Abstract
The role of the endogenous opioid system in modulation of urinary bladder activity by spinal nerve (SN) stimulation was studied in anesthetized female rats, using the rat model of isovolumetric bladder contraction. SN stimulation at a fixed frequency of 10 Hz attenuated bladder contraction frequency; the magnitude of the inhibition was directly proportional to the current intensity. Neither the κ-opioid antagonist nor-binaltorphimine (2 mg/kg iv) nor the δ-opioid antagonist naltrindole (5 mg/kg iv) attenuated the bladder inhibitory response to SN stimulation. In contrast, the μ-opioid receptor antagonist naloxone (NLX; 0.03 mg/kg iv) blocked the inhibitory responses evoked by SN stimulation at therapeutic current intensities at ≤1 × motor threshold current (Tmot). An action at spinal and supraspinal centers was further confirmed by the ability of intrathecal or intracerebroventricular administration of NLX methiodide to attenuate the bladder inhibitory effects of 1 × Tmot SN stimulation. The magnitude of SN-mediated neuromodulation using therapeutically relevant stimulation intensity (Tmot) is equivalent to 0.16 mg/kg of systemically administered morphine, which produces 50% inhibition of bladder contraction frequency. These results suggest that the inhibitory effects of lower intensity SN stimulation may be mediated through the release of endogenous μ-opioid peptides. Additionally, these data suggest that neuromodulation may offer a mode of treating the symptoms of overactive bladder with efficacy equal to the opioid drugs but without their liability for abuse and dependence.
Keywords: electrical stimulation, bladder, micturition, spinal nerve, opioid
stimulation of spinal nerves (SN, S3) is a proven clinical therapy for bladder hyperactivity. However, the neural pathways and neurotransmitters involved have not been studied in detail. Only recently have significant advances been made in identifying optimal stimulation parameters (22, 25) and understanding differences in sensitivity of particular nerve targets to neuromodulation (26). A major challenge for extending the clinical utility of neuromodulation has been the need for knowledge regarding which neural circuits and neurotransmitters participate. The experiments described here were designed to address these questions.
The opioid system has been reported to play a critical role in regulation of bladder contractility. At the level of the spinal cord, the parasympathetic outflow to the urinary bladder is subject to a tonic enkephalinergic inhibitory control, which can in turn be blocked by the nonselective opioid receptor antagonist naloxone (NLX; Ref. 19). At the supraspinal level, intracerebroventricular morphine (6) and β-endorphin (8) produce NLX-reversible inhibition of bladder motility. Specifically, opioid receptors in the dorsolateral tegmental-subceruleus region of the pons represent a highly sensitive locus for endogenous and exogenous inhibitory modulation of the micturition reflex arc (29). With the use of selective opioid agonists and antagonists in urodynamic studies, both μ- and δ-, but not κ-, opioid receptors activate descending inhibitory systems to suppress of micturition (7, 9), while κ-opioid receptor activation may suppress of activity in the ascending pathway to disinhibit the supraspinal inhibitory action, resulting in a facilatory effect (10, 13, 23). Peripherally, activation of opioid receptors triggers recurrent inhibition from bladder parasympathetic preganglionic neurones to prevent prolonged bladder contractions (17). It is not known whether endogenous opioid peptides are released during either normal or pathologic micturition or whether endogenous opioids can produce a net inhibitory action on a pathologic micturition reflex.
Studies in the cat have shown that neuromodulation-induced inhibition of bladder contractions may involve endogenous opioids (5, 16, 27). Additional studies are required to determine whether the contribution of the opioid pathway differs depending on the specific nerve target (e.g., SN) or stimulation parameters and which receptor subtypes are involved. Our previous work in the rat (24, 25) demonstrated an intensity-dependent bladder inhibitory effect in response to electrical stimulation of the L6 SN. In the present study, NLX was used to evaluate the role of endogenous opioid system in the inhibition of the micturition reflex by different intensities of SN stimulation. The selective δ-opioid receptor antagonist naltrindole, and the selective κ-opioid receptor antagonist nor-binaltorphimine (nor-BNI; Ref. 12) were used to determine the opiate receptor subtype(s) involved. The role of central nervous system (CNS) opiate receptors was evaluated by local administration (it or icv) of NLX methiodide (NLX Meth), an μ-opioid receptor antagonist with poor penetration through the blood-brain barrier (1).
MATERIALS AND METHODS
Female Sprague-Dawley rats weighing 200–300 g were anesthetized with urethane (2 ip injections, 4 min apart, total 1.2 g/kg). To record bladder contractions, a cannula (PE50) was inserted into the bladder via the urethra and secured with a suture tie. The urethral cannula was connected via a T-connector to a pressure transducer (MLT0380/D; ADInstruments, Colorado Springs, CO) and data acquisition system (ML880/P; ADInstruments). The intravesical pressure signal was amplified for recording (ML228; ADInstruments). The other end of the T-connector was attached to a syringe pump. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Medtronic and the Non-Clinical Research Board of Medtronic (Minneapolis, MN).
One jugular vein was cannulated with polyethylene tubing for intravenous administration of test drugs. For intrathecal or intracerebroventricular administration, rats with preimplanted intrathecal or intracerebroventricular cannulae were purchased (Charles River Laboratories) and housed for 3–7 days before testing. The placement for intrathecal and intracerebroventricular cannulae was confirmed by showing abolishment of bladder contractions by local injection of lidocaine (10%, 20 μl) or by staining with malachite green dye (Alfa Aesar), after completion of studies in some experiments.
