Abstract
Transient receptor potential (TRP) subfamily M member 5 (TRPM5) cation channel is involved in sensing sweet, bitter, umami, and fat taste stimuli, complex-tasting divalent salts, and temperature-induced changes in sweet taste. To investigate if the amiloride- and benzamil (Bz)-insensitive NaCl chorda tympani (CT) taste nerve response is also regulated in part by TRPM5, CT responses to 100 mM NaCl + 5 μM Bz (NaCl + Bz) were monitored in Sprague-Dawley rats, wild-type (WT) mice, and TRP vanilloid subfamily member 1 (TRPV1) and TRPM5 knockout (KO) mice in the presence of resiniferatoxin (RTX), a TRPV1 agonist. In rats, NaCl + Bz + RTX CT responses were also monitored in the presence of triphenylphosphine oxide, a specific TRPM5 blocker, and capsazepine and N-(3-methoxyphenyl)-4-chlorocinnamid (SB-366791), specific TRPV1 blockers. In rats and WT mice, RTX produced biphasic effects on the NaCl + Bz CT response, enhancing the response at 0.5–1 μM and inhibiting it at >1 μM. The NaCl + Bz + SB-366791 CT response in rats and WT mice and the NaCl + Bz CT response in TRPV1 KO mice were inhibited to baseline level and were RTX-insensitive. In rats, blocking TRPV1 by capsazepine or TRPM5 by triphenylphosphine oxide inhibited the tonic NaCl + Bz CT response and shifted the relationship between RTX concentration and the magnitude of the tonic CT response to higher RTX concentrations. TRPM5 KO mice elicited no constitutive NaCl + Bz tonic CT response. The relationship between RTX concentration and the magnitude of the tonic NaCl + Bz CT response was significantly attenuated and shifted to higher RTX concentrations. The results suggest that pharmacological or genetic alteration of TRPM5 activity modulates the Bz-insensitive NaCl CT response and its modulation by TRPV1 agonists.
Keywords: triphenylphosphine oxide, resiniferatoxin, capsazepine, SB-366791, TRPV1
appetitive and aversive neural and behavioral responses to NaCl are most likely transduced by a variety of mechanisms (35). Several studies suggest that in the anterior tongue Na+ from a Na+ salt taste stimulus enters a subset of taste bud cells by at least two types of cation channels located in taste cell apical membranes. One channel type is the Na+-specific epithelial Na+ channel (ENaC), in which Na+ transport is inhibited by amiloride and benzamil (Bz) (1, 3, 8). The second involves Na+ transport through a putative transient receptor potential (TRP) vanilloid subfamily member 1 (TRPV1t)-nonspecific cation channel (26, 27). The TRPV1t channel is insensitive to amiloride and Bz, permeable to Na+, K+, NH4+, and Ca2+, and contributes to cell depolarization (31). These conclusions are supported by the observations that the phasic (transient) and tonic (steady-state) components of the chorda tympani (CT) taste nerve response to NaCl are inhibited to the baseline rinse level in TRPV1 knockout (KO) mice stimulated with NaCl + Bz and in wild-type (WT) mice and rats in the presence of NaCl + Bz + N-(3-methoxyphenyl)-4-chlorocinnamid (SB-366791, a TRPV1 blocker) (26, 27). Several agonists and antagonists of TRPV1 modulate the Bz-insensitive NaCl CT responses in animal models and alter human salt taste (11, 20, 26–30, 40, 41, 48). TRPV1 expression has been observed immunohistochemically in primary afferents and also in oral epithelial cells in the rat tongue (18, 21) and in human taste cell lines from lingual epithelium by RT-PCR (16, 17). A recent study identified TRPV1 rs8065080 (C > T) single-nucleotide polymorphism as a likely modifier of an individual's perception of salt at suprathreshold levels (12).
We previously showed that changes in intracellular Ca2+, protein kinase C, calcineurin, and phosphatidylinositol 4,5-bisphosphate (PIP2) in fungiform taste receptor cells (TRCs) regulate the Bz-insensitive NaCl CT response (9, 31, 32). However, whether one or more bitter, sweet, and umami taste-specific intracellular signaling intermediates also modulate the Bz-insensitive salt response has not been tested. The enzyme PLCβ2 and the TRP subfamily M member 5 (TRPM5) cation channel are expressed in a subset of taste cells and are necessary for the perception of sweet, bitter, and umami taste stimuli (7, 50). However, the role of TRPM5 is by no means restricted to the sensing of sweet, bitter, and umami taste. It is suggested that the sensory attributes of complex-tasting divalent salts are also mediated by TRPM5 and TRPV1 channels (42). TRPM5 is also essential for fat taste (23) and for linoleic acid-induced CCK secretion from the enteroendocrine cell line STC-1 (44). TRPM5 is a highly temperature-sensitive, heat-activated channel that may underlie known effects of temperature on perceived taste, including the enhanced sweetness perception at high temperatures, in humans (46).
To better understand the intracellular signals involved in the neural responses to NaCl, we tested if the Bz-insensitive NaCl response is also partially dependent on TRPM5 and PLCβ2 activity. We monitored the CT response to 100 mM NaCl + 5 μM Bz in Sprague-Dawley rats, WT mice, TRPV1 KO mice, TRPM5 KO mice, and PLCβ2 KO mice in the absence and presence of varying concentrations of the TRPV1 agonist resiniferatoxin (RTX). In rats, CT responses were also monitored after topical lingual application of triphenylphosphine oxide (TPPO), a specific blocker of the TRPM5 cation channel (38), and in the presence of capsazepine (CZP) and SB-366791, specific blockers of TRPV1 and the Bz-insensitive NaCl CT response (15, 26, 27). Our results show that the CT response at 100 mM NaCl was significantly smaller in TRPM5 KO than WT mice. However, at high NaCl concentration (300 mM), the CT response was not different between the two genotypes. The difference in the CT response to 100 mM NaCl between WT and TRPM5 KO mice was due to the absence of the Bz-insensitive component of the NaCl CT response in TRPM5 KO mice. There was no difference in the Bz-sensitive component of the NaCl CT response between the two genotypes. The dose-response relationship between RTX concentration and the magnitude of the Bz-insensitive tonic NaCl CT response was significantly attenuated and was shifted to higher RTX concentrations in TRPM5 KO than WT mice. A similar shift in the RTX dose-response relationship was observed when TRPM5 activity was inhibited with TPPO or when TRPV1 activity was inhibited with CZP. Taken together, our results suggest that pharmacological or genetic alteration of TRPM5 activity in TRCs modulates the Bz-insensitive NaCl CT response at low NaCl concentrations in the absence and presence of TRPV1 modulators.
MATERIALS AND METHODS
CT taste nerve recordings.
Animals were housed in the Virginia Commonwealth University animal facility in accordance with institutional guidelines. All animal protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. Female Sprague-Dawley rats (150–200 g body wt; Charles River, Wilmington, MA) were anesthetized by intraperitoneal injection of pentobarbital sodium (60 mg/kg; Sigma-Aldrich, St. Louis, MO), and supplemental pentobarbital sodium (20 mg/kg) was administered as necessary to maintain surgical anesthesia. The animal's corneal reflex and toe-pinch reflex were used to monitor the depth of surgical anesthesia. Body temperatures were maintained at 37°C with a Deltaphase isothermal pad (model 39 DP, Braintree Scientific, Braintree, MA). The left CT nerve was exposed laterally as it exited the tympanic bulla and placed onto a 32-gauge platinum-iridium wire electrode. Stimulus solutions maintained at room temperature were injected into a Lucite lingual perfusion chamber (3 ml at 1 ml/s) affixed by vacuum to a 28-mm2 patch of anterior dorsal lingual surface. CT responses were recorded under zero lingual current clamp and analyzed as described previously (20).
CT responses were also monitored in WT (C57BL/6J) mice and homozygous TRPV1 KO mice (B6.129S4-Trpv1tmijul, Jackson Laboratory, Bar Harbor, ME), TRPM5 KO mice (obtained from Dr. Charles Zuker, University of California, San Diego), and PLCβ2 KO mice (obtained from Dr. Stephen D. Roper, University of Miami). Mice (30–40 g body wt) were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg), and supplemental pentobarbital sodium (10 mg/kg) was administered as necessary to maintain surgical anesthesia. Because our lingual perfusion chamber is too large for the mouse tongue, CT recordings were made in mice while the anterior tongue was stimulated by perfusion of the rinse solution or taste stimuli (3 ml) at the rate of 1 ml/s. The rest of the procedure was the same as described above for rats. At the end of each experiment, animals were humanely killed by an intraperitoneal overdose of pentobarbital sodium (∼195 mg/kg body wt for rats and 150 mg/kg body wt for mice).
The rinse solution (R) was 10 mM KCl, and stimulating solutions contained 100 mM NaCl; 100 mM NaCl + 5 μM Bz, a specific blocker of ENaC; 100 mM NaCl + 5 μM Bz + 1 μM SB-366791 (SB), a specific blocker of TRPV1; 100 mM NaCl + 5 μM Bz + RTX (0–10 μM), a specific agonist of TRPV1 (26, 27); and 100 mM NaCl + 5 μM Bz + RTX (0–10 μM) + 10 μM CZP, a blocker of TRPV1. Bz, SB, CZP, and RTX were obtained from Sigma-Aldrich. In some experiments, CT responses were monitored in rats before and after the topical lingual application of TPPO (Fisher Scientific, Pittsburgh, PA), a specific blocker of the TRPM5 cation channel (38). TPPO was directly dissolved in DMSO (Sigma-Aldrich) and applied topically to the dorsal surface of the rat tongue for 20 min at a concentration of 2 mM. After 20 min, the tongue was thoroughly washed with the rinse solution for 10 min before the CT recordings. We previously showed that topical application of DMSO alone does not alter CT responses to taste stimuli (24). Typically, stimulus solutions remained on the tongue for 1 min. Control stimuli consisting of 300 mM NH4Cl and 300 mM NaCl applied at the beginning and end of the experiment were used to assess preparation stability (31, 32). As in our previous studies, the control responses did not differ by more than 2–5% at the beginning and end of the experiment (11, 48). The stimulus series used in the CT experiments to generate the relationship between RTX concentration and the magnitude of the NaCl + Bz CT response was as follows: R → 300 mM NH4Cl → R → 300 mM NaCl → R → 100 mM NaCl → R → (100 mM NaCl + 5 μM Bz) → R → (100 mM NaCl + 5 μM Bz + RTX) → R.
