Abstract
A multidomain, multifunctional 230-kDa extracellular matrix (ECM) protein, hensin, regulates the adaptation of rabbit kidney to metabolic acidosis by remodeling collecting duct intercalated cells. Conditional deletion of hensin in intercalated cells of the mouse kidney leads to distal renal tubular acidosis and to a significant reduction in the number of cells expressing the basolateral chloride-bicarbonate exchanger kAE1, a characteristic marker of α-intercalated cells. Although hensin is secreted as a monomer, its polymerization and ECM assembly are essential for its role in the adaptation of the kidney to metabolic acidosis. Galectin-3, a unique lectin with specific affinity for β-galactoside glycoconjugates, directly interacts with hensin. Acidotic rabbits had a significant increase in the number of cells expressing galectin-3 in the collecting duct and exhibited colocalization of galectin-3 with hensin in the ECM of microdissected tubules. In this study, we confirmed the increased expression of galectin-3 in acidotic rabbit kidneys by real-time RT-PCR. Galectin-3 interacted with hensin in vitro via its carbohydrate-binding COOH-terminal domain, and the interaction was competitively inhibited by lactose, removal of the COOH-terminal domain of galectin-3, and deglycosylation of hensin. Galectin-9, a lectin with two carbohydrate-recognition domains, is also present in the rabbit kidney; galectin-9 partially oligomerized hensin in vitro. Our results demonstrate that galectin-3 plays a critical role in hensin ECM assembly by oligomerizing secreted monomeric hensin. Both the NH2-terminal and COOH-terminal domains are required for this function. We suggest that in the case of galectin-3-null mice galectin-9 may partially substitute for the function of galectin-3.
Keywords: metabolic acidosis, collecting duct, intercalated cell, extracellular matrix, clone C
galectins are a family of carbohydrate-binding proteins that have affinity for β-galactoside-containing glycoconjugates and share similar amino acid sequences. Galectins bind to a wide array of cell surface and extracellular matrix (ECM) glycoproteins mediating cell-cell and cell-matrix adhesion as well as interacting with cytoplasmic and nuclear proteins modulating signaling pathways (16). Fifteen mammalian galectins have been identified so far, and all galectins contain conserved carbohydrate-recognition domains (CRDs) of ∼130 amino acids, which are responsible for carbohydrate binding (5). Some galectins such as galectin-8 and galectin-9 have two distinct CRDs in tandem connected by a short linker of up to 70 amino acids. Galectin-3 is a unique galectin with an extended binding site for long oligosaccharides in its COOH-terminal CRD and a long proline- and glycine-rich NH2-terminal domain (7). Upon ligand binding by CRD the NH2-terminal domain promotes the oligomerization of galectin-3 up to pentamers, and this enables galectin-3 to form heterogeneous, cross-linked lattices with various glycoproteins including laminins (1, 2, 8).
The cortical collecting duct (CCD) of the kidney plays a crucial role in acid-base homeostasis by regulating the expression of acid-base transporters in the intercalated cells. Two distinct populations of intercalated cells have been definitively established to be present in the kidney, the β-intercalated cell expressing the apical chloride-bicarbonate exchanger pendrin and basolateral V-ATPase and the α-intercalated cell expressing basolateral chloride-bicarbonate exchanger (kAE1) and apical V-ATPase (14). In rabbits, CCDs generally secrete bicarbonate, but metabolic acidosis causes this segment to absorb bicarbonate (22). Using in vitro microperfusion studies on acid-loaded rabbit kidney tubules, we discovered that the reversal in bicarbonate flux in acid-loaded rabbits is mediated by the presence of the novel multidomain protein hensin in the ECM (24). When an immortalized cell line derived from rabbit kidney β-intercalated cells (clone C) was seeded at high density (HD), the confluent monolayers exhibited a phenotype with apical endocytic activity and robust apical actin cytoskeleton similar to that of α-intercalated cells. The ECM of these HD cells had abundant hensin, whereas the ECM of monolayers derived from cells seeded at low density (LD) had very little hensin and the cells did not resemble the α-intercalated cell phenotype (29). We confirmed the pivotal role of ECM hensin in mediating the adaptation of rabbit kidney collecting ducts to acidosis and in regulating the phenotypic changes in the β-intercalated cell line, using function-blocking antibody studies (23, 28). Recently, it has been reported that the conditional deletion of hensin in the mouse collecting duct leads to a dramatic decrease in the expression of basolateral chloride-bicarbonate exchanger kAE1, further confirming the role of ECM hensin in the adaptation of the kidney to metabolic acidosis (9).
In the course of our investigations of the ECM assembly of hensin, we discovered that galectin-3 had a specific interaction with hensin in the ECM of HD clone C cells (10). Furthermore, we observed that hensin colocalized with galectin-3 in the ECM of kidney tubules isolated from acid-loaded rabbits (22). In this study, we investigated the nature of the interaction between hensin and galectin-3 and explored the specific role of galectin-3 in ECM hensin assembly. While our studies were ongoing, another research group published their results that demonstrate DMBT1 (a human ortholog of hensin expressed in mucosal epithelia) interacts with galectin-3, using oligosaccharide-mediated interactions. However, the molecular mass of the glycosylated DMBT1 is 300 kDa whereas the molecular mass of glycosylated hensin is only 230 kDa, suggesting substantial difference in the glycosylation pattern of hensin and DMBT1 (21).
MATERIALS AND METHODS
Cell culture.
Stock cultures of immortalized β-inercalated cells (clone C) established from rabbit kidney cortex were maintained at 32°C as described previously (7). The cells were trypsinized, seeded on polycarbonate filters (pore size 0.4 μm; Costar) at a density of 2 × 104 cells/cm2 (low density, LD) or 5 × 105 cells/cm2 (high density, HD), and transferred to 40°C to inactivate the T antigen. Under these conditions the cells resemble α-intercalated cells.
Kidney tissue preparation and generation of metabolic acidosis in vivo.
Protocols were reviewed and approved by the University of Rochester University Committee on Animal Resources. Female New Zealand White rabbits weighing 1.5–2.5 kg were maintained on laboratory chow and tap water. Acid loading was accomplished by providing 75 mM NH4Cl in 5% sucrose drinking solution and limiting food intake to 2% of body weight for 3 days. To determine the expression levels of galectin-3 mRNA in the kidney, total RNA was extracted from cortical slices from six normal and six acid-treated rabbits with the RNeasy Mini Kit (Qiagen, Valencia, CA). In the experiment to determine the expression of galectin-9 protein, slices of cortical, medullary, and papillary regions of normal rabbit kidney were homogenized in the presence of protease inhibitors in NP-40 lysis buffer with 0.1% SDS as above and centrifuged at 12,000 rpm for 30 min, and the protein concentration of the supernatant was determined with the Non-Interfering Protein Assay Kit (EMD Biosciences, La Jolla, CA).
Antibodies.
Polyclonal anti-galectin-3 antibodies were generated in guinea pig as described by Hikita et al. (10). The antisera recognized a single band of 30-kDa molecular mass (the same as authentic His-tagged galectin-3) in bacterial lysates, clone C cell extracts, and kidney tissues (10, 22). New polyclonal antisera to galectin-3 were also generated for this study by immunizing a goat with purified recombinant galectin-3 (Pocono Rabbit Farm and Laboratory, Canadensis, PA) as described in Hikita et al. (10). In addition, the rabbit polyclonal antibody to Galectin-1 (sc-28248) and the goat polyclonal antibody to Galectin-9 (sc-19292) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Guinea pig anti-hensin antibodies against SRCR6–7 domains were obtained as described previously (26). For immunostaining kAE1, the monoclonal antibody IVF12 (Developmental Studies Hybridoma Bank) and rabbit polyclonal AE11-A (Alpha Diagnostics, Harrisburg, PA) were used. In Western blotting studies, 1:200 dilutions of goat polyclonal actin antibody (sc-1616) and mouse monoclonal antibody to GAPDH (sc-66163) from Santa Cruz Biotechnology were used. Rabbit polyclonal serum to cyclophilin A (CypA) was a gift of Cordelia Schiene-Fischer (Max Planck Research Unit for Enzymology of Protein Folding, Halle/Saale, Germany) and was used at 1:2,000 dilution for Western blotting (19).
Western blots of clone C cell lysates and conditioned media containing secreted hensin.
