Abstract
The function of the endothelial isoform of nitric oxide synthase (eNOS) and production of nitric oxide (NO) is altered in a number of disease states. Pharmacological approaches to enhancing NO synthesis and thus perhaps endothelial function could have substantial benefits in patients. We analyzed the effect of cicletanine, a synthetic pyridine with potent vasodilatory characteristics, on eNOS function and NO production in normal (liver) and injured rat sinusoidal endothelial cells, and we studied the effect of cicletanine-induced NO on stellate cell contraction and portal pressure in an in vivo model of liver injury. Sinusoidal endothelial cells were isolated from normal and injured rat livers. After exposure to cicletanine, eNOS phosphorylation, NO synthesis, and the signaling pathway regulating eNOS activation were measured. Cicletanine led to an increase in eNOS (Ser1177) phosphorylation, cytochrome c reductase activity, l-arginine conversion to l-citrulline, as well as NO production. The mechanism of the effect of cicletanine appeared to be via the protein kinase B (Akt) and MAP kinase/Erk signaling pathways. Additionally, cicletanine improved NO synthesis in injured sinusoidal endothelial cells. NO production induced by cicletanine in sinusoidal endothelial cells increased protein kinase G (PKG) activity as well as relaxation of stellate cells. Finally, administration of cicletanine to mice with portal hypertension induced by bile duct ligation led to reduction of portal pressure. The data indicate that cicletanine might improve eNOS activity in injured sinusoidal endothelial cells and likely activates hepatic stellate cell NO/PKG signaling. It raises the possibility that cicletanine could improve intrahepatic vascular function in portal hypertensive patients.
Keywords: protein kinase B, stellate cell, portal hypertension, signaling, endothelial isoform of nitric oxide synthase, mitogen-activated protein kinase, extracellular/signal-regulated kinase
the endothelium plays a central role in the maintenance of vascular homeostasis largely by virtue of its synthesis of nitric oxide (NO). It is now well appreciated that the endothelial isoform of nitric oxide synthase (eNOS) produces endothelium-derived NO. Furthermore, endothelial dysfunction contributes to the development and clinical course of vascular diseases such as pulmonary and portal hypertension, both characterized by reduced NO bioactivity (19, 23–24, 27).
(±)3-(4-Chlorophenyl)-1,3-dihydro-7-hydroxy-6-methylfuro-(3,4-c)pyridine 3-(4-chlorophenyl)-1,3-dihydro-7-hydroxy-6-methylfuro-(3,4-c) pyridine (cicletanine), a substituted synthetic pyridine developed as an antihypertension agent, has been shown to exert direct relaxant effects on vascular smooth muscle and has potent pulmonary vasodilatory effects in animal models and in pilot studies in humans (5, 9–10, 13, 26, 31). Cicletanine may exert beneficial effects on both endothelial (1, 16) and vascular smooth muscle (14, 29) cells. Furthermore, in vascular smooth muscle cells, cicletanine has been reported to have effects on prostanoid synthesis (10) and cGMP phosphodiesterases (28), whereas, in endothelial cells, cicletanine may regulate eNOS by virtue of coupling to tetrahydrobiopterin (BH4), and appears to lead to Ca2+/K+ channel opening (3) with NO/superoxide release (16).
Given the potential role of cicletanine on endothelium-dependent relaxation, we hypothesized that cicletanine may have a direct effect on eNOS activity in sinusoidal endothelial cells. In this study, we have demonstrated that cicletanine increases nitric oxide synthase (NOS) activity and NO production in both normal sinusoidal endothelial cells and injured endothelial cells, in part via protein kinase B (Akt) and MAP kinase/Erk signaling. Furthermore, paracrine NO regulates stellate cell contractility and appears to reduce portal pressure.
MATERIALS AND METHODS
Cell isolation and culture.
Sinusoidal endothelial cells were isolated from male Sprague-Dawley rats (450–500 g) (Harlan, Indianapolis, IN). In brief, after in situ perfusion of the liver with 20 mg/100 mg pronase (Roche Molecular Biochemicals, Indianapolis, IN), followed by collagenase (Worthington Biochemical, Lakewood, NJ), dispersed cell suspensions were removed from a layered discontinuous density gradient of 8.2 and 15.6% Accudenz (Accurate Chemical and Scientific, Westbury, NY), further purified by centrifugal elutriation (18 ml/min flow), and were grown in medium containing 20% serum (10% horse/calf). The purity of endothelial cells was documented by their uptake of fluorescently labeled di-I-acetoacetylated low-density lipoprotein as described (21). Only primary sinusoidal endothelial isolates of >95% purity were used for study. Experiments were performed with cells from a minimum of three different isolations (for all experiments).
For coculture experiments, freshly isolated stellate cells and freshly isolated sinusoidal endothelial cells were isolated separately and mixed together on collagen-coated plates and cultured for 5 days. Sinusoidal endothelial cells were plated at a density of 200,000 cells/cm2, and stellate cells were at a density of 65,000 cells/cm2.
Adenovirus.
Adenovirus (Ad) control empty vector (Ad-EV), constitutively active Akt (Ad-myrAkt), and dominant-negative Akt (Ad-dnAkt) have been described previously (17). Sinusoidal endothelial cells were exposed to Ad in 2% serum for 16 h, and medium was exchanged; cells were then harvested at the specified time points.
Immunoblotting.
