Abstract
Hepatocyte nuclear factor-1β (HNF-1β) is an epithelial tissue-specific transcription factor that regulates gene expression in the kidney, liver, pancreas, intestine, and other organs. Mutations of HNF-1β in humans produce renal cysts and congenital kidney anomalies. Here, we identify the LIM-domain protein zyxin as a novel binding partner of HNF-1β in renal epithelial cells. Zyxin shuttles to the nucleus where it colocalizes with HNF-1β. Immunoprecipitation of zyxin in leptomycin B-treated cells results in coprecipitation of HNF-1β. The protein interaction requires the second LIM domain of zyxin and two distinct domains of HNF-1β. Overexpression of zyxin stimulates the transcriptional activity of HNF-1β, whereas small interfering RNA silencing of zyxin inhibits HNF-1β-dependent transcription. Epidermal growth factor (EGF) induces translocation of zyxin into the nucleus and stimulates HNF-1β-dependent promoter activity. The EGF-mediated nuclear translocation of zyxin requires activation of Akt. Expression of dominant-negative mutant HNF-1β, knockdown of zyxin, or inhibition of Akt inhibits EGF-stimulated cell migration. These findings reveal a novel pathway by which extracellular signals are transmitted to the nucleus to regulate the activity of a transcription factor that is essential for renal epithelial differentiation.
Keywords: cell motility, epidermal growth factor, protein-protein interactions, transcription
hepatocyte nuclear factor-1β (HNF-1β) belongs to the POU/homeodomain-containing superfamily of transcription factors that regulate tissue-specific gene expression in the kidney, liver, pancreas, and other epithelial organs (20). HNF-1β contains an N-terminal dimerization domain, DNA-binding POU/homeodomain, and a C-terminal transactivation domain (20). HNF-1β binds DNA as a homodimer or as a heterodimer with the paralogous protein HNF-1α and recognizes the consensus sequence 5′-GTTAATNATTAAC-3′ that is found in the promoters of epithelial-specific genes (1, 20).
HNF-1β is highly expressed in the developing and mature kidney (7). In the adult mouse kidney, HNF-1β is expressed in all tubular epithelial cells composing the nephrons and collecting ducts. During embryonic development, HNF-1β is expressed beginning at embyronic day 12.5 in the ureteric bud that will give rise to the renal collecting system and in comma- and S-shaped bodies that will form the nephron proper. Studies in Xenopus, zebrafish, and mice have shown that HNF-1β is essential for normal kidney development (25, 27, 31, 35). Expression of a dominant-negative HNF-1β mutant in renal epithelial cells inhibits HGF-dependent tubulogenesis through dysregulation of store-operated cation channel 3 (SOCS3; Ref. 26).
Humans with mutations of HNF-1β (TCF2) develop congenital kidney abnormalities, including multicystic renal dysplasia, glomerulocystic kidney disease, oligomeganephronia, renal agenesis, renal hypoplasia, and familial juvenile hyperuricemic nephropathy (4, 12, 14, 15). In addition, mutations of HNF-1β produce extrarenal phenotypes, such as maturity-onset diabetes of the young (MODY), malformations of the genital tract, and pancreatic atrophy. Mice with kidney-specific inactivation of HNF-1β develop polycystic kidney disease due to downregulation of cystic disease genes including UMOD, PKHD1, and PKD2 (13). Expression of disease-causing HNF-1β mutants in transgenic mice interferes with DNA binding or coactivator recruitment, inhibits target gene transcription, and produces kidney cysts (17, 18). Collectively, these findings indicate that HNF-1β plays a central role in kidney development and the regulation of cystic disease genes that are essential for epithelial differentiation.
The various processes controlled by HNF-1β during development and in human diseases could be modulated by interacting proteins. For example, binding of coactivators and corepressors, dimerization cofactor for HNF-1 (DCoH), CREB binding protein (CBP), P/CAF, and histone deacetylase (HDAC1), can regulate the transcriptional activity of HNF-1β (2, 18, 29). In the Xenopus kidney, two zinc-finger proteins, E4F1 and ZFP36L, have been shown to interact with HNF-1β (11). Overexpression of E4F1 and ZFP36L reduces the transactivation potential of HNF-1β and interferes with nephrogenesis. Here, we identify a member of the zyxin family as a novel HNF-1β-interacting protein in the mammalian kidney. Zyxin is a LIM-domain protein that is concentrated in focal adhesions at sites of cell-matrix interaction. Zyxin can also shuttle from the cytoplasm to the nucleus, and we show that the nuclear translocation of zyxin in renal epithelial cells can be stimulated by epidermal growth factor (EGF) via Akt activation. In the nucleus, zyxin interacts with HNF-1β, stimulates its transcriptional activity, and upregulates target gene expression. These findings reveal a new pathway in which extracellular signals regulate the activity of a transcription factor that is essential for epithelial morphogenesis.
MATERIALS AND METHODS
Yeast transformation and growth selection.
LexA-HNF-1β fusion vectors were transformed into Saccharomyces cerevisiae (strain L40, genotype MATa trp1 leu2 his3 LYS2::lexA-HIS3 URA3::LexA-LacZ). Yeast were grown in YPDA (2% Difco peptone, 1% yeast extract, 2% glucose, and 0.1% adenine hemisulfate) or in synthetic minimal Trp dropout medium (SD-Trp) complemented with 0.1% adenine hemisulfate. For transformation, cultures were grown overnight to an optical density (OD600) of ∼1, centrifuged for 3 min at 1,200 g, washed with 40 ml distilled H2O, and resuspended in 1 ml H2O. Aliquots of 50 μl were added to 240 μl PEG 3350 (50% wt/vol), 50 μl distilled H2O, 36 μl 1 M LiOAc, 50 μg single-stranded DNA, and 1 μg plasmid. Proper expression of the bait proteins was confirmed by immunoblotting of total cell lysates with an antibody against LexA. As a control, the absence of autonomous L40 reporter gene activation by the bait proteins was verified.
Library screening.
An adult mouse kidney cDNA library (Matchmaker, Clontech, Madison, WI) was used. One-hundred milliliters of SD-Trp medium were inoculated with S. cerevisiae, grown to OD600 >2 at 30°C, and used to inoculate 1 l YPDA. The culture was further grown to an OD600 of 0.7 divided in fourths and centrifuged at 4,200 g for 15 min at 4°C. The pellets were washed with distilled H20 and transfected with 50 μg cDNA (1 mg/ml). After incubation at 30°C followed by heat shock at 42°C for 30 min, cells were harvested by centrifugation at 1,900 g for 3 min, washed with 80 ml distilled H2O, resuspended in 20 m distilled H2O, and plated in 50- to 14-cm Petri dishes. After 3 days, permeabilized cells were transferred onto Whatman filters and overlaid with 0.2 mg/ml X-gal, 50 mM Tris·Cl pH 7.4, 150 mM NaCl, and 0.8% agarose. X-gal-positive colonies were restreaked in selective medium and rescreened by colony-lift assays. Yeast DNA was purified from positive clones, and prey plasmids were isolated by transformation into KC8 cells. Prey plasmids were digested with BglII to determine insert size and sequenced.
