Abstract
We have shown that alcohol increases Caco-2 intestinal epithelial cell monolayer permeability in vitro by inducing the expression of redox-sensitive circadian clock proteins CLOCK and PER2 and that these proteins are necessary for alcohol-induced hyperpermeability. We hypothesized that alcohol metabolism by intestinal Cytochrome P450 isoform 2E1 (CYP2E1) could alter circadian gene expression (Clock and Per2), resulting in alcohol-induced hyperpermeability. In vitro Caco-2 intestinal epithelial cells were exposed to alcohol, and CYP2E1 protein, activity, and mRNA were measured. CYP2E1 expression was knocked down via siRNA and alcohol-induced hyperpermeability, and CLOCK and PER2 protein expression were measured. Caco-2 cells were also treated with alcohol or H2O2 with or without N-acetylcysteine (NAC) anti-oxidant, and CLOCK and PER2 proteins were measured at 4 or 2 h. In vivo Cyp2e1 protein and mRNA were also measured in colon tissue from alcohol-fed mice. Alcohol increased CYP2E1 protein by 93% and enzyme activity by 69% in intestinal cells in vitro. Alcohol feeding also increased mouse colonic Cyp2e1 protein by 73%. mRNA levels of Cyp2e1 were not changed by alcohol in vitro or in mouse intestine. siRNA knockdown of CYP2E1 in Caco-2 cells prevented alcohol-induced hyperpermeability and induction of CLOCK and PER2 proteins. Alcohol-induced and H2O2-induced increases in intestinal cell CLOCK and PER2 were significantly inhibited by treatment with NAC. We concluded that our data support a novel role for intestinal CYP2E1 in alcohol-induced intestinal hyperpermeability via a mechanism involving CYP2E1-dependent induction of oxidative stress and upregulation of circadian clock proteins CLOCK and PER2.
Keywords: oxidative stress, ethanol, clock genes, cytochrome P450 isoform 2E1, leaky gut
alcohol (ethanol, EtOH) is a widely used and often abused substance resulting in a significant healthcare burden globally and in the United States (57, 83). Chronic alcohol use can result in many pathological effects, including alcoholic steatohepatitis (ASH), leading to progressive alcoholic liver disease (ALD) (16, 39). Although alcohol is necessary for the development of ALD, only 20–30% of alcoholics develop ASH, progressive liver disease leading to cirrhosis and liver failure (ALD) (46, 55). This observation indicates that chronic alcohol consumption is not sufficient to induce clinically relevant liver damage, and an additional predisposing factor(s) must also be present. Preclinical and clinical studies provide compelling evidence that gut-derived bacterial endotoxin is necessary to initiate the sustained inflammatory cascades that are required for the development of alcohol-induced liver injury (28, 77, 83). The major source of endotoxin is the intestine, and, therefore, alcohol-induced intestinal hyperpermeability (i.e., leaky gut), which results in endotoxemia (4, 5, 29, 52), is particularly relevant in the etiology of ASH and progressive ALD. Alcohol induces intestinal hyperpermeability and endotoxemia in only a subset of alcoholics (5, 29), but the mechanisms that cause intestinal hyperpermeability are not fully established. Because most deleterious effects of alcohol are thought to be the consequence of metabolism byproducts, it is plausible that variability in alcohol metabolism pathways dictates susceptibility to alcohol-induced gut leakiness.
Several recent studies have shown that alcohol and/or alcohol metabolism products such as acetaldehyde (54) (produced as a byproduct of alcohol dehydrogenase, ADH) or stimulation of inducible nitric oxide synthase (iNOS) (1, 2, 14) disrupt intestinal barrier integrity via several mechanisms, including alterations in tight junction proteins (12, 53, 70). Despite these detrimental effects, ADH-induced effects are unlikely to fully explain alcohol-induced intestinal hyperpermeability because Caco-2 cells do not express ADH (30), yet low-dose alcohol exposure results in marked disruption of Caco-2 cell monolayer barrier function (14, 76). Cytochrome P450 isoform 2E1 (CYP2E1)-mediated metabolism is another major alcohol metabolism pathway that becomes increasingly engaged during chronic or excessive alcohol exposure (33, 38, 73) and results in the production of reactive oxygen species (ROS) (oxidative stress) and other damaging products (32, 36, 37). Despite CYP2E1 being present in the intestinal epithelial cells (3, 17, 61), there have been no studies to determine the possible contribution of alcohol metabolism via CYP2E1 to alcohol-induced intestinal barrier dysfunction.
Considerable evidence supports a role for liver CYP2E1-mediated alcohol metabolism in the pathogenesis of alcoholic liver disease via the production of oxidative stress (15, 33, 34, 36, 38, 40, 41). It is also well established that 1) CYP2E1 is expressed in the small intestine and colon tissue (3, 61), 2) Cyp2e1 protein is induced in intestinal tissue by chronic alcohol feeding in rodents and humans (17, 61, 69, 72), 3) CYP2E1 is one of the most highly expressed of the CYP450 isoforms in the human intestine (3, 79), and 4) activated CYP2E1 produces oxidative stress products (36) that can contribute to alcohol-induced tissue damage including ROS/reactive nitrogen species (RNS) that could mediate disruption of intestinal epithelial permeability (1, 2). However, despite these observations, the role of intestinal CYP2E1 in alcohol-induced intestinal hyperpermeability has not been investigated. Oxidative stress can impact intestinal barrier function in a variety of ways, but one potentially important pathway is via influencing redox-sensitive cellular signaling mechanisms. One intriguing and unexplored pathway is the role of redox-sensitive mechanisms that influence circadian clock genes. Circadian rhythms are 24-h biological patterns of function that synchronize humans and other organisms with the daily environmental patterns of light and dark and feeding (18, 59, 60) and are essential for the regulation of a wide range of metabolic and biological pathways. These rhythms are controlled by the cyclic pattern of circadian clock genes (11). Disruption of circadian rhythms has been implicated as a mechanism for a variety of inflammatory disorders such as metabolic syndrome, obesity, cardiovascular disease, and cancer (49, 56, 81). This hypothesis is particularly attractive because circadian clock genes and circadian rhythms regulate gastrointestinal function (23, 25, 50, 67), and our laboratory has demonstrated that perturbation of these rhythms makes the intestine susceptible to damage by injurious agents (47, 51, 78).
Specifically, we have also recently shown that the circadian clock genes Clock and Per2 in Caco-2 cells are critical for alcohol-induced intestinal hyperpermeability in vitro and that alcohol stimulates increases in both the CLOCK and PER2 proteins in intestinal Caco-2 cells. In addition, we have shown that chronic alcohol contained in Nanji diet feeding in BL/6 mice results in increased intestinal permeability and significantly elevated levels of PER2 protein in rat duodenum and colon in vivo (14, 28, 76). The circadian clock is influenced by the NAD/NADH redox ratio; thus, it is possible that CYP2E1-mediated oxidative stress affects circadian gene expression/function, resulting in intestinal hyperpermeability.
However, despite these observations, the role of intestinal CYP2E1 and its integration with clock genes in alcohol-induced intestinal hyperpermeability has not been studied. Accordingly, the aim of our study was to fill this gap in our knowledge by testing the hypothesis that CYP2E1 metabolism of alcohol and its oxidative stress products is central to alcohol-induced disruption of intestinal permeability via influencing intestinal circadian gene expression. To this end, we used in vitro Caco-2 intestinal epithelial cell monolayers (1, 20) as well as tissue from chronic (8 wk) alcohol-containing Nanji diet-fed mice that we have already shown to have gut leakiness (14). We sought to 1) determine whether CYP2E1 protein and activity or mRNA are induced in human intestinal Caco-2 cells by alcohol, 2) investigate whether chronic Nanji diet alcohol feeding affects intestinal (colonic) Cyp2e1 protein and/or mRNA in mice, 3) investigate whether alcohol-induced hyperpermeability of intestinal epithelial cell monolayers is CYP2E1 dependent by determining whether CYP2E1 siRNA knockdown prevents monolayer leakiness, 4) determine whether CYP2E1-mediated alcohol-induced disruption of intestinal barrier integrity results in the stimulation of circadian proteins CLOCK and PER2 by alcohol, and 5) investigate the role of alcohol-CYP2E1-mediated oxidative stress in alcohol modulation of circadian clock proteins CLOCK and PER2 in vitro.
MATERIALS AND METHODS
Intestinal epithelial cell monolayer permeability measurement.
We have used in vitro Caco-2 cell monolayers to model alcohol-induced intestinal barrier dysfunction. The alcohol concentration (i.e., 0.2%, 43 mM) was selected to reflect blood alcohol concentration after heavy drinking in alcoholics, and the duration (i.e., 2–4-h exposure) was selected based on the duration of time that colonic mucosa are exposed to alcohol via the blood. We have also demonstrated that 4–8 wk of daily alcohol Nanji diet feeding is sufficient to cause significant intestinal hyperpermeability in BL/6 mice (14).