Immunoblotting was performed by using specified primary antibodies, including anti-eNOS antibody (1:1,000; BD Transduction Laboratories, San Jose, CA), anti-phospho-eNOS-Ser1177 (1:1,000; BD Transduction Laboratories or Cell Signaling Technology), anti-phospho-Akt, anti-Akt (Cell Signaling Technology, Beverly, MA), and horseradish peroxidase-conjugated secondary antibody. Specific signals were visualized using enhanced chemiluminescence as per the manufacturer's instructions and were scanned and quantitated with standard software.
Animal model of liver injury and portal hypertension.
Liver injury and portal hypertension were induced by performing bile duct ligation in 450- to 500-gram male retired breeder Sprague-Dawley rats as described (17) and 20- to 25-gram male BALB/c mice. Briefly, bile duct ligation was performed by surgical isolation and ligation of the common bile duct. This model creates a portal-based fibrogenic response and portal hypertension 10–14 days after surgery (17). For study of injured sinusoidal endothelial cells, we used the rat model of bile duct ligation (10–14 days postsurgery); for in vivo study (primarily to conserve the use of active compound), we used the mouse model of bile duct ligation (examining portal pressure 4 wk postsurgery). In sham-operated rats or mice, laparotomy without isolation and section of the bile duct was performed. Studies were approved by the local Institutional Animal Care and Use Committees.
Portal pressure measurement.
Portal hypertension was induced in male BALB/c mice by bile duct ligation as above. Cicletanine (5 mg·kg−1·day−1) or vehicle was given by gavage as a single daily dose for 10 days before portal pressure measurement as described (17). In brief, mice were anesthetized, and an intravenous catheter (Becton Dickinson Vascular Access, Sandy, UT) was introduced in the portal vein and secured with a silk tie. A calibrated low-pressure transducer (Digi-Med, Louisville, KY) was connected to the portal vein catheter, and portal pressure was recorded continuously using the Digi-Med Integrator 200 System (Digi-Med).
NO measurement.
To assess NO production, we analyzed the release of nitrite, the stable breakdown product of NO, using a nitrite/nitrate assay kit (Cayman Chemicals, Ann Arbor, MI), following the manufacturer's instructions. Briefly, conditioned medium from sinusoidal endothelial cells with or without cicletanine treatment were loaded on a 96-well plate and mixed with both enzyme cofactor and nitrate reductase. After incubation at room temperature for 3 h, 100 μl Griess reagent mix were added to each well. After 10 more minutes at room temperature, the absorbance of each well was determined on a microplate reader at 540 nm, and the nitrite concentrations were deduced from a standard curve.
NOS activity assay.
NOS activity from sinusoidal endothelial cell lysates was assessed by measuring the conversion of l-[3H]arginine to l-[3H]citrulline as described (6) per the manufacturer's instructions (Cayman Chemical). In brief, triplicate samples of cell lysates were incubated with a reaction buffer containing 3 pmol of l-[3H]arginine (45 Ci/mmol), 0–50 μM arginine, 1 mM NADPH, 100 nM calmodulin (CaM), 2 mM CaCl2, and 30 μM BH4 in a volume of 50–100 μl at room temperature for 30 min and at 37°C for an additional 30 min in the presence and absence of 1 mM NG-nitro-l-arginine methyl ester (l-NAME). The reaction mixture was terminated by the addition of stop buffer and passed over a Dowex AG 50WX-8 resin column. Radiolabeled counts per minute of generated l-citrulline were measured and used to determine l-NAME-inhibitable NOS activity (thus, each experiment included total counts and background counts).
Cytochrome c reductase assay.
The reductase activities of sinusoidal endothelial cells with or without cicletanine exposure were determined using the NADPH-dependent cytochrome c assay. The reaction was carried out in a total volume of 1.1 ml containing cell lysates (30 μg) in 50 mM Tris·HCl, pH 7.2, 1 μM CaM, 0.2 mM CaCl2, manganese superoxide dismutase (Mn-SOD, 400 U/ml), and 100 μM cytochrome c. The reaction was initiated by the addition of NADPH to a final concentration of 0.5 mM. The reduction of cytochrome c was monitored at 550 nm at 25°C (Beckman Spectrophotometer-Model DU650). Mn-SOD (400 U/ml) was included to eliminate the cytochrome c reduction contributed by O2. The linear portion of the kinetic traces was used to calculate the rate of cytochrome c reduction and reductase activity of eNOS. Turnover number was calculated using the absorbance change during this 30-s interval and an extinction coefficient of 0.021/μM.
Collagen lattice assay.
Contraction of hepatic stellate cells was performed as previously described with minor modifications (25). Briefly, individual wells of a 24-well culture dish were incubated with PBS containing 1% BSA (500 μl/well) for 1 h at 37°C and then washed two times with PBS and allowed to air dry. Collagen gels were prepared by mixing 60% type I tail collagen (Upstate Laboratories), 10% 10× MEM (GIBCO), 10% 0.2 HEPES, and 20% DMEM (GIBCO) to make a final concentration of collagen of 2.4 mg/ml. The solution was added to the culture wells and incubated for 1 h at 37°C, and hepatic stellate cells and sinusoidal endothelial cells were isolated separately from normal rat livers and cocultured (each at a density of 100,000 cells/lattice) on collagen lattices for 5 days. Cells were changed to serum-free medium overnight, then exposed to cicletanine, and then stimulated with endothelin-1 (ET-1, 10 nM). Collagen lattices were released from their substrata, and gel contraction was measured from 0 to 30 min.