To deliver electrical stimulation, the skin around the dorsal sacral and thoracic area was shaved and a dorsal midline incision was made from approximately the L3 to the S2 vertebral body, and the L6/S1 posterior processes were exposed. The S1 processes were removed, and the L6 nerve trunks localized caudal and medial to the sacroiliac junction. Electrodes were made from Teflon-coated, 40-gauge, stainless steel wire with the coating removed from a 0.5-cm section (Cooner Wire, Chatsworth, CA) and placed under both sides of the L6 SN. After the wire electrode(s) were positioned, silicone adhesive (Kwik-Cast; World Precision Instruments) was applied to cover the wire around the nerve, and the skin incision was sutured shut. The electrode(s) were connected to a Grass S88 stimulator (Grass Medical Instruments), through stimulus isolation unit(s) (SIU-BI; Grass Medical Instruments). A needle electrode under the skin of the tail served as the ground.
Electrical stimulation of the SN evoked hind-toe twitches and/or pelvic floor muscle contraction. In each rat, the motor threshold current (Tmot) was defined as the lowest current required to evoke the first, barely observable, muscle contraction. Biphasic pulses (pulse width: 0.1 ms) of different intensities (0.8 × Tmot − 2 × Tmot) were used to stimulate the SN at 10 Hz.
To induce bladder rhythmic contractions (BRC), saline was infused into the bladder via the syringe pump at a rate of 50 μl/min to induce a micturition reflex. The infusion rate was then lowered to 10 μl/min and continued until three to five consecutive contractions were established. At this time, the saline infusion was terminated, and the BRC continued.
Saline or test compound was administered after a 10-min control period. After an additional 5-min control period (total 15-min control period), nerve stimulation was applied for 10 min. The BRC was recorded for 20 min poststimulation. The effects of stimulation on BRC were tested in 26 rats following different doses of NLX and 55 rats following different routes of administration of NLX Meth. In 33 experiments, the intensity-dependent effect of SN stimulation was tested and stimulation was applied after a 15-min control period using an increasing intensity paradigm. Current intensities were increased at 5-min intervals giving 0.8 × Tmot, Tmot, and 2 × Tmot for a total of 15 min. The BRC was recorded for 20 min poststimulation.
In nine rats, morphine was administered iv after 10-min control period using a cumulative dose paradigm. Drug doses were increased at 20-min intervals. Cumulative dose-response relationships were obtained by giving 0.01, 0.02, 0.07, 0.2, and 0.7 mg/kg (total cumulative dose: 1 mg/kg) of drug. The effects of a single dose (0.1 or 0.3 mg/kg) of morphine were also studied for 30 min after a 5-min pretreatment with either saline (in 10 rats) or NLX (0.3 mg/kg iv in 11 rats).
Data analysis.
Both frequency and amplitude of bladder contractions were analyzed. In cases of “shutdown” of the BRC induced by high-intensity SN stimulation, the amplitude of the bladder contractions was “0” and data were excluded from the analysis. Data were calculated in 5-min bins, corresponding to three control periods, two stimulation periods (10-min stimulation protocol) or three stimulation periods (15-min stimulation protocol) during stimulation, and four periods after stimulation. In the dose-response experiments with intravenous morphine, mean responses were determined at 5-min intervals during exposure to each dose. The mean value for each dose was recorded as the average of the four 5-min bins during the 20-min drug exposure.
All data were normalized to the mean response during the 5 min immediately before stimulation or dosing. All data are expressed as means ± SE. Time course for the BRC response was analyzed using repeated-measures ANOVA (Prism 5; GraphPad Software, San Diego, CA). Individual trials were one of the factors, and responses were measured at different time points. Bonferroni posttest was used to determine the statistical significance between individual time points. Student t-test was utilized to compare mean responses during stimulation among the different groups. The inhibitory dose 50 (ID50; dose to produce 50% inhibition of the bladder contraction) and 95% confidence interval (CI) were calculated from the 20–80% regression component of the cumulative dose-response curve (GraphPad Software). The sigmoidal dose-response curves in a variable slope mode were fitted with a four parameter logistic equation, Y = bottom + (top − bottom)/{1 + 10[(logID50X)*HillSlope]}, where X is the logarithm of the dose and Y is the modified response. A value of P < 0.05 was considered statistically significant.
Compounds.
Urethane [molecular weight (MW): 89.09], NLX hydrochloride (MW: 399.87), NLX Meth (MW: 469.31), morphine sulphate salt (MW: 758.83), nor-BNI dihydrochloride (MW: 734.71), and naltrindole hydrochloride (MW: 450.96) were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in water.
RESULTS
Effects of opioids.
Figure 1A shows an example of a recording of intravesical pressure in a rat showing BRC before and after a single intravenous dose of morphine (0.3 mg/kg).
Fig. 1.

Effects of morphine on bladder rhythmic contraction. A and B: raw traces of bladder rhythmic contraction. Intravenous injection of morphine (0.3 mg/kg) attenuated bladder contractions (A) in one rat and had no effect on bladder contractions in another rat that received naloxone (NLX; 0.3 mg/kg iv), 5 min before morphine administration (B). C and D: time-course response of frequency (C) and amplitude (D) of the bladder reflex contraction to intravenous administration of morphine without or with pretreatment of NLX (0.3 mg/kg iv). *P < 0.05, vs. control, repeated-measures ANOVA, Bonferroni posttest. E: effect of cumulative intravenous morphine on the response of bladder contractions. *P < 0.05 vs. control, #P < 0.05, frequency vs. amplitude, Student's t-test. Responses are represented as a percentage of predosing values, where the baseline response before morphine injection is defined as 100%.
