Abstract
Ventricular trabeculation and compaction are two of the many essential steps for generating a functionally competent ventricular wall. A significant reduction in trabeculation is usually associated with ventricular compact zone deficiencies (hypoplastic wall), which commonly leads to embryonic heart failure and early embryonic lethality. In contrast, hypertrabeculation and lack of ventricular wall compaction (noncompaction) are closely related defects in cardiac embryogenesis associated with left ventricular noncompaction (LVNC), a genetically heterogenous disorder. Here we review recent findings through summarizing several genetically engineered mouse models that have defects in cardiac trabeculation and compaction.
Keywords: heart development, cardiac compaction, trabeculation, signaling
INTRODUCTION
Left ventricular noncompaction (LVNC, OMIM300183) is a unique type of inherited cardiomyopathy and has gained increasing attention in the past decade [Jenni et al., 2001; Towbin 2010; Oechslin and Jenni 2011]. LVNC, first described in 1926, was previously known as spongy myocardium [Grant 1926; Freedom et al., 2005]. Its prevalence was estimated from 4.5 to 26 per 10,000 adult patients referred for echocardiographic diagnosis [Ritter et al., 1997; Aras et al., 2006; Sandhu et al., 2008; Hoedemaekers et al., 2010]. In the pediatric population, LVNC is the third most common cardiomyopathy after dilated cardiomyopathy and hypertrophic cardiomyopathy [Nugent et al., 2003; Nugent et al., 2005; Daubeney et al., 2006]. A recent article by Oechslin and Jenni presented a thorough clinical view of LVNC, including the current progress of diagnostic criteria, the epidemiology, the clinical presentation, outcome and management, the use of cardiac magnetic resonance imaging (MRI) to overcome potential misdiagnosis by commonly used echocardiography, as well as the genetic heterogeneity of LVNC [Oechslin and Jenni, 2011]. However, despite these important clinical observations over the past 25 years, the etiological cause of LVNC is largely unknown. The reasons could be three-fold: 1) the majority of clinical investigation is based on the anatomical features of isolated LVNC in the adult patients. It is therefore impossible to retrospectively track the abnormal developmental process at the embryonic stage; 2) the poor genotype-phenotype association in LVNC patients prevents a clear understanding of specific genetic pathways relevant to the pathogenesis of LVNC; 3) there is a lack of sufficient knowledge on the molecular control of normal trabeculation and compaction during ventricular myocardial development. To overcome these challenges, it is critical to establish animal models to analyze LVNC at both genetic and molecular level and use the knowledge gained from animal studies to interpret the clinical findings and eventually to find preventative and therapeutic measures for LVNC.
THE DEVELOPMENT OF VENTRICULAR WALL
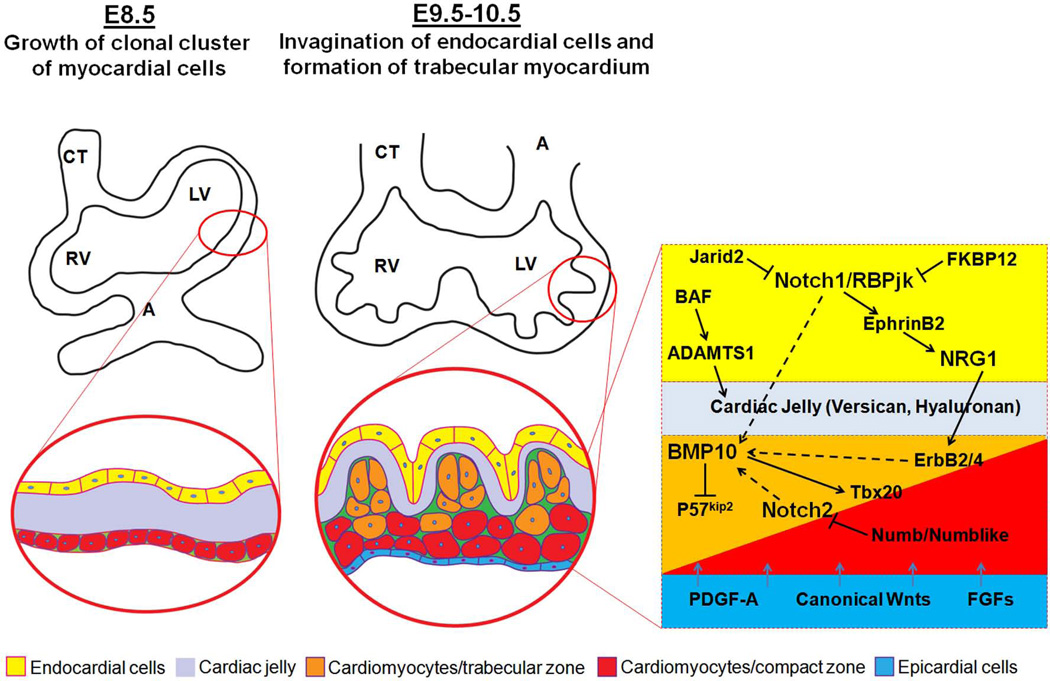
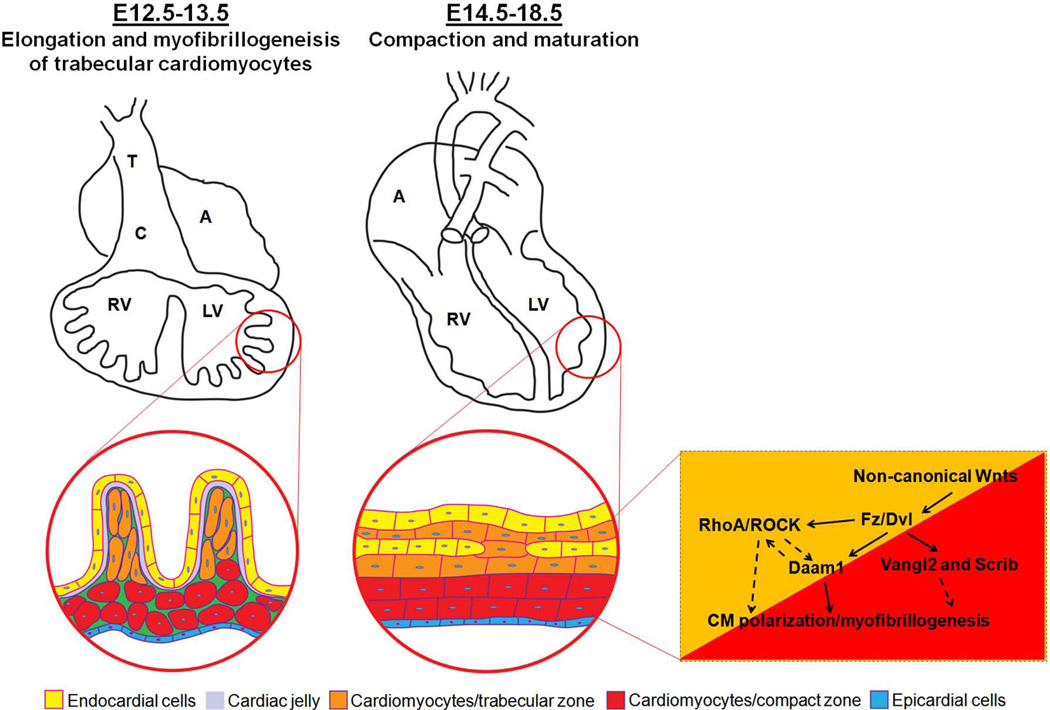
During mammalian heart development, the ventricles undergo a series of morphogenetic events [Taber, 1998; Bartman and Hove, 2005; Moorman et al., 2007]. Ventricular trabeculation and compaction are two of the many steps among those events essential for creating a functionally competent ventricular wall [Sedmera et al., 2000]. The development of ventricular wall can be viewed as a 4-step process. The first step involves the formation of a single cell layer of myocardium at an early developmental stage. Following induction via adjacent endoderm, the lateral mesoderm gives rise to an early tubular heart, which is composed of one cell layer of myocardium and one cell layer of endocardium lining the lumen with extracellular matrix (cardiac jelly) in between (Fig 1) [Brutsaert and Andries, 1992; Bartman and Hove, 2005]. The second step involves the formation of a trabeculated and compact myocardium at early midgestation stage. As the myocardium thickens, endocardial cells invaginate and cardiomyocytes in specific regions along the inner wall of the heart form sheet-like protrusions into the lumen to give rise to trabecular myocardium, while the outside layer of myocardium becomes the base for forming the compact myocardium later in development (Fig 1). Ventricular trabeculation has been suggested to facilitate oxygen and nutrient exchange in the heart muscle and to enhance heart muscle force generation to match the increasing blood and oxygen demand in developing embryos [Sedmera et al., 2000]. The third step regards myocardial compaction at late midgestation stage. As development proceeds, the trabecular myocardium collapses towards the myocardial wall in a process termed compaction, which contributes to forming a thicker, compact ventricular wall. The majority of trabeculae have become compacted after E14.5 in mouse embryos (Fig 2) [Sedmera et al., 2000; Risebro and Riley, 2006]. The fourth step is the formation of a mature and multilayered spiral myocardium during late fetal and neonatal stage [Taber, 1998]. Unlike the skeletal muscle, the ventricular myocardium consists of aggregated myocytes within a three-dimensional mesh. A series of elegant studies by Anderson and colleagues demonstrated that myocytes in the subendocardial and subepicardial layers of the myocardium form helical sheets with reciprocal angulation, whereas the myocytes at the middle layer form a circular sheet [Torrent-Guasp et al., 2005; Anderson et al., 2006; Lunkenheimer et al., 2006; Lunkenheimer et al., 2006; Anderson et al., 2007; Dorri et al., 2007; Schmid et al., 2007; Anderson et al., 2008]. This unique myocardial structure is believed to be essential for normal contractile function of the heart. Whether ventricular trabeculation and compaction is part of this dynamic process is unknown and requires further investigations.
