Abstract
We tested the hypothesis that low luminal K+ inhibits the activity of ROMK channels in the rat cortical collecting duct. Whole-cell voltage-clamp measurements of the component of outward K+ current inhibited by the bee toxin Tertiapin-Q (ISK) showed that reducing the bath concentration ([K+]o) to 1 mM resulted in a decline of current over 2 min compared with that observed at 10 mM [K+]o. However, maintaining tubules in 1 mM [K+]o without establishing whole-cell clamp conditions did not affect ISK. The [K+]o-dependent decline was not prevented by increasing cytoplasmic-side pH or by inhibition of phosphatase activity. It was, however, abolished by the inclusion of 0.5 mM DTT in the pipette solution to prevent oxidation of the intracellular environment. Conversely, treatment of intact tubules with the oxidant H2O2 (100 μM) decreased ISK in a [K+]o-dependent manner. Treatment of the tubules with the phospholipase C inhibitor U73122 prevented the effect of low [K+]o, suggesting the involvement of this enzyme in the process. We examined these effects further using Xenopus oocytes expressing ROMK2 channels. A 50-min exposure to the permeant oxidizing agent tert-butyl hydroperoxide (t-BHP; 500 μM) did not affect outward K+ currents with [K+]o = 10 mM but reduced currents by 50% with [K+]o = 1 mM and by 75% with [K+]o = 0.1 mM. Pretreatment of the oocytes with U73122 prevented the effects of t-BHP. Under conditions of low dietary K intake, K+ secretion by distal nephron segments may be suppressed by a combination of low luminal [K+]o and oxidative stress.
Keywords: ROMK channels, K+ secretion, oxidation, phospholipase C, U73122
modulation of the rate of K+ secretion by the cortical collecting duct (CCD) and related nephron segments is important for matching renal K excretion with dietary K intake. When K intake is high, several adaptations occur that stimulate K+ secretion including increased activity of apical ROMK channels (13, 24, 37, 39), activation of epithelial Na+ channels (12, 24), upregulation of basolateral Na-K-ATPase (24, 32), and suppression of the upstream NaCl cotransporter NCC (11, 16, 35). The last response will increase Na+ delivery to the more distal segments, reducing NaCl reabsorption and enhancing Na+/K+ exchange.
The mechanisms involved in limiting K+ secretion when dietary intake is low may be different from those that upregulate K+ transport. ROMK activity was reported to be reduced in some (1, 13) but not other (25, 39) studies. The downregulation is thought to be mediated, at least in part, by activation of the tyrosine kinase c-src (4, 22, 39). ROMK protein expression is also reduced by low K intake (11, 36). However, these decreases are relatively modest, suggesting that other events may be essential for preventing K+ secretion and minimizing K losses.
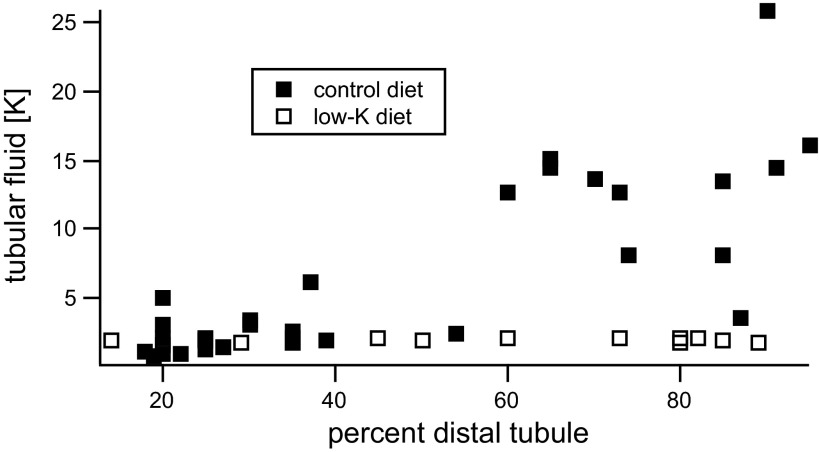
Measurements of ROMK activity in heterologous expression systems indicate that the channels are sensitive to changes in the extracellular K+ concentration ([K+]o); decreases in [K+]o downregulate the channels (7, 28, 29, 31). One aspect of this response is a shift in the dependence of channel activity on intracellular pH, with low [K+]o moving the titration curve for inhibition of the channels toward a higher, more physiological pH range. Such a mechanism could be relevant in vivo, since under conditions of K deprivation the luminal K+ concentration in distal nephron segments can fall to 1–2 mM (23; see Fig. 1). However, the effect of reducing [K+]o on K+ channels in renal tubules has not been examined.
Fig. 1.
Data from Malnic et al. (23) are replotted to show that with a low-K diet luminal K+ concentration ([K+]) is ≤ 2 mM.
In this study, we test the hypothesis that lowering [K+]o in K+-secreting cells can directly reduce ROMK channel activity. We provide evidence for such a reduction, which occurs most particularly under conditions of oxidative stress.
METHODS
Animals.
All procedures using animals were approved by the Institutional Animal Care and Use Committee of Weill-Cornell Medical College or the Chicago Medical College. Sprague-Dawley rats (150–200 g) of either gender (Charles River Laboratories, Kingston, NY) raised free of viral infections were used for all experiments. Animals were fed a conventional rodent diet (Purina). Stage V–VI oocytes were obtained by partial ovariectomy of female Xenopus laevis (NASCO, Ft. Atkinson, WI), while animals were anesthetized with tricaine methanesulfonate (1.5 g/l, adjusted to pH 7.0). Oocytes were defolliculated by incubation (on a Vari-Mix rocker) in Ca-free modified Barth's solution (82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES, adjusted to pH 7.5 with NaOH) containing 2 mg/ml collagenase type IA (cat no. C9891; Sigma Chemical, St. Louis, MO) for 90 min and (if necessary) another 90 min in a fresh enzyme solution at 23°C. Oocytes were injected with 5–10 ng of cRNA and incubated at 19°C in 2× diluted Leibovitz medium (Life Technologies, Grand Island, NY) for 1–3 days before measurements were made.
Electrophysiology.
