Abstract
Decreased bone mineral density (BMD) represents an extraintestinal complication of inflammatory bowel disease (IBD). Vitamin D3 has been considered a viable adjunctive therapy in IBD. However, vitamin D3 plays a pleiotropic role in bone modeling and regulates the bone formation-resorption balance, depending on the physiological environment, and supplementation during active IBD may have unintended consequences. We evaluated the effects of vitamin D3 supplementation during the active phase of disease on colonic inflammation, BMD, and bone metabolism in an adoptive IL-10−/− CD4+ T cell transfer model of chronic colitis. High-dose vitamin D3 supplementation for 12 days during established disease had negligible effects on mucosal inflammation. Plasma vitamin D3 metabolites correlated with diet, but not disease, status. Colitis significantly reduced BMD. High-dose vitamin D3 supplementation did not affect cortical bone but led to a further deterioration of trabecular bone morphology. In mice fed a high vitamin D3 diet, colitis more severely impacted bone formation markers (osteocalcin and bone alkaline phosphatase) and increased bone resorption markers, ratio of receptor activator of NF-κB ligand to osteoprotegrin transcript, plasma osteoprotegrin level, and the osteoclast activation marker tartrate-resistant acid phosphatase (ACp5). Bone vitamin D receptor expression was increased in mice with chronic colitis, especially in the high vitamin D3 group. Our data suggest that vitamin D3, at a dose that does not improve inflammation, has no beneficial effects on bone metabolism and density during active colitis or may adversely affect BMD and bone turnover. These observations should be taken into consideration in the planning of further clinical studies with high-dose vitamin D3 supplementation in patients with active IBD.
Keywords: inflammatory bowel disease; bone mineral density; 1,25-dihydroxyvitamin D3; bone turnover
inflammatory bowel disease (IBD) is characterized by an inappropriate and persistent immune response against commensal intestinal microbiota and by intestinal inflammation and mucosal damage. However, this chronic inflammatory disease can also affect bone metabolism and bone mineral density (BMD), with osteopenia and osteoporosis as the two major extraintestinal symptoms. Low BMD has been reported in Crohn's disease (CD) and ulcerative colitis (UC). The relative risk of fracture is 40% higher in the IBD patient than in the general population (3), and although this has not been systematically studied, it is expected that children with IBD do not reach the optimal peak bone mass in early adulthood. The prevalence of osteopenia and osteoporosis in IBD varies depending on the population studied, geographic location, and study design, with a range of 22–77% for osteopenia and 17–41% for osteoporosis (3).
Proinflammatory mediators, poor nutritional status, and malabsorption affecting vitamin D3, as well as vitamin K and Ca2+ homeostasis and hormonal factors, have been postulated to be the cause of altered bone morphology and metabolism. Vitamin D3 insufficiency or deficiency {based on 25-hydroxyvitamin D3 [25(OH)D3] measurement} has been reported in children and adult patients with CD and UC (40, 43).
Vitamin D3 (cholecalciferol) is supplied from the diet or synthetized in the skin as pre-vitamin D3 from 7-dehydrocholesterol by UV irradiation. Pre-vitamin D3 spontaneously isomerizes into vitamin D3, which is transported in the blood by vitamin D-binding protein to the liver, where it is hydroxylated to 25(OH)D3. 25(OH)D3 is transported to the kidneys and further hydroxylated by 1α-hydroxylase (Cyp27b1), resulting in formation of the bioactive form, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]. This process is tightly regulated by parathyroid hormone (PTH), which is regulated by serum Ca2+ concentration. Catabolism of 1,25(OH)2D3 and 25(OH)D3 by renal 24-hydroxylase (Cyp24a1) leads to formation of 1,24,25(OH)3D3 and 24,25(OH)2D3, respectively, which decreases 1,25(OH)2D3 biological activity or the pool of available 25(OH)D3.
Vitamin D3 increases intestinal and renal Ca2+ and Pi absorption and is typically considered an anabolic hormone that positively affects osteoblast differentiation and bone matrix synthesis. Moreover, its immunomodulatory effects, including promotion of macrophage antimicrobial responses to pathogens, regulation of dendritic cell maturation, and indirect and direct effects on effector and regulatory T cell functions, recently extensively reviewed by Hewison (20), suggest that innate and adaptive immune functions may be altered under conditions of vitamin D3 insufficiency and that vitamin D3 may be considered a viable adjunctive therapy in IBD. However, a more appropriate view of the role of vitamin D3 in bone metabolism is that of a factor regulating bone remodeling that can direct the bone formation-resorption balance, depending on the physiological/pathophysiological context. Vitamin D3 induces expression of the receptor activator of NF-κB (RANK) during macrophage-osteoclast differentiation (23), as well as RANK ligand (RANKL) on osteoblasts, and this latter effect is potentiated by IL-1β (29). While vitamin D3 also induces the expression of osteoprotegerin (OPG), which serves as a decoy receptor for RANKL and reduces its effects on osteoclasts and bone resorption, it can also induce expression of factors facilitating the attachment of multinucleated osteoclasts to the mineral matrix, such as αvβ3-integrin (33), osteopontin (32), and matrix metalloproteinase-13 (45). Since cytokines typically associated with active inflammation (IL-1β, IL-6, and TNFα) may act synergistically with vitamin D3 to negatively regulate bone turnover, there is a risk that high-dose vitamin D3 supplementation in active UC or CD may lead to a paradoxical BMD loss. Elevated levels of circulating 1,25(OH)2D3 and increased extrarenal expression of CYP27B1 in a significant fraction of adult (1) and pediatric (13) IBD patients with osteopenia or osteoporosis strongly suggest the need for further research.
In this report, we describe negligible effects on mucosal inflammation, no improvement in loss of cortical bone, and further loss of trabecular bone density as a result of 12 days of vitamin D3 supplementation during established disease in an adoptive IL-10−/− CD4+ T cell transfer model of chronic colitis. Adverse effects of vitamin D3 on BMD in this murine model should be taken into consideration in planning further clinical trials of high-dose vitamin D3 supplementation in IBD patients.
MATERIALS AND METHODS
Diets
The standard open formula mouse diet (7017 NIH-31, Harlan Teklad, Madison, WI) contains 4,200 IU/kg vitamin D3, which when calculated to human equivalency dose (HED) would be considered high. Therefore, the experimental diets (also based on the NIH-31 modified open formula) contained 1,000 or 5,000 IU/kg vitamin D3 (Harlan Teklad); on the basis of the estimated food consumption, vitamin D3 daily dose was calculated as HED of 730 and 3,648 IU/60 kg, respectively (35). Although daily intake of 730 IU of vitamin D3 would be considered adequate in humans, for the purpose of clear distinction, these two diets are referred to as “low vitamin D3” and “high vitamin D3,” respectively.
Experimental Animals
Specific pathogen-free Rag1−/− mice on a C57BL/6 background (Jackson Laboratory, Bar Harbor, ME) were obtained at 4 wk of age and maintained on the low vitamin D3 diet for 4 wk prior to induction of colitis. Adoptive T cell transfer colitis was induced by intraperitoneal injection of 0.5 × 106 CD4+ T cells magnetically selected (Miltenyi Biotech, Auburn, CA) from the spleen of IL-10−/− (C57BL/6) mice (Jackson Laboratory) (7). Control (PBS-injected) and colitic mice were maintained on the low vitamin D3 diet for 2 wk. T cell-transferred mice were then fed the low vitamin D3 diet supplemented with 240 ppm piroxicam for 7 days to enhance and synchronize colitis. After piroxicam treatment, mice were randomized to two groups receiving the same low or high vitamin D3 diet (both without piroxicam) and euthanized 12 days later. A schematic of the model is included in Fig. 1. Over the course of the experiment, body weight was monitored and serum was collected from the tail vein for serum amyloid A (SAA) assay. At the time of euthanization, tissues were collected for analyses, and the terminal bleed was carried out by cardiac puncture. Sentinel mice were routinely monitored and determined to be free from common murine pathogens (murine hepatitis virus, murine parvovirus, murine minute virus, Theiler's murine encephalomyelitis virus, Mycoplasma pulmonis, Sendai virus, epizootic diarrhea of infant mice, murine norovirus, and ecto- and endoparasites). Animal use protocols were approved by the University of Arizona Animal Care and Use Committee.
