Abstract
Effector T cells control intracellular infection by secreting cytokines and through contact-dependent cytolysis. Since cytokines can diffuse and act at a distance, we determined whether cytokine diffusion is sufficient to control M. tuberculosis, or whether direct recognition of infected cells by CD4 T cells is required. Using MHC II mixed bone marrow chimeras, we compared the bacterial burdens in lung myeloid cells capable of recognition by CD4 T cells (MHC-II+/+) or not (MHC-II−/−). MHC-II+/+ cells had lower bacterial burdens than did MHC-II−/− cells. CD4 T cell depletion increased the number of bacteria associated with MHC-II+/+, but not MHC-II−/− cells, indicating that direct recognition of infected cells by CD4 T cells is required for control of intracellular M. tuberculosis. These results show that the effector mechanisms required for CD4 T cell control of distinct intracellular pathogens differ, and that long range cytokine diffusion does not contribute to control of M. tuberculosis.
Introduction
CD4 and CD8 T cells contribute to immunity through cytolytic activity and by secretion of cytokines. Cytolytic activity requires direct recognition of an infected cell and formation of an immunological synapse, the site of antigen-specific CTL activation as well as the site of delivery of cytolytic effector molecules which kill the target cell (1, 2). Cytokine-secreting CD4 and CD8 T cells also require activation through immunological synapses, followed by vectorial or multidirectional release of cytokines (3). Secreted cytokines may act directly on the infected cell bearing the peptide-MHC complexes that provide the signal for T cell activation (3, 4), or may diffuse within a tissue to activate ‘bystander’ cells which themselves were not involved in engaging and activating antigen-specific T cells (5, 6). A recent study reported that Leishmania major, an intracellular pathogen of macrophages and dendritic cells, can be controlled by ‘bystander’ activation of antigen-specific CD4 T cells through secretion and diffusion of IFNγ, which caused killing of intracellular L. major up to 80 μm from the activated T cell (5).
Mycobacterium tuberculosis is also an intracellular pathogen of macrophages and dendritic cells, and requires CD4 T cells and IFNγ to restrict its growth and dissemination (7). Unlike L. major, M. tuberculosis can persist and cause disease with high pathogen burdens despite the expansion, differentiation, and trafficking of CD4 T cells to the site of infection (8). However, it remains unclear precisely how CD4 T cells contribute to control of M. tuberculosis (9). In mice, administration of Th1-polarized TCR transgenic CD4 T cells specific for the M. tuberculosis antigen, ESAT-6, provides potent antimycobacterial effects (10), yet these effects are independent of the ability of the transferred T cells to produce either IFNγ or TNF (11). Similarly, reconstitution of RAG2−/− mice by transfer of CD4 memory T cells from mice previously infected with M. tuberculosis conferred comparable control of subsequent infection whether the transferred T cells were competent to produce IFNγ or not (12).
In humans, the mechanisms used by CD4 T cells to control progression of M. tuberculosis are similarly poorly understood. In a recent clinical trial of BCG vaccination, infants that were protected from tuberculosis (TB) did not differ in their mycobacterial antigen-induced CD4 or CD8 T cell secretion of IFNγ, TNF, IL-2, or IL-17 from that in infants that developed TB (13). Likewise, adults can exhibit antigen-specific polyfunctional CD4 and CD8 T cell responses, yet still progress to active TB (14–16). Therefore, while CD4 T cells are essential for immunity to M. tuberculosis, the effector mechanisms they use to control M. tuberculosis are incompletely defined.
To further understand the contributions of CD4 T cells to immunity in TB, we determined whether immune control of M. tuberculosis requires direct recognition of infected cells, or whether bystander activation of CD4 effector cells is sufficient to restrict infection.