Caco-2 cells (ATCC no. CRL2101, human colorectal adenocarcinoma; Manassas, VA) (20) were grown to confluence (37°C, 5% CO2, 10% fetal bovine serum media with 5 mM penicillin-streptomycin) on Type 1 collagen-coated 12-mm/0.4-μM pore tissue culture plate inserts (Transwell; Corning, Corning, NY) as described previously (1, 14, 19). Cell viability is routinely measured by live/dead assay (Invitrogen, Life Technologies, Grand Island, NY) or Trypan blue staining (>95% cell viability for all assays). Caco-2 cell monolayers were treated with a physiologically relevant dose of alcohol (0.2% vol/vol, 43 mM; ∼2–3 drinks) for 30 min to 4 h as indicated. Media alcohol concentration was verified via an alcohol testing kit (Pointe Scientific, Canton, MI). Signaling experiments were terminated by removal of media and addition of PBS for scraping and mRNA expression analysis, SDS/RIPA buffer for whole cell lysates (Western blot), or Qiagen lysis buffer (gene expression) (Qiagen, Valencia, CA).
Intestinal permeability was measured using Caco-2 cells as transepithelial electrical resistance (TER) as previously described (14, 76). TER was determined using a dual electrode system designed for cell culture insert analysis (EVOM; World Precision Instruments, Sarasota, FL) in which naked culture inserts were used to blank for baseline values, which are subtracted from all values using inserts with living cells.
CYP2E1 knockdown with SiRNA.
Caco-2 cells were treated with gene-specific siRNA directed at human CYP2E1 or control nontargeting siRNA to control for “off target” (non-gene-specific) effects of siRNA using a modification of our previously published methods (13, 76). Briefly, 105 cells were combined with 11 picomoles of siRNA in 50 μl Lipofectamine (Invitrogen) and 50 μl Optimem (Invitrogen), mixed by gentle shaking, and then plated. Permeability studies were conducted 72–96 h after plating, once the cells were confluent. CYP2E1 siRNA was On Target Smartpool from Dharmacon (Dharmacon, Lafayette, CO). The siRNA sequences for human CYP2E1 (no. L-010134–01-0005) were as follows: UGAAAUACCCUGAGAUCGA, UGCAUGAGAUUCAGCGGUU, UGGUGGUGAUGCACGGCUA, and UGUACACAAUGGACGGUAU, whereas control (nontargeting) siRNA was from Santa Cruz Biotechnology (Santa Cruz, CA), and the sequence is proprietary (no. 37007). Control nontargeting siRNA was used to control for off-target (non-gene-specific) effects of siRNA on gene expression.
Experimental diet and animals.
Our methods were modified slightly from those published previously. Final alcohol concentration was changed from 5.15% to 4.5%, and all fat calories were in the form of fish oil rather than a combination of corn oil, fish oil, and vegetable oil (10, 80). Components of the Nanji liquid diet (44) included mineral mix, vitamin mix, choline bitartrate, DL-methionine, lactalbumin, xanthan gum, dextrose (all from Dyets, Bethlehem, PA), fish oil from menhaden, ethanol (both from Sigma, St. Louis, MO), and Hersey's chocolate syrup (Hershey, PA). The caloric composition of the diet was 36% protein, 29% carbohydrate/alcohol, and 35% fat (fish oil). The caloric composition of the control dextrose diet was the same except that alcohol calories were replaced with dextrose.
Colonic mucosa used to measure Cyp2e1 protein and mRNA in this study were collected from the mice who developed intestinal hyperpermeability after 8 wk of daily consumption of alcohol-containing Nanji diet (14). In brief, male C57BL/6 mice (Jackson Laboratory, Bar harbor, ME) weighing 20–25 g at the start of the experiments were housed individually in a climate-controlled environment on a standard 12-h:12-h light/dark cycle (lights on 7 AM, lights off 7 PM; ZT0 = 7 AM). ZT (Zeitgeber time) is a circadian biology term that arbitrarily divides 24 h into 24 ZT times beginning with ZT0 = lights on and ZT12 = lights off in a traditional 12-h:12-h light/dark cycle. Following 12 wk of habituation to the facility on a standard chow diet, the mice were switched to a liquid Nanji diet described above. In addition, half of the mice began the alcohol diet acclimation period for 2 wk followed by 8 wk of 4.5% vol/vol alcohol Nanji liquid diet. For the first 2 wk, the alcohol concentration was gradually increased as a percentage of total daily calories until the maximum of 29% of total daily caloric intake came from alcohol (4.5% vol/vol). The mice were then maintained on this diet for 8 wk before death and tissue collection. Control mice were maintained on an isocaloric dextrose diet throughout (14, 80). Colonic tissue was harvested immediately after death by decapitation at ZT6. Tissue was snap frozen in liquid nitrogen and stored at −80°C in RNAlater (Qiagen). RNAlater was removed for Cyp2e1 protein or qPCR analysis.
Housing facilities were accredited through the Association for Assessment and Accreditation of Laboratory Animal Care, and all experiments were carried out in accordance with the conditions set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and with the approval of the Institutional Animal Care and Use Committee (IACUC) at Rush University Medical Center.
Caco-2 cell oxidative stress experiments.
Caco-2 cells were grown to confluence on 24-mm Transwell inserts (Corning) in six-well plates in complete DMEM media with 10% serum. For experiments with NAC, some cells were pretreated for 24 h with 10 mM NAC, and then cells were stimulated with 0.2% alcohol for the indicated times. Complete cell lysates were prepared for Western blot analysis as described (14) at the indicated time points of 2 and 4 h. For stimulation with H2O2, Caco-2 cells grown on six-well inserts were stimulated with control media or media + 0.5 mM H2O2 and cell lysates made at the time points of 2 and 4 h for Western blot analysis of CLOCK and PER2 proteins.
Gene expression analysis with qRT-PCR.
Analysis of mRNA expression was carried out as previously described (13). Briefly, RNA was isolated from Caco-2 cells or mouse intestinal tissue (ZT6) using the Qiagen RNeasy kit (Qiagen). RNA was converted to cDNA using the high-capacity cDNA kit (Applied Biosystems, Life Technologies, Carlsbad, CA) and PCR amplified using fast Sybr green master mix (Applied Biosystems) using a 7500 fast real-time PCR system (Applied Biosystems). PCR primer sequences were as follows: for human CYP2E1: F-5′-CCTCCTGCTGGTGTCCATGT-3′, R-5′-CTTGGGCTTGGGTCTTCCTGAGTGCT-3′; for mouse Cyp2e1: F-5′-GAGGTGCTACTGAACCACAAG-3′; R-5′-ACGGAGGATACTTAGGGAAAACC-3′. Primers for human β-actin were as follows: F-5′-GCCAGGTCCAGACGCAGG-3′, R-5′-TGCTATCCAGGCTGTGCTATCC-3′; for mouse β-actin: F-5′-GTGACGTTGACATCCGTAAAGA-3′, R-5′-GCCGGACTCATCGTACTCC-3′. Expression was determined relative to the respective species β-actin using the ΔΔCt method (13).
Western blot and slot blot protein analysis.
For Western blot, total protein was determined (Bio-Rad, Hercules, CA), and samples were prepared with Laemmli sample buffer with 2-ME (Bio-Rad). Thirty micrograms of protein/lane was loaded into a 4%/10% stacking acrylamide Tris gel and electrophoresed at 100 V for 2 h as described (14). Protein was then transferred to a nitrocellulose membrane (GE Healthcare Limited, Buckinghamshire, UK) for 1.5 h at 130 V. Nonspecific binding was blocked by incubation of the membrane with 5% milk TBST for 1 h. Membranes were then incubated overnight at 4°C with antibodies for hCYP2E1 (rabbit anti-human; Abcam, Cambridge, MA), hCLOCK (rabbit anti-human; Santa Cruz Biotechnology, Santa Cruz, CA), hPER2 (rabbit anti-human; Abcam), or h-actin (rabbit anti-human; Sigma) in TBST and 5% nonfat dry milk. Membranes were subsequently washed with TBST for 1 h and incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for CYP2E1, CLOCK, PER2, nitrotyrosine, or β-actin (goat anti-rabbit; Cell Signaling Technology, Danvers, MA). Membranes were subsequently washed with TBST for 1 h. Chemiluminescent substrate (ECL, GE Healthcare) was applied to the membrane for protein visualization using autoradiography film (HyBlot CL, Denville Scientific, Metuchen, NJ). Optical density was determined via densitometric analysis with Image J Software (NIH, Bethesda, MD).
Slot blotting of Caco-2 cell lysates for nitrotyrosine was performed using PVDF membranes as previously described (1, 2) using cell lysates prepared as described above for Western blotting and primary rabbit antibody to 3-nitrotyrosine (no. 06–284; EMD Millipore, Billerica, MA) and anti-rabbit HRP. Blots were developed on film as described above and analyzed for densitometric data using Image J software.
CYP2E1 activity measurement.
CYP2E1 activity was measured as the spectrophotometric oxidation of p-nitrophenol at 546 nm as described (58, 85). CYP2E1 activity was measured in Caco-2 cell microsome fractions by the spectrophotometric analysis at 546 nm of the oxidation of p-nitrophenol to p-nitrocatechol in the presence of NADPH and oxygen as described (58, 85). The reaction mixture consisted of 100 μg of microsomal protein in 100 μl of solution volume containing 100 mM potassium phosphate buffer (pH 7.4), 0.2 mM PNP, and 1 mM NADPH. Reactions were carried out at 37°C for 30 min. CYP2E1 activity data are expressed as pmol/mg per minute of oxidized p-nitrophenol/mg protein per minute.