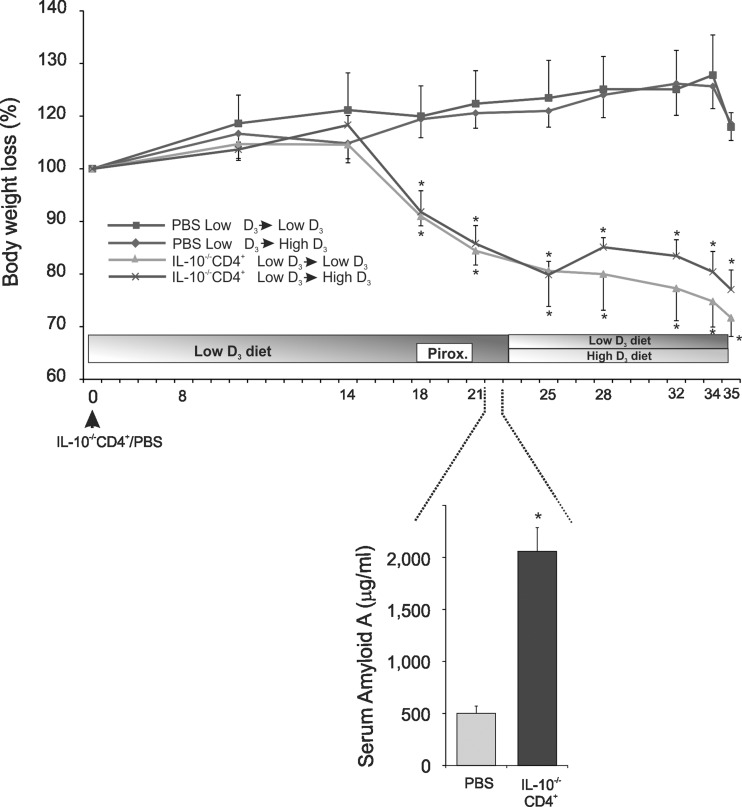
Fig. 1.
Body weight loss and serum amyloid A concentration in control (PBS-injected) mice (n = 5) and IL-10−/− CD4+ T cell-transferred mice (n = 7) maintained on the low vitamin D3 diet or switched to the high vitamin D3 diet. Top: timeline of the experimental design. Bottom: serum amyloid A concentration as a systemic marker of inflammation at the end of piroxicam treatment. Values are means ± SE. *P < 0.05 (by t-test).
Histology and Scoring
Proximal and distal colons from experimental mice were harvested and fixed in 10% neutral buffered formalin (Fisher Scientific, Tustin, CA). Tissues were then embedded in paraffin, and 5-μm-thick sections were stained with hematoxylin and eosin for microscopic examination (magnification ×100). Sections were graded by a veterinary pathologist blinded to the study design according to previously published criteria (24).
Real-Time RT-PCR
Real-time RT-PCR was used to evaluate colonic expression of TNF, IFNγ, and IL-1β mRNA and bone (femur) expression of RANKL, OPG, tartrate-resistant acid phosphatase (ACp5), osteocalcin [bone γ-carboxyglutamate (gla) protein (BgLap)], bone alkaline phosphatase (Alpl), vitamin D receptor (VDR), and Cyp24a1, as well as renal Cyp27b1 and Cyp24a1. Total RNA was isolated from mouse colon, kidneys, or bones, pulverized under liquid nitrogen using TRIzol reagent (Life Technologies), checked for integrity, and subjected to reverse transcription using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) followed by PCR with IQ Supermix (Bio-Rad) and TaqMan primer/probe sets (Applied Biosystems, Foster City, CA) using the Bio-Rad CFX96 real-time PCR detection system. Data were analyzed using the comparative cycle threshold (Ct) method as the means of relative quantification, normalized to an endogenous reference (GAPDH) and relative to a calibrator (normalized Ct value obtained from control mice), and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin 2, Revision B: “Relative Quantification of Gene Expression”).
Blood Plasma Analysis
SAA protein was evaluated by ELISA in individual mice according to the manufacturer's instruction (Life Technologies, Grand Island, NY). Plasma concentrations of osteocalcin and OPG were evaluated using the xMAP assay (Millipore, Billerica, MA) and Luminex 100 platform (Liquichip, Qiagen, Valencia, CA). Plasma levels of intact PTH-(1–84) and fibroblast growth factor 23 (FGF23) were evaluated by ELISA according to the manufacturer's protocols [Ids (Scottsdale, AZ); Immutopics (San Clemente, CA) and Kamiya Biomedical (Seattle, WA), respectively].
Liquid Chromatography-Tandem Mass Spectroscopy Analysis of Vitamin D3 Metabolites
The method for quantitative analysis of vitamin metabolites by liquid chromatography-tandem mass spectroscopy (LC-MS/MS) was adapted from Ding et al. (11).
Sample preparation.
Five hundred microliters of precipitation solution [4:1 (vol/vol) acetonitrile-isopropanol] with stable isotope (deuterium)-labeled D6–1,25(OH)2D3 (final concentration 10 ng/l), D3–25(OH)D3 (final concentration 10 μg/l), D3–25(OH)D2 (final concentration 10 μg/l), and 0.1% formic acid were placed in a 5-ml glass tube, and 100 μl of plasma were added to each tube. The solution was extracted with 1-chlorobutane and centrifuged. The supernatant was dried down under nitrogen at 35°C. The dry residue was resuspended in 200 μl of acetonitrile containing 0.25 g/l 4-phenyl-1,2,4-triazoline-3,5-dione. The solution was mixed carefully and left for 30 min at room temperature in darkness. After transfer to autosampler vials, the samples were analyzed for 1,25(OH)2D3 and then diluted 10-fold for quantification of 25(OH)D3 and 24,25(OH)D3.
LC-MS/MS analysis.
LC-MS/MS was performed on a HPLC system equipped with a Quattro TQ-S and an analytical column (100 mm × 2.1 mm × 1.7 μm; Acquity CSH, Waters, Eschborn, Germany).
Ultraperformance LC separation.
We used two buffers: 1) an aqueous buffer with 430 μl of methylamine and 100 μl of formic acid per liter and 2) methanol with 430 μl of methylamine and 100 μl of formic acid per liter. The gradient was developed to obtain a partial separation of both isomers generated by the derivatization, with 4-phenyl-1,2,4-triazoline-3,5-dione as an indicator of chromatography quality.
Micro-CT evaluation of cortical and trabecular bone density.