Monolayers of HD and LD cells were washed with PBS and extracted with NP-40 lysis buffer (1% NP-40, 50 mM Tris·HCl, pH 7.4, 150 mM NaCl, and 5 mM EDTA) with Complete protease inhibitor pellets (Roche, Indianapolis, IN). After a brief sonication and centrifugation at 8,000 rpm for 15 min in a microcentrifuge, the supernatant was collected and protein concentration was determined with the BCA assay (Pierce, Rockford, IL). Equal amounts of cell lysates (typically 30 μg) were loaded on 7.5% or 4–20% SDS-PAGE precast gels and transferred onto nitrocellulose membrane, which were then exposed to the appropriate primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. Membranes were then exposed to the chemiluminescent Pierce ECL Western Blotting Substrate (Thermo Scientific, Rockford, IL) reagent and developed with an X-ray developer. For conditioned medium, clone C cells seeded at low density were cultured to confluence at 40°C, washed with PBS, and cultured in serum-free culture medium for another 6 h. Conditioned medium from both apical and basal compartments of the transwell filters were collected after the 6 h and concentrated with 5,000-kDa-cutoff Centricon centrifugal filter units (Millipore), and their protein concentration was determined with the Non-Interfering Protein Assay Kit (EMD Biosciences). Equal amounts of conditioned medium were loaded onto SDS-PAGE gels, transferred, and blotted as above.
Densitometry.
Densitometry of Western blots was performed with ImageJ (National Institutes of Health) software using the Analyze/Gels drop-down menus and finding the area under the peaks obtained from the Plot Lane command. Densitometry values were normalized with the densitometry values of corresponding actin Western blots (cell lysate) and CypA Western blots (conditioned medium). Two-tailed Student t-test was performed on the densitometric results with Microsoft Excel. The n (number of Western blots) and P (statistical significance) values are indicated in figures.
Real-time PCR.
After RNA integrity was verified, first-strand cDNA was synthesized from 500 ng of total RNA with a SuperScript III first-strand cDNA synthesis kit (Invitrogen, Grand Island, NY). Rabbit galectin-3 forward/reverse primer (5′-GGCGCCAGCCCTTACAGCGC-3′, 5′-GGCTTCACCGTGCCCACAAT-3′) and rabbit GAPDH forward/reverse primer (5′-ACTCTGGCAAAGTGGATGTTGTCG-3′, 5′-TTGATGACCAGCTTCCCGTTCTCA-3′) sets were designed with Primertime QPCR software (IDT, Coralville, IA) and were synthesized by Integrated DNA Technologies. After the annealing and melting temperatures of the primers were optimized, galectin-3 mRNA levels of normal and acid-loading kidneys were determined by quantitative real-time PCR (SYBR Green method) normalized to GAPDH with the Bio-Rad MyiQ2 Two Color Real-Time PCR detection system (Bio-Rad, Hercules, CA). ΔCt values were obtained by subtracting the threshold cycle (Ct) values of the sample from that of GAPDH, and relative quantity (RQ) was determined with the ΔΔCt method. Unpaired t-test with two-tailed P value was performed on the RQs of normal and acidotic samples with GraphPad Instat software.
For the investigation of expression levels of various galectins in clone C cells, total RNA was extracted from three independent monolayer cultures of LD and HD with Tripure reagent (Roche) and RNA integrity was analyzed with the Agilent Bioanalyzer Nano 6000 kit. Total RNA was then treated with TURBO DNase (Ambion, Grand Island, NY), and first-strand cDNA was synthesized from 1–2 μg of total RNA with the High Capacity Reverse Transcriptase Kit (Applied Biosystems, Carlsbad, CA). Real-time PCR was performed with a TaqMan method in a 7900HT Sequence Detection System (Applied Biosystems) with TaqMan Universal Master Mix. Predesigned forward/reverse primers and fluorogenic probes for rabbit galectin-3, galectin-4, HPRT1, and GAPDH were from ABI (rabbit LGALS3: Oc03398084_m1, rabbit LGALS4: Oc03398870_m1, rabbit HPRT1: Oc03399461_m1, rabbit GAPDH: Oc03823402_g1). Primers and probes for rabbit galectin-1, galectin-7, and galectin-8 were designed based on the Ensembl rabbit sequence for these genes. Rabbit galectin-9 primers and probes were designed based on the predicted rabbit galectin-9 sequence (NCBI accession no. XM_002718781). The primers and probes developed and used in the investigation of the expression levels of various galectins in clone C cells are shown in Table 1. The real-time PCR results were analyzed in SDS RQ Manager 1.2, and RQ was determined with the ΔΔCt method.
Table 1.
TaqMan real-time PCR primers and probes developed and used in this study
| Sequence 5′ to 3′ | |
|---|---|
| Rabbit galectin-1 | |
| Forward primer | GTGTGCAACAGCAAGGAGAAG |
| Reverse primer | CCCGGCTGGAAGGGAAAC |
| Probe | CCACGCCCCAGACGC |
| Rabbit galectin-7 | |
| Forward primer | GTCCGAGGTGGTCTTCAACAG |
| Reverse primer | CGATGAGCAGCACCTCGAA |
| Probe | CCGCGCTCCTCCTTG |
| Rabbit galectin-8 | |
| Forward primer | CCCACAGGATAAGTCTGGAGAAAAT |
| Reverse primer | GAGCTGAAGCTAAAACCAATTGAGT |
| Probe | CACTCTGGGCATTTAT |
| Rabbit galectin-9 | |
| Forward primer | CTGGACAGATGTTCCCAAATCCT |
| Reverse primer | CCGGGATGCTGGTGAAGAAA |
| Probe | CACCCATGCTGTACCC |
Rabbit galectin-3, galectin-4, GAPDH, and HPRT1 are proprietary (Applied Biosystems) and are referenced in materials and methods.
Construction of full-length galectin-3, galectin-9, and galectin-3 NH2-terminal and COOH-terminal expression plasmids.
Total RNA was extracted from rabbit kidney tissue with the RNeasy Mini Kit (Qiagen). The cloning PCR primers were designed based on the GenBank sequence database (accession nos. NM001082338 and XM002718781). The full-length rabbit galectin-3 (1–242 aa) cloning forward/reverse primers are 5′-GGAATTCCATATGGCGGATGGTTTTTCG-3′ and 5′-CCGCTCGAGTATCATAGCATGTGA-3′, galectin-9 (1–322 aa) cloning forward/reverse primers are 5′-GGGAATTCCATATGGCGTTCCAACGCCCCCA-3′ and 5′-CCGCTCGAGTATCTGCACGTGGGTCAGCTG-3′, galectin-3 NH2-terminal (GNT; 1–108 aa) cloning forward/reverse primers are 5′-GGAATTCCATATGGCGGATGGTTTTTCG-3′ and 5′-CCGCTCGAGCACAGGCAGTGGTCCAGCAGA-3′, and galectin-3 COOH-terminal (GCT; 108–242 aa) cloning forward/reverse primers are 5′-GGAATTCCATATGCCTTATGACCTGCCTCTG-3′ and 5′-CCGCTCGAGTATCATAGCATGTGA-3′. All galectin cDNAs were generated with the SuperScript III One-Step RT-PCR system with Platinum Taq High Fidelity (Invitrogen) and cloned into Escherichia coli expression vectors pET26b and pET15b (Novagen, Madison, WI) by using NdeI and XhoI restriction enzyme (New England Biolabs, Beverly, MA) cloning sites, respectively. All recombinant constructs (pET26galectin-3, pET15galectin9, pET26GCT, and pET15GNT) were transformed into subclone-efficiency DH5α-competent cells (Invitrogen), screened and confirmed by sequencing.
Production of His-tagged full-length galectin-3, galectin-9, GNT, and GCT proteins.
All recombinant galectin expression constructs were transformed into E. coli Rosetta 2(DE3) pLysS expression strain (Novagen, Madison, WI) and induced with 1 mM isopropyl-1-thio-d-galactopyranoside (IPTG) for 4–6 h. Cultured cells were centrifuged, and the pellet was lysed in lysis buffer (in mM: 50 NaH2PO4, 300 NaCl, and 10 imidazole, with 1% EDTA-free Halt protease inhibitor cocktail, pH 8.0). The lysate was then incubated with lysozyme (1 mg/ml) for 30 min, sonicated on ice, and centrifuged at 10,000 g for 20 min. The supernatant was removed and incubated with 50% Ni-NTA agarose slurry (Qiagen) at 4°C for 1 h, and then the His-tagged proteins were eluted with elution buffer (in mM: 50 NaH2PO4, 300 NaCl, 100–300 imidazole). Eluates were dialyzed by PBS buffer at 4°C overnight and characterized with 4–20% Mini-protein precast gels (Bio-Rad), and galectin proteins were confirmed by immunoblotting with anti-galectin-3, galectin-9, and anti-His tag antibodies.