DNA constructs, antibodies, and reagents.
cDNAs encoding full-length mouse zyxin and deletion mutants were amplified by PCR and cloned into a pCMV plasmid containing a MYC epitope tag. Deletion mutants of HNF-1β were produced by PCR amplification from full-length HNF-1β and cloned into pCMV-Flag plasmid (Sigma, St. Louis, MO). MK-2206 was purchased from Sellekchem (Houston, TX). Leptomycin B and EGF were obtained from Sigma.
Cell culture, DNA transfection, and reporter gene assays.
HEK293 cells, HeLa cells, and mIMCD3 cells were routinely cultured in DMEM containing 10% FBS at 37°C in 5% CO2. Transfection was performed using Effectene (Qiagen, Valencia, CA) following the manufacturer's instructions. Cells were cotransfected with 20 ng pRL plasmid encoding Renilla luciferase to control for differences in transfection efficiency. After growth for 48 h, the cells were lysed in 500 μl passive lysis buffer (Promega, Madison, WI), freeze-thawed once, and centrifuged. Supernatants (20 μl) were added to 96-well plates, and firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega), according to the manufacturer's directions. Luciferase Assay Reagent II (100 μl) was added, and light output was measured for 10 s using a Wallac VICTOR V multilabel counter (Perkin Elmer, Wellesley, MA). Firefly luciferase activity was normalized to Renilla luciferase activity, which was measured by adding 100 μl of Stop & Glo reagent and measuring light output for 5 s.
In vitro wound healing assay.
Cells (1 × 106) were plated in 60-mm dishes and grown to confluence at 37°C. A scratch wound was created by manually scraping the cell monolayer with a P100 pipet tip. Cells were washed once and incubated with 4 ml of growth medium containing EGF, MK-2206, or vehicle. Images were acquired immediately and 15 h later using an AxioObserver FL microscope at ×100 magnification. The width of the wound was measured in three locations, and the percent wound healing was calculated using ImageJ software. Each experiment was performed in triplicate.
Immunoprecipitation, Western blot analysis, and cell fractionation.
Cells were lysed with immunoprecipitation buffer (50 mM Tris·Cl, 150 mM NaCl, 5 mM EDTA, 0.1% Triton X-100, 0.1% deoxycholic acid, 100 mM PMSF, and 1 tablet/10 ml protease inhibitor cocktail; Roche, Indianapolis, IN). Extracts were clarified by centrifugation at 12,000 g for 1 min. Cell extracts were mixed with primary antibody or control IgG at 4°C overnight and then incubated with 20 ml protein AG/slurry (1:1, vol/vol) for 1 h with gentle rotation at 4°C. Immunoprecipitates were washed three times with immunoprecipitation buffer and boiled in SDS-loading buffer. After SDS-PAGE under reducing conditions, proteins were transferred to nitrocellulose membranes and subjected to Western blot analysis using the ECL detection reagent (Pierce, Rockford, IL). Primary antibodies used were as follows: zyxin, lamin A, α-actin, HNF-1β, tubulin, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA); FLAG and MYC (Sigma); and Akt, p-Akt, pZyxin, and Zyxin (Cell Signaling, Danvers, MA).
Small interfering RNA transfection.
Small interfering RNA (siRNA) oligonucleotides targeting zyxin and control siRNA oligonucleotides were purchased from Santa Cruz Biotechnology and Dharmacon (Lafayette, CO). mIMCD3 cells were transfected with zyxin siRNA (10, 50, and 100 nM) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). Expression of zyxin was measured by Western blotting with anti-zyxin antibody.
Real-time RT-PCR.
Total RNA was isolated from cells using TRIzol reagent according to the manufacturer's protocol (Invitrogen). For RT-PCR, cDNAs were synthesized using the Super Script first-strand synthesis system (Invitrogen) and then amplified by PCR with Pkhd1 primers. Real-time PCR was performed in triplicate using iCycler and SYBR green Supermix reagents (Bio-Rad Laboratories, Hercules, CA). β2-Microglobulin was used as the control gene for normalization. Collected data were analyzed using IQ5 software (Bio-Rad Laboratories).
Chromatin immunoprecipitation and re-chromatin immunoprecipitation assays.
Chromatin immunoprecipitation (ChIP) and re-ChIP assays were performed using Re-ChIP-IT kits (Active Motif, Carlsbad, CA). mIMCD3 cells were cross-linked with 1% formaldehyde for 10 min at room temperature, and the cross-linked chromatin was extracted and sheared by sonication. Thirty micrograms of sheared chromatin were immunoprecipitated with 3 μg of anti-HNF-1β antibody (Santa Cruz Biotechnology) or rabbit IgG (Active Motif, Carlsbad, CA) as a negative control. The immunoprecipitates were washed three times, eluted, and desalted. Ten microliters from the first ChIP reaction were reserved, and the remaining chromatin was immunoprecipitated with anti-pZyxin antibody or rabbit IgG. The immunoprecipitates were washed and eluted, and cross-linking was reversed. After treatment with protease K and RNase A, the purified DNA was amplified using PCR primers derived from the 5′-flanking region of Pkhd1 (forward: 5′-CACATGCAGTAAGTGGAGAAAAATGTAAG-3′ and reverse: 5′-CGTTATTCGGTGACAGGCCAG-3′).
Antibody staining.
Cells were fixed with 4% paraformaldehyde and washed with PBS. The cells were incubated with a primary antibody against FLAG and HNF-1β, washed and then incubated with secondary antibodies. Secondary antibodies were conjugated to Alexa Fluor 594 or Alexa Fluor 488 (Molecular Probes, Eugene, OR). Fluorescence signals were visualized and digitally captured using a Zeiss fluorescence microscope.
Cell proliferation.
Cells were seeded at their optimal cell density (2 × 103 cells/well) into 96-well plates and were incubated overnight to allow cell attachment. Proliferation was measured with a CellTiter 96 AQ assay kit (Promega) according to the manufacturer's directions.
Statistical analysis.
Statistical analysis was performed using Student's t-test or ANOVA with Dunnett's correction for multiple comparisons as appropriate. P <0.05 was considered significant.
RESULTS
Zyxin interacts with HNF-1β in the kidney.