Micro-CT (μCT) analysis of mouse femurs was performed by the Rush University μCT Core Lab (Chicago, IL). Briefly, a desktop cone-beam μCT scanner (model 40, Scanco Medical, Brüttisellen, Switzerland) with μCT Evaluation Program version 6.0 was used to gather trabecular and cortical bone data; 70% ethanol was used as scanning medium. Samples were scanned horizontally in a 20.5-mm tube (with plastic holder; 5 femurs per tube, ∼300 slices per sample). Femurs were then rotated to transverse slices and analyzed. Calculations were made to analyze trabecular bone in the femur region between an arbitrary line at the distal 30% of the femur and the distal growth plate (∼250–450 slices). Cortical bone was measured at the midshaft (50% of the length of the femur), and then 50 slices were included on either side (100 slices total). Contours (endocortical surface, counterclockwise) for trabecular bone were drawn by hand, every 15 slices, and then morphed. Trabecular bone was analyzed with the trabecular bone script (#38) at a threshold of 250. Contours for cortical bone were drawn using the midshaft contour script (#16), and each was inspected and modified for best fit before analysis. Cortical bone was analyzed with the midshaft cortical bone script (#23) with multiple thresholds to gauge best fit, and a threshold of 300 was selected as the most appropriate.
Statistical Analysis
Statistical significance was determined by Student's t-test or ANOVA followed by Fisher's protected least significant difference post hoc test, as appropriate, using SigmaPlot version 12 (Systat, Chicago, IL). Values are means ± SE.
RESULTS
Vitamin D Supplementation Has a Limited Protective Effect in Established Colitis
Adoptive transfer of CD4+ T cells from IL-10−/− mice into syngeneic T and B cell-deficient recipients and synchronization by dietary exposure to piroxicam induces moderate-to-severe colitis within 6–8 wk, with symptoms resembling human IBD. Body weight loss and SAA (an early marker of the acute phase of inflammation) concentration were used as an indicator of established disease prior to randomization into dietary groups (Fig. 1).
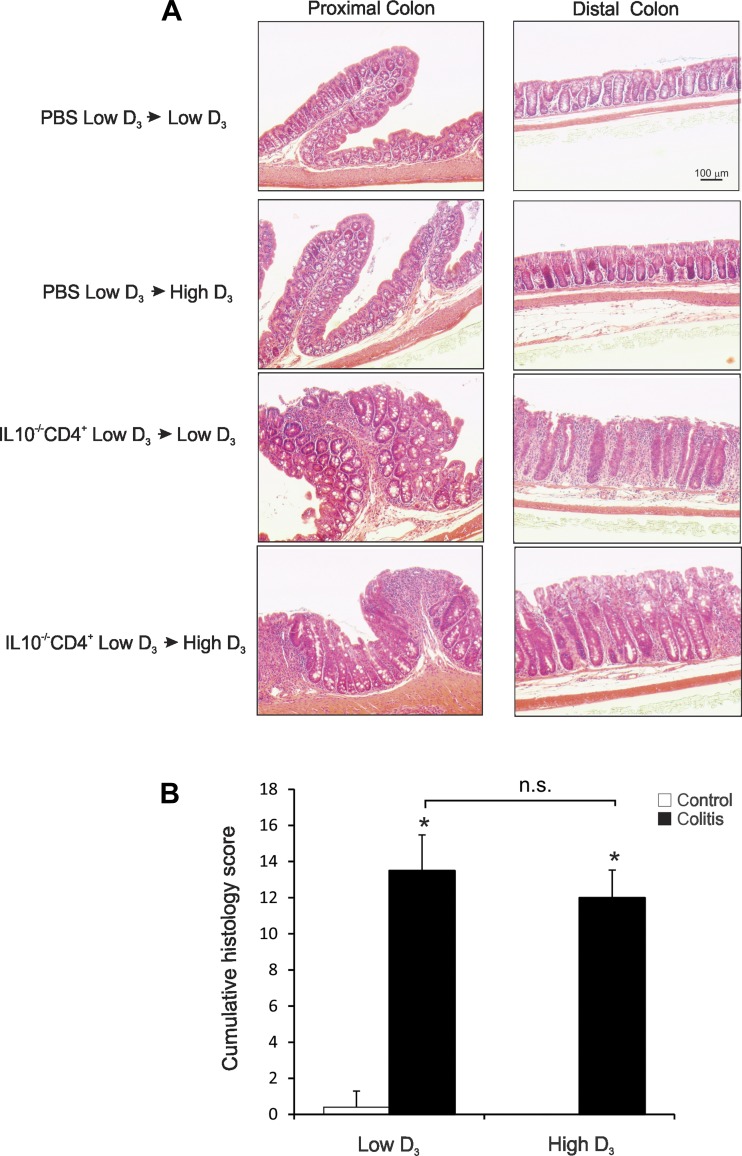
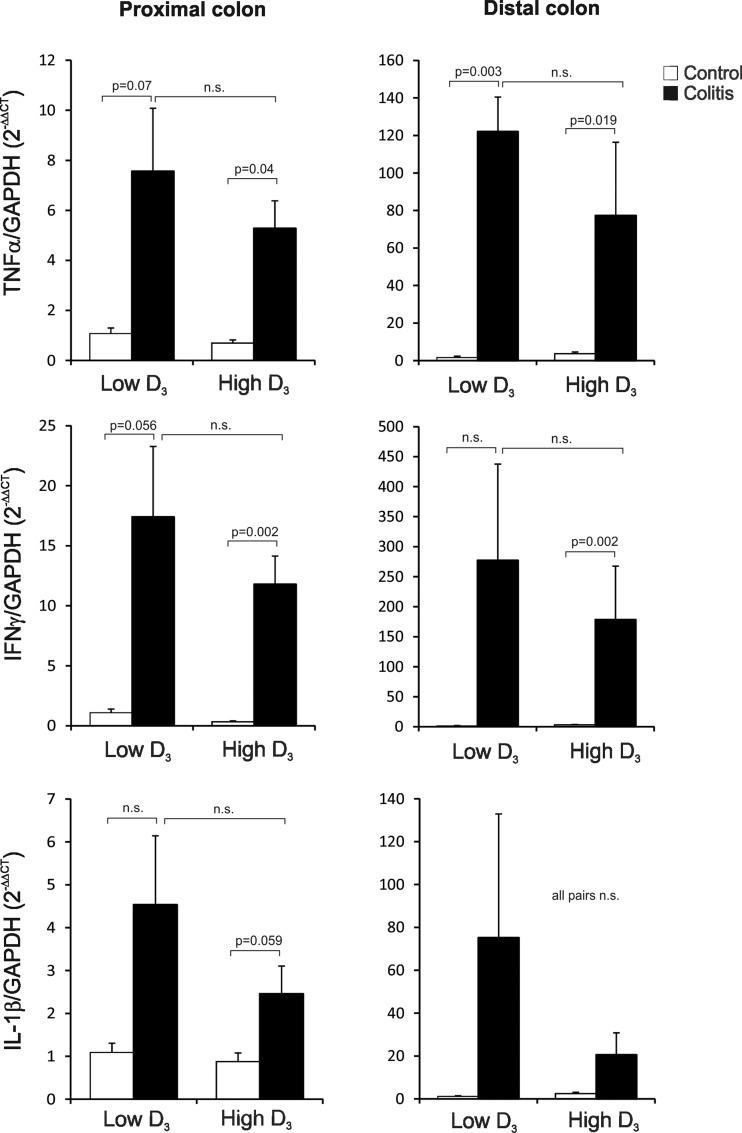
Development of robust colitis at the end of the study was confirmed by colonic histology scoring (Fig. 2) and colonic expression of TNF, IFNγ, and IL-1β mRNA (Fig. 3). The high vitamin D3 diet did not affect body weight in control mice and did not significantly improve body weight loss in colitic mice (Fig. 1). Morphology of the proximal and distal colon was analyzed. Proximal and distal colon of the control mice demonstrated a very mild “physiological inflammation” typical of mice housed under conventional conditions. Histological analysis of the proximal and distal colon of IL-10−/− T cell recipients showed a characteristic pattern associated with crypt hyperplasia, lymphocytic and granulocytic infiltration, and occasional mucosal ulcerations (Fig. 2A). The high vitamin D3 diet did not improve the histological signs of colonic inflammation, as evaluated by an unbiased pathologist (Fig. 2). Moreover, high-dose vitamin D3 supplementation had no statistically significant effect on colonic TNF, IFNγ, and IL-1β transcript levels in proximal and distal colon (Fig. 3).