Measurement of whole-cell K+ currents in principal cells of the CCD followed procedures described previously (10, 13). Split-open tubules were superfused with solutions prewarmed to 37°C containing the following (in mM): 5 NaCl, 130 Na methanesulfonate, 10 K methanesulfonate, 2 Ca methanesulfonate, 1 MgCl2, 2 glucose, and 10 HEPES adjusted to pH 7.4 with NaOH. [K+]o was reduced to 1 mM by substitution of K methanesulfonate with Na methanesulfonate. Methanesulfonate was used to minimize whole-cell anion currents.
For measurements of K+ currents through ROMK channels, the patch-clamp pipettes were filled with solutions containing the following (in mM): 7 KCl, 123 aspartic acid, 5 EGTA, and 10 HEPES, with the pH adjusted to 7.4 with KOH. The total concentration of K+ was ∼145 mM. Tertiapin-Q (TPNQ; Sigma-Aldrich) was dissolved in H2O at a concentration of 100 μM and diluted into the bath solution to a final concentration of 100 nM. Ba acetate was added to the bath solution to a final concentration of 5 mM.
Pipettes were pulled from hematocrit tubing, coated with Sylgard, and fire polished with a microforge. Pipette resistances ranged from 2 to 5 MΩ. Currents were measured with a List EPC-7 amplifier (Heka Elektronik, Lambrecht, Germany). Voltages were controlled and currents were recorded using pClamp software and a Digidata 1320 interface (Molecular Devices, Sunnyvale, CA).
Xenopus oocytes.
Whole-cell currents and conductances were measured in intact oocytes using a two-electrode voltage clamp (Model CA-1; Dagan, Minneapolis, MN) with 16 command pulses of 30-ms duration between −110 and +50 mV, centered around the resting potential. Upward deflections from the zero current line (dashed) denote outward current.
Oocytes expressing Kir1.1b (ROMK2) were bathed in permeant acetate buffers to control their internal pH as previously described (5). Variations in external (bath) [K] at constant ionic strength were accomplished with 0.1 mM KCl + 50 mM NaCl, 1 mM KCl + 49 mM NaCl, or 10 mM KCl + 40 mM NaCl, with the remainder of bathing solution being 50 mM Na-acetate, 1 mM MgCl2, 2 mM CaCl2, and 5 mM HEPES. The pH of the bath was adjusted to control internal oocyte pH at 7.8 with the acetate buffer system.
A membrane-permeant analog of hydrogen peroxide [tert-butyl hydroperoxide (t-BHP)] was obtained from Sigma Chemical (no. 19997) and used at a concentration of 500 µM. The PLC inhibitor U73122 (cat. no. 662035) and the related compound U73343, lacking PLC activity (cat. no. 662041), were both obtained from EMD Millipore (Billerica, MA).
Statistics.
Statistical differences were evaluated using the two-tailed Student's t-test. The threshold for statistical significance was P < 0.05.
RESULTS
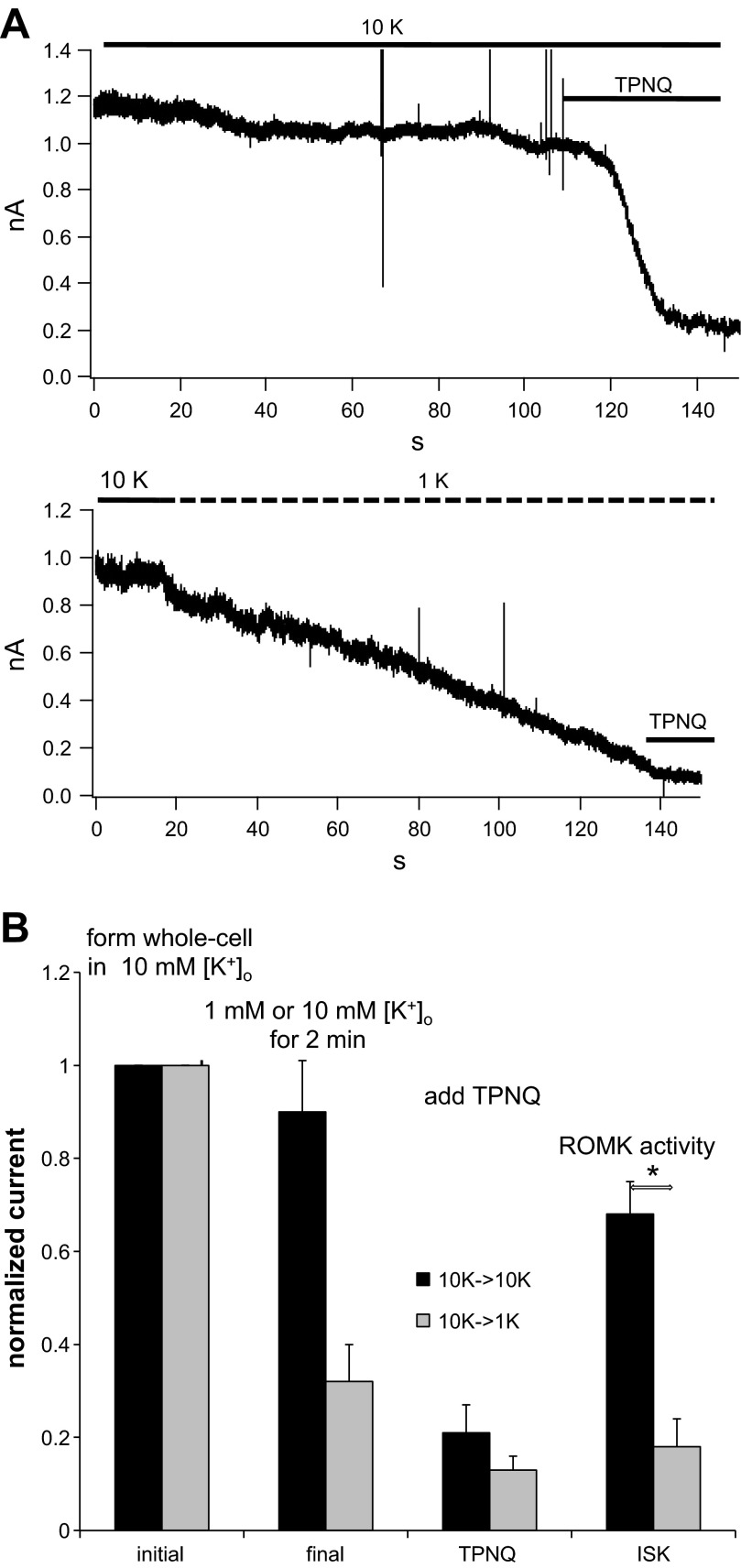
