Abstract
Transforming growth factor-β1 (TGF-β1) is thought to drive fibrogenesis in numerous organ systems. However, we recently established that ectopic expression of TGF-β1 abrogates collagen accumulation via canonical SMAD signaling mechanisms in a shear-induced model of kidney fibrosis. We herein delineate the temporal control of endogenous TGF-β1 signaling that generates sustained synchronous fluctuations in TGF-β1 cascade activation in shear-stimulated proximal tubule epithelial cells (PTECs). During 8-h exposure to physiological shear stress (0.3 dyn/cm2), PTECs experience in situ oscillatory concentrations of active endogenous TGF-β1 that are ∼10-fold greater than those detected under higher stress regimes (2–4 dyn/cm2). The elevated levels of intrinsic TGF-β1 maturation observed under physiological conditions are accompanied by persistent downstream SMAD3 activation. Pathological shear stresses (2 dyn/cm2) first elicit temporal variations in phosphorylated SMAD3 with an apparent period of ∼6 h, whereas even higher stresses (4 dyn/cm2) abolish SMAD3 activation. These divergent patterns of SMAD3 activation are attributed to varying levels of Notch4-dependent phospho-SMAD3 degradation. Depletion of Notch4 in shear-stimulated PTECs eventually increases the levels of active TGF-β1 protein by approximately fivefold, recovers stable SMAD phosphorylation and ubiquitinated SMAD species, and attenuates collagen accumulation. Collectively, these data establish Notch4 as a critical mediator of shear-induced fibrosis and further reinforce the renoprotective effects of canonical TGF-β1 signaling.
Keywords: fluid shear, chronic kidney disease, tubulointerstitial fibrosis, frequency-dependent signaling
transforming growth factor-β1 (TGF-β1) is a potent growth factor with many diverse functions. TGF-β1 exerts these pleiotropic effects via a fairly complex signaling cascade, which exhibits multiple points of regulation. Newly synthesized TGF-β1 protein is secreted possessing a latency peptide that effectively blocks activity until properly acted upon by a multitude of molecules (44). Cleaved, active TGF-β1 then binds a type II receptor, which subsequently recruits one of many type I receptors responsible for activating downstream ligands. Canonical signaling involves phosphorylation and activation of the receptor-regulated SMAD transcription factors SMAD2 and/or SMAD3, but MAP kinases can also be stimulated. However, activation of the TGF-β1 cascade does not necessarily initiate a downstream transcriptional response as the levels of both total and phosphorylated SMAD proteins are also subject to tight regulation via ubiquitin-mediated proteosomal degradation. Indeed, a large number of different ubiquitin ligases, such as the SMURF proteins, WWP1 and NEDD4L, are capable of recognizing inactive and/or phosphorylated SMAD proteins and targeting them for destruction (28, 55).
In the kidney, signaling events downstream of TGF-β1 have been hypothesized to drive the development of fibrosis leading to end-stage renal disease and eventual organ failure, potentially by initiating an epithelial-to-mesenchymal transition (EMT) (20). However, we recently demonstrated that not only are EMT and fibrosis mutually exclusive events in sheared proximal tubular epithelial cells (PTECs), but also that ectopic overexpression of TGF-β1 in these cells abrogates EMT-independent matrix accumulation via SMAD-dependent mechanisms (19). Consistent with these observations, recent work by Fragiadaki and colleagues (16) identified dynamic dosage-dependent effects of TGF-β1, whereby low concentrations stimulate collagen synthesis but high concentrations suppress it. Furthermore, we demonstrated that TGF-β1-mediated EMT may be kinetically limited via oscillatory activation of the downstream ERK signaling cascade (5); thus, accumulating evidence suggests that dynamic context-dependent TGF-β1 signaling defines unique cellular responses.
Although it has been discovered that SMAD1, a TGF-β family transcription factor, can exhibit oscillations in response to serum (57), no reports to date have demonstrated sustained oscillations in other TGF-β1 family members. In contrast, the oscillations of the transcription factors NF-κB, p53, and Hes/Her, the most prevalent signaling systems with such dynamics, have been somewhat extensively studied and characterized (37, 42, 53). Additionally, periodic behavior is evident in many important biological processes: the natural circadian rhythms that govern organism behavior (60), the regimented proliferation of the cell cycle (30), and even the complex spatiotemporal expression patterns in systems of genes that drive embryonic development (14, 23). The simplest of these dynamic responses are driven by the presence of a negative feedback loop operating with a delay, although their exact structure can comprise many different network motifs and the resulting output can vary dramatically (18). Because of the inherent interconnectedness of many circuit elements, elucidating key regulatory components of the oscillatory machinery is often both theoretically and experimentally difficult. Here, we discover that a physiologically relevant mechanical stimulus in kidney metabolism, fluid shear stress, induces novel oscillatory variations in both transcriptional expression of TGF-β1 and downstream cascade activation, which are in turn responsible for the regulation of matrix synthesis. Fluid shear stress is an important physical stimulus in kidney homeostasis (25). The physiological magnitudes of shear stress in the proximal tubule range from 0.06 to 0.3 dyn/cm2 (8, 17). Following surgical renal mass reduction, flow in the remaining nephrons has been shown to increase approximately threefold (24). Furthermore, systemic hypertension (33) and diabetes (43) also increase hydrodynamic forces in the kidney. Consequently, understanding the basis for the intrinsic oscillatory TGF-β1 response could highlight complex mechanisms of signal integration in kidney cells and provide new insights into fundamental aspects of renal physiology.
To study the effects of fluid shear on PTECs, an immortalized human PTEC line, HK-2, was subjected to prescribed levels of either physiological (0.3 dyn/cm2) or pathological (2 or 4 dyn/cm2) shear stresses. Because renal damage is the result of chronic insult occurring over decades, we used very high pathological shear stresses (2 or 4 dyn/cm2) to examine fundamental aspects of renal cell function in an experimentally feasible time scale. In other words, we chose the standard approach employed by toxicologists to evaluate the potential toxicity of lifetime exposure of humans to a chemical substance; that is, the investigation of supraphysiological concentrations of the chemical for an experimentally feasible time window.
Prior work in our laboratory demonstrated that collagen accumulation in shear-activated PTECs increases with the magnitude of applied shear stress, further corroborating the critical role of hyperfiltration on the progression of renal fibrosis (19). Moreover, ectopic expression of TGF-β1 evinced distinctly anti-fibrotic effects in shear-stimulated cells (19). To more fully characterize the role of canonical TGF-β1 signaling in shear-induced fibrosis, we examined the time evolution of endogenous TGF-β1-mediated SMAD activation in the absence of an ectopic expression vector. Importantly, we demonstrate shear stress dynamically regulates the SMAD3 signaling axis. Moreover, Notch4, a cell surface receptor important in the well-characterized synchronous oscillatory signaling system of the segmentation clock (12, 20), represents a key regulatory module responsible for the temporal variations of SMAD3 activity observed in shear-stimulated PTECs with direct effects on downstream matrix accumulation. Our data present a novel and important cross-talk between the Notch and TGF-β1 pathways and present new insights regarding the role of these molecules in the progression of renal fibrosis.