Fig. 2.
Histology of the proximal and distal colon of control and IL-10−/− CD4+ T cell-transferred mice maintained on the low vitamin D3 diet or switched to the high vitamin D3 diet. A: histological analysis (hematoxylin-eosin staining) of colon morphology in control and T cell-transferred mice on the low or high vitamin D3 diet. Scale bar applies to all images. B: cumulative histology score for the proximal and distal colon, on a scale of 0–5 based on the degree of lamina propria mononuclear cell infiltration, crypt hyperplasia, and architectural distortion, by an unbiased pathologist according to previously described criteria (24). Values are means ± SE; n = 5 and 7 for control and colitis groups, respectively. *P ≤ 0.05 vs. control [by ANOVA followed by Fisher's protected least significant difference (PLSD) post hoc test]; ns, not significant.
Fig. 3.
Real-time PCR analysis of TNFα, IFNγ, and IL-1β mRNA expression in the proximal and distal colon of control and IL-10−/− CD4+ T cell-transferred mice maintained on the low vitamin D3 diet or switched to the high vitamin D3 diet. Data were normalized to GAPDH mRNA as an internal control and calculated using the ΔΔCt method, with control mice on the low vitamin D3 diet used as a calibrator. Values are means ± SE; n = 5 and 7 for control and colitis groups, respectively. Statistical analysis was performed with ANOVA followed by Fisher's PLSD post hoc test, with P ≤ 0.05 considered significant.
Vitamin D Metabolism
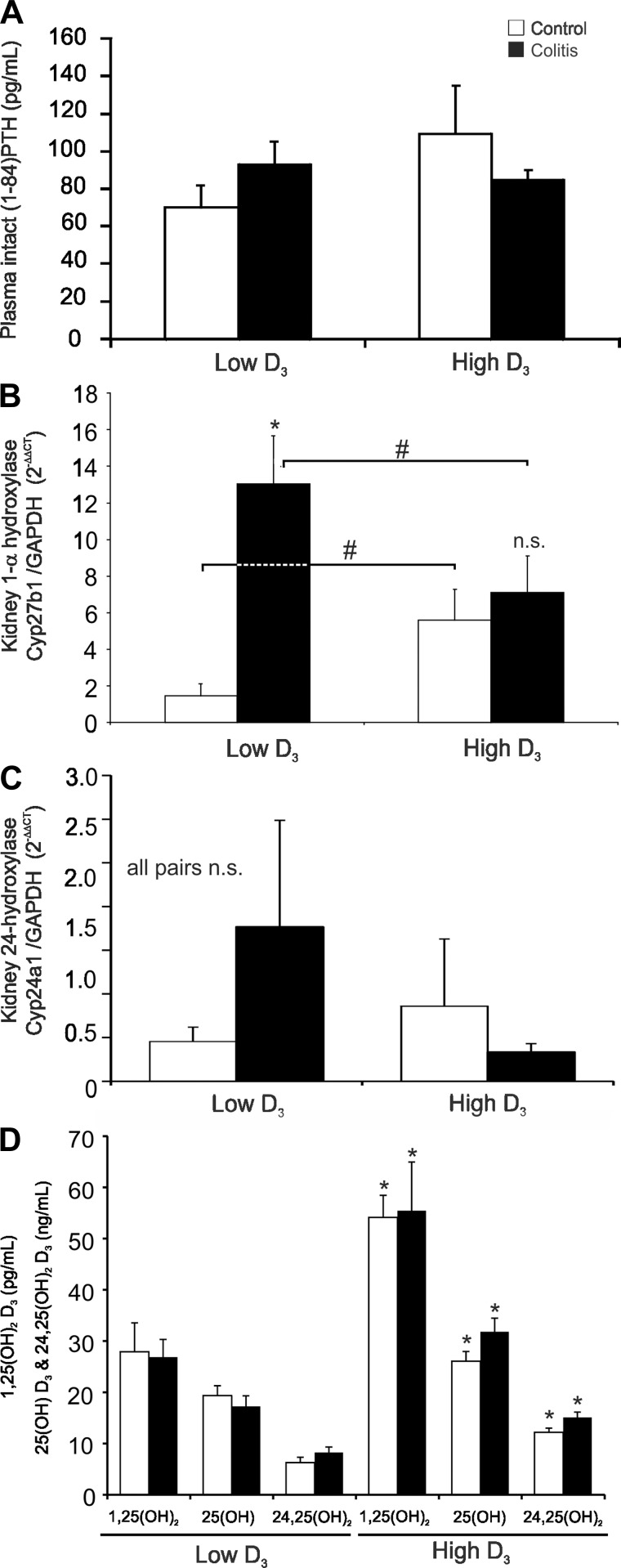
Similar to results from studies of IBD patients (1, 39), we did not observe statistically significant differences in plasma levels of intact PTH-(1–84) among the dietary or treatment groups (Fig. 4A) or significant changes in serum Ca2+ level among treatment groups (data not shown).
Fig. 4.
Vitamin D3 metabolism in control and IL-10−/− CD4+ T cell-transferred mice maintained on the low vitamin D3 diet or switched to the high vitamin D3 diet. A: measurement of plasma concentration of parathyroid hormone (PTH) by ELISA. B and C: real-time RT-PCR analysis of 1α-hydroxylase (Cyp27b1) and 24-hydroxylase (Cyp24a1) mRNA expression in the kidney. Data were normalized to GAPDH mRNA as an internal control and calculated using the ΔΔCt method, with control mice on the low vitamin D3 diet used as a calibrator. *Control vs. colitis; #low D3 vs. high D3 in control or colitic mice, respectively. D: quantitative assessment of vitamin D3 metabolites by liquid chromatography-tandem mass spectroscopy in the plasma of control and IL-10−/− CD4+ T cell-transferred mice on the low or high vitamin D3 diet. *Low D3 vs. high D3 for the respective metabolite. Values are means ± SE; n = 5 and 7 for control and colitis groups, respectively. ANOVA followed by Fisher's PLSD post hoc test.