Statistical analysis.
The data are presented as means ± SE. Group means were compared by analysis of variance and post hoc analysis. Significance was set at α < 0.05. All analyses were conducted using SPSS (SPSS, Chicago, IL).
RESULTS
Alcohol treatment increased CYP2E1 protein and enzymatic activity but not mRNA in Caco-2 cells and in mouse intestine.
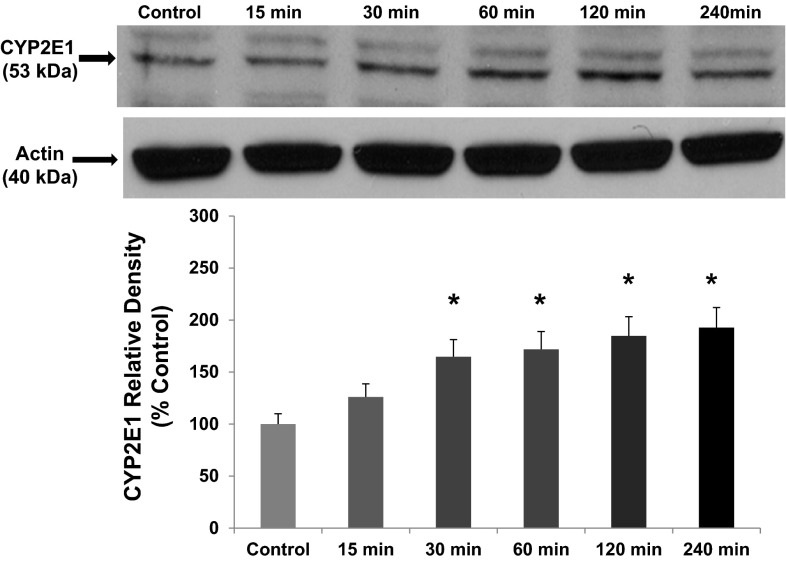
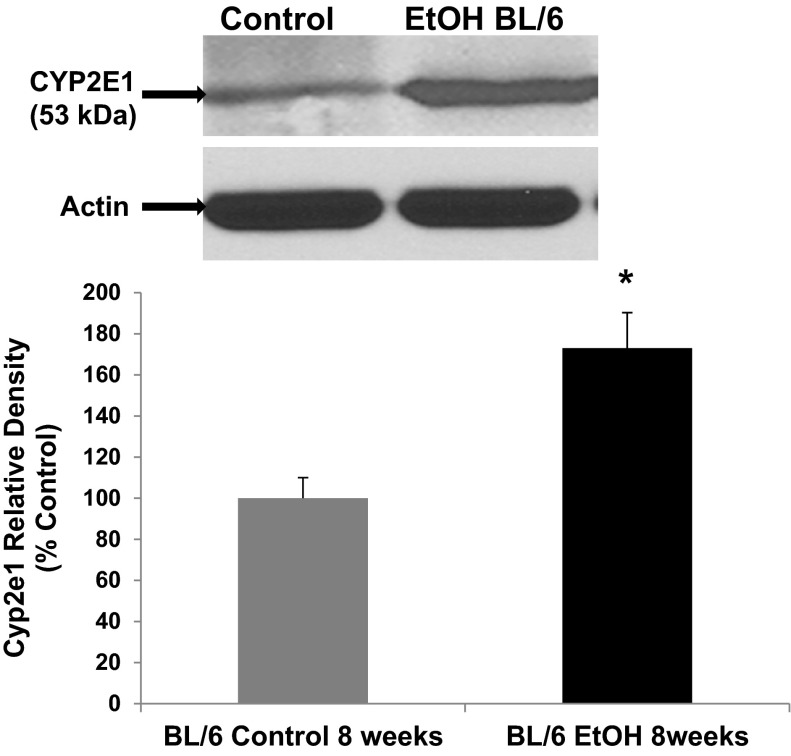
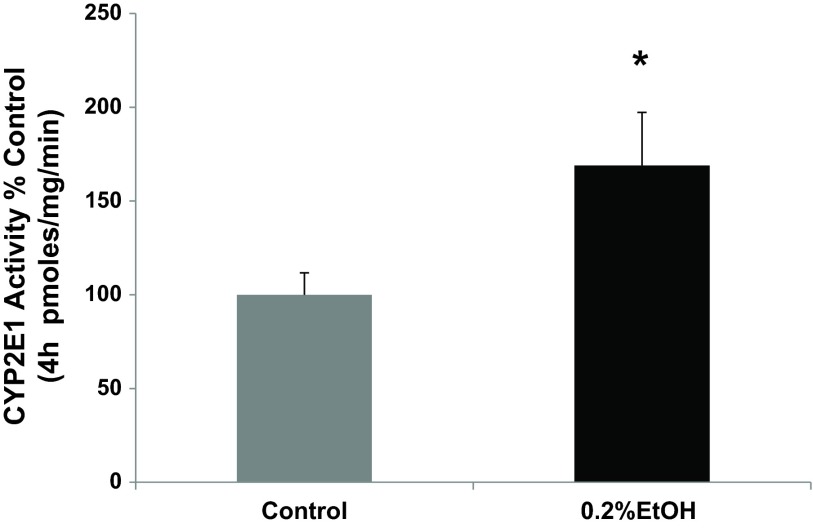
One hallmark of hepatic CYP2E1 is its induction by alcohol (36). We evaluated whether intestinal epithelial cell CYP2E1 protein and activity was induced by alcohol in our models similar to that observed by others for hepatic and intestinal tissue CYP2E1 (17, 61, 62, 69, 72). Our laboratory has shown that a physiologically relevant concentration of alcohol (i.e., 0.2%, 240 min) increases Caco-2 monolayer permeability (14, 76). Using this model, total cell lysates from Caco-2 cells exposed to alcohol were analyzed for the expression of CYP2E1 protein by Western blotting with a major CYP2E1 band at 53 kDa and an additional minor band at higher molecular weight probably due to ubiquitination of Cyp2e1 (62) (Fig. 1). By 30 min, alcohol had significantly increased intestinal epithelial cell CYP2E1 protein (increased 65%, P < 0.05 vs. control), an effect that was further increased (93%) by 240 min (time point controls were unchanged and are not shown). To determine whether this was also true in vivo, we used Western blotting to assess Cyp2e1 protein levels in lysates of colon tissue from mice fed either an alcohol-containing diet, 4.5% vol/vol, (Nanji alcohol diet) (14), or control dextrose diet for 8 wk (Fig. 2). As shown in Fig. 2, chronic alcohol resulted in a significant increase in Cyp2e1 protein levels in the colonic mucosa (increased 73%, P < 0.05) vs. dextrose-fed controls. Thus alcohol stimulated significant induction of intestinal cell CYP2E1 protein levels both in vitro and in vivo, where it also increased permeability of the epithelial cell monolayer (Fig. 5 below) and intestine (14), respectively. To determine whether the increase in protein was accompanied by a functional increase in CYP2E1 enzymatic activity, we measured p-nitrophenol oxidation in the Caco-2 cells microsomal fraction. As shown in Fig. 3, treatment of Caco-2 cells with alcohol (0.2% for 240 min) significantly increased CYP2E1 activity (increased 69%, P < 0.05) compared with controls. Thus the alcohol-stimulated induction of CYP2E1 protein corresponded closely with increased CYP2E1 activity in Caco-2 intestinal epithelial cells. To our knowledge, this is the first demonstration of alcohol induction of CYP2E1 protein and activity in human intestinal epithelial cells in vitro.
Fig. 1.
Alcohol treatment induces increased Cytochrome P450 isoform 2E1 (CYP2E1) protein in Caco-2 intestinal epithelial cells. Human Caco-2 intestinal epithelial cells were grown to confluence on tissue culture inserts in complete medium (controls) or complete medium containing alcohol (EtOH, 0.2%, 43 mM) as described in materials and methods. At the designated time points over 240 min (4 h), cells were lysed, and CYP2E1 protein expression was assessed by Western blotting. Blots were then stripped and reprobed for actin expression to control for equal loading. A representative blot from 1 of 5 independent experiments is shown. Histogram data are means ± SE of all N = 5 experiments. Calculated means are relative to controls for each time point that are not shown. *P < 0.05 vs. control for that time point.
Fig. 2.
Chronic alcohol feeding induces increased intestinal Cyp2e1 protein in BL/6 mice. C57BL/6 mice were pair fed a complete liquid diet (detailed in materials and methods) for 8 wk containing alcohol (EtOH BL/6; 4.5% vol/vol, 1 M) or control diet with calories matched with dextrose. Cyp2e1 protein expression was analyzed in proximal colon tissue by Western blotting tissue from N = 4 mice for each condition. Representative blot for tissue from 1 mouse for either control or alcohol-fed mice. Blot was stripped and reprobed for actin as a loading control. Histogram data depicts summarized data for all mice N = 4 for each condition. *P < 0.05 vs. control.