Materials and Methods
Generation and infection of MHC II mixed bone marrow chimeric mice and CD4 T cell depletion
Mixed bone marrow chimeras were generated as described (17) using T cell-depleted MHC II+/+ (CD45.1+) and MHC II−/−(CD45.2+) bone marrow cells. Chimeras were infected 7 weeks later by aerosol (~100 cfu/mouse) with M. tuberculosis H37Rv that expresses FACS-optimized green fluorescent protein (18, 19). Selected animals were treated every 5 days with 500 μg of CD4 depleting antibody (GK1.5) or isotype control (LTF-2) starting 18 d post infection until the day of harvest (day 35). Single-cell intracellular bacterial burdens were determined in sorted GFP+ cell subsets by fluorescence microscopy and manual counting. Bacterial loads in lung homogenates and sorted cell populations were determined by plating serial dilutions on 7H11 agar. Procedures involving mice were approved by the NYU School of Medicine IACUC.
Cell sorting
Lung cells from MHC II mixed chimeras infected with M. tuberculosis H37Rv-GFP were stained with CD11c, CD11b and Gr-1 antibodies as described (19, 20). Following staining, samples were pooled (2–4 mice/pool; 3 pools per group) and live cells were sorted using an iCyt Synergy™ sorter in BSL-3 containment into MHC II+/+ and MHC II−/− CD11chiCD11bhi DC and CD11clo/intCD11bmed recruited macrophages (RM). GFP+ cells were sorted from each of the DC and RM subsets, and either fixed for microscopic determination of single-cell bacterial burdens or plated for quantitation of live bacteria.
Statistical analysis
Statistical comparisons used Prism 4 for Macintosh (GraphPad, San Diego, CA), using the tests specified in figure legends. P values < 0.05 were considered significant.
Results and Discussion
To determine whether direct recognition of infected cells by CD4 effector T cells is required for optimal control of M. tuberculosis, we prepared mixed bone marrow chimeric mice using equal numbers of cells from MHC class II+/+ (CD45.1) and MHC class II−/− (CD45.2) mice, and infected them with M. tuberculosis that expresses FACS-optimized green fluorescent protein (GFP) (17–19). This allows assessment of the number of infected cells and the number of bacteria associated with cells that can (MHC II+/+) or cannot (MHC II−/−) be directly recognized by CD4 T cells, after isolation from the same lung environment.
Following infection with M. tuberculosis, mice reconstituted with 50% MHC II+/+ and 50% MHC II−/− bone marrow responded with equivalent (by frequency; Fig. 1A) or slightly greater (by cell number; Fig. 1B) CD4 T cell recruitment to the lungs compared with those in mice reconstituted with 100% MHC II+/+ marrow, indicating that MHC II+/+ cells were present in sufficient numbers in the II+/+:II−/− mixed chimeras to accomplish initial priming and trafficking of CD4 T cells to the lungs. Despite the presence of CD4 T cells at equivalent frequencies in the lungs of the two groups of mice, IFNγ concentrations were approximately 20% lower in lung homogenates from the II+/+:II−/− mixed chimeras (Fig. 1C), consistent with less effector T cell activation in the lungs. Concordant with the evidence of less effector T cell activation, the II+/+:II−/− mice had 5-fold more bacteria in the lungs on day 35 postinfection (Fig. 1D).
Figure 1. CD4 T cells, IFNγ, and bacterial burdens in mice reconstituted with MHC II+/+, or mixed MHC II+/+ and MHC II−/−, bone marrow and infected with M. tuberculosis.
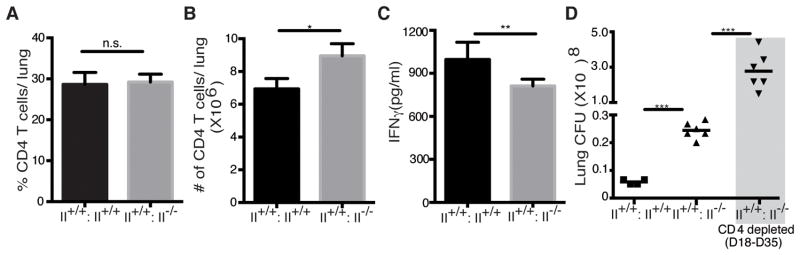
Irradiated MHC class II wild-type (45.1) mice were reconstituted with either 100% MHC II wild-type (II+/+: II+/+) (CD45.1+) or a 1:1 mixture of MHC II+/+ (CD45.1+) and MHC II−/− (CD45.2+) bone marrow cells (II+/+:II−/−) and infected with M. tuberculosis H37Rv expressing GFP (100 cfu/mouse) 7 weeks later. A group of II+/+:II−/− chimeras were depleted of CD4 T cells beginning on day 18 postinfection. (A) Frequency of CD4 T cells in lungs of mice reconstituted with wild-type (II+/+: II+/+) or mixed II+/+:II−/− bone marrow. (B) Total number of CD4 T cells in lungs of II+/+: II+/+ and II+/+:II−/− chimeras. (C) IFNγ in lung homogenates from mice reconstituted with wild-type (II+/+: II+/+) or mixed (II+/+:II−/−) bone marrow. (D) Effect of CD4 T cell depletion on lung bacterial burdens in II+/+: II+/+ and II+/+:II−/− bone marrow chimeras. Data represent 4–6 mouse replicates *p < 0.05; **p < 0.01; ***p < 0.001 (Student’s t-test).