We chose to investigate the influence of [K+]o over the range of 1 to 10 mM based on the micropuncture data from rat kidney of Malnic et al. (23) obtained from superficial distal convoluted tubules, redrawn in Fig. 1. These measurements indicate that with a low-K diet, [K+] in the luminal fluid is maintained <2 mM along the micropuncture-accessible distal tubule while concentrations rise to 10–15 mM in the second half of the tubules, with a normal K intake. The effect of reducing [K+]o during a whole-cell clamp recording is illustrated in Fig. 2A. Whole-cell clamp conditions were established in two separate principal cells, both in the presence of 10 mM [K+]o. The holding potential was zero mV, and the K+ concentration in the pipette, and by extension that in the cell (140 mM), was much larger than that of the bath, establishing a driving force for outward current carried by K+. In the top trace, 10 mM [K+]o were maintained for ∼2 min as a time control. At the end of this period, TPNQ (10−7M) was added to the bath and outward K+ current (ISK), the current passing through ROMK channels, was measured as that inhibited by the toxin. The residual current was blocked by Ba2+ (13) and presumably is carried by basolateral K+ channels. In the bottom trace, after a period of 10–15 s to establish baseline, the bath solution was exchanged for one containing 1 mM [K+]o. Under these conditions, the outward current fell progressively over a 2-min period, despite an increased driving force. After this period there was only a small response to TPNQ. Figure 2B shows a summary of this and similar experiments. The data indicate a strong inhibition of ISK in response to lowering [K+]o. In both cases TPNQ abolished a large fraction of the total outward K+ current. This suggests that the basolateral K+ currents, assumed to be TPNQ-insensitive, are small. This is due in part to the rapid rundown of these currents as described previously (13). It is also possible that the basolateral K+ channels are affected by low [K+]o and contribute to the decline in currents observed in Fig. 2A. We have not explored this systematically.
Fig. 2.
Effect of decreasing extracellular K+ concentration ([K+]o) on ROMK currents in the rat cortical collecting duct (CCD). A: whole-cell recording showing outward currents at a holding potential of 0 mV. Whole-cell clamps were formed in the presence of 10 mM [K+]o. Currents were stable in the presence of 10 mM [K+]o but declined with 1 mM [K+]o. At the end of the recording Tertiapin-Q (TPNQ; 100 nM) was added to the medium to measure the ROMK-specific currents. B: summary of 8 similar pairs of experiments. Data represent means ± SE. *P < 0.05, significant difference.
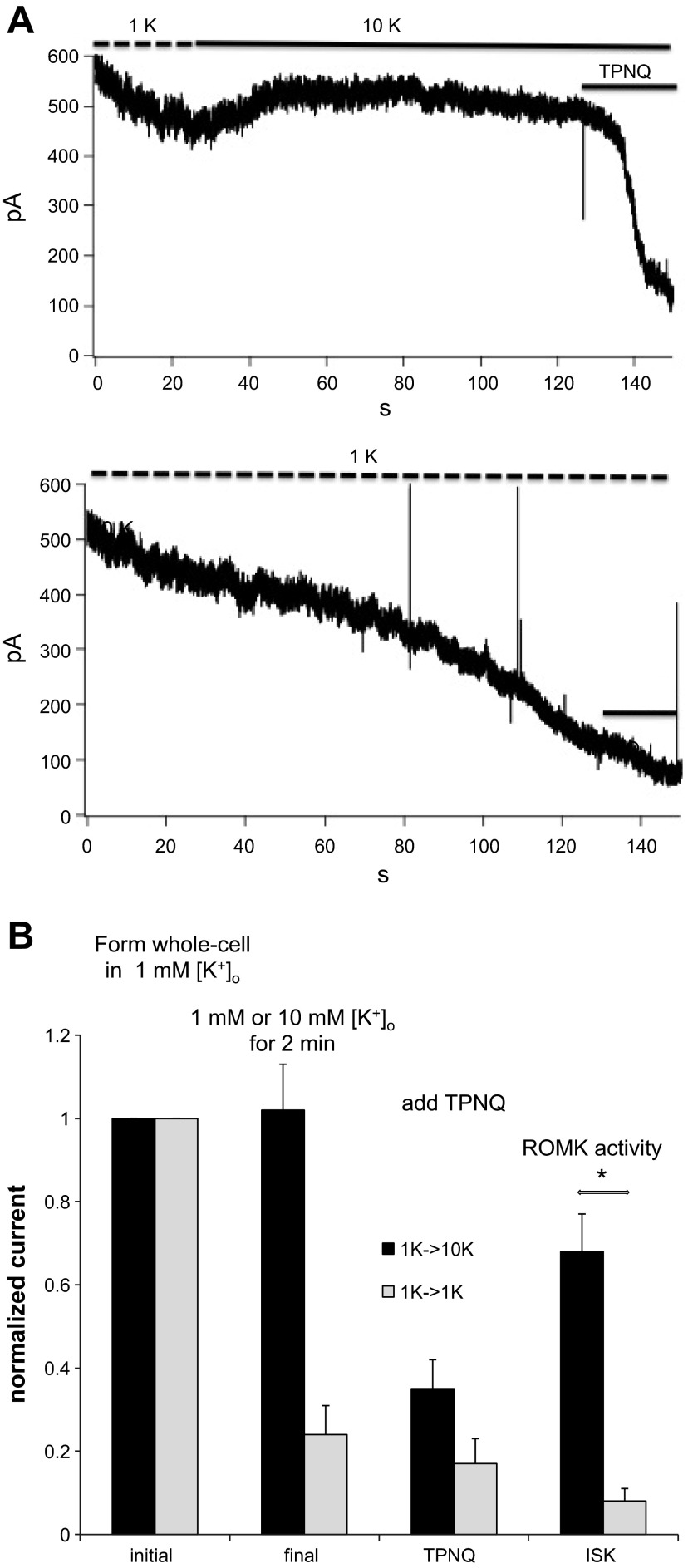
Figure 3 shows the converse experiment. Here the whole-cell recordings were established in the presence of 1 mM [K+]o and the concentration was either maintained or raised to 10 mM after a baseline was established. When [K+]o was raised to 10 mM, there was a slight increase in outward current seen in some but not all experiments, despite a decreased driving force, and the level was well maintained for 2 min, and there was a robust inhibition by TPNQ at the end of this period. In contrast, in the continued presence of 1 mM [K+]o the outward current decreased progressively with only a small response to TPNQ after 2 min. Figure 3B summarizes the results of this and similar experiments. As in the previous protocol, 1 mM [K+]o suppressed ISK.