In vitro binding assay or pull-down assay.
Different concentrations of purified recombinant His-tagged full-length galectin-3, GCT, and GNT proteins (0, 5, 10, 30, 50 μg/ml corresponding to 0, 0.15, 0.33, 1, and 1.7 μM) were dialyzed against Tris-buffered saline (TBS) to remove imidazole and immobilized on equilibrated 50% Ni-NTA agarose slurry beads (Qiagen) with a gentle rocking motion on a rotating platform for 1 h at 4°C. After extensive washes with TBS, conditioned media from clone C cultures (400 μg of total protein) were then added to these beads and incubated with gentle rocking on a platform at 4°C for 1 h. After the beads were washed with TBS to remove nonspecific binding partners, galectin-hensin binding proteins were eluted with elution buffer (in mM: 50 NaH2PO4, 300 NaCl, 250 imidazole buffer, pH 8.0). Eluates were then examined by electrophoresis on 4–20% Mini-protein precast gels followed by immunoblot with anti-hensin antibody.
Immunocytochemistry.
Clone C cells were plated at low density (5 × 105 cells/cm2) on Transwell filters and cultured in the presence or absence of 10, 20, 30, and 60 μg (0.33, 0.7, 1, and 2 μM) recombinant full-length galectin-3 for 4–5 days at 40°C. For F-actin staining, monolayers were fixed in 4% paraformaldehyde, blocked and permeabilized with PBS-0.3% bovine serum albumin-0.075% saponin, and stained with rhodamine phalloidin (R-415; Invitrogen) and nuclear stain SYTOX Green (Invitrogen). Stained monolayers were imaged with a FV1000 Olympus Laser Scanning Confocal Microscope and depicted as color images with Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
Competitive inhibition studies of galectin-3-hensin interaction.
Recombinant His-tagged full-length galectin-3 (20 μg/ml, 0.7 μM) was immobilized on equilibrated 50% Ni-NTA agarose slurry beads (Qiagen). After extensive washes with TBS to remove unbound galectin-3, the beads were incubated with culture medium with and without lactose (20, 50, and 100 mM), sucrose (100 mM), and mannose (100 mM) at 4°C for 1 h with a gentle rocking motion. Conditioned media from clone C cultures (400 μg of total protein) were then added to these beads and incubated with gentle rocking on a platform at 4°C for 1 h. Lactose serves as an effective competitive inhibitor in the range of 10–100 μM, and a lactose concentration up to 100 μM has been utilized in other competitive inhibition studies (11, 15). After the beads were washed with TBS to remove nonspecific binding partners, galectin-3 binding proteins were eluted with elution buffer (in mM: 50 NaH2PO4, 300 NaCl, 250 imidazole buffer, pH 8.0). Eluates were then examined by electrophoresis on 4–20% Mini-protein precast gels followed by immunoblot with anti-hensin antibody.
Deglycosylation of conditioned medium from clone C LD cultures.
We utilized the GlycoPro Enzymatic Deglycosylation Kit (Prozyme, Hayward, CA) to completely remove all N-linked and simple O-linked carbohydrates from the glycoproteins of clone C conditioned media per the manufacturer's protocol. Briefly, 100 μg total protein of LD clone C medium was concentrated to 35 μl, mixed with 10 μl of 5× incubation buffer, and treated with 1 μl of N-glycanase (5 U/ml), 1 μl of sialidase A (5 U/ml), and 1 μl of O-glycanase (1.25 U/ml) at 37°C for 16–18 h. The normal and deglycosylated LD media were incubated with 0.7 μM recombinant full-length galectin-3 agarose beads as described above and examined by electrophoresis on 4–20% Mini-protein precast gels followed by immunoblot with anti-hensin antibody.
OptiPrep density gradient analysis.
Concentrated conditioned media from LD cultures (1 ml, 100 μg/ml) were loaded on 11 ml of 5–40% OptiPrep density gradient medium (Sigma-Aldrich, St. Louis, MO) and ultracentrifuged at 100,000 g for 3 h at 4°C. Proteins in each fraction (1 ml) were precipitated by 10% TCA, dissolved in a sample buffer, and subjected to 8% SDS-PAGE followed by Western blotting with anti-hensin antibody. In studies that tested the effect of galectin-3 and galectin-9 in oligomerizing hensin, the concentrated conditioned medium (100 μg) was incubated with 20 μg (0.7 μM) of galectin-3 in either the presence or the absence of 100 mM lactose and with 32 μg (0.7 μM) galectin-9 for 1 h at room temperature before the samples were analyzed with OptiPrep density gradients as described above. OptiPrep density gradient analyses of standard proteins γ-globulin (158 kDa), catalase (232 kDa), and thyroglobulin (670 kDa) were performed to determine the approximate molecular masses of proteins that sediment in the different fractions of the OptiPrep density gradient (data not shown).
RESULTS
Galectin-3 synthesis is upregulated in acidotic rabbit kidneys.
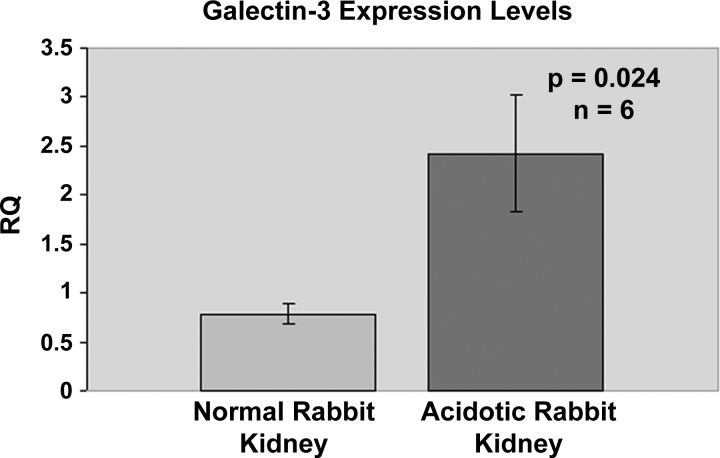
In a previous study, we demonstrated that the intensity of immunostaining and the number of CCD cells expressing galectin-3 were significantly upregulated in acid-loaded rabbits compared with control rabbits (22). Additionally, immunostaining revealed a similar expression pattern for galectin-3 in rabbit, mouse, and rat kidneys, suggesting that galectin-3 may play a similar role in acid-base homeostasis in diverse species. In this study we compared the expression levels of galectin-3 from six different cortical kidney tissues of acid-loaded and normal rabbits, using real-time PCR. Ct values for GAPDH expression were not significantly different between the two groups (∼21 cycles for all samples), and hence GAPDH was used as the normalization control for determining the relative quantity of galectin-3 expression. Statistical analysis of the relative expression levels of galectin-3 showed that there was a significant (P < 0.05) threefold increase in the expression levels of galectin-3 in acid-loaded kidney tissues compared with control rabbit kidney cortical tissues (Fig. 1).
Fig. 1.
Real-time PCR results for galectin-3 mRNA levels in normal and acidotic rabbit kidneys. Real-time PCR was performed on RNA extracted from 6 normal and acidotic rabbit kidney cortical tissues with galectin-3 SYBR Green primers as described in materials and methods. Relative quantity (RQ) was obtained from threshold cycle (Ct) values with the ΔΔCt method. GAPDH Ct values of corresponding samples were used to normalize the expression levels. Two-tailed Student t-test was performed with Microsoft Excel to obtain P values.
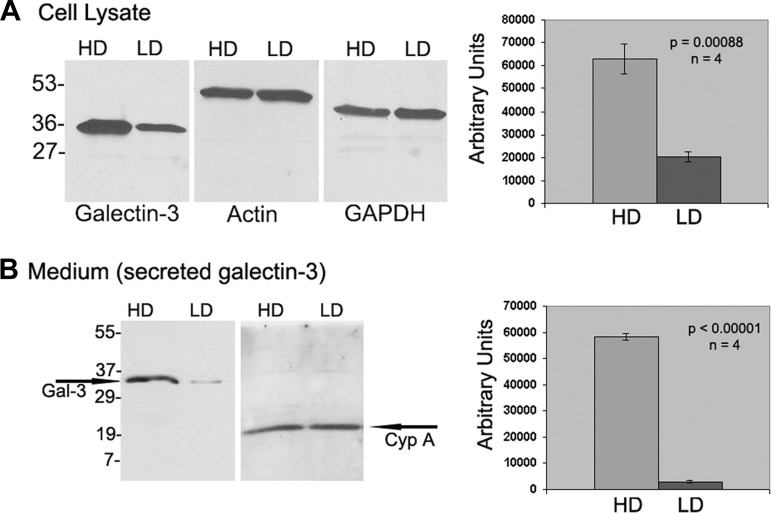
High-density clone C cells synthesize and secrete considerably more galectin-3 compared with LD cells.
