Abstract
Cell line studies have previously demonstrated that hypoxia-reoxygenation (H/R) leads to the production of NADPH oxidase 1 and 2 (NOX1 and NOX2)-dependent reactive oxygen species (ROS) required for the activation of c-Src and NF-κB. We now extend these studies into mouse models to evaluate the contribution of hepatocytes to the NOX- and c-Src-dependent TNF-α production that follows H/R in primary hepatocytes and liver ischemia-reperfusion (I/R). In vitro, c-Src-deficient primary hepatocytes produced less ROS and TNF-α following H/R compared with controls. In vivo, c-Src-KO mice also had impaired TNF-α and NF-κB responses following partial lobar liver I/R. Studies in NOX1 and p47phox knockout primary hepatocytes demonstrated that both NOX1 and p47phox are partially required for H/R-mediated TNF-α production. To further investigate the involvement of NADPH oxidases in the production of TNF-α following liver I/R, we performed additional in vivo experiments in knockout mice deficient for NOX1, NOX2, p47phox, Rac1, and/or Rac2. Cumulatively, these results demonstrate that NOX2 and its activator subunits (p47phox and Rac) control the secretion of TNF-α by the liver following I/R. Interestingly, in the absence of Kupffer cells and NOX2, NOX1 played a dominant role in TNF-α production following hepatic I/R. However, NOX1 deletion alone had little effect on I/R-induced TNF-α. Thus Kupffer cell-derived factors and NOX2 act to suppress hepatic NOX1-dependent TNF-α production. We conclude that c-Src and NADPH oxidase components are necessary for redox-mediated production of TNF-α following liver I/R and that hepatocytes play an important role in this process.
Keywords: c-Src, hepatocyte, NADPH oxidase, redox, reperfusion injury
partial lobar hepatic ischemia followed by reperfusion (I/R) is one established model to study hepatic redox-mediated injury observed immediately following liver transplantation (22). Reactive oxygen species (ROS) production following reperfusion of the ischemic liver plays important roles in modulation of inflammation and hepatocyte damage (22). Major sources of hepatic I/R-induced ROS include Kupffer cells, neutrophils, and dysfunctional mitochondria (22). The downstream consequences of I/R-induced hepatic ROS include the activation of several transcription factors including AP-1 and NF-κB, which control hepatocyte cell fate decisions and inflammation (2, 7, 30, 32, 34, 44–46). Kupffer cell- and neutrophil-derived NADPH oxidases, which generate superoxide, appear to be one major source of hepatic I/R-induced ROS (18, 22, 30, 32, 34). Ablation of Kupffer cells prior to hepatic ischemia reduces both injury and ROS production following reperfusion (4, 9, 18, 22, 25, 34). Kupffer cells are also a predominant source of the hepatic I/R-associated TNF-α and IL-1 that drive neutrophil infiltration (22, 25). The contribution of hepatocytes in the direct production of NADPH oxidase-dependent ROS and TNF-α remains predominantly unknown. However, alterations in glutathione metabolism and mitochondrial function in hepatocytes have been suggested to be major sources of ROS following I/R injury (22).
Activation of hepatic TNF-α production following I/R injury appears to be largely controlled by the transcriptional activation of NF-κB through a noncanonical pathway controlled by c-Src-dependent phosphorylation of IκBα on tyrosine 42 (7). This unique pathway of IκBα-mediated NF-κB activation occurs in the absence of canonical ubiquitin-dependent IκBα degradation and is not conserved with IκBβ, which lacks the tyrosine phosphorylation site found in IκBα (6, 7, 20). Knockin mice containing an IκBβ cDNA in place of the IκBα gene (AKBI mice) demonstrate protection from liver I/R damage, reduced reperfusion-dependent induction of plasma TNF-α and hepatic TNF-α mRNA, and reduced neutrophil recruitment following reperfusion (7). Furthermore, hepatic I/R injury induces c-Src kinase activity to tyrosine phosphorylate recombinant IκBα but not IκBβ. Indeed, tyrosine 42 of IκBα is required for NF-κB activation following hypoxia-reoxygenation (H/R) in primary hepatocytes (7). These findings identify c-Src-mediated activation of NF-κB as critical in the production of hepatic TNF-α following I/R and subsequent neutrophil infiltration.
NADPH oxidases generate superoxide through the transfer of an electron from NADPH to molecular oxygen. Seven known NADPH oxidase catalytic subunits exist (NOX1, NOX2, NOX3, NOX4, NOX5, Duox1, and Duox2) (23, 24). The most widely characterized NADPH oxidase is phagocytic gp91phox (NOX2). The phagocytic NADPH oxidase is a multisubunit enzyme complex with both membrane (gp91phox and p22phox) and cytosolic components (p47phox, p67phox, p40phox, and the small GTPase Rac1/2). Other NADPH oxidases share several of the coactivator subunits with NOX2 (i.e., p22phox, p47phox) but can also use unique regulators (i.e., NOXo1 and NOXa1) (23, 24). The spatial regulation of NADPH oxidase activation plays very important roles in redox signaling by inflammatory cytokines such as TNF-α and IL-1 (26, 27, 33).
Src family kinases also play important roles in the spatial control of ROS production by cells through regulation of NADPH oxidases (11, 12, 14, 24). For example, c-Src phosphorylates several NOX organizers including Tks4, Tks5, and p47phox required for NOX-dependent ROS production (13, 43). Very little is known about the importance of c-Src in mediating NADPH oxidase activation following I/R injury. However, cell line studies have demonstrated that c-Src is partially required for NOX1- and NOX2-dependent ROS production following H/R and the activation of NF-κB (28). Following H/R, an activated phosphorylated form of c-Src (P-Y416) is rapidly recruited to the endosomal compartment with Rac1 and p47phox in a dynamin-dependent manner to produce redoxosomes (i.e., redox-active endosomes containing activated NOX). This activated c-Src is capable of tyrosine phosphorylating IκBα and signaling NF-κB activation (28, 33). Interestingly, knockdown of c-Src with siRNA blocks Rac1 recruitment to the endosomal compartment required for NOX activation. These findings suggest that c-Src may play an important role in the activation of NADPH oxidases following I/R injury and the proinflammatory consequences mediated by redox-dependent NF-κB activation (6, 7, 31).
In contrast to the Kupffer cell (22, 25), it is less clear whether the hepatocyte participates in ROS-dependent c-Src activation of NF-κB and subsequent production of TNF-α. In cell lines, Rac1, p47phox, NOX1, and NOX2 appear to be important targets of c-Src-mediated ROS production in the endosomal compartment following H/R (28). In the present study, we sought to evaluate importance of c-Src, Rac, p47phox, NOX1, and NOX2 in TNF-α production by primary hepatocytes and the liver following H/R and I/R, respectively. As hypothesized, endosomal ROS, serum TNF-α, and hepatic NF-κB activity are all greatly decreased in c-Src knockout (KO) animals following partial liver I/R injury. Studies in primary mouse hepatocytes demonstrated that deletion of c-Src, NOX1, and p47phox all significantly impair H/R-induced TNF-α secretion. In vivo deletion of NOX2, NOX1/2, Rac1/2, Rac1, or p47phox significantly reduced plasma TNF-α levels following liver I/R injury. NOX1 deletion alone had no impact on I/R induction of plasma TNF-α, suggesting NOX2 may compensate for loss of NOX1 in vivo. Interestingly, in the absence of Kupffer cells and NOX2, NOX1-dependent plasma TNF-α levels following I/R significantly increased, suggesting that Kupffer cells and non-Kupffer cell-derived NOX2 act to modulate NOX1-mediated TNF-α production by the liver. Thus these studies demonstrate that c-Src and its known downstream targets (NOX2, Rac, p47phox) play significant roles in vivo in liver I/R production of ROS, NF-κB, and/or TNF-α. Furthermore, our in vitro studies demonstrate that c-Src pathways also induce and control TNF-α production by hepatocytes.
EXPERIMENTAL PROCEDURES
Animals.
All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Iowa. Several genetically defined KO strains of mice were used, including c-Src KO mice (39), NOX1 KO mice (10), NOX2 KO mice (35), NOX1/2 double-KO mice (40), p47phox KO mice (21), Rac1flx/flx conditional KO mice (15), Rac2 KO mice (37), and AlbCre-Rac1flx/flx/Rac2 KO mice (40) [which are deleted for Rac2 in all cells and also for Rac1 in hepatocytes using the previously described albumin promoter-CRE strain (36)]. The NOX2 and p47phox KO mice were bred as homozygotes and were congenic for C57BL6; thus C57BL6 mice were used as wild-type (WT) controls. The Rac1/2, NOX1, and NOX1/2 lines were on a mixed background and used sibling controls. The inbred C57BL6 c-Src KO mice are growth stunted, in part because they lack teeth. Thus this line was outbred onto an ICR background (Harlan) and sibling controls were used. The ICR backcrossed c-Src KO mice still lacked teeth, so they were supplemented with gruel daily.