Fig. 3.
Effect of increasing [K+]o on ROMK currents in the rat CCD. A: whole-cell recording showing outward currents at a holding potential of 0 mV. Whole-cell clamps were formed in the presence of 1 mM [K+]o. Currents declined with 1 mM [K+]o but stabilized in the presence of 10 mM [K+]o. At the end of the recording, TPNQ (100 nM) was added to the medium to measure the ROMK-specific currents. B: summary of 5 similar pairs of experiments. Data represent means ± SE. *P < 0.05, significant difference.
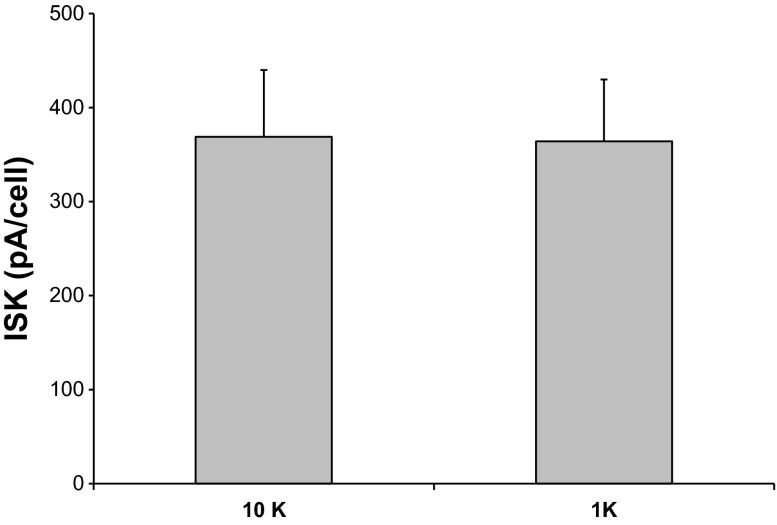
Although 1 mM [K+]o clearly inhibited ROMK channels in the protocols of both Figs. 2 and 3, several aspects of the results were puzzling. First, the baseline outward currents in cells preincubated in 1 or 10 mM [K+]o were not very different (compare initial currents in Figs. 2 and 3). Second, even though the cells in Fig. 3 were preincubated in low [K+]o, maintaining this concentration after the whole-cell clamp was formed led to a decline in the current. Thus it appeared that reduced [K+]o had a stronger effect on the channels under whole-cell conditions than when the cells were intact. To test this further, we measured TPNQ-sensitive currents in paired cells of the same tubule after preincubation for at least 5 min in either 10 or 1 mM [K+]o. The order was alternated to account for time-dependent changes in channel activity. There was no discernible effect of [K+]o under these conditions (Fig. 4). This suggests that conditions influenced by the formation of the whole-cell clamp predispose the channels to downregulation by low [K+]o.
Fig. 4.
TPNQ-sensitive outward currents (ISK) in paired principal cells after preincubation for >5 min in 1 or 10 mM [K+]o. Data represent means ± SE for 10–11 measurements. Incubation of intact cells in low [K+]o. did not diminish ROMK currents.
While the whole-cell conditions are unphysiological since cytoplasmic constituents are removed and replaced with those of the pipette solution, we felt that examination of what factors are involved in the [K+]o -dependent downregulation of the channels might give some insight into the physiological regulation of the channels. We examined several possible factors that might exert such an influence as shown in Fig. 5.
Fig. 5.
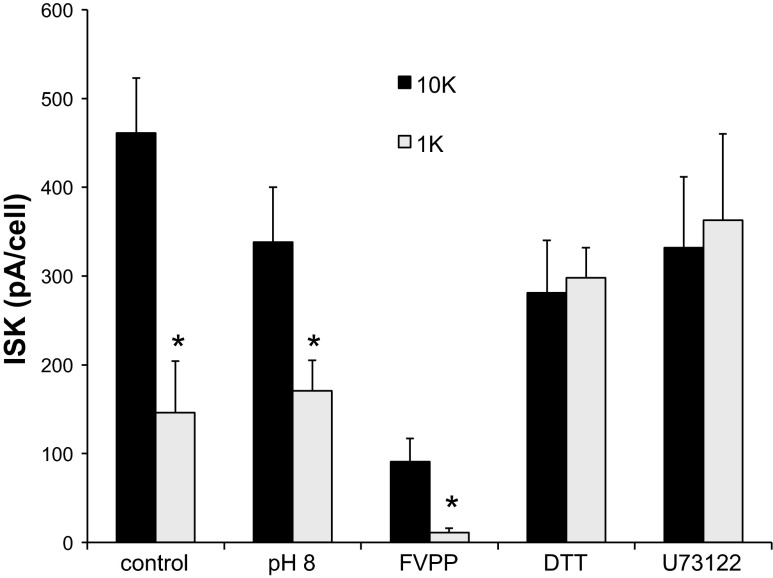
[K+]o-dependent decreases in ROMK current. TPNQ-sensitive outward currents were measured after maintaining the whole-cell clamp for 2 min with [K+]o = 1 or 10 mM. Differences in current persisted with pH 8.0 in the pipette solution and with FVPP pipette solution. Inclusion of 0.5 mM DTT in the pipette solution or preincubation of tubules with U73122 (5 μM) abolished the differences. Data represent means ± SE for 17 pairs of cells (control), 13 pairs (pH 8), 9 pairs (FVPP), 18 pairs (DTT), or 11 pairs (U73122). *P < 0.05, significant difference.
First, as mentioned in the Introduction, in oocytes inhibition by [K+]o depends on the intracellular pH, and the effect of low [K+]o appears as a shift in the pH dependence such that the channels are closed with a more alkaline pKi. To evaluate whether this could account for the observations in Figs. 2 and 3, we increased the pH of the pipette solution, and presumably also of the cytoplasm that is dialyzed by this solution under whole-cell conditions, to 8.0. In oocytes this fully opens the pH gate of the channels even with 1 mM [K+]o (29). The response to 1 mM [K+]o under these whole-cell clamp conditions was somewhat reduced but was still evident (Fig. 5). We therefore decided to pursue other possible mechanisms.