MATERIALS AND METHODS
Reagents.
Notch4 siRNA and scramble oligonucleotides as well as antibodies specific for p-SMAD2/3, SMAD2/3, and Notch4 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The proteosomal inhibitor MG-132 and an antibody directed toward β-actin were obtained from Cell Signaling Technology (Danvers, MA). All other reagents for SDS-PAGE experiments were purchased from Bio-Rad Laboratories (Hercules, CA). Mouse monoclonal antibody (mAb) specific to native collagen I was purchased from Abcam (Cambridge, MA). The anti-mouse IgG (H+L) antibody (Alexa Fluor 488 conjugate) as well as Lipofectamine 2000 were acquired through Invitrogen (Carlsbad, CA). The Notch4 intracellular domain (NICD) expression construct was a generous gift from Dr. Mary J. Hendrix at the Children's Hospital of Chicago Research Center (Chicago, IL) (21). Neutralizing α-TGF-β1 antibody was acquired from Genetex (Irvine, CA).
Cell culture and shear stress exposure.
The immortalized human proximal tubular epithelial cells HK-2 (ATCC CRL-2190, Manassas, VA) were grown (37°C in 5% CO2) on untreated glass slides (7.5 × 2.5 cm) in keratinocyte serum-free media (Invitrogen) supplemented with epidermal growth factor (5 ng/ml) and bovine pituitary extract (50 μg/ml). Before shear exposure, cells were incubated for 18 h in media free of exogenous growth factors. Cells were then subjected to a shear stress level of 0.3, 2, or 4 dyn/cm2 for prescribed periods of time in medium lacking growth factor supplements using a streamer flow device (Flexcell International, Hillsborough, NC) (22, 48, 51, 61). In select experiments, the proteosomal inhibitor MG-132 was added to the medium at the indicated concentrations just before the onset of shear exposure.
Transient transfection and reporter assay.
All transfections were carried out employing Lipofectamine 2000 according to the manufacturer's instructions. In RNA interference assays, HK-2 cells were transfected with 100 nM siRNA specific to Notch4 or a scramble control oligonucleotide sequence. For the SMAD reporter experiments, cells were transfected with 5 μg/slide of reporter construct. Transfected cells were allowed to recover for ≥12 h in growth medium and then incubated overnight in shear medium before their exposure to shear or static conditions.
qRT-PCR.
qRT-PCR assays were performed on the iCycler iQ detection system (Bio-Rad) using total RNA (Qiagen RNEasy kit; Qiagen, Valencia, CA), the iScript one-step RT-PCR kit with SYBR green (Bio-Rad), and primers (49, 62). The GenBank accession numbers and forward (F) and reverse (R) primers are as follows: GAPDH (NM_002046.3), F-GGCCTCCAAGGAGTAAGAC, R-AGGGGTCTACATGGCAACT; TGF-β1 (NM_000660.4), F-CAACTCCGGTGACATCAAAA, R-ACGTGGAGCTGTACCAGAAA. The specificity of primers was determined by dissociation curve and 1% agarose electrophoresis (data not shown). GAPDH was used as an internal control. Reaction mixtures were incubated at 50°C for 15 min, followed by 95°C for 5 min, and then 40 PCR cycles were performed with the following temperature profile: 95°C, 15 s; 58°C, 30 s; 68°C, 1 min; and 77°C, 20 s (48, 51, 61). Data were collected at the 77°C, 20-s step to remove possible fluorescent contribution from dimer primers. Gene expression values were normalized to GAPDH.
Western blot analysis.
HK-2 cells from sheared and matched static control specimens were lysed in RIPA buffer (25 mM Tris·HCl, pH 7.6; 150 mM NaCl; 1% Nonidet P-40; 1% sodium deoxycholate; and 0.1% SDS) containing a cocktail of proteinase inhibitors (Pierce Chemical, Rockford, IL) (47, 50). The protein content of the cell lysates was determined using bicinchoninic acid (BCA) protein assay reagent (Pierce Chemical). Total cell lysates (10 μg) were subjected to SDS-PAGE, transferred to a PVDF membrane, and probed with a panel of specific antibodies. β-Actin was used as a loading control. All Western hybridizations were performed at least in triplicate with independent cell samples.
Immunoprecipitation.
Shear-activated PTECs were washed twice with ice-cold PBS and homogenized in RIPA buffer with complete protease inhibitor mixture for 30 min on ice (9). After centrifugation for 10 min at 16,000 g at 4°C, the supernatant was collected. Protein concentrations were determined by BCA. A total of 300 μg protein was used for each immunoprecipitation experiment. Antibody (2 μg) was added to 0.4-ml protein extracts. The mixtures were rotated at 4°C overnight. Then, 25 μl of protein G agarose beads (Invitrogen) were added to the mixtures, and they were rotated for another 12 h at 4°C. The beads were harvested by centrifugation and washed 5× with RIPA buffer, and 5× with PBS. Finally, samples were boiled for 5 min in PBS to elute bound protein. After centrifugation, the eluants were subjected to immunoblot analysis.
Immunofluorescence staining.
Following the indicated static or shear treatments, HK-2 cells were fixed with 3.7% formaldehyde for 10 min, permeabilized with 0.1% Triton X-100 for 10 min at 4°C, washed 2× with PBS, and incubated in buffer containing 5% BSA/PBS for 60 min at room temperature (RT) (1). Cells were then incubated with a mouse mAb to collagen I overnight at 4°C, washed 2× with PBS, and incubated in buffer containing Alexa Fluor 488-labeled goat anti-mouse IgG for 60 min at RT. Finally, cells were washed 2× with PBS and photographed using a fluorescent microscope (Nikon Eclipse Ti).
Enzyme-linked immunosorbent assay.
Quantification of secreted, active TGF-β1 was performed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (eBiosciences, San Diego, CA) according to the manufacturer's instructions. Following shear for the indicated periods of time, confluent cell monolayers were carefully scraped into PBS and centrifuged before analysis of supernatant for levels of active TGF-β1. Data were then normalized to the total cell lysate protein concentration of each sample as measured via a BCA assay and corrected for background against static control samples. The experiments were repeated three times in triplicates, and data are shown as means ± SE.
Statistics.
Data represent means ± SE of ≥3 independent experiments. Statistical significance of differences between means was determined by Student's t-test.
RESULTS
Fluid shear stress dynamically regulates TGF-β1-mediated SMAD3 activation.