To determine whether the higher bacterial burdens in the lungs of II+/+:II−/− chimeras were attributable to the presence of bacteria in a larger proportion of MHC II−/− than MHC II+/+ cells, we took advantage of bacterial GFP expression to identify and quantitate infected lung leukocytes by flow cytometry. We concentrated on two cell subsets that are the most frequently infected in the lungs after the onset of adaptive immunity: CD11chiCD11bhi dendritic cells (DC) and CD11clo/intCD11bmed recruited macrophages (RM) (19). This revealed that 8–11% of the cells in each subset (DC and RM) contained bacteria as determined by flow cytometry after isolation from the lungs of mixed chimeras (Fig. 2); this did not differ significantly by the presence or absence of MHC II in either subset (DC: II+/+ 9.1±0.8, II−/− 10.8±0.9, p=0.4; RM: II+/+ 8.2±0.5, II−/− 11±1.1, p=0.1). These results indicate that the absence of MHC II expression on lung DC or RM has little or no effect on the proportion of cells that contain bacteria.
Figure 2. Similar frequencies of M. tuberculosis-infected MHC II+/+ and MHC-II−/− dendritic cells (DC) and recruited macrophages (RM) in lungs of MHC II mixed chimeras.
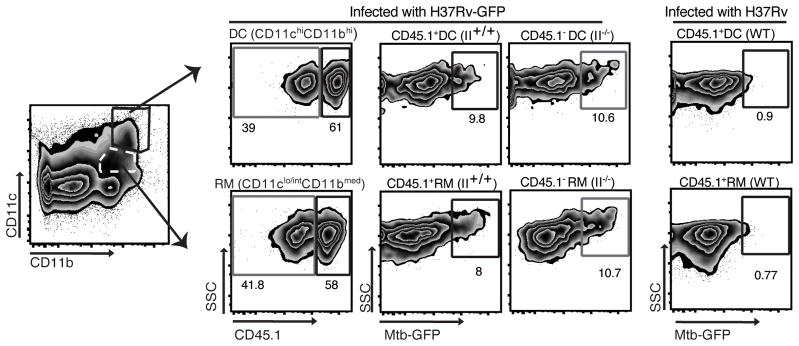
Single cell suspensions from lungs of MHC II mixed bone marrow chimeras (II+/+:II−/−) that had been infected with M. tuberculosis-GFP were stained to identify myeloid cell subsets and analyzed for infected (GFP+) wild-type (CD45.1+) and MHC-II−/− (CD45.1-) cells by flow cytometry.
Flow cytometry profile of GFP+ (infected) MHC II+/+ and MHC II−/− CD11chiCD11bhi DC (upper row) and CD11clo/intCD11bmed recruited macrophages (RM; lower row) in lungs of MHC II mixed bone marrow chimeras. Numbers near gates show the frequency of cells in each gate. Neutrophils (Gr-1hi CD11bhi) were gated out before defining cells into CD11c/CD11b subsets. The right panel in each row shows fluorescence of the same cell subsets from mice infected with the parental strain of M. tuberculosis H37Rv that does not express GFP. Data were obtained by preparing triplicate pools prior to sorting, with each pool containing cells from 2–4 mice. Comparable results were obtained in 3 independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001 (Student’s t-test and Mann-Whitney test).