In excised inside-out patches ROMK channels run down rapidly. The loss of activity can be slowed by a cocktail of phosphatase inhibitors (FVPP solution) containing F−, vanadate, and pyrophosphate (20). To our surprise, this solution by itself appeared to reduce ROMK currents under whole-cell conditions. We do not understand this difference between the whole-cell and excised-patch configurations. However, even with these reduced currents an inhibitory effect of low [K+]o was documented (Fig. 5), suggesting that phosphatase activity is not essential for the observed [K+]o dependence.
ROMK channels in excised patches from oocytes are sensitive to redox conditions. In particular, when the channels are closed by low pH on the cytoplasmic side of the patch, recovery is greatly accelerated by addition of a reducing agent such as DTT (30, 46). We therefore examined the effect of inclusion of DTT (500 μM) in the pipette solution on the response to [K+]o. As seen in Fig. 5, DTT abrogated the response to 1 mM [K+]o applied during whole-cell recording. This suggests that oxidative stress induced by dialysis of the cell with pipette solution combines with low [K+]o to close the channels.
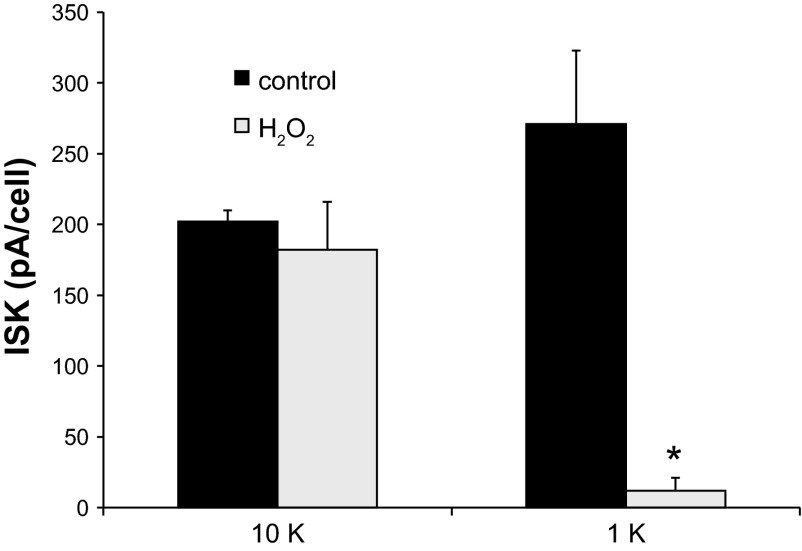
To test this further, we incubated intact tubules with an oxidizing agent, H2O2, added to the bath. This compound was previously shown to inhibit ROMK channels in cell-attached patches from the CCD (40). TPNQ-sensitive currents were then measured immediately after formation of whole-cell conditions to assess the response of the intact cell to the oxidant. As shown in Fig. 6, exposure of the cells to H2O2 at a concentration of 100 μM for 10–20 min reduced ISK in a [K+]o-dependent manner. With 10 mM [K+]o, the effect was minimal, while in 1 mM [K+]o. there was a consistent and dramatic inhibition. The higher [K+]o does not completely abolish the response to the oxidant; higher concentrations (≥1 mM) of H2O2 strongly decreased ISK even at 10 mM [K+]o (data not shown). Nevertheless, these results confirm the sensitivity of the channels to an oxidizing agent and demonstrate that high [K+]o protects the channels against this inhibition.
Fig. 6.
H2O2 decreases ROMK current in a [K+]o-dependent manner. TPNQ-sensitive outward currents were measured in cells preincubated for >10 min with [K+]o = 1 or 10 mM with or without 0.1 mM H2O2. Data represent means ± SE for 4 experiments, with measurements in 2–3 cells for each condition. *P < 0.05, significant difference.
We then examined the possibility that changes in the membrane content of phosphatidylinositol bisphosphate (PIP2) may be involved in these responses. This phospholipid stabilizes the activity of ROMK (15, 20), and oxidative stress has been shown to activate its breakdown through activation of PLC (26, 38). Tubules were preincubated for 10 min or more with PLC inhibitor U73122 (33) at a concentration of 5 μM. This compound was previously shown to prevent the stimulation of TRPC3 channels by t-BHP, an effect thought to involve activation of PLCγ and depletion of plasma-membrane PIP2 (14). Whole-cell clamps were then established in the presence of 10 mM [K+]o and maintained for 2 min with either 10 or 1 mM [K+]o. ISK was then assessed by addition of TPNQ. As shown in Fig. 5, PLC inhibition did not have a strong effect on the baseline ROMK current but abolished the inhibition observed with low [K+]o.