Statistical analyses.
All experiments were performed in replicate using cells isolated from different rats. All results were expressed as means ± SE. We performed statistical analysis using the two-tailed Student's t-test, and P < 0.05 was considered statistically significant.
RESULTS
Cicletanine stimulates eNOS in sinusoidal endothelial cells.
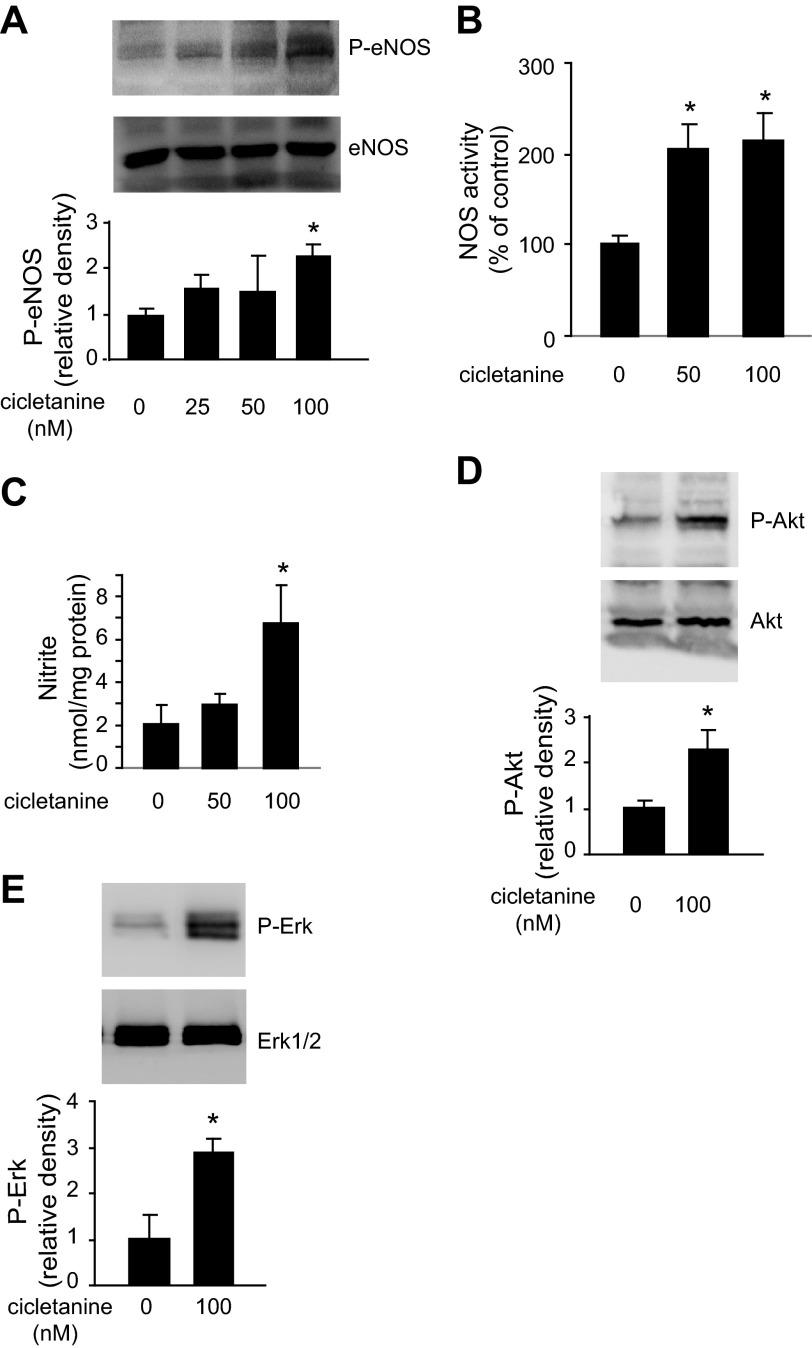
We examined whether cicletanine is capable of activating eNOS in sinusoidal endothelial cells; since eNOS is typically phosphorylation dependent, we initially examined eNOS phosphorylation (Ser1177). After exposure to cicletanine (100 nM), total eNOS expression was unchanged, whereas phosphorylation at Ser1177 was stimulated (Fig. 1A). Consistent with eNOS phosphorylation and activation, exposure of sinusoidal endothelial cells to cicletanine (100 nM) led to robust eNOS activity (i.e., conversion of arginine to citrulline), inhibitable by l-NAME (Fig. 1B). In addition, nitrite levels in conditioned medium were significantly increased after exposure to cicletanine, S-cicletanine, or R-cicletanine (Fig. 1C), consistent with the results of phosphorylation and citrulline activity assays.
Fig. 1.
Effect of cicletanine on the function of endothelial isoform of nitric oxide synthase (eNOS) in sinusoidal endothelial cells. A: sinusoidal endothelial cells were isolated from rat livers and exposed to cicletanine (100 nM) for 2 h, cell lysates were harvested, and phosphor (P)-eNOS (Ser1177), total eNOS, and β-actin were detected by immunoblotting. In the graph shown on the bottom, bands corresponding to phospho-eNOS were quantified (n = 4, *P < 0.01 vs. control). B: the activity of nitric oxide synthase (NOS) in normal sinusoidal endothelial cells was examined in the same cells. NOS activity without exposure to cicletanine was arbitrarily set to 100; n = 3, *P < 0.01 vs. control (no cicletanine), **P < 0.01 vs. cicletanine. l-NAME, NG-nitro-l-arginine methyl ester. C: cells were treated with cicletanine, S-cicletanine, and R-cicletanine as indicated for 2 h, conditioned medium was harvested, and nitrite levels were measured as in materials and methods (n = 3, *P < 0.001 and **P < 0.05 vs. control).