Figure 1.
Early growth and development of ventricular wall. The interactive regulation of endocardial-, myocardial- and epicardial-derived growth factors and signaling networks is critical to the ventricular wall growth and trabeculation.
Figure 2.
Ventricular wall trabeculation and compaction. Wnt/PCP signaling components are important in cardiomyocyte polarization and myofibrillogenesis and ventricular compaction.
Following the formation of primitive trabecular ridges (at about E9.5 in mouse embryo), the myocardium undergoes extensive expansion by either recruiting cardiomyocytes from myocardial wall into the trabecular ridges or via cellular proliferation within the trabecular cardiomyocytes. In support of the cellular recruitment mechanism, proliferative activity is consistently higher within the compact myocardium, and there is a gradient of decreasing proliferation and increasing differentiation of myocytes from the compact towards the trabecular zone of the myocardium [Icardo and Fernandez-Teran 1987; Icardo, 1988; Rumyantsev and Krylova, 1990; Pasumarthi and Field, 2002]. P57kip2, a cyclin-dependent kinase inhibitor of the p21 family, is specifically expressed in the trabecular myocardium, suggesting an active process to suppress cell proliferation in the trabeculae [Kochilas et al., 1999]. This balance of proliferation and differentiation is critical to the formation of a functionally competent ventricular wall. Fate mapping experiments using retroviral tagging with β-galactosidase in developing chick hearts provided important insights into the interplay between cellular proliferation and differentiation during ventricular wall formation [Mikawa et al., 1992; Mikawa et al., 1992]. Upon myogenic differentiation, single labeled cardiomyocytes give rise to transmural and cone-shaped growth units. This pattern of growth is closely associated with the formation of trabecular myocardium [Gourdie et al., 1999; Meilhac et al., 2003].
Several growth factors and their intracellular signaling pathways have been demonstrated to be critical to ventricular growth and trabeculation. Neuregulin 1 is produced in endocardial cells and acts through myocardial receptors ErbB2 and ErbB4. Mice deficient in either neuregulin 1 or ErbB2/4 develop hypoplastic wall lacking normal trabeculation [Gassmann et al., 1995; Lee et al., 1995; Kramer et al., 1996; Lai et al., 2010]. A recent study of the highly trabeculated zebrafish heart demonstrates that the endocardial neuregulin 1, in addition to its role in promoting cell proliferation, has another important function in regulating cardiomyocyte delamination to initiate ventricular trabeculation [Liu et al., 2010]. Bone morphogenetic protein 10 (BMP10), enriched in trabecular myocardium, is identified as a potent growth factor for maintaining cardiomyocyte proliferation via its activity in inhibiting p57kip2 (Fig 1) [Chen et al., 2004; Pashmforoush et al., 2004].Vascular endothelial growth factor (VEGF) and angiopoietin likely signal from myocardium to endocardium to regulate ventricular trabeculation [Sato et al., 1995; Ferrara et al., 1996; Suri et al., 1996]. For a proper interaction between endocardium and myocardium, the acellular cardiac jelly plays a key role in modifying these growth signals to be transmitted between these cell layers. Cardiac jelly is rich in extracellular matrix. Mice-deficient in either hyaluronan synthase-2 (Has-2, an enzyme required for production of the mucopolysaccharide hyaluronan) or versican (a chondroitin sulfate proteoglycan) reduce the level of trabeculation remarkably [Yamamura et al., 1997; Mjaatvedt et al., 1998; Camenisch et al., 2000]. This close association of reduced level of cardiac jelly with reduced trabeculation suggests an important contribution of cardiac jelly to ventricular trabeculation. The composition of cardiac jelly is controlled by chromatin-remodeling factor Brg1. Chromatin can be modified non-covalently by the rearrangement of nucleosomes catalyzed by ATP-dependent chromatin remolding complexes to facilitate both transcriptional activation and repression [Dunaief et al., 1994]. The Brm/Brg-associated-factor (BAF) complex is a ten subunit complex in which the ATPase component is encoded by either Brg1 or Brm. BAF complex was shown to be involved in cardiac development [Lickert et al., 2004]. Endocardial-restricted ablation of Brg1 leads to significant defect in ventricular trabeculation in mice [Stankunas et al., 2008]. Detailed molecular analyses have revealed that the absence of Brg1 results in the derepression of ADAMTS1 (a disintegrin and metalloproteinase with thrombospondin motif), which normally increases in expression later in development to prevent excessive trabeculation. The abnormal up-regulation of ADAMTS1 in Brg1 mutant embryos causes premature breakdown of the cardiac jelly and termination of trabeculation [Stankunas et al., 2008] and further demonstrates the critical role of cardiac jelly in ventricular wall development (Fig 1).
In addition to endocardium, epicardium is another important resource for mitogenic factors influencing ventricular wall growth and formation, which includes TGFβ/BMPs, PDGF-A, Wnts, Hh, and FGFs [Sucov et al., 2009]. The epicardium is derived from the proepicardial organ originated in the septum transversum and covers over the surface of the developing heart [Viragh and Challice, 1981; Komiyama et al., 1987; Manner, 1993]. Many of these growth factors are involved in epicardial development and coronary vasculature development. Among them, members of fibroblast growth factors (FGFs) are closely related to ventricular wall formation [Lavine et al., 2005]. Mice deficient in FGF9 or FGF16 develop thin myocardium phenotype [Lavine et al., 2005; Lu et al., 2008]. Myocardial-specific knockout of FGF receptor s1 and 2 (FGFR1 and FGFR2) results in a similar thin wall phenotype, further confirming the role of epicardial FGF-mediated signaling in ventricular wall development. Most interestingly, the epicardial-derived growth signals are particularly important to regulate compact myocardium development, while endocardial-derived growth signals are more relevant to trabecular myocardium formation. Using a neonatal cardiomyocyte cell culture system, Sucov and colleagues analyzed cardiomyocyte response to epicardial- and endocardial-derived factors [Kang and Sucov 2005]. VCAM1, a cell adhesion molecule, is specifically expressed in compact myocardium [Kwee et al., 1995; Terry et al., 1997], which is opposed to BMP10 and atrial natriuretic factor (ANF) as trabecular myocardial markers [Christoffels et al., 2000; Chen et al., 2004]. VCAM1 is specifically up-regulated in cells cultured in conditioned media containing epicardial-derived factors, whereas BMP10 and ANF are up-regulated only by endocardial growth factor neuregulin 1 [Kang and Sucov, 2005]. This finding demonstrates an interactive regulation of ventricular wall growth and development by the endocardial-, myocardial- and epicardial-derived signals (Fig 1).
VENTRICULAR COMPACTION AND LVNC
A significant reduction in trabeculation is usually associated with ventricular compact zone deficiencies (hypoplastic wall), which commonly leads to embryonic heart failure and early embryonic lethality. In contrast, abnormal trabecular remodeling (i.e., compaction) during ventricular wall formation is thought to be associated with ventricular noncompaction. In the past decade, a series of genetically engineered mouse models producing ventricular noncompaction phenotypes have been generated. Although “ventricular noncompaction” has been used in describing their common abnormal ventricular phenotypes, the actual ventricular wall abnormalities are not homogeneous, and they clearly vary from model to model, which may reflect the dynamic complexity of ventricular trabeculation and compaction controlled by distinct signaling pathways. “Hypertrabeculation” is another widely used terminology to describe noncompaction, and it has been suggested as an inadequate terminology for noncompaction phenotype in clinic [Oechslin and Jenni, 2011]. However, recent analyses of various of mouse models demonstrate that some mouse models having ventricular noncompaction are associated with the increased level of trabeculation (number and thickness of the trabeculae) at early embryonic stage, while some not. This suggests that there are two independent biological events leading to abnormal trabeculation and compaction. Thus, we use “hypertrabeculation” to refer to the phenotype with increased number and thickness of the trabeculae at embryonic stage (e.g., mouse E12.5-E14.5) and “noncompaction” to refer to the lack of trabecular remodeling towards the compact wall during and after the trabeculation. Detailed molecular analyses on these mouse models have helped us in determining the genetic pathways that are involved in the ventricular wall formation and maturation as well as the potential pathogenesis pathways contributing to LVNC.