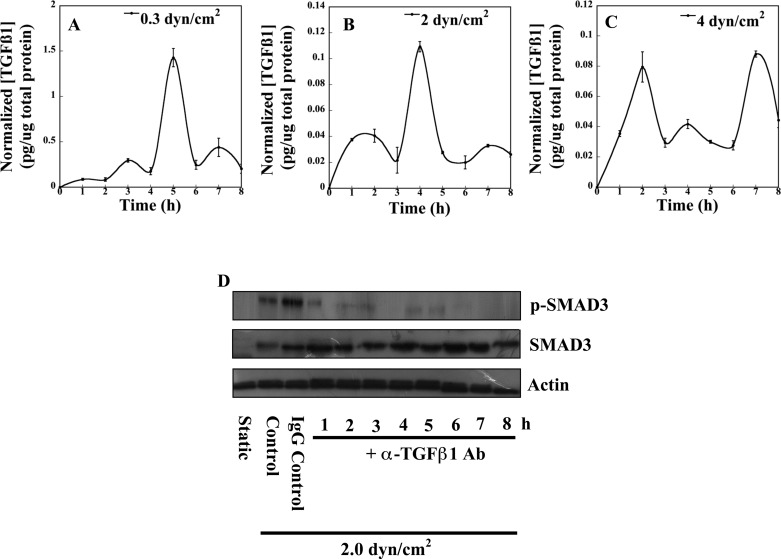
We recently reported that pathological levels of fluid shear induce renal fibrosis and that ectopic TGF-β1 expression attenuated fibrogenesis in sheared PTECs via SMAD-dependent mechanisms (19). To better understand how fluid shear stress modulates endogenous TGF-β1 signaling and thus potentiates collagen accumulation in PTECs, we quantified TGF-β1 gene expression and assessed activation of the canonical TGF-β1 transcription factor SMAD3 in shear-activated PTECs. Interestingly, we discovered that temporal expression of TGF-β1 mRNA displayed distinct oscillatory variations over the entire range of shear stresses examined herein (Fig. 1). In particular, application of a physiologically relevant shear stress level (0.3 dyn/cm2) rapidly induced low-amplitude bursts of TGF-β1 mRNA expression at 1 and 3 h that subsequently dissipated (Fig. 1A). In contrast, PTECs subjected to a pathological shear stress level of 2 dyn/cm2 exhibited relatively more pronounced TGF-β1 mRNA expression with bursts of transcription separated by ∼4 h (Fig. 1B). At higher shear stresses (4 dyn/cm2), dynamic repression of TGF-β1 transcript expression occurred, although the overall temporal characteristics of the oscillatory response were qualitatively similar to those observed in specimens subjected to 2 dyn/cm2 (Fig. 1C). Immunoblotting assays, using antibodies that recognize both SMAD2 and SMAD3 species, also revealed unique patterns of downstream SMAD3 protein phosphorylation that varied inversely to total protein levels of NICD, the cleaved portion of the activated Notch cell surface receptor responsible for transcriptional induction of Notch-responsive genes (15). Specifically, a polyclonal antibody directed to the COOH terminus region of Notch4 detected a low molecular weight band (∼40 kDa) indicative of cleaved Notch4 intracellular protein resulting from successful receptor-ligand interactions. Additionally, increasing shear stress decreased the persistence of TGF-β1-mediated SMAD3 activation (Fig. 1, D–F). For example, in PTECs subjected to 0.3 dyn/cm2, phosphorylation of SMAD3 protein remained relatively persistent over the time period examined, disappearing only ∼7 h when a marked increase in the protein levels of NICD occurred (Fig. 1D). To the contrary, SMAD3 phosphoprotein displayed more transient oscillatory dynamics in specimens subjected to 2 dyn/cm2 (Fig. 1E). Of note, protein levels of the intracellular domain of Notch4 varied anti-phase to these fluctuations in SMAD3 activation, which reinforced its role as a potential negative regulator of the TGF-β1 cascade (Fig. 1E). Finally, a shear stress level of 4 dyn/cm2 resulted in negligible amounts of both phosphorylated and total SMAD3 protein, while NICD reached dramatic and sustained levels under these conditions (Fig. 1F). In light of our recent observations establishing the anti-fibrotic effects of SMAD-dependent signaling (19), we hypothesized that Notch4 acts as a critical mediator of collagen accumulation in shear-activated PTECs by interfering with TGF-β1-mediated SMAD activation.
Fig. 1.
Fluid shear stress dynamically regulates both transforming growth factor (TGF)-β1 gene expression and downstream SMAD3 activation via Notch4 involvement. Confluent monolayers of proximal tubule epithelial cells (PTECs) were subjected to either static or fluid shear (0.3, 2, or 4 dyn/cm2) conditions for the prescribed periods of time. TGF-β1 gene expression in shear-activated PTECs was quantified via qRT-PCR over the time period examined relative to static controls (A–C). Data represent means ± SE of n = 3 independent experiments. P < 0.05. The activation of both canonical SMAD3 signaling and Notch4 was assessed via immunoblotting (D–F). SC, static control. Western blots are representative of 3 independent experiments, all revealing similar results.
Due to the complex nature of the TGF-β1 signaling pathway, Notch4-dependent regulation may occur over multiple levels of TGF-β1 signaling. Shear-stimulated cleavage of Notch4 and liberation of the intracellular domain may decrease extracellular activation of latent TGF-β1 and/or directly modulate the stability of downstream intracellular signaling components. We first quantified the levels of active TGF-β1 in shear-stimulated PTECs via an ELISA directed specifically toward mature TGF-β1 protein. Overall, a marked decrease in the levels of accumulating mature TGF-β1 was noted with increasing levels of fluid shear (Fig. 2, A, B, C). These data suggest that, to some degree, the observed variations in canonical signaling may be due to tight regulatory control of TGF-β1 latency complex processing. Interestingly, although PTECs exposed to high shear stresses (4 dyn/cm2) experienced repression of TGF-β1 gene expression and displayed less overall SMAD3 protein (Fig. 1, C and F), active TGF-β1 was still detected in the cell supernatant albeit at low levels (Fig. 2C). This apparent paradox suggests that PTECs under high shear exposure may access preexisting pools of latent TGF-β1 in the extracellular milieu. Furthermore, PTECs subjected to a shear stress level of 4 dyn/cm2 displayed more significant bursts of active TGF-β1 (Fig. 2C) compared with specimens exposed to 2 dyn/cm2 (Fig. 2B), although overall SMAD3 activation declined (compare Fig. 1, E and F). To confirm that the observed SMAD3 phosphorylation was solely a result of TGF-β1-mediated signaling events, confluent PTECs were sheared in the presence or absence of a neutralizing TGF-β1 antibody and assessed for phosphorylated SMAD3 protein. Blocking binding of TGF-β1 to its receptor successfully abolished SMAD3 protein phosphorylation (Fig. 2D). These observations link expression of active TGF-β1 directly to downstream SMAD3 activation. Collectively, our data clearly demonstrate that PTECs exert precise temporal control over the activation of endogenous TGF-β1.
Fig. 2.
Role of endogenous TGF-β1 in SMAD3 activation in shear-activated PTECs. Confluent monolayers of PTECs were subjected to shear conditions (0.3, 2, or 4 dyn/cm2) for the indicated periods of time and subsequently harvested in PBS. Cell supernatants were then analyzed via ELISA utilizing a mAb directed specifically toward active TGF-β1 protein. Secreted TGF-β1 concentrations were then adjusted for background signal and normalized to the total cellular protein of each sample as measured via a bicinchoninic acid assay (A–C). In separate experiments, PTECs were subjected to a shear stress level of 2 dyn/cm2 in the presence of a neutralizing antibody specific to TGF-β1. Immunoblotting using specific antibodies for total and phosphorylated SMAD3 was performed to examine changes in downstream SMAD3 activation (D). β-Actin served as a loading control. Western blots are representative of 3 independent experiments, all revealing similar results.
