Abstract
Members of the epidermal growth factor (EGF)-family bind to ErbB (EGFR)-family receptors that play an important role in the regulation of various fundamental cell processes in many organs including the kidney. In this field, most of the research efforts are focused on the role of EGF-ErbB axis in cancer biology. However, many studies indicate that abnormal ErbB-mediated signaling pathways are critical in the development of renal and cardiovascular pathologies. The kidney is a major site of the EGF-family ligands synthesis, and it has been shown to express all four members of the ErbB receptor family. The study of kidney disease regulation by ErbB receptor ligands has expanded considerably in recent years. In vitro and in vivo studies have provided direct evidence of the role of ErbB signaling in the kidney. Recent advances in the understanding of how the proteins in the EGF-family regulate sodium transport and development of hypertension are specifically discussed here. Collectively, these results suggest that EGF-ErbB signaling pathways could be major determinants in the progress of renal lesions, including its effects on the regulation of sodium reabsorption in collecting ducts.
Keywords: epidermal growth factor, ErbB receptor, EGFR, ENaC, salt-sensitive hypertension
members of the epidermal growth factor (EGF) family are small mitogenic proteins involved in a number of mechanisms such as normal cell growth and differentiation. Their effects are mediated via autocrine, paracrine, or endocrine mechanisms. ErbB receptors have been recognized as targets in anticancer therapy and are now used in the treatment of breast and colon malignancies (93). The name and gene symbol ErbB (Table 1) was derived from a viral oncogene to which these receptors are homologous: erythroblastic leukemia viral oncogene. Multiple epithelial cancers, including kidney carcinoma, are associated with the EGFR-EGF axis (61, 73, 111, 134). In addition to cancer biology, EGF receptor (EGFR) activation is critical in acute kidney injury (AKI) and chronic kidney diseases (CKD; Refs. 71, 108, 130). Here we discuss recent results supporting functional interactions between EGF-family proteins and blood pressure control in the kidney, as well as provide details of some mechanisms underpinning such interactions and the clinical consequences of these modulations. Particular emphasis is placed onto the mechanisms of regulation of sodium transport in the kidney by the EGF family growth factors. Hence we aim to provide critical insight into the physiological control of kidney function by EGF and its related growth factors.
Table 1.
Gene/chromosome locations of ErbBs
| Protein | Gene/Chromosome Location (Human) | Gene/Chromosome Location (Mouse) | Gene/Chromosome Location (Rat) |
|---|---|---|---|
| EGFR (ErbB1/HER-1) | Chr 7 | Chr 11 | Chr 14 |
| 55,086,725–55,275,031 | 16,652,206–16,813,910 | 97,617,358–97,788,211 | |
| ErbB2 (neu/HER-2) | Chr 17 | Chr 11 | Chr 10 |
| 37,844,393–37,884,915 | 98,273,798–98,299,030 | 87,219,157–87,242,919 | |
| ErbB3 (HER-3) | Chr 12 | Chr 10 | Chr 7 |
| 56,473,809–56,497,291 | 128,006,424–128,026,557 | 1,858,057–1,877,353 | |
| ErbB4 (HER-4) | Chr 2 | Chr 1 | Chr 9 |
| 212,240,442–213,403,352 | 68,086,540–69,154,633 | 66,843,898–67,967,970 |
Medical College of Wisconsin Rat Genome Database website (http://rgd.mcw.edu/) was used as a source for gene/chromosome locations.
EGFR, epidermal growth factor receptor.
ErbB Receptors
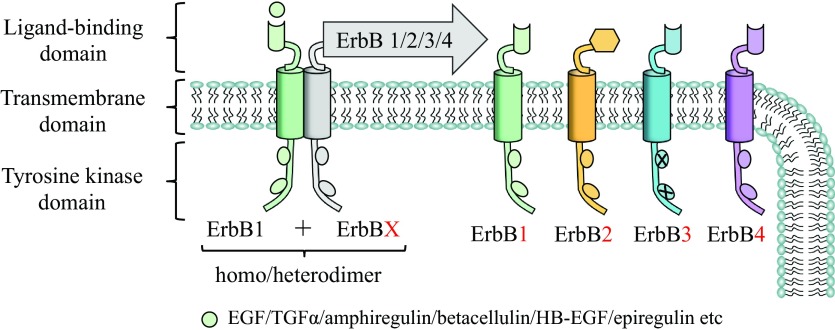
The EGFR family, also known as ErbB receptors (91), consists of four transmembrane receptors that belong to the receptor tyrosine kinase superfamily and includes EGFR (ErbB1/HER-1), ErbB2 (neu/HER-2), ErbB3 (HER-3), and ErbB4 (HER-4). EGFR as well as other receptors in this family is a transmembrane receptor that consists of an extracellular domain with conserved two cysteine-rich domains necessary for ligand binding; a single membrane-spanning domain, which has a passive role in signaling and functions as an “anchor” of receptor in plasma membrane (53), and a cytoplasmic protein tyrosine kinase domain, where six tyrosine autophosphorylation sites are located (Fig. 1). After ligand binding, ErbB receptors dimerize, which is a required critical step for intrinsic receptor tyrosine kinases to be activated and thereby specific tyrosine-containing residues to become autophosphorylated (12). Phosphorylated ErbB receptors can recruit different adapter proteins with Src homology domain-2 (SH2) or phosphotyrosine binding domains (PTB; Ref. 8). Signals from dimerized, activated ErbB receptors lead to activation of multiple intracellular signal transduction pathways. Depending on the pairing of ErbB family receptors (homodimers or heterodimers; Fig. 1), there can be downstream stimulation of different combinations of signaling pathways (43).
Fig. 1.
A schematic representation of ErbB receptors and their ligands. ErbB receptor is a transmembrane receptor that consists of an extracellular ligand binding domain, a transmembrane region, and an intracellular domain that includes an inactive (in the absence of ligand) tyrosine kinase region. Binding of a specific growth factor to ErbB transforms its extracellular domain into a configuration that enables formation of a homodimer or a heterodimer and activates the intracellular kinase domain. TGF-α, transforming growth factor-α. EGFR, epidermal growth factor receptor; HB-EGF, heparin binding-epidermal growth factor.
To some extent, EGFR is expressed in every nephron segment. Although EGFR is the predominant ErbB receptor found in the normal adult mammalian kidney tubule, ErbB2, ErbB3, and ErbB4 are also expressed in the kidney but mainly localize to the distal and connecting tubules and collecting ducts (87, 130, 131, 133). Figure 2 demonstrates representative immunohistochemical staining for ErbB2 receptor in the kidney cortex of Sprague-Dawley rat. A number of various mechanisms control ErbB receptors trafficking and degradation (64, 99) as well as polarized distribution (18, 127) in the renal epithelial cells.