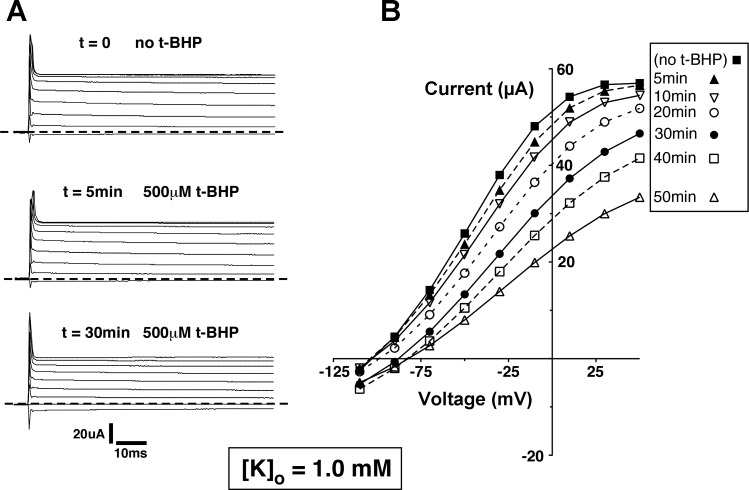
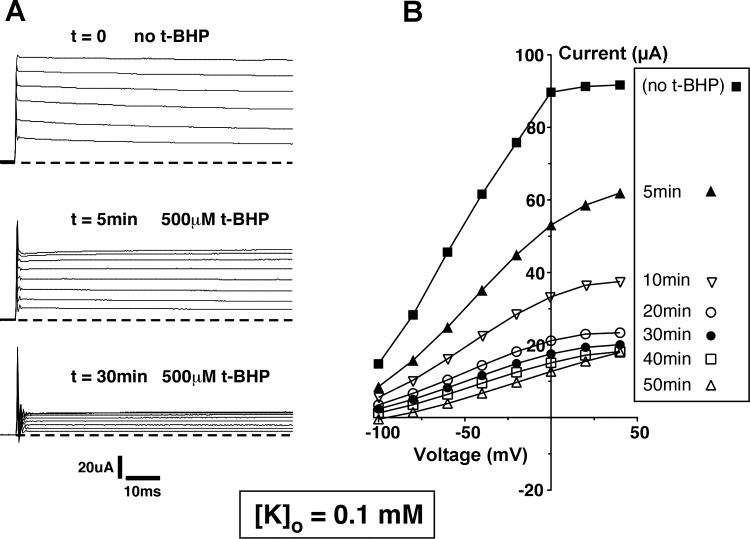
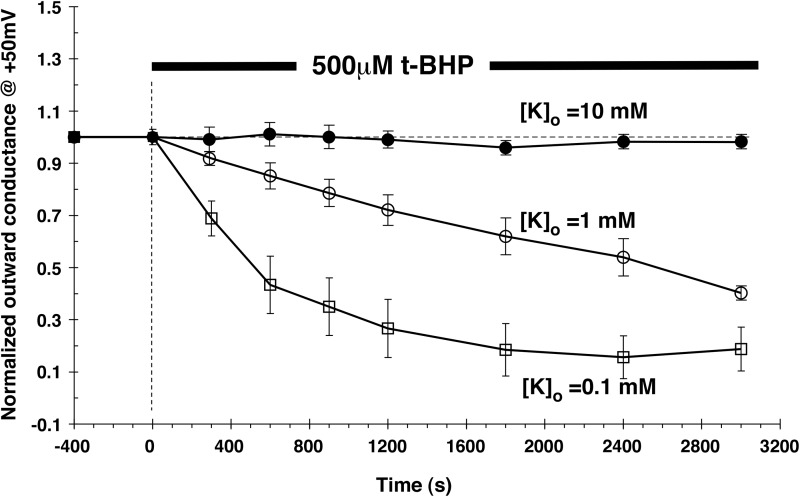
We then tested whether these effects could be reproduced in Xenopus oocytes expressing ROMK channels. This system is more stable than the principal cell under whole-cell voltage clamp and enables the continuous measurement of ROMK-mediated currents since the background conductance is very low. We first examined the effects of H2O2 added to the bathing medium. In agreement with previous results (3), this produced no substantial inhibition of the channels, even when used at higher concentrations (1 mM) and for longer times (up to 50 min) than were used with the CCD. This could have been due to a low permeability of the oocyte plasma membrane to the reagent. We therefore tested t-BHP, a membrane-permeant analog of H2O2. At a concentration of 500 μM, this compound did not affect ROMK currents in 10 mM [K+]o. However, substantial inhibition was observed at lower [K+]o concentrations. Figure 7 shows current traces and I–V relationships measured in the presence of 1 mM [K+]o before and at various times after addition of t-BHP. There was a progressive fall in outward currents to a level ∼50% of baseline after 50 min. Figure 8 illustrates a similar experiment with 0.1 mM [K+]o Here the inhibition by t-BHP was faster and more complete. A summary of the effects of t-BHP with the three different [K+]o is plotted in Fig. 9. These results suggest that ROMK channels are affected by oxidizing agents in the CCD and in the oocyte by similar mechanisms.
Fig. 7.
Effect of t-BHP on whole-cell currents in Kir1.1b (ROMK2)-expressing Xenopus oocytes with [K+]o =1.0 mM, pHi ∼ 7.8. A: representative current traces, measured in response to voltages applied between −110 and +50 mV. Dashed line denotes zero current. Upward deflections from the dashed line denote outward current. B: current-voltage relations recorded in the same oocyte at different times after application of 500 μM tert-butyl hydroperoxide (t-BHP).
Fig. 8.
Effect of t-BHP on whole-cell currents in Kir1.1b (ROMK2)-expressing Xenopus oocytes with [K+]o =0.1 mM, pHi ∼ 7.8. A: representative current traces, measured in response to voltages applied between −100 and +40 mV. Dashed line denotes zero current. Upward deflections from the dashed line denote outward current. B: current-voltage relations recorded in the same oocyte at different times after application of 500 μM t-BHP.
Fig. 9.
Effect of t-BHP (500 μM) on outward conductance in ROMK2-expressing oocytes with [K+]o = 10, 1, and 0.1 mM. Outward conductance was normalized to that measured just before addition of t-BHP. Data are means ± SE for n = 5 oocytes at each [K+]o. Acetate buffers maintained pHi =7.8, throughout.
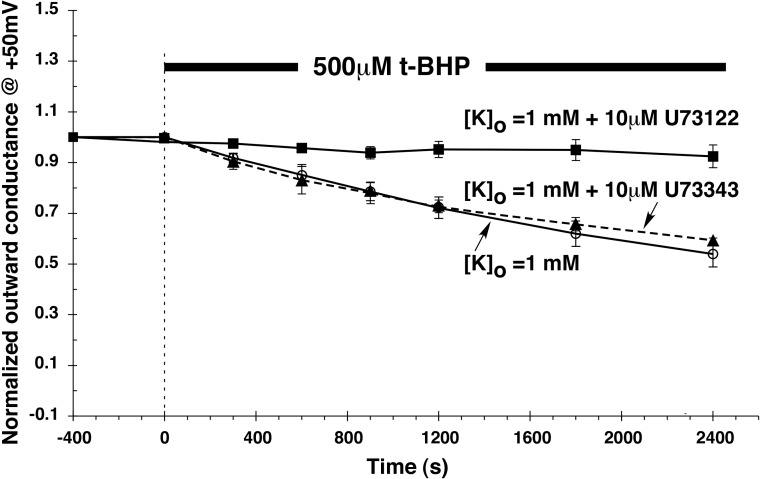
To examine the role of PLC in this system, oocytes incubated in 1 mM [K+]o were treated with 500 μM t-BHP in the presence and absence of U73122 (Fig. 10). The addition of 10 μM U73122 nearly abolished the response to t-BHP. In contrast, a related compound U73343 that lacks activity against PLC (33) did not interfere with the inhibition by t-BHP. This is consistent with results with the CCD and suggests that reduction in membrane PIP2 content represents at least one mechanism through which oxidative stress can modulate ROMK channels in a [K+]o-dependent manner.