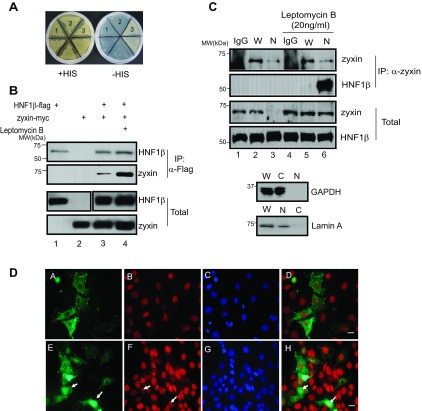
To identify novel HNF-1β interacting proteins, we performed yeast two-hybrid screening. We used full-length HNF-1β as the bait to screen a cDNA library derived from adult mouse kidney. Twenty-seven positive clones were identified. Sequence analysis and database comparison revealed that one positive clone contained the coding sequence of zyxin (GenBank accession number NM_011777; Ref. 8). The interaction between HNF-1β and zyxin was verified with yeast two-hybrid assays (Fig. 1A). Yeast were transformed with plasmids encoding HNF-1β fused to the lexA DNA-binding domain, zyxin fused to an activation domain, or both proteins and streaked on synthetic drop-out plates. Only yeast expressing both HNF-1β and zyxin fusion proteins grew on synthetic drop-out plates lacking histidine (Fig. 1A, 3). To confirm the in vivo interaction between HNF-1β and zyxin, expression plasmids encoding FLAG-tagged HNF-1β and MYC-tagged zyxin were cotransfected into HEK293T cells. Cells transfected with zyxin-MYC alone were used as a negative control. Western blot analysis of total cell lysates showed that both FLAG- and MYC-tagged proteins were expressed in transfected cells (Fig. 1B, lanes 3 and 4, bottom). Following immunoprecipitation with an anti-FLAG antibody, zyxin-MYC was detected in the precipitates (Fig. 1B, lanes 3 and 4, top), indicating coimmunoprecipitation of zyxin and HNF-1β. Treatment with leptomycin B, which blocks CRM-dependent nuclear export, resulted in greater immunoprecipitation of zyxin-MYC (Fig. 1B, lane 4, top), suggesting that zyxin interacts with HNF-1β in the nucleus. To confirm these results, mouse kidney epithelial cells (mIMCD3 cells) that endogenously express HNF-1β and zyxin were used for the immunoprecipitation experiments (Fig. 1C). mIMCD3 cells were incubated in the absence or presence of leptomycin B, separated into cytosolic and nuclear fractions, and subjected to immunoprecipitation. The endogenous HNF-1β and zyxin were detected (Fig. 1C, lanes 1 and 2, bottom). Zyxin was detected in the nuclear fraction derived from leptomycin B-treated cells (Fig. 1C, lanes 3 and 6, bottom). Immunoprecipitation of endogenous zyxin resulted in coprecipitation of endogenous HNF-1β in leptomycin B-treated cells (Fig. 1C, lane 6, top), further suggesting an interaction between these two proteins in the nucleus. mIMCD3 cells were transfected with GFP-zyxin and stained with an HNF-1β antibody. HNF-1β and zyxin were colocalized in the nucleus in leptomycin B-treated cells (Fig. 1D). Taken together, these results suggest a physical interaction between HNF-1β and zyxin in the nuclei of renal epithelial cells.
Fig. 1.
Zyxin interacts with hepatocyte nuclear factor-1β (HNF-1β). A: yeast were transformed with plasmids encoding LexA-HNF-1β alone (1), zyxin-activation domain (AD) alone (2), or both LexA-HNF-1β and zyxin-AD (3) and grown on histidine-containing plates (+His) or medium lacking histidine (-His). B: HEK293T cells were cotransfected with plasmids encoding MYC-tagged zyxin and/or FLAG-tagged HNF-1β and incubated in the absence or presence of leptomycin B (5 h, 20 ng/ml). Cell lysates were immunoprecipitated (IP) with anti-FLAG antibody, and coprecipitated products were detected by Western blotting with anti-MYC and anti-FLAG antibodies. Input controls are shown at bottom. MW, molecular mass. C: mIMCD3 cells were incubated in the presence or absence of leptomycin B (20 ng/ml) for 5 h, and whole cell lysates (W) and nuclear extracts (N) were prepared. Endogenous zyxin was immunoprecipitated with anti-zyxin antibody, and coprecipitated HNF-1β was detected by Western blotting with anti-HNF-1β. Western blot was performed with anti-GAPDH as a cytosolic marker and anti-lamin A antibody as a nuclear marker to verify fractionation. C, cytosolic. D: mIMCD3 cells were transfected with plasmids encoding GFP-zyxin and were treated with leptomycin B (20 ng/ml) for 2 h (bottom) or were left untreated (top). DA–DH: GFP fluorescence (DA and DE), antibody staining with anti-HNF-1β (DB and DF), nuclei counterstained with DAPI (DC and DG), and merged images (DD and DH). Arrows indicate colocalization of zyxin and HNF-1β in the nuclei of leptomycin B-treated cells. Scale bars = 20 μm.
Second LIM domain of zyxin mediates the interaction with HNF-1β.
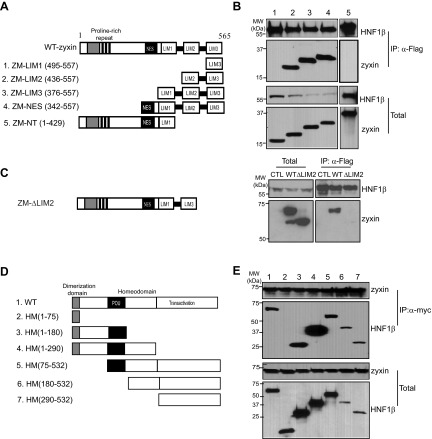
To identify the interacting domains of HNF-1β and zyxin, we generated five MYC-tagged truncated forms of zyxin, ZM-LIM1 (aa 495–557), ZM-LIM2 (aa 436–557), ZM-LIM3 (aa 376–557), ZM-NES (aa 342–557), and ZM-NT (aa 1–429; Fig. 2A). We also generated six FLAG-tagged deletion mutants of HNF-1β (aa 1–75, aa 1–180, aa 1–290, aa 75–532, aa 180–532, and aa 290–532; Fig. 2D). The zyxin deletion mutants were individually cotransfected with full-length FLAG-HNF-1β into HEK293T cells, and whole cell extracts were immunoprecipitated with anti-FLAG antibody. As shown in Fig. 2B, FLAG-tagged HNF-1β was detected in all the precipitates (lanes 1–5, top). However, only the zyxin mutants containing the second LIM domain (aa 436–557, aa 376–557, and aa 342–557) were detected in the immunoprecipitates (lanes 2–4, top). To confirm that the second LIM domain (LIM2) is necessary for the interaction with HNF-1β, we generated a zyxin mutant that lacks the second LIM domain (ZM-ΔLIM2; Fig. 2C). Immunoprecipitation assays in transfected mIMCD3 cells showed that HNF-1β interacts with full-length zyxin (wild type) but does not bind to the ZM-ΔLIM2 mutant. Western blot analysis showed that the deletion mutant and wild-type zyxin were expressed at similar levels. These results suggest that the interaction of zyxin with HNF-1β is mediated by the second LIM domain. However, we cannot exclude the possibility that the absence of the second LIM domain allosterically inhibits the interaction of HNF-1β with another region of the protein.