To establish that the effects of morphine were opioid receptor mediated, the effect of morphine on BRC was tested after the systemic administration of NLX (0.3 mg/kg iv). Figure 1B illustrates that this dose of morphine has no effect on BRC in a NLX-treated rat. In 10 control animals, the frequency of BRC following treatment with morphine at 0.1 and 0.3 mg/kg was reduced to 60.48 ± 16 and 30.92 ± 19% of predosing values 10 min postinjection, respectively. After administration of NLX in 11 rats, the corresponding values were 97.14 ± 5 and 95.22 ± 6% showing nearly complete blockade of the morphine response (Fig. 1C). Morphine (0.1 and 0.3 mg/kg) did not alter amplitude of BRC in the absence or presence of NLX (Fig. 1D).
The effect of cumulative iv morphine (1 mg/kg total dose) was evaluated on the response of BRC in nine rats (Fig. 1E). Morphine produced a dose-dependent decrease in the frequency of BRC and elicited a relatively weak inhibition on the amplitude of the bladder contractions at the highest dose of 1 mg/kg (P < 0.05, Student's t-test). The mean ID50 values were 0.16 ± 0.04 (CI: 0.09–0.24) and 0.94 ± 0.20 (CI: 0.53–1.34) mg/kg, respectively. An unpaired t-test showed a stronger inhibition by morphine at 1 mg/kg on the frequency than on the amplitude of contraction (P < 0.001).
To examine whether endogenous opioids are involved in BRC, the effect of the selective μ-opioid receptor antagonist NLX was evaluated. Of the total of 63 animals treated with NLX, 5 were dosed at 0.01, 20 at 0.03, 4 at 0.1, and 34 at 0.3 mg/kg. Interestingly, four types of responses were observed following NLX: 1) no change in BRC (Fig. 2A); 2) shutdown of contractions, lasting ∼5 min (Fig. 2B); 3) prolonged bladder contraction, as reflected by an increase in duration of the contraction (Fig. 2C); and 4) an increase in the frequency of BRC (Fig. 2D).
Fig. 2.
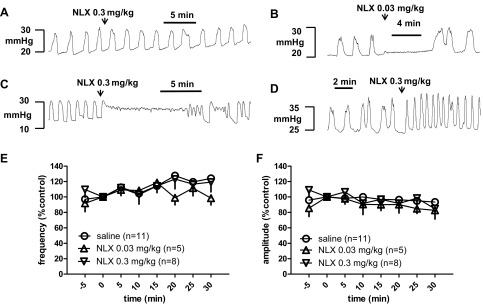
Effects of naloxone on bladder rhythmic contraction. A–D: typical responses following intravenous administration of NLX including no change in bladder contraction (A), transient shutdown of contractions (B), prolonged bladder contraction (C), and an increase in the frequency of contractions (D). E and F: time-course response of frequency (E) and amplitude (F) of the bladder reflex contraction to intravenous administration of NLX. Overall, NLX had no effect on the frequency or amplitude of the bladder rhythmic contraction. Responses are represented as a percentage of predosing values, where the baseline response before NLX administration is defined as 100%.
Following 0.01 mg/kg NLX, one rat responded with a long contraction followed by a long-term shutdown of contraction and the remaining four rats did not respond. Following 0.03 mg/kg NLX, one rat responded with a prolonged contraction and another rat responded with a 5-min shutdown of contraction, and the remaining 18 rats did not respond. Following 0.1 mg/kg NLX, one rat responded with a prolonged contraction and the remaining three rats did not respond. Of the 34 rats tested with 0.3 mg/kg NLX, 10 (29%) responded with a prolonged detrusor contraction, 2 (6%) exhibited a high frequency of rhythmic contraction, and 22 (65%) did not respond to NLX. Of the 63 rats, 47 (75%) did not respond, 15 (24%) showed activation by an increase in either contraction duration or BRC frequency, and only 1 (1%) showed a clear inhibitory response. From these data, it is evident that the effect of NLX in the BRC model was highly variable, suggesting that the role of the opioid system differs between individual rats. The opioid system exerts an inhibitory modulation of micturition in about one-fourth of the rats tested.
The frequency and amplitude of BRC were also followed for 30 min after NLX injection in the control (without stimulation) group for the neuromodulation study (see below). In these 13 rats, NLX had no effect on the frequency or amplitude of the BRC (Fig. 2, E and F).
Endogeneous opioids in neuromodulation.
Figure 3 depicts the effects of stimulation on BRC following different doses of NLX that are representative of the overall data (summarized in Fig. 3, E–G). Electrical stimulation of SN at Tmot intensity 10 Hz, 5 min following 0.01 mg/kg NLX eliminated bladder contractions (Fig. 3A). On the other hand, the same parameter of SN stimulation following 0.03 mg/kg (Fig. 3B), 0.10 mg/kg (Fig. 3C), and 0.30 mg/kg NLX (Fig. 3D) produced no reduction of the frequency or amplitude of bladder contractions.
Fig. 3.