The R → (NaCl + Bz + RTX) → R step was repeated for each concentration of RTX between 0 and 10 μM. At the end of the RTX concentration series, the control stimuli were again applied (R → 300 mM NH4Cl → R → 300 mM NaCl → R). In additional series, the stimulating solutions were 100 mM NaCl + 5 μM Bz + 1 μM SB + RTX or 100 mM NaCl + 5 μM Bz + 10 μM CZP + RTX.
In CT experiments, the tonic (steady-state) part of the NaCl CT responses was quantified. For quantification of the tonic part of a response, the area under the response vs. time curve was taken over the final 30 s of the response. To normalize this result, this area was divided by the area under the 300 mM NH4Cl response curve over the final 30 s of the tonic response period. In some CT experiments, we also quantified the transient (phasic) part of the NaCl CT response. For quantification of the phasic part of the CT response, the height of the stimulus-induced maximum CT response relative to the baseline response was divided by the mean steady-state (tonic) response to 300 mM NH4Cl. We also quantified the transient (phasic) response to the application of rinse solution to a tongue already superfused with the rinse solution (rinse artifact). The normalized data are reported as means ± SE of the number of animals (n) or percent change in tonic NaCl CT response relative to the response of NaCl + Bz in the control (6). Student's t-test was employed to analyze the differences between sets of data. Since we are comparing the normalized CT responses to NaCl + Bz before and after RTX in the same CT preparation, a paired t-test was used to evaluate statistical significance. Comparisons between two strains of mice were made using an unpaired t-test (GraphPad).
For clarity, the points on the graphs of the mean normalized tonic responses vs. the logarithm of the RTX concentration were connected by smooth curves. The curves were generated using a fitting function that models the characteristic biphasic property of the agonists of TRPV1. The biphasic property has been observed with every agonist of TRPV1t thus far examined (6, 11, 20, 26–30, 48). The fitting function was
| 1 |
where
| 2 |
and
| 3 |
where R is the response, x is the logarithm of the RTX concentration (mol/l), and a, b, d, m, n, and r are parameters chosen by least-squares criteria.
RESULTS
Effect of RTX on the NaCl + Bz CT response in rats.
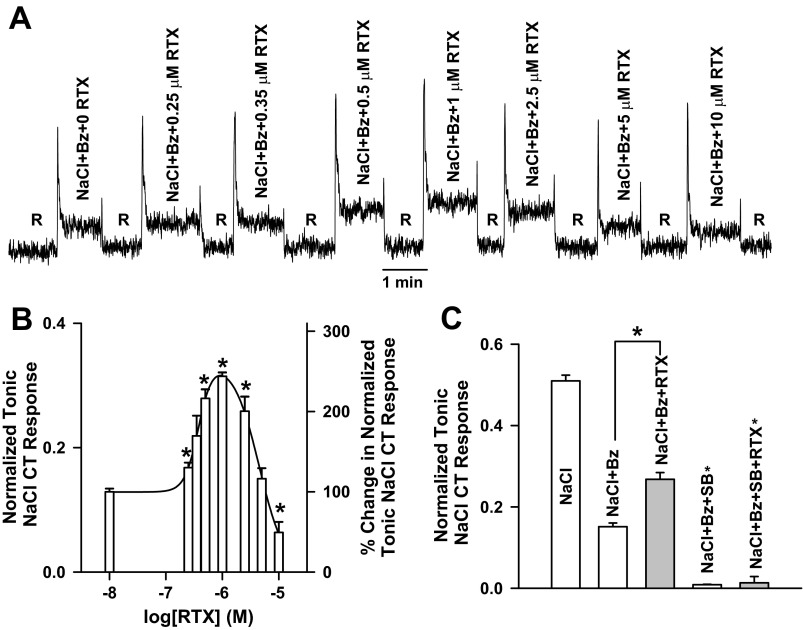
Consistent with previous studies (20, 26, 27, 29–32, 48), RTX, a specific TRPV1 agonist, produced a biphasic effect on the rat CT response to 100 mM NaCl + 5 μM Bz. RTX at 0.5–1 μM enhanced the tonic CT response (Fig. 1, A and B). At >1 μM RTX, the NaCl + Bz CT response was inhibited. At 5 μM RTX, the response was the same as control. At 10 μM RTX, the response was below the control value. As expected, Bz inhibited the NaCl CT response (Fig. 1C; P = 0.0001 by paired t-test, n = 3). In the presence of Bz + 1 μM SB-366791, the entire NaCl tonic CT response was inhibited to the baseline rinse level (Fig. 1C). RTX at 1 μM increased the NaCl + Bz tonic CT response (Fig. 1C; P = 0.0036); however, it did not enhance the CT response above the baseline rinse level to lingual stimulation with NaCl + Bz + SB-366791 (Fig. 1C). These results show that SB-366791 inhibits the constitutive Bz-insensitive NaCl CT response and the RTX-induced enhancement of the NaCl + Bz CT response.
Fig. 1.
Effect of benzamil (Bz), resiniferatoxin (RTX), and SB-366791 (SB) on the rat NaCl chorda tympani (CT) response. A: representative rat CT response to lingual application of a rinse solution (R; 10 mM KCl), 100 mM NaCl + 5 μM Bz, and 100 mM NaCl + 5 μM Bz + RTX (0.25–10 μM). B: Bz-insensitive NaCl CT responses in each rat normalized to the corresponding CT responses obtained with 300 mM NH4Cl. Values are means ± SE of 3 animals. *P = 0.016, 0.007, 0.001, 0.006, and 0.0217 at 0.25, 0.50, 1.0, 2.5, and 10 μM RTX, respectively, relative to 0 RTX (paired). C: rat CT responses to lingual application of a rinse solution (10 mM KCl), 100 mM NaCl, 100 mM NaCl + 5 μM Bz, 100 mM NaCl + 5 μM Bz + 1 μM RTX, 100 mM NaCl + 5 μM Bz + 1 μM SB, and 100 mM NaCl + 5 μM Bz + 1 μM SB + 1 μM RTX. In each rat, the Bz-insensitive NaCl CT responses were normalized to the corresponding CT responses obtained with 300 mM NH4Cl. Values are means ± SE of 3 animals. *P = 0.0036 (paired).
Effect of TPPO on the CT response to quinine.