Fig. 2.
Mapping the interacting domains of HNF-1β and zyxin. A: schematic diagram of full-length zyxin and deletion mutants. B: HEK293T cells were cotransfected with plasmids encoding FLAG-tagged HNF-1β and MYC-tagged zyxin deletion mutants. Cell lysates were immunoprecipitated with anti-FLAG antibody, and the zyxin fragments associated with HNF-1β were identified by Western blotting with anti-MYC antibody. Numbering of the lanes corresponds to the deletion mutants in A. Bottom: levels of expression of the proteins in whole cell lysates. C: schematic diagram of a zyxin mutant lacking the second LIM domain is shown at left. mIMCD3 cells were cotransfected with plasmids encoding FLAG-tagged HNF-1β and MYC-tagged zyxin deletion mutant. Cell lysates were immunoprecipitated with anti-FLAG antibody and blots were probed with anti-MYC antibody. Cells transfected with only FLAG-tagged HNF-1β plasmid were used as a control (CTL). D: schematic diagram of full-length HNF-1β and deletion mutants. E: HEK293T cells were cotransfected with plasmids encoding MYC-tagged full-length zyxin and FLAG-tagged HNF-1β deletion mutants. Zyxin was immunoprecipitated with anti-MYC antibody and the coprecipitated HNF-1β fragments were detected by Western blotting with anti-FLAG antibody. Expression of the proteins in whole cell lysates is shown at bottom.
To identify the zyxin-binding domain in HNF-1β, the HNF-1β deletion mutants were cotransfected with MYC-tagged zyxin into HEK293T cells, and whole cell lysates were subjected to immunoprecipitation with anti-MYC antibody. As shown in Fig. 2D, five nonoverlapping HNF-1β deletion mutants were detected in immunoprecipitates pulled down by anti-MYC antibody (Fig. 2E, lanes 3–7, top). These findings indicate that at least two domains of HNF-1β can interact with zyxin: an N-terminal domain extending from aa 75–180 including the POU domain and the C-terminal transactivation domain extending from aa 290–532.
Zyxin potentiates the transcriptional activity of HNF-1β.
Zyxin has been reported to shuttle from the cytoplasm into the nucleus where it may regulate gene expression (5, 23). We studied whether the interaction with zyxin affects the transcriptional activity of HNF-1β. Previously, we identified Pkhd1, the gene that is mutated in autosomal recessive polycystic kidney disease, as an HNF-1β target gene in the kidney (17). HNF-1β activates Pkhd1 transcription by directly binding to the promoter region and recruiting coactivators via its C-terminal activation domain. A reporter plasmid containing 1.9 kb of the Pkhd1 promoter driving the expression of a luciferase reporter gene was cotransfected into HeLa cells with HNF-1β and/or zyxin, and luciferase activity was measured. Figure 3A shows that HNF-1β stimulated Pkhd1 promoter activity, as reported previously (17). Cotransfection of zyxin together with HNF-1β produced a greater stimulation of promoter activity than HNF-1β by itself. Zyxin by itself had no effect on the promoter, indicating that the stimulation was HNF-1β dependent. To confirm these results, luciferase reporter assays were performed in mIMCD3 cells that endogenously express HNF-1β. As shown in Fig. 3B, transfection of increasing amounts of zyxin produced a dose-dependent increase in Pkhd1 promoter activity. However, the activity of a mutant Pkhd1 promoter containing mutations of the HNF-1β binding site was not increased, further indicating that zyxin stimulates Pkhd1 promoter activity via its interaction with HNF-1β. Previous studies showed that the C-terminal transactivation domain of HNF-1β interacts with the coactivator CBP (18). Therefore, we examined whether zyxin is contained in a transcriptional complex with CBP and HNF-1β. As shown in Fig. 3C, CBP was detected in immunoprecipitates pulled down by anti-zyxin antibody (lane 2), indicating that zyxin is present in a transcriptional complex with HNF-1β and CBP in kidney epithelial cells.
Fig. 3.
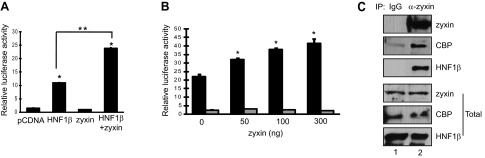
Zyxin potentiates the transcriptional activity of HNF-1β. A: HeLa cells were cotransfected with a luciferase reporter plasmid containing the Pkhd1 promoter and expression plasmids encoding HNF-1β alone, zyxin alone, or both HNF-1β and zyxin. Luciferase activity was measured 48 h after transfection. Luciferase activity was increased in cells transfected with both HNF-1β and zyxin but not zyxin alone. *P < 0.05, compared with pcDNA; **P < 0.05. B: mIMCD3 cells were cotransfected with increasing amounts of plasmids encoding zyxin and a constant amount of luciferase reporter plasmid containing the wild-type Pkhd1 promoter (solid bars) or a mutated promoter containing point mutations that prevent HNF-1β binding (gray bars). Zyxin produced a dose-dependent stimulation of the wild-type promoter but had no effect on the mutant promoter. *P < 0.05. C: endogenous zyxin was immunoprecipitated from mIMCD3 cells using an anti-zyxin antibody, and coprecipitated proteins were detected by Western blotting with anti-HNF-1β or anti-CREB binding protein (CBP) antibodies. Bottom: expression in whole cell lysates. Results represent the means of 3 independent experiments. Error bars indicate SD.
siRNA-mediated knockdown of zyxin reduces transcriptional activity of HNF-1β.
To address whether zyxin is required for HNF-1β-mediated gene expression, we used siRNA to silence the expression of zyxin. Transfection of mIMCD3 cells with siRNA against zyxin caused a substantial decrease in the protein levels of zyxin (Fig. 4A). The activity of the Pkhd1 promoter was then examined using reporter gene assays. Treatment of siRNA against zyxin reduced Pkhd1 promoter activity (Fig. 4B). To confirm that zyxin is involved in the regulation of the transcriptional activity of HNF-1β, we tested whether the expression of an HNF-1β target gene, Pkhd1, is affected by changes in zyxin levels. Control siRNA and zyxin siRNA were transfected into mIMCD3 cells, and mRNA was isolated. mRNA was reverse transcribed and analyzed by quantitative real-time PCR using primers that amplify Pkhd1. As shown in Fig. 4C, knockdown of zyxin reduced the expression of endogenous Pkhd1, indicating that zyxin is involved in HNF-1β-dependent gene expression. Collectively, these loss-of-function approaches complement the overexpression studies and demonstrate that zyxin plays a role in HNF-1β-mediated transcriptional activation.