Partial lobar hepatic I/R.
Liver medial lobe I/R was performed as previously described under isoflurane anesthesia (7). In brief, mice were anesthetized and injected with heparin (100 μg/kg) to prevent clotting of blood during lobar ischemia. The largest medial lobe of the liver was clamped at its base with a microaneurysm clamp, followed by placement of the liver and clamp back into the peritoneal cavity for 45 or 60 min. Animals were kept warm during the surgery and I/R. Following surgically implemented ischemia, the clamp was removed and the abdominal wall sutured, and the animals were returned to their cages. Removal of the clamp signified the start of reperfusion. Animals were rapidly euthanized at the indicated time points and blood was drawn from the heart into Li-heparin tubes. For studies comparing KO to WT mice, 3-h postreperfusion time points were used. c-Src KO mice were more susceptible to prolonged times of ischemia and thus 45 min of ischemia was used for this line. All other lines received 60 min of ischemia.
Gadolinium chloride ablation of Kupffer cells.
In vivo Kupffer cell depletion was performed as previously described by our laboratory. Briefly, mice were intravascularly injected through the tail (3 injections in total on consecutive days) with gadolinium chloride (GdCl3) at 7 mg/kg in PBS. On the fourth day after initiating GdCl3 animals were used for partial liver I/R (60 min ischemia and 3 h reperfusion). Animals were rapidly euthanized at the indicated time points and blood was drawn from the heart into Li-heparin tubes for TNF-α measurements. Kupffer cell depletion was confirmed by immunostaining of frozen liver sections using a FITC-conjugated anti-F4/80 antibody (Caltag Laboratories, RM2901). In brief, tissue sections were fixed in 4% paraformaldehyde in PBS for 20 min, washed in PBS, and then blocked for 30 min with blocking buffer (PBS containing 20% normal donkey serum, 0.3% Triton X-100, and 1 mM CaCl2). Sections were then stained overnight at 4°C with FITC-conjugated anti-F4/80 antibody (1:50 dilution) in blocking buffer containing 2% donkey serum. Sections were then washed and mounted in Vectashield mounting medium with DAPI (Vector Laboratories, H-1500) and imaged by fluorescent microscopy.
Hypoxia-reoxygenation experiments in primary hepatocytes.
Primary hepatocytes were generated as previously described by our laboratory (7). In brief, mouse livers were perfused through the portal vein first with perfusion buffer solution and then with liver digest medium (both from GIBCO-BRL) at a flow rate of 4–6 ml/min for 5 min. Hepatocyte suspensions were separated by Percoll centrifugation at 500 g for 10 min and washed three times, and then 1 × 106 cells were plated onto 60-mm collagen-coated tissue culture plates in DMEM with 10% fetal bovine serum, 100 μg/ml penicillin, and 100 μg/ml streptomycin. After overnight culture, the medium was replaced with F-12/DMEM medium containing insulin (10 μg/ml), dexamethasone (67 ng/ml), EGF (50 ng/ml), luteotropin (20 U/l), linoleic acid (500 μg/ml), transferrin (10 μg/ml), and triiodothyronine (67.3 ng/ml) for 2 additional days prior to H/R experiments. At the start of H/R experiments, hepatocytes were ∼70–80% confluent. Hepatocyte growth medium equilibrated in 95% N2-5% CO2 or 95% O2-5% CO2 was used as hypoxia or reoxygenation medium, respectively. Cells were covered with 1 ml hypoxia medium at 37°C for 5 h in an airtight chamber equilibrated with 95% N2-5% CO2 and containing water at the bottom of the chamber for humidity. The medium was then replaced with 1 ml reoxygenation medium, and cells were further incubated at 37°C in a 95% O2-5% CO2 atmosphere for up to 10 h. At 2-h intervals, culture plates were removed from the chamber, all the medium was harvested, and the chamber was reperfused with a 95% O2-5% CO2 atmosphere for additional time points. Each plate was used for only a single experimental time point, to avoid subtle changes to the volume and concentrations of secreted factors during the reoxygenation period.
TNF-α measurements.
Plasma and cell culture supernatant TNF-α levels were determined by using R&D TNF-α immunoassay ELISA kit (SMTA00) following the manufacturer's instructions.
Isolation of liver endomembranes and chemiluminescence assay for NADPH-dependent ROS production.
Ischemic liver lobes were washed in phosphate-buffered saline and then homogenized in 2 ml of homogenization buffer [0.3 M sucrose, 10 mM HEPES (pH 7.6), 10 mM KCl, 0.74 mM spermidine, 0.15 mM spermine, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, and 1 complete protease inhibitor mixture tablet (Roche Applied Science) per 50 ml]. Liver homogenate was then lysed by nitrogen cavitation. Crude lysate (600 μg of protein) was centrifuged at 3,000 g to remove the heavy mitochondria and nuclei, and the postnuclear supernatant was subsequently centrifuged at 100,000 g for 1 h to pellet total endomembranes. The membrane pellets were rinsed three times in homogenization buffer and then resuspended in 100 μl of homogenization buffer. NADPH oxidase activity was analyzed by measuring the rate of ROS generation with a chemiluminescent, lucigenin-based system as described previously (26, 27). In brief, 5 μM lucigenin in 50 μl of endomembrane fraction was used to calculate the relative change in rate of ROS production following the addition of β-NADPH at a final concentration of 100 μM.
Western blotting.
Western blotting was performed with standard protocols. Protein concentrations were determined by use of the Bio-Rad protein quantification kit. c-Src levels were evaluated from 500 μg of liver lysate protein following immunoprecipitation with a rabbit anti-c-Src antibody (Abcam) and Western blotting with a mouse anti-c-Src antibody (Santa Cruz). Immunoreactive protein was detected by using peroxidase-labeled anti-mouse antibody and enhanced chemiluminescence (ECL; GE Healthcare, Piscataway, NJ).
NF-κB activity.
An NF-κB reporter recombinant adenovirus (38) was used to infect mice by tail injection at a dose of 2 × 1011 particles/mouse. At 72 h after infection, mice were used for biophotonic imaging and I/R injury experiments. Mice were injected (ip) with firefly luciferin (Xenogen) at 150 mg/kg body wt 10–15 min before imaging on an IVIS instrument. Baseline recordings were taken 6 h prior to initiating I/R surgery and again at 4, 6, 12, and 18 h after reperfusion.
Quantification of Nox transcripts.
Total RNA was extracted from the liver of WT C57BL6, NOX2 KO, NOX1 KO, and NOX1/2 double-KO mice by a standard TRIzol procedure, and Poly(A) mRNA was purified with Dynabeads mRNA purification kit (Invitrogen). Reverse transcription of 200 ng mRNA was performed by using the SuperScript III first-strand synthesis kit for RT-PCR (Invitrogen) with oligo(dT) priming in a reaction volume of 25 μl. Each sample was evaluated by real-time PCR for NOX1, NOX2, and GAPDH mRNA copies by using the Power SYBR Green PCR Master Mix (Applied Biosystems). The primers used were as follows: Nox1 5′-CTGAGAAAGCCATTGGATCACA-3′ and 5′-TGCGGATAAACTCCATAGCTGA-3′ giving an amplicon of 322 bp; Nox2 5′-GGTTTATGATGATGGGCCTA-3′ and 5′-GAGCTATTGAATACCGGTCA-3′ giving an amplicon of 329 bp; and GAPDH 5′-AGCAATGCATCCTGCACCACCA-3′ and 5′-CGGCACGTCAGATCCACGACGG-3′ giving an amplicon of 302 bp. Samples were run on a Bio-Rad MyIQ real-time machine (step 1, heat at 95°C for 10 min; step 2, 45 cycles alternating between 95°C for 15 s and 60°C for 60 s). For each cDNA sample, duplicate real-time reactions were prepared with each of the three sets of primers. The average cycle threshold (Ct) values were used to calculate the relative abundance of NOX/GAPDH transcripts for samples via a ΔCt calculation [relative abundance of NOX/GAPDH transcripts = 2−(Average NOX:Ct − Average GAPDH:Ct)]. Additionally, a series of standards were created by using known copy numbers of NOX1 and NOX2 cDNA containing plasmids to calculate the PCR efficiency. The efficiencies of the PCR amplification with both NOX primer sets were 100% (i.e., 2-fold changes in signal within the linear range).