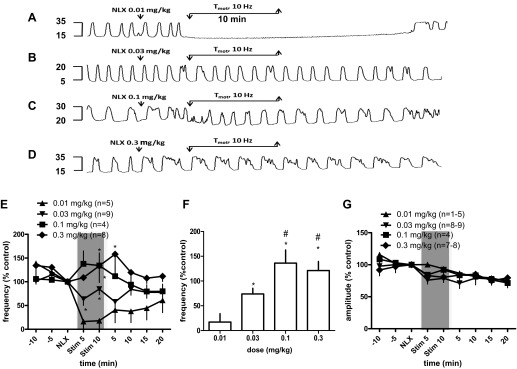
Effects of spinal nerve stimulation at motor threshold (Tmot; 10 Hz, pulse width 0.1 ms) for 10 min on bladder rhythmic contractions following different doses of NLX. A–D: typical experimental records showing the bladder reflex contraction (mmHg). Electrical stimulation, 5 min following 0.01 mg/kg NLX, eliminated bladder contractions (A) but produced little or no reduction of the frequency or amplitude of bladder contractions following 0.03 mg/kg (B), 0.1 mg/kg (C), and 0.3 mg/kg NLX (D). E: summary of bladder rhythmic contraction frequency to spinal nerve stimulation for 5 min (Stim 5) and 10 min (Stim 10) following NLX injection. *P < 0.05, vs. 0.01 mg/kg, repeated-measures ANOVA, Bonferroni posttest. F: dose-dependent blockade of NLX on bladder inhibitory response of spinal nerve stimulation. *P < 0.05 vs. 0.01 mg/kg dosing, #P < 0.05 vs. 0.03 mg/kg dosing, Student's t-test. Response of contraction frequency is the mean value as a percentage of pretreatment values (%control) during stimulation (the shaded areas in E). G: summary of bladder rhythmic contraction amplitude to spinal nerve stimulation for 5 min (Stim 5) and 10 min (Stim 10) following NLX injection. Responses are represented as a percentage of pretreatment values, where the baseline response before stimulation is defined as 100%.
Figure 3E summarizes BRC responses to SN stimulation post-NLX injection. Repeated-measures ANOVA analysis demonstrates that a significant difference of BRC frequency by SN stimulation at Tmot intensity is produced post 0.03, 0.1, or 0.3 mg/kg of NLX (P < 0.05, vs. 0.01 mg/kg; Fig. 3E).
Following 0.01, 0.03, 0.1 and 0.3 mg/kg NLX injection, SN stimulation changed the frequency of contractions to 17.07 ± 17% (n = 5, vs. 97.86 ± 9% control without stimulation; P < 0.05, Student's t-test), 74 ± 11% (n = 9; P > 0.05), 136 ± 27% (n = 4; P > 0.05), and 121.2 ± 18% of controls (n = 8; P > 0.05), respectively. The 0.1 and 0.3 mg/kg doses of NLX were more effective in blocking the effect of SN stimulation than 0.03 mg/kg. (Fig. 3F; P < 0.05, Student's t-test). Consistently, SN stimulation at Tmot intensity produced no reduction of the amplitude of bladder contractions during electrical stimulation (Fig. 3G).
A dose of 0.3 mg/kg NLX was selected for studies aimed at identifying intensity dependent effects of opioid receptor blockade on the response to SN stimulation. There was no significant change in BRC frequency or amplitude over the duration of the experiment if electrical stimulation was not applied (Fig. 4A). Depending on the current intensity, electrical stimulation of the SN attenuated the frequency of bladder contractions, either eliminating bladder contractions or reducing the contraction frequency during electrical stimulation (Fig. 4B). Following NLX injection (0.3 mg/kg), SN stimulation at 0.8 × Tmot and 1 × Tmot intensity produced mild reduction of bladder contractions but the ability of stimulation at 2 × Tmot to abolish bladder contraction in this model was not antagonized by NLX (Fig. 4C).
Fig. 4.
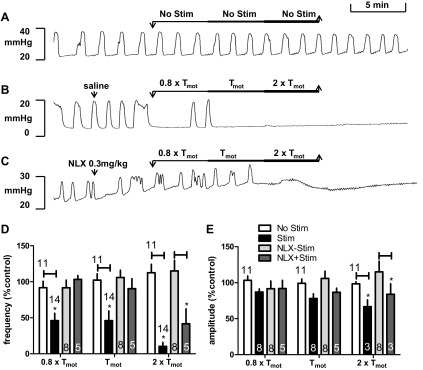
Intensity-dependent effects of spinal nerve stimulation on bladder contractions. A–C: typical experimental records showing the BRC. There was no significant change in BRC frequency or amplitude over the duration of the experiment if electrical stimulation was not applied (A, No Stim). Ten-Hertz spinal nerve stimulation (Stim) at 3 intensities relative to motor threshold (Tmot) for a total of 15 min stimulation (5-min duration of each intensity) attenuated bladder contractions (B). Following NLX injection, spinal nerve (SN) stimulation at 0.8 × Tmot and 1 × Tmot intensity produced mild reduction of bladder contractions and stimulation at 2 × Tmot abolished bladder contraction (C). D and E: summary of intensity- dependent effects of spinal nerve stimulation on frequency (D) and amplitude (E) of bladder rhythmic contraction without or with systemic administration of NLX (0.3 mg/kg). *P < 0.05, Student's t-test. Number of animals is indicated either above or in each bar. Responses are represented as a percentage of pretreatment values (%control), where the baseline response before stimulation is defined as 100%.
Figure 4D shows the mean responses of BRC frequency to different intensities of SN stimulation at 10 Hz without or with systemic administration of NLX (0.3 mg/kg). All intensities tested produced significant reductions of the frequency of bladder contractions, but only the inhibitory effects from 0.8 × Tmot and Tmot stimulation were antagonized by NLX. In control animals, stimulation at Tmot reduced the frequency of contractions during stimulation to 46.09 ± 10% of the prestimulation value (n = 14, vs. 102.34 ± 12%, without stimulation, n = 11; P < 0.05). In animals treated with NLX, stimulation at Tmot only reduced frequency to 90.40 ± 14% of the prestimulation value (n = 5, vs. 105.94 ± 10%, without stimulation, n = 8; P > 0.05).