FKBP12-deficient mouse model and the role of BMP10 in ventricular trabeculation
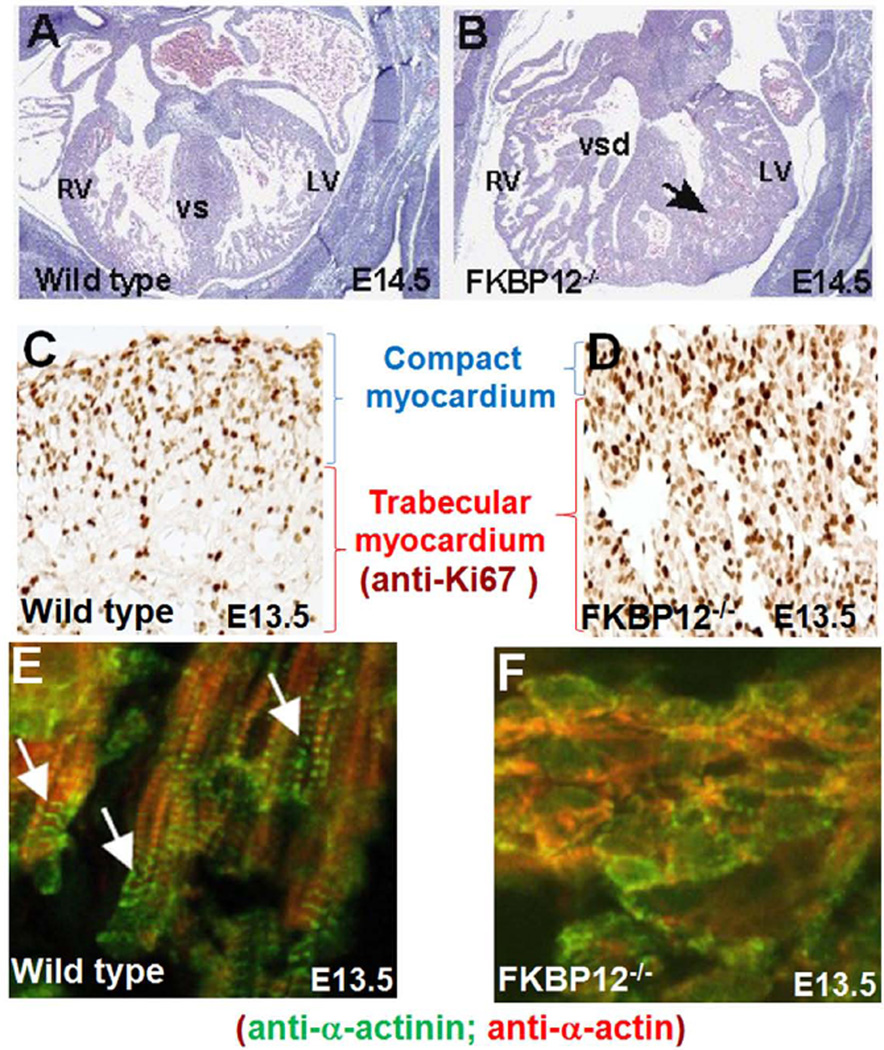
FKBP12 (also known as Fkbp1a) is a ubiquitously expressed 12kDa cytoplasmic protein and belongs to the immunophilin family[Bierer et al., 1990; Schreiber and Crabtree 1995]. FKBP12 has been shown to be associated with multiple intracellular protein complexes, including BMP/activin/TGFβ type-I receptors [Wang et al., 1996] and Ca2+-release channels, such as inositol trisphosphate receptor (IP3R) and ryanodine receptor (RyR) [Jayaraman et al., 1992; Cameron et al., 1995; Cameron et al., 1995; Cameron et al., 1997]. FKBP12-deficient mice die between E14.5 and birth due to severe cardiac defects including a characteristic increase in the number and thickness of ventricular trabeculae, deep inter-trabecular recesses, lack of compaction, thin ventricular wall, and a prominent ventricular septal defect (VSD) [Shou et al., 1998], representing many important clinical features of LVNC. As FKBP12-deficient mouse is the first mouse genetic model for ventricular noncompaction, it has been extensively used to study the underlying mechanisms regulating ventricular trabeculation and compaction.
Using a dual fluorescence imaging technique, we have established a method to quantify the thickness of trabecular ridges and sheets, the overall thickness of the compact wall, and the ratio of trabecular myocardium to compact wall thickness [Chen et al., 2009]. FKBP12-deficient mice are abnormal in both the processes of trabeculation and compaction. The thickening of trabecular myocardium become apparent at E11.5 (hypertrabeculation), followed by the thinning of the compact wall at later stage (noncompaction) (Figs 3A and 3B). There are two major altered cellular phenotypes associated with FKBP12-deficient hearts: 1) increased level of cardiomyocyte cell cycle activity in mutant myocardium, particularly remarkable in trabecular myocardium (Figs 3C and 3D); 2) disrupted cardiomyocyte polarization and myofibrillogenesis in mutant myocardium (Figs 3E & 3F). In normal early developing ventricles, cardiomyocytes are polygonal-shaped [Hirschy et al., 2006; Henderson and Chaudhry, 2011]. Cardiomyocytes are elongated in the trabecular myocardium to become polarized cardiomyocytes with clear structure of myofibrils (Fig 2 and Fig 3E), while cardiomyocytes in compact zone remain polygonal-shaped without distinct structure of myofibril [Hirschy et al., 2006; Henderson and Chaudhry 2011]. In FKBP12-deficient hearts, the majority of trabecular cardiomyocytes remain sphere-shaped, and the myofibrillogenesis is markedly impaired (Fig 3F).
Figure 3.
Hypertrabeculation and noncompaction in FKBP12-deficient hearts. A and B, Cardiac histology of wild-type (A) and FKBP12-deficient heart (B) at E14.5. Black arrow denotes persistent trabecular myocardium in FKBP12 mutant heart. LV, left ventricle; RV, right ventricle; VS, ventricular septum; VSD, ventricular septal defect. C and D, Marked increase of cardiomyocyte proliferation in FKBP12 mutant heart. Immunohistochemical analysis of anti-Ki67 immune reactivity; the dark-brown nuclear signals are positive for Ki67, indicating proliferating cells. E and F, Disrupted cardiomyocyte polarization and myofibrillogenesis in FKBP12 mutant trabecular myocardium. Immunofluorescence staining using anti-α-actinin and anti-α-actin antibody, white arrows denote well organized sarcomeres in elongated normal trabecular cardiomyocytes.
By transcriptome profile analysis, BMP10 is identified as one of the genes dramatically up-regulated in FKBP12-deficient hearts [Chen et al., 2004]. BMP10 is a peptide growth factor that belongs to the TGF-β superfamily [Chen et al., 2004] and is expressed transiently in the ventricular trabecular myocardium from E9.0 to E13.5, a critical time window when cardiac development shifts from patterning to growth and chamber maturation. By E16.5-E18.5, BMP10 is only detectable in the atria, and then is retracted to the right atria in postnatal hearts. Interestingly, the up-regulation of BMP10 expression in developing myocardium is associated with hypertrabeculation phenotype on other genetic mouse models, which include the Nkx2.5-myocardial specific knockout [Pashmforoush et al., 2004] and the Numb/Numblike-deficient mice [Yang et al., 2012]. This suggests that BMP10 is a key morphogenetic growth factor involved in the regulation of cardiac trabeculation and/or compaction.
To determine whether up-regulated BMP10 expression would directly impact trabeculation and/or compaction in the developing myocardium, the human atrial natriuretic factor (hANF) promoter is used to drive exogenous BMP10 expression in mouse embryonic hearts (hANF-BMP10). The transgene positive hearts have a significantly increased thickness of trabecular myocardium [Pashmforoush et al., 2004]. These data demonstrate that overexpression of BMP10 alone in the embryonic heart is sufficient to cause cardiac trabeculation abnormality. Consistently, BMP10-deficient embryos die in utero at E10.5 display cardiac dysgenesis with profound hypoplastic ventricular walls and an absence of ventricular trabeculae. Further analyses demonstrate that there is a marked reduction of proliferation in BMP10-deficient cardiomyocytes [Chen et al., 2004; Chen et al., 2009] and suggest that BMP10, although not critical for the initiation of cardiac trabeculation, is essential for subsequent trabecular growth. Consistent with this finding, cardiomyocyte proliferation is profoundly increased in FKBP12-deficient hearts and Nkx2.5 mutant hearts [Chen et al., 2004; Pashmforoush et al., 2004; Chen et al., 2009].
p57kip2 is a critical negative cell cycle regulator [Besson et al., 2008]. Interestingly, p57kip2 expression is first detectable in the developing mouse heart at E10.5 and is restricted to the ventricular trabecular myocardium [Kochilas et al., 1999]. Therefore, p57kip2 is considered a key negative regulator involved in cardiac cell cycle exit within the developing ventricular trabeculae during cardiac chamber maturation. Immunohistochemistry staining reveals that p57kip2 is up-regulated and ectopically expressed throughout the ventricular wall in BMP10-null hearts at E9.5 compared to wild type littermate controls. Conversely, FKBP12-deficient hearts exhibit significantly lower p57kip2 levels in trabecular myocardium [Chen et al., 2004]. Taken together, the analysis of FKBP12-deficient hearts demonstrated the first time that the increased level of cardiomyocyte proliferation is closely associated with the genesis of ventricular hypertrabeculation and noncompaction.