Fig. 2.
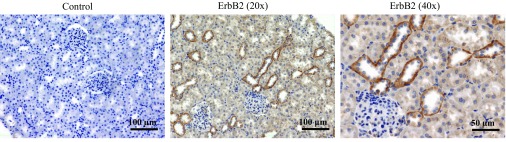
Immunohistochemical staining of ErbB2 receptor distribution in the kidney cortex. Representative images of kidney sections of Sprague-Dawley rats are shown at ×20 and ×40 magnifications. ErbB2 is predominantly distributed at basolateral surface of the collecting ducts.
ErbB Ligands
Regulation of ErbB receptors function is controlled by their ligands, members of the EGF-related peptide growth factor family (39). All EGF-family members, like many other growth factors, are derived from membrane-bound precursor proteins (97, 128). There are at least 12 ligands identified so far that can bind ErbBs and induce dimerization of distinct functional receptor pairs. EGF, transforming growth factor-α (TGF-α), and amphiregulin are specific for EGFR and primarily induce the formation of EGFR/EGFR homodimers and EGFR/ErbB2 heterodimers (27). Heparin binding-EGF (HB-EGF), betacellulin, and epiregulin can bind to either EGFR or ErbB4. Neuregulins-1 and −2 can bind both ErbB3 and ErbB4; neuregulins-3 and -4 are specific only for ErbB4 (71). Currently, no ligands have been identified for ErbB2 homodimers. However, ErbB2 is the preferred heterodimerization partner of other ErbB family members and ErbB2-containing heterodimers have the strongest signaling output (36, 114). Interestingly, the sequence of the ErbB3 catalytic domain suggests that this receptor does not have receptor tyrosine kinase activity (17). Thus ErbB3 may function as a platform for heterodimerization and subsequent transphosphorylation by other members of the ErbB family. Hence ErbB2 and ErbB3 can be activated through heterodimerization (6, 17).
Most of the ErbB-family ligands are expressed in the kidney (see Table 2 for some examples), and their expression could be dramatically changed during renal development or in pathological conditions (49, 71, 130). EGF and its related growth factors are present in the lumen at levels much higher than in plasma. However, ErbB receptors are expressed at the basolateral surfaces of tubules in normal adult kidneys (see Fig. 2). This may serve as a protective mechanism for the distal nephron, which can be disturbed under some pathophysiological conditions. One of the examples of apicobasal polarity abnormalities is mislocalization of ErbB receptors that is observed in a variety of genotypic and phenotypic animal models as well as in humans with polycystic kidney disease (PKD; Ref. 127). Another potential mechanism that emerges under some pathological conditions is a disturbance of epithelial cellular integrity. Following tubular injury, tight junctions can be disrupted and ErbB receptor ligands can traverse to the basolateral surface and consequently activate corresponding receptors.
Table 2.
EGF family growth factor expression in the kidney
| Protein | Expression Reported | References |
|---|---|---|
| EGF | TAL, DCT, CNT, CDs, papilla | (11, 30, 34, 49, 79, 81, 89, 118) |
| TGF-α | TAL, DCT, CDs | (11, 35, 68, 79, 117) |
| HB-EGF | Podocytes, mesangial cells, parietal epithelial cells, DCT, PCT, CDs | (10, 29, 44, 68, 74, 77, 78, 88, 98, 116) |
| Amphiregulin | PCT, CDs, podocytes, mesangial cells | (58, 68, 74) |
| Epiregulin | mesangial cells | (74) |
TGF-α, transforming growth factor-α; HB-EGF, heparin binding-epidermal growth factor; PCT, proximal convoluted tubule; TAL, thick ascending limb of Henle's loop; DCT, distal convoluted tubule; CNT, connecting tubule; CDs, collecting ducts.
Activation of ErbB Receptors and Consequent Physiological Functions
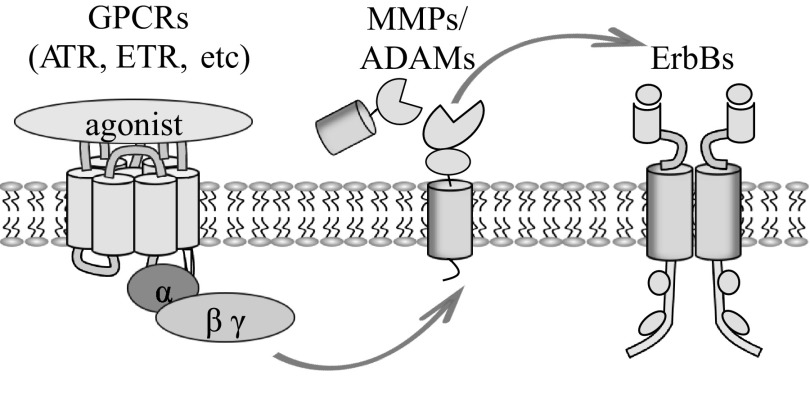
As ErbB ligands exist as inactive transmembrane precursors, they need proteolytic cleavage of their ectodomain to be released as mature soluble ligands. All EGF-family members are synthesized as membrane-anchored precursors that can be processed by specific metalloproteases to release the soluble bioactive factors from the cell surface. This cleavage is performed by a disintegrin and metalloprotease (ADAM) family members (9, 72, 80, 90) and matrix metalloproteinases (MMPs; Refs. 55, 104) and tightly regulated by different factors. ADAM-dependent EGFR ligand shedding can be induced by activation of G protein-coupled receptors (GPCRs), such as ANG II (ATR) or endothelin-1 (ETR) receptors (Fig. 3). A crucial role for ANG II-dependent EGFR transactivation in chronic kidney disease was demonstrated in mice overexpressing the dominant negative form of EGFR (57). MMPs, a large family of proteolytic enzymes that have the capability to degrade extracellular matrix proteins, are also implicated in regulating nephron formation and the pathogenesis of kidney diseases (13, 107, 121). For instance, it was shown recently that chronic administration of MMP inhibitors delays the progression of, and may even reverse, hypertension and diabetic nephropathy (125).
Fig. 3.
Transactivation of ErbBs by G protein-coupled receptors (GPCR). GPCR activation triggers intracellular signaling cascades that induce transactivation of matrix metalloproteinases (MMPs)/a disintegrin and metalloproteases (ADAMs) family proteins, which are able to cleave ErbB ligands into soluble active moieties. EGF family ligands can then bind to and activate ErbB and facilitate receptor dimerization and downstream activation of the intracellular protein kinases cascades of the same or of the other cell.