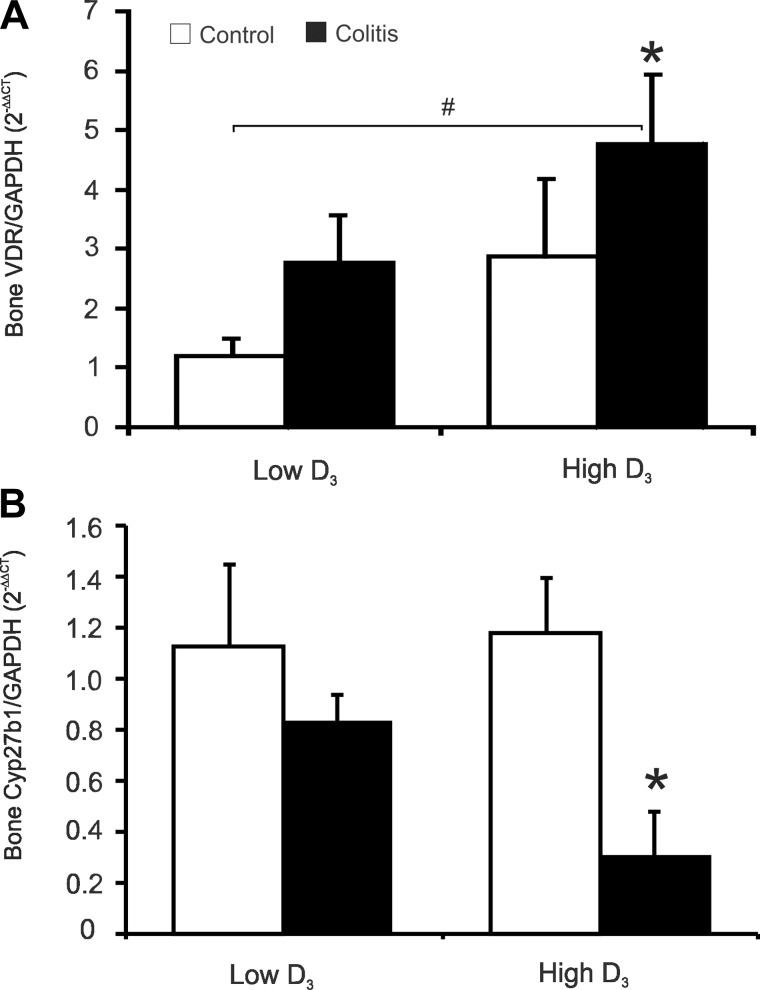
Abreu et al.(1) demonstrated increased expression of Cyp27b1 in the inflamed intestinal tissue, which was postulated to drive the higher conversion rate and elevated levels of 1,25(OH)2D3 in a significant subset of patients, which correlated with low lumbar BMD. However, renal expression of Cyp27b1 in colitis patients has not been evaluated. In PBS-injected Rag1−/− mice switched to the high vitamin D3 diet, expression of Cyp27b1 increased (Fig. 4B). Intriguingly, development of colitis was associated with a greater increase in renal Cyp27b1 mRNA expression, but only in mice fed the low vitamin D3 diet (Fig. 4B). We also analyzed mRNA expression of the renal catabolic hydroxylase Cyp24a1, but because of high animal-to-animal variation, we were not able to demonstrate statistically significant differences or trends. To evaluate whether this increase in renal Cyp27b1 expression would translate into higher plasma levels of 1,25(OH)2D3 as a function of colitis, we analyzed selected vitamin D3 metabolites by LC-MS/MS in our mice. The levels of 1,25(OH)2D3, 25(OH)D3, and 24,25(OH)2D3 were consistently increased in mice switched to the high vitamin D3 diet but were unrelated to the development of colitis (Fig. 4C).
Colitis Reduces BMD and High Vitamin D3 Diet Negatively Affects Trabecular Bone Morphology
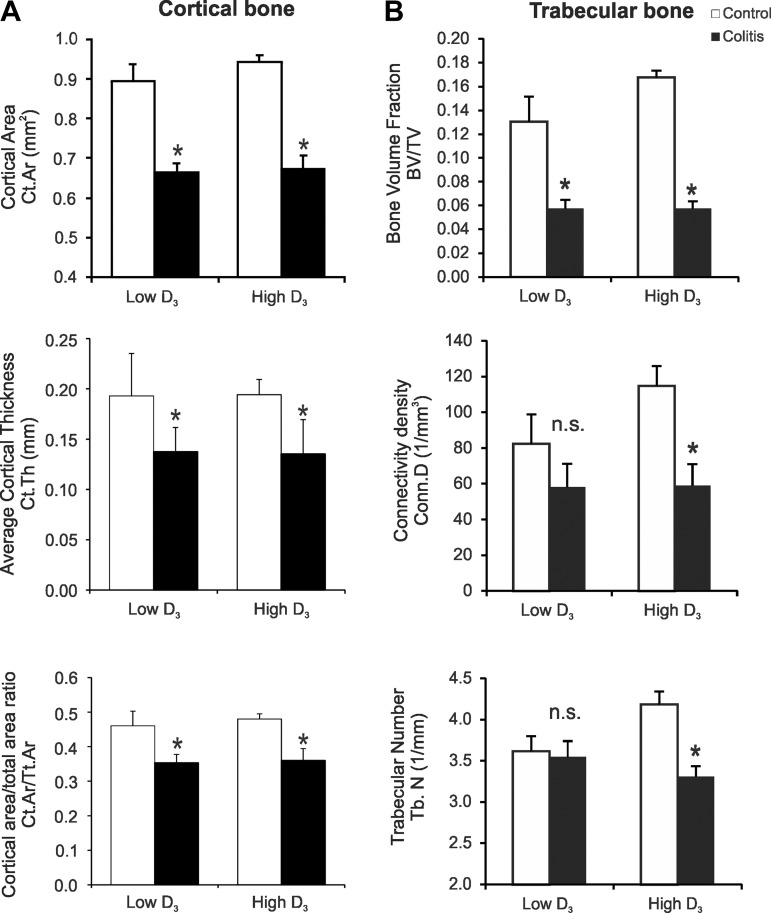
Using μCT, we analyzed the microarchitecture of control and colitic mice maintained on the low vitamin D3 diet or switched to the high vitamin D3 diet. Selection of parameters and terminology were based on recently published guidelines for μCT assessment of bone microstructure in rodents (8). Colitis was associated with a significant loss of cortical bone density (Fig. 5A), as evaluated by total cortical area (Ct.Ar), average cortical thickness, and cortical area-to-total bone area ratio (Ct.Ar/Tt.Ar) (Fig. 6A). There were no significant changes in total bone area (Tt.Ar), thus confirming a uniform initial group of animals (data not shown). Administration of the high vitamin D3 diet for 12 days did not affect cortical bone morphology in control or colitic mice (Fig. 6A). Changes in trabecular bone are usually more dynamic. The high vitamin D3 diet did not significantly affect trabecular bone parameters (Fig. 6B). In mice fed the low vitamin D3 diet, colitis significantly affected trabecular bone volume fraction (ratio of bone volume to trabecular volume), but it remained without effect on connectivity density or trabecular number (Figs. 5B and 6B). In colitic mice switched to the high vitamin D3 diet, however, connectivity density and trabecular number significantly decreased (Fig. 6B), thus demonstrating that introduction of the high vitamin D3 diet during established and active inflammation is detrimental to the trabecular bone.
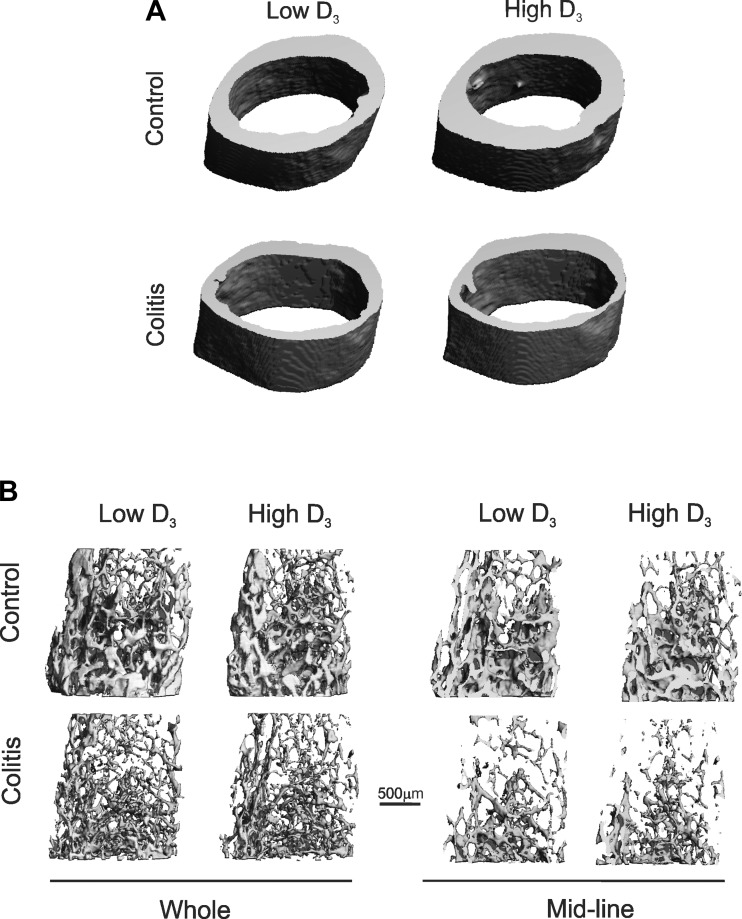
Fig. 5.