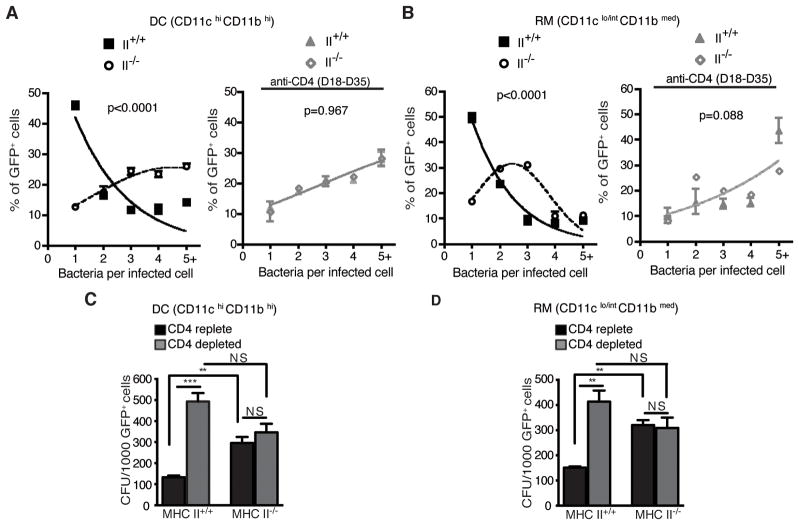
As an alternative explanation for the larger bacterial burden in the lungs of II+/+:II−/− mixed chimeras, we determined whether the absence of MHC II expression resulted in poorer control of infection manifest as a larger number of bacteria per infected cell. We sorted lung cells from M. tuberculosis-GFP-infected MHC II mixed chimeras on the basis of cell phenotype (DC or RM), the presence or absence of the ability to express MHC class II (using CD45.1+ to identify MHC II+/+, and CD45.1- to identify MHC II−/− cells (from CD45.2 donors)), and the presence of GFP fluorescence. The number of bacteria per cell in GFP+ DC and RM were then quantitated by fluorescence microscopy. This revealed that the number of bacteria per infected DC (Fig. 3A) or RM (Fig. 3B) was greater in MHC II−/− cells than in MHC II+/+ cells of either myeloid phenotype. Since the absence of MHC II may have effects other than the loss of CD4 T cell recognition (21), we depleted CD4 T cells from the II+/+:II−/− mixed chimeras and found that this increased the total lung bacterial burden (Fig. 1D), increased the number of GFP+ bacteria per infected MHC II+/+ cell, and abolished the difference in the frequency distribution of bacteria in MHC II+/+ compared with MHC II−/− cells (Fig. 3A and 3B, right panels). These results indicate that MHC II+/+ DC and RM control intracellular M. tuberculosis more effectively than MHC II−/− cells, and this difference is CD4 T cell dependent. This suggests that direct recognition of infected DC or RM by CD4 T cells is necessary for optimal control of M. tuberculosis.
Figure 3. Direct recognition of infected lung cells by CD4 T cells is required for control of intracellular M. tuberculosis.
(A) Frequency distribution of the number of bacteria per infected CD11chiCD11bhi DC, expressed as the percentage of infected cells containing the stated number of bacteria after flow sorting from MHC II mixed bone marrow chimeras treated with CD4-depleting antibody (right panel) or isotype control (left panel). (B) Analysis as in (A), for CD11clo/intCD11bmed recruited macrophages (RM). For each subset, 200–300 sorted GFP+ cells were analysed for the number of bacteria per cell by fluorescence microscopy. (C) Quantitation of live M. tuberculosis in FACS-sorted infected (GFP+) MHC II+/+ or MHC II−/− CD11chiCD11bhi DC from II+/+: II−/− mixed chimeras treated with CD4-depleting antibody or isotype control. (D) Analysis as in (C), for CD11c lo/intCD11bmed recruited macrophages.
Data in panels A–D represent 3 sample pools from 6–9 mice. Statistical comparisons for panels A and B were done with the extra sum-of-squares F test, and confirmed with Aikaike’s Information Criterion. *p < 0.05; **p < 0.01; ***p < 0.001.