Shear-mediated fibrosis requires Notch4-dependent degradation of phosphorylated SMAD3.
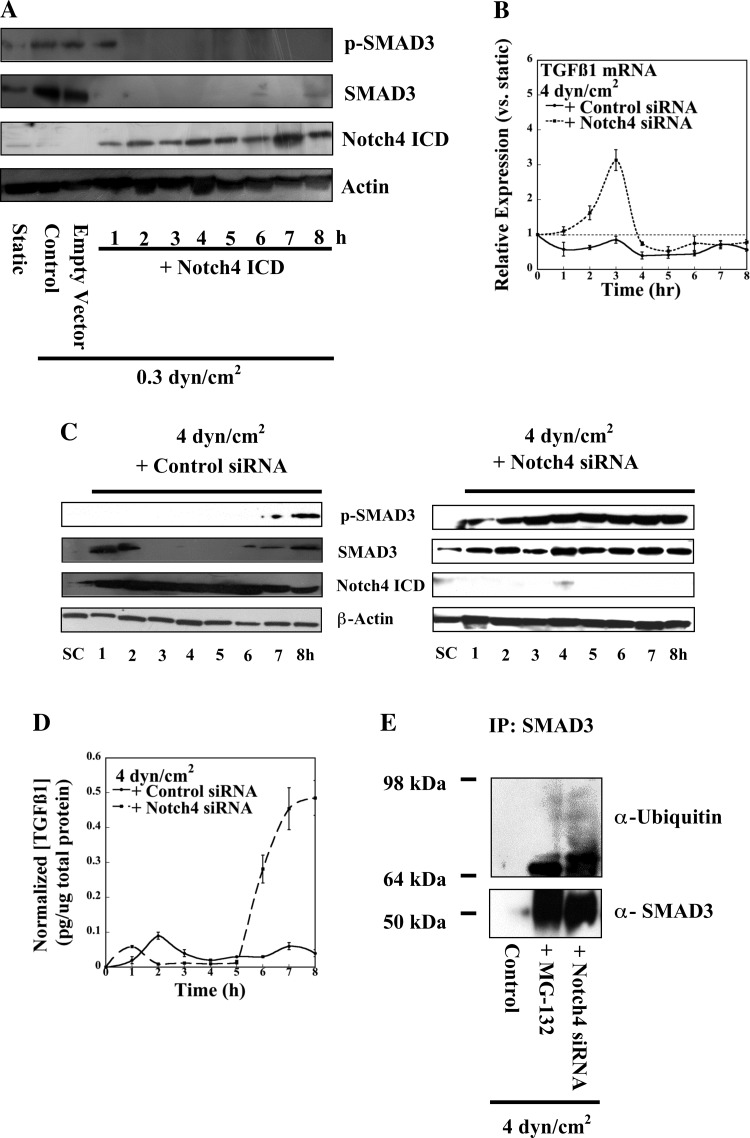
To establish whether ligand-mediated Notch4 cleavage and successful release of the intracellular domain regulate SMAD3 activation, PTECs transfected with a NICD expression construct (21) were subjected to a shear stress level of 0.3 dyn/cm2. This molecular intervention resulted in a dramatic decline in both total and phosphorylated SMAD3 protein levels (Fig. 3A), which is consistent with Notch-mediated suppression. We next wished to elucidate the mechanism by which Notch4 regulates the TGF-β1 cascade. To this end, confluent PTECs were transfected with either Notch4 siRNA or control oligonucleotides and subjected to static or shear stress conditions (4 dyn/cm2). Not only did Notch4 knockdown remove transcriptional repression of TGF-β1 (Fig. 3B), but it also recovered persistent SMAD3 phosphorylation in PTECs sheared at 4 dyn/cm2 (Fig. 3C). This response closely resembles our observations at the physiologically relevant shear stress regime where cleaved NICD was relatively absent (Fig. 1D). Importantly, even complete depletion of the NICD did not alter the oscillatory expression profile of the TGF-β1 transcript (Fig. 3B). These data point to the potential existence of additional oscillatory pathways controlling TGF-β1 signaling. Since pulsatile expression of TGF-β1 mRNA resulted in a concomitant plateau of SMAD phosphorylation (Fig. 3, B and C), we examined whether NICD directly increased the levels of active TGF-β1 protein or whether Notch4 perhaps controlled downstream SMAD degradation. To this end, we examined the levels of circulating active TGF-β1 in Notch4 knockdown samples (Fig. 3D). Although early during shear exposure depletion of NICD had little to no effect, active, mature TGF-β1 levels surged dramatically ∼6 h (Fig. 3D). We also performed an immunoprecipitation assay using an anti-SMAD3 antibody along with cell lysates from PTECs subjected to high stress (4 dyn/cm2) in the presence of the proteosome inhibitor MG-132, vehicle control transfection, or Notch4 siRNA transfection. Both MG-132 and Notch4 depletion successfully recovered SMAD3 and ubiquitinated SMAD3 species in shear-activated PTECs (Fig. 3E). Additional immunoprecipitations performed employing an IgG control clearly revealed that the observed immunopurified species did not reflect nonspecific binding contaminants (data not shown). These findings establish that liberation of the NICD plays an important role in regulating the levels of active TGF-β1 protein and degradation of downstream SMAD3 protein. Furthermore, given the antagonism of SMAD-dependent signaling to extracellular collagen deposition (19), these data suggest Notch4 may be a critical mediator of shear-induced fibrosis.
Fig. 3.
Notch4 regulates TGF-β1 and downstream SMAD3 expression. PTECs, transfected with either Notch4 intracellular domain construct or the appropriate empty vector, were exposed to low shear stress (0.3 dyn/cm2) for the indicated time points. Levels of Notch4 intracellular domain as well as total and phosphorylated SMAD3 species were assessed via SDS-PAGE (A). In separate experiments, PTECs were transfected with either control or Notch4-specific siRNA oligonucelotides before shear exposure (4 dyn/cm2) and assessed for TGF-β1 transcript levels via qRT-PCR (B) or for the activation of canonical SMAD components and Notch4 (C). Changes in the levels of endogenous TGF-β1 activation in transfected PTECs were also assessed via ELISA (D). In select experiments, PTECs were subjected to shear stress (4 dyn/cm2) either alone or in the presence of the proteosomal inhibitor MG-132 or Notch4 siRNA oligonucleotides. Cell lysates were immunoprecipitated with a SMAD3 antibody and analyzed by SDS-PAGE using antibodies specific for SMAD3 and ubiquitin (E). Western blots are representative of 3 independent experiments, all revealing similar results. Data in B and D represent means ± SE of n = 3 independent experiments. P < 0.05.