DHE measurements of ROS in primary hepatocyte cultures.
Hepatocytes were subjected to hypoxia for 5 h as described above. During the hypoxia period, dihydroethidium (DHE) was dissolved in DMSO at 10 mM and kept in the dark on ice until use. Immediately before the 5-h hypoxia was completed, DHE was diluted 1:10,000 into oxygen-bubbled reoxygenation media to a final concentration of 1 μM DHE. The reoxygenation medium, containing DHE, was pipetted onto hepatocytes. Plates were incubated in reoxygenation medium (with DHE) for 10 or 20 min inside sealed chambers containing 95% oxygen-5% CO2 at 37°C. Cells that did not receive hypoxia were treated similarly but maintained in ambient oxygen-5% CO2 at 37°C during the DHE incubation period. Following reoxygenation, cultures were placed on a preheated 37°C stage of a Leica spinning disk confocal microscope and fluorescent images were rapidly captured. Images were analyzed with Metamorph image analysis software to measure the average cellular fluorescence intensity in each culture. The relative average fluorescent intensity of ∼50 cells from each culture was used to calculate means and SE for each experimental point (N = 3–4 plates for ICR cultures and N = 4–5 plates for c-Src cultures).
RESULTS
c-Src influences hepatic ROS production, NF-κB activation, and TNF-α secretion following I/R injury.
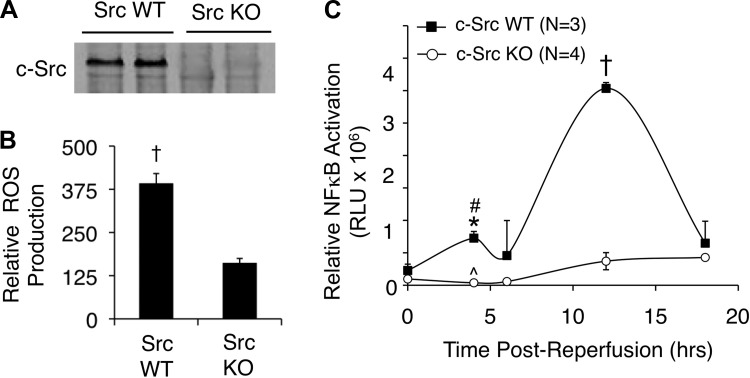
On the basis of previous findings in cell lines (13, 28, 43), we hypothesized that c-Src might control hepatic I/R-induced ROS and NF-κB activation through NADPH oxidases. Since we have previously demonstrated that newly formed endosomes following H/R injury are crucial for NOX1/2 signaling and ROS production (28), we first investigated whether endomembranes isolated from c-Src KO and littermate control livers (Fig. 1A) had altered ROS production following I/R. Indeed, c-Src deficiency significantly decreased NADPH-dependent ROS production by hepatic endomembranes following ischemia and 30 min of reperfusion (Fig. 1B). This reduction in endomembrane NADPH-dependent ROS production correlated with significantly impaired NF-κB transcriptional activation in vivo as determined by biophotonic imaging with an NF-κB-luciferase reporter (Fig. 1C). Two peaks of NF-κB transcriptional activation occurred in WT animals at 4 and 12 h following reperfusion, most likely representing the acute redox-dependent and later subacute inflammatory phases of NF-κB activation, respectively. Interestingly, in c-Src KO mice, the acute-phase peak of NF-κB activation was completely absent, whereas the subacute-phase peak was significantly blunted (Fig. 1C). Cumulatively, these findings are consistent with the early involvement of c-Src in the redox-dependent activation of NF-κB. They also demonstrate for the first time in vivo that c-Src is important for activation of NADPH-dependent endosomal ROS production following liver I/R.
Fig. 1.
c-Src deficiency reduces hepatic reactive oxygen species (ROS) production and NF-κB activation following hepatic ischemia-reperfusion (I/R). A: immunoprecipitation and Western blot for c-Src in liver lysates from wild-type (WT) or c-Src knockout (KO) mice. B: NADPH-dependent ROS production by hepatic endomembranes by lucigenin detection following liver I/R in WT and c-Src KO mice (45 min ischemia and 30 min reperfusion, N = 3 mice, ±SE). A statistically significant difference in ROS production between genotypes was observed by use of the Student's t-test (†P < 0.002). C: animals were preinfected with a recombinant adenoviral NF-κB-luciferase reporter vector 72 h prior to partial lobar hepatic I/R. Then 45 min of partial lobar ischemia was performed and NF-κB-luciferase reporter activity was assessed by biophotonic imaging on an IVIS imaging system. The zero time point represents pre-I/R luciferase activity and was not significantly different between genotypes (P = 0.1447). In all panels results depict means ± SE. The N independent animals are given. Statistically significant differences between genotypes were determined by a 2-tailed Student's t-test (*P < 0.001; †P < 0.0001). Statistically significant differences between the same genotypes comparing pre-I/R to post-I/R at the 4-h time point were determined by a 2-tailed paired Student's t-test (#P < 0.026; ^P = 0.1615).
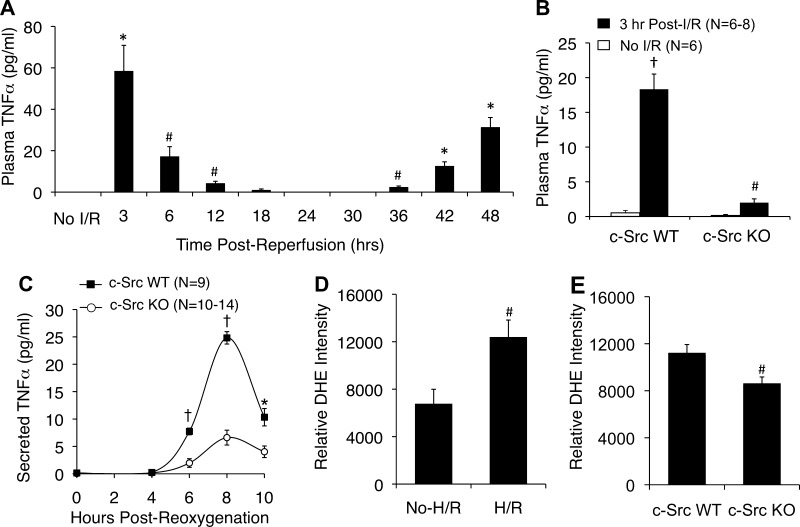
TNF-α is a major NF-κB responsive gene that controls inflammatory responses following I/R (3, 22). In addition to being responsive to NF-κB, TNF-α can also activate NF-κB in an autocrine and paracrine manner. TNF-α KO mice are protected from both acute and subacute liver damage following I/R and fail to activate NF-κB during both acute and subacute phases of I/R damage (41). Thus early acute TNF-α production appears to prime the liver for subsequent proinflammatory responses. We hypothesized that early acute-phase c-Src-mediated activation of NF-κB in response to I/R may impact acute-phase TNF-α production. Since to our knowledge, the kinetics of plasma TNF-α following liver I/R has not been evaluated in a single study, we first sought to evaluate this directly in WT animals so we could determine the optimal time to study the peak of the acute-phase TNF-α response. We hypothesized that TNF-α would rise during both the acute (3–4 h) and subacute (12–18 h) phases of NF-κB transcriptional activation (Fig. 1C). As anticipated, there was an acute-phase rise in plasma TNF-α (Fig. 2A) that overlapped with NF-κB transcriptional activation (Fig. 1C). However, to our surprise, the subacute phase of NF-κB transcriptional activation (Fig. 1C) demonstrated a significant decline in plasma TNF-α (Fig. 2A), with plasma TNF-α rising again at 2 days after reperfusion. Given the concordance in TNF-α levels and the NF-κB response early following I/R, we focused on the acute-phase response at 3 h and evaluated whether c-Src KO mice had impaired plasma TNF-α coincident with absent NF-κB responses (Fig. 1C). Indeed, as hypothesized, c-Src KO mice had significantly lower (9.2-fold) plasma TNF-α levels at 3 h of reperfusion compared with controls (Fig. 2B).