Activation of ErbB receptors by binding to their ligands promotes several biological responses. The physiological role of ErbB receptors is well established, especially in cancer. What is more, the role of ErbB-family members in renal development, physiology, and pathophysiology is also well recognized (130). Under physiological conditions, ErbB activation appears to play an important role in the regulation of renal hemodynamics and electrolyte handling by the kidney, while in different pathophysiological states ErbB activation may mediate either beneficial or detrimental effects to the kidney (130). ErbB signaling is critically involved in cell signaling, cell growth, proliferation, and renal electrolyte homeostasis. Once activated by site-specific phosphorylation, ErbB receptors serve as molecular integrators through either direct phosphorylation of target molecules or by serving as scaffolds for adaptor proteins. The great diversity of ligands and receptor dimer pairs allows activation of numerous signaling pathways that coordinately regulate complex processes including developmental growth control and adult homeostasis.
Effects of EGF-Family Members in the Kidney
The involvement of EGFR signaling in AKI and CKD development has been recently thoroughly reviewed (108). Although the precise mechanisms underlying effects of EGF signaling are not completely clear, numerous studies evidence abnormal EGF-EGFR axis functionality during progression and development of these diseases. Changes in EGFR expression and EGFR phosphorylation were detected in a variety of experimental models of AKI (15, 41, 44, 108) and CKD (16, 42, 56, 63, 109). Pivotal role of HB-EGF expression and EGFR signaling is also demonstrated in rapidly progressive glomerulonephritis (10, 31); abnormalities of apico-basal polarity and expression of EGF-EGFR axis were also described in the PKD epithelia (68, 126, 133).
What is more, EGF-family growth factors are involved in regulation of various epithelial ion channels in the kidney. For instance, EGF stimulates store-operated Ca2+ channels in human mesangial cells through an intracellular signaling mechanism involving tyrosine kinase and PKC (66). Later it was shown that EGF activates store-operated Ca2+ channels by a PLC-dependent, but inositol 1,4,5-trisphosphate receptor-independent, pathway (60). Polycistin-2, critical in PKD, can be also activated in response to EGF in the kidney epithelial cells (67). The physiological relevance of EGF-induced activation of TRPP2 was supported by animal studies in which homozygous deletion of the Egfr gene resulted in cystic dilatation of collecting ducts (112, 113). A role for ErbB receptor activation in the regulation of Mg2+ channels in the kidney has also been demonstrated. Genetic analysis has revealed that the melastatin transient receptor potential 6 (TRPM6) channel is mutated in patients with primary hypomagnesemia and secondary hypocalcemia (92, 122). Furthermore, the direct effects of EGF on TRPM6 channels were reported (46, 47, 110) and it was demonstrated that the EGFR inhibitor erlotinib is capable of affecting TRPM6 regulation and thereby altering Mg2+ handling in vivo (22).
EGF-Dependent Regulation of Epithelial Na+ Channel-Mediated Sodium Transport in the Kidney
The epithelial Na+ channel (ENaC) activity is the rate-limiting step for Na+ reabsorption across many epithelia, including the distal nephron (7, 101, 102). Dysfunction and aberrant regulation of this channel lead to a spectrum of diseases including hyper- and hypotension. For at least two decades, EGF has been known to regulate ENaC-mediated sodium transport. It was previously shown that EGF decreases active sodium transport in the isolated and perfused rabbit CD (75, 119, 120, 123) and immortalized epithelial cells (25, 26, 37, 94). Interestingly, EGF-family members cause a biphasic response of ENaC. EGF transiently increases sodium transport in Xenopus laevis kidney A6 cells in a phosphatidylinositol 3-kinase-dependent manner, and this stimulation is mediated by the inhibition of the MAPK pathway (70). Later it was reported that acute treatment with EGF and TGF-α enhanced ENaC activity in A6 cells by affecting channel open probability via phosphatidylinositol 3-kinase signaling pathway (62). However, the same study demonstrated that continuous treatment with EGF and TGF-α inhibited ENaC activity by decreasing the number of channels in the plasma membrane through MAPK1/2 pathways (62). Our data are also consistent with the idea that EGF-family members have a biphasic effect on sodium absorption in the mammalian kidney (59). Four members of the EGF-family (EGF, TGF-α, HB-EGF, and amphiregulin) were tested in cultured mpkCCDc14 principal cells. Basolateral addition of growth factors to these cells initially increased Na+ reabsorption above basal levels in a time-dependent manner. A significant increase was detected after 30 min, the earliest time point measured, with a maximum reached by 2 h. After 4 h of treatment, a slow and continuous decrease of ENaC-mediated transepithelial current was observed. Thus chronic treatment of monolayers with EGF-family growth factors leads to significant inhibition of ENaC-mediated current (59).
EGF-family ligands, as many other signaling molecules, activate corresponding receptors in a dose-responded manner. Thus establishing the dose-response curves for the effects of EGF and other members of this family is critical for understanding physiological role of these growth factors. As we previously demonstrated, using immortalized mouse mpkCCDc14 principal cells, both EGF and TGF-α dose dependently modulated ENaC activity. The EC50 value for EGF activation (acute phase) was 3.7 ± 1.5 ng/ml. Chronic treatment with various concentrations of EGF (0.1, 0.5, 1, 10, and 100 ng/ml) and TGF-α (0.5, 1, 10, and 100 ng/ml) also resulted in dose-dependent decreases in ENaC-mediated transepithelial current (59). Liu et al. (62) also reported that EGF-family ligands have dose-dependent biphasic effects on amphibian A6 cells. Both TGF-α and EGF chronically decreased sodium transport in a dose-dependent manner. The IC50 was 4.7 ng/ml for TGF-α-induced inhibition and 110 ng/ml for EGF-induced inhibition (62). A number of studies analyzed concentrations of EGF-family ligands in plasma and urine of rodents and human. For instance, it was shown that concentrations of EGF in serum of 60-day-old male mice ranged between 0.264 and 0.503 ng/ml (84); significant age and gender variability of EGF level was also noted by the authors. Considering the fact that ErbB receptors localize at the basolateral side and concentration in the lumen is at least 10-fold higher, these concentrations could represent the same range as levels required for ENaC modulation.
At least acute effects of EGF correlate with reactive oxygen species (ROS) production since pretreatment with the nonselective NADPH oxidase activity inhibitor apocynin blunted both generation of ROS and increase in ENaC-mediated current in response to EGF (48). Most likely, an increase in hydrogen peroxide (H2O2) levels mediates this effects since it was shown that H2O2 is critical for ENaC activity (23, 65, 69, 103). Furthermore, EGF had no effect in Rac1 knockdowned principal cells (48). It appears that small GTPase Rac1, which has been shown to be implicated in the development of many renal and cardiovascular diseases (54, 76, 95, 96), is a critical protein in transmission of the signal from EGF to ENaC. Rac1 might mediate its effects on ENaC either through the MAPK pathway (62, 100), WAVE proteins (52), or ROS production (48), since Rac1 is also one of the key subunits of the NADPH oxidase complex.