Neuromodulation elicited relatively weak inhibition on the amplitude of the bladder contractions. Figure 4E summarizes intensity-dependent effects of SN stimulation on amplitude of BRC. Stimulation at 2 × Tmot significantly decreased the amplitude of contractions during stimulation to 66.72 ± 10% of the prestimulation value (n = 3, vs. 98.44 ± 3%, without stimulation, n = 11; P < 0.05). This effect was not attenuated in rats treated with NLX, with stimulation reducing amplitude of BRC to 83.95 ± 15% of the prestimulation value (n = 3, vs. 115.02 ± 15%, without stimulation, n = 8; P < 0.05), respectively.
Since we observed an opioid mechanism of inhibitory effects to SN stimulation at ≤Tmot intensity, an intensity of Tmot SN stimulation was selected for studies to identify the sites of opioid actions by using site-specific administrations (iv, it, or icv) of NLX Meth, a derivative of NLX having a quaternary nitrogen atom that reduces its ability to cross the blood brain barrier. Although systemic NLX Meth (0.1 and 0.3 mg/kg iv) had no effects on its own, it partially reversed the effects of SN stimulation on bladder contractions (Fig. 5A). We then used NLX Meth (0.01 mg it or icv) to determine if there is a role of CNS opioid pathways in neuromodulation of bladder activity. SN stimulation reduced BRC frequency to 24.07 ± 12% of the prestimulation value following intrathecal saline injection (vs. 84.75 ± 20%, without stimulation; P < 0.05; Fig. 5B). NLX Meth (it) completely blocked the inhibitory effect of SN stimulation (124.7 ± 8 vs. 111.20 ± 23%, without stimulation). Similarly, NLX Meth (icv) reversed the inhibitory effect of neuromodulation (Fig. 5C). In this experimental series, SN stimulation reduced BRC in saline-treated animals to 38.7 ± 11% of the prestimulation value (vs. 90.45 ± 13%, without stimulation; P < 0.05). In animals treated with NLX Meth (icv), SN stimulation had no effect (144.7 ± 44 vs. 119.5 ± 19%, without stimulation; P > 0.05), Neuromodulation had no effects on amplitude of contractions following any routes (iv, it, or icv) of injection of either saline or NLX Meth. Data are summarized in Fig. 5, D–F.
Fig. 5.
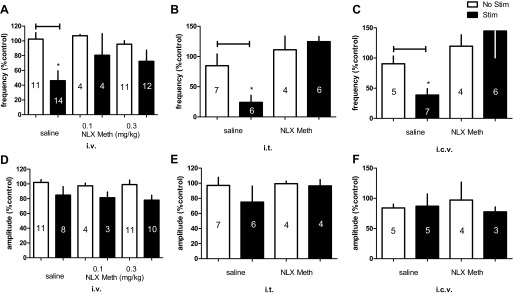
Summary of inhibitory effects of spinal nerve stimulation (Stim) on frequency (A–C) and amplitude (D–F) of bladder rhythmic contraction without or with intravenous (0.1 and 0.3 mg/kg iv), intrathecal (0.01 mg it), or intracerebroventricular (0.01 mg icv) administration of naloxone methiodide (NLX Meth). *P < 0.05, Student's t-test. Number of animals is indicated in each bar. Responses are represented as a percentage of pretreatment values (%control), where the baseline response before stimulation is defined as 100%.
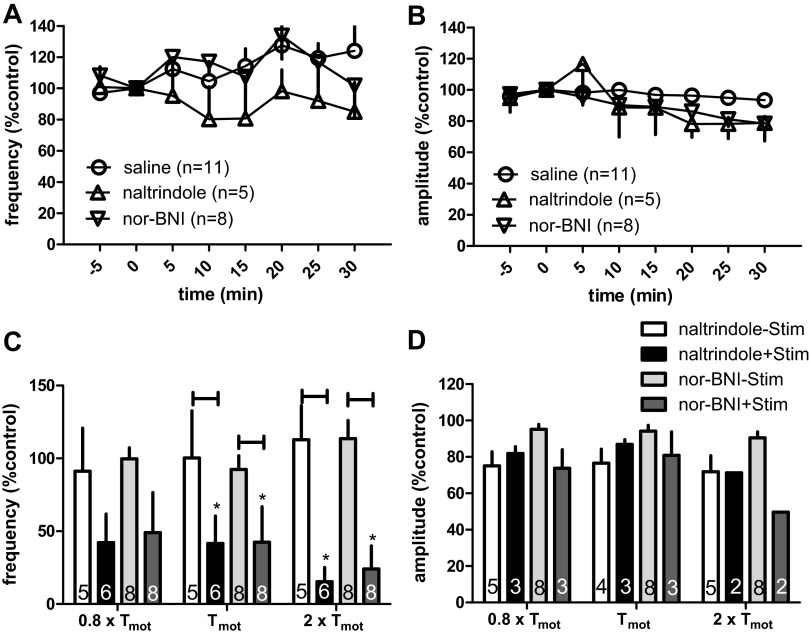
The role of opioid receptor subtypes in the neuromodulatory action of SN stimulation was investigated by examining the effects of the δ-opioid receptor antagonist naltrindole (5 mg/kg iv) or the κ-opioid receptor antagonist nor-BNI (2 mg/kg iv). Neither antagonist alone produced any significant change in the frequency and amplitude of BRC. The data are summarized in Fig. 6, A and B.
Fig. 6.