Fig. 2.
c-Src deficiency inhibits hepatic and hepatocyte secretion of TNF-α following I/R and hypoxia-reoxygenation (H/R), respectively. A: kinetics of plasma TNF-α levels in C57BL6 mice following 60 min of partial lobar ischemia followed by the various time points of reperfusion. Blood was collected by terminal cardiac bleed (N = 3 independent animals for each data point). The first point represents nonischemic controls (labeled No I/R). B: plasma TNF-α levels prior to and following 45 min of partial lobar ischemia and 3 h of reperfusion. C: primary hepatocytes were generated from c-Src KO and c-Src WT littermates. TNF-α secretion into the media of these cultures was then evaluated following 5-h hypoxia and the indicated times of oxygenation. In all panels results depict means ± SE. The N independent animals or replicates are given in B and C. D and E: dihydroethidium (DHE) determination of ROS production in ICR (D) and c-Src WT and KO (E) cultured primary hepatocytes following 5 h of hypoxia and 20 min of reoxygenation. No H/R controls were also treated with DHE for 20 min. Fluorescent photomicrographs were quantified by use of Metamorph software; graphs depict the relative DHE intensity per cell (±SE) from N = 3–5 independent primary cultures. Statistically significant differences were determined by a 2-tailed Student's t-test (#P < 0.05, *P < 0.005; †P < 0.0001). Comparisons in A are between the preischemia and various postreperfusion time points. Comparisons in B are as follows: #c-Src KO preischemia vs. c-Src KO postreperfusion; †c-Src KO postreperfusion vs. c-Src WT postreperfusion, and c-Src WT preischemia vs. c-Src WT postreperfusion. Comparisons in C are between genotypes at each specific time point.
We hypothesized that hepatocytes represent a source of TNF-α during this acute phase of I/R injury and that c-Src controls the induction of TNF-α. To test this hypothesis, we developed an H/R model using purified primary hepatocytes from c-Src KO mice and control littermates. We cultured these hepatocytes and then treated them with 5 h of hypoxia. Following reoxygenation, we measured TNF-α secreted into the medium. WT primary hepatocytes produced a significant amount of TNF-α following H/R with peak levels appearing in the media at 8 h following reoxygenation. By contrast, c-Src KO hepatocytes produced significantly less TNF-α than did control WT hepatocytes (Fig. 2C). Given that c-Src has been shown to be required for ∼30% of NOX-mediated ROS production in HeLa cells following H/R (28), we sought to evaluate whether reductions in H/R-induced TNF-α production observed in c-Src KO hepatocytes also correlated with reduced redox stress. In WT ICR hepatocytes, H/R significantly induced ROS production compared with no-H/R controls (Fig. 2D). Importantly, c-Src KO hepatocytes produced ∼25% less (P < 0.0199) ROS than c-Src WT littermate controls at 20 min following H/R. Similar results were also observed at 10 min post-H/R, with a 28% decrease in ROS observed in c-Src KO hepatocytes vs. littermate controls (P < 0.0073) (data not shown). These results led to two important conclusions. First, hepatocytes themselves can produce TNF-α in response to H/R without paracrine action from other cell types. Second, c-Src is important for the production of both ROS and TNF-α by hepatocytes after H/R in vitro.
NOX1 and p47phox influence TNF-α production by primary hepatocytes in response to H/R injury.
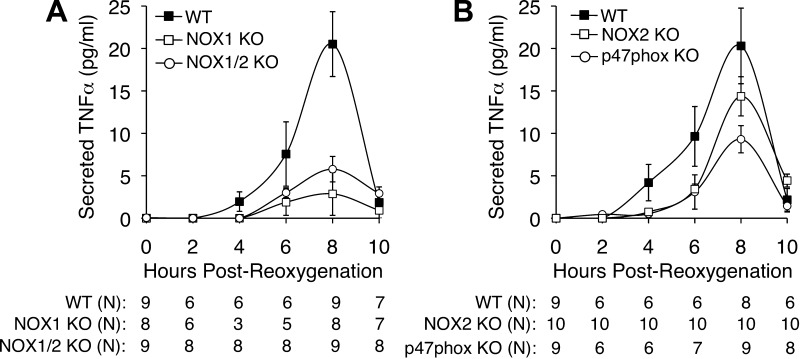
Previous studies have demonstrated that c-Src plays an important role in the activation of NOX1 by phosphorylating p47phox and other NOX1 organizers (13, 28, 43). Thus we hypothesized that if NOX1 and p47phox were acting downstream to c-Src in primary hepatocytes to induce TNF-α, H/R-induced TNF-α would be blunted in the absence of NOX1 or p47phox. As hypothesized, we observed that NOX1 KO hepatocytes did indeed produce significantly less TNF-α than did WT hepatocytes (Fig. 3A). Similarly, p47phox KO hepatocytes also produced significantly less TNF-α than did WT controls (Fig. 3B). The reduction in peak TNF-α production was greater for NOX1 KO (7.2-fold) than for p47phox KO (2.2-fold) hepatocytes, suggesting that other NOX organizers may also participate in the NOX1-mediated TNF-α response by hepatocytes. To investigate the contribution of NOX2 to the production of TNF-α following H/R, we also performed studies with NOX2 KO and NOX1/2 double-KO primary hepatocytes. Deletion of NOX2 led to a small decline in TNF-α production at 8 h following reoxygenation that was not significantly different from WT control hepatocytes (Fig. 3B). Furthermore, we found no significant difference between the TNF-α secretion by NOX1/2 double-KO hepatocytes and NOX1 KO hepatocytes following H/R (Fig. 3A). In combination with previous studies (7, 28), these results strongly implicate c-Src and NOX1 as major H/R signaling effectors important for TNF-α production by the hepatocyte.
Fig. 3.
Nox1 and p47phox deficiency inhibits hepatocellular secretion of TNF-α following H/R. A: primary hepatocytes were generated from NOX1 WT, NOX1 KO, and NOX1/2 double-KO littermates. TNF-α secretion into the media was then studied following 5 h hypoxia and the indicated times of oxygenation. B: primary hepatocytes were generated from C57BL6 inbred p47phox KO mice, NOX2 KO mice, or WT C57BL6 mice. TNF-α secretion into the media was then measured following 5 h hypoxia and the indicated times of reoxygenation. In both A and B, results depict means ± SE. The N independent replicates (number of plates of hepatocytes treated with H/R) are given below each graph. Statistically significant differences between genotypes at the 8-h time point were determined by a 2-tailed Student's t-test (WT vs. NOX1 KO, P < 0.0020; WT vs. NOX1/2 KO, P < 0.0025; WT vs. NOX2 KO, P = 0.2270; WT vs. p47phox KO, P < 0.0278).
NADPH oxidase components are required for acute-phase TNF-α production in vivo following liver I/R injury.
