of STEP, and STEP-mediated regulation of behavioral and synaptic plasticity, we generated STEP KO mice. These mice do not express any of the STEP isoforms normally found in wild-type animals (Venkitaramani et al., 2009). Using immunohistochemistry we have shown that STEP KO mice have increased baseline phosphorylation of ERK1/2 and other STEP targets (i.e., fyn, NR2B and GluR2) in regions of the brain involved in learning and memory, such as the hippocampus, amygdala and cortex (Zhang et al., 2008, 2010; Venkitaramani et al., 2009). Here we further confirmed the increase in amygdala ERK activity using Western blot to allow for quantitative analysis. We determined that knocking down STEP expression results in enhanced activation of ERK1/2 without changes in total protein expression of ERK in the CeN and LA, supporting the previously reported immunohistochemistry results. These data confirm that a reduction of STEP has immediate consequences for the function of ERK1/2, and other downstream targets of STEP such as Fyn, NR2B, and GluR2 within multiple regions (c.f., Goebel-Goody et al., 2012a,b).
However, like other studies that use developmental knockout mice, there are potential limitations, such as the possibility of compensatory changes that are not directly related to STEP. These issues have been investigated extensively in previous studies (Venkitaramani et al., 2009, 2011; Kurup et al., 2010; Zhang et al., 2010; Carty et al., 2012; Goebel-Goody et al., 2012a,b). Nevertheless, the current data may be particularly relevant to those circumstances where the levels or the activity of STEP is chronically altered such as is expected in progressive or permanent clinical disorders. Indeed, STEP is elevated in brains of individuals suffering from several chronic disorders with cognitive impairments such as Alzheimer’s disease, fragile × syndrome and schizophrenia, as well as in genetic animal models that reproduce these disorders (Zhang et al., 2010; Carty et al., 2012; Goebel-Goody et al., 2012a,b). In these studies, we identified a common outcome of STEP deletion that results in increased phosphorylation of its primary targets ERK, Fyn, NR2B, and GluR2. Importantly, we have not found any evidence of decreases of Tyr phosphorylation, total protein levels, or localization of these substrates, suggesting that compensatory regulation is limited. The increased Tyr phosphorylation of STEP substrates occurs within several brain regions, and while the current study focuses on the amygdala, parallel effects of STEP deletion on neuroplasticity and cognition dependent on the hippocampus and prefrontal cortex have been demonstrated (Hicklin et al., 2011; Venkitaramani et al., 2011; Zhang et al., 2011; Goebel-Goody et al., 2012a,b). To date, our work using the STEP KO shows no evidence of significant compensation by other tyrosine phosphatases, thus making the STEP KO mouse a valuable model for understanding STEP function in the brain.
The increase in ERK1/2 phosphorylation within the LA suggested that STEP KO mice would display facilitated Pavlovian fear conditioning, as this form of learning requires experience-induced activation of ERK1/2 within this region. Consistent with this hypothesis, we found that the STEP KO mice showed augmented conditioned suppression after training on Pavlovian fear conditioning. Conditioned suppression and conditioned freezing show a high degree of correlation (Bouton and Bolles, 1980). At the level of fear memory formation and expression in the amygdala, conditioned suppression is likely analogous to conditioned freezing behavior. Specifically, lesions of the LA and CeA blocks both conditioned freezing behavior and conditioned suppression (LeDoux et al., 1990; Killcross et al., 1997). However, the expression of freezing and conditioned suppression also involves dissociable neural circuits as lesions of the periaqueductal gray blocks freezing but only partially affects conditioned suppression (Amorapanth et al., 1999). Based on the role of LA in both behaviors, the reduction in motivated instrumental performance during presentation of a fear-associated CS can thus be used as an index of LA-dependent fear memory strength, i.e., the greater response suppression, the stronger the fear memory.
Deletion of STEP augmented the formation of Pavlovian fear memory and these behavioral observations provide evidence that one important function of STEP may be to regulate neuroplasticity and memory formation or expression. This notion is supported by the augmented LTP in amygdala slices from STEP KO mice compared to WT controls, without significant effects on baseline electrophysiological measures or paired-pulse facilitation. The induction of LTP in the LA has been demonstrated to be an essential step in the formation of a long-term fear memory (Sigurdsson et al., 2007), and this enhancement in amygdala neuroplasticity is consistent with the present behavioral observations.