Effects of the δ-opioid receptor antagonist naltrindole and the κ-opioid receptor antagonist nor-binaltorphimine (nor-BNI) on neuromodulation of bladder contraction. A and B: time-course response of frequency (A) and amplitude (B) of the bladder reflex contraction to intravenous administration of nor-BNI or naltrindole. Overall, naltrindole and nor-BNI had no effect on the frequency or amplitude of the bladder contractions. C and D: effects of spinal nerve stimulation (Stim) on bladder contraction frequency (C) and amplitude (D) in rats pretreated with either naltrindole or nor-BNI. *P < 0.05, Student's t-test. Number of animals is indicated in each bar. Responses are represented as a percentage of pretreatment values (%control), where the baseline response before stimulation is defined as 100%.
The ability of SN stimulation to produce neuromodulation of BRC frequency was not attenuated by treatment with rats with either naltrindole or nor-BNI. Stimulation at 0.8 × Tmot, 1 × Tmot, and 2 × Tmot decreased the frequency of BRC to 42.17 ± 20% (n = 6, vs. 91.19 ± 30%, without stimulation, n = 5; P > 0.05), 41.57 ± 9% (vs. 100.34 ± 32%, without stimulation; P < 0.05), and 15.29 ± 11% (vs. 112.83 ± 23%, without stimulation; P < 0.05) of prestimulation values, after naltrindole injection, respectively. Similarly, stimulation deceased contraction frequency to 49.07 ± 27% (n = 8, vs. 99.69 ± 8%, without stimulation, n = 8; P > 0.05), 42.49 ± 24% (vs. 92.50 ± 9%, without stimulation; P < 0.05) and 24.04 ± 16% (vs. 113.51 ± 13%, without stimulation; P < 0.05) of prestimulation values, after nor-BNI injection, respectively.
As in previous experiments, neuromodulation usually had little effect on the amplitude of BRC contraction (Fig. 6, C and D).
DISCUSSION
In this study, stimulation was applied at a fixed frequency of 10 Hz, which has been shown to be optimal for inhibition of bladder contractions by both low- and high-intensity stimulation (25); stimulation intensity ranged from 0.8 to 2 × Tmot. In agreement with our previous reports (24, 25), SN stimulation inhibited the frequency of volume induced BRC, with the magnitude of the inhibition directly proportional to the applied current (stimulus intensity). Bladder inhibitory responses evoked by SN stimulation at therapeutic current intensities of 0.8 × Tmot and 1 × Tmot, but not the higher intensity of 2 × Tmot, were blocked by the opioid receptor antagonist NLX. This effect appears to involve activation of the μ-opioid receptor since neither the κ (nor-BNI, 2 mg/kg iv)- nor δ (naltrindole, 5 mg/kg iv)-opioid receptor antagonists affected the bladder inhibitory responses to SN stimulation. Opioid receptor-mediated neuromodulation of bladder activity at threshold SN stimulation occurs at both spinal and supraspinal levels since spinal (it) or supraspinal (icv) pretreatment with NLX Meth attenuated the inhibitory effect. Consistent with this premise, morphine inhibits bladder activity by mimicking endogenous processes.
Opioid control of bladder micturition.
Administration of μ-opioid receptor agonist morphine dose-dependently inhibited or decreased the frequency of the BRCs (ID50 of 0.16 mg/kg). Morphine produced weak inhibition of the amplitude of the bladder contractions only at the relatively high dose of 1 mg/kg. The effect of morphine on contraction frequency is comparable with previous reports (18, 29). NLX antagonized the inhibitory effect of single doses (0.1 or 0.3 mg/kg) of morphine on bladder contractions, confirming that the effects of morphine were produced by activation of opioid receptors.
The effects of morphine have been proposed to be centrally mediated (30). Both the inhibitory effect of morphine and its blockade by NLX demonstrate the ability of these compounds to rapidly gain access to the CNS after systemic administration. In contrast, an equal systemic dose of NLX Meth produced only partial blockade of the inhibitory effect of morphine (data not shown).
The effect of NLX on micturition in the BRC model suggests that the role of endogenous opioids in the control of micturition is highly variable. This is different from the effect of morphine and other opiate agonists, which show a consistent inhibitory action. Although a majority of the rats showed no effect, a significant number showed either an increase in bladder contraction frequency or bladder contraction duration. These stimulatory responses to opioid antagonists are consistent with an inhibitory action of endogenous opioid peptides in ∼25% of the animals tested.
Why are opiate receptors activated during micturition in some but not all rats? At this time, we have no explanation. Since female rats were used, it is possible that the role of opiate receptors is dependent on the estrous cycle. Johnson and Berkley (15) reported that micturition thresholds in female rats showed cycle-dependent differences in acute responsiveness to an intravesical irritant turpentine. Bon et al. (4) found no differences in cyclophosphamide-induced bladder irritation between male and female rats, and, in female rats, no time- or cycle-dependent influence on the response to this bladder irritant. However, Bon et al. did observe that a subset of animals who were in estrous early on the morning of the experiment showed the most severe bladder irritation but not the behavioral impairment observed in most of the other rats. This might suggest a release and action of endogenous opiate peptides in this group of rats. Finally, Ball et al. (2) measured the estrous cycle effects on the nociceptive visceromotor reflex to bladder distention in intrathecal naloxone-treated rats. Naloxone enhanced the reflex response in estrus, diestrus, and metestrus but not in proestrus, suggesting that the endogenous opioid modulatory system can vary as a function of the estrous cycles in rats.