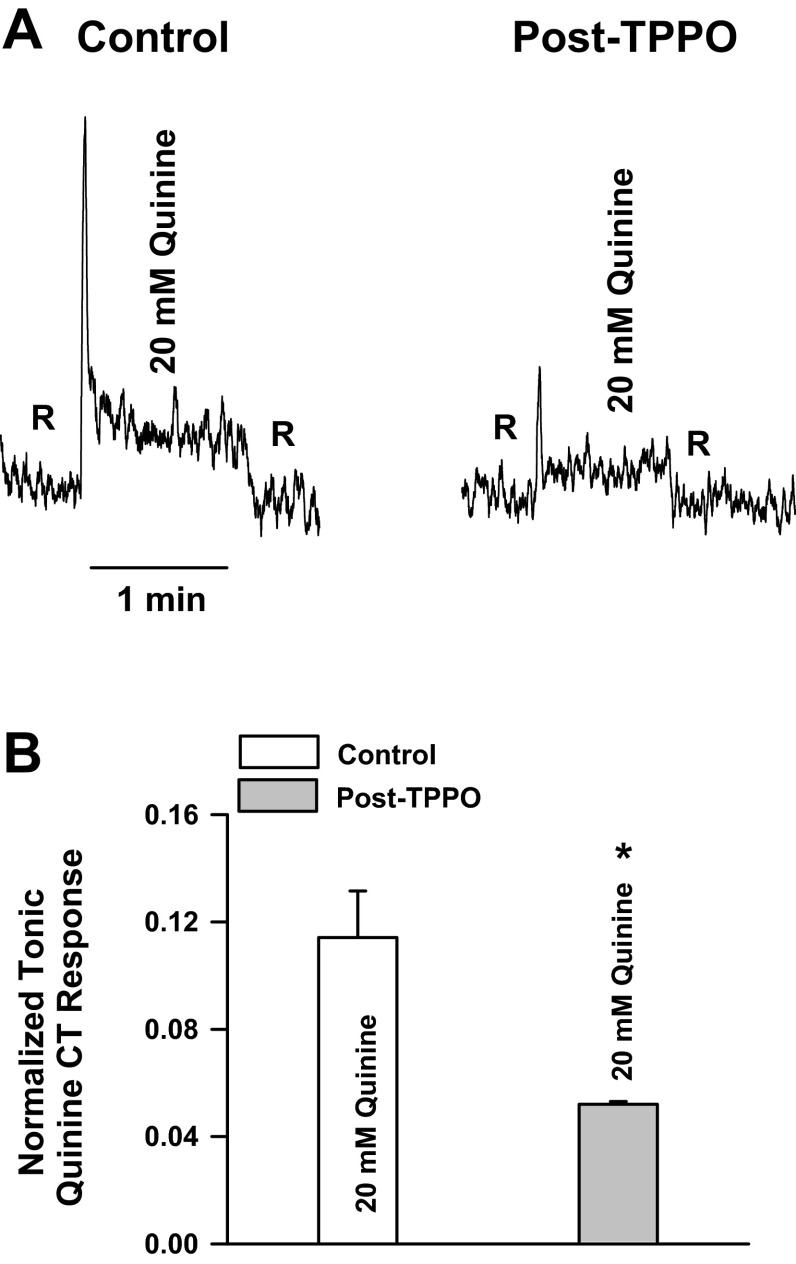
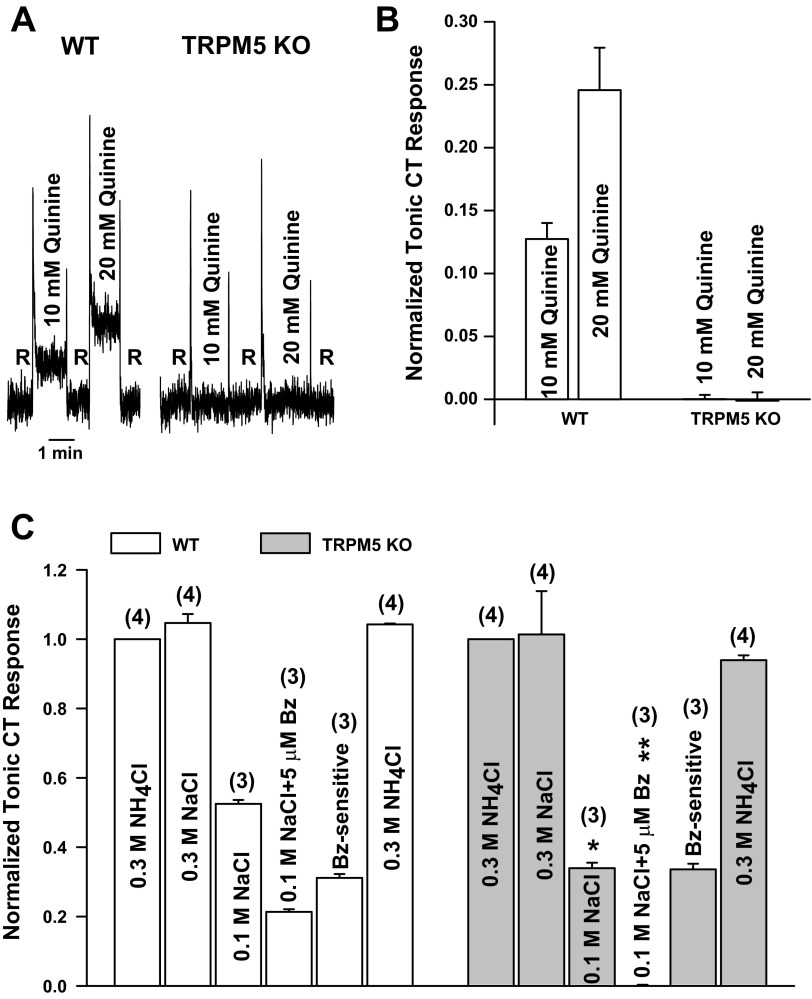
Topical lingual application of 2 mM TPPO, a specific blocker of TRPM5 (38), partially inhibited the CT response to 20 mM quinine (Fig. 2; P = 0.023 by unpaired t-test, n = 3). These results indicate that topical lingual application of 2 mM TPPO is sufficient for the drug to diffuse below the tight junctions to partially inhibit TRPM5 localized in the basolateral membrane of a subset of TRCs (19).
Fig. 2.
Effect of triphenylphosphine oxide (TPPO) on the CT response to quinine. A: representative CT responses to 20 mM quinine relative to the rinse solution (10 mM KCl) in the same rat before (control) and after topical lingual application of 2 mM TPPO (post-TPPO). B: quinine CT response in each rat normalized to the corresponding CT responses obtained with 300 mM NH4Cl. Values are means ± SE of 3 animals. *P = 0.023 (paired).
Effect of TPPO and CZP on the Bz-insensitive NaCl CT responses in the absence and presence of RTX.
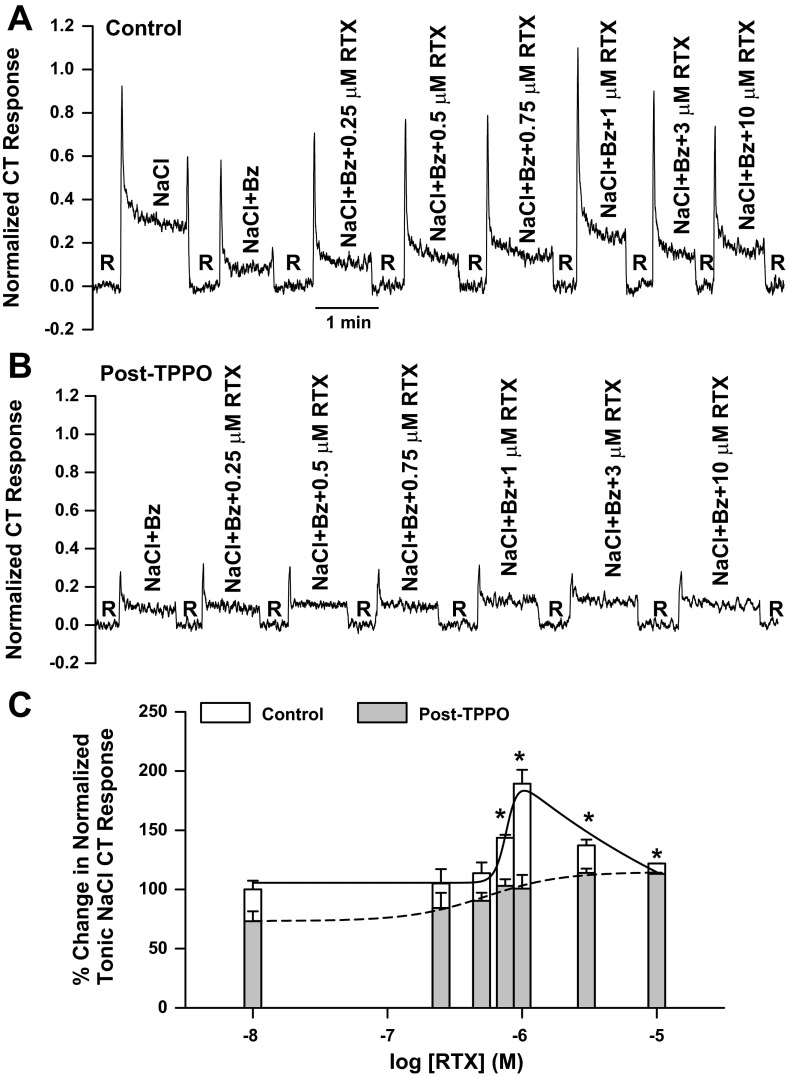
As shown in a representative CT trace, after topical lingual application of 2 mM TPPO (Fig. 3B), the mean normalized tonic CT response to 100 mM NaCl + 5 μM Bz increased in the presence of RTX (Fig. 3C). While under control conditions the maximum increase in the Bz-insensitive NaCl CT response was observed at 1 μM RTX (Fig. 3, A and C), the maximum increase in the CT response following TPPO treatment was observed at 3 and 10 μM RTX relative to 0 RTX. P values at 0.75, 3, and 10 μM RTX were 0.04, 0.011, and 0.009, respectively, relative to 0 RTX. We hypothesize that, after TPPO treatment, higher concentrations of RTX may be needed to inhibit the CT response to the level at 0 RTX. TPPO decreased the magnitude of the NaCl + Bz + RTX tonic CT response and shifted the relationship between the RTX concentration and the magnitude of the tonic CT response to the right on the RTX concentration axis relative to control (Fig. 3C). These results suggest that modulation of the Bz-insensitive NaCl CT response by RTX is partially dependent on TRPM5 activity in a subset of TRCs.
Fig. 3.
Effect of TPPO on the CT response to NaCl + Bz. A and B: representative normalized CT responses to 100 mM NaCl and 100 mM NaCl + 5 μM Bz + RTX (0.25–10 μM) relative to the rinse solution (10 mM KCl) in the same rat before (control) and after topical lingual application of 2 mM TPPO (post-TPPO). C: Bz-insensitive NaCl CT responses in each rat normalized to the corresponding CT responses obtained with 300 mM NH4Cl. Values are means ± SE of 3 animals. Mean normalized tonic NaCl + Bz CT responses at each RTX concentration were compared before (control) and after TPPO treatment (post-TPPO). Under control conditions, the mean normalized tonic CT responses at 0.75, 1, 3, and 10 μM RTX were significantly different from the response at 0 RTX: P = 0.005, 0.003, 0.0136, and 0.0411, respectively. After TPPO treatment, the mean normalized tonic CT responses at 3 and 10 μM RTX were significantly different from the response at 0 RTX: P = 0.011 and 0.0086, respectively. After TPPO treatment, the mean normalized tonic CT responses at 0.75, 1, 3, and 10 μM RTX were significantly smaller than control: *P = 0.0027, 0.0058, 0.0185, and 0.0001, respectively.