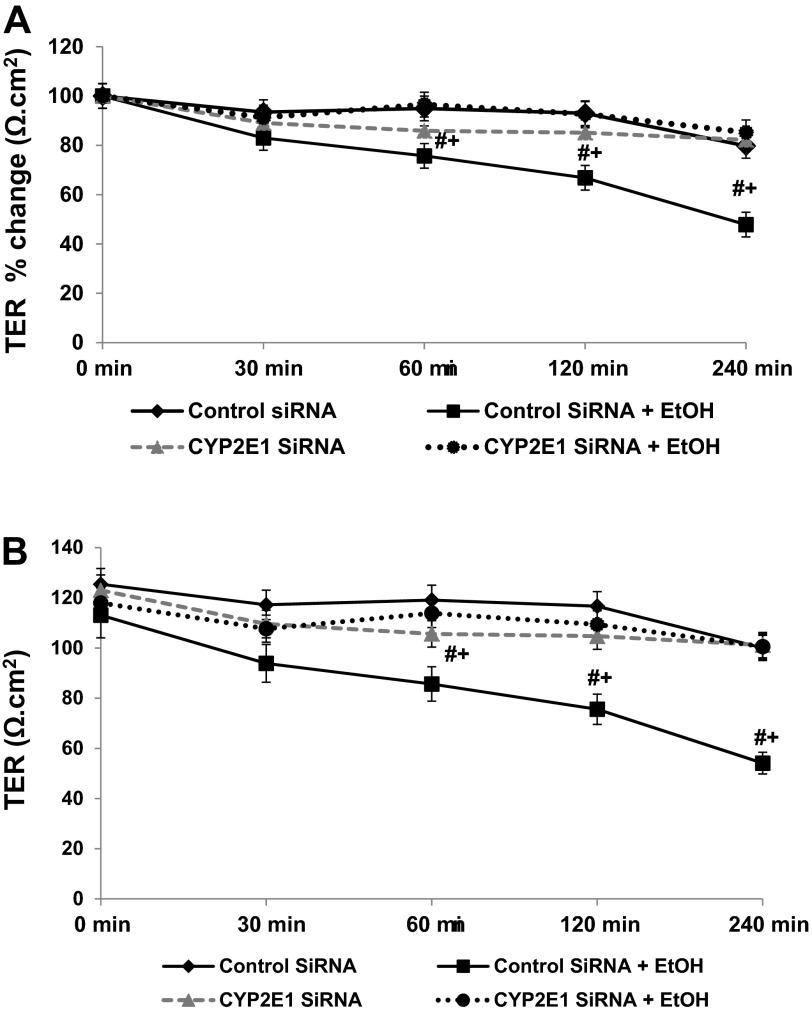
Fig. 5.
siRNA inhibition of CYP2E1 expression prevents alcohol-induced permeability in Caco-2 monolayers. Caco-2 cells grown to confluence on Transwell inserts were treated with control or alcohol containing (EtOH, 0.2%, 43 mM) media and assessed over time (30–240 min) for alcohol-induced paracellular permeability by measurement of transepithelial electrical resistance (TER) changes as described in materials and methods. To investigate the role of CYP12E1, cells were pretreated with either control nontargeting siRNA or siRNA specific for CYP2E1 as described in materials and methods. A: data are TER means (of triplicate wells) ± SE (Ω cm2) for N = 4 separate experiments expressed as percent change from original baseline (set as 100%). B: data are actual TER means (of triplicate wells) ± SE (Ω cm2; with background subtracted) for N = 4 experiments. Cells measured for TER were lysed on membranes at the conclusion of each experiment to provide lysates for the Western blotting analysis shown in Fig. 6. #P < 0.05 vs. control siRNA alone; +P < 0.05 vs. CYP2E1 siRNA + EtOH.
Fig. 3.
Alcohol treatment induces increased CYP2E1 activity in Caco-2 intestinal epithelial cells. Caco-2 cells grown on Transwell inserts were treated with complete media containing alcohol (EtOH, 0.2%, 43 mM) or no alcohol (control) for 240 min (4 h). as described for Fig. 1. CYP2E1 activity was measured in Caco-2 cell microsome fractions by the spectrophotometric analysis at 546 nm of the oxidation of p-nitrophenol to p-nitrocatechol in the presence of NADPH and oxygen as described in materials and methods. CYP2E1 activity data are expressed as the means of pmol/mg per minute of oxidized p-nitrophenol/mg protein per minute. Means ± SE for N = 4 experiments. *P < 0.05 vs. control.
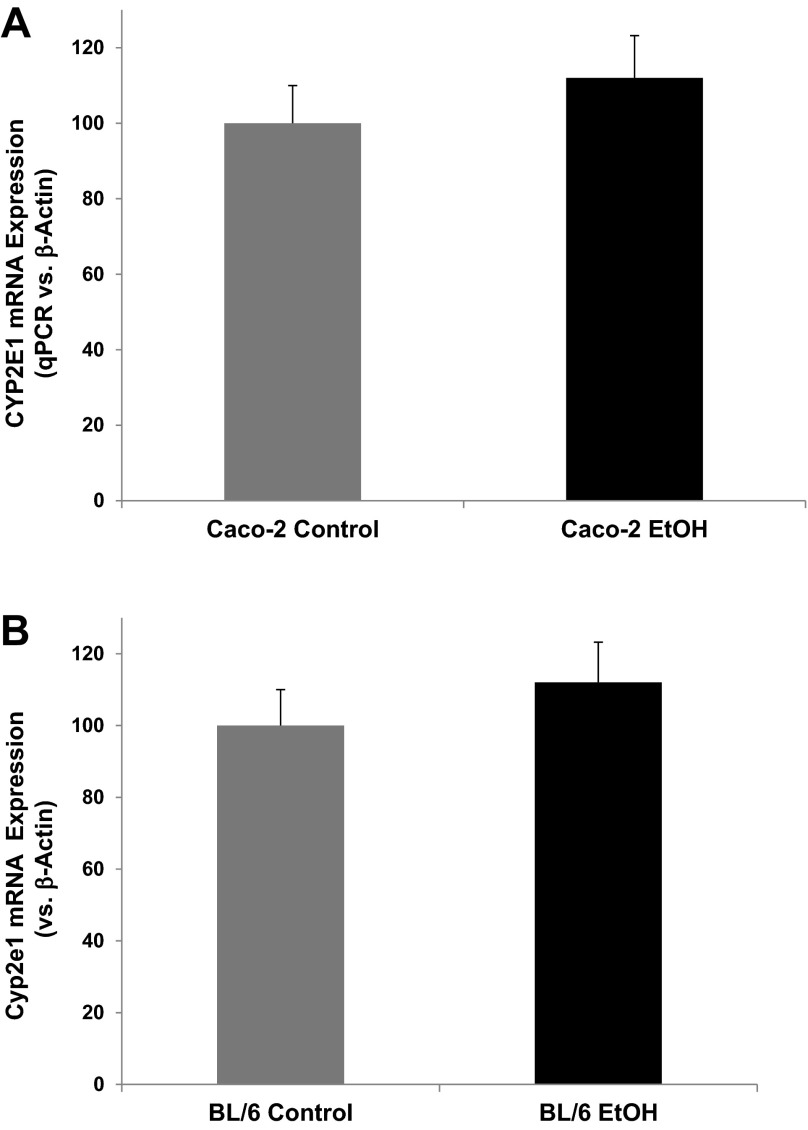
We next sought to determine whether the alcohol-induced increase in CYP2E1 protein was a consequence of increased mRNA expression using RT-qPCR analysis. mRNA was isolated from control and alcohol-treated Caco-2 cells (0.2% for 240 min) and from control and alcohol-fed mouse colon tissue (i.e., Nanji alcohol diet for 8 wk) and was analyzed for CYP2E1 mRNA expression by qPCR. Both in vitro (Fig. 4A) and in vivo (Fig. 4B) data demonstrate a slight, but nonsignificant, increase in CYP2E1 mRNA expression (each increased 12%, P > 0.05) in response to alcohol. These data support the fact that the increased CYP2E1 protein and functional activity is not the consequence of increased CYP2E1 transcription in our models.
Fig. 4.
Alcohol-induced CYP2E1 mRNA expression in Caco-2 cells and mouse colon. A: alcohol-induced CYP2E1 mRNA expression in Caco-2 cells. Caco-2 cells grown on Transwell inserts were treated with alcohol (EtOH, 0.2%, 43 mM) or complete media alone (control) for 4 h as described in Fig. 1 were subjected to mRNA extraction and RT-PCR analysis for CYP2E1 expression as described in materials and methods with expression normalized to β-actin expression using the ΔΔCT method and then expressed as percentage of control. Data are means ± SE for N = 4 experiments. B: chronic alcohol feeding induced Cyp2e1 mRNA expression in proximal colon tissue of BL/6 mice. BL/6 mice were pair fed a complete Nanji (fish oil) liquid diet as described in materials and methods for 8 wk containing either alcohol (EtOH, 4.5% vol/vol, 1 M) or control diet with calories matched with dextrose. Colon tissue stored in RNAlater at −80°C was then analyzed for Cyp2e1 mRNA expression by qRT-PCR as described above for Caco-2 cells. ΔΔCT data relative to β-actin were then expressed as percentage of control for N = 4 mice as means ± SE.