Notch signaling in venricular trabeculation and LVNC
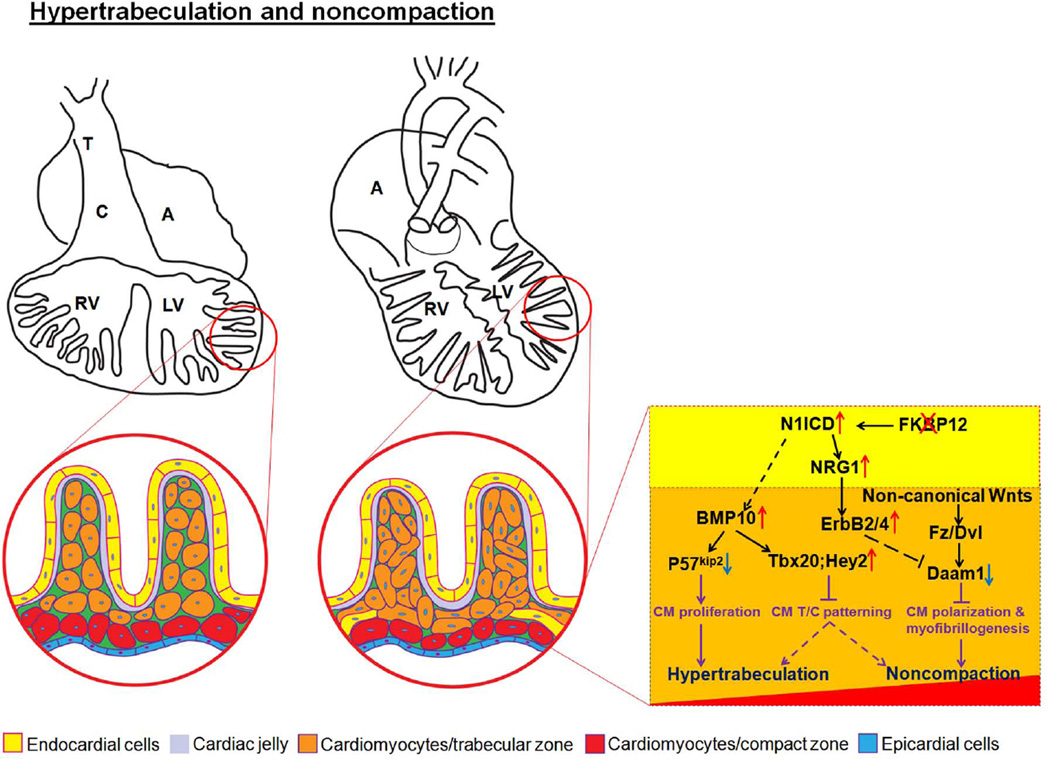
Earlier work by Watanabe et al used an inducible transgenic system to demonstrate that overexpression of activated Notch 1 (N1ICD) in early cardiac cell lineages led to several abnormal cardiac phenotypes, including abnormal ventricular morphology similar to ventricular noncompaction [Watanabe et al., 2006], suggesting that Notch signaling is a potential contributor to ventricular hypertrabeculation and/or noncompaction. Interestingly, while Notch2 and Jag1 expression are restricted to the developing trabecular myocardium, Notch1, Notch4 and Delta4 are transcribed in the endocardium from gastrulation onward [Del Monte et al., 2007]. Consistently, activated Notch1 protein (N1ICD) is found predominantly in the endocardial cells proximal to the base of the developing trabeculae [Del Monte et al., 2007; Grego-Bessa et al., 2007]. Endothelial/endocardial restricted ablation of Notch 1 or its co-factor RBPjk lead to a defect in ventricular trabeculation, strongly supporting the role of Notch signaling in the development of ventricular wall. Molecular analysis suggests that Notch–dependent signaling regulates trabeculation via neuregulin 1, EphrinB2, and BMP10 [Grego-Bessa et al., 2007]. Subsequent analysis using embryo cultures with conditioned media supplemented with either neuregulin 1 or BMP10 further demonstrate that BMP10 is required for Notch-mediated regulation of cardiomyocyte proliferation, while neuregulin 1 likely mediates ventricular trabeculation independent of cell proliferation [Grego-Bessa et al., 2007], which is consistent with other published work [Chen et al., 2004; Liu et al., 2010]. In addition, our recent work demonstrated that FKBP12 is a novel regulator for endothelial Notch1 activity [Chen et al., 2013] and that the upstream regulator for BMP10 expression lies within the developing endocardium [Chen et al., 2009]. Endothelial-restricted ablation of FKBP12 enhanced N1ICD activity; the inhibition of Notch activity partly normalizes abnormal hypertrabeculation phenotype in FKBP12-deficient hearts [Chen et al., 2013]. Interestingly, Tbx20 and Hey2 expression are expanded from compact wall to trabecular myocardium in FKBP12 mutant hearts, demonstrating a loss of trabecular/compact myocardium patterning (Fig 4) [Chen et al., 2013]. Consistently, earlier work also shows that BMP10 regulates Tbx20 myocardial expression [Zhang et al., 2011]. These findings implie that Notch signaling has a critical role in regulating trabecular/compact myocardium patterning. Other mouse models with enhanced Notch activity are also associated with hypertrabeculation and/or noncompaction, including Jarid2/Jumonji mutants [Lee et al., 2000; Mysliwiec et al., 2011; Mysliwiec et al., 2012], and the numb/numblike compound mutant mice [Yang et al., 2012]. Collectively, these data strongly support that Notch signaling is essential for normal ventricular wall development (Fig 1).
Figure 4.
Disrupted morphogenetic patterning of trabecular and compact myocardium. In situ hybridization analysis of Hey2 and Tbx20 expression; both Hey2 and Tbx20 expression are significantly higher in compact myocardium of wild-type hearts, but are expanded to trabecular myocardium in FKBP12 mutant hearts. Red arrows denote trabecular myocardium; blue arrows denote compact wall.
Noncanonical Wnt/PCP signaling and ventricular noncompaction
Planar cell polarity (PCP) signaling is an essential molecular mechanism by which epithelial cells establish polarity in planes orthogonal to apical-basal axis [Wang and Nathans, 2007; Simons and Mlodzik, 2008]. The key features for PCP signaling include the control of cellular alignment and orientation in polarized tissues [Wang and Nathans, 2007; Simons and Mlodzik, 2008]. The major PCP signaling components are mainly demonstrated in the Drosophila by using genetic mutagenesis and clonal analysis, which has revealed an essential role of noncanonical Wnt signaling, independent of β-catenin, is critical for PCP [Wang and Nathans, 2007; Simons and Mlodzik, 2008]. This Wnt/PCP signaling pathway has an essential function in embryonic patterning and organogenesis, more specifically in vertebrates including the regulation of gastrulation, neurulation, and hair cell polarity within the inner ear in mouse and within the lateral line in zebrafish [Wang and Nathans, 2007; Simons and Mlodzik, 2008]. This noncanonical Wnt/PCP signaling pathway in Drosophila is relatively well understood [Wang and Nathans, 2007; Simons and Mlodzik, 2008], which is composed of core signaling components [e.g., Frizzled (Fz), Dishevelled (Dvl), Prickle (Pk), Vangl, Celsr1], PCP regulators [e.g., Casein kinase 1ε (CK1ε)] and a large number of PCP effectors (e.g., Daam1, Rac1, PhoA) [Simons and Mlodzik, 2008]. Interestingly, many of the core signaling components are well conserved in vertebrates and mammals. However, the degree of mechanistic signaling conservation between flies and vertebrates and mammals is still unclear. Cardiomyocytes are not a type of epithelial cells, thus they may not have a classic PCP phenotype. But the mature cardiomyocytes are highly polarized with the majority of cell-cell junction proteins located at intercalated disc, joining cell end to end, while the attachment to the extracellular matrix are on their lateral surface [Hirschy et al., 2006; Henderson and Chaudhry, 2011]. Interestingly, the immature cardiomyocytes at early embryonic hearts are mostly polygonal or spherical [Hirschy et al., 2006; Henderson and Chaudhry, 2011]. The mechanism of cardiomyocytes polarization during cardiac development, and more specifically whether Wnt/PCP signaling has a role in cardiomyocyte polarization, is unknown.
