Abstract
Chronic infection of the gastric mucosa by Helicobacter pylori is associated with an increased risk of developing gastric cancer; however, the vast majority of infected individuals never develop this disease. One H. pylori virulence factor that increases gastric cancer risk is the cag pathogenicity island, which encodes a bacterial type IV secretion system. Cyclooxygenase-2 (COX-2) expression is induced by proinflammatory stimuli, leading to increased prostaglandin E2 (PGE2) secretion by gastric epithelial cells. COX-2 expression is increased in gastric tissue from H. pylori-infected persons. H. pylori also activates the epidermal growth factor receptor (EGFR) in gastric epithelial cells. We now demonstrate that H. pylori-induced activation of COX-2 in gastric cells is dependent upon EGFR activation, and that a functional cag type IV secretion system and direct bacterial contact are necessary for full induction of COX-2 by gastric epithelial cells. PGE2 secretion is increased in cells infected with H. pylori, and this induction is dependent on a functional EGFR. Increased apoptosis in response to H. pylori occurs in cells treated with a COX-2 inhibitor, as well as COX-2−/− cells, indicating that COX-2 expression promotes cell survival. In vivo, COX-2 induction by H. pylori is significantly reduced in mice deficient for EGFR activation compared with wild-type mice with a fully functional receptor. Collectively, these findings indicate that aberrant activation of the EGFR-COX-2 axis may lower the threshold for carcinogenesis associated with chronic H. pylori infection.
Keywords: cyclooxygenase-2, EGFR, Helicobacter pylori
helicobacter pylori selectively colonizes human gastric mucosa and induces an inflammatory response that persists for decades. In a subset of infected individuals, the chronic inflammatory process progresses to gastric atrophy, followed by intestinal metaplasia, dysplasia, and finally adenocarcinoma (5). Both bacterial strain and host constituents contribute to the development of H. pylori-induced cancer (17). Chronic infection with H. pylori strains harboring the cag pathogenicity island, which encodes a type IV secretion system (T4SS), is characterized by more severe inflammation and an increased risk for gastric cancer compared with infection with cag-negative strains (9).
Cyclooxygenase-2 (COX-2) catalyzes the conversion of arachidonic acid to prostaglandins (PGs), the biological effects of which are mediated by prostanoid receptors (EP receptors). Levels of COX-2 expression are low under resting conditions in most cells, but can be induced by proinflammatory and mitogenic stimuli (25). Overexpression of COX-2 has been implicated in the development of gastric cancer, since COX-2 inhibits apoptosis and increases invasiveness of malignant cells (23). The most abundant PG produced by COX-2 in gastric cancer is PGE2 (22). PGE2 binds to four subtypes of receptors (EP1-EP4) and promotes tumor growth by stimulating cell proliferation, promoting angiogenesis, inhibiting apoptosis, inducing invasion, and suppressing immune activation (25).
Expression of COX-2 is increased within gastric mucosa of patients infected with H. pylori and in experimentally infected Mongolian gerbils (7, 18, 19). Treatment of H. pylori-infected animals with specific COX-2 inhibitors reduces the intensity of mucosal inflammation induced by the bacteria; similarly, long-term ingestion of nonsteroidal anti-inflammatory drugs that inhibit COX activity decreases the risk for gastric cancer (24, 26). H. pylori also upregulates the expression of epidermal growth factor receptor (EGFR) in gastric epithelial cells through transactivation of EGFR (12). Recently, we demonstrated that transactivation of EGFR by H. pylori results in activation of protein kinase B/phosphatidylinositol 3-kinase activity as well as upregulation of Bcl-2, which inhibits gastric epithelial cell apoptosis (31). EGFR has been implicated in mediating the oncogenic activity of PGE2, and, conversely, PGE2 can transactivate EGFR in colon cancer and gastric epithelial cells (29).
In this study, we investigated the role of EGFR activation and downstream signaling cascades triggered by H. pylori in the induction of COX-2 expression using conditionally immortalized mouse gastric epithelial cells as a model. These cells are unique in that they mimic primary gastric epithelial cells when grown under nonimmortalizing conditions. Our results show that direct interaction between H. pylori and gastric epithelial cells is necessary for full induction of COX-2 expression, and a functional cag T4SS is required for this process. Soluble factors derived from H. pylori also induce COX-2 expression, but to a lesser extent than direct contact of the bacterium with host cells. Inhibition of COX-2 expression increases apoptosis in response to H. pylori. Using wild-type (WT) and EGFR-deficient (Egfrwa5) mice as an in vivo model, we also demonstrate that a fully functional EGFR is necessary for COX-2 expression in response to H. pylori.
MATERIALS AND METHODS
Cell lines.
Conditionally immortalized stomach (ImSt) epithelial cells were harvested from the gastric epithelium of H-2Kb-tsA58 mice as previously described (10, 28). EGFR−/− and COX-2−/− ImSt were isolated from the corresponding knockout mice crossed with the Immortomouse (H-2Kb-tsA58) at the Vanderbilt University Digestive Disease Research Center Novel Cell Line Development Core as described (27, 28). Cells were maintained in RPMI 1640 media containing 10% fetal bovine serum and 5 U/ml of murine interferon-γ (IFN-γ) at 33°C and 5% CO2. Cells were transferred to 37°C (nonpermissive temperature) in 0.5% fetal bovine serum-RPMI without IFN-γ for 18–24 h before treatment or infection.