CYP2E1 is required for EtOH-induced hyperpermeability in Caco-2 monolayers.
To determine whether intestinal CYP2E1 is critical for alcohol-induced intestinal hyperpermeability, we used siRNA-mediated gene-specific knockdown of CYP2E1 (or nontargeting siRNA for controls) in Caco-2 cells. Cells treated with the control nontargeting siRNA or the CYP2E1 siRNA alone demonstrated virtually no change in permeability assessed with TER over the 240-min time period (Fig. 5., A and B) However, cells treated with control nontargeting siRNA plus alcohol showed a significant decrease in TER (increase in permeability), consistent with our previous publications (14, 76). The alcohol-induced increase in permeability became statistically significant after 60 min and further increased throughout the duration of the experiment. This alcohol-induced effect was prevented by knocking down expression of intestinal epithelial cell CYP2E1 with siRNA for CYP2E1 (Fig. 5, A and B). These data support the fact that intestinal cell CYP2E1 is critical for alcohol-induced effects on intestinal permeability in this model.
Intestinal CYP2E1 is required for alcohol-induced circadian clock gene expression that promotes intestinal hyperpermeability.
The selection of Clock and Per2 circadian clock genes was hypothesis driven and based on our previous studies (76). CLOCK protein is a circadian transcription factor known to bind to the promoter of Per2 and drive PER2 protein expression. Furthermore, Per2 has been associated with the physiological response to alcohol in mice and humans (74, 75). We have recently shown that alcohol-induced expression of the circadian clock gene proteins CLOCK and PER2 in Caco-2 cells is required for alcohol-induced intestinal permeability (76). We therefore sought to determine whether CYP2E1 stimulation by alcohol was involved in circadian regulation of alcohol-induced intestinal hyperpermeability. As depicted in Fig. 6, treatment of Caco-2 cells with control nontargeting siRNA alone had little effect on CYP2E1 protein and was set as 100% (N = 4 separate experiments summarized in histograms, blot is from a single representative experiment). Treatment with CYP2E1-specific siRNA alone had a measureable but not significant effect on CLOCK or PER2 protein levels. However, treatment of cells with alcohol and with the control nontargeting siRNA resulted in increased CLOCK and PER2 protein levels (*P < 0.05 vs. control), an effect that correlated with increased permeability (Fig. 5) and is in agreement with our previously published results (76). In contrast, pretreatment of cells with siRNA specific for CYP2E1 (with CYP2E1 knockdown ≥70% for alcohol-treated cells, see Fig. 6) prevented the alcohol-induced increase in CLOCK or PER2 proteins as well as the corresponding hyperpermeability (**P < 0.05 vs. alcohol-treated control) (Fig. 6). These data demonstrate, for the first time, that CYP2E1 is critical for the alcohol-induced increase in intestinal cell CLOCK and PER2 circadian clock proteins and for alcohol-induced increased intestinal hyperpermeability. Thus the increases in CLOCK and PER2 proteins we have previously shown required for alcohol-induced intestinal permeability are dependent on alcohol stimulation of CYP2E1 in intestinal epithelial cells.
Fig. 6.
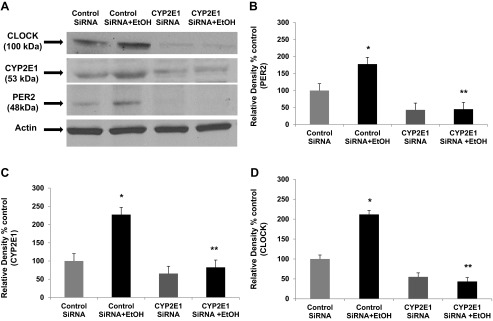
siRNA inhibition of CYP2E1 expression prevents alcohol-induced increases in the circadian gene proteins CLOCK and PER2 in Caco-2 cells. The same Caco-2 cells grown on Transwell inserts and tested for alcohol-induced permeability (EtOH, 0.2%, 43 mM) shown in Fig. 5 were lysed at the end of each experiment (N = 4) and used for Western blotting analysis (as described in Fig. 1) for protein expression of CLOCK, PER2, CYP2E1, and β-actin. Representative blots from a single experiment are shown in A. B–D: histograms showing summarized means ± SE for densitometry data compiled from analysis of blots from all 4 separate experiments for expression of PER2, CYP2E1, and CLOCK. *P < 0.05 vs. control siRNA alone; **P < 0.05 vs. control siRNA + EtOH.
Role of alcohol-CYP2E1-induced oxidative stress in stimulation of CLOCK and PER2 proteins.
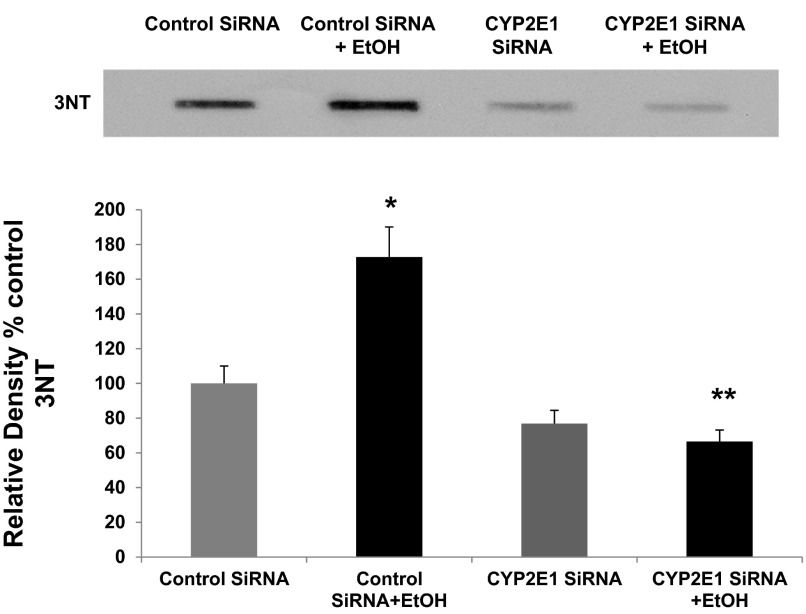
Finally, we wished to investigate whether oxidative stress resulting from CYP2E1-mediated metabolism of alcohol could be stimulating increased expression of redox-sensitive CLOCK and PER2 proteins by alcohol. Recent studies in mice have shown in liver that oxidative stress induced by alcohol requires Cyp2e1 for production of 3-nitrotyrosine (3NT), a marker for oxidative stress (89). To examine this possibility, we used slot-blotting of total cellular proteins to test for 3NT production in the same siRNA-treated lysates tested in Fig. 6 for expression of CLOCK and PER2 and CYP2E1. As seen in Fig. 7, siRNA inhibition of CYP2E1 expression significantly reduced alcohol-induced production of 3NT by 85% (N = 6; P < 0.05). These data strongly support a role for CYP2E1 in alcohol-induced oxidative stress in this Caco-2 model of intestinal permeability.
Fig. 7.
siRNA inhibition of CYP2E1 dramatically reduces alcohol-induced oxidative stress (3-nitrotyrosine, 3NT) in Caco-2 cells. The same total cell lysates from the SiRNA permeability experiments tested in Fig. 6 above (EtOH, 0.2%, 43 mM) were also analyzed by slot blotting, as described in materials and methods, for 3NT formation with antibody specific for 3NT, a measure of oxidative stress. Top: representative blot (from 1 experiment). Bottom: histogram summarizing blot densitometry means ± SE data from N = 6 complete experiments. *P < 0.05 vs. control siRNA; **P < 0.05 vs. control siRNA + EtOH.
We next sought to determine whether oxidative stress resulting from CYP2E1 metabolism of alcohol was directly responsible for the observed increase in the circadian CLOCK and PER2 proteins. To test this, we preincubated Caco-2 cells with NAC (10 mM), a well-established inhibitor of alcohol-Cyp2e1-mediated oxidative stress in liver cells in vitro (88) and in mice (8, 91). As seen in Fig. 8, treatment of Caco-2 cells with alcohol stimulated increased levels of CLOCK by 173% (P < 0.001) and PER2 of 64% (P < 0.026), consistent with our published data (76); pretreatment with the antioxidant NAC significantly inhibited alcohol induction of CLOCK protein by 68% (P < 0.001) and inhibited alcohol-induced PER2 protein levels by 44% (P < .019) (N = 6 experiments). These data thus directly support further a role for alcohol-induced oxidative stress in upregulating protein levels of CLOCK and PER2. NAC has also been shown to inhibit alcohol-Cyp2e1-induced peroxynitrite and nitrotyrosine formation, as we have shown in Fig. 7 above (8). As further proof of this principle, we stimulated Caco-2 cells directly with one type of oxidative stress, H2O2 (0.5 mM) ± NAC (10 mM) and measured the changes in CLOCK and PER2 proteins. As seen in Fig. 9, H2O2-mediated oxidative stress stimulation resulted in a significant increase in Caco-2 intestinal cell CLOCK protein (93%; P < 0.014) as well as increased PER2 protein (120%; P < 0.011). Preincubation with NAC inhibited H2O2 stimulation of CLOCK protein by 41% (P < 0.048) and inhibited PER2 protein levels by 35% (P < 0.034). Thus, when taken together, our data strongly support a model in which oxidative stress resulting from alcohol metabolism by CYP2E1 results in stimulation of increased CLOCK and PER2 circadian clock proteins that in turn mediate increased intestinal hyperpermeability.