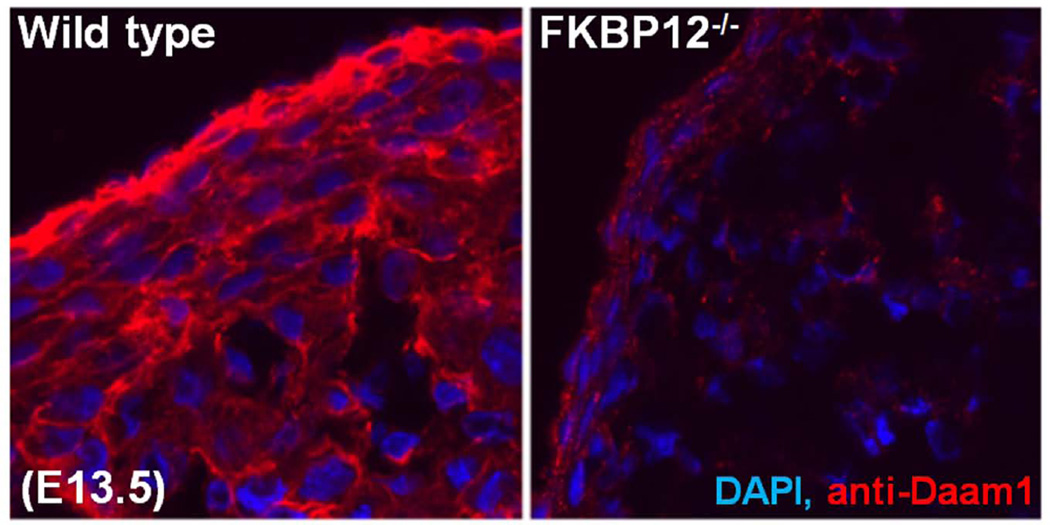
Interestingly, mutant mouse models deficient in several key Wnt/PCP signaling components develop ventricular noncompaction. Vangl2 is expressed in the developing myocardium and adult myocardium [Phillips et al., 2005]. Mice-deficient in Vangl2 (loop-tail mutant, lp/lp) develop thin ventricular wall with noncompaction characteristics [Phillips et al., 2008]. lp/lp cardiomyocytes are spherical in shape, whereas normal cardiomyocytes are elongated [Phillips et al., 2008]. Another key Wnt/PCP molecule Scrib may also contribute to cardiomyocyte polarization [Murdoch et al., 2003; Phillips et al., 2007]. Examination of Scrib mutant mice (Circletail mutant, Crc/Crc) demonstrate altered ventricular chamber formation that resembles ventricular noncompaction. Importantly, the mutations in both of these genes result in abnormalities in the polarization and organization of cardiomyocyte in ventricular myocardium as well as outflow myocardium; the latter gives rise to outflow defects such as Double Outlet Right Ventricle (DORV). This strongly suggests that cardiomyocyte polarization is critical for cardiac development and that Wnt/PCP signaling is a key signaling pathway in regulating cardiomyocyte polarization (Fig 2). In addition, other recent experimental evidence for a role of Wnt/PCP signaling in ventricular development comes from the studies of dishevelled (Dvl, the core Wnt/PCP signaling element) and disheveled-associated activator of morphogenesis 1 (Daam1, the potential effector of Wnt/PCP signaling) mutant mice (Figure 2). Similar to Vangl2 and Scrib mutants, mutant mice-deficient in Dvl1/2 develop severe defects in both outflow tract development and ventricular structure [Sinha et al., 2012]. Daam1 is expressed in the developing hearts and adult hearts. The first indication of Daam1 has a role in ventricular wall development comes from our initial screening of differentially expressed genes in FKBP12-deficient hearts when compared to normal control hearts. Daam1 is markedly down-regulated in FKBP12-deficient ventricles (Fig 5). Thus, we have generated Daam1-deficient mice to explore the potential function of Daam1 in ventricular formation [Li et al., 2011]. In most part, Daam1-deficient mice resemble abovementioned mutant mice and have defects in cardiomyocyte polarization, alignment, and junction, which results in ventricular noncompaction, DORV, and ventricular septal defect (VSD) [Li et al., 2011]. Importantly, Daam1-deficient hearts have normal cardiomyocyte proliferation and BMP10 expression, suggesting that altered cell cycle activity is not required for ventricular noncompaction phenotype [Li et al., 2011] and that two independent signaling pathways underlie the pathogenesis of hypertrabeculation and noncompaction (Fig 6). A recent case report revealed that a human copy number variation (CNV) in DAAM1 gene was associated with multiple cardiac congenital defects (CHDs) and embryonic heart failure [Bao et al., 2012], further suggesting the role of Wnt/PCP signaling in the pathogenesis of CHDs and inherited cardiomyopathies.
Figure 5.
Daam1 down-regulated in FKBP12 mutant hearts (E14.5). Immunofluorescence staining using anti-Daam1 antibody.
Figure 6.
Hypothetical model for the pathogenesis of ventricular hypertrabeculation and noncompaction. Two independent signaling pathways are associated with ventricular hypertrabeculation and noncompaction, in which abnormal regulation of cardiomyocyte proliferation (i.e., BMP10-mediated pathway) underlies the development of hypertrabeculation, while abnormal regulation of cardiomyocyte polarity and myofibrillogenesis (i.e., Daam1-mediated function) underlies the development of ventricular noncompaction.
LVNC mouse models for human mutations
In the past decade, genetic screening of LVNC patients (mostly isolated form) identified a handful of gene mutations, mostly sarcomere proteins, such as β-myosin heavy chain (βMHC/MYH7), α-cardiac actin (ACTC), cardiac troponin T (TNNT2), Tafazzin (TAZ/G4.5), α-dystrobrevin, lamin A/C (LMNA), ZASP/LBD3, and dystrophin [Xing et al., 2006; Klaassen et al., 2008]. Interestingly, most of these genes are part of myofibril structure and are associated with contractile function. However, it is puzzling how these genetic variations and mutations lead to ventricular noncompaction that is presumably developed at embryonic stage. One recent report suggests that the presence or absence of a sarcomere gene mutation in LVNC does not predict the clinical phenotypes [Probst et al., 2011], further clouding the genetic etiology and the pathogenesis pathways for LVNC in patients.
Several attempts have been made to mimic human LVNC in mice. Barth syndrome (OMIM302060) is a rare-X-linked multisystem genetic disorder due to mutations in G4.5 (tafazzin) gene [Schlame et al., 2002; Towbin 2010]. Tafazzin catalyzes cardiolipin biosynthesis [Xu et al., 2006; Xu et al., 2006]. Normal cardiolipin is critical to mitochondrial bioenergetic function. LVNC is one of several clinical hallmarks of Barth syndrome. Phoon and his colleagues used an inducible shRNA transgenic approach to knock-down tafazzin in mice (TAZKD) and studied the pathogenesis of LVNC [Phoon et al., 2012]. Tafazzin knockdown leads to altered cardiolipin and mitochondrial ultrastructure, as well as prenatal and perinatal lethality due to altered cardiac function at embryonic stage [Phoon et al., 2012]. Importantly, the defects of TAZKD hearts mimic clinical phenotype of ventricular noncompaction, which can be seen as early as E13.5, thus providing an important animal model for Barth syndrome and the LVNC [Phoon et al., 2012].
Another interesting missense mutation at codon 96 (from GAG to AAG) in exon 10 of the cardiac troponin T gene (TNNT2) is found to associate with a familial case of LVNC. Mouse transgenic approach was used to mimic the clinical condition by introducing this mutation into the mouse heart using the αMHC promoter [Luedde et al., 2010]. Although the transgenic overexpression of the mutant TNNT2 leads to cardiomyopathy and severely altered cardiac function, histological findings have excluded LVNC phenotype [Luedde et al., 2010]. This discrepancy is likely due to transgenic overexpression system and/or the genetic difference between humans and mice. The highly variable outcomes of cardiomyopathy phenotypes in association with mutations of TNNT2 and other genes critical to cardiac contractile function strongly suggest the genetic modifiers are involved in the pathogenesis of LVNC. Excitingly, a recent report describes a human mutant in mindbomb homolog 1 (MIB1) is associated with a LVNC familial case. Myocardial specific knockout of Mib1 apparently recapitulates the clinical hallmarks of LVNC [Luxan et al., 2013]. MIB1 is an E3 ubiquitin ligase that regulates the endocytosis of the Notch ligands Delta and Jagged. Affected individuals show altered NOTCH1 activity and altered expression of target genes, which leads to the loss of trabecular/compact myocardial patterning similarly seen in FKBP12 mutant hearts (Figure 4 and Figure 6). This mutation and the subsequent confirmation using a mouse model further strengthen the notion that Notch signaling is a critical contributor to the pathogenesis of LVNC, and it may also reflect the complexity of Notch signaling pathways in which the right combination of spatial, temporal and dosage is essential for regulating ventricular wall formation.