Role of EGF-EGFR Axis in the Development of Hypertension
A link between EGF-EGFR signaling pathway and renal vasculature and nephron functions was identified in several studies. Previous studies revealed that renal cortical expression of EGFR was increased in both prehypertensive and hypertensive salt-sensitive (SS) rats (129). Immunohistochemical analysis demonstrated that EGFR expression was increased in SS rats compared with Sprague-Dawley and Dahl/Rapp salt-resistant rats. Following addition of EGF, autophosphorylation of the EGFR was further enhanced in the primary cells derived from SS rats and this effect was especially strengthened in the renal vasculature (129). Previous EGF binding studies also revealed that SS rats (105) and the spontaneously hypertensive Lyon rats exhibited increased levels of EGFR in freshly prepared kidney and aortic tissue membranes compared with normotensive or hypotensive strains (106). Microarray-based global gene expression analysis in deoxycorticosterone acetate (DOCA)-salt-induced hypertensive rats further demonstrated that chronic treatment with an EGFR inhibitor AG1478 leads to inhibition of the majority of genes associated with development of renal dysfunction and hypertension in this model (5). Thus expression of EGFR is associated with hypertensive pathology and can potentially play a role in kidney damage associated with high blood pressure. However, it is hard to define whether changes in gene/protein expression are a consequence of hypertension or the primary basis of the diseases. Previous excellent review by Cowley and Roman (19) discussed potential lines of evidence, which could help to evaluate this question. Additional studies are required for better understanding of the mechanisms resulting in development of hypertension, including salt-sensitive hypertension.
Several possible mechanisms may account for the role of ErbB signaling in the development of hypertension (4). EGF induces constriction of both preglomerular and postglomerular arterioles, resulting in acute major reductions in the rates of glomerular filtration and perfusion (40). EGF and TGF were described as vasoconstrictors on endothelium-denuded thoracic aortas dissected from two models of hypertension: 1) DOCA-salt; and 2) one-kidney, one-clip hypertensive rats (32). HB-EGF was also characterized as a vasoconstrictor released under influence of GPCRs, such as adrenoceptors and angiotensin receptors, in isolated mesenteric arteries (38). Similarly, HB-EGF is required for endothelin-1-induced vasoconstriction and increase in blood pressure (14). Transactivation of EGFRs by GPCRs was also shown in the thoracic aorta smooth muscle cell line (24) and rat aorta (115). Moreover, it was shown that ANG II-induced hypertension and hypertrophy are attenuated by EGFR antisense (50). Briefly, a number of lines of evidence suggest that EGF-EFGR signaling mediates vasoconstriction and arterial hypertension in response to various stimuli. However, most of the studies of the role of EGF-EGFR axis in the vasculature are performed in the heart and conduit vessels and outside of the focus of current review.
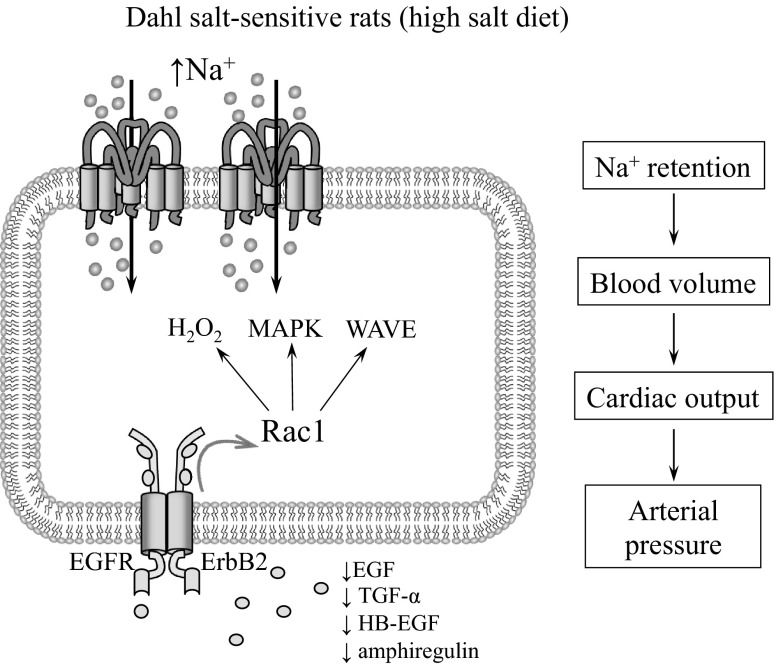
As discussed above, in the collecting ducts EGF has a biphasic effect on ENaC-mediated sodium reabsorption. While acute changes to ErbB ligands are critical for the fast regulation of channel activity in response to alterations in cells environment under certain pathological conditions, chronic inhibition of ENaC is relevant to changes in the blood pressure and development of hypertension. Our recent data indicate that ENaC contributes to the development of hypertension in the Dahl SS rat strain (82), which is a commonly used model for the studies of salt-sensitive hypertension (20, 21, 28, 45, 132). Although a high-salt diet was found to downregulate ENaC in salt-resistant animals (33, 86), it was previously demonstrated that ENaC expression is upregulated in SS rats (1–3, 51). Our studies provide evidence that ENaC is abnormally regulated by dietary sodium in SS hypertensive rats, and this abnormal activity is one of the major factors causing salt-sensitive hypertension. Thus, as we recently demonstrated, ENaC activity is significantly enhanced in SS rats fed a high-salt diet compared with salt-resistant SS.13BN consomic strain and SS rats fed a low-salt diet (82). Importantly, the servo-controlled approach, which allowed us to determine the contribution of renal perfusion pressure (RPP) in the development of hypertension and renal injury in SS rats, provides evidence that ENaC is essential for the development of salt-sensitive hypertension. In the servo-controlled studies, the system maintains the RPP to the left kidney of the instrumented SS rat at control level, whereas RPP to the right kidney increases in response to a high-salt diet. Therefore, both left and right kidneys are exposed to an identical systemic neurohormonal and metabolic environment, but controlled levels of RPP within the left kidney protected it from the high pressure. At least, β-ENaC expression (as analyzed by immunohistochemistry) was increased in the uncontrolled kidneys compared with controlled left kidneys (82).