Fig. 8.
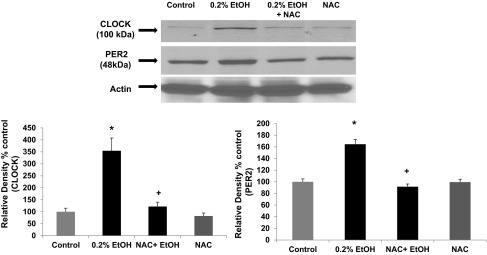
N-acetylcysteine (NAC) significantly inhibits alcohol-induced expression of CLOCK and PER2 circadian proteins in Caco-2 intestinal cells. Caco-2 human intestinal cells were grown to confluence on Transwell inserts and stimulated with alcohol (EtOH, 0.2%, 43 mM) for 4 h. Cell lysates were then used to measure CLOCK and PER2 protein expression with Western blotting (30 μg protein/lane). Blots were stripped and reprobed with antibody to β-actin to validate equal lane loading. Blots shown are representative of N = 6 experiments (from 1 experiment), and histogram data are means ± SE for summarized data from all N = 6 experiments. *P < 0.05 vs. control; +P < .05 vs. 0.2% EtOH alone.
Fig. 9.
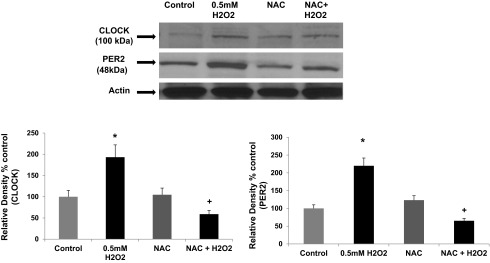
Stimulation of Caco-2 intestinal cells with H2O2 results in increased expression of CLOCK and PER2 circadian proteins. Caco-2 human intestinal cells were grown to confluence on Transwell inserts and stimulated with H2O2 (0.0017%, 0.5 mM) for 2 h. Cell lysates were then used to measure CLOCK and PER2 protein expression with Western blotting (30 μg protein/lane). Blots were stripped and reprobed with antibody to β-actin to validate equal lane loading. Blots shown are representative of N = 6 experiments (all from 1 experiment), and histogram data are means ± SE for summarized data from all N = 6 experiments. *P < 0.05 vs. control; +P < 0.05 vs. 0.5 mM H2O2 alone.
DISCUSSION
Several previous studies have shown that alcohol can disrupt circadian rhythms at the behavioral level (63, 64, 68) as well as at the level of gene transcription in the suprachiasmatic nuclei of the brain (9, 75), the central clock. However, the mechanisms for these effects are not established. In addition, a growing number of studies has suggested a connection between oxidative stress and circadian rhythms (65, 87). One recent review states that the circadian system probably evolved in response to the “Great Oxidation Event” in response to oxidative stress 2.5 billion years ago (35). Furthermore, alcohol is widely acknowledged to exert many of its tissue-damaging effects though oxidative stress-related mechanisms (36, 83). Indeed, we have now for the first time linked alcohol-mediated oxidative stress and disruption of circadian rhythms together and shown that oxidative stress from CYP2E1 metabolism of alcohol is associated with upregulation of circadian clock proteins and disruption of intestinal barrier function induced by alcohol.
We are reporting several novel findings in this study that provide significant new information toward understanding of mechanisms by which alcohol induces intestinal hyperpermeability. First, we show for the first time that alcohol induces increased CYP2E1 protein and activity in human intestinal epithelial cells (Caco-2) in vitro (Figs. 1 and 3). A very recent study showed that chronic alcohol use induced CYP2E1 protein in the colons of human subjects (69). Our results are also in agreement with data showing that CYP2E1 is expressed in a human colon cancer cell line (LS174T), is found in nonalcoholic human colon tissue (3, 86), and is induced in the colonic mucosa of alcoholics (69). Second, we demonstrate that Cyp2e1 is present in intestinal tissue of the mouse colon in vivo (C57BL/6 mice; Fig. 2) and that intestinal Cyp2e1 protein levels are induced in the colonic mucosa of mice after 8 wk of daily consumption of alcohol containing Nanji diet when they also had increased intestinal permeability (14). Our data confirmed prior studies that alcohol intake induced intestinal Cyp2e1 protein in mice and rats with other alcohol diets (17, 61). However, those prior studies did not assess intestinal permeability after alcohol consumption. We did not find any significant alcohol-induced changes in mRNA in either intestinal Caco-2 cell CYP2E1 mRNA levels or in intestinal tissue of alcohol-fed mice. Our finding is similar to studies that reported none or only minor alcohol-induced changes in CYP2E1 mRNA in human liver cells (62) or intestine in mice (61). In the liver, alcohol inhibits degradation of CYP2E1, resulting in increased protein levels (62). Although not tested in the present study, a similar posttranslational mechanism would be expected to be responsible for the observed alcohol-induced increases in intestinal CYP2E1 levels we show in vitro and in vivo.
Third, we found that alcohol-induced intestinal hyperpermeability in Caco-2 cells requires CYP2E1 (Fig. 5), implicating CYP2E1-mediated alcohol metabolism in this mechanism. This interpretation is compatible with several prior studies demonstrating deleterious effects of CYP2E1-mediated alcohol metabolism and oxidative stress on other organs such as the liver and brain (31, 73, 77, 83). One major tissue effect of CYP2E1-mediated alcohol metabolism is the production of oxidative stress (Fig. 7) (ROS/RNS) (32, 36, 73). Our laboratory and others have shown that oxidative damage and oxidative regulation of tight junction proteins are key mechanisms by which alcohol disrupts intestinal epithelial barrier function (1, 2, 14). Thus it is possible that CYP2E1 metabolism of alcohol in intestinal epithelial cells in the colon, and the resulting production of oxidative stress, may be a mechanism by which CYP2E1 mediates hyperpermeability. Indeed, free radical production would be augmented as a consequence of CYP2E1-induction by alcohol, which would be expected to overwhelm cellular antioxidant protective mechanisms (36).
Fourth, we report that siRNA knockdown of CYP2E1 expression, not only prevented alcohol-induced monolayer hyperpermeability (Fig. 5), it also prevented the alcohol-induced increase in two key circadian clock proteins CLOCK and PER2 within Caco-2 intestinal epithelial cells (Fig. 6). This outcome supports our recent study showing in the in vitro Caco-2 model CLOCK and PER2 proteins were found to be required for alcohol-induced hyperpermeability (76). Our data support a model in which oxidative stress resulting from alcohol metabolism by CYP2E1 drives the increased circadian protein expression required for alcohol-induced intestinal hyperpermeability. To further support this model, we show that the antioxidant NAC (known to prevent CYP2E1-alcohol-mediated oxidative stress) (89) significantly inhibited alcohol-induced increases of CLOCK and PER2 proteins in Caco-2 cells (Fig. 8). Finally, we also show that another exogenous source of oxidative stress, H2O2, also stimulates increased levels of CLOCK and PER2 proteins that can be significantly inhibited by NAC pretreatment (Fig. 9). Stimulation with H2O2 has also been shown to increase Per2 mRNA levels in zebrafish cells that were inhibitable with the antioxidant catalase (21). In those and other studies, the authors show that the circadian clock in zebrafish can be entrained by oxidative stress stimuli though a MAPK-dependent pathway (21, 22). Their data thus strongly support our model for similar circadian clock effects from alcohol-induced oxidative stress. In Fig. 10, we summarize these in vitro studies and propose a working model for these observed effects in our Caco-2 in vitro cell model. Our in vivo mouse and rat data support the observed alcohol-induced intestinal permeability as well as alcohol-induced increases in intestinal circadian clock proteins (14, 76). Studies are presently underway in our laboratory to establish the role of Cyp2e1-mediated oxidative stress in alcohol-induced intestinal hyperpermeability in vivo.
Fig. 10.