Trabecular and cortical bone morphology in control and IL-10−/− CD4+ T cell-transferred mice on the low or high vitamin D3 diet. Representative micro-CT (μCT) 3-dimensional reconstruction images show cortical bone of the femoral midshaft (A) and trabecular bone of the distal femoral metaphysis [B: complete thickness (whole) and midline (sagittal)].
Fig. 6.
Bone mineral density in control and IL-10−/− CD4+ T cell-transferred mice on the low or high vitamin D3 diet. A: evaluation of cortical bone density by measurement of cortical area (Ct.Ar.), average cortical thickness (Ct.Th), and cortical area-to-total area ratio (Ct.Ar/Tt.Ar.). B: evaluation of trabecular bone density by quantitative assessment of bone volume fraction [ratio of bone volume to trabecular volume (BV/TV)], connectivity density (Conn.D), and trabecular number (Tb.N). Values are means ± SE; n = 5 and 7 for control and colitis groups, respectively. *P ≤ 0.05 vs. control (by ANOVA followed by Fisher's PLSD post hoc test).
Decoupling of Bone Formation and Resorption During Colitis is Exacerbated by High Vitamin D3 Diet
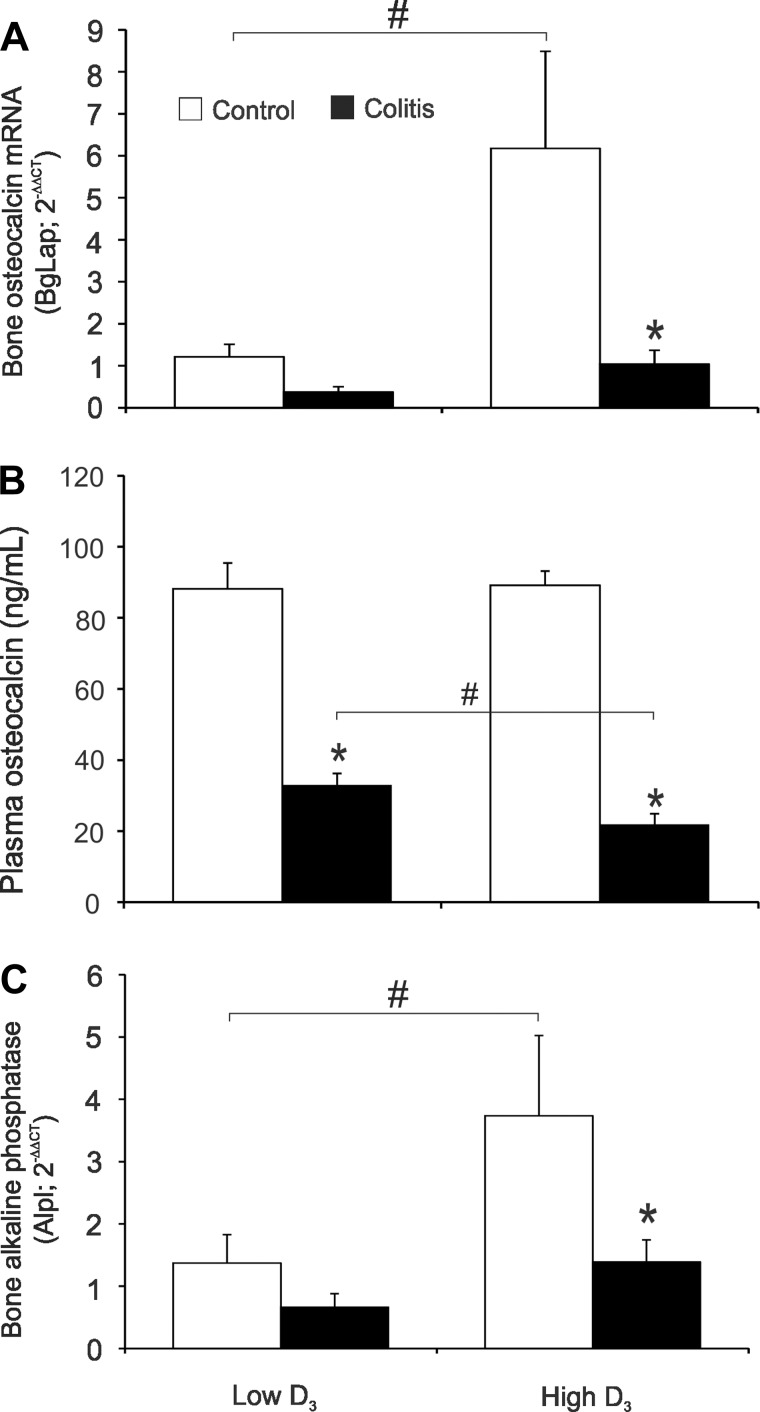
Bone is continuously being remodeled by coordinated bone formation and resorption. This coupled process is highly regulated to ensure the maintenance of skeletal integrity. Osteocalcin is the most abundant (∼15%) noncollagenous protein of the bone extracellular matrix secreted by osteoblasts and is accepted as a reliable marker of bone formation (10). Bone osteocalcin (BgLap) mRNA significantly increased in healthy mice switched to the high vitamin D3 diet (Fig. 7A). Colitis decreased osteocalcin mRNA expression in both dietary groups, although a statically significant decrease was documented only in colitic mice switched to the high vitamin D3 diet (Fig. 7A). The effect of the high vitamin D3 diet on bone osteocalcin mRNA was not reflected in circulating plasma osteocalcin (Fig. 7B). However, in mice with colitis, the high vitamin D3 diet more potently downregulated circulating osteocalcin (Fig. 7B). Bone expression of another marker of bone formation, bone alkaline phosphatase (Alpl), followed the same pattern as osteocalcin transcript (Fig. 7C), thus indicating that, during active inflammation, vitamin D3 negatively affects bone formation.
Fig. 7.
Bone formation in control and colitic mice maintained on the low vitamin D3 diet or switched to the high vitamin D3 diet. A and C: real-time RT-PCR analysis of bone osteocalcin [bone γ-carboxyglutamate protein (BgLap)] and bone alkaline phosphatase (Alpl) mRNA expression. Data were normalized to GAPDH mRNA as an internal control and calculated using the ΔΔCt method, with control mice on the low vitamin D3 diet used as a calibrator. B: plasma concentration of osteocalcin. Values are means ± SE; n = 5 and 7 for control and colitis groups, respectively. *P ≤ 0.05 vs. control; #P ≤ 0.05, low D3 vs. high D3 (by ANOVA followed by Fisher's PLSD post hoc test).