In the cat, systemic NLX increases the frequency of the contractions and in some cases also prolongs the contraction, which is associated with the postganglionic nerve firing (19). Such an action is likely to be mediated within the CNS since NLX had no effects on bladder contractions induced by stimulation of peripheral parasympathetic nerves (29). These results indicate that opioid peptides may have differential effects at the synapses comprising the micturition reflex arc. If endogenous opioids are released at different sites to different degrees under various conditions, the overall effect of opiate receptor blockade on micturition could vary substantially between different animals.
Opioid mechanisms of neuromodulation.
Consistently, SN stimulation at 1 × Tmot intensity inhibited bladder contraction by 46%; a similar effect was produced by an ID50 dose (0.16 mg/kg) of morphine. As the effects of morphine were antagonized by NLX, bladder inhibitory effects to stimulation at therapeutic intensities (0.8 × Tmot and 1 × Tmot) were also antagonized by NLX. The present observations broadly confirm the previous conclusions regarding the contribution of opioid mechanisms to pudendal or foot stimulation (5, 16, 28). High-intensity SN stimulation abolished bladder contraction; this effect could not be blocked by NLX. Although the mechanism of this effect was not determined, the insensitivity to NLX shows that nonopioid actions are involved. The inhibitory effects of neuromodulation on the BRC at intensities at or below Tmot, like those of morphine, are probably opioid receptor mediated.
It is not clear at present whether the bladder inhibitory effect results from an interaction of opioid peptides on the peripheral nervous system, the supraspinal micturition center in the brain stem, or the spinal sacral parasympathetic motor outflow to the bladder. All have been postulated as possible sites for opioid-mediated changes in bladder activity. It is tempting to speculate that the inhibitory effects of neuromodulation on the supraspinal/spinal reflex may be of therapeutic value in the treatment of overactive bladder.
Opiate-mediated inhibition of micturition probably involves an action within the CNS, since the effective intrathecal or intracerebroventricular dose of NLX Meth (0.01 mg) was much smaller than that required for a partial blockade following systemic injection (0.1 and 0.3 mg/kg). Even the partial blockade produced by systemic NLX Meth may be due to contamination of the NLX Meth with small quantities of NLX (3). The main sites of action for opioid peptides released by SN stimulation would thus seem to be the spinal nerves or supraspinal micturition center (8, 19, 21).
It is presently unclear whether each site (supraspinal or spinal cord) is also separately involved in the neuromodulation. However, it is notable that both sites have been recognized in the neurogenic control of bladder motility and opioid mechanisms related to bladder control have been firmly established in those regions (29). Based on this neurochemical and neuroanatomical findings, it is reasonable to hypothesize that opioids are released by SN stimulation and inhibit the micturition reflex by depressing the activity of neurons descending from the pons to the autonomic nuclei in the lower lumbar and upper sacral spinal cord, which, in turn, are believed to activate preganglionic pelvic nerve fibers innervating the urinary bladder.
The subtype selective opiate receptor antagonists naltrindole (δ-receptor) and nor-BNI (κ-receptor) were used to characterize the receptor selectivity of the interactions observed. Both failed to antagonize the inhibitory effects of SN stimulation at all intensities tested. These observations suggested that neither δ- nor κ-opioid receptors are important for neuromodulation of bladder activity. The release of endogenous opioids is differentially regulated by electrical stimulation. From examination of a diagnostic lumbar cerebrospinal fluid sample, it appears that low-frequency nerve stimulation (2 Hz) results in a marked increase Met-enkephalin-Arg-Phe while high-frequency (100 Hz) stimulation produces an increase in dynorphin A (14). It is likely that multiple neurotransmitters and peptides are released during neuromodulation. Since 10-Hz stimulation of the SN is optimal for bladder inhibitory effects, functionally important mechanisms of bladder control by SN stimulation are associated with a selective change in the release of μ-opioid agonist peptides, which interact with CNS μ-opioid receptors in a similar manner as the exogenous agonist morphine.
The present observation provides direct support for the hypothesis that the release of endogenous opioid peptides is involved in the central regulation of urinary bladder activity by neuromodulation of the SN. Exogenously administered morphine also produces its central effects on bladder activity by mimicking endogenous processes.
Opioid sensitivity may be particularly appreciated in view of the recent evidence linking endogenous opioid peptides to the regulation of bladder capacity. Chronic treatment of overactive bladder with opiate drugs would probably not be desirable due to the potential for tolerance and physical/psychological dependence, as well as the other well-known side effects of these drugs (e.g., sedation, dizziness, nausea, vomiting, constipation, and respiratory depression; Refs. 11, 20). However, these problems would not be associated with neuromodulation therapy.
In summary, SN stimulation attenuates bladder contractions. The inhibitory effects on bladder contraction to SN stimulation may be mediated by both opioid and nonopioid mechanisms. Therapeutic intensities of stimulation (≤Tmot) may act by stimulating endogenous μ-opioid release at the CNS. The magnitude of the inhibitory effects of neuromodulation at the therapeutic range of intensities (Tmot) is equivalent to that of 0.16 mg/kg (ID50 dose) systemic administration of morphine. Finally patients with high sensitivity to opiate drugs may be especially good candidates for InterStim therapy.
DISCLOSURES
Support for this study was provided by Medtronic.
AUTHOR CONTRIBUTIONS
Author contributions: X.S. conception and design of research; X.S. and A.N. analyzed data; X.S. and D.E.N. interpreted results of experiments; X.S. prepared figures; X.S. drafted manuscript; X.S. and D.E.N. edited and revised manuscript; A.N. performed experiments; D.E.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Greg Molnar for helpful comments and Matt Kelly for study coordination. The manuscript was edited by J. Paul Hieble Scientific Writing.