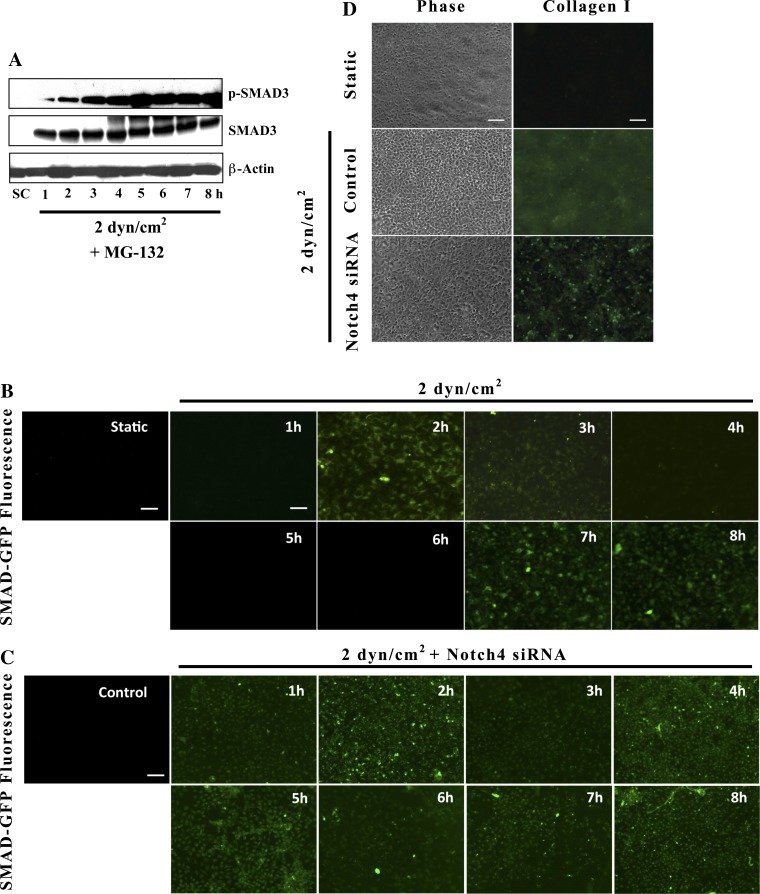
We next wanted to examine whether these results extend to the shear stress level of 2 dyn/cm2, where SMAD ubiquitination was less dramatically apparent. In particular, we wished to determine whether targeted degradation of phosphorylated SMAD3 could explain the temporal variations of SMAD3 activation observed in PTECs subjected to this stress level. Indeed, in the presence of the proteosomal inhibitor MG-132, shear-activated PTECs (2 dyn/cm2) exhibited persistent SMAD3 activation that accumulated with time (Fig. 4A). Despite sporadic degradation of the phosphorylated SMAD3 species in PTECs exposed to 2 dyn/cm2, total SMAD3 remained relatively stable. This may reflect specific targeting or preference of degradation for the phosphorylated form as previously described in the literature (12, 38). Total SMAD protein may disappear at higher stresses, then, due to an overall higher ratio of the activated SMAD species to unphosphorylated form. Together, these findings identify ubiquitination as a key regulatory mechanism contributing to the dynamic responses of SMAD3 activity in shear-activated PTECs.
Fig. 4.
Notch4 depletion results in uniform SMAD transcriptional activity and attenuates collagen deposition in shear-activated PTECs. Confluent PTECs were subjected to shear stress (2 dyn/cm2) in the presence of the proteosomal inhibitor MG-132. Activation of canonical SMAD3 components was then assessed via SDS-PAGE (A). In separate experiments, PTECs were transfected with a GFP-SMAD2/3 transcriptional reporter construct alone (B) or simultaneously with scramble control or Notch4 siRNA (C) before exposure to fluid stress (2 dyn/cm2) for the prescribed periods of time. SMAD3 transcriptional activity was sequentially assessed via fluorescence microscopy. In other experiments, PTECs, transfected with either Notch4 siRNA or control oligonucleotides, were exposed to either static or shear stress conditions (2 dyn/cm2) for 72 h, and subsequently examined for type I collagen deposition by immunofluorescence using a collagen I-specific mAb (D). Scale bars are 100 μm.
Following SMAD3 phosphorylation by TGF-β1 receptor complexes, the transcription factor partners with SMAD4 and translocates to the nucleus where numerous proteins and cofactors are capable of binding SMAD molecules and regulating downstream transcriptional activation (40, 56). To assess whether the variations in SMAD3 phosphorylation detected via Western blotting correlate with direct differences in SMAD transcriptional activity, we transfected confluent monolayers of PTECs with a green fluorescent protein (GFP) reporter construct under the control of SMAD2/3 transcriptional elements. Transfected cells were subsequently subjected to a shear stress level of 2 dyn/cm2 for prescribed periods of time, and GFP expression levels were evaluated via fluorescence microscopy (Fig. 4B). Our results confirmed that SMAD transcriptional activity in shear-stimulated PTECs directly corresponded to increased SMAD3 phosphorylation as detected via Western blotting (Fig. 1E). More importantly, SMAD transcriptional activity in each individual cell fluctuated in concert with the surrounding cells. For such synchrony to occur, the cells themselves must be coupled either directly, as is the case in voltage-controlled synchronous Ca2+ oscillations (26, 54), or indirectly entrained via a “pacemaker” pathway as with the segmentation clock in vertebrates (14, 23). Thus, these data support the involvement of multiple pathways, such as Notch, in the complex temporal regulation of TGF-β1 signaling in sheared PTECs.