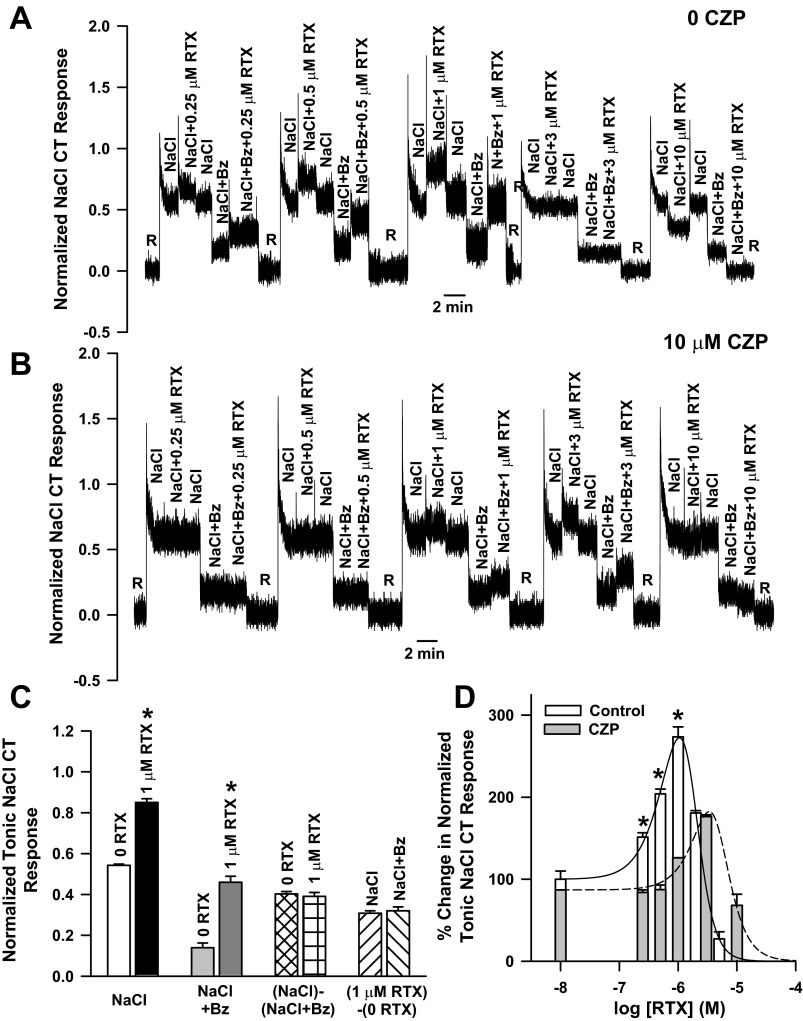
To directly test if the inhibition of the putative TRPV1t cation channel results in a shift in the relationship between the agonist concentration and the magnitude of the Bz-insensitive NaCl CT response, further experiments were performed in 100 mM NaCl and 100 mM NaCl + 5 μM Bz solutions containing 10 μM CZP. As shown by the representative CT trace (Fig. 4A) and our earlier studies (26, 27), under control conditions, RTX produced a biphasic effect on the CT response in the presence of NaCl alone or NaCl + Bz. We compared normalized CT responses to NaCl alone or to NaCl+Bz at two RTX concentrations, 0 and 1 μM. As shown in Fig. 4C, the Bz-sensitive component of the NaCl CT response [NaCl-(NaCl + Bz)] and the RTX-induced enhancement of the NaCl and NaCl + Bz CT response were not different from each other. Similarly, we did not observe differences in the Bz-sensitive component of the NaCl CT response and the RTX-induced increase in the CT response in the presence of NaCl or NaCl + Bz at other RTX concentrations (data not shown). In our previous studies (6), modulators of TRPV1/TRPV1t did not alter CT responses in the presence of NaCl + SB-366791, again demonstrating that TRPV1/TRPV1t modulators do not alter the Bz-sensitive component of the NaCl CT response. These results demonstrate that RTX modulates only the Bz-insensitive NaCl CT response.
Fig. 4.
Effect of capsazepine (CZP) on the CT response to NaCl and NaCl + Bz. A and B: representative CT response to stimulation of the rat tongue first stimulated with a rinse solution (10 mM KCl) and then with 100 mM NaCl + RTX (0.25–10 μM) or 100 mM NaCl + 5 μM Bz + RTX (0.25–10 μM) in the absence (0 CZP) and presence of 10 μM CZP. C: in each case, the CT response was normalized to the corresponding tonic CT responses obtained with 300 mM NH4Cl. Values are means ± SE of 3 animals. Mean normalized values at 0 and 1 μM RTX for 100 mM NaCl, 100 mM NaCl + 5 μM Bz, and 100 mM NaCl-(100 mM NaCl + 5 μM Bz) (the Bz-sensitive component of the NaCl CT response) and the RTX-induced increase in the CT response in the presence of 100 mM NaCl and 100 mM NaCl + 5 μM Bz are shown. *P < 0.001 vs. 0 RTX. D: mean normalized tonic CT responses at each RTX concentration in the absence (control) and presence of CZP. In the presence of CZP, the mean normalized tonic CT responses at 0.25, 0.5, and 1.0 μM RTX were significantly smaller than control: *P = 0.0004, 0.0001, and 0.0003, respectively.
In the presence of 10 μM CZP, the RTX-induced increase in the CT response to NaCl or NaCl + Bz was attenuated and the relationship between RTX concentration and the magnitude of the CT response was shifted to the right on the RTX concentration axis (Fig. 4B). The mean normalized rat CT responses to NaCl + Bz + RTX in the absence and presence of 10 μM CZP are summarized in Fig. 4D. In the presence of CZP, RTX still produced biphasic effects on the NaCl + Bz tonic CT response. However, the relationship between RTX concentration and the magnitude of the NaCl + Bz tonic CT response is shifted downward and to the right on the RTX concentration axis relative to control (Fig. 4D).
Studies with WT and KO mice.
We recorded CT responses to 10 and 20 mM quinine in WT and TRPM5 KO mice. As expected (36), the WT mice responded with a concentration-dependent increase in the CT response to 10 and 20 mM quinine (Fig. 5A). In contrast, TRPM5 KO mice elicited only transient phasic CT responses to 10 and 20 mM quinine. No tonic CT response to 10 and 20 mM quinine above the baseline rinse level was observed in TRPM5 KO mice (Fig. 5, A and B). In TRPM5 KO mice, the tonic CT response to quinine was eliminated. Furthermore, the phasic CT response to quinine was TRPM5-independent. These studies are consistent with the observation that neural responses to bitter compounds are significantly attenuated in TRPM5 KO mice (7, 50). In contrast to TRPM5 KO mice, even at high TPPO concentrations (2 mM), only partial inhibition of the quinine CT response was observed in rats (Fig. 2). Since TRPM5 is localized in the basolateral membrane of a subset of TRCs (19), significantly higher concentrations of TPPO may have to be applied topically to the tongue for it to diffuse below the tight junctions to completely inhibit the TRPM5 activity and the CT response to quinine to the baseline rinse level. We did not test the effect of TPPO in WT mice. We predict that TPPO will have the same effect in WT mice.
Fig. 5.
CT responses in WT and transient receptor potential (TRP) subfamily M member 5 (TRPM5) knockout (KO) mice. A: CT responses of the mouse tongue during stimulation first with a rinse solution (10 mM KCl) and then with 10 and 20 mM quinine. B: quinine CT responses in WT and TRPM5 KO mice normalized to the corresponding CT responses obtained with 300 mM NH4Cl. Values are means ± SE of 3 animals. C: CT responses during stimulation of the mouse tongue first with a rinse solution (10 mM KCl) and then with 300 mM NH4Cl, 300 mM NaCl, 100 mM NaCl, and 100 mM NaCl + 5 μM Bz. In each mouse, the Bz-insensitive NaCl CT responses were normalized to the corresponding tonic CT responses obtained with 300 mM NH4Cl. Values are means ± SE of number of animals in parentheses. *P = 0.0007; **P = 0.0001 (paired).
We recorded CT responses to 300 and 100 mM NaCl in WT and TRPM5 KO mice. While the mean normalized tonic CT response to 300 mM NaCl was not different between WT and TRPM5 KO mice (Fig. 5C), the tonic response to 100 mM NaCl was significantly lower in TRPM5 KO than WT mice. To determine if the difference between the CT responses is due to the Bz-sensitive or Bz-insensitive component of the NaCl CT response, neural responses were monitored in the presence of 5 μM Bz, a potent blocker of ENaC. At 100 mM NaCl, the WT mice demonstrated a Bz-sensitive and a Bz-insensitive component of the CT response (Fig. 6A). However, in TRPM5 KO mice, Bz inhibited the NaCl tonic CT response to the baseline rinse level (Figs. 5C and 6B). These results demonstrate that, at low NaCl concentrations (100 mM), TRPM5 KO mice lack the constitutive Bz-insensitive NaCl CT response. In contrast, the normalized Bz-sensitive (ENaC-dependent) component of the NaCl CT response was not different between the TRPM5 KO and WT mice (Fig. 5C).
Fig. 6.