Our recent studies identified EGF as a key molecular substrate for a new positive feedback mechanism that diminishes development of salt-sensitive hypertension (82). First, we found that EGF concentration in the kidney cortex of SS rats fed a high-salt diet was significantly lower compared with SS rats on a low-salt diet or salt-resistant SS.13BN consomic rats on both diets (82), which is consistent with upregulation of EGFR expression (105, 129). To directly evaluate the role of EGF in the development of hypertension and its effect on ENaC activity, EGF was intravenously infused and blood pressure was continuously monitored. In addition, ENaC activity was assessed at the end of experiments, when EGF was continuously infused during several days. Infusion of EGF decreased ENaC activity, prevented the development of hypertension and attenuated renal glomerular and tubular damage (82). As shown on Fig. 4, in vivo EGF infusion prevents development of salt-induced hypertension (Fig. 4A) in this strain and decreases ENaC activity in collecting ducts, which might mediate EGF effects on blood pressure (Fig. 4, B and C). We believe that these findings advance our understanding of salt-sensitive hypertension and provide some insight into its molecular basis (Fig. 5). Importantly, recent studies in mice demonstrated that the EGFR monoclonal antibody Cetuximab given intraperitoneally for 8–10 days raised the blood pressure of mice on normal-salt diet by 25 mmHg and that of those on high-salt diets by 34 mmHg (85). The authors proposed that downregulation of ENaC by arachidonic acid metabolites (83, 124) mediates these effects (85). Thus it appears that EGF-EGFR signaling is involved in blood pressure regulation not only in salt-sensitive but also in salt-resistant animals.
Fig. 4.
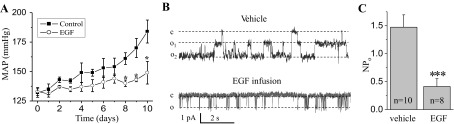
Chronic infusion of EGF prevents development of hypertension in salt-sensitive (SS) rats fed a high-salt diet via downregulation of ENaC activity. A: effect of continuous intravenous EGF infusion on the mean arterial pressure in the SS rats fed a high-salt diet. B and C: representative single channel traces (B) and summary graph (C) for ENaC activity from freshly isolated split opened collecting ducts derived from SS rats after chronic treatment with EGF or vehicle. MAP, mean arterial pressure; NPo, average channel activity. [Reproduced from Ref. 82 with permission].
Fig. 5.
Scheme illustrating the hypothesized mechanisms responsible for involvement of EGF and ENaC in development of salt-sensitive hypertension.
Conclusion
ErbB family members are implicated in the development of cancer and cardiac and renal diseases such as AKI, CKD, PKD, and hypertension. Therefore, the therapeutic potential of targeting the ErbB receptors and EGF-family signaling pathways needs further detailed investigations. Undoubtedly, many questions remain regarding regulation of sodium reabsorption and hypertension by ErbB ligands. For instance, receptor specificity plays the key role in these mechanisms. Furthermore, it is not clear if different ligands targeting the same homo- or heteromer work as a complex and whether they have different affinities and different efficacies depending on the target conformation. Also, transactivation of ErbB receptors and the crucial roles of ADAM and MMP proteases are understudied. In addition, we would like to emphasize that special caution should be paid during the treatment of cancer patients with antibodies vs. ErbBs of inhibitors of EGF-ErbB signaling since inhibition of EGF-ErbBs axis could result in development of hypertension as a side effect in patients with predisposition to salt-sensitive hypertension.
GRANTS
Part of this work was supported by National Heart, Lung, and Blood Institute Grant HL-108880 (to A. Staruschenko) and American Heart Association Grant 13SDG14220012 (to T. S. Pavlov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S. conception and design of research; A.S. drafted manuscript; A.S., O.P., D.V.I., and T.S.P. edited and revised manuscript; A.S., O.P., D.V.I., and T.S.P. approved final version of manuscript; O.P. and D.V.I. prepared figures.
ACKNOWLEDGMENTS
We apologize to the investigators whose work was not directly discussed due to space limitations.
REFERENCES
- 1.Amin MS, Reza E, El-Shahat E, Wang HW, Tesson F, Leenen FH. Enhanced expression of epithelial sodium channels in the renal medulla of Dahl S rats. Can J Physiol Pharmacol 89: 159–168, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Aoi W, Niisato N, Sawabe Y, Miyazaki H, Marunaka Y. Aldosterone-induced abnormal regulation of ENaC and SGK1 in Dahl salt-sensitive rat. Biochem Biophys Res Commun 341: 376–381, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Aoi W, Niisato N, Sawabe Y, Miyazaki H, Tokuda S, Nishio K, Yoshikawa T, Marunaka Y. Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt-sensitively hypertensive rats. Cell Biol Int 31: 1288–1291, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Beltowski J, Lowicka E. EGF receptor as a drug target in arterial hypertension. Mini Rev Med Chem 9: 526–538, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Benter IF, Canatan H, Benboubetra M, Yousif MH, Akhtar S. Global upregulation of gene expression associated with renal dysfunction in DOCA-salt-induced hypertensive rats occurs via signaling cascades involving epidermal growth factor receptor: a microarray analysis. Vascul Pharmacol 51: 101–109, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Berger MB, Mendrola JM, Lemmon MA. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett 569: 332–336, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Blaikie P, Immanuel D, Wu J, Li N, Yajnik V, Margolis B. A region in Shc distinct from the SH2 domain can bind tyrosine-phosphorylated growth factor receptors. J Biol Chem 269: 32031–32034, 1994 [PubMed] [Google Scholar]
- 9.Blobel CP, Carpenter G, Freeman M. The role of protease activity in ErbB biology. Exp Cell Res 315: 671–682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollee G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, Schordan E, Sabaa N, Vandermeersch S, Galaup A, Rodenas A, Casal I, Sunnarborg SW, Salant DJ, Kopp JB, Threadgill DW, Quaggin SE, Dussaule JC, Germain S, Mesnard L, Endlich K, Boucheix C, Belenfant X, Callard P, Endlich N, Tharaux PL. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 17: 1242–1250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carev D, Saraga M, Saraga-Babic M. Expression of intermediate filaments, EGF and TGF-alpha in early human kidney development. J Mol Histol 39: 227–235, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem 265: 7709–7712, 1990 [PubMed] [Google Scholar]
- 13.Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol 292: F905–F911, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Chansel D, Ciroldi M, Vandermeersch S, Jackson LF, Gomez AM, Henrion D, Lee DC, Coffman TM, Richard S, Dussaule JC, Tharaux PL. Heparin binding EGF is necessary for vasospastic response to endothelin. FASEB J 20: 1936–1938, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int 82: 45–52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res 284: 54–65, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Cotton CU, Hobert ME, Ryan S, Carlin CR. Basolateral EGF receptor sorting regulated by functionally distinct mechanisms in renal epithelial cells. Traffic 14: 337–354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA 275: 1581–1589, 1996 [PubMed] [Google Scholar]