Fig. 4.
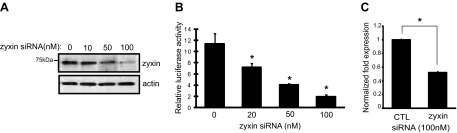
Small interfering (si)RNA-mediated knockdown of zyxin reduces the transcriptional activity of HNF-1β. A: mIMCD3 cells were transfected with increasing amounts of zyxin siRNA and subjected to Western blot analysis with anti-zyxin antibody. Bottom: Western blotting for α-actin as a control. B: mIMCD3 cells were transfected with increasing amounts of siRNA targeting zyxin. After 24 h, the cells were transfected with a Pkhd1 promoter-luciferase reporter plasmid, and luciferase activity was measured 36 h later. C: mIMCD3 cells were transfected with control siRNA or zyxin siRNA. After 60 h, the expression of endogenous Pkhd1 mRNA transcripts was quantified by real-time RT-PCR. Results represent the means of 3 independent experiments. Error bars indicate SD. *P < 0.05.
Zyxin translocates into the nucleus in response to cGMP and EGF.
It has been suggested that altering the subcellular localization of zyxin might affect cell migration, cell death, proliferation, and gene regulation (6, 23). Recent studies have shown that the translocation of zyxin into the nucleus can be mediated by cGMP, and nuclear accumulation of zyxin can be induced by the growth factors NGF and EGF (6, 23). To identify factors that induce the translocation of zyxin into the nuclei of kidney epithelial cells, mIMCD3 cells were treated with cGMP, cAMP, or EGF. Cell lysates were separated into nuclear and cytosolic fractions and subjected to Western blot analysis. As shown in Fig. 5A, zyxin accumulated in nuclear extracts derived from cells treated with cGMP and EGF but not cAMP. Next, we examined whether EGF affects zyxin-dependent transcriptional activation. As shown in Fig. 5B, Pkhd1 promoter activity was increased in EGF-treated cells. Moreover, transfection with zyxin and treatment with EGF produced a greater than additive stimulation of promoter activity. It has been reported that Akt phosphorylates zyxin, and phosphorylated zyxin is located in the nucleus (6). Therefore, we examined whether the translocation of zyxin into the nucleus that is mediated by EGF depends on Akt activation. Phosphorylated Akt was detected in mIMCD3 cells treated with EGF (Fig. 5C, lanes 2, 3, and 4), and phosphorylation of Akt was blocked in cells pretreated with LY294002, a phosphatidylinositol 3-kinase inhibitor (Fig. 5C, lanes 6, 7, and 8), indicating that EGF induces phosphorylation of Akt in kidney epithelial cells. To determine whether activation of Akt is important for the translocation of zyxin into the nucleus, mIMCD3 cells and LY294002-pretreated cells were incubated with EGF, and cell lysates were separated into nuclear and cytosolic fractions. As shown in Fig. 5D, zyxin was increased in the nuclear fraction of EGF-treated cells (lanes 3 and 4). However, in LY294002-pretreated cells, nuclear zyxin was not increased by EGF treatment (Fig. 5D, lanes 7 and 8). Taken together, these results suggest that EGF induces translocation of zyxin into the nucleus, which is mediated by activation of Akt.
Fig. 5.
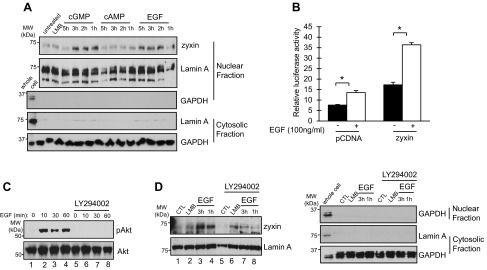
Zyxin translocates to the nucleus in response to cGMP and EGF. A: mIMCD3 cells were treated with 8-bromo-cGMP (100 nM), dibutyryl-cAMP (100 nM), or EGF (100 ng/ml) for the indicated time periods. Nuclear extracts were subjected to Western blotting with anti-zyxin and anti-lamin A antibodies. LMB, leptomycin B. B: stably transfected mIMCD3 cells containing a chromosomally integrated Pkhd1-Gal4-VP16 transgene were cotransfected with either pcDNA or pCMV-MYC-zyxin and a Gal4-luciferase reporter plasmid. After 24 h, the cells were treated with EGF (100 ng/ml) for 16 h (open bars) or vehicle (closed bars). Luciferase activity was measured 40 h after transfection. C: mIMCD3 cells were treated with EGF (100 ng/ml) for the indicated time period in the presence or absence of LY294002 (50 μM). Cell lysates were subjected to Western blotting with anti-Akt and anti-phospho-Akt antibodies. D: mIMCD3 cells were treated with EGF in the presence or absence of LY294002 (50 μM). Accumulation of zyxin in nuclear extracts was measured by Western blotting with an anti-zyxin antibody. Lamin A was used as a nuclear marker protein. Anti-GAPDH and anti-lamin A antibodies were used as a marker for the purity of cytosolic and nuclear fractions (A and D). Results represent the means of 3 independent experiments. Error bars indicate SD. *P < 0.05.
Mutation of HNF-1β and knockdown of zyxin inhibit cell migration in response to EGF.
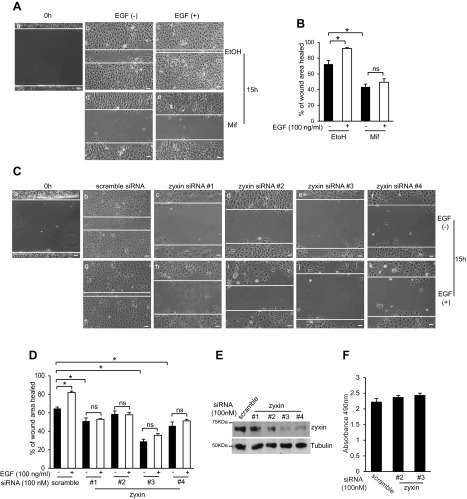
Next, we examined the roles of zyxin and HNF-1β in mediating the cellular responses to EGF. In the developing kidney, EGF induces tubulogenesis and branching morphogenesis through stimulation of epithelial proliferation and cell migration (22). Our previous studies have shown that HNF-1β is also required for renal tubulogenesis (26). To determine whether HNF-1β is required for EGF-dependent cell migration, wound healing assays were performed using renal epithelial cells (53A cells) that express dominant-negative mutant HNF-1β (DN-HNF-1β; Refs. 18, 26). 53A cells were treated with mifepristone to induce the expression of DN-HNF-1β, and confluent monolayers were scratched with a pipet tip. Cells were then incubated in the presence or absence of EGF and photographed 15 h later. A representative image of the wound healing assay is shown in Fig. 6A, and quantitative data from three independent experiments are summarized in Fig. 6B. In the absence of mifepristone, 53A cells migrated steadily across the wound from both sides, moving as an orderly sheet of cells with a smooth leading edge. This cell movement was accelerated by treatment with EGF. However, DN-HNF-1β-expressing cells showed much slower wound healing, and cell migration was not stimulated by EGF (Fig. 6, A and B). Similarly, mIMCD3 cells transfected with siRNA against zyxin showed reduced protein expression (Fig. 6E) and slower wound healing compared with control cells (Fig. 6C). The greatest inhibition of wound healing was observed in cells with the lowest levels of zyxin (siRNA #3 and #4). Treatment with EGF did not accelerate wound healing in zyxin knockdown cells but stimulated the migration of control siRNA transfected cells (Fig. 6D). mIMCD3 cells transfected with siRNA against zyxin showed identical rates of proliferation as control cells transfected with a scrambled siRNA, indicating that the difference in wound healing rates was not due to differences in cell proliferation (Fig. 6F). Collectively, these results suggest that the promigratory effects of EGF may be mediated by zyxin-dependent activation of HNF-1β.