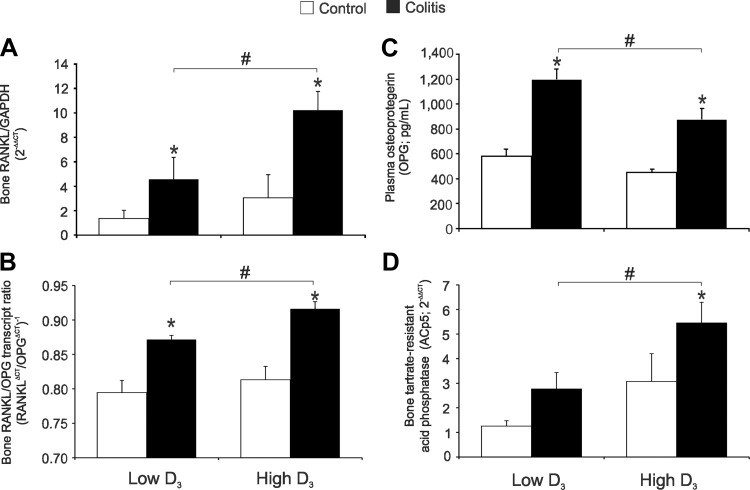
The RANK/RANKL/OPG pathway is critical in controlling the deposition of bone matrix and osteoclast function. RANKL is produced by osteoblasts, immune cells, and some cancer cells and binds to RANK (an osteoclast cell surface receptor) to stimulate osteoclastogenesis and bone resorption. OPG is secreted by osteoblasts as a decoy receptor for RANKL, prevents RANKL binding to RANK on osteoclasts, and reduces bone resorption. The RANK/RANKL/OPG system was recently reviewed by Silva and Branco (38). In our experimental system, colitis significantly increased expression of bone RANKL mRNA (Fig. 8A). Consistent with μCT analysis and bone turnover markers, this induction was significantly enhanced in mice switched to the high vitamin D3 diet (Fig. 8A). The RANKL-to-OPG transcript ratio followed a similar pattern (Fig. 8B). Moreover, although plasma OPG concentration was elevated in colitic mice fed the low vitamin D3 diet, this increase was significantly less prominent in mice fed the high vitamin D3 diet (Fig. 8C). In our model of colitis, soluble plasma RANKL level did not correlate with bone loss or disease severity (data not shown); therefore, the ratio of plasma RANKL to OPG proteins was intentionally not calculated. Consistent with this observation, bone expression of the osteoclast activation marker tartrate-resistant acid phosphatase (ACp5) increased as a result of colitis, but to a greater and statistically significant extent only in mice fed the high vitamin D3 diet (Fig. 8D). Collectively, the data imply that the high vitamin D3 diet is either not effective in improving bone metabolism or may lead to decreased bone formation and increased bone resorption during chronic active colitis.
Fig. 8.
Analysis of bone resorption markers in control and colitic mice maintained on the low vitamin D3 diet or switched to the high vitamin D3 diet. A, B, and D: real-time RT-PCR analysis of bone receptor activator of NF-κB ligand (RANKL), bone RANKL-to-osteoprotegrin (OPG) transcript ratio, and bone tartrate-resistant acid phosphatase (ACp5). Data were normalized to GAPDH mRNA as an internal control and calculated using the ΔΔCt method, with control mice on the low vitamin D3 diet used as a calibrator. C: plasma OPG concentration. Values are means ± SE; n = 5 and 7 for control and colitis groups, respectively. *P ≤ 0.05 vs. control; #P ≤ 0.05, low D3 vs. high D3 (by ANOVA followed by Fisher's PLSD post hoc test).
FGF23
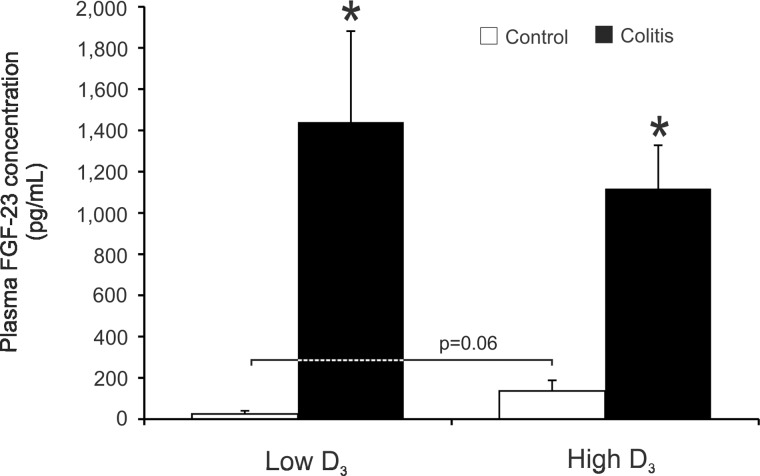
FGF23 is a phosphaturic hormone produced by osteoblasts under the control of vitamin D3 (26), and its serum levels correlate with 25(OH)D3 and 1,25(OH)2D3 concentrations (31, 36). In a recent report, serum FGF23 was significantly higher among pediatric patients with IBD during flare than in controls (13). Along with elevated serum 1,25(OH)2D3 and urinary phosphorus, FGF23 was a statistically significant determinant of low BMD in IBD patients (13). We observed a trend toward elevated plasma FGF23 concentration (5-fold increase in mean FGF23 level) in control mice fed the high vitamin D3 diet (Fig. 9; P = 0.06). Colitis resulted in a much greater (100-fold) increase in blood plasma FGF23 concentration and was not further affected by introduction of the high vitamin D3 diet in colitic mice (Fig. 9). The latter observation suggests that, at least in our animal model, there is no synergism between the effects of inflammatory mediators and vitamin D3 on FGF23 secretion.
Fig. 9.
Fibroblast growth factor 23 (FGF23) concentration in plasma of control and colitic mice maintained on the low vitamin D3 diet or switched to the high vitamin D3 diet evaluated by ELISA. Values are means ± SE; n = 5 and 7 for control and colitis groups, respectively. *P ≤ 0.05 vs. control; P = 0.06 indicates a statistical trend in low D3 vs. high D3 in the control group (by ANOVA followed by Fisher's PLSD post hoc test).
Bone VDR Expression During Chronic Colitis
Polymorphisms in the human VDR gene have been reported to be associated with IBD (48), and VDR gene knockout in mice has been shown to lead to exacerbated experimental colitis (15, 16, 25, 27). Wada et al. (47) also demonstrated decreased VDR expression in mucosal biopsies from UC patients without and with dysplasia or colorectal cancer, thus suggesting that inflammation leads to decreased mucosal VDR and may lead to exacerbated disease and, over the long-term, may contribute to IBD-associated colorectal cancer. The changes we observed in the bones of colitic mice switched to the high vitamin D3 diet suggest, however, that expression of bone VDR is not negatively affected by colitis. Consistent with this hypothesis, real-time quantitative PCR demonstrated an increase in bone VDR mRNA in mice with chronic colitis that was greater and statistically significantly different only in mice fed the high vitamin D3 diet (Fig. 10).
Fig. 10.
Effect of intestinal inflammation and dietary vitamin D3 on bone vitamin D receptor (VDR) and 1α-hydroxylase (Cyp27b1) expression. Real-time RT-PCR was used to analyze VDR and Cyp27b1 mRNA expression in bone of control or colitic mice maintained on the low vitamin D3 diet or switched to the high vitamin D3 diet. Data were normalized to GAPDH mRNA as an internal control and calculated using the ΔΔCt method, with control mice on the low vitamin D3 diet used as a calibrator. Values are means ± SE; n = 5 and 7 for control and colitis groups, respectively. *P ≤ 0.05 vs. control; #P ≤ 0.05, low D3 control vs. high D3 colitis (by ANOVA followed by Fisher's PLSD post hoc test).