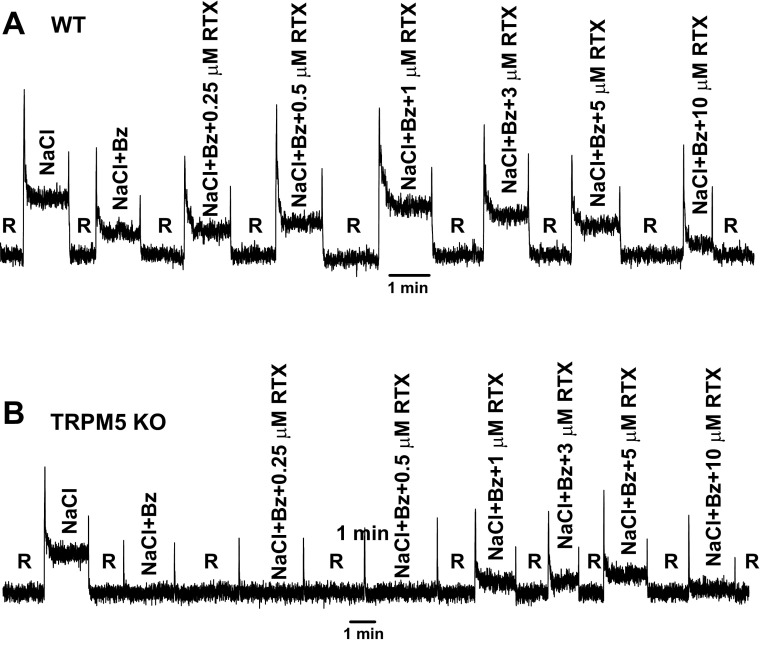
Effect of RTX on the NaCl + Bz CT response in WT and TRPM5 KO mice. Representative CT responses of a WT (A) and a TRPM5 KO (B) mouse tongue were recorded during stimulation first with a rinse solution (10 mM KCl) and then with 100 mM NaCl + 5 μM Bz and 100 mM NaCl + 5 μM Bz + RTX (0.25–10 μM).
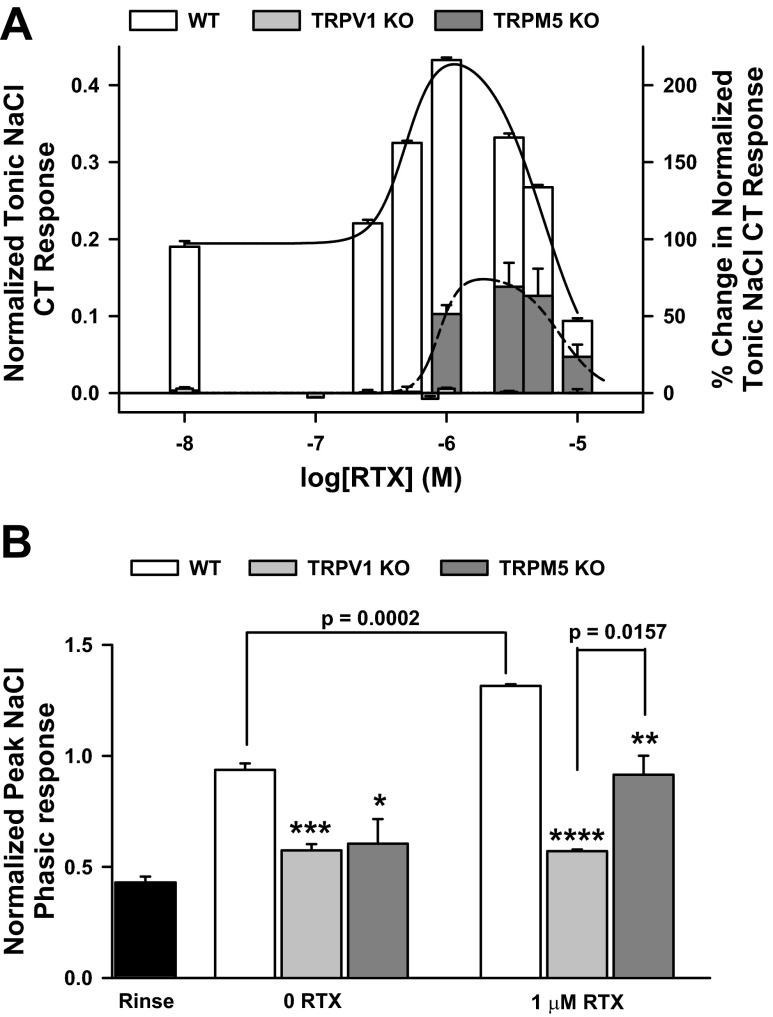
To further characterize the differences in the Bz-insensitive NaCl CT responses in WT and TRPM5 KO mice in situ, we generated the relationship between RTX concentration and the magnitude of the tonic CT response to 100 mM NaCl + 5 μM Bz in the two mouse strains (Figs. 6 and 7). Consistent with our previous data (20, 26, 27, 29–32, 48), in WT mice (Fig. 6A), RTX produced a biphasic effect on the NaCl + Bz CT response. Similar to the case with rats (Fig. 1, A and B), in WT mice, 1 μM RTX produced a maximum enhancement of the NaCl + Bz CT response. In contrast to the WT mice (Figs. 6A and 7A), TRPM5 KO mice elicited no Bz-insensitive NaCl CT response (Figs. 6B and 7A), and the relationship between RTX concentration and the magnitude of the NaCl + Bz response was shifted downward and to the right on the RTX concentration axis. As shown in Fig. 7B, the normalized peak NaCl + Bz phasic CT response in TRPM5 KO mice was significantly smaller than its value in WT mice but was not significantly different from the rinse artifact (Fig. 7B). At 1 μM, RTX enhanced the phasic CT response in WT and TRPM5 KO mice; however, the enhancement was significantly smaller in TRPM5 KO than WT mice. These results show that, at 100 mM NaCl, phasic and tonic components of the Bz-insensitive NaCl CT response are diminished in TRPM5 KO mice relative to their values in the WT mice.
Fig. 7.
Summary of the normalized Bz-insensitive NaCl CT responses in WT, transient receptor potential vanilloid subfamily member 1 (TRPV1) KO, and TRPM5 KO mice. From similar experiments shown in Fig. 6, the Bz-insensitive NaCl CT responses were normalized to the corresponding CT responses obtained with 300 mM NH4Cl. A: tonic responses of WT and TRPM5 KO mice and WT and TRPV1 KO mice to RTX. All mean normalized tonic 100 mM NaCl + 5 μM Bz CT responses in TRPM5 KO and TRPV1 KO mice in the absence and presence of RTX were significantly smaller than their corresponding values in WT mice (P < 0.0001, paired). B: normalized phasic 100 mM NaCl + 5 μM Bz CT responses of WT, TRPM5 KO, and TRPV1 KO mice in the absence and presence of 1 μM RTX. Values are means ± SE of 3 animals. *P = 0.0434; **P = 0.0094; ***P = 0.0009; ****P = 0.0001 (unpaired). Also shown is the mean normalized value of the mechanical rinse artifact (Rinse) in 3 TRPM5 KO mice. Normalized phasic 100 mM NaCl + 5 μM Bz CT responses in the absence of RTX in TRPV1 KO and TRPM5 KO mice and in the presence of 1 μM RTX in TRPV1 KO mice were not significantly different from the normalized value of the mechanical rinse artifact (P > 0.05).
Similar to TRPM5 KO mice, TRPV1 KO mice also did not elicit a CT response to 100 mM NaCl + 5 μM Bz. However, in TRPV1 KO mice, the NaCl + Bz + RTX tonic CT response remained at the baseline rinse level over the entire RTX concentration range (0–10 μM) (Fig. 7A) (48). In TRPV1 KO mice, the phasic CT response decreased to the rinse artifact level and was not altered in the presence of 1 μM RTX (Fig. 7B). These results show that phasic and tonic components of the Bz-insensitive NaCl CT response are inhibited to the baseline rinse levels in TRPV1 KO mice (31).
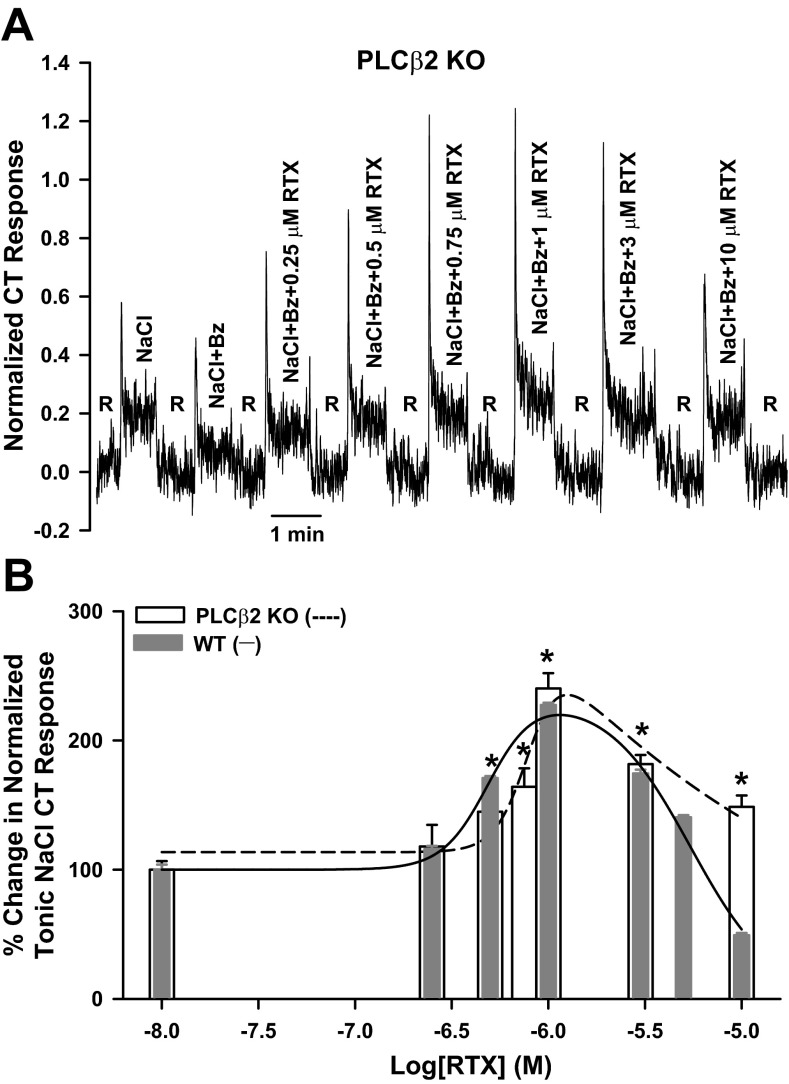
Since PLCβ2 and TRPM5 are essential downstream intracellular signaling effectors in sweet, bitter, and umami taste transduction, we next tested if Bz-insensitive NaCl CT responses in the absence and presence of RTX are also dependent on PLCβ2 activity. Unlike TRPV1 and TRPM5 KO mice, PLCβ2 KO mice elicited a Bz-sensitive and Bz-insensitive NaCl CT response. Varying RTX concentrations modulated the NaCl + Bz CT responses in a biphasic manner (Fig. 8A). Similar to rats and WT mice, in PLCβ2 KO mice, 1 μM RTX produced a maximum increase in the NaCl + Bz tonic CT response (Fig. 8B). These studies suggest that the Bz-insensitive NaCl CT response is specifically inhibited by pharmacological blocking or genetic silencing of the TRPM5 cation channel in TRCs but is not affected by genetic elimination of the PLCβ2 enzyme activity.