Fig. 6.
Expression of dominant-negative mutant HNF-1β or knockdown of zyxin inhibits EGF-stimulated cell migration. A: 53A cells were grown to confluence and treated with mifepristone for 48 h to induce expression of DN-HNF-β or ethanol as a control. A scratch wound was created using a pipet tip (a), and cells were incubated for 15 h in the absence (b and d) or presence (c and e) of EGF (100 ng/ml). B: area of the wound was measured and the percent wound healing was calculated using ImageJ software. C: confluent monolayers of mIMCD3 cells transfected with control siRNA (b and g) or zyxin siRNA (c–f and h–k) were wounded by scraping with a pipet tip (a) and incubated for 15 h in the absence (b–f) or presence (g–k) of EGF (100 ng/ml). D: area of the wound was measured and the percent wound healing was calculated using ImageJ software. E: mIMCD3 cells were transfected with scrambled siRNA or four different siRNAs targeting zyxin and subjected to Western blot analysis with anti-zyxin antibody and anti-tubulin antibody. F: rates of proliferation of mIMCD3 cells transfected with a scrambled siRNA or 2 different siRNAs against zyxin. Results represent the means of 3 independent experiments. Error bars indicate SD. Photomicrographs were acquired using an AxioObserver FL microscope at ×10 magnification. *P < 0.05, compared with controls. Scale bars = 30 μm.
Inhibition of Akt inhibits HNF-1β transcriptional activity cell migration and interaction with zyxin.
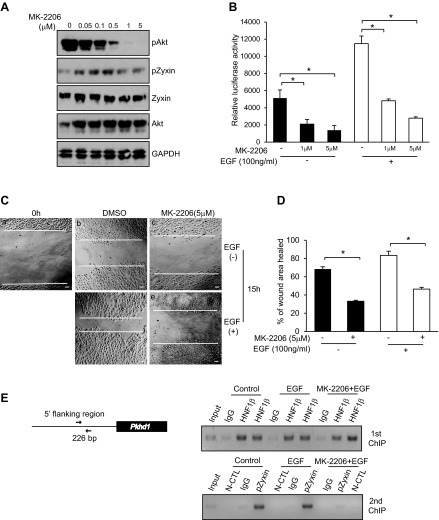
We demonstrated that EGF treatment leads to Akt activation, which induces the translocation of zyxin into the nucleus (Fig. 5). To further explore the role of Akt, we treated mIMCD3 cells with a highly specific allosteric Akt inhibitor, MK-2206 (10). Cells were treated with MK-2206 for 24 h, and cell lysates were subjected to Western blot analysis with antibodies against pAkt, Akt, pZyxin, zyxin, and GAPDH as a control. MK-2206 reduced the phosphorylation of Akt in a dose-dependent manner and led to reduced phosphorylation of zyxin (Fig. 7A). Luciferase assays showed that treatment with MK-2206 produced a dose-dependent inhibition of basal and EGF-stimulated HNF-1β transcriptional activity (Fig. 7B). Similarly, MK-2206 inhibited wound healing compared with control cells (Fig. 7C).
Fig. 7.
Inhibition of Akt inhibits HNF-1β transcriptional activity cell migration and zyxin interaction with the Pkhd1 promoter. A: mIMCD3 cells were incubated with increasing concentrations of MK-2206 for 24 h. Cell lysates were subjected to Western blot analysis with antibodies against pAkt, Akt, Zyxin, pZyxin, and GAPDH. B: mIMCD3 cells were transfected with a Pkhd1 promoter-luciferase reporter plasmid. Cells were incubated with MK-2206 alone or in combination with EGF for 16 h. Luciferase activity was measured 48 h after transfection. C: confluent monolayers of mIMCD3 cells treated with MK-2206 or vehicle were wounded by scraping with a pipet tip (a) and incubated for 15 h in the absence (b and c) or presence (d and e) of EGF. D: area of the wound was measured and the percent wound healing was calculated using ImageJ software. E, left: Pkhd1 gene and its upstream promoter. Arrows depict the primers that flank the HNF-1β binding site. mIMCD3 cells were incubated with EGF (100 ng/ml) alone or in the presence of MK-2206 (5 μM) for 20 h. Chromatin immunoprecipitation (ChIP) was performed with anti-HNF-1β antibody or IgG as a negative control, followed by a second round of immunoprecipitation with an anti-phospho-zyxin (pZyxin) antibody. First round (right top) and 2nd round (right bottom) immunoprecipitated DNA was analyzed by PCR. Immunoprecipitation with IgG in the 1st round and anti-pZyxin antibody in the 2nd round was used as an additional negative control (N-CTL). Representative results of 3 independent experiments are shown. Error bars indicate SD. Photomicrographs were acquired using an AxioObserver FL microscope at ×10 magnification. *P < 0.05. Scale bars = 30 μm.
Coimmunoprecipitation experiments indicated that zyxin interacts with HNF-1β (Fig. 3C). To determine if zyxin and HNF-1β assemble in a transcriptional complex on target genes, we performed sequential ChIP/re-ChIP assays. mIMCD3 cells were treated with EGF in the presence or absence of MK-2206, and chromatin was isolated and subjected to a first round of immunoprecipitation with anti-HNF-1β antibody or control rabbit IgG. The immunoprecipitated chromatin was eluted and subjected to a second round of immunoprecipitation with anti-pZyxin antibody or control IgG. PCR was used to amplify a region in the Pkhd1 promoter that contains a conserved HNF-1β binding site (Fig. 7E, left). ChIP/re-ChIP showed that phosphorylated zyxin and HNF-1β co-occupy the HNF-1β binding site in the Pkhd1 promoter (Fig. 7E, right). Moreover, treatment with MK-2206 had no effect on the binding of HNF-1β but inhibited the binding of phosphorylated zyxin. Taken together, these results indicate that zyxin translocates to the nucleus via an Akt-dependent mechanism and stimulates transcriptional activity by associating with HNF-1β on target promoters.