H. pylori coculture and cell stimulation assays, preparation of cell lysates, and Western blot analysis.
The H. pylori cag+ rodent-adapted strain 7.13 and its isogenic cagA− and cagE− mutants (16) were grown in Brucella broth with 5% FBS for 18 h, harvested by centrifugation, and used to infect gastric epithelial cells at a multiplicity of infection of 100:1 (H. pylori-gastric cells) for the times indicated. For ligand stimulation, cells were treated with 10 ng/ml of epidermal growth factor (EGF) (Peprotech). For some experiments, direct contact between gastric epithelial cells and H. pylori was prevented by the use of Transwell filters (Corning) with a pore size of 0.2 μm. AG-1478 (Calbiochem) was added to the cells 1 h before infection at a final concentration of 1 μM. After coculture or ligand stimulation, cell monolayers were lysed and prepared for SDS-PAGE. Denatured cell lysates were resolved by SDS-PAGE and used for Western blot analysis. Primary and secondary antibodies used in Western blot analyses included rabbit anti-phospho-EGFR Y1068 (Cell Signaling), rabbit anti-EGFR (Millipore), mouse anti-total actin (Sigma), horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit polyclonal (Cell Signaling Technologies), and goat anti-COX-2 and HRP-conjugated anti-goat polyclonal (Santa Cruz Biotechnologies) antibodies.
Mice.
The Institutional Animal Care and Use Committee at Vanderbilt University approved all animal experiments. C57BL/6 WT and Egfrwa5 mice were used at 8–12 wk of age. Egfrwa5 mice harbor a dominant-negative mutation in one of the egfr alleles, which is located within the region encoding for the activation loop of the EGFR kinase (13). Mice were gavaged with 1 × 109 H. pylori strain 7.13 or Brucella broth alone as a control. After 48 h, mice were killed, and stomachs were removed and used for isolation of gastric epithelial cells as previously described (4).
Small-interfering RNA transfections.
ImSt cells were plated at a seeding density of 3–6 × 105 cells/well in six-well plates and grown for 24 h at 33°C in cell growth media without IFN-γ. Cells were then transfected either with 200 nM siGenome SMARTpool number 2 nontargeting (NT) small-interfering RNA (siRNA) or 200 nM mouse EGFR siGenome SMARTpool siRNA (Dharmacon) using the Lipofectamine RNAiMax (Invitrogen) transfection reagent according to the manufacturer's instructions. Cells were then grown at 37°C for 16–18 h in serum-free RPMI before experiments.
PGE2 ELISA.
ImSt cells were cocultured with WT H. pylori strain 7.13 in 0.5% FBS-RPMI for the time points indicated. Conditioned media was harvested, centrifuged, and analyzed using a PGE2 ELISA (Assay Designs) according to the manufacturer's instructions.
Apoptosis.
ImSt cells were cocultured with WT H. pylori for 16 and 24 h, and cells were processed as previously described for annexin-V and propidium iodide staining and analyzed by flow cytometry (30).
Statistical analysis of experimental data.
All data shown are representative of at least three experimental replicates. Results were analyzed using GraphPad Prism data analysis software (GraphPad Software, La Jolla, CA) by one-way ANOVA with Newman-Keuls multiple-comparison test.
RESULTS
H. pylori stimulates the production of COX-2 by gastric epithelial cells in a cag T4SS-dependent manner.
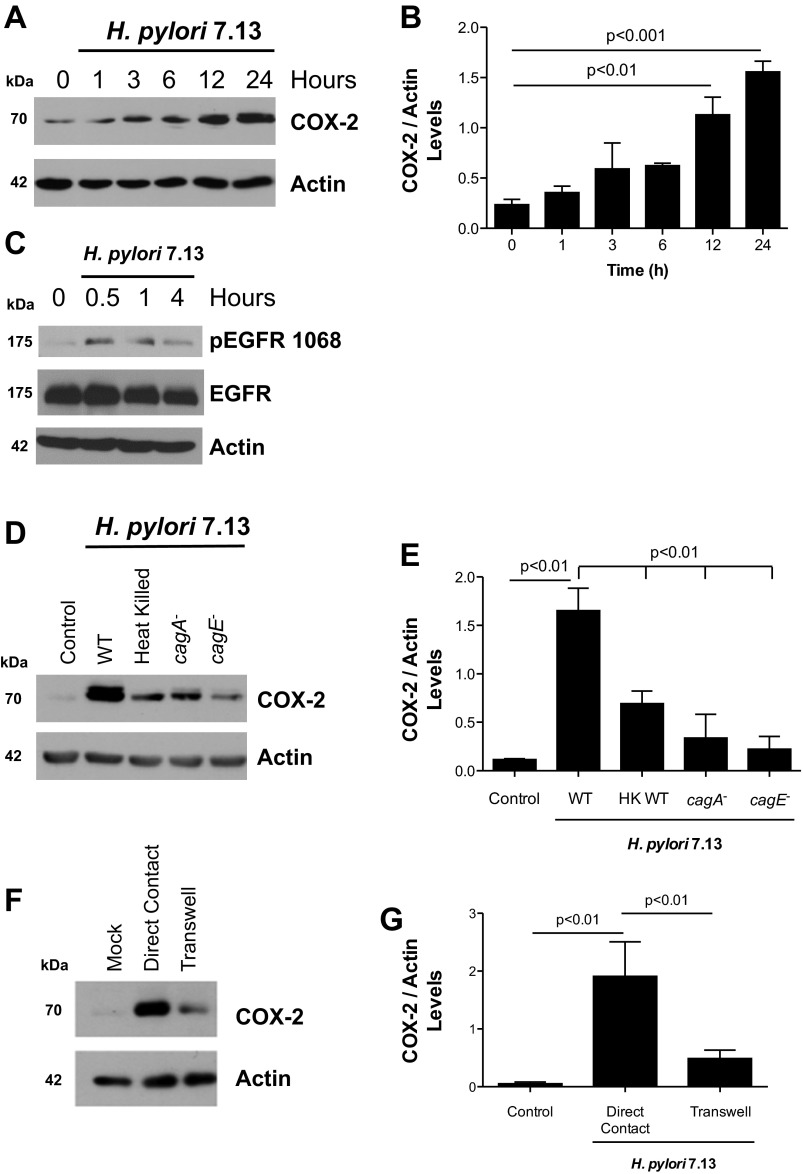