Fig. 8.
Effect of RTX on the NaCl + Bz CT response in PLCβ2 KO mice. A: representative normalized CT response to lingual stimulation first with a rinse solution (R; 10 mM KCl) and then with 100 mM NaCl + 5 μM Bz and 100 mM NaCl + 5 μM Bz + RTX (0.25–10 μM) in a PLCβ2 KO mouse. B: Bz-insensitive NaCl CT responses in each PLCβ2 KO mouse normalized to the corresponding CT responses obtained with 300 mM NH4Cl. Values are means ± SE of 3 animals. *P = 0.0193, 0.0153, 0.0005, 0.0011, and 0.0115 for 0.5, 0.75, 1, 3, and 10 μM RTX, respectively, relative to NaCl + Bz alone (paired). Data points for the WT mice in Fig. 7A (solid line) are plotted for easy comparison.
DISCUSSION
Relationship between TRPV1/TRPV1t and TRPM5 and Bz-insensitive NaCl CT responses.
We observed significant differences in the CT response between TRPM5 KO and WT mice at low (100 mM), but not high (300 mM), NaCl concentration (Fig. 5C). Consistent with these results, Damak et al. (7) also reported significant differences in the CT responses between TRPM5 KO and C57BL/6J control mice at lower (30 and 100 mM), but not higher (300 and 1,000 mM), NaCl concentrations. In our studies, we used TRPM5 KO mice with a partial deletion of TRPM5, such that they retain most of the amino-terminal portion of the gene (50). In contrast, Damak et al. used TRPM5 KO mice null for TRPM5 protein expression. Thus, despite slight differences in the TRPM5 KO mice construct, in both studies TRPM5 KO mice show significantly smaller NaCl CT responses at low (100 mM), but not high (300 mM), NaCl concentrations. In contrast, in two other studies, TRPM5 KO mice gave a normal CT response to 100 mM NaCl normalized to the CT response to 100 mM citric acid (50) or to 100 mM NH4Cl (46) relative to C57BL/6J control mice. Since the difference in the magnitude of the NaCl CT response between the two genotypes is small (Fig. 5C; ∼25–30%), several factors, including large variations in the CT response between individual animals, differences in the normalization, and the method used to record the CT responses, may have contributed to masking the difference between the NaCl CT responses in the above-mentioned studies.
In earlier studies (7, 50), in WT and TRPM5 KO mice, amiloride was not used while CT responses to NaCl were recorded. Thus the magnitudes of the Bz-sensitive and Bz-insensitive components of the NaCl CT response were not quantified in the two genotypes in earlier studies (7, 46, 50). Our results using Bz show that the difference in the CT response between TRPM5 KO and WT mice at 100 mM NaCl is due to the absence of the Bz-insensitive component of the NaCl CT response in TRPM5 KO mice (Figs. 5C, 6, and 7). We did not observe any differences in the Bz-sensitive ENaC-dependent component of the NaCl CT response between the two genotypes. These results indicate that TRPM5 KO mice demonstrate a specific decrease in the Bz-insensitive NaCl CT response, while the Bz-sensitive NaCl CT response remains the same as in WT mice. These results suggest that the Bz-sensitive and Bz-insensitive NaCl CT responses not only originate in different taste cells within the taste bud but are also regulated independently of each other (3, 35).
WT and TRPM5 KO mice also showed marked differences in the relationship between RTX concentration and the magnitude of the tonic CT response to NaCl + Bz (Figs. 6 and 7). While in WT mice RTX produced biphasic changes in the NaCl + Bz tonic CT response with a maximum increase in the CT response at 1 μM RTX, in TRPM5 KO mice the RTX dose-response curve was shifted to higher RTX concentrations and the enhancement of the CT response in the presence of RTX was significantly blunted relative to control. The results in TRPM5 KO mice were confirmed using TPPO, a pharmacological blocker of TRPM5 in rats. It is important to note that although the concentration of TPPO used in our experiments (2 mM) did not inhibit the CT response to quinine (Fig. 2) or to 100 mM NaCl + 5 μM Bz (Fig. 3) to the baseline rinse level, the RTX dose-response curve was shifted to higher RTX concentrations and the RTX-induced enhancement of the CT response was significantly blunted relative to control. Taken together, the data from TRPM5 KO mice and rats treated with TPPO suggest that inhibition of TRPM5 produces significant changes in the constitutive Bz-insensitive NaCl CT response and its modulation by RTX. As stated above, exposure of the lingual surface to significantly higher concentrations of TPPO for longer durations may be necessary for it to diffuse below the tight junctions to completely inhibit the TRPM5 activity in the basolateral membrane of TRCs (19).
The constitutive Bz-insensitive NaCl response is blocked by submicromolar concentrations of the TRPV1 blockers SB-366791 and CZP in WT mice (Fig. 1), Sprague-Dawley rats, alcohol-preferring (P) rats, and alcohol-non-preferring (NP) rats (6, 26). In addition, several low-affinity blockers that inhibit the Bz-insensitive NaCl CT response in the micromolar-to-millimolar range include RTX, capsaicin, cetylpyridinium chloride, Maillard reacted peptides (MRPs), N-geranylcyclopropylcarboximide, ethanol, and nicotine (6, 11, 20, 26, 27, 29, 30, 40, 41, 48). Our whole nerve data are supported by the effect of TRPV1 agonists on single CT nerve fibers. In the CT nerve, a subset of the broadly tuned E-type fibers (presumably the amiloride-insensitive fibers) demonstrated enhancement and another subset of E-type fibers showed suppression of the NaCl response when the rat tongue was stimulated with a mixture containing 100 mM NaCl + 326 μM capsaicin (37). It is likely that single units in the CT nerve have variable capsaicin dose-response relationships. In our earlier studies (26), capsaicin produced maximum enhancement and inhibition of the Bz-insensitive NaCl CT response at 40 and 200 μM, respectively. In contrast to the above studies, Breza and Contreras (2) reported that SB-366791 and cetylpyridinium chloride did not alter CT nerve or single-cell responses. The lack of effect of these antagonists may be related to significant differences in the methodology used: 1) in the study of Breza and Contreras, the CT responses were recorded while the tongue was perfused at a significantly slower flow rate (50 μl/s vs. 1 ml/s in our study); 2) the rinse solution was artificial saliva vs. 10 mM KCl in our study; 3) the tongue was superfused with solutions maintained at 35°C vs. room temperature in our study; and 4) a short sampling time of 5 s or 60 s in conjunction with slow flow rate. We previously showed that flow rate and temperature have significant effects on the NaCl CT response in the absence and presence of Bz (26). In our studies, at slow flow rates (∼133 μl/s), the phasic component of the CT response to NaCl and HCl was not observed and the tonic CT response reached its maximum value ∼2 min after the stimulation onset (25, 26).
The constitutive Bz-insensitive NaCl response is modulated by changes in TRC Ca2+ concentration, protein kinase C, calcineurin, and membrane PIP2 levels (31, 32). It is spontaneously upregulated in P rats relative to NP rats. Exposure of NP rats to oral ethanol in a no-choice paradigm upregulated the Bz-insensitive NaCl CT response relative to that of the naive NP rats (6). Naive P rats and NP rats exposed to ethanol demonstrated enhancement of the NaCl + Bz CT response in the presence of RTX, with the maximum enhancement of the neural response at 1 μM. At low NaCl concentrations (2–32 mM), the amiloride-insensitive NaCl CT response was enhanced in the A/J mouse (5, 34). TRPM5 KO mice also show a deficit in the CT response at low NaCl concentrations only (30 and 100 mM) (7).
Consistent with our previous studies (26, 27), the spontaneous Bz-insensitive NaCl CT response was absent in TRPV1 KO mice (Fig. 1). Unlike WT mice, TRPV1 KO mice did not respond to RTX, ethanol, GalA-MRPs, and nicotine with an increase in the CT response above the baseline rinse level (6). However, in another study, CT responses to a concentration series of NaCl with and without amiloride did not differ between the two genotypes (45). As stated above, the differences between our results and the findings reported by Smith et al. (45) may be related to significant differences in the methods used in the two studies. In our studies, putative TRPV1/TRPV1t agonists and antagonists show similar effects in WT mice, PLCβ2 KO mice, Sprague-Dawley rats, P rats, and NP rats (6). The nonpungent agonists of TRPV1/TRPV1t-dependent Bz-insensitive NaCl CT responses modulate human salt taste in a biphasic manner (11, 20). Thus the effects of TRPV1/TRPV1t agonists/antagonists are strain- and agonist-independent. The constitutive Bz-insensitive NaCl CT response is not observed in TRPV1 and TRPM5 KO mice, and in rats and WT mice it is inhibited by a variety of low- and high-affinity antagonists. In our studies, phasic and tonic components of the Bz-insensitive NaCl CT response are inhibited in TRPM5 and TRPV1 KO mice. It is likely that phasic and tonic components of the salt response originate in different cell types and have different transduction mechanisms (9, 10, 33).