Cicletanine induces eNOS activity in a time- and dose-dependent manner.
We next investigated the effect of cicletanine at different times after its exposure to sinusoidal endothelial cells. We exposed sinusoidal endothelial cells to cicletanine (100 nM) for 0–4 h before harvesting cells and conditioned medium (Fig. 2A). Cicletanine's effects on nitrite production were also dose dependent (Fig. 2B). Cicletanine induced eNOS phosphorylation to the greatest extent at 1 h after exposure; nitrite levels started to increase at 30 min and continued to accumulate over time (Fig. 2, A and C). The NOS inhibitor l-NAME inhibited nitrite production (Fig. 2D). To determine the extent to which cicletanine increased eNOS activity above the basal level, we collected cell lysates at 2 and 6 h after exposure to cicletanine (100 nM) and measured NOS activity. Cicletanine led to consistent increases in eNOS activity at both time points, and was greater at 6 h (Fig. 2E).
Fig. 2.

Cicletanine induces eNOS activity in a time- and dose-response manner. A: sinusoidal endothelial cells were isolated from rat livers and exposed to cicletanine (100 nM) from 1 to 4 h, and cell lysates were subjected to immunoblotting to detect phosphor- or total eNOS. Blots shown are representative of 3 others. In the lower panel, nitrite levels were measured in conditioned medium form the same cells, and quantitative data are shown in the graph below. Medium from cells without treatment served as a control (n = 3, *P < 0.05 and **P < 0.01 vs. time “0”). B: sinusoidal endothelial cells were exposed to 50 or 100 nM cicletanine for 2 h, and cell lysates were subjected to immunoblotting to detect phosphor- or total eNOS. Blots shown are representative of 3 others. In the lower panel, nitrite levels were measured in conditioned medium form the same cells, and quantitative data are shown in the graph below; medium from cells without treatment served as a control (n = 3, *P < 0.01 vs. control). C: normal sinusoidal endothelial cells were exposed to indicated concentrations of cicletanine, and nitrite was measured in conditioned medium at the indicated times; medium from cells without treatment served as controls (n = 3, *P < 0.01 and **P < 0.05 vs. control). D: normal sinusoidal endothelial cells were preexposed to l-NAME (1 μM) or not for 4 h and then exposed to the cicletanine (at the indicated concentrations) for a further 1 or 2 h. In the upper panel is shown a representative immunoblot of cell lysates. Conditioned medium was collected, and nitrite levels were measured as in materials and methods (n = 3, *P < 0.05 vs. 1-h control, ** P < 0.01 vs. 2-h control, #P < 0.01 indicates significant difference between indicated groups). E: sinusoidal endothelial cells were exposed to cicletanine (100 nM) for either 2 or 6 h, cells were harvested, and NOS activity was measured in cell lysates, which was normalized to the level observed at the 2-h time point without cicletanine (n = 3, *P < 0.05 vs. 2-h exposure control, **P < 0.001 vs. 6-h exposure controls).
Increased eNOS activity caused by cicletanine can be attributed to enhanced reductase activity.
We found a significant increase in cytochrome c reductase activity after exposure of sinusoidal endothelial cells to cicletanine (100 nM) (Fig. 3).
Fig. 3.
Cicletanine potentiates eNOS cytochrome c reductase activity. Sinusoidal endothelial cells were isolated from rat livers and exposed to cicletanine (100 nM) for 2 h, cell lysates were harvested, and NADPH cytochrome c reductase activity was measured as in materials and methods. Cells without treatment served as a control. The rate of basal cytochrome c reductase activity and the cytochrome c reductase activity in the presence of calmodulin (CaM) are shown graphically (n = 3, *P < 0.01 vs. control).
Cicletanine-induced eNOS activity is Akt dependent.
To understand the mechanism by which cicletanine stimulates eNOS, we performed studies examining the known eNOS-signaling partner Akt. Again, cicletanine increased eNOS phosphorylation at Ser1177 as well as Akt phosphorylation at Ser473 without a change in total Akt expression (Fig. 4, A and B). To determine whether eNOS phosphorylation by cicletanine was Akt dependent, we transduced sinusoidal endothelial cells with Ad-myrAkt, Ad-dnAkt, or Ad-EV for 24 h and exposed cells to cicletanine (100 nM) for an additional 2 h. Cicletanine stimulated nitrite production while the dominant active Akt further increased it and the dominant negative Akt blocked cicletanine's effects (Fig. 4C). These data suggest that cicletanine's mechanism of action requires Akt signaling.
Fig. 4.