DISCUSSION
We identified the focal adhesion component zyxin as a novel protein that interacts with the transcription factor HNF-1β in the nuclei of renal epithelial cells. We found that the second LIM domain of zyxin was essential for interacting with HNF-1β. In addition, we demonstrated that zyxin is present in a transcriptional complex with the coactivator CBP and stimulates the transcriptional activity of HNF-1β. Zyxin is an 80-kDa protein that is concentrated in focal adhesions where it regulates integrin-mediated signaling (3, 16). Zyxin contains an actin-binding region in the N terminus, a proline-rich Src homology 3 (SH3)-binding domain, and a C-terminal LIM domain involved in protein-protein interactions. Zyxin has been shown to interact with focal adhesion proteins and integrin signaling molecules including α-actinin, Ena/vasodilator-stimulated phosphoprotein (VASP), Vav, p130Cas, CRP, LATS1, and LASP1. Loss of zyxin results in depletion of Ena/VASP from actin filaments, which alters cell-matrix adhesion and enhances cell migration (19). Zyxin interacts directly with the stretch-sensitive protein p130cas, which suggests that it may play a role in the cytoskeletal remodeling that occurs upon the application of mechanical load (38).
In addition to focal adhesions, zyxin can be present in the nucleus. Zyxin contains a functional nuclear export signal and accumulates in the nucleus following treatment with leptomycin B, which blocks CRM1-mediated nuclear export (16, 34). We found that zyxin and HNF-1β are colocalized in the nucleus after leptomycin B treatment. In the nucleus, zyxin has been shown to interact with the E6 protein and ZNF384, a DNA-binding transcription factor that is involved in bone metabolism (9, 21). These findings suggest that zyxin can shuttle between focal adhesions where it regulates actin remodeling and the nucleus where it interacts with nuclear proteins. However, the movement of zyxin into the nucleus and its spectrum of functions in the nucleus are poorly understood. Our findings indicate that zyxin regulates gene expression through physical interaction with the transcription factor HNF-1β. However, the mechanism by which zyxin regulates HNF-1β activity is not known. The N terminus of zyxin can function as a transactivation domain when fused to a GAL4 DNA-binding domain, which suggests that zyxin may act as a coactivator for HNF-1β. Alternatively, zyxin might function as a scaffolding protein that promotes the assembly of transcription factors and coactivators on gene promoters.
Zyxin functions as a mechanosensor by altering its subcellular distribution and has been implicated in the regulation of cell adhesion, spreading, and motility (16). Mechanical force, such as unidirectional cyclic stretch, redistributes zyxin from focal adhesions to actin filaments or the nucleus (5, 37), suggesting the involvement of zyxin in mechanosensitive gene expression. Although several studies suggest that zyxin might be involved in regulating gene expressions in the nucleus, the physiological signals that induce zyxin translocation to the nucleus remain poorly understood. In cardiac myocytes, Akt-mediated phosphorylation of zyxin forms a complex with acinus to prevent apoptosis, and the complex between zyxin and acinus is induced by the growth factors NGF and EGF (6). Our results suggest that the nuclear translocation of zyxin in renal epithelial cells is physiologically regulated by EGF. Furthermore, the EGF-induced translocation of zyxin is mediated by the activation of Akt.
The regulation of HNF-1β by zyxin family members may play an important role in kidney development and cystic kidney diseases. Signaling from the cell-extracellular matrix interface is important for epithelial polarity, tubular morphogenesis, and vectorial transport of solutes in kidney tubules (36). Accumulating evidence suggests that abnormalities of focal adhesions may play important roles in the pathogenesis of polycystic kidney disease. For example, loss of the focal adhesion protein tensin results in renal failure and the formation of multiple, large cysts in the proximal tubules in the kidney (24). Our studies suggest that focal adhesion proteins may translocate to the nucleus where they regulate the HNF-1β-dependent expression of cystic disease genes. The involvement of EGF in the translocation of zyxin may be particularly relevant to the functions of HNF-1β. EGF and EGF receptor (EGFR) are highly expressed in the kidney and are required for the branching morphogenesis of tubular epithelial organs including lung, mammary gland, and kidney (22). Overactivation of the EGF/EGFR axis contributes to the pathogenesis of PKD, since EGFR inhibition slows disease progression in animal models of PKD (32). EGF is also increased in the C57BL/6J-cpk mouse, which develops an infantile form of PKD.
Our previous studies showed that HNF-1β is required for renal tubulogenesis by controlling SOCS3 gene expression (26). The current study reveals a second pathway by which HNF-1β may regulate tubulogenesis during kidney development. Renal epithelial cells are susceptible to injury caused by ischemia-reperfusion, oxidative stress, nephrotoxins, and hyperosmotic stress. The injured cells detach from the tubular surface, and autocrine/paracrine growth factors secreted at the site of injury stimulate the repair processes (33). EGFR-mediated proliferation and migration appear crucial in the wound healing process, and EGFR ligands are potent promigratory factors in mammalian cells (28). In the current study, wound healing was impaired in DN-HNF-1β-expressing cells. Moreover, DN-HNF-1β-expressing cells did not show the promigratory effect of EGF, suggesting that HNF-1β regulates EGF-mediated cell migration in the kidney. Similarly, zyxin knockdown cells had slower wound healing than control siRNA transfected cells, and wound healing was not accelerated by EGF. Zyxin has been shown to affect cell migration by mediating dynamic actin rearrangement (30). Our findings suggest a second mechanism by which zyxin controls cell migration through the regulation of gene transcription. In this pathway, EGF stimulates gene expression controlled by the transcription factor HNF-1β by inducing the translocation of zyxin into the nucleus. Collectively, our findings reveal a novel mechanism for how signals from the cell surface regulate gene expression through a transcription factor that is essential for epithelial differentiation and morphogenesis.
GRANTS
Support for this work was provided by National Institute of Diabetes and Digestive and Kidney Diseases Grant R37DK042921 and University of Texas Southwestern O'Brien Kidney Research Core Center (National Institute of Diabetes and Digestive and Kidney Diseases Grant P30DK079328).
ACKNOWLEDGMENTS
We thank Serge Gisler for assistance with the yeast two-hybrid screen, Sachin Hajarnis for critically reviewing the manuscript, and Yimei Gong for expert technical assistance.
Present address of B. T. McNally: Qiagen, 6951 Executive Way, Frederick, MD 21703.