- 20.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 57: 269–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimke H, van der WJ, Alexander TR, Meijer IM, Mulder GM, van GH, Tejpar S, Hoenderop JG, Bindels RJ. Effects of the EGFR inhibitor erlotinib on magnesium handling. J Am Soc Nephrol 21: 1309–1316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downs CA, Kumar A, Kreiner LH, Johnson NM, Helms MN. H2O2 regulates lung ENaC via ubiquitin-like protein Nedd8. J Biol Chem 288: 8136–8145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escano CS, Jr, Keever LB, Gutweiler AA, Andresen BT. Angiotensin II activates extracellular signal-regulated kinase independently of receptor tyrosine kinases in renal smooth muscle cells: implications for blood pressure regulation. J Pharmacol Exp Ther 324: 34–42, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Falin R, Veizis IE, Cotton CU. A role for ERK1/2 in EGF- and ATP-dependent regulation of amiloride-sensitive sodium absorption. Am J Physiol Cell Physiol 288: C1003–C1011, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Falin RA, Cotton CU. Acute downregulation of ENaC by EGF involves the PY motif and putative ERK phosphorylation site. J Gen Physiol 130: 313–328, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feigin ME, Muthuswamy SK. ErbB receptors and cell polarity: new pathways and paradigms for understanding cell migration and invasion. Exp Cell Res 315: 707–716, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Feng D, Yang C, Geurts A, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor PM, Cowley AW., Jr Increased expression of NAD(P)H oxidase subunit p67phox in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15: 201–208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng L, Garcia GE, Yang Y, Xia Y, Gabbai FB, Peterson OW, Abraham JA, Blantz RC, Wilson CB. Heparin-binding EGF-like growth factor contributes to reduced glomerular filtration rate during glomerulonephritis in rats. J Clin Invest 105: 341–350, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher DA, Salido EC, Barajas L. Epidermal growth factor and the kidney. Annu Rev Physiol 51: 67–80, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Flamant M, Bollee G, Henique C, Tharaux PL. Epidermal growth factor: a new therapeutic target in glomerular disease. Nephrol Dial Transplant 27: 1297–1304, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Florian JA, Watts SW. Epidermal growth factor: a potent vasoconstrictor in experimental hypertension. Am J Physiol Heart Circ Physiol 276: H976–H983, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Frindt G, Ergonul Z, Palmer LG. Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol 131: 617–627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gesualdo L, Di PS, Calabro A, Milani S, Maiorano E, Ranieri E, Pannarale G, Schena FP. Expression of epidermal growth factor and its receptor in normal and diseased human kidney: an immunohistochemical and in situ hybridization study. Kidney Int 49: 656–665, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Goodyer PR, Fata J, Mulligan L, Fischer D, Fagan R, Guyda HJ, Goodyer CG. Expression of transforming growth factor-alpha and epidermal growth factor receptor in human fetal kidneys. Mol Cell Endocrinol 77: 199–206, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J 16: 1647–1655, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grossmann C, Freudinger R, Mildenberger S, Krug AW, Gekle M. Evidence for epidermal growth factor receptor as negative-feedback control in aldosterone-induced Na+ reabsorption. Am J Physiol Renal Physiol 286: F1226–F1231, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res 94: 68–76, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res 284: 2–13, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Harris RC, Hoover RL, Jacobson HR, Badr KF. Evidence for glomerular actions of epidermal growth factor in the rat. J Clin Invest 82: 1028–1039, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He S, Liu N, Bayliss G, Zhuang S. EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury. Am J Physiol Renal Physiol 304: F356–F366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helle F, Jouzel C, Chadjichristos C, Placier S, Flamant M, Guerrot D, Francois H, Dussaule JC, Chatziantoniou C. Improvement of renal hemodynamics during hypertension-induced chronic renal disease: role of EGF receptor antagonism. Am J Physiol Renal Physiol 297: F191–F199, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol 44: 195–217, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Homma T, Sakai M, Cheng HF, Yasuda T, Coffey RJ, Jr, Harris RC. Induction of heparin-binding epidermal growth factor-like growth factor mRNA in rat kidney after acute injury. J Clin Invest 96: 1018–1025, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong NJ, Garvin JL. Angiotensin II type 2 receptor-mediated inhibition of NaCl absorption is blunted in thick ascending limbs from Dahl salt-sensitive rats. Hypertension 60: 765–769, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikari A, Okude C, Sawada H, Yamazaki Y, Sugatani J, Miwa M. TRPM6 expression and cell proliferation are up-regulated by phosphorylation of ERK1/2 in renal epithelial cells. Biochem Biophys Res Commun 369: 1129–1133, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Ikari A, Sanada A, Okude C, Sawada H, Yamazaki Y, Sugatani J, Miwa M. Up-regulation of TRPM6 transcriptional activity by AP-1 in renal epithelial cells. J Cell Physiol 222: 481–487, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin and IGF-1. Am J Physiol Cell Physiol 304: C102–C111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung JY, Song JH, Li C, Yang CW, Kang TC, Won MH, Jeong YG, Han KH, Choi KB, Lee SH, Kim J. Expression of epidermal growth factor in the developing rat kidney. Am J Physiol Renal Physiol 288: F227–F235, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Kagiyama S, Eguchi S, Frank GD, Inagami T, Zhang YC, Phillips MI. Angiotensin II-induced cardiac hypertrophy and hypertension are attenuated by epidermal growth factor receptor antisense. Circulation 106: 909–912, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Kakizoe Y, Kitamura K, Ko T, Wakida N, Maekawa A, Miyoshi T, Shiraishi N, Adachi M, Zhang Z, Masilamani S, Tomita K. Aberrant ENaC activation in Dahl salt-sensitive rats. J Hypertens 27: 1679–1689, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Karpushev AV, Levchenko V, Ilatovskaya DV, Pavlov TS, Staruschenko A. Novel role of Rac1/WAVE signaling mechanism in regulation of the epithelial Na+ channel. Hypertension 57: 996–1002, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Kashles O, Szapary D, Bellot F, Ullrich A, Schlessinger J, Schmidt A. Ligand-induced stimulation of epidermal growth factor receptor mutants with altered transmembrane regions. Proc Natl Acad Sci USA 85: 9567–9571, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawarazaki W, Nagase M, Yoshida S, Takeuchi M, Ishizawa K, Ayuzawa N, Ueda K, Fujita T. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol 23: 997–1007, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kramarenko II, Bunni MA, Raymond JR, Garnovskaya MN. Bradykinin B2 receptor interacts with integrin α5β1 to transactivate epidermal growth factor receptor in kidney cells. Mol Pharmacol 78:126–134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laouari D, Burtin M, Phelep A, Martino C, Pillebout E, Montagutelli X, Friedlander G, Terzi F. TGF-alpha mediates genetic susceptibility to chronic kidney disease. J Am Soc Nephrol 22: 327–335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Lee SB, Huang K, Palmer R, Truong VB, Herzlinger D, Kolquist KA, Wong J, Paulding C, Yoon SK, Gerald W, Oliner JD, Haber DA. The Wilms tumor suppressor WT1 encodes a transcriptional activator of amphiregulin. Cell 98: 663–673, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Levchenko V, Zheleznova NN, Pavlov TS, Vandewalle A, Wilson PD, Staruschenko A. EGF and its related growth factors mediate sodium transport in mpkCCDc14 cells via ErbB2 (neu/HER-2) receptor. J Cell Physiol 223: 252–259, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Li WP, Tsiokas L, Sansom SC, Ma R. Epidermal growth factor activates store-operated Ca2+ channels through an inositol 1,4,5-trisphosphate-independent pathway in human glomerular mesangial cells. J Biol Chem 279: 4570–4577, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Liang L, Li L, Zeng J, Gao Y, Chen YL, Wang ZQ, Wang XY, Chang LS, He D. Inhibitory effect of silibinin on EGFR signal-induced renal cell carcinoma progression via suppression of the EGFR/MMP-9 signaling pathway. Oncol Rep 28: 999–1005, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Liu L, Duke BJ, Malik B, Yue Q, Eaton DC. Biphasic regulation of ENaC by TGF-α and EGF in renal epithelial cells. Am J Physiol Renal Physiol 296: F1417–F1427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu W, Fan LX, Zhou X, Sweeney WE, Jr, Avner ED, Li X. HDAC6 regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation in renal epithelial cells. PLoS ONE 7: e49418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma HP. Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J Biol Chem 286: 32444–32453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma R, Sansom SC. Epidermal growth factor activates store-operated calcium channels in human glomerular mesangial cells. J Am Soc Nephrol 12: 47–53, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacRae DK, Nemo R, Sweeney WE, Jr, Avner ED. EGF-related growth factors in the pathogenesis of murine ARPKD. Kidney Int 65: 2018–2029, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in the distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markadieu N, Crutzen R, Blero D, Erneux C, Beauwens R. Hydrogen peroxide and epidermal growth factor activate phosphatidylinositol 3-kinase and increase sodium transport in A6 cell monolayers. Am J Physiol Renal Physiol 288: F1201–F1212, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Melenhorst WB, Mulder GM, Xi Q, Hoenderop JG, Kimura K, Eguchi S, van Goor H. Epidermal growth factor receptor signaling in the kidney: key roles in physiology and disease. Hypertension 52: 987–993, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Melenhorst WB, Visser L, Timmer A, van den Heuvel MC, Stegeman CA, van Goor H. ADAM17 upregulation in human renal disease: a role in modulating TGF-α availability? Am J Physiol Renal Physiol 297: F781–F790, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Minner S, Rump D, Tennstedt P, Simon R, Burandt E, Terracciano L, Moch H, Wilczak W, Bokemeyer C, Fisch M, Sauter G, Eichelberg C. Epidermal growth factor receptor protein expression and genomic alterations in renal cell carcinoma. Cancer 118: 1268–1275, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Mishra R, Leahy P, Simonson MS. Gene expression profiling reveals role for EGF-family ligands in mesangial cell proliferation. Am J Physiol Renal Physiol 283: F1151–F1159, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Muto S, Furuya H, Tabei K, Asano Y. Site and mechanism of action of epidermal growth factor in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 260: F163–F169, 1991 [DOI] [PubMed] [Google Scholar]
- 76.Nagase M, Fujita T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol 9: 86–98, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Nakagawa T, Hayase Y, Sasahara M, Haneda M, Kikkawa R, Higashiyama S, Taniguchi N, Hazama F. Distribution of heparin-binding EGF-like growth factor protein and mRNA in the normal rat kidneys. Kidney Int 51: 1774–1779, 1997 [DOI] [PubMed] [Google Scholar]
- 78.Nguyen HT, Bride SH, Badawy AB, Adam RM, Lin J, Orsola A, Guthrie PD, Freeman MR, Peters CA. Heparin-binding EGF-like growth factor is up-regulated in the obstructed kidney in a cell- and region-specific manner and acts to inhibit apoptosis. Am J Pathol 156: 889–898, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nouwen EJ, de Broe ME. EGF and TGF-alpha in the human kidney: identification of octopal cells in the collecting duct. Kidney Int 45: 1510–1521, 1994 [DOI] [PubMed] [Google Scholar]
- 80.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol 291: C1–C10, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Oka Y, Fujiwara K, Endou H. Intranephron distribution of epidermal growth factor immunoreactivity in the mouse. Renal Physiol 10: 283–288, 1987 [DOI] [PubMed] [Google Scholar]
- 82.Pavlov TS, Levchenko V, O'Connor PM, Ilatovskaya DV, Palygin O, Mori T, Mattson DL, Sorokin A, Lombard JD, Cowley AW, Jr, Staruschenko A. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol 2013 Apr 18 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pavlov TS, Ilatovskaya DV, Levchenko V, Mattson DL, Roman RJ, Staruschenko A. Effects of cytochrome P450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC). Am J Physiol Renal Physiol 301: F672–F681, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perheentupa J, Lakshmanan J, Hoath SB, Beri U, Kim H, Macaso T, Fisher DA. Epidermal growth factor measurements in mouse plasma: method, ontogeny, and sex difference. Am J Physiol Endocrinol Metab 248: E391–E396, 1985 [DOI] [PubMed] [Google Scholar]
- 85.Pidkovka N, Rao R, Mei S, Gong Y, Harris RC, Wang WH, Capdevila JH. Epoxyeicosatrienoic acids (EETs) regulate epithelial sodium channel activity by extracellular signal-regulated kinase 1/2 (ERK1/2)-mediated phosphorylation. J Biol Chem 288: 5223–5231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J 24: 2056–2065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 5: 953–962, 1990 [PubMed] [Google Scholar]
- 88.Sakai M, Zhang M, Homma T, Garrick B, Abraham JA, McKanna JA, Harris RC. Production of heparin binding epidermal growth factor-like growth factor in the early phase of regeneration after acute renal injury. Isolation and localization of bioactive molecules. J Clin Invest 99: 2128–2138, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salido EC, Barajas L, Lechago J, Laborde NP, Fisher DA. Immunocytochemical localization of epidermal growth factor in mouse kidney. J Histochem Cytochem 34: 1155–1160, 1986 [DOI] [PubMed] [Google Scholar]