To examine whether there was a significant difference in the synthesis levels of galectin-3 cells in HD clone C monolayers compared with their LD counterparts, equal amounts (40 μg) of cell lysates extracted from HD and LD monolayers were probed with galectin-3 antibody. A typical Western blot result shown in Fig. 2A, left, reveals a significantly more intense galectin-3 band in HD cells compared with LD cells. However, immunoblotting the same samples with anti-GAPDH or anti-actin antibodies revealed no significant difference in their expression levels. Densitometry results of galectin-3 bands from four independent experiments were normalized with those of actin immunoblots. The results showed that galectin-3 levels in the cell lysates of HD cells were three times higher than those of the LD cells (Fig. 2A, right). Also, we immunoblotted 30 μg of concentrated conditioned media obtained from LD and HD cell cultures with galectin-3 antibody and with a control, CypA antibody, and a typical result is shown in Fig. 2B. The data shown in Fig. 2B demonstrate that HD cells secreted significantly more galectin-3 into the medium compared with their LD counterparts. Densitometric analysis of four independent studies indicated that the average value of galectin-3 in HD medium was 58,249 ± 1,242 arbitrary units, whereas in the LD medium it was 3,034 ± 610 arbitrary units, a significant reduction (P < 0.0001). In summary, HD cells synthesize and secrete more galectin-3 than LD cells.
Fig. 2.
Western blot results for galectin-3 levels in total cell lysates (A) and conditioned medium (B) of low-density (LD) and high-density (HD) clone C cells. A, left: 40 μg of cell lysates extracted from HD and LD monolayers was probed with guinea pig polyclonal sera against rabbit galectin-3, and the same amount of total cell lysates was probed with actin and GAPDH antibodies. Right: densitometric values obtained from 4 independent Western blots. B, left: 30 μg of concentrated conditioned medium obtained from HD and LD cell cultures was probed with galectin-3 antibody, and in control studies the same amount of conditioned medium was probed with cyclophilin A (CypA) antibody. Right: densitometric values obtained from 4 independent Western blots.
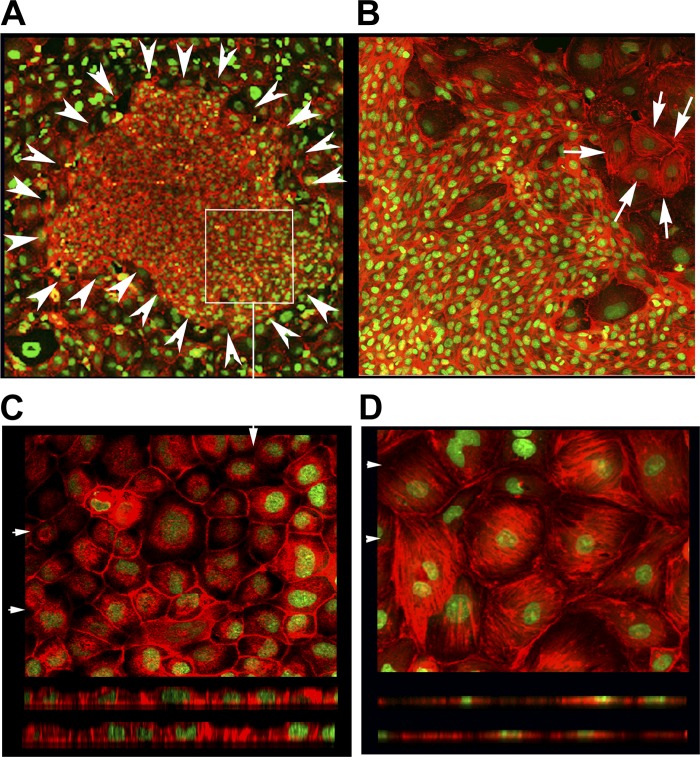
Addition of recombinant galectin-3 during culture modulates LD phenotype.
We have previously demonstrated that LD monolayers exhibited exuberant basal actin stress fibers, but the apical actin staining was absent. Additionally, these cells did not possess the apical endocytic property and only had sparse microvilli. In contrast, the HD cells, which showed active apical endocytosis, exhibited exuberant apical actin staining and microvilli but diminished basal actin stress fibers. In addition, the cell shapes of these two phenotypes were different, with HD cells being tall with a small cross-sectional area whereas LD cells were low and flat (28). To test whether galectin-3 had a role in this phenotype remodeling, we cultured LD clone C cells with various doses (10, 20, 30, 60 μg/ml) of recombinant rabbit galectin-3 for 4–5 days in parallel with LD cells with no added galectin-3. The cells were then stained with rhodamine-phalloidin (F-actin) and a nuclear stain (SYTOX Green, Invitrogen) and inspected for morphology. This experiment was repeated three times. In LD cells cultured with 30 and 60 μg/ml galectin-3, we observed patches of cells that resembled HD cells (both in terms of size and shape and in terms of the absence of stress fibers). The patches did not cover the entire monolayer but were observed in ∼30–40% of the monolayer. Two such patches of HD phenotype cells are shown in Fig. 3, A and B. Detailed investigation of these patches using optical sectioning (laser confocal microscopy) revealed that the cells in these patches were tall and cuboidal with a distinct apical actin cytoskeleton (Fig. 3C). However, a typical LD monolayer lacks a distinct apical actin cytoskeleton and has more basal stress fibers, and the cells are flat (Fig. 3D).
Fig. 3.
Typical F-actin staining results of clone C monolayers seeded at low density in the presence of 30 and 60 μg/ml galectin-3. A: clone C cells seeded at low density were cultured in the presence of 30 μg/ml recombinant rabbit galectin-3 for 4–5 days and stained with rhodamine phalloidin (F-actin, depicted in red) and SYTOX Green nuclear stain (×10 field). Arrowheads circumscribe a typical patch of HD phenotype cells that formed out of the LD monolayer in the presence of recombinant galectin-3 during culture. B: representative ×20 field of a LD clone C monolayer cultured in the presence of 60 μg/ml recombinant rabbit galectin-3 for 4 days shows the presence of a patch of HD phenotype cells adjacent to LD phenotype cells exhibiting stress fibers (the latter marked by arrows). C: high-magnification (×40) field of the patch area marked with a rectangular box in A. Top: XY projection of the top 4 optical sections (of 0.6 μm) revealing the presence of punctate apical actin. Bottom: actin staining (red) could be easily seen above the nucleus (apical portions) in the XZ projections taken at 2 places marked by arrowheads at top. D, top: typical XY projection of an LD monolayer field (×40) reveals the presence of exuberant stress fibers. Bottom: XZ sections of this field; note the absence of red (actin) staining above the nucleus.
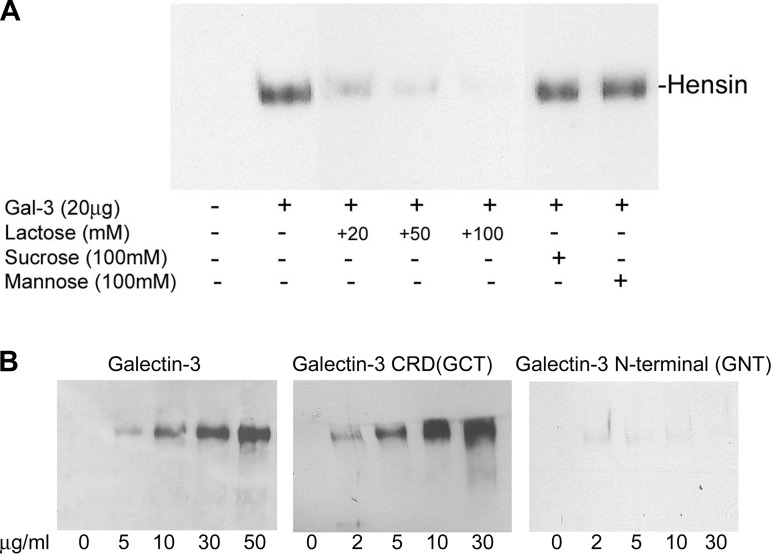
Galectin-3 interacts with hensin via its COOH-terminal carbohydrate-recognition domain.