Cicletanine-induced eNOS activity is protein kinase B (Akt) dependent. A: sinusoidal endothelial cells were isolated from rat livers and were exposed to 50 or 100 nM cicletanine for 2 h, and cell lysates were harvested and subjected to immunoblotting to detect phospho (Ser473)- and total Akt. In the graph shown on bottom, bands corresponding to phospho-Akt were quantified (n = 3, *P < 0.01 vs. control). B: sinusoidal endothelial cells were exposed to 50 or 100 nM cicletanine for 1 or 2 h, and cell lysates were harvested and subjected to immunoblotting to detect phospho-eNOS, total eNOS, phospho-Akt (Ser473), total Akt, and β-actin. Blots representative of 3 others are shown. C: normal sinusoidal endothelial cells were infected with adenovirus (Ad, multiplicity of infection 250) encoding constitutively active Akt (Ad-myrAkt), dominant-negative Akt (Ad-dnAkt), or an empty vector (Ad-EV) for 24 h before exposure to cicletanine (100 nM) for an additional 2 h. Conditioned medium was harvested, and nitrite levels were measured and presented in the graph; medium from cells treated with Ad-EV alone served as a control (n = 3, *P < 0.05 and **P < 0.01 vs. control).
Erk activation is involved in cicletanine-induced eNOS activity.
We also explored the possibility that eNOS activation induced by cicletanine involved the MAP kinase signaling partners, including Erk1 and Erk2. First, we examined the relationship between Erk and eNOS phosphorylation. We found that phosphorylation of Erk and eNOS was increased in a dose-dependent manner after exposure of sinusoidal endothelial cells to cicletanine for 2 h (Fig. 5A). Additionally, the Erk inhibitor PD-98059 blocked cicletanine-induced eNOS phosphorylation (Fig. 5B) and NO production (Fig. 5C).
Fig. 5.
Cicletanine-induced eNOS activity is Erk dependent. A: sinusoidal endothelial cells were isolated from rat livers and were exposed to 50 or 100 nM cicletanine for 2 h, and cell lysates were subjected to immunoblotting to detect phospho-eNOS, total eNOS, phospho-Erk, total Erk1/2, and β-actin. In the graphs shown on bottom, bands corresponding to phospho-Erk were quantified (n = 3, *P < 0.05 vs. control). B: sinusoidal endothelial cells as in A were pretreated with the MAP kinase inhibitor PD-98059 (10 μM) for 30 min and then stimulated with cicletanine (100 nM) for an additional 2 h; eNOS and Erk phosphorylation were detected by immunoblotting. In the graph shown on bottom, bands corresponding to phospho-Erk were quantified (n = 3, *P < 0.01 vs. no cicletanine control, **P < 0.005 vs. cicletanine control). C: conditioned supernatants from cells as in B were collected, and nitrite levels were measured (n = 3, *P < 0.05 vs. no cicletanine control, **P < 0.01 vs. cicletanine control).
Cicletanine stimulates eNOS activity in injured sinusoidal endothelial cells.
We and others have previously shown that Akt phosphorylation and eNOS activity is reduced after liver injury and in portal hypertensive rats and mice (17, 24, 27). We next tested whether cicletanine could rescue the abnormal endothelial cell/eNOS phenotype typical of liver injury. Cicletanine restored eNOS phosphorylation to above basal levels in sinusoidal endothelial cells injured by bile duct ligation (note that there is little to no phosphorylated eNOS in unstimulated cells; Fig. 6A), concomitant with an increase in eNOS activity [eNOS activity in injured sinusoidal endothelial cells is significantly reduced compared with that from normal endothelial cells (24, 27); Fig. 6B] and nitrite production (Fig. 6C). Cicletanine also restored Akt phosphorylation to above normal levels (Fig. 6D) and increased Erk1/2 phosphorylation (Fig. 6E).
Fig. 6.
Effects of cicletanine in injured sinusoidal endothelial cells. A: sinusoidal endothelial cells were isolated from rat livers 10 days after bile duct ligation (BDL), allowed to adhere overnight, and then exposed to the indicated concentrations of cicletanine for 2 h; cells were harvested, and cell lysates were subjected to immunoblotting to detect phospho- and total eNOS. In the graph shown on bottom, bands corresponding to phospho-eNOS were quantified (n = 3, *P < 0.01 vs. control). B: sinusoidal endothelial cells as in A were harvested, and NOS activity was measured in cell lysates; NOS activity was normalized to that of control cells and is presented graphically (n = 3, *P < 0.005 vs. control). C: conditioned medium as in A was harvested, and nitrite levels were measured as in materials and methods (n = 3, *P < 0.05 vs. control). D and E: sinusoidal endothelial cells as in A were harvested, and cell lysates were immunoblotted to detect phospho- and total Akt and phospho- and total Erk1/2, respectively. Bands corresponding to phospho-Akt and phospho-Erk were quantitated, normalized, and shown in the lower graphs (D: n = 3, *P < 0.01 vs. control; E: n = 3, *P < 0.005 vs. control).
Cicletanine-induced NO inhibits stellate cell contractility via activation of NO/protein kinase G.
To determine whether cicletanine-induced NO has a physiological function, we developed a coculture model in which we cultured sinusoidal endothelial cells with hepatic stellate cells, the latter of which generate considerable contractile force (25). We used a model in which stellate cells were cultured on thick collagen lattices; after their activation by ET-1, they contract. Cicletanine inhibited stellate cell contraction in a dose-dependent manner (Fig. 7A), consistent with a relevant physiological effect. Next, we studied protein kinase G (PKG) activation by assaying phospho-vasodilator-stimulated phosphoprotein (VASP) in stellate cells cocultured with or without sinusoidal endothelial cells. VASP, a key downstream target of the NO/cGMP signaling pathway, is a vasodilator-stimulated phosphoprotein. We found that cicletanine did not alter VASP phosphorylation in stellate cells, but it stimulated VASP phosphorylation in stellate cells cocultured with sinusoidal endothelial cells (Fig. 7B) in a dose-dependent manner and was inhibited by the eNOS inhibitor l-NAME (Fig. 7C).