In addition to abovementioned mouse models, there are many other mouse models having LVNC phenotypes, such as 14-3-3ε/Ywhae knockout mouse [Kosaka et al., 2012; Chang et al., 2013], transgenic mice overexpressing SHP2-Q79R (the Noonan Syndrome SHP2 gain-of-function mutation) [Nakamura et al., 2007], and transgenic mice overexpressing βMHC-Met531Arg [Kaneda et al., 2008]. However, the complexity of genetic heterogeneity and clinical phenotypes in LVNC patients suggest that multiple mechanisms at molecular and cellular levels are involved in the pathogenesis of LVNC. Figure 6 provides our currently understanding and hypothesis of two independent signaling pathways associated with ventricular hypertrabeculation and noncompaction using FKBP12 mutant mice as a model. It is understandable that the current study may have only revealed a subset of signaling pathways and networks. Thus, continued efforts to model clinical and genetic findings in animals will advance our understanding of the molecular mechanisms by which regulate ventricular trabeculation and compaction, and will eventually provide solutions to the medical challenges of LVNC.
ACKNOWLEDGMENTS
The work is supported in part by the National Institute of Health to W.S. and C-P.C. and by the Riley Children’s foundation to W.Z and W.S. C-P C is also supported by the American Heart Association Established Investigator Award, March of Dimes Foundation, CHARGE syndrome Foundation, Oak Foundation, Stanford Child Health Research Institute.
Biographies
Wenjun Zhang, M.D. is a research assistant professor of Pediatrics, Riley Heart Research Center, Indiana University School of Medicine. His research focuses on molecular signaling in regulating of mammalian heart development and heart failure.
Hanying Chen, M.D., is a research assistant professor of Pediatrics, Riley Heart Research Center, Indiana University School of Medicine. His research focuses on TGFβ/BMP signaling in regulating of ventricular wall development and maturation.
Xiuxia Qu, Ph.D., is a research associate at Department of Medical and Molecular Genetics, Indiana University School of Medicine. Her research interests is FGF signaling and morphogens in mammalian embryonic development and organogenesis.
Ching-Pin Chang, M.D., Ph.D. is Associate Professor of Medicine, Division of Cardiovascular Medicine, Department of Medicine, Stanford University School of Medicine. His research focuses on the epigenetic regulation of cardiovascular development and heart failure, and the translation of the bench findings to clinical applications.
Weinian Shou, Ph.D. is Professor of Pediatrics, Biochemistry & Molecular Biology, Medical & Molecular Genetics and Pharmacology & Toxicology, Indiana University School of Medicine. His research focuses on the molecular signaling regulating cardiac development and the pathogenesis of congenital heart defects, with particular emphasis on ventricle wall formation and maturation.
Footnotes
The authors do not have conflict of interest to declare.
REFERENCES
- Anderson RH, Ho SY, Sanchez-Quintana D, Redmann K, Lunkenheimer PP. Heuristic problems in defining the three-dimensional arrangement of the ventricular myocytes. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:579–586. doi: 10.1002/ar.a.20330. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Sanchez-Quintana D, Niederer P, Lunkenheimer PP. Structural-functional correlates of the 3-dimensional arrangement of the myocytes making up the ventricular walls. J Thorac Cardiovasc Surg. 2008;136:10–18. doi: 10.1016/j.jtcvs.2007.09.083. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Sanchez-Quintana D, Redmann K, Lunkenheimer PP. How are the myocytes aggregated so as to make up the ventricular mass? Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007:76–86. doi: 10.1053/j.pcsu.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Aras D, Tufekcioglu O, Ergun K, Ozeke O, Yildiz A, Topaloglu S, Deveci B, Sahin O, Kisacik HL, Korkmaz S. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail. 2006;12:726–733. doi: 10.1016/j.cardfail.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Bao B, Zhang L, Hu H, Yin S, Liang Z. Deletion of a single-copy DAAM1 gene in congenital heart defect: a case report. BMC Med Genet. 2012;13:63. doi: 10.1186/1471-2350-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartman T, Hove J. Mechanics and function in heart morphogenesis. Developmental Dynamics. 2005;233:373–381. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- Bartman T, Hove J. Mechanics and function in heart morphogenesis. Dev Dyn. 2005;233:373–381. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Bierer BE, Mattila PS, Standaert RF, Herzenberg LA, Burakoff SJ, Crabtree G, Schreiber SL. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990;87:9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert DL, Andries LJ. The endocardial endothelium. Am J Physiol. 1992;263:H985–H1002. doi: 10.1152/ajpheart.1992.263.4.H985. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AM, Nucifora FC, Jr, Fung ET, Livingston DJ, Aldape RA, Ross CA, Snyder SH. FKBP12 binds the inositol 1,4,5-trisphosphate receptor at leucine-proline (1400–1401) and anchors calcineurin to this FK506-like domain. J Biol Chem. 1997;272:27582–27588. doi: 10.1074/jbc.272.44.27582. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. 1995;83:463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Sabatini DM, Kaplin AI, Walensky LD, Snyder SH. Immunophilin FK506 binding protein associated with inositol 1,4,5-trisphosphate receptor modulates calcium flux. Proc Natl Acad Sci U S A. 1995;92:1784–1788. doi: 10.1073/pnas.92.5.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Gorbea C, Lezin G, Li L, Shan L, Sakai N, Kogaki S, Otomo T, Okinaga T, Hamaoka A, Yu X, Hata Y, Nishida N, Yost HJ, Bowles NE, Brunelli L, Ichida F. 14-3-3epsilon Gene variants in a Japanese patient with left ventricular noncompaction and hypoplasia of the corpus callosum. Gene. 2013;515:173–180. doi: 10.1016/j.gene.2012.12.049. [DOI] [PubMed] [Google Scholar]
- Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, Chen Z, Yang Z, Schneider MD, Chien KR, Conway SJ, Yoder MC, Haneline LS, Franco D, Shou W. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang W, Li D, Cordes TM, Mark Payne R, Shou W. Analysis of ventricular hypertrabeculation and noncompaction using genetically engineered mouse models. Pediatr Cardiol. 2009;30:626–634. doi: 10.1007/s00246-009-9406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang W, Sun X, Yoshimoto M, Chen Z, Zhu W, Liu J, Shen Y, Yong W, Li D, Zhang J, Lin Y, Li B, Vandusen NJ, Snider P, Schwartz RJ, Conway SJ, Field LJ, Yoder MC, Firulli AB, Carlesso N, Towbin JA, Shou W. Fkbp1a controls ventricular myocardium trabeculation and compaction by regulating endocardial Notch1 activity. Development. 2013;140:1946–1957. doi: 10.1242/dev.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels VM, Keijser AG, Houweling AC, Clout DE, Moorman AF. Patterning the embryonic heart: identification of five mouse Iroquois homeobox genes in the developing heart. Dev Biol. 2000;224:263–274. doi: 10.1006/dbio.2000.9801. [DOI] [PubMed] [Google Scholar]
- Daubeney PE, Nugent AW, Chondros P, Carlin JB, Colan SD, Cheung M, Davis AM, Chow CW, Weintraub RG. Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation. 2006;114:2671–2678. doi: 10.1161/CIRCULATIONAHA.106.635128. [DOI] [PubMed] [Google Scholar]
- Del Monte G, Grego-Bessa J, Gonzalez-Rajal A, Bolos V, De La Pompa JL. Monitoring Notch1 activity in development: evidence for a feedback regulatory loop. Dev Dyn. 2007;236:2594–2614. doi: 10.1002/dvdy.21246. [DOI] [PubMed] [Google Scholar]
- Dorri F, Niederer PF, Redmann K, Lunkenheimer PP, Cryer CW, Anderson RH. An analysis of the spatial arrangement of the myocardial aggregates making up the wall of the left ventricle. Eur J Cardiothorac Surg. 2007;31:430–437. doi: 10.1016/j.ejcts.2006.11.040. [DOI] [PubMed] [Google Scholar]