REFERENCES
- 1. Bai Y, Pontoglio M, Hiesberger T, Sinclair AM, Igarashi P. Regulation of kidney-specific Ksp-cadherin gene promoter by hepatocyte nuclear factor-1β. Am J Physiol Renal Physiol 283: F839–F851, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Barbacci E, Chalkiadaki A, Masdeu C, Haumaitre C, Lokmane L, Loirat C, Cloarec S, Talianidis I, Bellanne-Chantelot C, Cereghini S. HNF1beta/TCF2 mutations impair transactivation potential through altered co-regulator recruitment. Hum Mol Genet 13: 3139–3149, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Beckerle MC. Zyxin: zinc fingers at sites of cell adhesion. Bioessays 19: 949–957, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noel LH, Velho G, Timsit J. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med 140: 510–517, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Cattaruzza M, Lattrich C, Hecker M. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension 43: 726–730, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Chan CB, Liu X, Tang X, Fu H, Ye K. Akt phosphorylation of zyxin mediates its interaction with acinus-S and prevents acinus-triggered chromatin condensation. Cell Death Differ 14: 1688–1699, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Coffinier C, Barra J, Babinet C, Yaniv M. Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev 89: 211–213, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Crawford AW, Beckerle MC. Purification and characterization of zyxin, an 82,000-dalton component of adherens junctions. J Biol Chem 266: 5847–5853, 1991 [PubMed] [Google Scholar]
- 9. Degenhardt YY, Silverstein S. Interaction of zyxin, a focal adhesion protein, with the e6 protein from human papillomavirus type 6 results in its nuclear translocation. J Virol 75: 11791–11802, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devlin JR, Hannan KM, Ng PY, Bywater MJ, Shortt J, Cullinane C, McArthur GA, Johnstone RW, Hannan RD, Pearson RB. AKT signalling is required for ribosomal RNA synthesis and progression of Emu-Myc B-cell lymphoma in vivo. FEBS J 2013 Jan 19 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11. Dudziak K, Mottalebi N, Senkel S, Edghill EL, Rosengarten S, Roose M, Bingham C, Ellard S, Ryffel GU. Transcription factor HNF1beta and novel partners affect nephrogenesis. Kidney Int 74: 210–217, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet 43: 84–90, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J 23: 1657–1668, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haumaitre C, Fabre M, Cormier S, Baumann C, Delezoide AL, Cereghini S. Severe pancreas hypoplasia and multicystic renal dysplasia in two human fetuses carrying novel HNF1beta/MODY5 mutations. Hum Mol Genet 15: 2363–2375, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Heidet L, Decramer S, Pawtowski A, Moriniere V, Bandin F, Knebelmann B, Lebre AS, Faguer S, Guigonis V, Antignac C, Salomon R. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hervy M, Hoffman L, Beckerle MC. From the membrane to the nucleus and back again: bifunctional focal adhesion proteins. Curr Opin Cell Biol 18: 524–532, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, Somlo S, Igarashi P. Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 113: 814–825, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P. Role of the hepatocyte nuclear factor-1beta (HNF-1beta) C-terminal domain in Pkhd1 (ARPKD) gene transcription and renal cystogenesis. J Biol Chem 280: 10578–10586, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hoffman LM, Jensen CC, Kloeker S, Wang CL, Yoshigi M, Beckerle MC. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. J Cell Biol 172: 771–782, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Igarashi P, Shao X, McNally BT, Hiesberger T. Roles of HNF-1beta in kidney development and congenital cystic diseases. Kidney Int 68: 1944–1947, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Janssen H, Marynen P. Interaction partners for human ZNF384/CIZ/NMP4–zyxin as a mediator for p130CAS signaling? Exp Cell Res 312: 1194–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Karihaloo A, Nickel C, Cantley LG. Signals which build a tubule. Nephron Exp Nephrol 100: e40–45, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Kato T, Muraski J, Chen Y, Tsujita Y, Wall J, Glembotski CC, Schaefer E, Beckerle M, Sussman MA. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt. J Clin Invest 115: 2716–2730, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lo SH, Yu QC, Degenstein L, Chen LB, Fuchs E. Progressive kidney degeneration in mice lacking tensin. J Cell Biol 136: 1349–1361, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lokmane L, Heliot C, Garcia-Villalba P, Fabre M, Cereghini S. vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development 137: 347–357 [DOI] [PubMed] [Google Scholar]
- 26. Ma Z, Gong Y, Patel V, Karner CM, Fischer E, Hiesberger T, Carroll TJ, Pontoglio M, Igarashi P. Mutations of HNF-1beta inhibit epithelial morphogenesis through dysregulation of SOCS-3. Proc Natl Acad Sci USA 104: 20386–20391, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Massa F, Garbay S, Bouvier R, Sugitani Y, Noda T, Gubler MC, Heidet L, Pontoglio M, Fischer E. Hepatocyte nuclear factor 1beta controls nephron tubular development. Development 140: 886–896, 2013 [DOI] [PubMed] [Google Scholar]
- 28. Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11: 633–643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soutoglou E, Viollet B, Vaxillaire M, Yaniv M, Pontoglio M, Talianidis I. Transcription factor-dependent regulation of CBP and P/CAF histone acetyltransferase activity. EMBO J 20: 1984–1992, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sperry RB, Bishop NH, Bramwell JJ, Brodeur MN, Carter MJ, Fowler BT, Lewis ZB, Maxfield SD, Staley DM, Vellinga RM, Hansen MD. Zyxin controls migration in epithelial-mesenchymal transition by mediating actin-membrane linkages at cell-cell junctions. J Cell Physiol 222: 612–624, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Sun Z, Hopkins N. vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev 15: 3217–3229, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sweeney WE, Chen Y, Nakanishi K, Frost P, Avner ED. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int 57: 33–40, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Toback FG. Regeneration after acute tubular necrosis. Kidney Int 41: 226–246, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Gilmore TD. LIM domain protein Trip6 has a conserved nuclear export signal, nuclear targeting sequences, and multiple transactivation domains. Biochim Biophys Acta 1538: 260–272, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Wild W, Pogge von Strandmann E., Nastos A., Senkel S, Lingott-Frieg A, Bulman M., Bingham C, Ellard S, Hattersley AT, Ryffel GU. The mutated human gene encoding hepatocyte nuclear factor 1beta inhibits kidney formation in developing Xenopus embryos. Proc Natl Acad Sci USA 97: 4695–4700, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson PD, Burrow CR. Cystic diseases of the kidney: role of adhesion molecules in normal and abnormal tubulogenesis. Exp Nephrol 7: 114–124, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Wojtowicz A, Babu SS, Li L, Gretz N, Hecker M, Cattaruzza M. Zyxin mediation of stretch-induced gene expression in human endothelial cells. Circ Res 107: 898–902, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Yi J, Kloeker S, Jensen CC, Bockholt S, Honda H, Hirai H, Beckerle MC. Members of the Zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J Biol Chem 277: 9580–9589, 2002 [DOI] [PubMed] [Google Scholar]