Fig. 7.
Cicletanine-induced nitric oxide (NO) production in sinusoidal endothelial cells causes protein kinase G (PKG) activation and stellate cell relaxation. A: hepatic stellate cells and sinusoidal endothelial cells were isolated separately from rat livers and cocultured (each at a density of 100,000 cells/lattice) on collagen lattices for 5 days as in materials and methods. Cells were then serum starved overnight, exposed to 50 or 100 nM cicletanine for 2 h, and then stimulated with endothelin-1 (ET-1, 10 nM). Collagen lattices were released from their substrata, and gel contraction was measured over time. A representative experiment with images of changes in gel area at the indicated time points is shown on left. Gel areas were measured, quantified, and depicted quantitatively in the graph on the right (n = 4, *P < 0.05 and **P < 0.001 vs. control). B: hepatic stellate cells (HSC) and sinusoidal endothelial cells (EC) were isolated, and HSC were cultured alone or cocultured (HSC + EC) as in materials and methods. Cells were harvested, and cell lysates were immunoblotted to detect phospho-vasodilator-stimulated phosphoprotein (VASP) for PKG activity. HSC or HSC + EC without cicletanine served as controls. Bands corresponding to phospho-VASP were quantitated, normalized, and shown in the lower graph (n = 3, *P < 0.01 and **P < 0.001 vs. HSC + EC control). C: cells (HSC + EC) as in B were preexposed to l-NAME (1 μM) for 4 h and then exposed to cicletanine (100 nM) for an additional 1–2 h. Phospho-VASP, PKG, and β-actin were detected in cell lysates by immunoblotting, and representative images are shown.
Cicletanine reduces portal pressure in vivo.
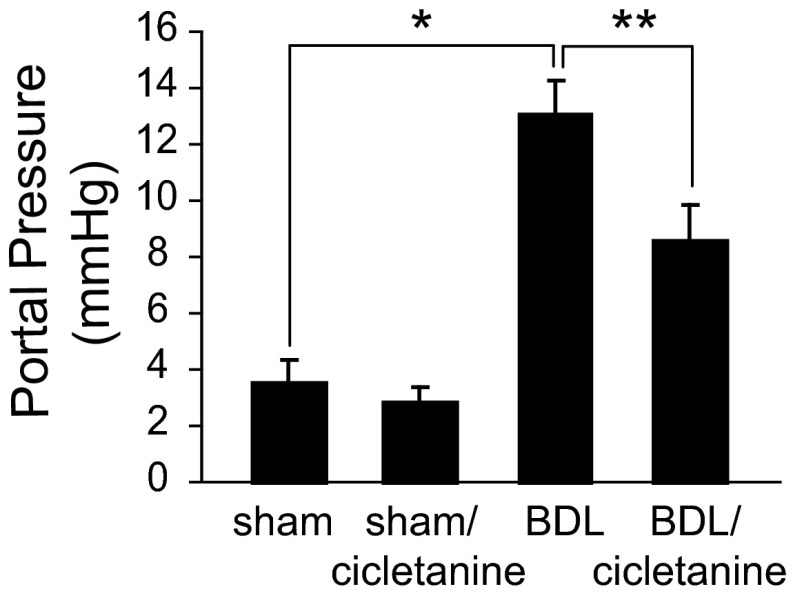
To investigate whether cicletanine may have an effect on intrahepatic relaxation in vivo, we measured portal pressure following cicletanine gavage in bile duct-ligated (BDL) mice. As expected, portal pressure was significantly elevated in BDL mice compared with sham-operated mice (Fig. 8). However, cicletanine treatment significantly reduced portal pressure after BDL. Slight and nonsignificant decreases in portal pressure were observed in sham-operated mice in response to cicletanine.
Fig. 8.
Cicletanine reduces portal pressure. Portal hypertension was induced by performing BDL in BALB/c mice; sham operations were also performed as described in materials and methods. Cicletanine (5 mg·kg−1·day−1) or vehicle was given by gavage in a single daily dose for 10 days before portal pressure measurement (which was performed at day 28) as described in materials and methods and presented graphically (n = 5 for each group; *P < 0.001 and **P < 0.005 for differences between indicated groups).
DISCUSSION
We have shown that cicletanine directly stimulates eNOS in sinusoidal endothelial cells in a concentration- and dose-dependent fashion; the mechanism of eNOS activation appears to be linked to an Akt/Erk pathway and regulation of stellate cell contractility via the NO/PKG signaling pathway (Fig. 9). Additionally, cicletanine stimulated eNOS in injured sinusoidal endothelial cells, suggesting that it may rescue abnormal signaling pathways.
Fig. 9.
A proposed model of cicletanine effects on sinusoidal endothelial cells. Cicletanine, a furopyridine derivative, stimulated eNOS phosphorylation in sinusoidal endothelial cells (SECs). The data suggest a dual role, including at the level of Akt-eNOS and Erk-eNOS signaling. Cicletanine-mediated NO generation via enhanced eNOS activity appears to stimulate soluble guanylate cyclase in stellate cells, which activates cGMP/PKG and (downstream) VASP, leading to stellate cell relaxation.