We have previously demonstrated that hensin is secreted into the conditioned medium of both LD and HD cultures, but it is present in a monomeric form in the conditioned medium of LD cultures and in higher-order oligomeric forms in the HD conditioned medium. To examine the nature of interaction between hensin and galectin-3 (i.e., to test whether the interaction is a protein-protein interaction or a carbohydrate-dependent interaction), we incubated concentrated conditioned media from LD cultures (100 μg total protein) with recombinant galectin-3 (20 μg) immobilized with Ni-NTA beads at 4°C in the presence or absence of various concentrations of lactose (20, 50, and 100 mM), a specific inhibitor of the carbohydrate binding activity of galectin-3. In control experiments, a similar procedure was performed but in the presence of nonspecific disaccharide inhibitors sucrose and mannose (13, 25). The bound proteins were eluted with SDS-PAGE sample buffer and investigated by immunoblotting with hensin antibody (Fig. 4A). The results showed that the interaction of galectin-3 with hensin was inhibited in the presence of lactose in a dose-dependent manner, but the presence of a moderately high concentration of sucrose and mannose (100 mM) did not prevent the interaction of galectin-3 with hensin, demonstrating that the interaction between galectin-3 and hensin is primarily carbohydrate mediated.
Fig. 4.
Anti-hensin antibody Western blots of eluates bound to recombinant galectin-3 immobilized on Ni-NTA agarose beads in the presence (+) or absence (-) of lactose, sucrose, and mannose (A) and eluates bound to Ni-NTA agarose beads with various amounts of galectin-3, COOH-terminal domain (GCT), and NH2-terminal domain (GNT) (B). A: 20 μg recombinant galectin-3-bound Ni-NTA agarose beads were incubated with 400 μg of concentrated LD conditioned medium at 4°C for 1 h in the presence of indicated amounts of lactose (competitive inhibitor of galectin-3) and nonspecific ligands sucrose and mannose, and the eluates were examined by Western blotting with hensin antibody. B: indicated amounts of recombinant galectin-3 (left), recombinant GCT (center), and recombinant GNT (right) immobilized on beads were incubated with 400 μg of concentrated LD conditioned medium as in A, and the eluates were examined by Western blotting with hensin antibody. Hensin bands were observed only in the eluates of galectin-3 and GCT beads and not in the GNT beads. CRD, carbohydrate-recognition domain.
To determine the role of galectin-3 CRD in its interaction with hensin more directly, we incubated concentrated conditioned media from LD cultures (100 μg total protein) with Ni-NTA beads immobilized with various amounts (as indicated in Fig. 4B) of recombinant galectin-3, the CRD of galectin-3 (GCT), and the NH2-terminal domain of galectin-3 (GNT). After extensive washing, proteins bound to the Ni-NTA beads were eluted and immunoblotted with hensin antibody (Fig. 4B). The results showed that a dose-dependent increase in hensin bands was observed in the eluates of galectin-3-bound beads, indicating specific interaction of the galectin-3 with hensin (Fig. 4B, left). Moreover, a similar dose-dependent increase in hensin bands was also observed in the eluates of the GCT-bound beads, confirming the role of galectin-3 CRD in the interaction with hensin (Fig. 4B, center). In contrast, no hensin band was observed in the eluates of the GNT-bound beads, suggesting that the NH2-terminal domain of galectin-3 has no significant role in mediating the interaction of galectin-3 with hensin (Fig. 4B, right).
Galectin-3 is unable to interact with deglycosylated hensin.
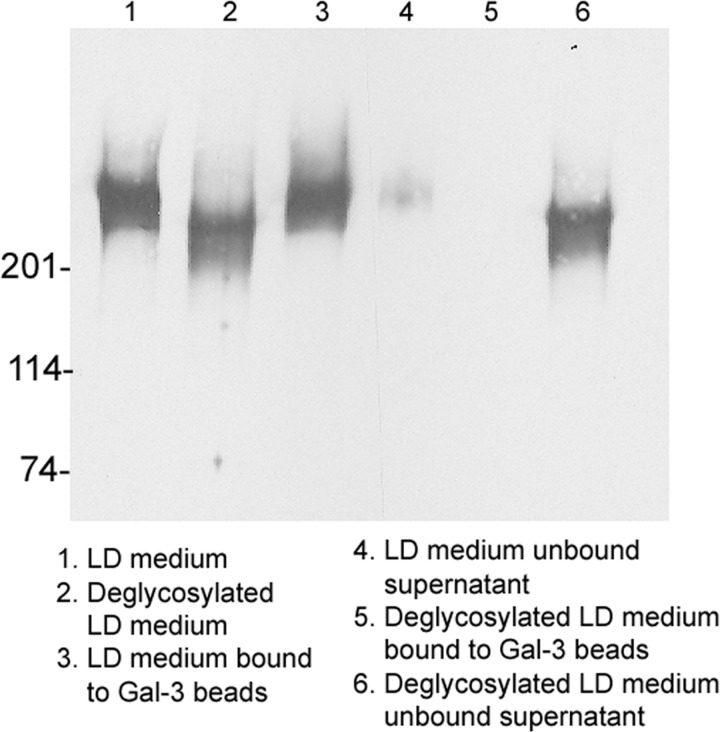
Finally, to conclusively establish that galectin-3 interacts with hensin through carbohydrate-mediated interactions, we subjected conditioned media from LD cultures to enzymatic deglycosylation (both O- and N-deglycosylation) and then incubated the deglycosylated medium with recombinant galectin-3 (20 μg) immobilized on Ni-NTA beads. In control studies, conditioned medium that was not subjected to deglycosylation was incubated with galectin-3 beads in a similar manner. The bound proteins eluted from the beads as well as the supernatant containing the unbound proteins were then investigated by immunoblotting with hensin antibody. The results showed that our deglycosylation procedure resulted in a considerable reduction in the molecular mass of hensin (Fig. 5, lanes 1 and 2), confirming the effectiveness of deglycosylation. In the case of nondeglycosylated conditioned medium (Fig. 5, lanes 3 and 4), most of the hensin appeared in the bound fraction (Fig. 5, lane 3) and only a small amount was present in the supernatant (Fig. 5, lane 4). In contrast, in the deglycosylated medium (Fig. 5, lanes 5 and 6) most of the hensin was present in the supernatant fraction (Fig. 5, lane 6) and no detectable hensin band was observed in the bound fraction (Fig. 5, lane 5). This result demonstrated that deglycosylated hensin was incapable of binding to galectin-3.
Fig. 5.
Anti-hensin antibody Western blots of galectin-3 bound and unbound fractions of deglycosylated and nondeglycosylated LD conditioned medium. Conditioned medium from LD monolayers (100 μg total protein) was subjected to enzymatic deglycosylation (to remove all N-linked and simple O-linked carbohydrates from glycoproteins) and incubated with 20 μg/ml galectin-3 containing Ni-NTA beads. After centrifugation and wash, the unbound supernatant and eluates from the beads were probed by Western blotting with anti-hensin antibody (lanes 4–6). In the nondeglycosylated conditioned medium most of the hensin is retrieved in the galectin-3 bound fraction (lane 3), whereas in the deglycosylated conditioned medium most of the hensin is retrieved in the unbound supernatant fraction (lane 6). The molecular mass of the conditioned medium that was subjected to deglycosylation is significantly reduced to ∼210 kDa (lane 2) from the normal size of nondeglycosylated hensin, 230 kDa (lane 1).
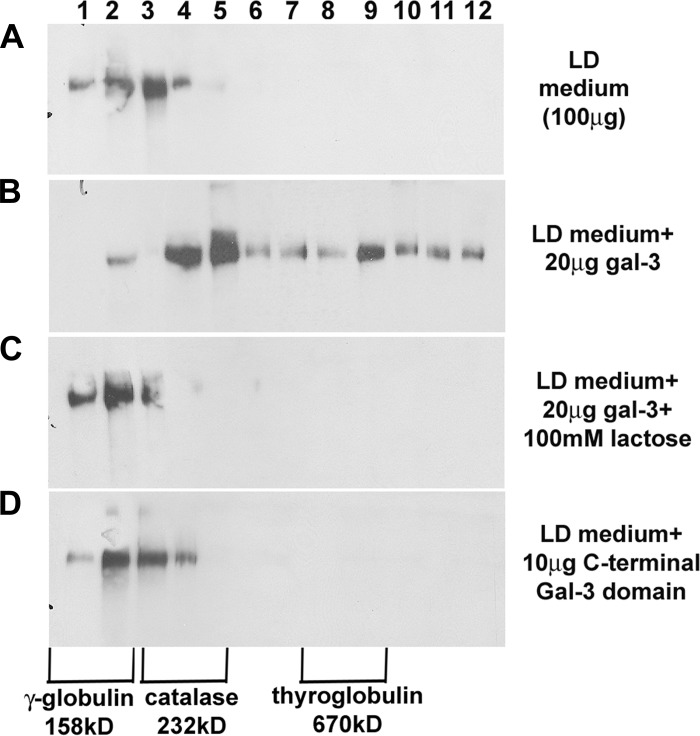
Galectin-3 oligomerizes secreted hensin.
