Abstract
To investigate the role of hepatic glucagon receptors in the hypersensitivity to glucagon observed in insulin-deprived diabetics, liver plasma membranes were prepared from control rats and from streptozotocin-induced diabetic rats some of whom were treated with high-dose and low-dose insulin. The untreated diabetic animals exhibited hyperglycemia, weight loss, hypoinsulinemia, and hyperglucagonemia. High-dose insulin treatment (2 U Protamine-zinc-insulin/100 g per day) resulted in normoglycemia, normal weight gain, mild hyperinsulinemia, and return of glucagon levels toward base line. The low-dose (1 U protamine-zinc-insulin/100 g per day) insulin-treated diabetic group demonstrated chemical changes intermediate between the untreated and the high-dose insulin-treated animals.
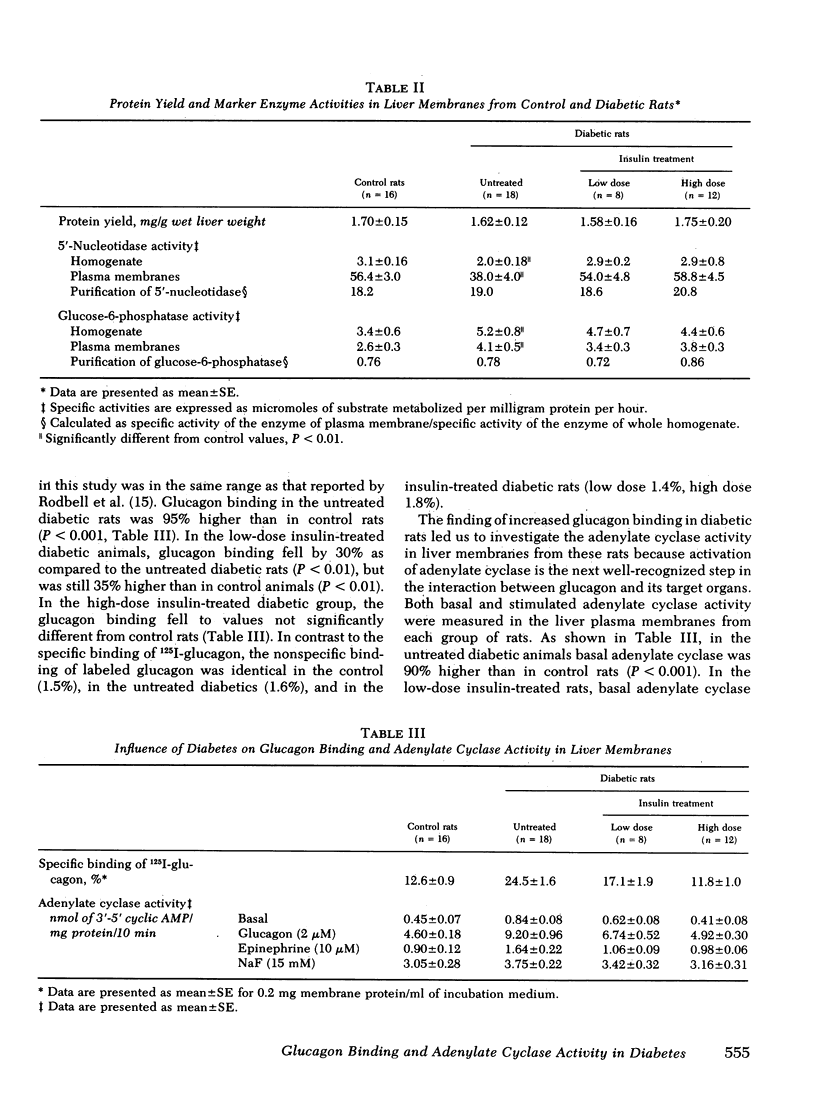
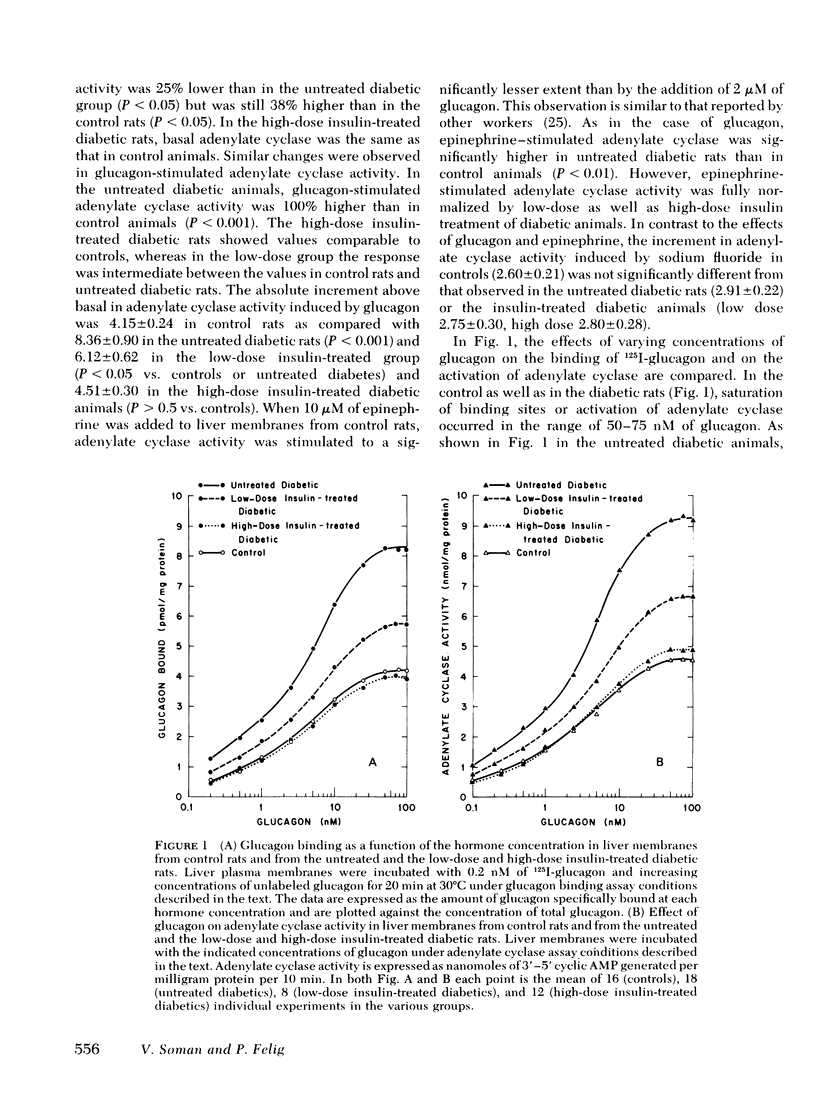
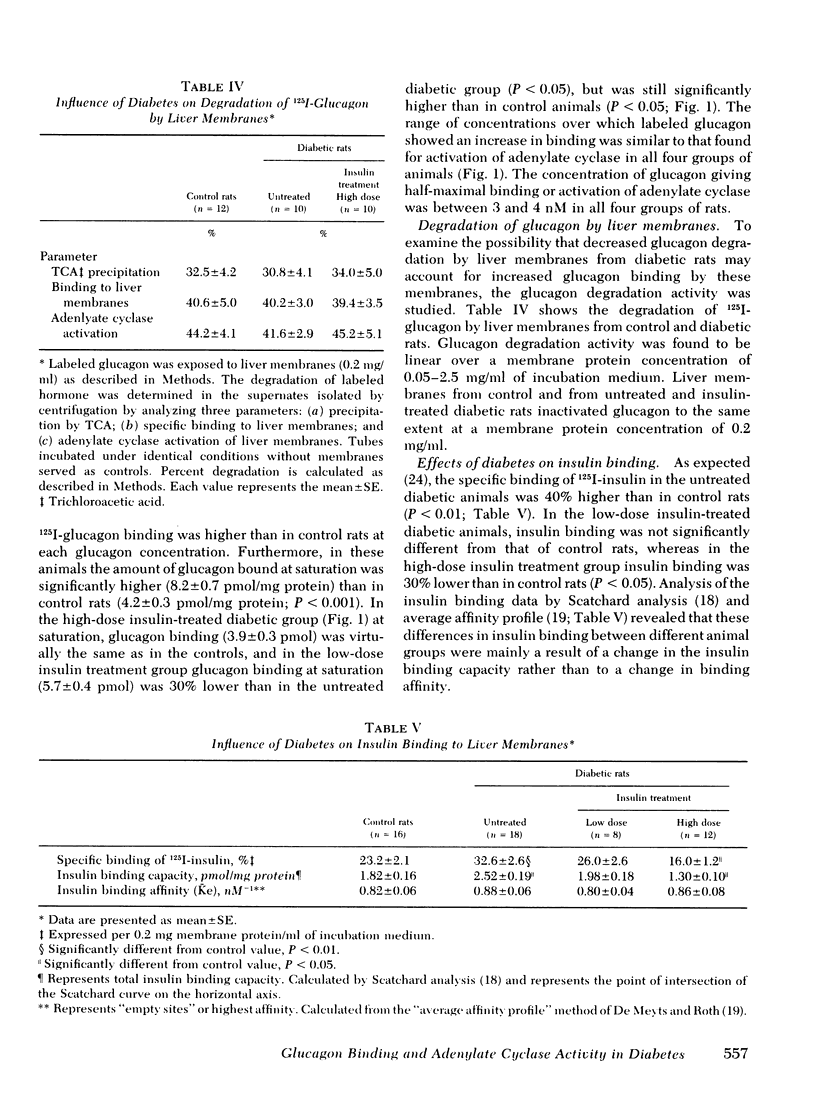
In liver plasma membranes from the untreated diabetic rats, specific binding of 125I-glucagon was increased by 95%. Analysis of binding data suggested that the changes in glucagon binding were a consequence of alterations in binding capacity rather than changes in binding affinity. Furthermore, in the untreated diabetic rats, both basal and glucagon (2 μM)-stimulated adenylate cyclase activity were twofold higher than in controls. In the high-dose insulin-treated diabetic rats, glucagon binding and basal and glucagon-stimulated adenylate cyclase activity were normalized to control values, whereas low-dose insulin treatment resulted in changes intermediate between control and untreated diabetic rats. In contrast to glucagon-stimulated adenylate cyclase activity, fluoride-stimulated adenylate cyclase activity was similar in all groups of rats. Liver plasma membranes from untreated and insulin-treated diabetic animals degraded 125I-glucagon to the same extent as control rats.