However, a major difference between TRPV1 KO mice and TRPM5 KO mice is that, in TRPV1 KO mice, the channel is not expressed globally and the Bz-insensitive NaCl response remains at the baseline rinse level in the presence of a wide range of RTX concentrations (48). Similarly, in rats and WT mice, in the presence of SB-366791, the spontaneous Bz-insensitive NaCl CT response is inhibited to the baseline rinse level and the CT response does not increase above the baseline rinse level in the presence of a wide range of agonist concentrations. In TRPM5 KO mice, the putative TRPV1t cation channel is inhibited, but its activity can be elevated to some extent in the presence of high concentrations of RTX (Figs. 6 and 7). In our previous studies, inhibiting the spontaneous putative TRPV1t activity by increasing TRC intracellular Ca2+ concentration, altering the phosphorylation state of the channel protein, or increasing membrane PIP2 levels (31, 32) did not result in a shift in relationship between the agonist concentration and the magnitude of the Bz-insensitive NaCl CT response. Therefore, the rightward shift in the RTX dose-response curve cannot be due to the changes in the phosphorylation/dephosphorylation state of the putative TRPV1 channel protein or changes in membrane PIP2 levels. However, in our studies, in the presence of a subthreshold concentration of a TRPV1 blocker, CZP (10 μM), the RTX dose-response curve was shifted to the right on the RTX concentration axis and the RTX-induced enhancement of the CT response was significantly blunted relative to control (Fig. 4). It is important to note that this concentration of CZP is significantly less than the CZP concentration (100 μM) that blocks 50% of the enhancement of the NaCl + Bz CT response in the presence of 0.75 μM RTX (26).
We previously showed that TRPV1 KO mice elicited CT responses to monosodium glutamate (MSG) + Bz + SB-366791 and MSG + Bz + SB-366791 + IMP that were not different from those observed in WT mice (32). In our earlier studies, 10 μM RTX inhibited the rat Bz-insensitive NaCl CT response to baseline but did not alter CT responses to quinine or sucrose (26). In addition, 1 μM SB-366791 inhibited the rat Bz-insensitive NaCl CT response to baseline but did not alter CT responses to quinine and sucrose and the IMP-induced increase in the CT response to MSG + Bz (32). These results would tend to support the notion that TRPV1t inhibition does not affect TRPM5 activity in type II cells within the taste bud. The results of the present study suggest that inhibition of TRPM5 activity attenuates the putative TRPV1/TRPV1t channel activity and results in the rightward shift of the RTX dose-response curve.
Relationship between neural and behavioral responses in WT and KO mice.
The differences in the Bz-insensitive NaCl CT responses are most likely related to the reported behavioral difference between WT and KO mice. In two-bottle 48-h preference tests, TRPM5 KO mice were indifferent to NaCl concentrations between 18 and 75 mM, whereas WT mice tended to prefer these concentrations of NaCl (7). Both genotypes avoided NaCl concentrations between 300 and 600 mM, with TRPM5 KO mice showing slightly diminished avoidance relative to WT mice. In brief-access tests, WT mice showed aversion to 100–1,000 mM NaCl, and TRPM5 KO mice showed aversion to ≥200 mM NaCl. The KO mice showed decreased aversion to 100–1,000 mM NaCl relative to the WT mice. This relationship is also observed between different strains of mice. A/J mice, which show spontaneously greater amiloride-insensitive NaCl CT responses than C57BL/6J mice, were also behaviorally different from control mice. C57BL/6J mice preferred 25 mM NaCl over water, whereas A/J mice consumed water and 25 mM NaCl equally. At higher concentrations (75 and 225 mM), both genotypes had identical NaCl preferences (47). Since differences between test and control animals are observed at low and high NaCl concentrations, it is likely that additional amiloride- and Bz-insensitive salt taste receptors are involved in sensing low and high salt concentrations. A transmembrane channel-like (tmc-1) gene that encodes a Na+-sensitive channel is required for salt chemosensation in Caenorhabditis elegans (4). In contrast, in mice, high salt sensing seems to involve the two primary aversive taste pathways as a result of activation of the sour and bitter taste-sensing taste bud cells (35).
In contrast to the above-mentioned studies, using brief-access tests, Zhang et al. (50) reported no differences between WT and TRPM5 KO mice in behavioral responses to 0.3 and 1.0 M NaCl. In a recent study (35), water-deprived WT and TRPM5 KO mice showed robust dose-dependent behavioral aversion to 250 and 500 mM NaCl in the presence of 30 μM amiloride and to 250 and 500 mM KCl. In a two-response operant discrimination test, WT and TRPV1 KO mice demonstrated similar detection thresholds for NaCl and KCl (48). Using a condition taste aversion generalization procedure, Smith et al. (45) showed that LiCl-injected WT and TRPV1 KO mice learned to avoid NaCl + amiloride relative to controls, but their generalization profiles did not differ; LiCl-injected mice avoided the non-Na+ salts and quinine. The lack of behavioral differences between WT and KO mice (TRPV1 and TRPM5) may be due to acquisition of salt quality information from other taste receptive fields that may also express different Bz-insensitive salt taste receptors. For example, NaCl responses in the glossopharyngeal nerve that originate in the posterior taste field containing circumvallate papillae are amiloride-insensitive (22). It is also likely that behavioral studies in the presence of TRPV1 and TRPM5 agonists may be useful in resolving the differences between various studies (41). In two-bottle 48-h preference tests, a “kokumi”-taste active peptide produced biphasic effects on NaCl preference in C57BL/6J mice and on the Bz-insensitive NaCl CT response (41). MRPs and N-geranylcyclopropylcarboximide produced biphasic effects on the Bz-insensitive NaCl CT response in rodents and also on salt sensory evaluation in humans (11, 20). However, significant behavioral differences from WT mice for high concentrations of NaCl and KCl were observed only in double-mutant mice in which polycystic kidney disease 2-like 1-expressing cells were silenced and TRPM5 was mutated (35). These double-mutant mice showed no salt aversion even at concentrations at which controls are strongly repelled.
Possible interactions between TRPV1/TRPV1t and TRPM5.
The amiloride-sensitive Na+ channels appear to be expressed in cells that lack voltage-gated inward currents, likely the type I taste cells (49). α-ENaC has been shown to be expressed in cells that are distinct from the sweet-, bitter-, and umami-sensing TRCs (3). The conditional α-ENaC KO mice lacking α-ENaC only in salt-sensing TRCs retained all responses to non-Na+ salts. These results demonstrate that amiloride-sensitive Na+-specific (ENaC-dependent) and amiloride-insensitive nonspecific salt taste responses are mediated by genetically separable components (3). Consistent with this, RTX only modulated the CT responses to NaCl + Bz and did not alter CT responses to bitter (quinine), sweet (sucrose), and acidic (HCl) stimuli (26). However, the paracrine or autocrine signaling events through which changes in TRPM5 activity modulate the Bz-insensitive NaCl CT response are not known. The Bz-insensitive NaCl CT response was unaffected by elimination of PLCβ2 enzyme activity (Fig. 8). This suggests that a subset of taste cells with type II cell membrane properties but without PLC signaling components (49) may be involved in the regulation of the putative TRPV1-dependent Bz-insensitive NaCl CT response.
In rats, TRPV1 protein expression has been observed in the epithelium facing the oral cavity, although taste cells seemed to be devoid of TRPV1. The fungiform papillae are rich in TRPV1-immunostained fibers, which are localized around the taste buds, and some of these fibers actually enter taste buds. Most TRPV1 fibers show immunoreactivity for substance P, while approximately half of the TRPV1 fibers are positive for calcitonin gene-related peptide (14, 18, 21). It has been reported that the neuropeptides CCK and substance P increase rat CT responses to NaCl (13). These and other peptides and modulators of the Bz-insensitive NaCl response released from TRPV1-containing nerve fibers around the taste buds could directly or indirectly modulate the Bz-insensitive NaCl CT response in the presence of TRPV1 agonists in rats and mice (43). In contrast, human taste cells have been shown to express TRPV1 (16, 17). This raises the possibility that TRPV1 agonists may be able to directly modulate the Bz-insensitive NaCl CT response specifically by interacting with TRPV1t in human taste cells. Further studies are needed to resolve these issues.
In summary, the data presented here suggest that, at low NaCl concentrations, the constitutive Bz-insensitive NaCl CT response and its modulation by putative TRPV1t agonists are dependent on TRPM5 activity but are independent of PLCβ2 activity in a subset of TRCs (49). This pathway seems to be different from the classical bitter, sweet, and umami taste transduction pathway, which shows a strict requirement for PLCβ2 and TRPM5 (50).
GRANTS
This work was supported by National Institute of Deafness and Other Communication Disorders Grants DC-000122, DC-005981, and DC-011569, a Korea Food Research Institute Grant, and a Jeffress Trust Grant to V. Lyall.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.R., T.-H.T.P., and S.M. performed the experiments; Z.R., T.-H.T.P., S.M., J.A.D., and V.L. analyzed the data; Z.R., S.M., and V.L. prepared the figures; Z.R., M.-R.R., S.M., K.S.M., J.R.G., J.A.D., and V.L. edited and revised the manuscript; Z.R., M.-R.R., T.-H.T.P., S.M., K.S.M., J.R.G., J.A.D., and V.L. approved the final version of the manuscript; M.-R.R., K.S.M., J.R.G., J.A.D., and V.L. are responsible for conception and design of the research; M.-R.R., K.S.M., J.R.G., J.A.D., and V.L. interpreted the results of the experiments; V.L. drafted the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gerard Heck for help with CT taste nerve recordings.