We have previously demonstrated that galectin-3 colocalizes with hensin only in the ECM of HD cells but not in LD ECM (10). Also, addition of galectin-3 to LD cultures led to the development of HD morphology (Fig. 3) and induced apical endocytosis. These results suggested that galectin-3 mediates the ECM assembly of hensin. To understand the mechanism by which galectin-3 could facilitate ECM assembly of hensin, we utilized the OptiPrep density gradient method to fractionate high-molecular-mass oligomers of hensin. We incubated concentrated conditioned medium from LD cultures (100 μg total protein) with recombinant galectin-3 (20 μg/ml or 0.7 μM) in the presence or absence of lactose for 1 h at room temperature. The samples were then placed on an OptiPrep gradient and ultracentrifuged, and all 12 of the 1-ml fractions of the OptiPrep gradient were analyzed by immunoblotting with anti-hensin antibody. A similar procedure was performed on concentrated LD medium incubated with 0.7 μM recombinant galectin-3 CRD (GCT; 10 μg). These experiments were performed independently at least three different times, and the results of the experiments were similar to each other. Typical results of this study are shown in Fig. 6. The presence of hensin in the LD medium was observed only in the first three fractions corresponding to 158–220 kDa, indicating it is primarily monomeric (Fig. 6A). However, in samples incubated with galectin-3, hensin existed in higher-order oligomeric forms as evidenced by its presence in all fractions up to fraction 12 (Fig. 6B). This change in the oligomeric status was not observed in samples treated with lactose in addition to galectin-3 or in samples treated with the recombinant galectin-3 CRD. These results demonstrated that although the CRD of galectin-3 was required for its interaction with hensin, both the NH2-terminal and COOH-terminal domains of galectin-3 were required for the oligomerization of hensin.
Fig. 6.
Anti-hensin antibody Western blots of OptiPrep density gradient fractions of LD conditioned medium incubated with galectin-3 in the presence or absence of lactose and GCT. A: 1 ml of concentrated conditioned medium from LD cultures (100 μg/ml) was placed on 11 ml of 5–40% OptiPrep density gradient medium and ultracentrifuged, and TCA-precipitated proteins from each 1-ml fraction were examined by Western blotting with anti-hensin antibody. Similar density gradient analyses of standard proteins γ-globulin (158 kDa), catalase (232 kDa), and thyroglobulin (670 kDa) were performed, and their locations in the different fractions of the density gradient are marked at bottom. The medium shows no hensin oligomerization. B: an anti-hensin Western blot of density gradient fractions of conditioned medium incubated with 20 μg/ml (0.7 μM) galectin-3 with oligomerization to fraction 12. C: anti-hensin Western blot of density gradient fractions of conditioned medium samples incubated with both galectin-3 and lactose; the lactose prevented hensin oligomerization. D: anti-hensin Western blot of the density gradient fractions of conditioned medium incubated with 0.7 μM (10 μg/ml) recombinant GCT alone; there was no hensin oligomerization.
Expression profile of other galectins in clone C cells.
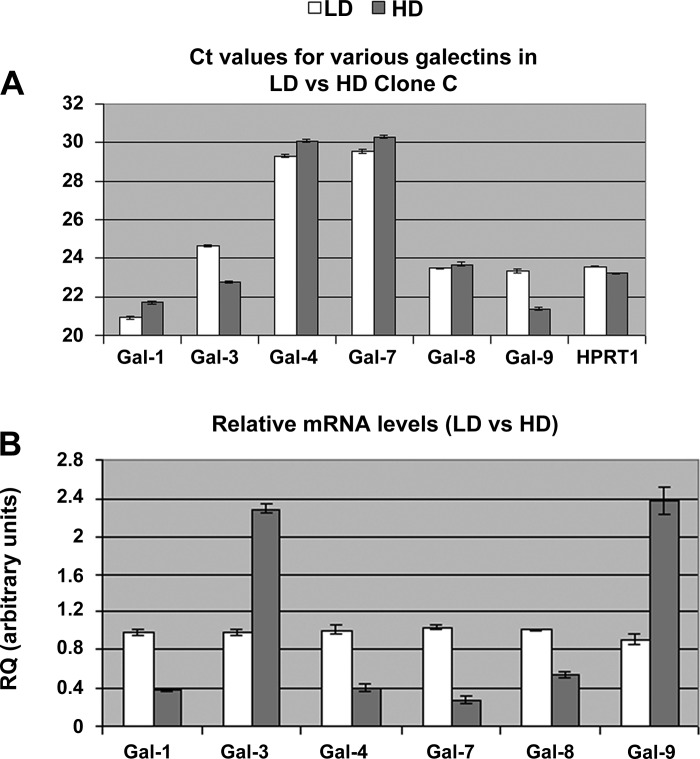
Poland et al. (20) performed a thorough investigation of the expression levels of various galectin transcripts in Madin-Darby canine kidney (MDCK) cells and found, in descending order of abundance, galectin-3, galectin-9, galectin-8, galectin-1, galectin-4, and galectin-7. We performed a real-time PCR investigation of these galectin transcripts in clone C cells seeded at high and low densities. Our results showed that in clone C cells there was a significant level of mRNA expression of galectin-1, galectin-8, and galectin-9 in addition to galectin-3, with average Ct values ranging from 21 to 24 cycles, whereas those of galectin-4 and galectin-7 were around 29–30 cycles (Fig. 7A). An examination of the RQ of mRNA transcripts showed that galectin-3 mRNA levels in HD cells were 2.3 times those of LD cells (RQ of galectin-3 in HD was 2.293 ± 0.047 compared with 0.985 ± 0.033 arbitrary units in LD; P < 0.001). Similarly, the galectin-9 transcript level in HD cells was 2.6 times that of LD cells (RQ of galectin-9 in HD was 2.382 ± 0.143 compared with 0.906 ± 0.055 arbitrary units in LD; P < 0.001). However, there was a significant decrease in the mRNA level of galectin-1 and galectin-8 in HD cells compared with LD cells (Fig. 7B).
Fig. 7.
Real-time PCR results showing mRNA levels of various galectins in LD and HD monolayers of clone C cells. A: total RNA was extracted from 3 independent monolayer cultures of LD and HD, and real-time PCR was performed with the TaqMan method. Average Ct values for various galectins investigated in this study as well as that of HPRT1 (control) are shown. Primers and probes that were developed and used in the investigation are shown in Table 1. B: real-time PCR results were analyzed in SDS RQ Manager 1.2, and RQ was determined with the ΔΔCt method. Average normalized RQ values of various galectins in LD and HD cells are shown. Error bars show SE. Two-tailed t-test analysis showed a significant increase (P < 0.001) in the mRNA levels of galectin-3 and galectin-9 in HD cells compared with LD cells.
Galectin-9 may replace galectin-3 function in galectin-3-null mice.
It has recently been shown that the collecting duct-specific conditional deletion of hensin in mice leads to a drastic reduction in the number of cells expressing the basolateral chloride-bicarbonate exchanger of the α-intercalated cells, kAE1 (9). In light of this observation, we examined frozen sections of galectin-3 knockout mice (B6.Cg-Lgals3tm1Poi/J; The Jackson Laboratory) by immunostaining with antibodies that recognize kAE1. The results did not show any significant difference in the number of cells expressing kAE1 between the wild-type and galectin-3 knockout kidneys (data not shown). This is consistent with the earlier observation that bicarbonate secretion and intercalated cell function in galectin-3-null mutant mice were similar to those of wild-type mice (3). These results suggested that in the absence of galectin-3 another lectin might replace its role in the oligomerization of hensin. Galectin-1 and galectin-9 are the other galectins primarily expressed in the kidney (12). Of these, galectin-1 is expressed in very low levels in the normal adult kidney, and its expression is restricted to kidney interstitial tissues (12, 31). In addition, galectin-1 is structurally similar to the galectin-3 CRD, and addition of galectin-3 CRD alone to the monomeric hensin-containing LD medium did not lead to hensin oligomerization (Fig. 6D); however, the CRD domain of galectin-3 is clearly required for hensin-galectin-3 interaction and subsequent oligomerization (Fig. 6, B and C).