- 90.Sanderson MP, Dempsey PJ, Dunbar AJ. Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors 24: 121–136, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110: 669–672, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Schlingmann KP, Weber S, Peters M, Niemann NL, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31: 166–170, 2002 [DOI] [PubMed] [Google Scholar]
- 93.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 12: 278–287, 2012 [DOI] [PubMed] [Google Scholar]
- 94.Shen JP, Cotton CU. Epidermal growth factor inhibits amiloride-sensitive sodium absorption in renal collecting duct cells. Am J Physiol Renal Physiol 284: F57–F64, 2003 [DOI] [PubMed] [Google Scholar]
- 95.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest 121: 3233–3243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal 17: 1183–1193, 2005 [DOI] [PubMed] [Google Scholar]
- 98.Smith JP, Pozzi A, Dhawan P, Singh AB, Harris RC. Soluble HB-EGF induces epithelial-to-mesenchymal transition in inner medullary collecting duct cells by upregulating Snail-2. Am J Physiol Renal Physiol 296: F957–F965, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res 315: 683–696, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Soundararajan R, Melters D, Shih IC, Wang J, Pearce D. Epithelial sodium channel regulated by differential composition of a signaling complex. Proc Natl Acad Sci USA 106: 7804–7809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem 285: 30363–30369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Staruschenko A. Regulation of transport in the connecting tubule and cortical collecting duct. Compr Physiol 2: 1541–1584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels (ENaC) in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem 272: 31730–31737, 1997 [DOI] [PubMed] [Google Scholar]
- 105.Swaminathan N, Sambhi MP. Induction of high affinity epidermal growth factor binding in the aorta of Dahl hypertensive rats fed with high salt diet. Hypertens Res 19: 65–68, 1996 [DOI] [PubMed] [Google Scholar]
- 106.Swaminathan N, Vincent M, Sassard J, Sambhi MP. Elevated epidermal growth factor receptor levels in hypertensive Lyon rat kidney and aorta. Clin Exp Pharmacol Physiol 23: 793–796, 1996 [DOI] [PubMed] [Google Scholar]
- 107.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol 302: F1351–F1361, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int 83: 804–810, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P, Friedlander G. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106: 225–234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thebault S, Alexander RT, Tiel Groenestege WM, Hoenderop JG, Bindels RJ. EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol 20: 78–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomasson M, Hedman H, Ljungberg B, Henriksson R. Gene expression pattern of the epidermal growth factor receptor family and LRIG1 in renal cell carcinoma. BMC Res Notes 5: 216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, Barnard JA, Yuspa HH, Coffey RJ, Magnuson T. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269: 230–234, 1995 [DOI] [PubMed] [Google Scholar]
- 113.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 94: 6965–6970, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 16: 5276–5287, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ulu N, Gurdal H, Landheer SW, Duin M, Guc MO, Buikema H, Henning RH. α1-Adrenoceptor-mediated contraction of rat aorta is partly mediated via transactivation of the epidermal growth factor receptor. Br J Pharmacol 161: 1301–1310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uttarwar L, Peng F, Wu D, Kumar S, Gao B, Ingram AJ, Krepinsky JC. HB-EGF release mediates glucose-induced activation of the epidermal growth factor receptor in mesangial cells. Am J Physiol Renal Physiol 300: F921–F931, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Vaughan TJ, James PS, Pascall JC, Brown KD. Molecular cloning and tissue distribution of pig transforming growth factor alpha. Biochem J 296: 837–842, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vaughan TJ, Pascall JC, James PS, Brown KD. Expression of epidermal growth factor and its mRNA in pig kidney, pancreas and other tissues. Biochem J 279: 315–318, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vehaskari VM, Hering-Smith KS, Moskowitz DW, Weiner ID, Hamm LL. Effect of epidermal growth factor on sodium transport in the cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F803–F809, 1989 [DOI] [PubMed] [Google Scholar]
- 120.Vehaskari VM, Herndon J, Hamm LL. Mechanism of sodium transport inhibition by epidermal growth factor in cortical collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 261: F896–F903, 1991 [DOI] [PubMed] [Google Scholar]
- 121.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003 [DOI] [PubMed] [Google Scholar]
- 122.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31: 171–174, 2002 [DOI] [PubMed] [Google Scholar]
- 123.Warden DH, Stokes JB. EGF and PGE2 inhibit rabbit CCD Na+ transport by different mechanisms: PGE2 inhibits Na+-K+ pump. Am J Physiol Renal Fluid Electrolyte Physiol 264: F670–F677, 1993 [DOI] [PubMed] [Google Scholar]
- 124.Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol 124: 719–727, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williams JM, Zhang J, North P, Lacy S, Yakes M, Dahly-Vernon A, Roman RJ. Evaluation of metalloprotease inhibitors on hypertension and diabetic nephropathy. Am J Physiol Renal Physiol 300: F983–F998, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilson PD. Polycystic kidney disease. N Engl J Med 350: 151–164, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Wilson PD. Apico-basal polarity in polycystic kidney disease epithelia. Biochim Biophys Acta 1812: 1239–1248, 2011 [DOI] [PubMed] [Google Scholar]
- 128.Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology 25: 85–101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ying WZ, Sanders PW. Enhanced expression of EGF receptor in a model of salt-sensitive hypertension. Am J Physiol Renal Physiol 289: F314–F321, 2005 [DOI] [PubMed] [Google Scholar]
- 130.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res 315: 602–610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeng F, Zhang MZ, Singh AB, Zent R, Harris RC. ErbB4 isoforms selectively regulate growth factor induced Madin-Darby canine kidney cell tubulogenesis. Mol Biol Cell 18: 4446–4456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheleznova NN, Yang C, Ryan RP, Halligan BD, Liang M, Greene AS, Cowley AW., Jr Mitochondrial proteomic analysis reveals deficiencies in oxygen utilization in medullary thick ascending limb of Henle in the Dahl salt-sensitive rat. Physiol Genomics 44: 829–842, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zheleznova NN, Wilson PD, Staruschenko A. Epidermal growth factor-mediated proliferation and sodium transport in normal and PKD epithelial cells. Biochim Biophys Acta 1812: 1301–1313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhong H, Bowen JP. Recent advances in small molecule inhibitors of VEGFR and EGFR signaling pathways. Curr Top Med Chem 11: 1571–1590, 2011 [DOI] [PubMed] [Google Scholar]