Some of the data reported here were presented as an abstract at the International Symposium on Olfaction and Taste Meeting in June 2012 (39).
REFERENCES
- 1.Bosak NP, Inoue M, Nelson TM, Hummler E, Ishiwatari Y, Bachmanov AA. Epithelial sodium channel (ENaC) is involved in reception of sodium taste: evidence from mice with a tissue-specific conditional targeted mutation of the ENaC gene (Abstract). Chem Senses 35: A2, 2010 [Google Scholar]
- 2.Breza JM, Contreras RJ. Anion size modulates salt taste in rats. J Neurophysiol 107: 1632–1648, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzigeorgiou M, Bang S, Hwang SW, Schafer WR. tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans. Nature 494: 95–99, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherukuri CM, Bachmanov AA, McCaughey SA. A/J and C57BL/6J mice differ in chorda tympani responses to NaCl. Neurosci Res 75: 283–288, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman J, Williams A, Phan TH, Mummalaneni S, Melone P, Ren Z, Zhou H, Mahavadi S, Murthy KS, Katsumata T, Desimone JA, Lyall V. Strain differences in the neural, behavioral and molecular correlates of sweet and salty taste in naive, ethanol- and sucrose-exposed P and NP rats. J Neurophysiol 106: 2606–2621, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31: 253–264, 2006 [DOI] [PubMed] [Google Scholar]
- 8.DeSimone JA, Heck GL, DeSimone SK. Active ion transport in dog tongue: a possible role in taste. Science 214: 1039–1041, 1981 [DOI] [PubMed] [Google Scholar]
- 9.DeSimone JA, Phan TH, Heck GL, Ren ZJ, Mummalaneni S, Lyall V. Changes in taste receptor cell [Ca2+]i modulate chorda tympani responses to salty and sour taste stimuli. J Neurophysiol 108: 3206–3220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeSimone JA, Phan TH, Ren ZJ, Mummalaneni S, Lyall V. Changes in taste receptor cell [Ca2+]i modulate chorda tympani responses to bitter, sweet and umami taste stimuli. J Neurophysiol 108: 3221–3232, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewis ML, Phan TH, Meng X, Cui M, Mummalaneni S, Rhyu M, DeSimone JA, Lyall V. N-geranylcyclopropyl-carboximide (NGCC) modulates salty and umami taste in humans and animal models. J Neurophysiol 109: 1078–1090, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dias AG, Rousseau D, Duizer L, Cockburn M, Chiu W, Nielsen D, El-Sohemy A. Genetic variation in putative salt taste receptors and salt taste perception in humans. Chem Senses 38: 137–145, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Esakov AE, Serova ON. Influence of substance P on taste receptor organ and salt intake in rats. Neurosciences 14: 321–327, 1988 [Google Scholar]
- 14.Finger TE. Peptide immunochemistry demonstrates multiple classes of perigemmal nerve fibers in the circumvallate papilla of the rat. Chem Senses 11: 135–143, 1986 [Google Scholar]
- 15.Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Luis Harris S, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterization of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology 46: 133–149, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hochheimer A, Krohn M, Zinke H. Endogenous gustatory response and properties of immortalized human taste cell lines from lingual epithelium (Abstract). Proc XVI Int Symp Olfaction Taste, Stockholm, Sweden, 23–27 June 2012 [Google Scholar]
- 17.Hochheimer A, Krohn M, Zinke H. Endogenous gustatory response and properties of immortalized human taste cell lines from lingual epithelium (Abstract). Chem Senses 38: 270, 2013 [Google Scholar]
- 18.Ishida Y, Ugawa S, Ueda T, Murakami S, Shimada S. Vanilloid receptor subtype-1 (VR1) is specifically localized to taste papillae. Brain Res Mol Brain Res 107: 17–22, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Kaske S, Krasteva G, König P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci 8: 49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsumata T, Nakakuki H, Tokunaga C, Fujii N, Egi M, Phan TH, Mummalaneni S, DeSimone JA, Lyall V. Effect of Maillard reacted peptides on human salt taste and the amiloride-insensitive salt taste receptor (TRPV1t). Chem Senses 33: 665–680, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kido MA, Muroya H, Yamaza T, Terada Y, Tanaka T. Vanilloid receptor expression in the rat tongue and palate. J Dent Res 82: 393–397, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Kitada Y, Mitoh Y, Hill DL. Salt taste responses of the IXth nerve in Sprague-Dawley rats: lack of sensitivity to amiloride. Physiol Behav 63: 945–949, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Liu P, Shah BP, Croasdell S, Gilbertson TA. Transient receptor potential channel type M5 is essential for fat taste. J Neurosci 31: 8634–8642, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyall V, Heck GL, DeSimone JA, Feldman GM. Effects of osmolarity on taste receptor cell size and function. Am J Physiol Cell Physiol 277: C800–C813, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, Montrose MH, Chu S, Heck GL, Feldman GM, DeSimone JA. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol 281: C1005–C1013, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive salt taste receptor is a vanilloid receptor-1 variant. J Physiol 558: 147–159, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, DeSimone JA. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem Senses 30 Suppl 1: i42–i43, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lyall V, Heck GL, Phan TH, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. I. Effect on TRC volume and Na+ flux. J Gen Physiol 125: 569–585, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyall V, Heck GL, Phan TH, Mummalaneni S, Malik SA, Vinnikova AK, Desimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J Gen Physiol 125: 587–600, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyall V, Phan TH, Mummalaneni S, Mansouri M, Heck GL, Kobal G, DeSimone JA. Effect of nicotine on chorda tympani responses to salty and sour stimuli. J Neurophysiol 98: 1662–1674, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Lyall V, Phan TH, Mummalaneni S, Melone P, Mahavadi S, Murthy KS, DeSimone JA. Regulation of amiloride-insensitive NaCl chorda tympani responses by intracellular Ca2+, protein kinase C and calcineurin. J Neurophysiol 102: 1591–1605, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyall V, Phan TH, Ren ZJ, Mummalaneni S, Melone P, Mahavadi S, Murthy KS, DeSimone JA. Regulation of the amiloride-insensitive NaCl chorda tympani responses by phosphatidylinositol 4,5-bisphosphate. J Neurophysiol 103: 1337–1349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaughey SA. Taste-evoked responses to sweeteners in the nucleus of the solitary tract differ between C57BL/6ByJ and 129P3/J mice. J Neurosci 27: 35–45, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaughey SA, Cherukuri CM, Bachmanov AA. The amiloride-insensitive component of the chorda tympani response to NaCl is larger in A/J than in C57BL/6J mice (Abstract). Chem Senses 31: A62–A63, 2010 [Google Scholar]
- 35.Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. High salt recruits aversive taste pathways. Nature 494: 472–475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan TH, Mummalaneni S, Melone P, Desimone JA, Nicolelis MA, Simon SA. Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci USA 106: 1596–601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osada K, Komai M, Bryant BP, Suzuki H, Goto A, Tsunoda K, Kimura S, Furukawa Y. Capsaicin modifies responses of rat chorda tympani nerve fibers to NaCl. Chem Senses 22: 249–255, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Palmer RK, Atwal K, Bakaj I, Carlucci-Derbyshire S, Buber MT, Cerne R, Cortés RY, Devantier HR, Jorgensen V, Pawlyk A, Lee SP, Sprous DG, Zhang Z, Bryant R. Triphenylphosphine oxide is a potent and selective inhibitor of the transient receptor potential melastatin-5 ion channel. Assay Drug Dev Technol 8: 703–713, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Ren ZJ, Phan TH, Rhyu M, Mummalaneni S, Murthy KS, Grider JR, DeSimone JA, Lyall V. Modulating TRPM5 activity alters benzamil (Bz)-insensitive NaCl chorda tympani (CT) taste nerve responses (Abstract). Proc XVI Int Symp Olfaction Taste, Stockholm, Sweden, 23–27 June 2012 [Google Scholar]
- 40.Rhyu MR, Song AY, Kim HY, Kim SS, Tokunaga C, Phan TH, Heck GL, DeSimone JA, Lyall V. Naturally occurring peptides in mature Korean soy sauce modulate TRPV1 variant salt taste receptor (Abstract). Chem Senses 32: A23, 2007 [Google Scholar]
- 41.Rhyu MR, Song A, Abe K, Lyall V. Effect of kokumi taste active peptides on amiloride-insensitive salt taste preference in C57BL/6J mice (Abstract). Chem Senses 34: A41–A42, 2009 [Google Scholar]
- 42.Riera CE, Vogel H, Simon SA, Damak S, le Coutre J. Sensory attributes of complex tasting divalent salts are mediated by TRPM5 and TRPV1 channels. J Neurosci 29: 2654–2662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roper SD. Signal transduction and information processing in mammalian taste buds. Pflügers Arch 454: 759–776, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah BP, Liu P, Yu T, Hansen DR, Gilbertson TA. TRPM5 is critical for linoleic acid-induced CCK secretion from the enteroendocrine cell line, STC-1. Am J Physiol Cell Physiol 302: C210–C219, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith KR, Treesukosol Y, Paedae AB, Contreras RJ, Spector AC. The contribution of TRPV1 channel to salt taste quality in mice as assessed by conditioned taste aversion generalization and chorda tympani nerve responses. Am J Physiol Regul Integr Comp Physiol 303: R1195–R1205, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of water and sodium intake. Physiol Behav 91: 620–631, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol 292: R1799–R1809, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci 9: 1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003 [DOI] [PubMed] [Google Scholar]