We have previously reported that infusion of a substrate-trapping inactive form of STEP into the LA impairs Pavlovian fear conditioning (Paul et al., 2007). Post-training infusions of this substrate-trapping STEP variant into the LA was sufficient to reduce fear memory expression, demonstrating an effect on memory consolidation. These observations suggest that the effect of STEP deletion may primarily be through enhancement of memory consolidation. In our previous study, we also found that training on the Pavlovian fear conditioning task increased STEP expression by the de novo translation of both the cytosolic STEP46 and the membrane-bound STEP61 through a mechanism dependent on ERK1/2 activation. The rapid translation of STEP in response to a significant learning event suggests that this is an important step in the temporal or spatial regulation of STEP targets during neuroplasticity, including ERK1/2 phosphorylation. It was suggested that the increase in STEP serves to constrain the duration of the cellular processes underlying experience-induced neuroplasticity during fear conditioning to promote the temporal and/or stimulus specificity of the fear memory.
The role of STEP in neuroplasticity is also mediated through the regulation of ionotropic glutamate receptor trafficking and function. STEP knockdown by RNAi increases the functional properties of NR2B-containing NMDARs (Pelkey et al., 2002; Braithwaite et al., 2006), and we recently established that the synaptic localization of NR1/NR2B and GluR1/GluR2 receptors are regulated by STEP. Indeed, STEP KO mice show increased levels of both NR1/NR2B (Venkitaramani et al., 2011) and GluR1/GluR2 in the synaptic membrane (Zhang et al., 2008). The tyrosine kinase Fyn phosphorylates tyrNR2B at tyr1472, leading to exocytosis of NMDARs to surface membranes (Dunah et al., 2004). STEP dephosphorylates and inactivates Fyn, thus suggesting that STEP works through two pathways to promote internalization of NMDARs: direct dephosphorylation of both tyr1472 of the NR2B subunit and tyr420 of Fyn. GluR2 is tyrosine phosphorylated by Src family kinases (Ahmadian et al., 2004; Hayashi and Huganir, 2004). STEP was recently shown to regulate GluR1/GluR2 trafficking (Zhang et al., 2008). GluR2 is known to be tyrosine dephosphorylated and internalized after dihydroxyphenylaglycine (DHPG)-induced long-term depression (LTD), although the identity of the PTP was unknown (Moult et al., 2006). We determined that STEP is required for DHPG-induced endocytosis of GluR1/GluR2 (Zhang et al., 2008). Moreover, GluR1/GluR2 receptors are no longer internalized in STEP KO cultures after DHPG stimulation, but are again internalized if STEP is acutely replaced using a TAT-STEP protein (but are not internalized by an inactive TAT-STEP control).
Importantly, the consequences of STEP deletion are persistent and not compensated for by other tyrosine phosphatases. The lack of redundancy may indicate unique and essential cellular functions for STEP that cannot be executed by other PTPs. This important aspect of STEP function may provide an opportunity to selectively modulate STEP activity without at the same time affecting additional intracellular signaling pathways. This is an exciting possibility since it indicates a potential role for STEP inhibitors as novel therapeutic agents to promote neuroplasticity in an effort to reduce cognitive deficits and aberrant emotional and motivational processes that emerge in neuropsychiatric disorders.
Acknowledgments
We would like to thank laboratory members for helpful discussions and critical reading of the manuscript. This work was financially supported by: National Institutes of Health Grants MH01527 (P.J.L.), MH52711 (P.J.L.), DA015333 (J.R.T.), MH066172 (subcontract to J.R.T.), the National Association of Research on Schizophrenia and Depression (NARSAD; P.J.L.), the Brown–Coxe Award (D.V.V.); the Canadian Institutes of Health Research (CIHR; MT-12682, M.W.S.). M.W.S. holds Canada Research Chairs and is an International Research Scholar of the Howard Hughes Medical Institute. The authors of this research article do not have any biomedical financial interests or potential conflict of interests.