Previous literature suggests that cicletanine leads to vasodilation by increasing intracellular Ca2+ concentrations and stimulating the formation of prostaglandins (5, 10) and cGMP phosphodiesterases in cultured vascular smooth muscle cells (28–29). Another study revealed that cicletanine enhanced eNOS coupling to BH4, leading to NO production. Cicletanine also stimulated Ca2+/K+ channel opening in cultured cells consistent with cell vasorelaxation (3, 20). Finally, cicletanine caused NO/O2− release in human umbilical vein endothelial cells (16). Here, our data extend these previous studies by indicating that the mechanism by which cicletanine acts is by posttranslational eNOS phosphorylation and activation.
Currently, posttranslational mechanisms of eNOS activation are an area of active investigation. eNOS catalytic function is influenced by phosphorylation, acylation, and protein interactions (4, 6, 11). Because phosphorylation of eNOS at Ser1177 by Akt is critical for activation of eNOS (7, 11), we investigated the effects of cicletanine in this regard; our results clearly indicate that cicletanine activates the protein kinase Akt, leading to posttranslational activation of eNOS via phosphorylation of Ser1177 (Figs. 4 and 6).
The interrelationships of eNOS and MAP kinase pathways are not entirely understood. Of note, eNOS has many putative MAP kinase phosphorylation consensus binding site sequences (2). Additionally, recent studies suggest that eNOS and Erk physically associate in the cell cytoplasm (2) and that Erk appears to promote eNOS activity (18). Therefore, we inquired as to whether cicletanine stimulates Erk and thus eNOS. We found that cicletanine robustly induced Erk activation in sinusoidal endothelial cells (Figs. 5 and 6). PD-098059, an MEK inhibitor and downstream inactivator of Erk, abolished cicletanine stimulation of Ser1177 phosphorylation and NO generation, suggesting a role for Erk. Thus, it is possible that cicletanine regulates eNOS in a dual pathway involving Akt and Erk.
The biochemical basis by which NOS functions is via the regulation of electron flux form NADPH through flavins in the reductase portion of NOS protein to the heme domain in the oxygenase domain and control the rate of NO synthesis (8, 22). We therefore examined eNOS cytochrome c reductase activity (12) and found that cicletanine is able to enhance the rate of cytochrome c reduction twofold more compared with no cicletanine treatment in both the basal level and CaM stimulation (Fig. 3), suggesting that cicletanine plays a critical role on the electron flux from the reductase domain to the oxygenase domain and contributes to increase NO generation in sinusoidal endothelial cells.
Portal hypertension is associated with deficient endothelial cell NO production, which results in increased intrahepatic resistance and portal pressure (24, 27). Thus, providing the liver with NO is a potentially novel therapeutic strategy. Here, we found that cicletanine, which has been shown to have effects on the pulmonary endothelium (15, 26, 30, 32), not only enhances eNOS activity in normal sinusoidal endothelial cells but also improves the reduced eNOS activity typical of injured sinusoidal endothelial cells (Fig. 6). The mechanism appears to involve enhancement of Akt-Erk-eNOS signaling in injured sinusoidal endothelial cells. Overall, our data substantially extend previous work and emphasize a mechanism involving ERK responsible for the impairment of sinusoidal endothelium-dependent NO production in injured sinusoidal endothelial cells. The data also raise the possibility that cicletanine may augment eNOS bioactivity and may be useful for treatment of portal hypertension (Fig. 9).
In summary, our data support a physiological role for the use of pharmacological agents such as cicletanine to stimulate eNOS function in sinusoidal endothelial cells, which had paracrine effects on stellate cells (Fig. 7), and additionally reduced portal pressure (Fig. 8). The data highlight the possibility that pharmacological intervention in patients with increased intrahepatic resistance and portal hypertension may be clinically feasible.
GRANTS
This work was supported by a grant from Gilead Pharmaceuticals, National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-57830 (to D. C. Rockey), and the American Liver Foundation (S. Liu is the recipient of an American Liver Foundation Liver Scholar Award).
DISCLOSURES
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: S.L. and D.C.R. conception and design of research; S.L. performed experiments; S.L. and D.C.R. analyzed data; S.L. and D.C.R. interpreted results of experiments; S.L. and D.C.R. prepared figures; S.L. and D.C.R. drafted manuscript; S.L. and D.C.R. edited and revised manuscript; S.L. and D.C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Yingyu Ren for valuable assistance with primary cell isolation and cell culture, Dr. Qing Yu for valuable assistance with collagen lattice assay and data collection, and Faquan Liang for helpful discussion.