REFERENCES
- 1. Al-Khrasani M, Lackó E, Riba P, Király K, Sobor M, Timár J, Mousa S, Schäfer M, Fürst S. The central versus peripheral antinociceptive effects of μ-opioid receptor agonists in the new model of rat visceral pain. Brain Res Bull 87: 238–243, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Ball CL, Ness TJ, Randich A. Opioid blockade and inflammation reveal estrous cycle effects on visceromotor reflexes evoked by bladder distention. J Urol 184: 1529–1535, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Bianchi G, Fiocchi R, Tavani A, Manara L. Quaternary narcotic antagonists' relative ability to prevent antinociception and gastrointestinal transit inhibition in morphine-treated rats as an index of peripheral selectivity. Life Sci 30: 1875–1883, 1982 [DOI] [PubMed] [Google Scholar]
- 4. Bon K, Lanteri-Minet M, Menetrey D, Berkley KJ. Sex, time-of-day and estrous variations in behavioral and bladder histological consequences of cyclophosphamide-induced cystitis in rats. Pain 73: 423–429, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Chen ML, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC, Tai C. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Exp Neurol 224: 282–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dray A, Metsch R. Inhibition of urinary bladder contractions by a spinal action of morphine and other opioids. J Pharmacol Exp Ther 231: 254–260, 1984 [PubMed] [Google Scholar]
- 7. Dray A, Metsch R. Opioid receptor subtypes involved in the central inhibition of urinary bladder motility. Eur J Pharmacol 104: 47–53, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Dray A, Metsch R, Davis TP. Endorphins and the central inhibition of urinary bladder motility. Peptides 5: 645–647, 1984 [DOI] [PubMed] [Google Scholar]
- 9. Dray A, Nunan L, Wire W. Central delta-opioid receptor interactions and the inhibition of reflex urinary bladder contractions in the rat. Br J Pharmacol 85: 717–726, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gear RW, Levine JD. Antinociception produced by an ascending spino-supraspinal pathway. J Neurosci 15: 3154–3161, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 43: 879–923, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Gross GJ, Baker JE, Hsu A, Wu HE, Falck JR, Nithipatikom K. Evidence for a role of opioids in epoxyeicosatrienoic acid-induced cardioprotection in rat hearts. Am J Physiol Heart Circ Physiol 298: H2201–H2207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gotoh A, Goto K, Sengoku A, Shirakawa T, Akao Y, Fujisawa M, Okada H, Arakawa S, Sasaki H, Kamidono S. Inhibition of urinary bladder motility by a spinal action of U-50488H in rats. J Pharm Pharmacol 54: 1645–1650, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Han JS, Chen XH, Sun SL, Xu XJ, Yuan Y, Yan SC, Hao JX, Terenius L. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 47: 295–298, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Johnson OL, Berkley KJ. Estrous influences on micturition thresholds of the female rat before and after bladder inflammation. Am J Physiol Regul Integr Comp Physiol 282: R289–R294, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol 189: 1574–1579, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mazières L, Jiang CH, Lindström S. Recurrent inhibition of the bladder C fibre reflex in the cat and its response to naloxone. J Physiol 575: 603–615, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morikawa K, Hashimoto S, Yamauchi T, Kato H, Ito Y, Gomi Y. Inhibitory effect of inaperisone hydrochloride (inaperisone), a new centrally acting muscle relaxant, on the micturition reflex. Eur J Pharmacol 213: 409–415, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Roppolo JR, Booth AM, De Groat WC. The effects of naloxone on the neural control of the urinary bladder of the cat. Brain Res 264: 355–358, 1983 [DOI] [PubMed] [Google Scholar]
- 20. Safarinejad MR, Hosseini SY. Safety and efficacy of tramadol in the treatment of idiopathic detrusor overactivity: a double-blind, placebo controlled, randomized study. Br J Clin Pharmacol 61: 456–463, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Sillén U, Rubenson A. Central and peripheral motor effects of morphine on the rat urinary bladder. Acta Physiol Scand 126: 181–187, 1986 [DOI] [PubMed] [Google Scholar]
- 22. Snellings AE, Grill WM. Effects of stimulation site and stimulation parameters on bladder inhibition by electrical nerve stimulation. BJU Int 110: 136–143, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Su X, Sengupta JN, Gebhart GF. Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 77: 1566–1580, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Su X, Nickles A, Nelson DE. Unilateral versus bilateral neuromodulation in a rat model of the bladder rhythmic contraction. Neurourol Urodyn 30: 963, 2011 [Google Scholar]
- 25. Su X, Nickles A, Nelson DE. Neuromodulation in a rat model of the bladder micturition reflex. Am J Physiol Renal Physiol 302: F477–F486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su X, Nickles A, Nelson DE. Comparison of neural targets for neuromodulation of bladder micturition reflex in the rat. Am J Physiol Renal Physiol 303: F1196–F1206, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Tai C, Ogagan PD, Chen G, Larson JA, Shen B, Wang J, Roppolo JR, de Groat WC. Involvement of opioid receptors in inhibition of bladder overactivity induced by foot stimulation in cats. J Urol 188: 1012–1016, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willette RN, Morrison S, Sapru HN, Reis DJ. Stimulation of opiate receptors in the dorsal pontine tegmentum inhibits reflex contraction of the urinary bladder. J Pharmacol Exp Ther 244: 403–409, 1988 [PubMed] [Google Scholar]
- 30. Wu D, Kang YS, Bickel U, Pardridge WM. Blood-brain barrier permeability to morphine-6-glucuronide is markedly reduced compared with morphine. Drug Metab Dispos 25: 768–771, 1997 [PubMed] [Google Scholar]