Abbreviations
- CeN
central nucleus of the amygdala
- DHPG
dihydroxyphenylaglycine3
- ERK1/2
extracellular-signal-regulated kinase 1/2
- fEPSP
field excitatory post-synaptic potential
- HET
heterozygous
- KIM
kinase interacting motif
- KO
knockout
- LA
lateral amygdala
- LTD
long-term depression
- LTP
long-term potentiation
- STEP
STriatal-Enriched protein tyrosine Phosphatase
- WT
wild-type
REFERENCES
- Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, Taghibiglou C, Wang Y, Lu J, Wong TP, Sheng M, Wang YT. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorapanth P, Nader K, LeDoux JE. Lesions of periaqueductal gray dissociate-conditioned freezing from conditioned suppression behavior in rats. Learn Mem. 1999;6:491–499. doi: 10.1101/lm.6.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Conditioned fear assessed by and by the suppression of three different baselines. Anim Learn Behav. 1980;8:429–434. [Google Scholar]
- Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: one STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult A, Zhao F, Dirkx R, Jr, Raghunathan A, Solimena M, Lombroso PJ. STEP: a family of brain-enriched PTPs. Alternative splicing produces transmembrane, cytosolic and truncated isoforms. Eur J Cell Biol. 1997;72:337–344. [PubMed] [Google Scholar]
- Bult A, Zhao F, Dirkx R, Jr, Sharma E, Lukacsi E, Solimena M, Naegele JR, Lombroso PJ. STEP61: a member of a family of brain-enriched PTPs is localized to the endoplasmic reticulum. J Neurosci. 1996;16:7821–7831. doi: 10.1523/JNEUROSCI.16-24-07821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty NC, Xu J, Kurup P, Goebel-Goody SM, Brouillette J, Austin DR, Yuan P, Chen P, Chen G, Correa PR, Pittenger C, Lombroso PJ. The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatry. 2012 doi: 10.1038/tp.2012.63. http://dx.doi.org/10.1038/tp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Lin SL, Lee B, Kurup P, Cho HY, Naegele JR, Lombroso PJ, Obrietan K. Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase. J Neurosci. 2007;27:2999–3009. doi: 10.1523/JNEUROSCI.4913-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-d-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol Pharmacol. 2004;65:121–129. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Baum M, Paspalas C, Carty NC, Fernandez S, Kurup P, Lombroso PJ. Therapeutic implications for STriatal-Enriched protein tyrosine Phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012a;64:65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Wilson-Wallis E, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile × syndrome mouse model. Genes Brain Behav. 2012b;11:586–600. doi: 10.1111/j.1601-183X.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin TR, Wu PH, Radcliffe RA, Freund RK, Goebel SM, Proctor WR, Lombroso PJ, Browning MD. STriatal Enriched protein tyrosine Phosphatase (STEP) mediates ethanol inhibition of NMDA receptor activity at hippocampal glutamatergic synapses. PNAS. 2011;108:6650–6655. doi: 10.1073/pnas.1017856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Murdoch G, Lerner M. Molecular characterization of a protein tyrosine phosphatase enriched in striatum. PNAS. 1991;88:7242–7246. doi: 10.1073/pnas.88.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, Goto J, Umemori H, Tezuka T, Iwakura Y, Watanabe M, Yamamoto T, Manabe T. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Oyama T, Goto S, Nishi T, Sato K, Yamada K, Yoshikawa M, Ushio Y. Immunocytochemical localization of the striatal enriched protein tyrosine phosphatase in the rat striatum: a light and electron microscopic study with a complementary DNA-generated polyclonal antibody. Neuroscience. 1995;69:869–880. doi: 10.1016/0306-4522(95)00278-q. [DOI] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, Mills E, Hakim S, Salter MW, Taylor JR, Lombroso PJ. The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry. 2007;61:1049–1061. doi: 10.1016/j.biopsych.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20:5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin-dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004;24:3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma E, Zhao F, Bult A, Lombroso PJ. Identification of two alternatively spliced transcripts of STEP: a subfamily of brain-enriched protein tyrosine phosphatases. Brain Res Mol Brain Res. 1995;32:87–93. doi: 10.1016/0169-328x(95)00066-2. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding L, Tressler L, Allen M, Sacca R, Picciotto MR, Lombroso PJ. Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63:69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Moura PJ, Picciotto MR, Lombroso PJ. STriatal-Enriched protein tyrosine Phosphatase (STEP) knockout mice have enhanced hippocampal memory. Eur J Neurosci. 2011;33:2288–2298. doi: 10.1111/j.1460-9568.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Bartos JA, Hell JW, Lombroso PJ. STriatal-Enriched protein tyrosine Phosphatase (STEP) regulates Pyk2 activity. JBC. 2012;87:20942–20956. doi: 10.1074/jbc.M112.368654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YF, Kurup P, Xu J, Carty N, Fernandez S, Nygaard HB, Pittenger C, Greengard P, Strittmatter S, Nairn AC, Lombroso PJ. Genetic reduction of the tyrosine phosphatase STEP reverses cognitive and cellular deficits in a mouse model of Alzheimer’s disease. PNAS. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YF, Kurup P, Xu J, Anderson G, Greengard P, Nairn AC, Lombroso PJ. Reduced levels of the tyrosine phosphatase STEP block beta amyloid-mediated GluA1/GluA2 receptor internalization. J Neurochem. 2011;119:472–664. doi: 10.1111/j.1471-4159.2011.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]