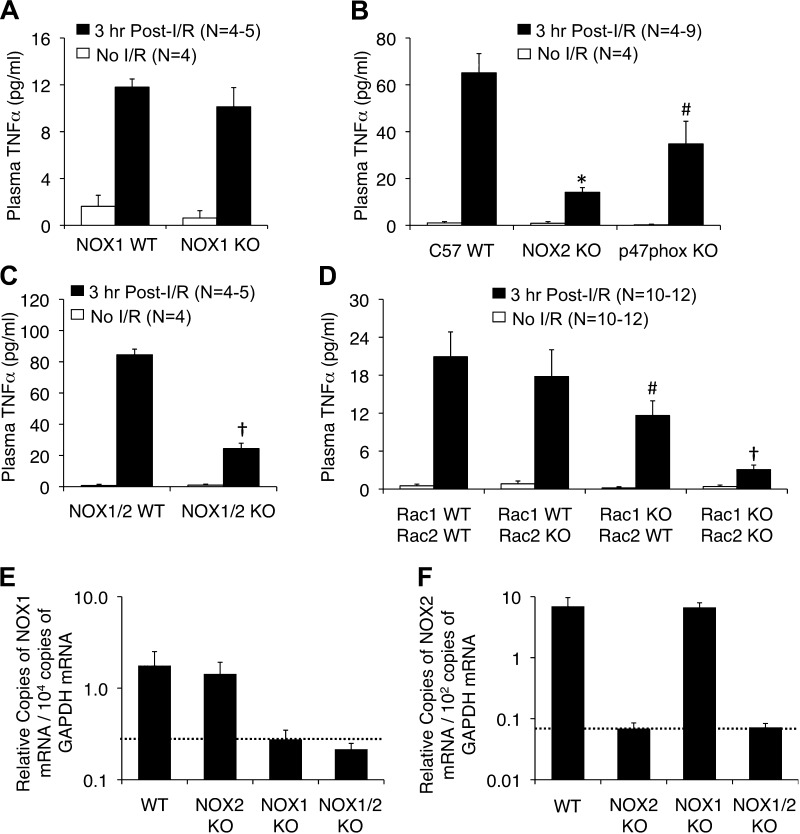
On the basis of these in vitro results in primary hepatocytes, we anticipated that both NOX1 and p47phox KO mice would also demonstrate impaired acute-phase plasma TNF-α levels following liver I/R compared with their WT controls. Surprisingly though, deletion of NOX1 had no effect on 3 h postreperfusion plasma TNF-α levels (Fig. 4A). However, similar to primary hepatocytes, deletion of p47phox significantly reduced the plasma TNF-α level by ∼2-fold during the acute phase after I/R (Fig. 4B). Since p47phox can serve as part of both the NOX1 and NOX2 complexes in certain cells (24), we hypothesized that NOX2 may compensate for the lack of NOX1 in vivo. Indeed in cell line studies, both NOX1 and NOX2 have been shown to be important for ROS production following H/R (28). Therefore, we repeated liver I/R experiments with single NOX2 KO as well as NOX1/NOX2 double-KO animals. Results from these studies demonstrated that deletion of NOX2 alone was sufficient to significantly decrease (4.6-fold) acute-phase plasma TNF-α levels (Fig. 4B). Deletion of both NOX1 and NOX2 produced a similar level of decrease in plasma TNF-α as observed with NOX2 deletion (Fig. 4C). Thus NOX2 is the major NOX in vivo that controls acute-phase TNF-α production following I/R. By contrast, in primary hepatocytes, NOX1 appears to be the primary NOX that facilitates the induction of TNF-α following H/R (Fig. 3, A and B). We hypothesized that the induction of the NOX2 gene at the mRNA level might occur in vivo on a NOX1 KO background, thus compensating for the loss of NOX1 in vivo. To evaluate this possibility we performed quantitative RT-PCR on liver total RNA from WT, NOX2 KO, NOX1 KO, and NOX1/2 KO mice. Results from these studies demonstrated no transcription compensation of NOX1 or NOX2 genes on the NOX2 KO or NOX1 KO backgrounds, respectively (Fig. 4, E and F). As expected, NOX2 mRNA expression predominated in the liver at a level ∼400-fold higher than NOX1 mRNA. Thus, if NOX2 compensation for NOX1 deletion does occur in vivo in the hepatocyte, it may be at a posttranscriptional level involving NOX2 regulators (i.e., activation of p47phox, p67phox, or Rac1). Alternatively, transcriptional compensation of the NOX2 gene in NOX1 KO hepatocytes in vivo may be such a small percentage of the overall NOX2 expression in the liver that it cannot be detected by RT-PCR. It is presently unclear why NOX1 does not compensate for the deletion of NOX2 in vivo. But results from double-NOX1/NOX2 KO mice (Fig. 4C) suggest that such compensation does not occur to any detectable level.
Fig. 4.
Nox2, Rac1/2, and p47phox deficiency inhibits hepatic secretion of TNF-α following partial lobar liver I/R. The indicated genetic strains were subjected to 60 min of partial lobar ischemia followed by 3 h of reperfusion. Blood was then harvested by cardiac bleeds and plasma was assessed for TNF-α levels. A: comparison of plasma TNF-α levels between Nox1 KO and Nox1 WT littermates. B: comparison of plasma TNF-α levels between C57 inbred mice with WT, NOX2 KO, or p47phox KO genotypes. C: comparison of plasma TNF-α levels between NOX1/2 WT and NOX1/2 double-KO mice. D: comparison of plasma TNF-α levels between Rac1flx/flx/Rac2-WT (Rac1-WT/Rac2-WT), Rac1flx/flx/Rac2-KO (Rac1-WT/Rac2-KO), AlbCRE-Rac1flx/flx/Rac2-WT (Rac1-KO/Rac2-WT), and AlbCRE-Rac1flx/flx/Rac2-KO (Rac1-KO/Rac2-KO) mice. In all panels results depict means ± SE. The N independent animals are given in each graph. Statistically significant differences in A–D were determined by a 2-tailed Student's t-test (#P < 0.05, *P < 0.005; †P < 0.0005). Marked comparisons are between the WT and KO postreperfusion time points. E and F: quantitative RT-PCR for NOX1 (E) and NOX2 (F) mRNA from total liver RNA generated from WT, NOX2 KO, NOX1 KO, and NOX1/2 double-KO mice. GAPDH was used as internal control using ΔCt calculations for relative abundance. Values show means (± SE, N = 5 independent animals) relative abundance of NOX transcripts normalized to GAPDH transcripts as 2−(Nox:Ct − GAPDH:Ct) values. Dotted lines indicate the background levels of detection.
Rac1 and Rac2 are requisite activators of both NOX1 and NOX2 (24). Both NOX1 and NOX2, as well as Rac1, have been identified in hepatocytes (5). Rac1 was also shown to be required for the recruitment of c-Src to NOX-active endosomes following H/R in cell line studies that used Rac1-siRNA knockdown (28). We sought to determine whether Rac also played a role in acute-phase TNF-α production in vivo following I/R injury. Constitutive Rac1 deletion is embryonic lethal, whereas Rac2 KO animals are viable. Given that Rac1 and Rac2 are 92% identical and have been shown to have many redundant functions (17), we focused on the hepatocyte-specific functions of Rac1 in the context of Rac2 KO and WT backgrounds. We generated mice with specific hepatocyte deletion of Rac1 by breeding Rac1flx/flx mice to albumin promoter-CRE transgenic animals. As previously shown (40), these Rac1flx/flx/Alb-CRE animals are devoid of Rac1 protein in the liver, demonstrating that hepatocytes are the predominant site of Rac1 expression in the liver. We first compared I/R-induced 3-h plasma TNF-α levels between Rac1flx/flx/Alb-CRE/Rac2-KO and Rac1flx/flx/Rac2-WT littermates (Fig. 4D). As expected, deletion of Rac1 and Rac2 in the liver significantly reduced acute-phase plasma TNF-α levels following I/R compared with control WT mice. These results demonstrate that Rac, a critical modulator of c-Src and NOX1/2, is important in acute-phase TNF-α secretion by the liver following I/R. We next evaluated Rac1flx/flx/Rac2-KO mice and found that these animals gave rise to similar levels of acute-phase TNF-α secretion as observed in Rac1flx/flx/Rac2-WT animals following I/R (Fig. 4D). Lastly, hepatic I/R studies in Rac1flx/flx/Alb-CRE/Rac2-WT mice demonstrated that hepatocyte-specific deletion of Rac1 significantly attenuated the acute-phase TNF-α response. Given that hepatic Rac1 expression is predominantly limited to hepatocytes (40), these findings strongly support the importance of hepatocyte Rac1/NOX activation in early TNF-α responses by the liver following I/R.
Kupffer cells dynamically regulate TNF-α secretion in a NOX-dependent fashion following I/R injury of the liver.
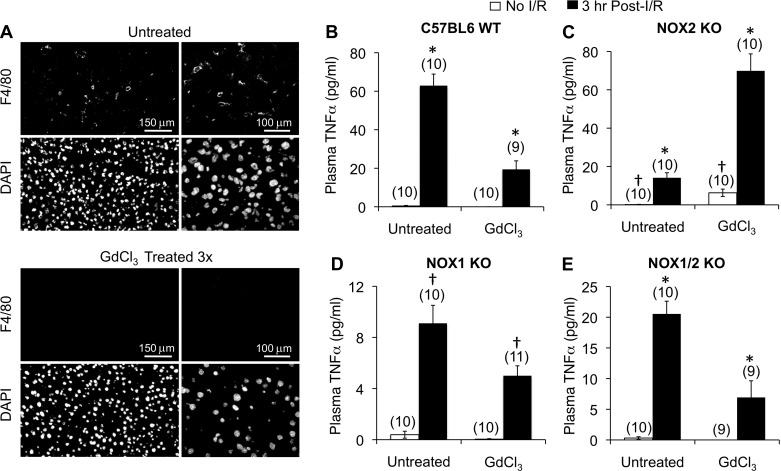
Previous studies have demonstrated that Kupffer cells play an important role in regulating hepatic post-I/R inflammatory responses including TNF-α production (25, 31). These studies have demonstrated that GdCl3 treatment of the liver prior to I/R, to deplete or inactivate Kupffer cells, leads to a significant reduction in TNF-α production following I/R. To investigate the contribution of hepatocyte- and Kupffer cell-derived NOX1 and/or NOX2 to the overall production of TNF-α following I/R, we performed Kupffer cell ablation with GdCl3 prior to hepatic I/R in WT, NOX2 KO, NOX1 KO, and NOX1/2 double-KO mice. The GdCl3 treatment ablated Kupffer cells from the liver as evidenced by immunostaining with a macrophage-specific marker (F4/80) (Fig. 5A). As previously observed (25, 31), Kupffer cell ablation significantly reduced (∼3-fold) the production of TNF-α in the plasma at 3 h following reperfusion (Fig. 5B). Interestingly, Kupffer cell ablation of NOX2 KO animals significantly enhanced (∼5-fold) TNF-α production following I/R compared with I/R control untreated NOX2 KO mice (Fig. 5C). Furthermore, there was a significant rise in baseline plasma TNF-α of GdCl3-treated NOX2 KO animals that did not undergo I/R, compared with untreated control animals (Fig. 5C). By contrast, GdCl3 treatment of NOX1 KO mice led to significant reduction (1.8-fold) in TNF-α production following I/R compared with untreated controls (Fig. 5D), albeit smaller than the reduction seen in WT animals (Fig. 5B). These findings suggested the intriguing hypothesis that in the absence of Kupffer cells and NOX2, NOX1 might promote excessive TNF-α production by the liver prior to and following I/R. To test this hypothesis, we also performed Kupffer cell ablation studies in NOX1/2 double-KO mice. Indeed, deletion of both NOX1 and NOX2 reversed both the elevated baseline and post-I/R production of TNF-α observed following Kupffer cell ablation in the NOX2 KO background (Fig. 5E): NOX1/2 KO mice ablated for Kupffer cells had a significant threefold reduction in plasma TNF-α levels following I/R compared with I/R control littermates that were not treated with GdCl3. In summary, these findings demonstrate a dynamic relationship between NOX1 and NOX2 in the regulation of TNF-α by the liver, which is mediated by Kupffer cells, hepatocytes, and potentially other cell types found in the liver.