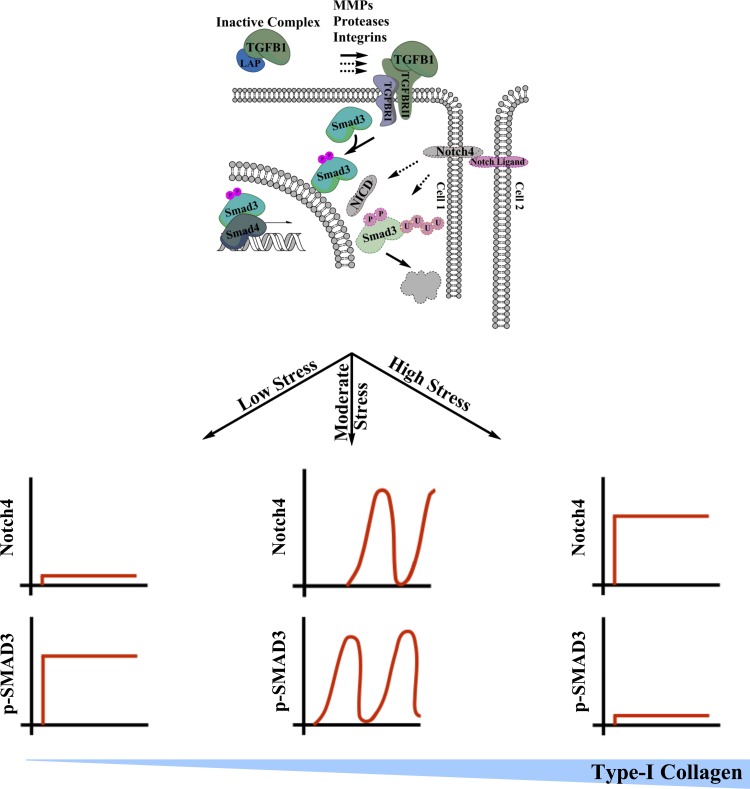
To exclude the possibility that Notch4 knockdown results in asynchronous cellular fluctuations that cumulatively hide a persistent oscillatory signal in a Western blot, PTECs were cotransfected with Notch4 siRNA and a GFP SMAD2/3 transcriptional reporter before exposure to 2 dyn/cm2. Our results indicate that Notch4 depletion resulted in persistent SMAD transcriptional activity within the confluent PTEC monolayer and no loss of synchrony was observed (Fig. 4C). Thus, knockdown of Notch4 and inhibition of the intracellular domain fundamentally alter the cyclic dynamics of TGF-β1-mediated SMAD signaling. Since our previous work (19) associated canonical TGF-β1 signaling with an overall anti-fibrotic response, the consequences of Notch4 knockdown on type I collagen deposition were also examined. While sheared PTECs transfected with scramble control displayed a diffuse fluorescent staining pattern consistent with extracellular collagen accumulation, shear-activated PTECs transfected with Notch4 siRNA exhibited primarily intracellular staining with clear demarcation of individual cells as assessed via immunofluorescence (Fig. 4D). The effects of Notch4 signaling on collagen expression were also examined via qRT-PCR analysis of mRNA from PTECs transfected with a NICD construct and subjected to a shear stress level of 2 dyn/cm2. Cumulatively, these data conclusively identify Notch4 as a key regulator of TGF-β1 signaling and a potential therapeutic target in renal fibrosis (Fig. 5).
Fig. 5.
Representation of our model of shear-induced kidney fibrosis whereby dynamic inhibition of canonical TGF-β1 signaling via Notch4 results in a fibrogenic response. At physiological shear stresses (0.3 dyn/cm2), elevated activation of secreted TGF-β1 coincides with negligible Notch4 activation resulting in stable SMAD3 phosphorylation and no discernible collagen deposition. In the pathological shear regime (2 dyn/cm2), overall TGF-β1 activation decreases and periodic Notch4 activation results in cyclic degradation of phosphorylated SMAD3. This oscillatory activation profile fails to prevent collagen deposition in shear-activated PTECs. Moreover, higher pathological shear stresses (4 dyn/cm2) result in persistent activation of Notch4, which in turn represses TGF-β1 gene expression and SMAD3 protein levels. Negligible activation of the canonical TGF-β1 signaling cascade results in maximal collagen accumulation. This model not only establishes a mechanism of shear-mediated fibrosis, but it also identifies novel oscillatory signaling dynamics in the TGF-β1 cascade.
DISCUSSION
Our findings clearly establish that exposure to pathological shear stresses results in diminished accumulation of active TGF-β1 in PTECs and, more importantly, inhibits canonical TGF-β1-mediated signaling via Notch4-dependent degradation of phosphorylated SMAD3 protein. Furthermore, we identify oscillatory variations in both the levels of TGF-β1 transcript and the availability of mature signaling factor at least partly due to Notch4 regulation. Although depletion of NICD results in uniform SMAD activation in shear-activated PTECs, it does not alter the fundamental pulsatile pattern of TGF-β1 gene expression, suggesting that other oscillating pathways may also regulate TGF-β1 signaling. Given the numerous reports that establish that TGF-β1 is capable of inducing its own transcription (58, 59), it is possible that recovery of TGF-β1 expression in Notch4 knockdown samples is partly due to recovery of active SMAD3 protein. The potential conversion of a plateau of SMAD phosphorylation into a spurt of TGF-β1 gene transcription could be accomplished through the additional temporal regulation of promoter binding via the partnering of phosphorylated SMAD3 with any one of a number of cofactors such as the AP-1, Ets, forkhead, and homeobox families (32). Similarly, the role of Notch4 in regulating the levels of active TGF-β1 secreted by shear-stimulated PTECs could be mediated via multiple different mechanisms. Integrins (43) and proteases, such as plasmin, thrombospondin, or matrix metalloproteinases, are each capable of liberating active TGF-β1 from the latency peptide (29), and thus they represent potential targets of Notch4 action. Additionally, a host of different molecules have also been shown to target SMAD3 for degradation that could lie downstream of ligand-mediated Notch4 cleavage of the intracellular domain (28, 55). In light of our recent observations showing that TGF-β1 exerts anti-fibrotic effects (19), our new data present striking evidence suggesting that dynamic temporal regulation of the TGF-β1-Notch4 signaling axis is a critical component of shear-induced fibrosis.
Although many other studies have shown a fibrogenic role for TGF-β1 (27, 35), we and others challenge the simplicity of this finding. For example, recent work has suggested overexpression of latent TGF-β1 exerts renoprotective effects, although the authors link these consequences to overall decreases in downstream TGF-β1 signaling via a negative feedback (52). A phase I/II trial of anti-TGF-β1 therapy in systemic sclerosis failed to note any efficacy in halting the development of fibrotic lesions (13). Perhaps TGF-β1 itself is a pleiotropic molecule capable of exerting both anti-fibrotic and profibrotic effects with the overall phenotypic consequence determined by the temporal characteristics of the stimulus. Along these lines, recent work has discovered that low doses of TGF-β1 stimulate expression of collagen while high doses of TGF-β1 trigger inhibition of collagen I via the negative transcriptional regulator CUX1 (16). These observations are in concert with our current data, wherein physiological shear stress is beneficial by inducing larger pulses of active TGF-β1 and persistent downstream SMAD3 activation, whereas pathological shear stresses result in smaller and less persistent TGF-β1 activity. Our ELISA data show distinct temporal profiles of low concentrations of active TGF-β1 at both 2.0 and 4.0 dyn/cm2, thereby suggesting frequency modulation as a potential regulatory mechanism. Furthermore, others have demonstrated this phenomenon whereby differences in pulse frequency of TNF-α result in observable differences in transcriptional activation of different classes of downstream genes (5). Finally, it is also possible that the involvement of an as of yet unidentified pathway acts in concert with TGF-β1 and directly modulates the signaling cascade. The end results of TGF-β1 signal transduction, or even the predominant signaling mechanism, whether it be via SMAD3 or some specific kinase cascade, could be entirely context dependent. There is already extensive evidence of the context-dependent, contradictory roles TGF-β1 plays in cellular physiology in the literature (2, 10).