REFERENCES
- 1.Akamatsu N, Sawada S, Komatsu S, Tamagaki T, Hiranuma O, Kawahara T, Tsuda Y, Kono Y, Higaki T, Tada Y, Yamasaki S, Imamura H, Sato T, Tsuji H, Nakagawa M. Effect of cicletanine on the nitric oxide pathway in human umbilical vein endothelial cells. J Cardiovasc Pharmacol 38: 174–182, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Bernier SG, Haldar S, Michel T. Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem 275: 30707–30715, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bukoski RD, Bo J, Xue H, Bian K. Antiproliferative and endothelium-dependent vasodilator properties of 1,3-dihydro-3-p-chlorophenyl-7-hydroxy-6-methyl-furo-(3,4c) pyridine hydrochloride (cicletanine). J Pharmacol Exp Ther 265: 30–35, 1993 [PubMed] [Google Scholar]
- 4.Busconi L, Michel T. Endothelial nitric oxide synthase. N-terminal myristoylation determines subcellular localization. J Biol Chem 268: 8410–8413, 1993 [PubMed] [Google Scholar]
- 5.Calder JA, Schachter M, Sever PS. Interaction between cicletanine and the eicosanoid system in human subcutaneous resistance arteries. J Pharm Pharmacol 44: 574–578, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC, Shah V. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem 276: 14249–14256, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest 120: 2319–2330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen PF, Tsai AL, Berka V, Wu KK. Endothelial nitric-oxide synthase. Evidence for bidomain structure and successful reconstitution of catalytic activity from two separate domains generated by a baculovirus expression system. J Biol Chem 271: 14631–14635, 1996 [PubMed] [Google Scholar]
- 9.Dobrucki LW, Marsh BJ, Kalinowski L. Elucidating structure-function relationships from molecule-to-cell-to-tissue: from research modalities to clinical realities. J Physiol Pharmacol 60, Suppl 4: 83–93, 2009 [PubMed] [Google Scholar]
- 10.Ebeigbe AB, Oguogho A, Ighoroje AD, Aloamaka CP. Differential effects of prostanoid synthesis inhibitors on cicletanine-induced relaxation of isolated human epigastric arteries. Clin Auton Res 4: 137–139, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh S, Gachhui R, Crooks C, Wu C, Lisanti MP, Stuehr DJ. Interaction between caveolin-1 and the reductase domain of endothelial nitric-oxide synthase. Consequences for catalysis. J Biol Chem 273: 22267–22271, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Hirawa N, Uehara Y, Kawabata Y, Akie Y, Ichikawa A, Funahashi N, Goto A, Omata M. Restoration of endothelial cell function by chronic cicletanine treatment in Dahl salt-sensitive rats with salt-induced hypertension. Hypertens Res 19: 263–270, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Izumi M, Mitsui-Saito M, Ozaki H, Karaki H. Cicletanine-induced decreases in cytosolic Ca2+ level and contraction in vascular smooth muscle. Jpn J Pharmacol 76: 57–63, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Jin H, Yang RH, Oparil S. Cicletanine blunts the pulmonary pressor response to acute hypoxia in rats. Am J Med Sci 304: 14–19, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Kalinowski L, Dobrucki IT, Malinski T. Cicletanine stimulates nitric oxide release and scavenges superoxide in endothelial cells. J Cardiovasc Pharmacol 37: 713–724, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med 11: 952–958, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem 278: 9142–9149, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 277: 44085–44092, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Noack T, Deitmer P. Effects of cicletanine on whole-cell currents of single smooth muscle cells from the guinea-pig portal vein. Br J Pharmacol 109: 164–170, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitas RE, Boyles J, Mahley RW, Bissell DM. Uptake of chemically modified low density lipoproteins in vivo is mediated by specific endothelial cells. J Cell Biol 100: 103–117, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Presta A, Liu J, Sessa WC, Stuehr DJ. Substrate binding and calmodulin binding to endothelial nitric oxide synthase coregulate its enzymatic activity. Nitric Oxide 1: 74–87, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Rabinovitch M, Bothwell T, Hayakawa BN, Williams WG, Trusler GA, Rowe RD, Olley PM, Cutz E. Pulmonary artery endothelial abnormalities in patients with congenital heart defects and pulmonary hypertension. A correlation of light with scanning electron microscopy and transmission electron microscopy. Lab Invest 55: 632–653, 1986 [PubMed] [Google Scholar]
- 24.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology 114: 344–351, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest 92: 1795–1804, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saadjian A, Philip-Joet F, Paganelli F, Arnaud A, Levy S. Long-term effects of cicletanine on secondary pulmonary hypertension. J Cardiovasc Pharmacol 31: 364–371, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, Groszmann RJ. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 117: 1222–1228, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Silver PJ, Buchholz A, Dundore RL, Harris AL, Pagani ED. Inhibition of low Km cyclic GMP phosphodiesterases and potentiation of guanylate cyclase activators by cicletanine. J Cardiovasc Pharmacol 16: 501–505, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Silver PJ, O'Connor B, Cumiskey WR, Van Aller G, Hamel LT, Bentley RG, Pagani ED. Inhibition of low Km cGMP phosphodiesterases and Ca+(+)-regulated protein kinases and relationship to vasorelaxation by cicletanine. J Pharmacol Exp Ther 257: 382–391, 1991 [PubMed] [Google Scholar]
- 30.Stenmark KR, Rabinovitch M. Emerging therapies for the treatment of pulmonary hypertension. Pediatr Crit Care Med 11: S85–S90, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Uehara Y, Kawabata Y, Hirawa N, Takada S, Nagata T, Numabe A, Iwai J, Sugimoto T. Possible radical scavenging properties of cicletanine and renal protection in Dahl salt sensitive rats. Am J Hypertens 6: 463–472, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Waxman AB, Lawler L, Cornett G. Cicletanine for the treatment of pulmonary arterial hypertension. Arch Intern Med 168: 2164–2166, 2008 [DOI] [PubMed] [Google Scholar]