Previous studies examining the induction of COX-2 by H. pylori in gastric epithelial cells have been performed using cancer cell lines, which may not fully recapitulate COX-2 function and/or regulatory signaling that occurs in vivo. Therefore, we investigated the regulation of COX-2 production in a more biologically relevant model of normal gastric epithelium by using conditionally immortalized mouse stomach (ImSt) epithelial cells. Coculture of ImSt with the H. pylori cag+ carcinogenic strain 7.13 induced COX-2 expression as early as 3 h postinfection, and levels of COX-2 continued to increase up to 24 h postinfection (Fig. 1, A and B). Additionally, we evaluated EGFR activation in gastric epithelial cells after infection with H. pylori. Phosphorylation of EGFR at tyrosine residue 1068 was observed 30 min after addition of the bacteria (Fig. 1C). To determine if increased COX-2 production was dependent on CagA and/or a functional T4SS, ImSt cells were cocultured with the WT H. pylori strain 7.13 or isogenic mutants lacking cagA and cagE (encoding a structural element of the T4SS) for 24 h, and cell lysates were analyzed by Western blotting. COX-2 levels were significantly decreased in ImSt cells cocultured with the isogenic cagA− and cagE− mutants, compared with levels observed in cells cocultured with H. pylori WT strain 7.13 (Fig. 1, D and E), indicating that a functional T4SS is necessary for the full induction of COX-2 by H. pylori in this model.
Fig. 1.
Helicobacter pylori stimulation of cyclooxygenase-2 (COX-2) production in mouse gastric epithelial cells is mediated in part by a functional cag pathogenicity island. A: mouse gastric epithelial cells [immortalized stomach (ImSt)] were cocultured with H. pylori at a multiplicity of infection (MOI) of 100 for the indicated times. Expression of COX-2 and actin was evaluated by Western blot analysis. B: densitometric analysis of replicate experiments measuring COX-2 levels induced at different time points after infection with H. pylori normalized to actin levels. C: ImSt cells were cocultured with H. pylori at MOI of 100 for the indicated times. Expression of phospho (p)-epidermal growth factor receptor (EGFR) Y1068, total EGFR, and actin was evaluated by Western blot analysis. D: ImSt cells were cocultured with H. pylori wild-type (WT), cagA−, or cagE− mutants or heat-killed (100°C for 30 min before coculture) bacteria for 24 h, and COX-2 levels were evaluated by Western blotting. E: densitometric analysis of replicate experiments measuring COX-2 levels induced by H. pylori WT and isogenic mutant strains normalized to actin levels. F: ImSt cells were cocultured either in direct contact with H. pylori or separated from the bacteria by a permeable membrane (0.2 μm Transwell) for 24 h, and COX-2 expression was determined by Western blotting. G: densitometric analysis of replicate experiments measuring COX-2 levels induced by H. pylori cocultured in direct contact with ImSt cells or separated by a Transwell filter support. Total actin was used as loading control for all of the experiments. Figures are representative of three independent experiments. HK, heat killed.
We next investigated whether live bacteria were necessary for the induction of COX-2 by coculturing ImSt cells with heat-killed H. pylori. Expression of COX-2 was lower in cells treated with heat-killed bacteria compared with ImSt cells cocultured with live H. pylori (Fig. 1, D and E). To test whether direct cell contact was required for COX-2 induction, we used a Transwell filter with a permeable membrane to separate H. pylori from epithelial cells and then quantified COX-2 levels by Western blotting. As shown in Fig. 1, F and G, H. pylori added to the upper chamber of the Transwell induced lower levels of COX-2 compared with cells that were in direct contact with H. pylori. These results suggest that direct contact between gastric epithelial cells and H. pylori cag+ strains is required for maximal COX-2 induction.
Induction of COX-2 by H. pylori requires EGFR expression and kinase activity.