- Dunaief JL, Strober BE, Guha S, Khavari PA, Alin K, Luban J, Begemann M, Crabtree GR, Goff SP. The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest. Cell. 1994;79:119–130. doi: 10.1016/0092-8674(94)90405-7. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Freedom RM, Yoo SJ, Perrin D, Taylor G, Petersen S, Anderson RH. The morphological spectrum of ventricular noncompaction. Cardiol Young. 2005;15:345–364. doi: 10.1017/S1047951105000752. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Kubalak S, Mikawa T. Conducting the embryonic heart: orchestrating development of specialized cardiac tissues. Trends Cardiovasc Med. 1999;9:18–26. doi: 10.1016/s1050-1738(98)00035-8. [DOI] [PubMed] [Google Scholar]
- Grant RT. An unusual anomaly of the coronary vessels in the malformed heart of a child. heart. 1926;13:273–283. [Google Scholar]
- Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, Perez-Pomares JM, de la Pompa JL. Notch signaling is essential for ventricular chamber development. Dev Cell. 2007;12:415–429. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DJ, Chaudhry B. Getting to the heart of planar cell polarity signaling. Birth Defects Res A Clin Mol Teratol. 2011;91:460–467. doi: 10.1002/bdra.20792. [DOI] [PubMed] [Google Scholar]
- Hirschy A, Schatzmann F, Ehler E, Perriard JC. Establishment of cardiac cytoarchitecture in the developing mouse heart. Dev Biol. 2006;289:430–441. doi: 10.1016/j.ydbio.2005.10.046. [DOI] [PubMed] [Google Scholar]
- Hoedemaekers YM, Caliskan K, Michels M, Frohn-Mulder I, van der Smagt JJ, Phefferkorn JE, Wessels MW, ten Cate FJ, Sijbrands EJ, Dooijes D, Majoor-Krakauer DF. The importance of genetic counseling, DNA diagnostics, and cardiologic family screening in left ventricular noncompaction cardiomyopathy. Circ Cardiovasc Genet. 2010;3:232–239. doi: 10.1161/CIRCGENETICS.109.903898. [DOI] [PubMed] [Google Scholar]
- Icardo JM. Heart anatomy and developmental biology. Experientia. 1988;44:910–919. doi: 10.1007/BF01939884. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Fernandez-Teran A. Morphologic study of ventricular trabeculation in the embryonic chick heart. Acta Anat (Basel) 1987;130:264–274. doi: 10.1159/000146455. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Brillantes AM, Timerman AP, Fleischer S, Erdjument-Bromage H, Tempst P, Marks AR. FK506 binding protein associated with the calcium release channel (ryanodine receptor) J Biol Chem. 1992;267:9474–9477. [PubMed] [Google Scholar]
- Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–671. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T, Naruse C, Kawashima A, Fujino N, Oshima T, Namura M, Nunoda S, Mori S, Konno T, Ino H, Yamagishi M, Asano M. A novel beta-myosin heavy chain gene mutation, p.Met531Arg, identified in isolated left ventricular non-compaction in humans, results in left ventricular hypertrophy that progresses to dilation in a mouse model. Clin Sci (Lond) 2008;114:431–440. doi: 10.1042/CS20070179. [DOI] [PubMed] [Google Scholar]
- Kang JO, Sucov HM. Convergent proliferative response and divergent morphogenic pathways induced by epicardial and endocardial signaling in fetal heart development. Mech Dev. 2005;122:57–65. doi: 10.1016/j.mod.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Klaassen S, Probst S, Oechslin E, Gerull B, Krings G, Schuler P, Greutmann M, Hurlimann D, Yegitbasi M, Pons L, Gramlich M, Drenckhahn JD, Heuser A, Berger F, Jenni R, Thierfelder L. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation. 2008;117:2893–2901. doi: 10.1161/CIRCULATIONAHA.107.746164. [DOI] [PubMed] [Google Scholar]
- Kochilas LK, Li J, Jin F, Buck CA, Epstein JA. p57Kip2 expression is enhanced during mid-cardiac murine development and is restricted to trabecular myocardium. Pediatr Res. 1999;45:635–642. doi: 10.1203/00006450-199905010-00004. [DOI] [PubMed] [Google Scholar]
- Komiyama M, Ito K, Shimada Y. Origin and development of the epicardium in the mouse embryo. Anat Embryol (Berl) 1987;176:183–189. doi: 10.1007/BF00310051. [DOI] [PubMed] [Google Scholar]
- Kosaka Y, Cieslik KA, Li L, Lezin G, Maguire CT, Saijoh Y, Toyo-oka K, Gambello MJ, Vatta M, Wynshaw-Boris A, Baldini A, Yost HJ, Brunelli L. 14-3-3epsilon plays a role in cardiac ventricular compaction by regulating the cardiomyocyte cell cycle. Mol Cell Biol. 2012;32:5089–5102. doi: 10.1128/MCB.00829-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R, Bucay N, Kane DJ, Martin LE, Tarpley JE, Theill LE. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc Natl Acad Sci U S A. 1996;93:4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- Lai D, Liu X, Forrai A, Wolstein O, Michalicek J, Ahmed I, Garratt AN, Birchmeier C, Zhou M, Hartley L, Robb L, Feneley MP, Fatkin D, Harvey RP. Neuregulin 1 sustains the gene regulatory network in both trabecular and nontrabecular myocardium. Circ Res. 2010;107:715–727. doi: 10.1161/CIRCRESAHA.110.218693. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Lee Y, Song AJ, Baker R, Micales B, Conway SJ, Lyons GE. Jumonji, a nuclear protein that is necessary for normal heart development. Circ Res. 2000;86:932–938. doi: 10.1161/01.res.86.9.932. [DOI] [PubMed] [Google Scholar]
- Li D, Hallett MA, Zhu W, Rubart M, Liu Y, Yang Z, Chen H, Haneline LS, Chan RJ, Schwartz RJ, Field LJ, Atkinson SJ, Shou W. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138:303–315. doi: 10.1242/dev.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert H, Takeuchi JK, Von Both I, Walls JR, McAuliffe F, Adamson SL, Henkelman RM, Wrana JL, Rossant J, Bruneau BG. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432:107–112. doi: 10.1038/nature03071. [DOI] [PubMed] [Google Scholar]
- Liu J, Bressan M, Hassel D, Huisken J, Staudt D, Kikuchi K, Poss KD, Mikawa T, Stainier DY. A dual role for ErbB2 signaling in cardiac trabeculation. Development. 2010;137:3867–3875. doi: 10.1242/dev.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SY, Sheikh F, Sheppard PC, Fresnoza A, Duckworth ML, Detillieux KA, Cattini PA. FGF-16 is required for embryonic heart development. Biochem Biophys Res Commun. 2008;373:270–274. doi: 10.1016/j.bbrc.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde M, Ehlermann P, Weichenhan D, Will R, Zeller R, Rupp S, Muller A, Steen H, Ivandic BT, Ulmer HE, Kern M, Katus HA, Frey N. Severe familial left ventricular non-compaction cardiomyopathy due to a novel troponin T (TNNT2) mutation. Cardiovasc Res. 2010;86:452–460. doi: 10.1093/cvr/cvq009. [DOI] [PubMed] [Google Scholar]
- Lunkenheimer PP, Redmann K, Kling N, Jiang X, Rothaus K, Cryer CW, Wubbeling F, Niederer P, Heitz PU, Ho SY, Anderson RH. Three-dimensional architecture of the left ventricular myocardium. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:565–578. doi: 10.1002/ar.a.20326. [DOI] [PubMed] [Google Scholar]
- Lunkenheimer PP, Redmann K, Westermann P, Rothaus K, Cryer CW, Niederer P, Anderson RH. The myocardium and its fibrous matrix working in concert as a spatially netted mesh: a critical review of the purported tertiary structure of the ventricular mass. Eur J Cardiothorac Surg. 2006;29(Suppl 1):S41–S49. doi: 10.1016/j.ejcts.2006.02.062. [DOI] [PubMed] [Google Scholar]
- Luxan G, Casanova JC, Martinez-Poveda B, Prados B, D'Amato G, MacGrogan D, Gonzalez-Rajal A, Dobarro D, Torroja C, Martinez F, Izquierdo-Garcia JL, Fernandez-Friera L, Sabater-Molina M, Kong YY, Pizarro G, Ibanez B, Medrano C, Garcia-Pavia P, Gimeno JR, Monserrat L, Jimenez-Borreguero LJ, de la Pompa JL. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat Med. 2013;19:193–201. doi: 10.1038/nm.3046. [DOI] [PubMed] [Google Scholar]