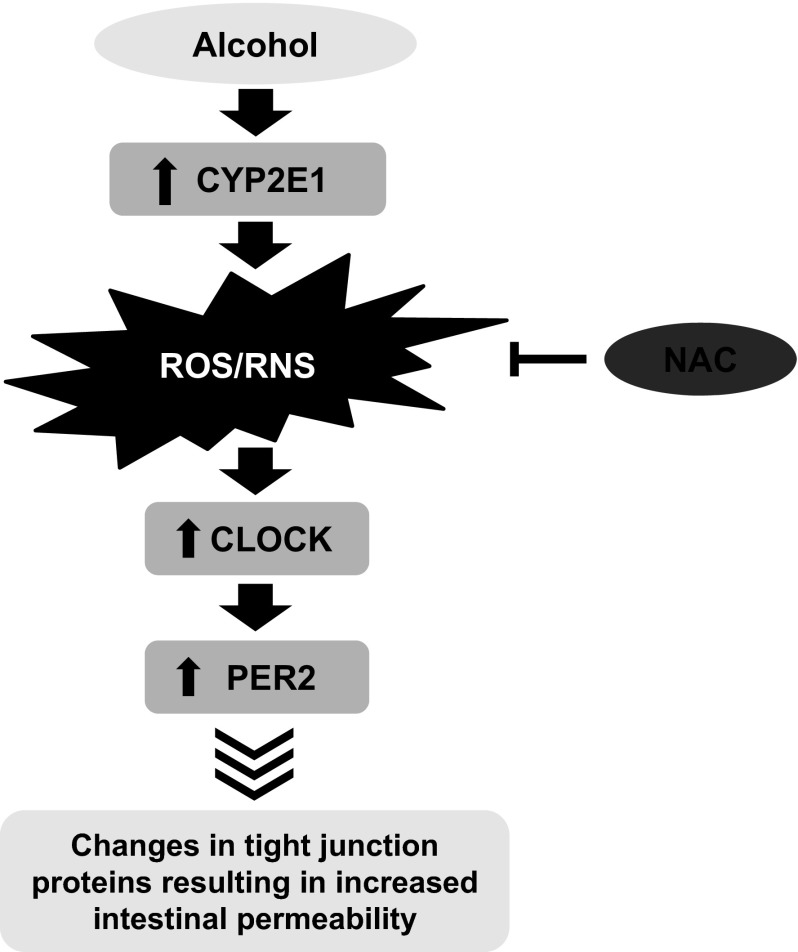
Proposed model for alcohol-mediated oxidative stress stimulation of CLOCK and PER2 circadian proteins and intestinal hyperpermeability. This cartoon summarizes the data in this study using the human intestinal epithelial Caco-2 cell model of intestinal permeability. We show that alcohol induces increased intestinal cell CYP2E1 protein and activity that results in increased oxidative stress and reactive oxygen species (ROS)/reactive nitrogen species (RNS). This oxidative stress can be inhibited by preincubation of cells with NAC. The oxidative stress then promotes increased levels of CLOCK and PER2 circadian clock proteins. The increase in CLOCK and PER2 then stimulates the observed increase in intestinal hyperpermeability, possibly by changes (decreases) in the tight junction proteins that regulate intestinal permeability.
One possible mechanism by which CYP2E1 metabolism of alcohol might affect circadian protein function/expression is through effects on the redox status of intestinal cells. Previous investigators have shown that function of several circadian genes, including Clock and Per2, is sensitive to changes in the cellular redox state resulting from cellular metabolism (65, 66). Recent studies show that redox state alone in the absence of transcription can regulate circadian rhythms in red blood cells (45) and cyanobacteria (82) and that redox state regulates neuron function within the brain central circadian clock suprachiasmatic nuclei (84). Alcohol metabolism by ADH, acetaldehyde dehydrogenase, and CYP2E1 increases cellular oxidative stress through several mechanisms including production of ROS as well as stimulation of iNOS and production of RNS (36–38, 89), along with alteration of the intracellular NADH/NAD ratio in liver cells (32, 36). Molecular oxygen itself is thought to be an important substrate for CYP2E1. CYP2E1, relative to several other P450 enzymes, displays high NADPH oxidase activity, as it appears to be poorly coupled with NADPH-cytochrome P450 reductase (7). Alternatively, another component of the circadian clock machinery, the class III deacetylase Sirtuin 1 (SIRT1), is closely tied to the cellular redox state as well (42, 43). Indeed, SIRT1 function and expression are inhibited by alcohol in alcoholic liver disease (71, 92). Thus alcohol-mediated oxidative stress could affect Clock and Per2 gene function via its effects on SIRT1. Further studies are needed to elucidate the potential role of SIRT1 in alcohol-induced, circadian gene-mediated oxidative stress and intestinal hyperpermeability.
We report, for the first time, that alcohol metabolism and oxidative stress via CYP2E1 may regulate clock gene expression/function and thus permeability by affecting the cellular redox state. In addition, our data show that pretreatment with the antioxidant NAC results in significant inhibition of alcohol-Cyp2e1-mediated induction of CLOCK and PER2 proteins. In further support of a direct role for oxidative stress in this model, we show that H2O2 directly stimulates increased levels of CLOCK and PER2 proteins, mimicking the effects of alcohol. However, these experiments only support the overall model for a role for oxidative stress. Further experiments will be needed to identify the specific molecular species that cause the alcohol-induced effect and how these circadian proteins modulate intestinal permeability.
Our data therefore support the hypothesis that CYP2E1-mediated alcohol metabolism and oxidative stress effects on circadian clock protein function are regulating intestinal hyperpermeability. This should not be a surprise because it is well established that 3–20% of the genome in each organ is directly controlled by circadian genes (6, 27, 48), and several tight junction proteins that are responsible for the regulation of epithelial barrier function in kidney such as E-cadherin and claudins are targets of circadian genes (90). Significantly, circadian genes including Per genes have recently been shown to regulate other gastrointestinal functions such as intestinal motility (23–27). Thus, alcohol-CYP2E1 oxidative stress modulation of circadian clock gene-controlled downstream genes regulating intestinal permeability may mediate the increase in intestinal permeability by alcohol. Further in vivo studies with whole animal or intestinal-specific Cyp2e1 knockout mice will be needed to establish this model in vivo. Further studies are also needed to identify candidate cell junctional proteins that may be circadian targets and thus directly involved in circadian clock gene-mediated alcohol-induced gut leakiness.
Taken together, these data support a model in which alcohol-induced induction of intestinal epithelial cell CYP2E1 increases CLOCK and PER2 circadian clock protein levels through an oxidative stress-mediated mechanism, which in turn stimulates alcohol-induced intestinal hyperpermeability. These studies have identified a new circadian gene-related mechanism by which alcohol metabolism affects intestinal hyperpermeability. Our studies have identified potential new therapeutic targets to prevent alcohol-induced hyperpermeability, consequent endotoxemia, and subsequent deleterious effects like liver injury and other alcohol-associated inflammatory pathologies.
GRANTS
This research was supported in part by grant AA020216 (A. Keshavarzian, F. Turek, C. Forsyth, and R. Voigt) and a gift from Mr. and Mrs. Larry Field and Mr. and Mrs. Silas Keehn (A. Keshavarzian).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.B.F., R.M.V., Y.T., A.I.C., F.W.T., and A.K. conception and design of research; C.B.F., R.M.V., M.S., A.I.C., F.W.T., and A.K. analyzed data; C.B.F., M.S., Y.T., A.I.C., F.W.T., and A.K. interpreted results of experiments; C.B.F., R.M.V., M.S., and A.K. prepared figures; C.B.F. drafted manuscript; C.B.F., R.M.V., M.S., Y.T., A.I.C., F.W.T., and A.K. edited and revised manuscript; C.B.F., R.M.V., M.S., Y.T., A.I.C., F.W.T., and A.K. approved final version of manuscript; R.M.V. and M.S. performed experiments.