Since GFP+ intracellular bacteria could include both live and dead bacteria, we determined whether the larger number of GFP+ bacteria associated with MHC II−/− DC and RM observed by microscopy reflected a larger number of live bacteria. Using the same sorting parameters as described for quantitating bacteria per infected cell by microscopy, we quantitated live bacteria (as colony-forming units) per 1,000 GFP+ cells in each sorted fraction. This revealed that the number of live M. tuberculosis is greater in MHC II−/− compared with MHC II+/+ DC and RM in CD4 T cell replete mice (Fig. 3C and 3D). Depletion of CD4 T cells resulted in significant increases in the number of live bacteria associated with MHC II+/+ DC and RM, confirming that CD4 T cells contribute to control of intracellular M. tuberculosis by restricting the number of bacteria per infected cell. In contrast, CD4 T cell depletion had no effect on the number of live bacteria associated with MHC II−/− DC or RM (Fig. 3C and 3D). This indicates that CD4 T cell control of intracellular M. tuberculosis depends on direct recognition of infected cells, and that this mechanism predominates over those that can occur as the consequence of bystander activation, such as through the action of diffusible cytokines.
The requirement for direct recognition of infected lung myeloid cells by CD4 effector T cells for optimal control of M. tuberculosis is clearly distinct from findings with L. major, in which bystander activation of CD4 effector cells is sufficient for control of intracellular parasites (5). There are three possible explanations for the differences in the results with L. major and M. tuberculosis. One is that infection with M. tuberculosis establishes a tissue environment in which diffusion of cytokines secreted by activated CD4 effector cells is restricted, thereby preventing secreted cytokines from acting on infected cells at a distance. For example, IFNγ binds to glycosaminoglycans, restricting the diffusion and the action of IFNγ (22), and glycosaminoglycans are deposited in high concentrations at the site of infection in TB (23). Related to this, another possibility is that the amount of IFNγ produced per CD4 effector cell is low in TB, and this restricts its effects to adjacent cells. The other potential explanation for the requirement for direct CD4 T cell recognition of M. tuberculosis-infected cells in vivo is that control of M. tuberculosis requires the action of one or more mechanisms that requires direct contact between CD4 effector cells and M. tuberculosis-infected cells. This is consistent with the recent observations that CD4 T cells contribute to control of M. tuberculosis in vivo, even when they are not competent to secrete IFNγ or TNF, express perforin, or interact with Fas (11, 12). The results reported here suggest that further investigation of CD4 effector mechanisms in tuberculosis must concentrate on mechanisms that require direct recognition and contact with infected cells.
Our results have implications for TB vaccine development. First, these results suggest that TB vaccines that promote development of CD4 T cell mechanisms that operate through direct recognition and contact of infected cells may have greater efficacy than vaccines that induce polyfunctional cytokine responses alone. Therefore, investigation of vaccine vectors and adjuvants should include determination of their ability to induce T cells with effector mechanisms that extend beyond cytokine secretion. Second, considering recent evidence that the frequency of antigen-specific CD4 effector T cell activation at the site of infection is low (4, 24), and that this limits the antimycobacterial efficacy of antigen-specific CD4 effector T cells (24), the results reported here indicate the need for better understanding of the mechanisms of antigen presentation and their limitations in cells infected with M. tuberculosis. In light of evidence that M. tuberculosis interferes with MHC class II antigen presentation by the cells that it infects (25–30), vaccine-induced CD4 T cells may have limited efficacy unless the mechanism(s) that limit antigen presentation can be overcome or bypassed. The evidence that optimal control of intracellular M. tuberculosis requires direct recognition of infected cells by CD4 effector T cells heightens the importance of identifying the mechanisms that attenuate MHC class II antigen presentation in M. tuberculosis-infected cells.
Acknowledgments
We thank Michael Gregory of the Flow Cytometry and Cell Sorting Center (partially supported by the NYU Cancer Institute Cancer Center Support Grant, 5P30CA016087-33) for expert assistance with cell sorting. We also thank Ludovic Desvignes for help with irradiation of mice, and members of the Ernst Laboratory for constructive discussions and suggestions.
Footnotes
Supported by NIH grants R01 AI051242 and R01 AI084041.