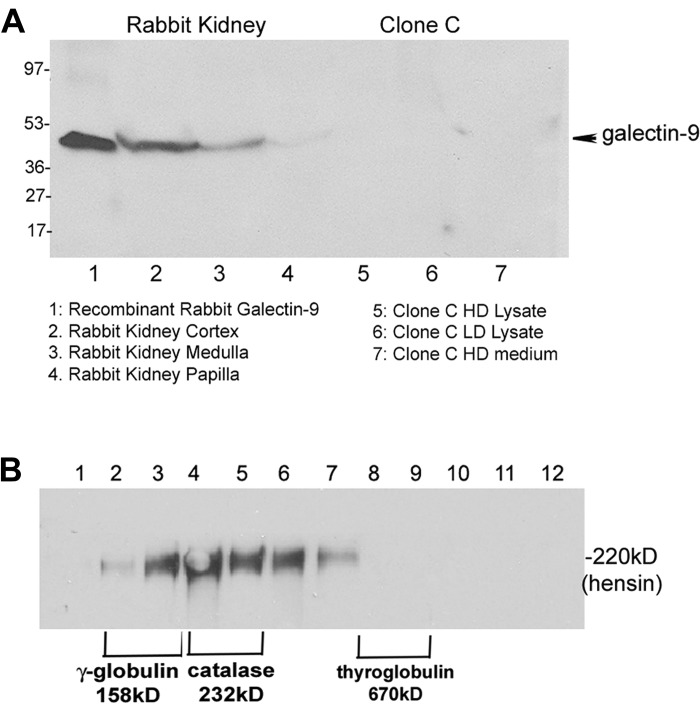
In contrast, galectin-9 is a tandem-repeat galectin containing two linked CRDs. Galectin-9 is expressed primarily in proximal tubules of the renal cortex, and its expression is upregulated in normal adult kidneys compared with embryonic kidneys (30). To test the possible role of galectin-9 in hensin oligomerization, we expressed recombinant rabbit galectin-9 in a bacterial expression system (Fig. 8A, lane 1). Western blotting of equal amounts of tissue lysates (20 μg) from normal rabbit kidney cortex, medulla, and papilla confirmed that galectin-9 is expressed in significant amounts in the rabbit kidney cortex and medulla (Fig. 8A, lanes 2 and 3). However, examination of 40 μg of cell lysates from clone C cells (LD and HD) or concentrated HD conditioned medium by Western blotting with galectin-9 antibody did not show any detectable bands. Since our real-time PCR studies showed a significant amount of galectin-9 expression at the transcript level we repeated our Western blotting studies with 100 μg of cell lysates from HD and LD cells, and we still did not detect any galectin-9 bands (data not shown). This result suggests the possibility that, although galectin-3 and galectin-9 transcripts are made in significant amounts in HD clone C cells, there may be significant differences in the translational efficiency or half-life of galectin-9, thus enabling galectin-3 to be the primary partner of hensin in its oligomerization.
Fig. 8.
Western blot of kidney tissue extract and clone C cell lysate with galectin-9 antibody (A) and OptiPrep density gradient fractions of conditioned medium incubated with recombinant galectin-9 (B). A: a goat polyclonal antibody to galectin-9 was used for Western blot of 20 μg of tissue extract from cortical, medullary, and papillary tissues of normal rabbit kidney (lanes 2–4) along with 40 μg of recombinant galectin-9 (lane 1) and 40 μg of clone C lysates (HD, lane 5; LD, lane 6) and conditioned medium (lane 7). The antibody recognized an ∼45-kDa band in lane 1 containing the recombinant galectin-3, confirming its specificity. B: anti-hensin Western blot of OptiPrep density gradient fractions of LD conditioned medium incubated with 0.7 μM (32 μg/ml) of recombinant galectin-9, showing oligomerization of hensin to fraction 7.
To investigate the effect of recombinant galectin-9 in mediating hensin oligomerization, we incubated concentrated conditioned medium from LD cultures (100 μg) with 0.7 μM recombinant galectin-9 (32 μg; molar equivalent to 20 μg galectin-3) at room temperature for 1 h and investigated the OptiPrep fractions by Western blotting with anti-hensin antibody as before. The result showed that hensin bands were detected in fractions 2–7, representing oligomeric forms up to 600 kDa (Fig. 8B). This result demonstrated that recombinant galectin-9 was able to convert monomeric hensin to oligomeric hensin, although not to the same extent as that of galectin-3.
DISCUSSION
In a previous study, in light of the observation that galectin-3 coeluted with hensin in an ion exchange column despite their tremendously different isoelectric points (hensin pI = 4.86, galectin-3 pI = 8.95), it was suggested that galectin-3 may be interacting with hensin via protein-protein interactions rather than carbohydrate-mediated interactions (10). Our studies clearly demonstrated that only the COOH-terminal CRD domain of galectin-3 interacted with hensin in a dose-dependent manner; the NH2-terminal domain did not interact. Furthermore, galectin-3 failed to interact with the deglycosylated form of hensin, and the galectin-3-hensin interaction was competitively inhibited by the addition of lactose. These results unequivocally established that galectin-3 interacted with hensin through its CRD. In this context, it is noteworthy that a recent study utilizing surface plasmon resonance technology (SPR) has established that the oligosaccharide side chains of DMBT1 (a human ortholog of hensin expressed in mucosal epithelia) are recognized by the CRD of galectin-3 and modification in the pattern of oligosaccharides modulates the binding parameters of DMBT1 with galectin-3 (21).
Our results demonstrated that although galectin-3 interacted with hensin through its lectin domain, full-length galectin-3 containing the nonlectin NH2-terminal domain was required for oligomerizing hensin. Several biochemical studies have shown that, when bound to multivalent glycoconjugates, galectin-3 self-associates through intermolecular interactions involving the NH2-terminal domain (11, 17). This NH2-terminal-dependent self-oligomerization of galectin-3 has been proposed to be critical for most of the extracellular functions of galectin-3 including cellular adhesion, signal transduction through receptor clustering, and lattice formation (18). Our observation that LD cultures formed HD phenotypes only in patches suggested that, in addition to mediating hensin oligomerization, galectin-3 might be mediating receptor clustering. In this context, it is significant to note that galectin-3 is observed in an ECM pattern in terminally differentiated cells of the intestine and prostate compared with its cytoplasmic pattern in less differentiated epithelia (6, 12). We have previously demonstrated that αvβ1-integrin clustering regulates ECM assembly of hensin and α6-integrin participates in the ECM hensin-triggered signal transduction in intercalated cells (28). In future studies, it will be interesting to directly test whether galectin-3 participates in clustering of these integrin receptors.
Although there was some confusion in the cell types expressing galectin-3 in the kidney collecting duct, results from several studies including our own have established that both principal cells and α-intercalated cells express galectin-3 in various species. In rabbits, using double-immunolabeling studies, we showed that galectin-3 was expressed in the α-intercalated cells and principal cells of the medullary collecting duct and CCD (22). Other studies have confirmed the expression of galectin-3 in the α-intercalated cells of the human medullary collecting duct and in the α-intercalated cells of the mouse inner medullary collecting duct (3, 32).
Many different lines of evidence support a role for galectin-3 in the adaptation of the kidney to metabolic acidosis. In a previous study, using immunostaining techniques, we observed that after 3 days of acid loading the intensity of cellular staining for galectin-3 and the number of cells expressing galectin-3 increased in the CCDs (22). The percentage of CCD cells expressing galectin-3 increased from ∼26% to 66% in the outer cortex and from 64% to 78% in the inner cortex. Many β-intercalated cells expressed galectin-3 after acidosis. Moreover, after acid loading both galectin-3 and hensin were found in the ECM of microdissected CCDs. Subsequent to our study, Cheval et al. (4) showed that galectin-3 was one of the most significant tags identified by serial analysis of gene expression (SAGE) analysis to be upregulated in outer medullary collecting ducts from mice with metabolic acidosis. They validated their SAGE analysis results by performing real-time PCR on RNA extracted from microdissected CCDs obtained from 3-day and 14-day acidotic mice. The real-time PCR studies showed a significant 2.8-fold increase in galectin-3 mRNA levels in 3-day acidotic mice and a 5.1-fold increase in galectin-3 mRNA levels in 14-day acidotic mice (4). Our new results from this study showing a significant threefold increase in the galectin-3 mRNA levels in the cortex of 3-day acidotic rabbits compared with those from normal rabbits are consistent with the results of Cheval et al. (4) and point to an important role for galectin-3 in acid-base regulation.