Fig. 5.
Kupffer cell depletion differentially affects hepatic secretion of TNF-α in a Nox1- and Nox2-dependent fashion following partial lobar liver I/R. The indicated genetic strains were treated with or without GdCl3 and subjected to 60 min of partial lobar ischemia followed by 3 h of reperfusion. Blood was then harvested by cardiac bleeds and plasma was assessed for TNF-α levels. A: macrophage marker (F4/80) staining of livers from animals untreated or treated with GdCl3 demonstrating the depletion of Kupffer cells. B–E: comparison of plasma TNF-α levels prior to and following I/R for untreated and GdCl3-treated C57BL6 WT mice (B), NOX2 KO mice (C), NOX1 KO mice (D), or NOX1/2 double-KO mice (E). In all panels results depict means ± SE. The N independent animals are given in brackets above each data point. Statistically significant differences were determined by a 2-tailed Student's t-test for the indicated marked comparisons (*P < 0.001, †P < 0.02).
DISCUSSION
TNF-α production by the liver following I/R plays important roles in the activation of NF-κB and proinflammatory responses causing liver damage. This is most clearly demonstrated by protection from I/R liver damage in TNF-α KO mice (41). However, low doses of TNF-α from either hepatic preconditioning or pre-I/R injection of TNF-α can also protect the liver from I/R injury through the activation of NF-κB-mediated protective genes (41, 42). Thus the timing (acute vs. late) and level of TNF-α production, as well as its cellular source, likely play important roles in both the inflammatory and the protective responses to liver I/R. The pathways and hepatic cell types (i.e., Kupffer cells, hepatocytes, endothelial cells) associated with the activation of TNF-α secretion by the liver following liver I/R are complex and likely also influence hepatocellular fates following I/R. For example, Kupffer cells are a recognized predominant source of TNF-α following liver I/R, and NOX2-dependent superoxide production is partially required for this TNF-α production (18, 22, 25). However, the contribution of hepatocytes to the production of TNF-α following liver I/R has remained unclear. Furthermore, signaling pathways that control the redox-dependent I/R responses by the hepatocyte have also not been fully delineated.
In the present study, we have demonstrated that c-Src significantly contributes to I/R-dependent induction of ROS, NF-κB activation, and acute-phase TNF-α production by the liver. Studies with primary cultures also clearly demonstrate, for the first time, that hepatocytes can produce TNF-α following H/R injury, and that c-Src plays an important role in this response. Given previous findings demonstrating that the inhibition of c-Src-mediated IκBα, NF-κB, and TNF-α activation is associated with a reduction of plasma ALT and liver inflammation in AKBI mice (in which IκBβ replaces IκBα) following hepatic I/R (7), we anticipated that c-Src KO mice would be protected from I/R injury. However, this was not the case; although c-Src knockout mice had decreased acute-phase TNF-α production and NF-κB activation (like AKBI mice), 6 h post-I/R plasma alanine aminotransferase (ALT) rose similarly in both c-Src WT and c-Src KO mice (c-Src WT: pre-I/R 162 ± 20 U/l, post-I/R 2,646 ± 274 U/l; c-Src KO: pre-I/R 142 ± 20 U/l, post-I/R 2,585 ± 142 U/l). There was also no significant difference in pre- or 6 h post-I/R values between genotypes for several other liver function tests (data not shown, but included alkaline phosphatase, γ-glutamyl transferase, bile acids, total bilirubin, blood urea nitrogen, and total cholesterol). The reason for a lack of protection from I/R liver damage in c-Src KO mice remains unclear but may involve functions of c-Src in the activation of NF-κB protective responses in the hepatocyte and/or other hepatic cell types. Additionally, it should be noted that AKBI mice demonstrated reduced, but not completely absent, acute-phase I/R-induced NF-κB activation (7), whereas c-Src KO mice had absent acute-phase NF-κB responses (Fig. 1C). For example, AKBI mice demonstrated ∼2.5-fold and ∼4-fold reductions in TNF-α levels and NF-κB activity, respectively, compared with WT animals following liver I/R. These reductions were much more significant in c-Src KO mice following I/R, with 9.2-fold and 19.3-fold reductions in TNF-α levels and NF-κB activity, respectively. Low levels of TNF-α and NF-κB activation have also been demonstrated to be protective to hepatocytes during hepatic ischemic preconditioning (19, 41, 42). Thus, in c-Src KO mice, these levels may be reduced below this protective threshold, whereas this may not occur in AKBI mice. Alternatively, c-Src may directly influence protective signaling pathways that are unrelated to TNF-α and NF-κB activation.
Our studies in primary hepatocytes clearly demonstrate that NOX1 and p47phox, two downstream targets of c-Src, play important roles in the activation of TNF-α secretion by hepatocytes following H/R (Fig. 3). Studies in NOX2 KO and NOX1/2 double-KO primary hepatocytes suggest that NOX2 in primary hepatocytes plays a relatively minor role in post-H/R TNF-α secretion. However, although post-H/R TNF-α secretion by NOX2 KO hepatocytes was not significantly different than WT controls, deletion of NOX2 marginally reduced TNF-α secretion, suggesting that NOX2 may also partially influence pathways that activate TNF-α secretion by hepatocytes. It was thus surprising that NOX1 KO mice demonstrated no differences in acute-phase plasma TNF-α levels following liver I/R injury (Fig. 4). Unlike NOX1 KO mice, p47phox KO mice and their primary hepatocytes demonstrated similar reductions in TNF-α production following I/R and H/R, respectively. In vivo, NOX2 appears to play a dominant role in I/R-induced TNF-α secretion, suggesting that NOX2 may compensate in vivo for the loss of NOX1 in hepatocytes. Since both NOX1 and NOX2 have been found in isolated hepatocyte membranes (5), the observation of compensation may not be surprising. It is interesting that NOX2 compensation does not occur in primary NOX1 KO hepatocytes, but given the findings that NOX1-dependent TNF-α production was elevated in Kupffer cell-depleted NOX2 KO mice (Fig. 5C), hepatocyte-extrinsic factors may also play a role in modulating NOX2 activity in the hepatocyte. Nonetheless, since both NOX1 and NOX2 have been shown to be responsible for c-Src-dependent ROS production following H/R in cell lines, and NOX1 and NOX2 can both utilize p47phox as an activator, our in vivo and primary hepatocyte data using c-Src KO mice are consistent with NOX2 compensation for loss of NOX1. Interestingly, NOX1 function was not redundant for NOX2 in the context of I/R-induced TNF-α secretion (Fig. 4B). The reason for this remains unclear but may involve the inability of NOX1 to compensate for the loss of NOX2 in neutrophils (35) and thus presumably also in Kupffer cells.