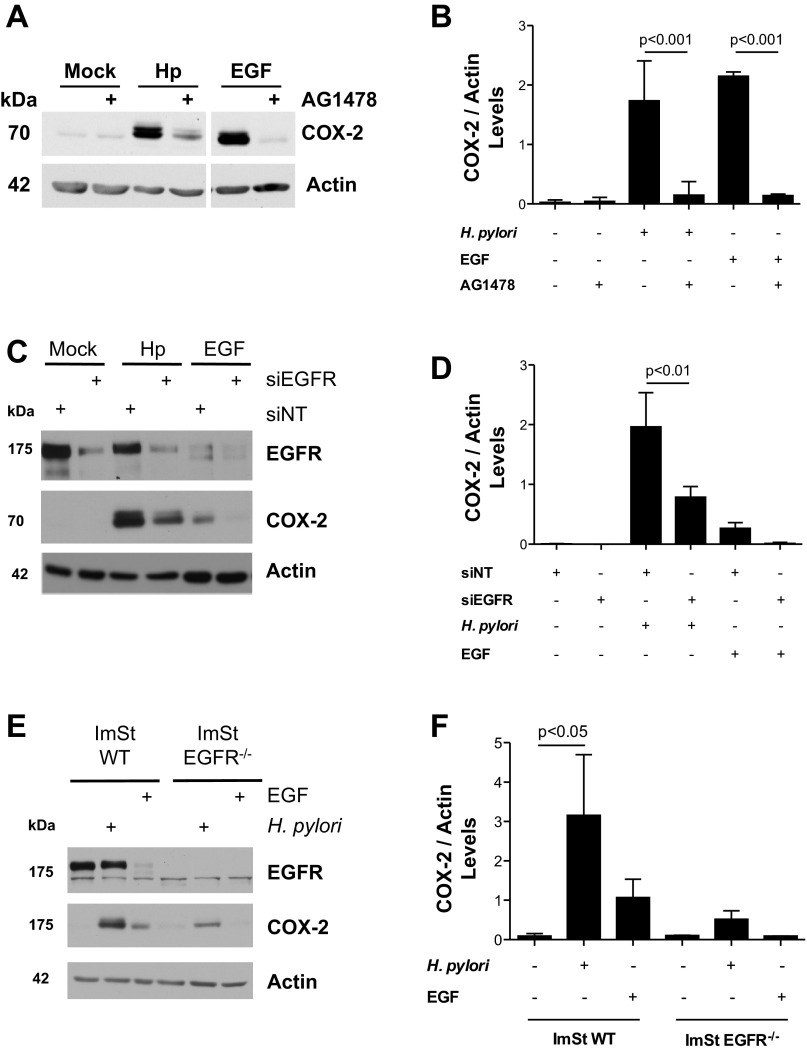
Previous studies have demonstrated that H. pylori transactivates EGFR in gastric epithelial cells (31). Because EGFR activation can induce COX-2 expression in other cell types, we determined whether H. pylori-stimulated COX-2 expression was mediated via transactivation of EGFR. ImSt cells were cocultured with H. pylori strain 7.13 in the presence or absence of AG-1478, a small-molecule inhibitor of EGFR kinase activity. As shown in Fig. 2, A and B, COX-2 production was significantly decreased in H. pylori-infected and EGF-treated ImSt cells that were pretreated with AG-1478. To further define a specific role for EGFR in the induction of COX-2, ImSt cells were transfected either with siRNA targeting EGFR or NT siRNA as a negative control. Following 18 h of transfection, cells were cocultured with H. pylori 7.13. Transfection of ImSt cells with EGFR siRNA significantly decreased EGFR expression levels in ImSt cells (Fig. 2, C and D). Transfection with EGFR siRNA also significantly reduced the expression of COX-2 in response to H. pylori or EGF compared with cells transfected with the NT siRNA (Fig. 2, C and D). We subsequently confirmed these data using EGFR-deficient mouse gastric epithelial cells (ImSt EGFR−/−). As shown in Fig. 2, E and F, the production of COX-2 by ImSt EGFR−/− cells cocultured with H. pylori was significantly lower compared with H. pylori-infected ImSt WT cells. Collectively, these results indicate that EGFR expression and activation is necessary for induction of COX-2 in ImSt cells in response to H. pylori infection.
Fig. 2.
Stimulation of COX-2 production in mouse gastric epithelial cells by H. pylori is mediated by EGFR activation. A: ImSt cells were cocultured with H. pylori (Hp, MOI 100), treated with 10 ng/ml of recombinant epidermal growth factor (EGF), or left untreated (Mock) for 24 h in the presence or absence of AG-1478 (1 μM). After 24 h, cell lysates were analyzed by Western blotting for COX-2 expression. Samples were run in a single gel but were not continuous, as indicated by a white space between lanes. B: densitometric analysis of replicate experiments measuring COX-2 levels induced by H. pylori in ImSt cells cultured in the presence or absence of AG-1478. C: ImSt cells transfected with either a nontargeting siRNA (siNT) or EGFR siRNA were cocultured with H. pylori (MOI 100) for 24 h. COX-2 and EGFR levels were assessed by Western blotting. D: densitometric analysis of replicate experiments measuring COX-2 levels induced by H. pylori in ImSt cells transfected with nontargeting or EGFR siRNA. E: WT ImSt cells or cells deficient in EGFR (EGFR−/−) were cocultured with H. pylori (MOI 100) for 24 h. The expression of EGFR and COX-2 was evaluated by Western blotting. F: densitometric analysis of replicate experiments measuring COX-2 levels induced by H. pylori in WT ImSt cells or EGFR−/− ImSt. Data are representative of three different experiments. Actin was used as loading control for all of the experiments.
Induction of COX-2 by H. pylori leads to increased PGE2 secretion by gastric epithelial cells.