REFERENCES
- 1.Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther 291: 1075–1085, 1999 [PubMed] [Google Scholar]
- 2.Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther 294: 997–1008, 2000 [PubMed] [Google Scholar]
- 3.Bergheim I, Bode C, Parlesak A. Distribution of cytochrome P450 2C, 2E1, 3A4, and 3A5 in human colon mucosa. BMC Clin Pharmacol 5: 4, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1: 179–182, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol 4: 8–14, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Bozek K, Relogio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H. Regulation of clock-controlled genes in mammals. PLoS One 4: e4882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 83: 519–548, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Cederbaum AI, Yang L, Wang X, Wu D. CYP2E1 sensitizes the liver to LPS- and TNF alpha-induced toxicity via elevated oxidative and nitrosative stress and activation of ASK-1 and JNK mitogen-activated kinases. Int J Hepatol 2012: 582790, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem 88: 1547–1554, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Donohue TM, Jr, Curry-McCoy TV, Todero SL, White RL, Kharbanda KK, Nanji AA, Osna NA. L-Buthionine (S,R) sulfoximine depletes hepatic glutathione but protects against ethanol-induced liver injury. Alcohol Clin Exp Res 31: 1053–1060, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dunlap JC. Molecular bases for circadian clocks. Cell 96: 271–290, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Ferrier L, Berard F, Debrauwer L, Chabo C, Langella P, Bueno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol 168: 1148–1154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Alcohol stimulates activation of snail, epidermal growth factor receptor signaling, and biomarkers of epithelial-mesenchymal transition in colon and breast cancer cells. Alcohol Clin Exp Res 34: 19–31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Role of snail activation in alcohol-induced iNOS-mediated disruption of intestinal epithelial cell permeability. Alcohol Clin Exp Res 35: 1635–1643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, Jr, French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med 224: 302–308, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Grant BF, Dufour MC, Harford TC. Epidemiology of alcoholic liver disease. Semin Liver Dis 8: 12–25, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Hakkak R, Korourian S, Ronis MJ, Ingelman-Sundberg M, Badger TM. Effects of diet and ethanol on the expression and localization of cytochromes P450 2E1 and P450 2C7 in the colon of male rats. Biochem Pharmacol 51: 61–69, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 4: 649–661, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Hidalgo I, Raub TJ, Bochardt RT. Characterization of human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96: 736–749, 1989 [PubMed] [Google Scholar]
- 20.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96: 736–749, 1989 [PubMed] [Google Scholar]
- 21.Hirayama J, Cho S, Sassone-Corsi P. Circadian control by the reduction/oxidation pathway: catalase represses light-dependent clock gene expression in the zebrafish. Proc Natl Acad Sci USA 104: 15747–15752, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirayama J, Miyamura N, Uchida Y, Asaoka Y, Honda R, Sawanobori K, Todo T, Yamamoto T, Sassone-Corsi P, Nishina H. Common light signaling pathways controlling DNA repair and circadian clock entrainment in zebrafish. Cell Cycle 8: 2794–2801, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Hoogerwerf WA. Role of biological rhythms in gastrointestinal health and disease. Rev Endocr Metab Disord 10: 293–300, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Hoogerwerf WA. Role of clock genes in gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol 299: G549–G555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoogerwerf WA, Hellmich HL, Cornelissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 133: 1250–1260, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Hoogerwerf WA, Shahinian VB, Cornelissen G, Halberg F, Bostwick J, Timm J, Bartell PA, Cassone VM. Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol Gastrointest Liver Physiol 298: G143–G150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoogerwerf WA, Sinha M, Conesa A, Luxon BA, Shahinian VB, Cornelissen G, Halberg F, Bostwick J, Timm J, Cassone VM. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology 135: 2019–2029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol 50: 538–547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 94: 200–207, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Koivisto T, Salaspuro M. Effects of acetaldehyde on brush border enzyme activities in human colon adenocarcinoma cell line Caco-2. Alcohol Clin Exp Res 21: 1599–1605, 1997 [PubMed] [Google Scholar]
- 31.Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J 6: 724–730, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev 77: 517–544, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968–1998)–a review. Alcohol Clin Exp Res 23: 991–1007, 1999 [PubMed] [Google Scholar]
- 34.Lieber CS, Rubin E, DeCarli LM. Hepatic microsomal ethanol oxidizing system (MEOS): differentiation from alcohol dehydrogenase and NADPH oxidase. Biochem Biophys Res Commun 40: 858–865, 1970 [DOI] [PubMed] [Google Scholar]
- 35.Loudon AS. Circadian biology: a 2.5 billion year old clock. Curr Biol 22: R570–R571, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med 44: 723–738, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Cederbaum AI. CYP2E1 potentiation of LPS and TNFalpha-induced hepatotoxicity by mechanisms involving enhanced oxidative and nitrosative stress, activation of MAP kinases, and mitochondrial dysfunction. Genes Nutr 5: 149–167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Wu D, Wang X, Ward SC, Cederbaum AI. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic Biol Med 49: 1406–1416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis 24: 217–232, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Morimoto M, Hagbjork AL, Nanji AA, Ingelman-Sundberg M, Lindros KO, Fu PC, Albano E, French SW. Role of cytochrome P4502E1 in alcoholic liver disease pathogenesis. Alcohol 10: 459–464, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Morimoto M, Hagbjork AL, Wan YJ, Fu PC, Clot P, Albano E, Ingelman-Sundberg M, French SW. Modulation of experimental alcohol-induced liver disease by cytochrome P450 2E1 inhibitors. Hepatology 21: 1610–1617, 1995 [PubMed] [Google Scholar]
- 42.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134: 329–340, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324: 654–657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res 18: 1280–1285, 1994 [DOI] [PubMed] [Google Scholar]
- 45.O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature 469: 498–503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology 51: 307–328, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Pan X, Hussain MM. Clock is important for food and circadian regulation of macronutrient absorption in mice. J Lipid Res 50: 1800–1813, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol Heart Circ Physiol 275: H2334–H2337, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Polidarova L, Sladek M, Sotak M, Pacha J, Sumova A. Hepatic, duodenal, and colonic circadian clocks differ in their persistence under conditions of constant light and in their entrainment by restricted feeding. Chronobiol Int 28: 204–215, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek FW. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am J Physiol Regul Integr Comp Physiol 295: R2034–R2040, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 42: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol 447: 171–183, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res 22: 1724–1730, 1998 [PubMed] [Google Scholar]
- 55.Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease. I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 286: G881–G884, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Reddy AB, O'Neill JS. Healthy clocks: healthy body, healthy mind. Trends Cell Biol 20: 36–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373: 2223–2233, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation: a microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos 13: 548–552, 1985 [PubMed] [Google Scholar]
- 59.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 418: 935–941, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63: 647–676, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Roberts BJ, Shoaf SE, Jeong KS, Song BJ. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochem Biophys Res Commun 205: 1064–1071, 1994 [DOI] [PubMed] [Google Scholar]
- 62.Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem 270: 29632–29635, 1995 [DOI] [PubMed] [Google Scholar]
- 63.Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav 84: 537–542, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol 297: R729–R737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem 71: 307–331, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293: 510–514, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Scheving LA. Biological clocks and the digestive system. Gastroenterology 119: 536–549, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms 24: 304–312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seitz HK, Wang XD. The role of Cytochrome P450 2E1 in ethanol-mediated carcinogenesis. Subcell Biochem 67: 131–143, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Seth A, Basuroy S, Sheth P, Rao RK. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol 287: G510–G517, 2004 [DOI] [PubMed] [Google Scholar]
- 71.Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 298: G364–G374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimizu M, Lasker JM, Tsutsumi M, Lieber CS. Immunohistochemical localization of ethanol-inducible P450IIE1 in the rat alimentary tract. Gastroenterology 99: 1044–1053, 1990 [DOI] [PubMed] [Google Scholar]
- 73.Song BJ. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol Clin Exp Res 20: 138A–146A, 1996 [DOI] [PubMed] [Google Scholar]
- 74.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11: 35–42, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body's biological clock. Alcohol Clin Exp Res 29: 1550–1557, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Swanson G, Forsyth CB, Tang Y, Shaikh M, Zhang L, Turek FW, Keshavarzian A. Role of intestinal circadian genes in alcohol-induced gut leakiness. Alcohol Clin Exp Res 35: 1305–1314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol 16: 1321–1329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Y, Preuss F, Turek FW, Jakate S, Keshavarzian A. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med 10: 597–603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thorn M, Finnstrom N, Lundgren S, Rane A, Loof L. Cytochromes P450 and MDR1 mRNA expression along the human gastrointestinal tract. Br J Clin Pharmacol 60: 54–60, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tipoe GL, Liong EC, Casey CA, Donohue TM, Jr, Eagon PK, So H, Leung TM, Fogt F, Nanji AA. A voluntary oral ethanol-feeding rat model associated with necroinflammatory liver injury. Alcohol Clin Exp Res 32: 669–682, 2008 [DOI] [PubMed] [Google Scholar]
- 81.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Ooijen G, Millar AJ. Non-transcriptional oscillators in circadian timekeeping. Trends Biochem Sci 37: 484–492, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol 16: 1304–1313, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang TA, Yu YV, Govindaiah G, Ye X, Artinian L, Coleman TP, Sweedler JV, Cox CL, Gillette MU. Circadian Rhythm of Redox State Regulates Excitability in Suprachiasmatic Nucleus Neurons. Science, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Cederbaum AI. S-adenosyl-L-methionine attenuates hepatotoxicity induced by agonistic Jo2 Fas antibody following CYP2E1 induction in mice. J Pharmacol Exp Ther 317: 44–52, 2006 [DOI] [PubMed] [Google Scholar]
- 86.White TB, Hammond DK, Vasquez H, Strobel HW. Expression of two cytochromes P450 involved in carcinogen activation in a human colon cell line. Mol Cell Biochem 102: 61–69, 1991 [DOI] [PubMed] [Google Scholar]
- 87.Wilking M, Ndiaye M, Mukhtar H, Ahmad N. Circadian rhythm connections to oxidative stress: implications for human health. Antioxid Redox Signal 19(2): 1–17, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu D, Wang X, Zhou R, Yang L, Cederbaum AI. Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radic Biol Med 53: 1346–1357, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu D, Xu C, Cederbaum A. Role of nitric oxide and nuclear factor-kappaB in the CYP2E1 potentiation of tumor necrosis factor alpha hepatotoxicity in mice. Free Radic Biol Med 46: 480–491, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Yamato M, Ito T, Iwatani H, Yamato M, Imai E, Rakugi H. E-cadherin and claudin-4 expression has circadian rhythm in adult rat kidney. J Nephrol 23: 102–110, 2010 [PubMed] [Google Scholar]
- 91.Yang L, Wu D, Wang X, Cederbaum AI. Cytochrome P4502E1, oxidative stress, JNK, and autophagy in acute alcohol-induced fatty liver. Free Radic Biol Med 53: 1170–1180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 294: G892–G898, 2008 [DOI] [PubMed] [Google Scholar]