Since oscillatory fluctuations of TGF-β1-mediated SMAD3 activity in shear-activated PTECs are dependent on variations in the levels of cleaved NICD, it is possible that our observations reflect an incarnation of the segmentation clock. In developing vertebrate embryos, oscillations in Notch signaling arise via feedback from the induction of inhibitory genes such as lunatic fringe (11). Coupled with similar oscillations in the WNT and FGF pathways, a synchronous periodic expression system develops whose spatiotemporal variations allow for precise tissue organization (14, 23). Because the TGF-β1 family of growth factors serves important roles during development (3, 36), it is not surprising for there to be significant cross-talk between these pathways. Indeed, Notch1 has been implicated in both tubulointerstitial fibrosis and EMT (4, 39), while both Notch2 and Notch3 have been shown to antagonize the effects of TGF-β1 (31, 41). The activated intracellular domain of Notch4, in particular, has been shown to bind to the receptor-regulated SMAD3 and block TGF-β1 transcriptional activity (46). Our results provide a new mode of interaction between these two important pathways and further highlight the importance of temporal control exerted by the Notch pathway. Although there is less evidence for its involvement, the circadian clock, the rhythmic expression of genes that control our day-night cycles, has also been shown to control periodic expression of a number of different genes in the kidney (45). Disruption of the natural period of these oscillators even disrupts blood pressure profiles and results in organ damage (34). At the very least, these studies further reinforce the importance of cyclic signaling events to kidney homeostasis.
Taken together, our data indicate that the sole focus on TGF-β1 as a therapeutic target in renal fibrosis may be inadequate. It is possible that some other growth factor or pathway occupies a more fundamental role in driving shear-induced fibrosis. The family of platelet-derived growth factor (PDGF) proteins is typically associated with stimulating growth of myofibroblast-like cells (6), and inhibition of the PDGF-D isoform, in particular, is capable of preventing tubulointerstitial fibrosis in a rat model (7). Finally, we identify synchronous oscillatory dynamics in the TGF-β1 signaling cascade. This discovery suggests that TGF-β1 signaling is subject to complex modes of behavior with potentially divergent downstream consequences. To this end, only a truly systems level analysis, as opposed to an approach focusing on one individual molecule operating on narrow time scales, could fully understand relevant signaling phenomena.
GRANTS
This work was supported by grants from the National Cancer Institute (U54-CA143868) and Kleberg Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.M.G. and K.K. conception and design of research; B.M.G. performed experiments; B.M.G. analyzed data; B.M.G. and K.K. interpreted results of experiments; B.M.G. prepared figures; B.M.G. and K.K. drafted manuscript; B.M.G. and K.K. edited and revised manuscript; B.M.G. and K.K. approved final version of manuscript.
REFERENCES
- 1. Abulencia JP, Gaspard R, Healy ZR, Gaarde WA, Quackenbush J, Konstantopoulos K. Shear-induced cyclooxygenase-2 via a JNK2/c-Jun-dependent pathway regulates prostaglandin receptor expression in chondrocytic cells. J Biol Chem 278: 28388–28394, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, Parenti AR, Rosato A, Bicciato S, Balmain A, Piccolo S. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 137: 87–98, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Andersson O, Reissmann E, Ibáñez CF. Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep 7: 831–837, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aoyagi-Ikeda K, Maeno T, Matsui H, Ueno M, Hara K, Aoki Y, Aoki F, Shimizu T, Doi H, Kawai-Kowase K, Iso T, Suga T, Arai M, Kurabayashi M. Notch induces myofibroblast differentiation of alveolar epithelial cells via TGF-β/Smad3 pathway. Am J Respir Cell Mol Biol 45: 136–144, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, Ryan S, Spiller DG, Unitt JF, Broomhead DS, Kell DB, Rand DA, Sée V, White MRH. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science 324: 242–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15: 255–273, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Boor P, Konieczny A, Villa L, Kunter U, van Roeyen CRC, LaRochelle WJ, Smithson G, Arrol S, Ostendorf T, Floege J. PDGF-D inhibition by CR002 ameliorates tubulointerstitial fibrosis following experimental glomerulonephritis. Nephrol Dial Transplant 22: 1323–1331, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol 279: F270–F274, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chen SH, Dallas MR, Balzer EM, Konstantopoulos K. Mucin 16 is a functional selectin ligand on pancreatic cancer cells. FASEB J 26: 1349–1359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung SW, Cooper CR, Farach-Carson MC, Ogunnaike BA. A control engineering approach to understanding the TGF-β paradox in cancer. J R Soc Interface 9: 1389–1397, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dale JK, Maroto M, Dequeant ML, Malapert P, McGrew M, Pourquie O. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature 421: 275–278, 2003 [DOI] [PubMed] [Google Scholar]
- 12. De Boeck M, ten Dijke P. Key role for ubiquitin protein modification in TGFβ signal transduction. Ups J Med Sci 117: 153–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, Silliman N, Streisand J, Powell J, Akesson A, Coppock J, Hoogen Fvd Herrick A, Mayes MD, Veale D, Haas J, Ledbetter S, Korn JH, Black CM, Seibold JR, Group CS, Consortium SCT. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum 56: 323–333, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Dequéant ML, Glynn E, Gaudenz K, Wahl M, Chen J, Mushegian A, Pourquié O. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science 314: 1595–1598, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Fouillade C, Monet-Leprêtre M, Baron-Menguy C, Joutel A. Notch signaling in smooth muscle cells during development and disease. Cardiovasc Res 95: 138–146, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Fragiadaki M, Ikeda T, Witherden A, Mason RM, Abraham D, Bou-Gharios G. High doses of TGF-β potently suppress type I collagen via the transcription factor CUX1. Mol Biol Cell 22: 1836–1844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedrich C, Endlich N, Kriz W, Endlich K. Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol 291: F856–F865, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Goh KI, Kahng B, Cho KH. Sustained oscillations in extended genetic oscillatory systems. Biophys J 94: 4270–4276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grabias BM, Konstantopoulous K. Epithelial-mesenchymal transition and fibrosis are mutually exclusive reponses in shear-activated proximal tubular epithelial cells. FASEB J 26: 4131–4141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ. An autocrine TGF-β/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell 22: 1686–1698, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hardy KM, Kirschmann DA, Seftor EA, Margaryan NV, Postovit LM, Strizzi L, Hendrix MJC. Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer Res 70: 10340–10350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Healy ZR, Lee NH, Gao X, Goldring MB, Talalay P, Kensler TW, Konstantopoulos K. Divergent responses of chondrocytes and endothelial cells to shear stress: cross-talk among COX-2, the phase 2 response, and apoptosis. Proc Natl Acad Sci USA 102: 14010–14015, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horikawa K, Ishimatsu K, Yoshimoto E, Kondo S, Takeda H. Noise-resistant and synchronized oscillation of the segmentation clock. Nature 441: 719–723, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol Renal Fluid Electrolyte Physiol 241: F85–F93, 1981 [DOI] [PubMed] [Google Scholar]