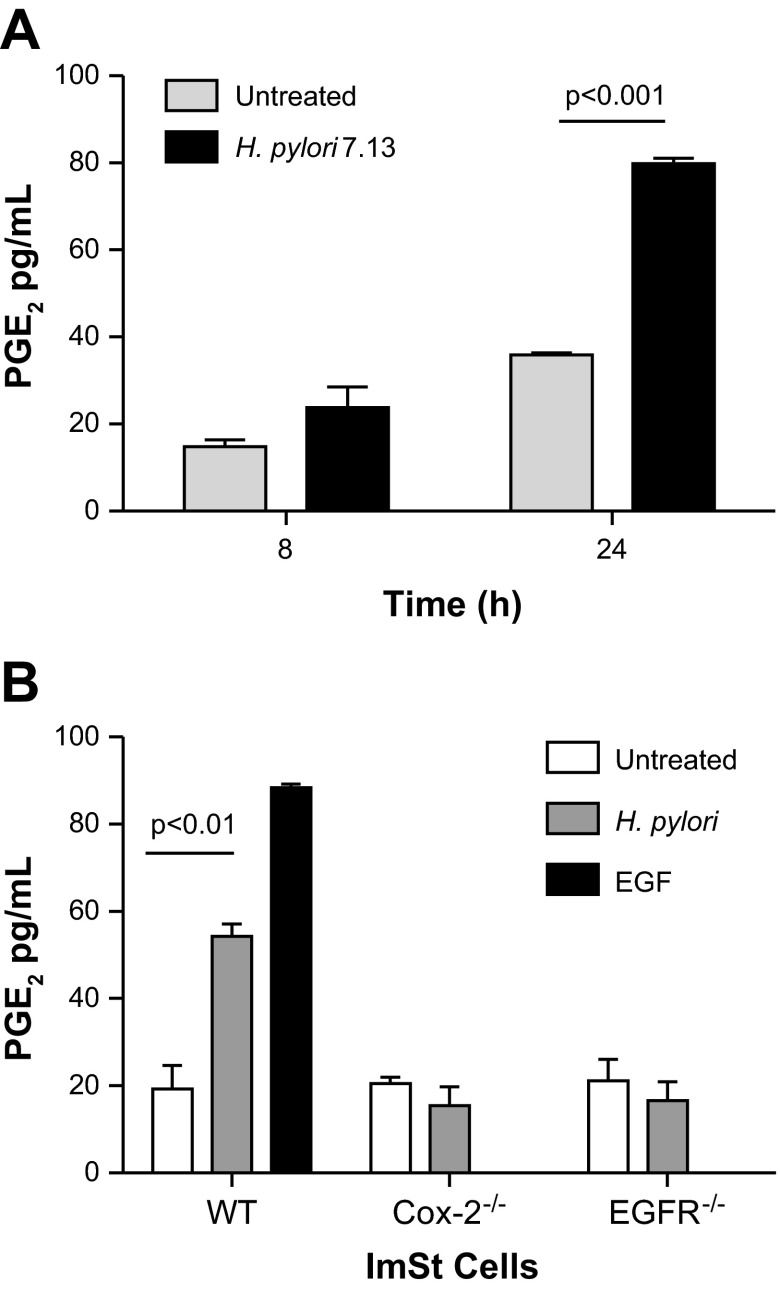
COX-2 catalyzes the production of PGE2 from arachidonic acid that is released from the plasma membrane by phospholipase A2; therefore, we quantified the production of PGE2 by ImSt cells cocultured with H. pylori. Following 8 h of infection, a slight increase in PGE2 production occurred in infected compared with untreated cells, but this significantly increased after 24 h of infection (Fig. 3A). Because PGE2 generation can be catalyzed by both COX-1 and COX-2, we also evaluated PGE2 production induced by H. pylori using COX-2-deficient cells (ImSt COX-2−/−). As shown in Fig. 3B, no detectable increases in PGE2 production were observed in ImSt COX-2−/− cells cocultured with H. pylori. We also investigated the role of EGFR in PGE2 production by measuring the secretion of PGE2 by ImSt EGFR−/− cells after coculture with H. pylori. No significant increases in levels of PGE2 production were observed in EGFR-deficient cells cocultured with H. pylori compared with untreated cells (Fig. 3B). As expected, treatment of WT ImSt cells with EGF increased the secretion of PGE2 (Fig. 3B). These results indicate that induction of COX-2 by H. pylori results in an increased production of PGE2 by gastric epithelial cells and that this production is dependent on EGFR.
Fig. 3.
H. pylori increases the release of prostaglandin E2 (PGE2) by mouse gastric epithelial cells. A: ImSt cells were cocultured with H. pylori strain 7.13 (MOI 100), and supernatants were recovered after 8 and 24 h for PGE2 quantification by ELISA. B: ImSt cells, either WT, COX-2−/−, or EGFR−/−, were cocultured with H. pylori for 24 h, and supernatants were recovered to assess PGE2 production. ImSt cells were treated with 10 ng/ml of recombinant EGF as a positive control. Data are expressed as means ± SE from three independent experiments.
Increased COX-2 production by H. pylori attenuates apoptosis in gastric epithelial cells.