References
- 1.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. The Journal of clinical investigation. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 3.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nature immunology. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 4.Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34:807–819. doi: 10.1016/j.immuni.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller AJ, Filipe-Santos O, Eberl G, Aebischer T, Spath GF, Bousso P. CD4+ T cells rely on a cytokine gradient to control intracellular pathogens beyond sites of antigen presentation. Immunity. 2012;37:147–157. doi: 10.1016/j.immuni.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Perona-Wright G, Mohrs K, Mohrs M. Sustained signaling by canonical helper T cell cytokines throughout the reactive lymph node. Nature immunology. 2010;11:520–526. doi: 10.1038/ni.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philips JA, Ernst JD. Tuberculosis Pathogenesis and Immunity. Annual review of pathology. 2011 doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 8.Cooper AM. Cell-mediated immune responses in tuberculosis. Annual review of immunology. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrado E, Cooper AM. What do we really know about how CD4 T cells control Mycobacterium tuberculosis? PLoS pathogens. 2011;7:e1002196. doi: 10.1371/journal.ppat.1002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. The Journal of experimental medicine. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallegos AM, van Heijst JW, Samstein M, Su X, Pamer EG, Glickman MS. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS pathogens. 2011;7:e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. The Journal of experimental medicine. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, Mahomed H, Hawkridge A, Hussey G, Kaplan G, Hanekom WA. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. American journal of respiratory and critical care medicine. 2010;182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O’Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol. 2011;187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, Cavassini M, Calandra T, Blanchet CL, Jaton K, Faouzi M, Day CL, Hanekom WA, Bart PA, Pantaleo G. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nature medicine. 2011;17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutherland JS, I, Adetifa M, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. European journal of immunology. 2009;39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 17.Kincaid EZ, Wolf AJ, Desvignes L, Mahapatra S, Crick DC, Brennan PJ, Pavelka MS, Jr, Ernst JD. Codominance of TLR2-dependent and TLR2-independent modulation of MHC class II in Mycobacterium tuberculosis infection in vivo. Journal of immunology. 2007;179:3187–3195. doi: 10.4049/jimmunol.179.5.3187. [DOI] [PubMed] [Google Scholar]
- 18.Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110–7119. doi: 10.4049/jimmunol.1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 20.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. The Journal of experimental medicine. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, Yao H, Cao X. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nature immunology. 2011;12:416–424. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Botran R, Yan J, Justus DE. Binding of interferon gamma by glycosaminoglycans: a strategy for localization and/or inhibition of its activity. Cytokine. 1999;11:313–325. doi: 10.1006/cyto.1998.0438. [DOI] [PubMed] [Google Scholar]
- 23.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycans in granulomatous lung diseases. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 1997;10:2731–2737. doi: 10.1183/09031936.97.10122731. [DOI] [PubMed] [Google Scholar]
- 24.Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS pathogens. 2011;7:e1002063. doi: 10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fortune SM, Solache A, Jaeger A, Hill PJ, Belisle JT, Bloom BR, Rubin EJ, Ernst JD. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J Immunol. 2004;172:6272–6280. doi: 10.4049/jimmunol.172.10.6272. [DOI] [PubMed] [Google Scholar]
- 26.Gercken J, Pryjma J, Ernst M, Flad HD. Defective antigen presentation by Mycobacterium tuberculosis-infected monocytes. Infection and immunity. 1994;62:3472–3478. doi: 10.1128/iai.62.8.3472-3478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hmama Z, Gabathuler R, Jefferies WA, de Jong G, Reiner NE. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers. J Immunol. 1998;161:4882–4893. [PubMed] [Google Scholar]
- 28.Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J Immunol. 2003;171:175–184. doi: 10.4049/jimmunol.171.1.175. [DOI] [PubMed] [Google Scholar]
- 29.Pancholi P, Mirza A, Bhardwaj N, Steinman RM. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993;260:984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 30.Sendide K, Deghmane AE, Pechkovsky D, Av-Gay Y, Talal A, Hmama Z. Mycobacterium bovis BCG attenuates surface expression of mature class II molecules through IL-10-dependent inhibition of cathepsin S. J Immunol. 2005;175:5324–5332. doi: 10.4049/jimmunol.175.8.5324. [DOI] [PubMed] [Google Scholar]