Our findings of reduced ROS production in the absence of c-Src, following hepatocyte H/R (using DHE, Fig. 2E) and hepatic I/R (using an NADPH-dependent lucigenin assay in endomembranes, Fig. 1B), are consistent with previous studies in HeLa cells following siRNA-mediated knockdown of c-Src (28). While the use of lucigenin for detection of superoxide may have limitations in live cells (8), others have suggested that lucigenin, when used at 5 μM concentrations or lower, can accurately index superoxide production compared with oxygen consumption and electron paramagnetic resonance (EPR) methods (29). We have found that in isolated endomembranes the NADPH-dependent ROS detected by lucigenin correlates well with NADPH-dependent superoxide production as determined by EPR (26, 27). Thus, although not formally proven in these studies, the c-Src-dependent ROS following I/R is likely NOX dependent and thus superoxide. Additionally, our DHE studies in live primary hepatocytes support c-Src involvement in the activation of ROS production by the hepatocyte following H/R. The mechanism by which c-Src enhances ROS production following I/R and H/R likely involves the ability of c-Src to phosphorylate several NOX organizers (including p47phox) required for NOX-dependent ROS production (13, 33, 43). Thus, as previously hypothesized (28), our data are consistent with c-Src having a dual role in reperfusion-induced redox signaling (i.e., enhancing ROS through NOX activation and as a target of ROS required for NF-κB activation and TNF-α production). Although it is widely accepted that c-Src is activated by ROS (1, 16, 28), the mechanism by which ROS activates c-Src remains unclear but may involve the H2O2-mediated inhibition of phosphatases that act on c-Src (28).
Given the potential for cell type-specific compensation between NOX1 and NOX2 (i.e., hepatocyte vs. Kupffer cells), the Kupffer cell depletion experiments on the various NOX KO backgrounds provided interesting insights into the biology of hepatic TNF-α regulation. These studies confirmed previous findings (25, 31) in WT mice by demonstrating that Kupffer cell ablation significantly decreased I/R-induced TNF-α in the plasma (Fig. 5B). However, the finding of elevated baseline and post-I/R plasma TNF-α in Kupffer cell depleted NOX2 KO mice (Fig. 5C), together with the reversal of this phenotype when both NOX1 and NOX2 are deleted (Fig. 5E), suggest the intriguing hypothesis that Kupffer cells and non-Kupffer cell-derived NOX2 act in concert to inhibit NOX1-dependent TNF-α production by the liver. Such a hypothesis is also consistent with NOX1 playing a dominant role in H/R-induced TNF-α production by primary hepatocytes when other extrinsic hepatic factors are absent. A working model that could explain these findings might include a secreted Kupffer cell-derived factor that represses a NOX2-dependent anti-inflammatory pathway in endothelial cells, stellate cells, cholangiocytes, and/or blood-derived cell types. This anti-inflammatory pathway could act to suppress NOX1-dependent TNF-α production by hepatocytes and/or other cell types in the liver (Fig. 6). Although the in vivo Kupffer cell depletion experiments cannot differentiate between the potential cellular sources of enhanced TNF-α production in the NOX2 KO Kupffer cell-depleted background, our in vitro studies in primary hepatocytes support the notion that these cells are potential sources of NOX1-dependent TNF-α production.
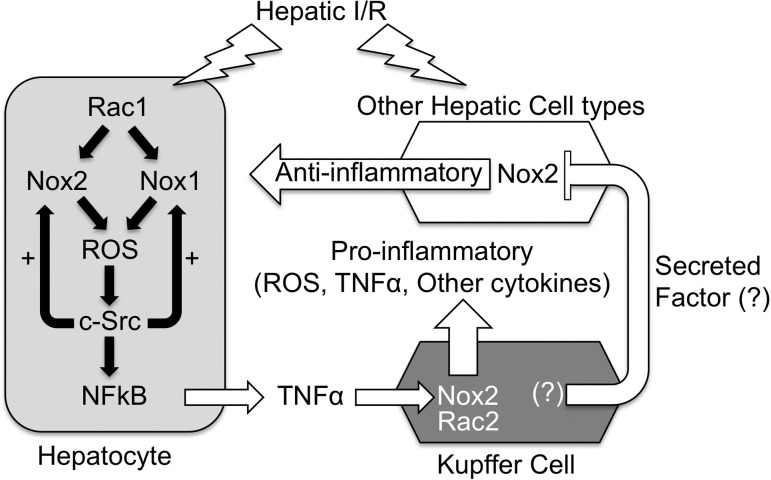
Fig. 6.
Working model for the NOX dependence of TNF-α production by the liver following I/R injury. We propose that the hepatocyte is one of the first cell types to respond to I/R injury by secreting low levels of NOX1/2-dependent TNF-α, priming Kupffer cells to mount an increased inflammatory response including high levels of TNF-α production. In this context, both hepatocyte Rac1/Nox1 and/or Rac1/Nox2 can contribute to ROS production by the hepatocyte, which activates c-Src and NF-κB pathways to induce TNF-α production by the hepatocyte. As in other cell types (28), c-Src in the hepatocyte appears to potentiate H/R-induced NOX-mediated ROS production in the hepatocyte. On the basis of our Kupffer cell-depletion experiments on the various NOX KO backgrounds, we propose that, in vivo, Kupffer cell-derived factors repress a partially NOX2-dependent pathway (labeled “Anti-inflammatory”) that otherwise would inhibit NOX1 activity in the liver. In vivo, this anti-inflammatory pathway may limit NOX1 activity in hepatocytes. In cultured primary hepatocytes (i.e., ex vivo), where this extrinsic factor is absent, NOX1 predominates as the pathway to activate TNF-α following H/R in the absence of other hepatic cell types. In NOX1 KO mice, NOX2 in the hepatocyte compensates for the loss of NOX1, and I/R responses are similar to WT animals. In NOX2 KO mice, NOX1 can still lead to low levels of acute-phase TNF-α production following I/R; however, the amplification of proinflammatory responses by the Kupffer cells is absent since NOX2 is the predominant NOX in this cell type. In Nox1/2 double-KO mice, ROS-dependent signaling that activates much of the TNF-α response following I/R is absent. In WT mice lacking Kupffer cells, a NOX2-dependent anti-inflammatory state is induced by the lack of Kupffer cell-secreted proinflammatory factors, and I/R-mediated TNF-α responses are attenuated. In NOX1 KO animals lacking Kupffer cells, the NOX2-dependent anti-inflammatory state is maintained, whereas Kupffer cell proinflammatory contributions are eliminated, leading to diminished I/R-mediated plasma TNF-α levels. In NOX2 KO Kupffer cell depleted animals, the NOX2-dependent anti-inflammatory state is no longer present and this transitions to a proinflammatory state raising both the baseline and I/R-induced TNF-α production by hepatocytes and potentially other non-Kupffer hepatic cell types. In this case (the absence of NOX2 and Kupffer cells), NOX1 activity may increase in the hepatocyte and/or other cell types, leading to enhanced NOX1-dependent production of TNF-α. In NOX1/2 double-KO animals depleted for Kupffer cells, the proinflammatory state caused by absence of both NOX2 and Kupffer cells is attenuated since NOX1 is not present to induce TNF-α production.
Rac plays important roles in the activation of NOX1, NOX2, and c-Src (11, 23, 24, 28). Thus it is not surprising that Rac1/Rac2 KO mice had significantly diminished I/R-induced acute-phase TNF-α secretion, given that NOX2 and c-Src KO mice demonstrated a similar phenotype. However, our findings in Rac1-WT/Rac2-KO mice demonstrated a lack of reduction in acute-phase TNF-α secretion following I/R (Fig. 4D). Given that neutrophils from Rac1-WT/Rac2-KO mice have significantly attenuated ROS production and proinflammatory properties (15, 37), this suggests that Rac1 cannot compensate for Rac2 function in leukocytes. The compensatory roles of Rac1 and Rac2 in Kupffer cells have not been previously studied, but it seems likely that a nonredundant role for Rac2 regulation of NOX2 is similar between leukocytes and Kupffer cells. If this is correct, our studies in Rac1-WT/Rac2-KO mice would essentially be similar to a Kupffer cell-specific ablation of NOX2 activity. Most notably, the significant decline in I/R-induced acute-phase TNF-α production in hepatocyte-specific Rac1-KO/Rac2-WT animals, compared with Rac1-WT/Rac2-WT controls, demonstrates the importance of hepatocyte Rac1 and thus also likely NOX activation in hepatic TNF-α responses following I/R. Overexpression of a dominant Rac1 mutant (N17Rac1), which will block both Rac1 and Rac2 activation, in the liver prior to I/R injury has been shown to be protective from liver damage (34). Our findings are consistent with this previous observation and now demonstrate for the first time that loss of Rac is associated with reductions in I/R-induced TNF-α secretion (Fig. 4D). Furthermore, our studies now show that endothelial and Kupffer cell-derived Rac1 does not play a significant role in the acute TNF-α response following hepatic I/R, since hepatocyte-specific deletion of Rac1 on a Rac2-KO background attenuates nearly all the I/R-induced TNF-α response (Fig. 4D). It is presently unclear whether Rac2 can partially compensate for the lack of Rac1 in hepatocytes, but this appears unlikely given that only Rac1, but not Rac2, is found in hepatocyte plasma membranes (5). Despite the fact that Rac2 deletion alone failed to attenuate acute-phase TNF-α following I/R, Rac2 and NOX2 in the Kupffer cell certainly play a role in modulating the TNF-α response. As discussed in Fig. 6, we hypothesize that early hepatocyte-derived TNF-α responses following I/R lead to amplification of hepatic TNF-α by Kupffer cells and potentially other hepatic sources. In this context, knocking out the hepatocyte-dominated early TNF-α response following I/R as demonstrated in AlbCRE-Rac1 KO animals attenuates downstream proinflammatory responses by Kupffer cells and/or other cell types in the liver.