Despite these strong indicators pointing to a key role for galectin-3 in acid-base regulation, galectin-3-null mutant mice exhibited no significant difference in urinary bicarbonate excretion or in serum bicarbonate concentration (3). In the present study, we observed no significant difference in the expression of α-intercalated cell chloride-bicarbonate exchanger kAE1 in galectin-3 null mice, although a significant reduction in its expression has been reported in hensin-null mice (9). These observations led us to explore other kidney galectins that could possibly substitute for the function of galectin-3 in its absence. Our results described in this report suggested that galectin-9 is a possible candidate that could replace the functions of galectin-3 in its role in acid-base regulation. Further studies, possibly using double-mutant mice (for galectin-3 and galectin-9), will be needed to confirm this possibility.
Our studies reveal the presence of galectin-9 protein in kidney lysates but not in the cell lysates of intercalated cell lines. However, a significant amount of galectin-9 expression at the transcript level is noted in the cell lines, consistent with previous observations in MDCK cells. Further investigation of the synthesis and half-life of galectin-9 protein in clone C cells would be important. It would also be interesting to determine what other cell types in the kidney synthesize galectin-9.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5 RO1 DK-050603-32 (to G. J. Schwartz) and support from the Department of Pediatrics, University of Rochester to S. Vijayakumar.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). G. J. Schwartz was a consultant for Novartis.
AUTHOR CONTRIBUTIONS
Author contributions: S.V. and G.J.S. conception and design of research; S.V. and H.P. performed experiments; S.V., H.P., and G.J.S. analyzed data; S.V., H.P., and G.J.S. interpreted results of experiments; S.V. prepared figures; S.V. and H.P. drafted manuscript; S.V. and G.J.S. edited and revised manuscript; S.V. and G.J.S. approved final version of manuscript.
REFERENCES
- 1. Ahmad N, Gabius HJ, André S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem 279: 10841–10847, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Barboni EA, Bawumia S, Hughes RC. Kinetic measurements of binding of galectin 3 to a laminin substratum. Glycoconj J 16: 365–373, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Bichara M, Attmane-Elakeb A, Brown D, Essig M, Karim Z, Muffat-Joly M, Micheli L, Eude-Le Parco I, Cluzeaud F, Peuchmaur M, Bonvalet JP, Poirier F, Farman N. Exploring the role of galectin 3 in kidney function: a genetic approach. Glycobiology 16: 36–45, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Cheval L, Morla L, Elalouf JM, Doucet A. Kidney collecting duct acid-base “regulon.” Physiol Genomics 27: 271–281, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Cooper DN. Galectinomics: finding themes in complexity. Biochim Biophys Acta 1572: 209–231, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Delacour D, Koch A, Ackermann W, Eude-Le Parco I, Elsässer HP, Poirier F, Jacob R. Loss of galectin-3 impairs membrane polarisation of mouse enterocytes in vivo. J Cell Sci 121: 458–465, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Dumic J, Dabelic S, Flogel M. Galectin-3: an open ended story. Biochim Biophys Acta 1760: 616–635, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Friedrichs J, Torkko JM, Helenius J, Teräväinen TP, Füllekrug J, Muller DJ, Simons K, Manninen A. Contributions of galectin-3 and -9 to epithelial cell adhesion analyzed by single cell force spectroscopy. J Biol Chem 282: 29375–29383, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Gao X, Eladari D, Leviel F, Tew BY, Miró-Julià C, Cheema F, Miller L, Nelson R, Paunescu TG, McKee M, Brown D, Al-Awqati Q. Deletion of hensin/DMBT1 blocks conversion of beta- to alpha-intercalated cells and induces distal renal tubular acidosis. Proc Natl Acad Sci USA 107: 21872–21877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hikita C, Vijayakumar S, Takito J, Erdjument-Bromage H, Tempst P, Al-Awqati Q. Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin-3. J Cell Biol 151: 1235–1246, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsu DK, Zuberi R, Liu FT. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem 267: 14167–14174, 1992 [PubMed] [Google Scholar]
- 12. Hughes RC. Galectins in kidney development. Glycoconj J 19: 621–629, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Jeon SB, Yoon HJ, Chang CY, Koh HS, Jeon SH, Park EJ. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol 185: 7037–7046, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Kim YH, Verlander JW, Matthews SW, Kurtz I, Shin W, Weiner ID, Everett LA, Green ED, Nielsen S, Wall SM. Intercalated cell H+/OH- transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol 289: F1262–F1272, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific β-1,2-linked mannans. J Immunol 17: 4718– 4726, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer 5: 29–41, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Mehul B, Bawumia S, Martin SR, Hughes RC. Structure of baby hamster kidney carbohydrate-binding protein CBP30, an S-type animal lectin. J Biol Chem 269: 18250–18258, 1994 [PubMed] [Google Scholar]
- 18. Nieminen J, Kuno A, Hirabayashi J, Sato S. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem 282: 1374–1383, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Peng H, Vijayakumar S, Schiene-Fischer C, Li H, Purkerson JM, Malesevic M, Liebscher J, Al-Awqati Q, Schwartz GJ. Secreted cyclophilin A, a peptidyl prolyl cis-trans isomerase, mediates matrix assembly of hensin, a protein implicated in epithelial differentiation. J Biol Chem 284: 6465–6475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poland PA, Rondanino C, Kinlough CL, Heimburg-Molinaro J, Arthur CM, Stowell SR, Smith DF, Hughey RP. Identification and characterization of endogenous galectins expressed in Madin Darby canine kidney cells. J Biol Chem 286: 6780–6790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossez Y, Coddeville B, Elass E, Quinchon JF, Vidal O, Corfield AP, Gosset P, Lacroix JM, Michalski JC, Robbe-Masselot C. Interaction between DMBT1 and galectin 3 is modulated by the structure of the oligosaccharides carried by DMBT1. Biochimie 93: 593–603, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Schwaderer AL, Vijayakumar S, Al-Awqati Q, Schwartz GJ. Galectin-3 expression is induced in renal β-intercalated cells during metabolic acidosis. Am J Physiol Renal Physiol 290: F148–F158, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Schwartz GJ, Tsuruoka S, Vijayakumar S, Petrovic S, Mian A, Al-Awqati Q. Acid incubation reverses the polarity of intercalated cell transporters, an effect mediated by hensin. J Clin Invest 109: 89–99, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimura K, Arata Y, Uchiyama N, Hirabayashi J, Kasai K. Determination of the affinity constants of recombinant human galectin-1 and -3 for simple saccharides by capillary affinophoresis. J Chromatogr B Analyt Technol Biomed Life Sci 768: 199–210, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Takito J, Hikita C, Al-Awqati Q. Hensin, a new collecting duct protein involved in the in vitro plasticity of intercalated cell polarity. J Clin Invest 98: 2324–2331, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Adelsberg J, Edwards JC, Takito J, Kiss B, Al-Awqati Q. An induced extracellular matrix protein reverses the polarity of band 3 in intercalated epithelial cells. Cell 76: 1053–1061, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Vijayakumar S, Erdjument-Bromage H, Tempst P, Al-Awqati Q. Role of integrins in the assembly and function of hensin in intercalated cells. J Am Soc Nephrol 19: 1079–1091, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vijayakumar S, Takito J, Hikita C, Al-Awqati Q. Hensin remodels the apical cytoskeleton and induces columnarization of intercalated epithelial cells: processes that resemble terminal differentiation. J Cell Biol 144: 1057–1067, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wada J, Ota K, Kumar A, Wallner EI, Kanwar YS. Developmental regulation, expression, and apoptotic potential of galectin-9, a beta-galactoside binding lectin. J Clin Invest 99: 2452–2461, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wasano K, Hirakawa Y, Yamamoto T. Immunohistochemical localization of 14 kDa galactoside-binding lectin in various organs of the rat. Cell Tissue Res 259: 43–49, 1990 [DOI] [PubMed] [Google Scholar]
- 32. Winyard PJ, Bao Q, Hughes RC, Woolf AS. Epithelial galectin-3 during human nephrogenesis and childhood cystic diseases. J Am Soc Nephrol 8: 1647–1657, 1997 [DOI] [PubMed] [Google Scholar]