The specific binding of 125I-insulin in the untreated diabetic animals was 40% higher than in control rats. In low-dose insulin-treated diabetic rats, insulin binding was not significantly different from that of control rats, whereas in the high-dose insulin-treated group in whom plasma insulin was 70% above control levels, insulin binding was 30% lower than in control rats.
These findings suggest that alterations in glucagon receptors may contribute to the augmented glycemic and ketonemic response to glucagon observed in insulin-deprived diabetics.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker L., Kaye R., Haque N. Metabolic homeostasis in juvenile diabetes mellitus. II. Increased ketone responsiveness to epinephrine. Diabetes. 1969 Jun;18(6):421–427. doi: 10.2337/diab.18.6.421. [DOI] [PubMed] [Google Scholar]
- Barnes A. J., Bloom S. R., Goerge K., Alberti G. M., Smythe P., Alford F. P., Chisholm D. J. Ketoacidosis in pancreatectomized man. N Engl J Med. 1977 Jun 2;296(22):1250–1253. doi: 10.1056/NEJM197706022962202. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Neufeld A. H. Selective effects of insulin on hepatic epinephrine responsive adenyl cyclase activity. Endocrinology. 1972 May;90(5):1331–1335. doi: 10.1210/endo-90-5-1331. [DOI] [PubMed] [Google Scholar]
- Chandramouli V., Carter J. R., Jr Cell membrane changes in chronically diabetic rats. Diabetes. 1975 Mar;24(3):257–262. doi: 10.2337/diab.24.3.257. [DOI] [PubMed] [Google Scholar]
- De Meyts P., Roth J. Cooperativity in ligand binding: a new graphic analysis. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1118–1126. doi: 10.1016/0006-291x(75)90473-8. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Bier D. M., Schneider V., Tsalikian E., Karam J. H., Forsham P. H. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med. 1975 May 8;292(19):985–989. doi: 10.1056/NEJM197505082921901. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hepp K. D. Adenylate cyclase and insulin action. Effect of insulin, nonsuppressible insulin-like material, and diabetes on adenylate-cyclase activity in mouse liver. Eur J Biochem. 1972 Dec 4;31(2):266–276. doi: 10.1111/j.1432-1033.1972.tb02529.x. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem. 1973 Jan 10;248(1):244–250. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pilkis S. J., Exton J. H., Johnson R. A., Park C. R. Effects of glucagon on cyclic AMP and carbohydrate metabolism in livers from diabetic rats. Biochim Biophys Acta. 1974 Mar 20;343(1):250–267. doi: 10.1016/0304-4165(74)90258-x. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Pohl S. L., Krans H. M., Birnbaumer L., Rodbell M. Inactivation of glucagon by plasma membranes of rat liver. J Biol Chem. 1972 Apr 25;247(8):2295–2301. [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Friesen H. G. Induction of a lactogenic receptor in rat liver: influence of estrogen and the pituitary. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2407–2410. doi: 10.1073/pnas.71.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin P., Fujita Y., Unger R. H. Effect of insulin-glucose infusions on plasma glucagon levels in fasting diabetics and nondiabetics. J Clin Invest. 1975 Nov;56(5):1132–1138. doi: 10.1172/JCI108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Sherwin R. S., Bastl C., Finkelstein F. O., Fisher M., Black H., Hendler R., Felig P. Influence of uremia and hemodialysis on the turnover and metabolic effects of glucagon. J Clin Invest. 1976 Mar;57(3):722–731. doi: 10.1172/JCI108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R. S., Fisher M., Hendler R., Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med. 1976 Feb 26;294(9):455–461. doi: 10.1056/NEJM197602262940901. [DOI] [PubMed] [Google Scholar]
- Soman V., Felig P. Glucagon and insulin binding to liver membranes in a partially nephrectomized uremic rat model. J Clin Invest. 1977 Jul;60(1):224–232. doi: 10.1172/JCI108759. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Unger R. H., Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975 Jan 4;1(7897):14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- Unger R. H. The Banting Memorial Lecture 1975. Diabetes and the alpha cell. Diabetes. 1976 Feb;25(2):136–151. doi: 10.2337/diab.25.2.136. [DOI] [PubMed] [Google Scholar]