- 25. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol Renal Fluid Electrolyte Physiol 241: F85–F93, 1981 [DOI] [PubMed] [Google Scholar]
- 26. Imtiaz MS, Katnik CP, Smith DW, van Helden DF. Role of voltage-dependent modulation of store Ca2+ release in synchronization of Ca2+ oscillations. Biophys J 90: 1–23, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inazaki K, Kanamaru Y, Kojima Y, Sueyoshi N, Okumura K, Kaneko K, Yamashiro Y, Ogawa H, Nakao A. Smad3 deficiency attenuates renal fibrosis, inflammation, and apoptosis after unilateral ureteral obstruction. Kidney Int 66: 597–604, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Inoue Y, Imamura T. Regulation of TGF-beta family signaling by E3 ubiquitin ligases. Cancer Sci 99: 2107–2112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol 40: 1068–1078, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Kang Q, Pomerening JR. Punctuated cyclin synthesis drives early embryonic cell cycle oscillations. Mol Biol Cell 2: 284–296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kennard S, Liu H, Lilly B. Transforming growth factor-beta (TGF- 1) down-regulates Notch3 in fibroblasts to promote smooth muscle gene expression. J Biol Chem 283: 1324–1333, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Koinuma D, Tsutsumi S, Kamimura N, Imamura T, Aburatani H, Miyazono K. Promoter-wide analysis of Smad4 binding sites in human epithelial cells. Cancer Sci 100: 2133–2142, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuo CC, Wu VC, Tsai CW, Wu KD. Relative kidney hyperfiltration in primary aldosteronism: a meta-analysis. J Renin Angiotensin Aldosterone Syst 2: 113–122, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Lemmer B, Witte K, Enzminger H, Schiffer S, Hauptfleisch S. Transgenic TGR(mREN2)27 rats as a model for disturbed circadian organization at the level of the brain, the heart, and the kidneys. Chronobiol Int 20: 711–738, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Medina C, Santos-Martinez MJ, Santana A, Paz-Cabrera MC, Johnston MJ, Mourelle M, Salas A, Guarner F. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J Pathol 224: 461–472, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Memon MA, Anway MD, Covert TR, Uzumcu M, Skinner MK. Transforming growth factor beta (TGFbeta1, TGFbeta2 and TGFbeta3) null-mutant phenotypes in embryonic gonadal development. Mol Cell Endocrinol 294: 70–80, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Momiji H, Monk NAM. Dissecting the dynamics of the Hes1 genetic oscillator. J Theor Biol 254: 784–798, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Nakano A, Koinuma D, Miyazawa K, Uchida T, Saitoh M, Kawabata M, Hanai Ji Akiyama H, Abe M, Miyazono K, Matsumoto T, Imamura T. Pin1 downregulates transforming growth factor-beta (TGF-beta) signaling by inducing degradation of Smad proteins. J Biol Chem 284: 6109–6115, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med 14: 290–298, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Okano K, Schnaper HW, Bomsztyk K, Hayashida T. RACK1 binds to Smad3 to modulate transforming growth factor-beta1-stimulated alpha2(I) collagen transcription in renal tubular epithelial cells. J Biol Chem 281: 26196–26204, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Ono Y, Sensui H, Okutsu S, Nagatomi R. Notch2 negatively regulates myofibroblastic differentiation of myoblasts. J Cell Physiol 210: 358–369, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Proctor CJ, Gray DA. Explaining oscillations and variability in the p53-Mdm2 system. BMC Syst Biol 2: 75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rudberg S, Persson B, Dahlquist G. Increased glomerular filtration rate as a predictor of diabetic nephropathy–an 8-year prospective study. Kidney Int 41: 822–828, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-β structure and activation. Nature 474: 343–349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol 22: 598–604, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun Y, Lowther W, Kato K, Bianco C, Kenney N, Strizzi L, Raafat D, Hirota M, Khan NI, Bargo S, Jones B, Salomon D, Callahan R. Notch4 intracellular domain binding to Smad3 and inhibition of the TGF-beta signaling. Oncogene 24: 5365–5374, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Wang P, Zhu F, Konstantopoulos K. The antagonistic actions of endogenous interleukin-1β and 15-deoxy-Δ12,14-prostaglandin J2 regulate the temporal synthesis of matrix metalloproteinase-9 in sheared chondrocytes. J Biol Chem 287: 31877–31893, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang P, Zhu F, Konstantopoulos K. Interleukin-6 synthesis in human chondrocytes is regulated via the antagonistic actions of prostaglandin (PG)E2 and 15-deoxy-Δ(12,14)-PGJ2. PLos One 6: e27630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49. Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-κB activation. Am J Physiol Cell Physiol 298: C1445–C1456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang P, Zhu F, Lee NH, Konstantopoulos K. Shear-induced interleukin-6 synthesis in chondrocytes: roles of E prostanoid (EP) 2 and EP3 in cAMP/protein kinase A- and PI3-K/Akt-dependent NF-κB activation. J Biol Chem 285: 24793–24804, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang P, Zhu F, Tong Z, Konstantopoulos K. Response of chondrocytes to shear stress: antagonistic effects of the binding partners Toll-like receptor 4 and caveolin-1. FASEB J 25: 3401–3415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, Wang XJ, Lan HY. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol 16: 1371–1383, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Wang Y, Paszek P, Horton CA, Kell DB, White MR, Broomhead DS, Muldoon MR. Interactions among oscillatory pathways in NF-kappa B signaling. BMC Syst Biol 5: 23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamashita M. Synchronization of Ca2+ oscillations: a capacitative (AC) electrical coupling model in neuroepithelium. FEBS J 277: 293–299, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Yan X, Liu Z, Chen Y. Regulation of TGF-beta signaling by Smad7. Acta Biochim Biophys Sin (Shanghai) 41: 263–272, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yau DM, Sethakorn N, Taurin S, Kregel S, Sandbo N, Camoretti-Mercado B, Sperling AI, Dulin NO. Regulation of Smad-mediated gene transcription by RGS3. Mol Pharmacol 73: 1356–1361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yoshiura S, Ohtsuka T, Takenaka Y, Nagahara H, Yoshikawa K, Kageyama R. Ultradian oscillations of Stat, Smad, and Hes1 expression in response to serum. Proc Natl Acad Sci USA 104: 11292–11297, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yu N, Kozlowski JM, Park II, Chen L, Zhang Q, Xu D, Doll JA, Crawford SE, Brendler CB, Lee C. Overexpression of transforming growth factor β1 in malignant prostate cells is partly caused by a runaway of TGF-β1 auto-induction mediated through a defective recruitment of protein phosphatase 2A by TGF-β type I receptor. Urology 76: 1519.e1518–1513, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang M, Fraser D, Phillips A. ERK, p38, and Smad signaling pathways differentially regulate transforming growth factor-beta1 autoinduction in proximal tubular epithelial cells. Am J Pathol 169: 1282–1293, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zheng X, Sehgal A. Probing the relative importance of molecular oscillations in the circadian clock. Genetics 178: 1147–1155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu F, Wang P, Kontrogianni-Konstantopoulos A, Konstantopoulos K. Prostaglandin (PG)D(2) and 15-deoxy-Delta(12,14)-PGJ(2), but not PGE(2), mediate shear-induced chondrocyte apoptosis via protein kinase A-dependent regulation of polo-like kinases. Cell Death Differ 17: 1325–1334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhu F, Wang P, Lee NH, Goldring MB, Konstantopoulos K. Prolonged application of high fluid shear to chondrocytes recapitulates gene expression profiles associated with osteoarthritis. PLos One 5: e15174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]