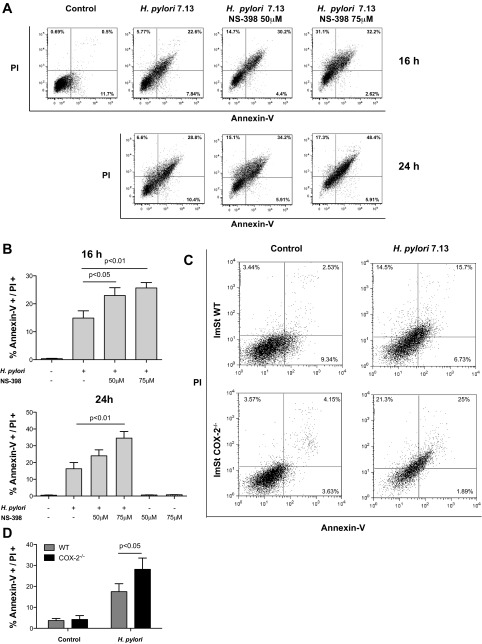
Increased expression of COX-2 is linked to tumor development, and PGE2 has been identified as the critical prostanoid promoting this phenotype in colorectal tumors (8). After PGE2 binds to its receptors, multiple cellular responses can be activated, including cell survival (8). Because infection by H. pylori is involved in the development of gastric cancer and our data indicate that COX-2 production is increased in gastric epithelial cells cocultured with H. pylori, we assessed the role of COX-2 in H. pylori-induced apoptosis. ImSt WT cells were treated with NS-398, a specific COX-2 inhibitor, or vehicle control for 1 h and then cocultured for 16 or 24 h with H. pylori. Apoptosis was assessed by annexin-V and propidium iodide staining, and the percentage of apoptotic cells was analyzed by flow cytometry (Fig. 4A). Inhibition of COX-2 induced a significant increase in apoptosis in ImSt cells infected with H. pylori compared with levels observed in infected ImSt cells that were not treated with NS-398 (Fig. 4B). We also assessed apoptosis in ImSt WT and COX-2−/− cells cocultured with H. pylori. The percentage of apoptotic cells was significantly increased in H. pylori-infected ImSt COX-2−/− cells compared with ImSt WT (Fig. 4, C and D). These results suggest that H. pylori-induced COX-2 activity inhibits apoptosis, which may play a role in carcinogenesis.
Fig. 4.
Increased expression of COX-2 by gastric epithelial cells in response to H. pylori attenuates apoptosis. A: ImSt cells were cocultured with H. pylori (MOI 100) for 16 and 24 h with or without pretreatment with NS-398, and cells were stained with annexin-V FITC and propidium iodide (PI). Samples were analyzed by flow cytometry, and the percentage of double-positive cells, representing late apoptosis, was determined. Representative flow dot plots are shown. B: percentages of double-positive cells from three experiments are expressed as means ± SE. C: ImSt cells, either WT or COX-2−/−, were cocultured with H. pylori for 24 h, and cells were stained with annexin-V FITC and PI. Representative flow dot plots are shown. D: percentages of double-positive cells from three experiments are expressed as means ± SE.
COX-2 expression in H. pylori-infected mouse gastric epithelial cells in vivo is mediated by EGFR activation.
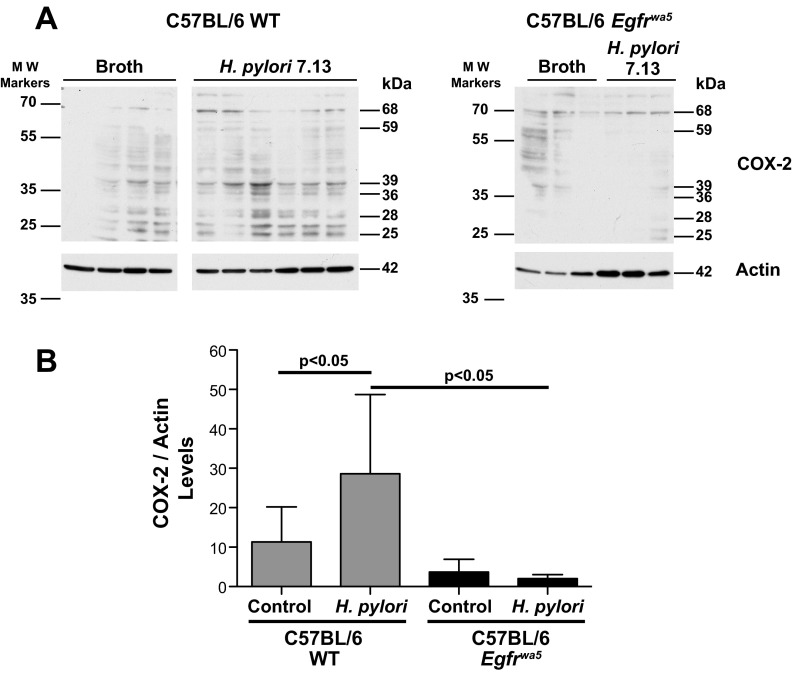
We next extended our results into a rodent model of H. pylori infection. To evaluate the ability of H. pylori to induce COX-2 through EGFR activation in vivo, we compared WT mice with Egfrwa5 mice. C57BL/6 WT and Egfrwa5 littermates were challenged with H. pylori for 48 h, and COX-2 expression was assessed by Western blotting in epithelial cells isolated from stomach specimens. We observed a significant increase in COX-2 expression in the gastric epithelium of WT mice challenged with H. pylori compared with Egfrwa5 mice (Fig. 5, A and B), results that mirrored our findings in vitro (Fig. 2, C and E) with the EGFR inhibitor (Fig. 2A) and with siRNA targeting EGFR (Fig. 2C).
Fig. 5.