Fig. 10.
Effect of PLC inhibition (U73122) on the response to t-BHP with [K+]o = 1 mM. Oocytes were treated with 500 μM t-BHP in the presence of 10 μM U73122, U73343, or without either drug. Outward conductance was normalized to that measured just before the addition of t-BHP. Data are means ± SE for 4 oocytes under each condition. Acetate buffer maintained pHi = 7.8 throughout.
DISCUSSION
Effects of [K+]o.
External K+ has several effects on ROMK channels, all of them stimulatory. Increasing [K+]o shifts the pH dependence of the channels, keeping them open in more acid conditions (29, 31). Furthermore, high [K+]o prevents the channels from entering a stable inactive state in the presence of low intracellular pH (27). In this study, we show that 10 mM [K+]o also protects the channels from closure in response to oxidative stress. This appears to involve, at least in part, a reduction in the amount of PIP2 in the membrane. This negatively charged phospholipid stabilizes the open state ROMK (15, 20), as well as that of other inward rectifiers (8), by interacting with positively charged lysine and arginine residues situated at the membrane/cytoplasm interface.
Molecular mechanism.
It is likely that oxidation, low-K, and PIP2 effects are all related. The simplest mechanism involves binding of K+ to sites at the outer mouth of the selectivity filter, stabilizing it in an open configuration. This interaction would antagonize any allosteric effects that favor a closed state in which the filter collapses (e.g., Ref. 47). In this way external K+ could antagonize a variety of inhibitory effects that act on the inside of the cell including cytoplasmic acidification, PIP2 depletion, and oxidative stress. Consistent with this idea, several Kir channels including Kir1.1 (ROMK) and 4.1 are selectively activated by cations that interact with the pore either as permeant ions or blockers (7, 9, 29). In this respect, regulation of Kir channels by external K+ is analogous to C-type inactivation of voltage-gated K+ channels, a process that is also inhibited by extracellular permeant ions (18, 21).
Similar effects of H2O2 on ROMK channels measured in cell-attached patches from rat CCD were previously reported (40). In that study inhibition of channel activity was also purported to be indirect, rather than through oxidation of the channel protein itself, since the effect was not observed in excised patches and could be prevented by simultaneous treatment with antagonists of tyrosine kinase, P38, and ERK pathways. However, this phenomenon was observed with the channels exposed to high (140 mM) external K+ and the mechanism may therefore be different from that reported here.
Physiological significance.
The main goal of this investigation was to test whether external K+ could regulate ROMK channels in renal tubules and over a physiological concentration range. This range is actually quite large; during antidiuresis, luminal K+ concentrations in the outer medullary collecting duct exceed 40 mM (42). We were more interested, however, in the lower end of the spectrum. Micropuncture measurements showed that luminal K+ in the DCT is maintained at <2 mM when dietary K intake is low but increases to 10–30 mM in the distal half of the DCT, with a normal K intake (23). Here the “DCT” was defined as the part of the distal nephron accessible to micropuncture and may include K+-secreting segments such as the DCT2 and connecting tubule. We therefore considered the question of whether changes in [K+]o from 1 to 10 mM could directly influence channel activity. The main finding was that indeed regulation could occur over this concentration range at least under conditions of oxidative stress. When this occurs, channel activity would be shut down when luminal [K+] is low, minimizing K+ secretion even when the driving force exerted by the concentration gradient across the apical membrane is large.
The oxidative stress imposed by the whole-cell clamp conditions may involve the removal of reducing agents from the cytoplasm as the constituents are exchanged with the solution in the patch-clamp pipette, although other factors such as removal of ATP may also contribute. This is not a physiological perturbation of the system but similar alterations in redox status may occur in vivo. There is evidence that a K-deficient diet not only decreases delivery of K+ to the distal nephron but also enhances oxidative stress. Superoxide concentrations in renal cortex and outer medulla (2), as well as in vascular tissue (44), are increased with chronic K-restriction. Conversely, a high-K diet reduced ROS production in vascular tissue and protected against deleterious effects of a high Na intake (17). These effects may be mediated in part by angiotensin II (ANG II). A low-K diet increases plasma renin activity (19) and may elevate circulating and/or renal ANG II levels. ANG II promotes oxidative stress in the kidney (43) as well as the heart and vascular system (6). Thus we envision a scenario in which restriction of K intake leads to conditions conducive to the inhibition of K+ channels through the combination of low luminal [K+] and a more oxidizing environment. Consistent with this idea, Babilonia et al. (2) showed that renal K losses in K-deficient rats were increased when superoxide formation was inhibited with tempol.
This is not the only mechanism through which low K intake influences ROMK channels. In CCDs isolated from K-depleted rats, ROMK activity is partially inhibited even when measured in vitro with high [K+]o and in the absence of oxidative stress (1, 13). This is due at least in part to a decreased expression of channel protein (11, 36) and/or expression at the cell surface (34, 45). In contrast, the effects of low [K+]o described here would serve to inhibit the channels remaining in the luminal membrane. Furthermore, a low-K diet enhanced the response of the CCD to inhibition by H2O2 in vitro (40). This chronic effect of K-depletion is distinct from the acute sensitization by low ambient [K+]o reported here. Finally, a low-K diet predisposes the channels to inhibition by ANG II in vitro (41). The mechanism that we propose here through which ROMK may be influenced by ANG II in vivo would be superimposed with this effect. The presence of multiple mechanisms for preventing K+ secretion when urinary K losses need to be minimized underscores the importance to the organism of maintaining K balance.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-27847 (to L. G. Palmer) and R01-DK-46950 (to H. Sackin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.F., H.S., and L.G.P. conception and design of research; G.F., H.L., H.S., and L.G.P. performed experiments; G.F., H.S., and L.G.P. interpreted results of experiments; G.F., H.S., and L.G.P. edited and revised manuscript; G.F., H.L., H.S., and L.G.P. approved final version of manuscript; H.S. and L.G.P. analyzed data; H.S. and L.G.P. prepared figures; L.G.P. drafted manuscript.