- Manner J. Experimental study on the formation of the epicardium in chick embryos. Anat Embryol (Berl) 1993;187:281–289. doi: 10.1007/BF00195766. [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Kelly RG, Rocancourt D, Eloy-Trinquet S, Nicolas JF, Buckingham ME. A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development. 2003;130:3877–3889. doi: 10.1242/dev.00580. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Borisov A, Brown AM, Fischman DA. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus: I. Formation of the ventricular myocardium. Dev Dyn. 1992;193:11–23. doi: 10.1002/aja.1001930104. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Cohen-Gould L, Fischman DA. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus. III: Polyclonal origin of adjacent ventricular myocytes. Dev Dyn. 1992;195:133–141. doi: 10.1002/aja.1001950208. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM, Anderson RH, van den Hoff MJ. The heart-forming fields: one or multiple? Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 2007;362:1257–1265. doi: 10.1098/rstb.2007.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, Arkell R, Stanier P, Copp AJ. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- Mysliwiec MR, Bresnick EH, Lee Y. Endothelial Jarid2/Jumonji is required for normal cardiac development and proper Notch1 expression. J Biol Chem. 2011;286:17193–17204. doi: 10.1074/jbc.M110.205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysliwiec MR, Carlson CD, Tietjen J, Hung H, Ansari AZ, Lee Y. Jarid2 (Jumonji, AT rich interactive domain 2) regulates NOTCH1 expression via histone modification in the developing heart. J Biol Chem. 2012;287:1235–1241. doi: 10.1074/jbc.M111.315945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Colbert M, Krenz M, Molkentin JD, Hahn HS, Dorn GW, 2nd, Robbins J. Mediating ERK 1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J Clin Invest. 2007;117:2123–2132. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AW, Daubeney PE, Chondros P, Carlin JB, Cheung M, Wilkinson LC, Davis AM, Kahler SG, Chow CW, Wilkinson JL, Weintraub RG. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- Nugent AW, Daubeney PE, Chondros P, Carlin JB, Colan SD, Cheung M, Davis AM, Chow CW, Weintraub RG. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation. 2005;112:1332–1338. doi: 10.1161/CIRCULATIONAHA.104.530303. [DOI] [PubMed] [Google Scholar]
- Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32:1446–1456. doi: 10.1093/eurheartj/ehq508. [DOI] [PubMed] [Google Scholar]
- Pashmforoush M, Lu JT, Chen H, Amand TS, Kondo R, Pradervand S, Evans SM, Clark B, Feramisco JR, Giles W, Ho SY, Benson DW, Silberbach M, Shou W, Chien KR. Nkx2–5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117:373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- Phillips HM, Hildreth V, Peat JD, Murdoch JN, Kobayashi K, Chaudhry B, Henderson DJ. Non-cell-autonomous roles for the planar cell polarity gene Vangl2 in development of the coronary circulation. Circ Res. 2008;102:615–623. doi: 10.1161/CIRCRESAHA.107.160861. [DOI] [PubMed] [Google Scholar]
- Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- Phillips HM, Rhee HJ, Murdoch JN, Hildreth V, Peat JD, Anderson RH, Copp AJ, Chaudhry B, Henderson DJ. Disruption of planar cell polarity signaling results in congenital heart defects and cardiomyopathy attributable to early cardiomyocyte disorganization. Circ Res. 2007;101:137–145. doi: 10.1161/CIRCRESAHA.106.142406. [DOI] [PubMed] [Google Scholar]
- Phoon CK, Acehan D, Schlame M, Stokes DL, Edelman-Novemsky I, Yu D, Xu Y, Viswanathan N, Ren M. Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction. J Am Heart Assoc. 2012:1. doi: 10.1161/JAHA.111.000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst S, Oechslin E, Schuler P, Greutmann M, Boye P, Knirsch W, Berger F, Thierfelder L, Jenni R, Klaassen S. Sarcomere gene mutations in isolated left ventricular noncompaction cardiomyopathy do not predict clinical phenotype. Circ Cardiovasc Genet. 2011;4:367–374. doi: 10.1161/CIRCGENETICS.110.959270. [DOI] [PubMed] [Google Scholar]
- Risebro CA, Riley PR. Formation of the ventricles. Scientific World Journal. 2006;6:1862–1880. doi: 10.1100/tsw.2006.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter M, Oechslin E, Sutsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72:26–31. doi: 10.4065/72.1.26. [DOI] [PubMed] [Google Scholar]
- Rumyantsev PP, Krylova MI. Ultrastructure of myofibers and cells synthesizing DNA in the developing and regenerating lymph-heart muscles. Int Rev Cytol. 1990;120:1–52. doi: 10.1016/s0074-7696(08)61598-3. [DOI] [PubMed] [Google Scholar]
- Sandhu R, Finkelhor RS, Gunawardena DR, Bahler RC. Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction. Echocardiography. 2008;25:8–12. doi: 10.1111/j.1540-8175.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Schlame M, Towbin JA, Heerdt PM, Jehle R, DiMauro S, Blanck TJ. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann Neurol. 2002;51:634–637. doi: 10.1002/ana.10176. [DOI] [PubMed] [Google Scholar]
- Schmid P, Lunkenheimer PP, Redmann K, Rothaus K, Jiang X, Cryer CW, Jaermann T, Niederer P, Boesiger P, Anderson RH. Statistical analysis of the angle of intrusion of porcine ventricular myocytes from epicardium to endocardium using diffusion tensor magnetic resonance imaging. Anat Rec (Hoboken) 2007;290:1413–1423. doi: 10.1002/ar.20604. [DOI] [PubMed] [Google Scholar]
- Schreiber SL, Crabtree GR. Immunophilins, ligands, and the control of signal transduction. Harvey Lect. 1995;91:99–114. [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anatomical Record. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Shou W, Aghdasi B, Armstrong DL, Guo Q, Bao S, Charng MJ, Mathews LM, Schneider MD, Hamilton SL, Matzuk MM. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature. 1998;391:489–492. doi: 10.1038/35146. [DOI] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha T, Wang B, Evans S, Wynshaw-Boris A, Wang J. Disheveled mediated planar cell polarity signaling is required in the second heart field lineage for outflow tract morphogenesis. Dev Biol. 2012;370:135–144. doi: 10.1016/j.ydbio.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang CP. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell. 2008;14:298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucov HM, Gu Y, Thomas S, Li P, Pashmforoush M. Epicardial control of myocardial proliferation and morphogenesis. Pediatr Cardiol. 2009;30:617–625. doi: 10.1007/s00246-009-9391-8. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Taber LA. Mechanical aspects of cardiac development. Prog Biophys Mol Biol. 1998;69:237–255. doi: 10.1016/s0079-6107(98)00010-8. [DOI] [PubMed] [Google Scholar]
- Taber LA. Mechanical aspects of cardiac development. Progress in Biophysics & Molecular Biology. 1998;69:237–255. doi: 10.1016/s0079-6107(98)00010-8. [DOI] [PubMed] [Google Scholar]
- Terry RW, Kwee L, Baldwin HS, Labow MA. Cre-mediated generation of a VCAM-1 null allele in transgenic mice. Transgenic Res. 1997;6:349–356. doi: 10.1023/a:1018475031852. [DOI] [PubMed] [Google Scholar]
- Torrent-Guasp F, Kocica MJ, Corno AF, Komeda M, Carreras-Costa F, Flotats A, Cosin-Aguillar J, Wen H. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg. 2005;27:191–201. doi: 10.1016/j.ejcts.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Towbin JA. Left ventricular noncompaction: a new form of heart failure. Heart Fail Clin. 2010;6:453–469. viii. doi: 10.1016/j.hfc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Viragh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kokubo H, Miyagawa-Tomita S, Endo M, Igarashi K, Aisaki K, Kanno J, Saga Y. Activation of Notch1 signaling in cardiogenic mesoderm induces abnormal heart morphogenesis in mouse. Development. 2006;133:1625–1634. doi: 10.1242/dev.02344. [DOI] [PubMed] [Google Scholar]
- Xing Y, Ichida F, Matsuoka T, Isobe T, Ikemoto Y, Higaki T, Tsuji T, Haneda N, Kuwabara A, Chen R, Futatani T, Tsubata S, Watanabe S, Watanabe K, Hirono K, Uese K, Miyawaki T, Bowles KR, Bowles NE, Towbin JA. Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab. 2006;88:71–77. doi: 10.1016/j.ymgme.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Xu Y, Condell M, Plesken H, Edelman-Novemsky I, Ma J, Ren M, Schlame M. A Drosophila model of Barth syndrome. Proc Natl Acad Sci U S A. 2006;103:11584–11588. doi: 10.1073/pnas.0603242103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. J Biol Chem. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mutant mouse. Dev Biol. 1997;186:58–72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- Yang J, Bucker S, Jungblut B, Bottger T, Cinnamon Y, Tchorz J, Muller M, Bettler B, Harvey R, Sun QY, Schneider A, Braun T. Inhibition of Notch2 by Numb/Numblike controls myocardial compaction in the heart. Cardiovasc Res. 2012;96:276–285. doi: 10.1093/cvr/cvs250. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen H, Wang Y, Yong W, Zhu W, Liu Y, Wagner GR, Payne RM, Field LJ, Xin H, Cai CL, Shou W. Tbx20 a downstream mediator for bone morphogenetic protein 10 in regulating cardiac ventricular wall development and function. J Biol Chem. 2011 doi: 10.1074/jbc.M111.279679. [DOI] [PMC free article] [PubMed] [Google Scholar]