Induction of COX-2 expression in gastric epithelial cells isolated from mice challenged with H. pylori. A: gastric epithelial cells were isolated from C57BL/6 WT and Egfrwa5 mice challenged with H. pylori for 48 h or nonchallenged controls, and lysates were prepared and analyzed by Western blotting for COX-2 expression. Each lane represents cells isolated from individual mice. The multiple bands shown represent the expected full-length COX-2 size of 68 kDa plus COX-2 sequence-specific proteolysis products, at 59, 39, 36, 28, and 25 kDa as previously reported (14a). Samples were run in a single gel but were not continuous as indicated where shown by a white space between lanes. B: densitometric analysis of COX-2 levels was performed using actin as a loading control. Full-length COX-2, as well as the cleaved products at the band sizes listed above in A, were considered for the densitometric analysis.
DISCUSSION
In this study, we demonstrated that an H. pylori cag+ isolate is able to induce the expression of COX-2 in primary gastric epithelial cells through transactivation of EGFR. Previous studies have shown that H. pylori strains isolated from gastric cancer patients induce higher expression levels of COX-2 in vitro (3). Consistent with those results, we observed increased expression of COX-2 in response to H. pylori strain 7.13, an isolate capable of inducing gastric cancer in Mongolian gerbils and INS-GAS mice (6). We also showed that the induction of COX-2 is dependent on a functional T4SS; however, deletion of cagE or lack of direct contact between cells and the bacteria did not completely eliminate the upregulation of COX-2 in response to H. pylori, suggesting that a soluble virulence factor(s) could be involved in this process. In support of this, a previous study using bacterial culture supernatants from H. pylori to stimulate MKN28 cells showed that γ-glutamyltranspeptidase present in the supernatant upregulates COX-2 expression (1). Conversely, other reports have indicated that the cag pathogenicity island is not involved in cox-2 gene expression induced by H. pylori (1, 11). The discrepancy with our data could be explained by differences in the H. pylori strains used as well as the use of transformed cell lines in prior studies, which is in contrast to the conditionally immortalized cell model system employed in the current study. Indeed, we performed all the experiments under nonimmortalized conditions to avoid confounding effects exerted by the transformed phenotype of cancer cell lines. The involvement of the cag pathogenicity island in the induction of COX-2 has been also confirmed in H. pylori-infected Mongolian gerbils, where COX-2 mRNA levels increased after infection with WT H. pylori, but which were significantly lower in animals infected with a cagE mutant strain (18).
H. pylori transactivates EGFR and upregulates its expression creating an autocrine loop (12). This continuous activation of EGFR by H. pylori initiates signaling pathways that are both pro-mitotic and anti-apoptotic, potentially leading to unrestrained proliferation and eventually to gastric neoplasia. COX-2 overexpression has been documented in numerous types of cancer, including lung, colon, stomach, pancreas, and breast (25). Additionally, overexpression of COX-2 has been shown to regulate tumor cell invasiveness and angiogenesis and also promote apoptosis resistance in colon cancer cells (14, 21). In this study, we showed that downregulation of EGFR expression by siRNA treatment or chemical inhibition of its kinase activity results in reduced expression of COX-2 in response to H. pylori. Additionally, by using a rodent model of infection, we demonstrated that COX-2 expression was increased in WT, but not in Egfrwa5, mice after challenge with H. pylori. These results indicate that stimulation of COX-2 production in gastric epithelial cells by H. pylori is mediated by EGFR activation.
The main product of COX-2 activation is PGE2, which binds to EP receptors and can promote cancer progression by stimulating cell motility, invasion, and tumor-associated angiogenesis as well as promoting cell survival (2). We found PGE2 secretion to be increased over time and to be mediated by COX-2 in gastric epithelial cells exposed to H. pylori, and this secretion was EGFR dependent. We also observed increased apoptosis in response to H. pylori infection when gastric epithelial cells were treated with a COX-2 inhibitor and also in ImSt COX-2−/− cells. Increased secretion of PGE2 may also affect adaptive immunity, since it is known to suppress the T helper 1 (Th1) immune response to bacterial infection (15). Th1-type responses are crucial to control the infection but, at the same time, can promote the development of gastric cancer precursor lesions through the production of IFN-γ in response to H. pylori (20). A recent report indicated that inhibition of COX-2, which leads to a concomitant reduction in PGE2 levels, accelerated the development of gastritis and premalignant lesions in a model of H. felis-induced, T cell-driven gastric preneoplasia (20). However, additional studies using other mouse models of H. pylori-induced gastric cancer are necessary to clarify the contribution of COX-2 to the development of preneoplasic lesions.
In summary, we have demonstrated that activation of EGFR in primary gastric epithelial cells by H. pylori leads to increased COX-2 expression and that this response is partially dependent on a functional T4SS. Augmented COX-2 expression results in increased PGE2 secretion, leading to attenuated apoptosis in cells infected with H. pylori, which may play a role in carcinogenesis induced by this pathogen over prolonged periods of colonization.