REFERENCES
- 1.Babilonia E, Li D, Wang Z, Sun P, Lin DH, Jin Y, Wang WH. Mitogen-activated protein kinases inhibit the ROMK (Kir 1.1)-like small conductance K channels in the cortical collecting duct. J Am Soc Nephrol 17: 2687–2696, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babilonia E, Wei Y, Sterling H, Kaminski P, Wolin M, Wang WH. Superoxide anions are involved in mediating the effect of low K intake on c-Src expression and renal K secretion in the cortical collecting duct. J Biol Chem 280: 10790–10796, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannister JP, Young BA, Main MJ, Sivaprasadarao A, Wray D. The effects of oxidizing and cysteine-reactive reagents on the inward rectifier potassium channels Kir2.3 and Kir11. Pflügers Arch 438: 868–878, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Chen P, Guzman JP, Leong PK, Yang LE, Perianayagam A, Babilonia E, Ho JS, Youn JH, Wang WH, McDonough AA. Modest dietary K+ restriction provokes insulin resistance of cellular K+ uptake and phosphorylation of renal outer medulla K+ channel without fall in plasma K+ concentration. Am J Physiol Cell Physiol 290: C1355–C1363, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Choe H, Zhou H, Palmer LG, Sackin H. A conserved cytoplasmic region of ROMK modulates pH sensitivity, conductance and gating. Am J Physiol Renal Physiol 273: F516–F529, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293: H2009–H2023, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Dahlmann A, Li M, Gao ZH, McGarrigle D, Sackin H, Palmer LG. Regulation of Kir channels by intracellular pH and extracellular K+: mechanisms of coupling. J Gen Physiol 123: 441–454, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J Biol Chem 279: 37271–37281, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Edvinsson JM, Shah AJ, Palmer LG. Kir4.1 K(+) channels are regulated by external cations. Channels (Austin) 5: 269–279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frindt G, Houde V, Palmer LG. Conservation of Na+ versus K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol 301: F14–F20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Renal Physiol 286: F669–F674, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Frindt G, Shah A, Edvinsson JM, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol 296: F347–F354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groschner K, Rosker C, Lukas M. Role of TRP channels in oxidative stress. Novartis Found Symp 258: 222–230; discussion 231–225, 263–226, 2004 [PubMed] [Google Scholar]
- 15.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391: 803–806, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Kido M, Ando K, Onozato ML, Tojo A, Yoshikawa M, Ogita T, Fujita T. Protective effect of dietary potassium against vascular injury in salt-sensitive hypertension. Hypertension 51: 225–231, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kiss L, Korn SJ. Modulation of C-type inactivation by K+ at the potassium channel selectivity filter. Biophys J 74: 1840–1849, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotchen TA, Guthrie GP, Jr, Galla JH, Luke RG, Welch WJ. Effects of NaCl on renin and aldosterone responses to potassium depletion. Am J Physiol Endocrinol Metab 244: E164–E169, 1983 [DOI] [PubMed] [Google Scholar]
- 20.Leung YM, Zeng WZ, Liou HH, Solaro CR, Huang CL. Phosphatidylinositol 4,5-bisphosphate and intracellular pH regulate the ROMK1 potassium channel via separate but interrelated mechanisms. J Biol Chem 275: 10182–10189, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Levy DI, Deutsch C. A voltage-dependent role for K+ in recovery from C-type inactivation. Biophys J 71: 3157–3166, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol 286: F881–F892, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malnic G, Klose RM, Giebisch G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol 211: 529–547, 1966 [DOI] [PubMed] [Google Scholar]
- 24.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Qin S, Inazu T, Yamamura H. Activation and tyrosine phosphorylation of p72syk as well as calcium mobilization after hydrogen peroxide stimulation in peripheral blood lymphocytes. Biochem J 308: 347–352, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sackin H, Palmer LG, Krambis M. Potassium dependent slow inactivation of Kir1.1 (ROMK) channels. Biophys J 86: 2145–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sackin H, Syn S, Palmer LG, Choe H, Walters E. Regulation of ROMK by extracellular cations. Biophys J 80: 683–697, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sackin H, Vasilyev A, Palmer LG, Krambis M. Permeant cations and blockers modulate pH gating of ROMK channels. Biophys J 84: 910–921, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulte U, Hahn H, Wiesinger H, Ruppersberg JP, Fakler B. pH-dependent gating of ROMK (Kir1.1) channels involves conformational changes in both N and C termini. J Biol Chem 273: 34575–34579, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Schulte U, Weidemann S, Ludwig J, Ruppersberg J, Fakler B. K-dependent gating of Kir1.1 channels is linked to pH gating through a conformational change in the pore. J Physiol 534: 49–58, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva P, Brown RS, Epstein FH. Adaptation to potassium. Kidney Int 11: 466–475, 1977 [DOI] [PubMed] [Google Scholar]
- 33.Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther 253: 688–697, 1990 [PubMed] [Google Scholar]
- 34.Sterling H, Lin DH, Wei Y, Wang WH. Tetanus toxin abolishes exocytosis of ROMK1 induced by inhibition of protein tyrosine kinase. Am J Physiol Renal Physiol 284: F510–F517, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300: F1385–F1393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Schwab A, Giebisch G. Regulation of small conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Wang XT, McCullough KD, Wang XJ, Carpenter G, Holbrook NJ. Oxidative stress-induced phospholipase C-gamma 1 activation enhances cell survival. J Biol Chem 276: 28364–28371, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol 281: F206–F212, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Wei Y, Wang Z, Babilonia E, Sterling H, Sun P, Wang W. Effect of hydrogen peroxide on ROMK channels in the cortical collecting duct. Am J Physiol Renal Physiol 292: F1151–F1156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem 282: 6455–6462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinstein AM. A mathematical model of rat cortical collecting duct; determinants of the transtubular potassium gradient. Am J Physiol Renal Physiol 280: F1072–F1092, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yang BC, Li DY, Weng YF, Lynch J, Wingo CS, Mehta JL. Increased superoxide anion generation and altered vasoreactivity in rabbits on low-potassium diet. Am J Physiol Heart Circ Physiol 274: H1955–H1961, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Zeng WZ, Babich V, Ortega B, Quigley R, White SJ, Welling PA, Huang CL. Evidence for endocytosis of ROMK potassium channel via clathrin-coated vesicles. Am J Physiol Renal Physiol 283: F630–F639, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Zhang YY, Sackin H, Palmer LG. Localization of the pH gate in Kir1.1 channels. Biophys J 91: 2901–2909, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature 414: 43–48, 2001 [DOI] [PubMed] [Google Scholar]