In summary, these studies have demonstrated the importance of NADPH oxidases and their known activators in acute-phase TNF-α production following partial hepatic I/R injury. Our studies in primary hepatocytes demonstrate that c-Src- and NOX-dependent pathways play important roles in secretion of TNF-α by the hepatocyte following reoxygenation injury. Thus redox-dependent pathways involving c-Src, Rac, p47phox, and NOX1/2 control, at least in part, acute-phase NF-κB and TNF-α responses following hepatic I/R injury, and the hepatocyte appears to be directly involved in these responses.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.Y.S., Z.Y., T.J.L., and J.F.E. conception and design of research; N.Y.S., W.Z., Q.L., Y.Z., M.L., T.J.L., and D.A. performed experiments; N.Y.S., W.Z., Q.L., M.L., Z.Y., D.A., B.B., and J.F.E. analyzed data; N.Y.S., Q.L., Z.Y., B.B., and J.F.E. interpreted results of experiments; N.Y.S., Z.Y., T.J.L., and J.F.E. prepared figures; N.Y.S. and J.F.E. drafted manuscript; N.Y.S., Z.Y., B.B., and J.F.E. edited and revised manuscript; N.Y.S., W.Z., Q.L., Y.Z., M.L., Z.Y., T.J.L., D.A., B.B., and J.F.E. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK051315 and K01 DK083429), the Animal Models Core funded through the Center for Gene Therapy (P30 DK54759), and the Roy J. Carver Chair in Molecular Medicine.
REFERENCES
- 1. Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem 272: 20389– 20394, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Bhogal RH, Curbishley SM, Weston CJ, Adams DH, Afford SC. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl 16: 1303– 1313, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia-reperfusion injury. Am J Transplant 6: 652– 658, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Chen YX, Sato M, Kawachi K, Abe Y. Neutrophil-mediated liver injury during hepatic ischemia-reperfusion in rats. Hepatobiliary Pancreat Dis Int 5: 436– 442, 2006 [PubMed] [Google Scholar]
- 5. Diaz-Cruz A, Vilchis-Landeros MM, Guinzberg R, Villalobos-Molina R, Pina E. NOX2 activated by α1-adrenoceptors modulates hepatic metabolic routes stimulated by β-adrenoceptors. Free Radic Res 45: 1366– 1378, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Fan C, Li Q, Ross D, Engelhardt JF. Tyrosine phosphorylation of IκBα activates NFκB through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem 278: 2072– 2080, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Fan C, Li Q, Zhang Y, Liu X, Luo M, Abbott D, Zhou W, Engelhardt JF. IκBα and IκBβ possess injury context-specific functions that uniquely influence hepatic NF-κB induction and inflammation. J Clin Invest 113: 746– 755, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faulkner K, Fridovich I. Luminol and lucigenin as detectors for O2. Free Radic Biol Med 15: 447– 451, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Frankenberg MV, Weimann J, Fritz S, Fiedler J, Mehrabi A, Buchler MW, Kraus TW. Gadolinium chloride-induced improvement of postischemic hepatic perfusion after warm ischemia is associated with reduced hepatic endothelin secretion. Transpl Int 18: 429– 436, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett 580: 497– 504, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell 19: 2984– 2994, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal 2: ra54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gianni D, Taulet N, DerMardirossian C, Bokoch GM. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell 21: 4287– 4298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med 49: 516– 527, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol 170: 5652– 5657, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20: 2175– 2183, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Guo F, Cancelas JA, Hildeman D, Williams DA, Zheng Y. Rac GTPase isoforms Rac1 and Rac2 play a redundant and crucial role in T-cell development. Blood 112: 1767– 1775, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harada H, Hines IN, Flores S, Gao B, McCord J, Scheerens H, Grisham MB. Role of NADPH oxidase-derived superoxide in reduced size liver ischemia and reperfusion injury. Arch Biochem Biophys 423: 103– 108, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal 14: 893– 907, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF. Tyrosine phosphorylation of IκB-α activates NF-κ B without proteolytic degradation of IκB-α. Cell 86: 787– 798, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med 182: 751– 758, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando) 26: 103– 114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181– 189, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30: 653– 661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li JY, Gu X, Zhang WH, Jia S, Zhou Y. GdCl3 abates hepatic ischemia-reperfusion injury by inhibiting apoptosis in rats. Hepatobiliary Pancreat Dis Int 8: 518– 523, 2009 [PubMed] [Google Scholar]
- 26. Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol 26: 140– 154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-κB by TNF-α. Antioxid Redox Signal 11: 1249– 1263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Q, Zhang Y, Marden JJ, Banfi B, Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J 411: 531– 541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem 273: 2015– 2023, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Liu PG, He SQ, Zhang YH, Wu J. Protective effects of apocynin and allopurinol on ischemia-reperfusion-induced liver injury in mice. World J Gastroenterol 14: 2832– 2837, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Llacuna L, Mari M, Lluis JM, Garcia-Ruiz C, Fernandez-Checa JC, Morales A. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-κB inactivation in prolonged ischemia-reperfusion. Am J Pathol 174: 1776– 1785, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marden JJ, Zhang Y, Oakley FD, Zhou W, Luo M, Jia HP, McCray PB, Jr, Yaniv M, Weitzman JB, Engelhardt JF. JunD protects the liver from ischemia-reperfusion injury by dampening AP-1 transcriptional activation. J Biol Chem 283: 6687– 6695, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal 11: 1313– 1333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ozaki M, Deshpande SS, Angkeow P, Bellan J, Lowenstein CJ, Dinauer MC, Goldschmidt-Clermont PJ, Irani K. Inhibition of the Rac1 GTPase protects against nonlethal ischemia-reperfusion-induced necrosis and apoptosis in vivo. FASEB J 14: 418– 429, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9: 202– 209, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J Biol Chem 274: 305– 315, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 10: 183– 196, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Jr, Ritchie TC, Hunninghake GW, Zandi E, Engelhardt JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-α secretion through IKK regulation of NF-κB. J Biol Chem 276: 30188– 30198, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64: 693– 702, 1991 [DOI] [PubMed] [Google Scholar]
- 40. Spencer NY, Yan ZY, Boudreau RL, Zhang YL, Luo MH, Li QA, Tian X, Shah AM, Davisson RL, Davidson B, Banfi B, Engelhardt JF. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J Biol Chem 286: 8977– 8987, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teoh N, Field J, Sutton J, Farrell G. Dual role of tumor necrosis factor-α in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-α gene knockout mice. Hepatology 39: 412– 421, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Teoh N, Leclercq I, Pena AD, Farrell G. Low-dose TNF-α protects against hepatic ischemia-reperfusion injury in mice: implications for preconditioning. Hepatology 37: 118– 128, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 23: 981– 987, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia-reperfusion. Hepatology 33: 902– 914, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Zwacka RM, Zhang Y, Zhou W, Halldorson J, Engelhardt JF. Ischemia-reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor κB independently of IκB degradation. Hepatology 28: 1022– 1030, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Zwacka RM, Zhou W, Zhang Y, Darby CJ, Dudus L, Halldorson J, Oberley L, Engelhardt JF. Redox gene therapy for ischemia-reperfusion injury of the liver reduces AP1 and NF-κB activation. Nat Med 4: 698– 704, 1998 [DOI] [PubMed] [Google Scholar]