GRANTS
This work was supported by National Institutes of Health Grants P01-CA-116087 (to K. T. Wilson, R. M. Peek, Jr., D. B. Polk), P01-CA-028842 (to K. T. Wilson), R01-DK-053620 (to K. T. Wilson), K01-AT-007324 (to R. Chaturvedi), RO1-CA-077955 and RO1-DK-058587 (to R. M. Peek, Jr.), RO1-DK-54993 and RO1-DK-56008 (to D. B. Polk); the Flow Cytometry Core of the Vanderbilt Digestive Disease Research Center supported by National Institutes of Health Grant P30-DK-058404; and Merit Review Grant 1I01-BX-001453 (to K. T. Wilson) from the Office of Medical Research, Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.C.S., S.S.H., R.C., F.Y., K.T.W., R.M.P.J., and D.B.P. conception and design of research; J.C.S., S.S.H., and R.C. performed experiments; J.C.S., S.S.H., and R.C. analyzed data; J.C.S., K.T.W., R.M.P.J., and D.B.P. interpreted results of experiments; J.C.S. prepared figures; J.C.S., F.Y., K.T.W., and R.M.P.J. drafted manuscript; J.C.S., S.S.H., R.C., F.Y., K.T.W., R.M.P.J., and D.B.P. edited and revised manuscript; J.C.S., S.S.H., R.C., F.Y., K.T.W., R.M.P.J., and D.B.P. approved final version of manuscript.
REFERENCES
- 1.Busiello I, Acquaviva R, Di Popolo A, Blanchard TG, Ricci V, Romano M, Zarrilli R. Helicobacter pylori gamma-glutamyltranspeptidase upregulates COX-2 and EGF-related peptide expression in human gastric cells. Cell Microbiol 6: 255–267, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med 58: 239–252, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol 66: 1465–1477, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo MB, Yan F, Israel DA, Casero RA, Jr, Correa P, Gobert AP, Polk DB, Peek RM, Jr, Wilson KT. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141: 1696–1708 e1691–E1692, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52: 6735–6740, 1992 [PubMed] [Google Scholar]
- 6.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, Whitehead R, Gaus K, O'Brien DP, Romero-Gallo J, Peek RM., Jr Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA 102: 10646–10651, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu S, Ramanujam KS, Wong A, Fantry GT, Drachenberg CB, James SP, Meltzer SJ, Wilson KT. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology 116: 1319–1329, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30: 377–386, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 125: 1636–1644, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88: 5096–5100, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juttner S, Cramer T, Wessler S, Walduck A, Gao F, Schmitz F, Wunder C, Weber M, Fischer SM, Schmidt WE, Wiedenmann B, Meyer TF, Naumann M, Hocker M. Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell Microbiol 5: 821–834, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Keates S, Keates AC, Katchar K, Peek RM, Jr, Kelly CP. Helicobacter pylori induces up-regulation of the epidermal growth factor receptor in AGS gastric epithelial cells. J Infect Dis 196: 95–103, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Lee D, Cross SH, Strunk KE, Morgan JE, Bailey CL, Jackson IJ, Threadgill DW. Wa5 is a novel ENU-induced antimorphic allele of the epidermal growth factor receptor. Mamm Genome 15: 525–536, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 276: 18563–18569, 2001 [DOI] [PubMed] [Google Scholar]
- 14a.(a)Mancini A, Jovanovic DV, He QW, Di Battista JA. Site-specific proteolysis of COX-2: a putative step in inflammatory prostaglandin E(2) biosynthesis. J Cell Biochem 101: 425–441, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Meyer F, Ramanujam KS, Gobert AP, James SP, Wilson KT. Cutting edge: cyclooxygenase-2 activation suppresses Th1 polarization in response to Helicobacter pylori. J Immunol 171: 3913–3917, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Nagy TA, Frey MR, Yan F, Israel DA, Polk DB, Peek RM., Jr Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J Infect Dis 199: 641–651, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer 10: 403–414, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai T, Fukui H, Franceschi F, Penland R, Sepulveda AR, Fujimori T, Terano A, Genta RM, Graham DY, Yamaoka Y. Cyclooxygenase expression during Helicobacter pylori infection in Mongolian gerbils. Dig Dis Sci 48: 2139–2146, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Takahashi S, Fujita T, Yamamoto A. Role of cyclooxygenase-2 in Helicobacter pylori-induced gastritis in Mongolian gerbils. Am J Physiol Gastrointest Liver Physiol 279: G791–G798, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Toller IM, Hitzler I, Sayi A, Mueller A. Prostaglandin E2 prevents Helicobacter-induced gastric preneoplasia and facilitates persistent infection in a mouse model. Gastroenterology 138: 1455–1467, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93: 705–716, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Uefuji K, Ichikura T, Mochizuki H. Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer. Clin Cancer Res 6: 135–138, 2000 [PubMed] [Google Scholar]
- 23.van Rees BP, Saukkonen K, Ristimaki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol 196: 171–179, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Walduck AK, Weber M, Wunder C, Juettner S, Stolte M, Vieth M, Wiedenmann B, Meyer TF, Naumann M, Hoecker M. Identification of novel cyclooxygenase-2-dependent genes in Helicobacter pylori infection in vivo (Abstract). Mol Cancer 8: 22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Dubois RN. Prostaglandins and cancer. Gut 55: 115–122, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang WH, Huang JQ, Zheng GF, Lam SK, Karlberg J, Wong BC. Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: a systematic review and meta-analysis. J Natl Cancer Inst 95: 1784–1791, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Whitehead RH, Robinson PS. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol 296: G455–G460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA 90: 587–591, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett 295: 7–16, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, Moss SF, Hacker A, Wang Y, Casero RA, Jr, Wilson KT. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res 64: 8521–8525, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Yan F, Cao H, Chaturvedi R, Krishna U, Hobbs SS, Dempsey PJ, Peek RM, Jr, Cover TL, Washington MK, Wilson KT, Polk DB. Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis. Gastroenterology 136: 1297–1307, e1291–e1293, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]