DISCUSSION
Numerous studies of pediatric and adult CD and UC patients have indicated a high prevalence of vitamin D insufficiency or deficiency, and population studies have suggested that lower levels of vitamin D associated with reduced exposure to UV-B radiation could account for a north-south gradient of global IBD distribution (30). Moreover, single-nucleotide polymorphisms in the VDR have been linked to increased susceptibility to CD and UC (48). In mice, 1,25(OH)2D3 and Ca2+ administration reduced the symptoms of colitis in IL-10−/− mice(9), while IL-10/VDR double-knockout mice demonstrated an exacerbated phenotype (17). These anti-inflammatory effects of vitamin D are believed to be, at least in part, mediated by promotion of macrophage antimicrobial responses, regulation of the maturation of dendritic cells, and indirect and direct effects on T cell (including regulatory T cell) functions (20). These mechanisms, as well as the well-known fact that chronic vitamin D deficiency results in secondary hyperparathyroidism and bone loss, leading to muscle weakness, mineralization defects, osteoporosis, and fractures, have made the use of vitamin D3 an attractive main or supplemental therapy in postmenopausal and inflammation-associated loss of BMD.
Current vitamin D intake recommendations of the Institute of Medicine suggest 1,000–2,000 IU daily in healthy adults, a dose embraced by the Endocrine Society's Clinical Practice Guidelines, which also considered 10,000 IU/day to be safe (21). Very high single annual doses of 300,000–500,000 IU have been tested as a means of prevention of osteoporosis or depression in the elderly, albeit to no effect (37, 42). The effects of vitamin D alone or in combination with bisphosphonates in BMD in IBD patients yielded inconclusive data, with some studies indicating significant benefits (46), limited effects (5), or no effect (6). In light of the increasing complexity of the gut-renal-skeletal axis and the associated regulatory networks, we have postulated that supplementation with a high dose of vitamin D3 may be detrimental or have a limited beneficial potential to bone health if used during the uncontrolled phase of inflammation in IBD patients, and we have suggested that, in patients at clear risk of osteopenia or osteoporosis or with proven osteopenia or osteoporosis, vitamin D3 be withheld until remission is achieved (18, 19). To test this hypothesis, we used an established and clinically relevant model of adoptive T cell transfer colitis induced under dietary supply of HED of ∼730 IU/day. While no animal model fully represents human IBD and the results should be interpreted with caution, the adoptive T cell transfer model is considered one of the best, combining T cell- and microbiota-driven progressive (not self-limiting) intestinal inflammation. After the disease was established, mice were randomized into two dietary groups receiving the same “low” or “high” dose of vitamin D3, equivalent to 3,648 IU/day in humans. Although colitis was not affected by vitamin D3 supplementation, we observed significant effects of chronic colitis on cortical and trabecular bone density independent of the diet, as well as early negative effects of high vitamin D3 supplementation on trabecular bone parameters in colitic mice. These changes were associated with altered bone metabolism, as indicated by decreased bone formation and increased bone resorption markers. Since trabecular (cancellous) bone undergoes more dynamic remodeling than cortical bone, it was not surprising that relatively short-term treatment of colitic mice with the high vitamin D3 diet affected this type of osseous tissue first, with the most striking effects on connectivity density (a 3-dimensional measure of the number of trabeculi per mm3) and trabecular number (a 2-dimensional equivalent, or the number of trabeluli per mm). Similarly, in pathological states such as postmenopausal osteoporosis, trabecular bone is more severely affected than cortical bone.
Many of the potential benefits of vitamin D3 have sources in the observed relationships between vitamin D uptake, sun exposure, and serum 25(OH)D3 concentration. Only recently, new views are being expressed that low 25(OH)D3 levels may not be related to decreased intake or UV exposure but, rather, other factors. In IBD, similar to granulomatosis and other inflammatory/infectious diseases (2), elevated production of 1,25(OH)2D3 has been reported and attributed to elevated extrarenal expression of Cyp27b1 (1, 13). It is conceivable that some IBD patients identified with vitamin D3 insufficiency/deficiency based on serum 25(OH)D3 level may have an elevated concentration of the most bioactive metabolite, 1,25(OH)2D3. We recently reported that experimental colitis results in transcriptional inhibition of renal expression of Klotho (4), a key player in mineral homeostasis and vitamin D metabolism (28), which, among other functions, serves as an obligatory coreceptor for FGF23 (31, 34, 36). Although in experimental colitis (Fig. 9), similar to recent data from pediatric IBD patients (13), circulating FGF23 is significantly elevated, decreased expression of Klotho may be responsible for the lack of its suppressive effects on Cyp27b1 expression and activity during active inflammation (22, 30). Interestingly, in our experimental model, colitis led to elevated renal expression of Cyp27b1 when mice were fed the low vitamin D3 diet, but when the mice were switched to the high vitamin D3 diet, expression of this enzyme was reduced. It is plausible that increased vitamin D3, which has been shown to stimulate Klotho expression (14, 44), partially reversed the effects of cytokine-mediated suppression of Klotho, thus allowing for inhibitory effects of FGF23 on renal Cyp27b1 expression.
Another intriguing observation derived from our study is that while expression of renal Cyp27b1 was elevated in colitic mice fed the low vitamin D3 diet, this did not translate into an elevated plasma level of 1,25(OH)2D3, as described in IBD patients (1). This could potentially be explained by elevated renal expression and activity of the catabolic 24-hydroxylase (Cyp24a1), although this scenario seems unlikely, as we were not able to demonstrate significant changes in Cyp24a1 expression, and the level of plasma 24,25(OH)2D3 was also unaltered. It remained to be determined whether this observation is model-specific and what the underlying mechanism is.
Even though the level of 25(OH)D3 is routinely monitored in standard clinical settings, its value in chronic active inflammation may be limited. Moreover, with increasing demand for testing, the accuracy and specificity of commonly used 25(OH)D3 immunoassays have recently been questioned (12, 41). On the other hand, measurement of 1,25(OH)2D3 in the clinical setting is considered impractical because of the cost and instability of 1,25(OH)2D3. However, with the overall safety and well-known benefits of vitamin D3 in the general population, as well as increasing evidence of immunomodulatory effects of vitamin D3, elevated intake of vitamin D3 is commonly recommended in IBD patients. The presented preclinical data with a mouse model of established active colitis can be interpreted in more than one way. They may argue that during active disease the effects of circulating inflammatory mediators may synergize with proresorptive effects of elevated levels of 1,25(OH)2D3, thus leading to further loss of BDM in IBD patients. Some of the data may also indicate that during active colitis, despite elevated expression of the VDR in the bone, increased vitamin D3 intake fails to improve BMD. Further increase in vitamin D3 dose or exposure time may clarify these hypotheses. While our observations require clinical confirmation, they address a potentially significant adverse effect of vitamin D3 on bone metabolism during active disease or the fact that active colitis prevents the beneficial effect of vitamin D3 supplementation.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5R37 DK-033209 (to F. K. Ghishan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.B.L., R.-M.T.M., F.M.H., R.S., and R.R. performed the experiments; C.B.L., R.-M.T.M., F.M.H., R.S., R.R., and D.G.B. analyzed the data; C.B.L., F.K.G., and P.R.K. interpreted the results of the experiments; C.B.L. prepared the figures; C.B.L. drafted the manuscript; C.B.L., F.K.G., and P.R.K. approved the final version of the manuscript; F.K.G. and P.R.K. are responsible for conception and design of the research; P.R.K. edited and revised the manuscript.
REFERENCES
- 1.Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, Chen S, Zehnder D, Lin YC, Yang H, Hewison M, Adams JS. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut 53: 1129–1136, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys 523: 95–102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med 122: 599–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthel TK, Mathern DR, Whitfield GK, Haussler CA, Hopper HA, Hsieh JC, Slater SA, Hsieh G, Kaczmarska M, Jurutka PW, Kolek OI, Ghishan FK, Haussler MR. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol 103: 381–388, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bartram SA, Peaston RT, Rawlings DJ, Francis RM, Thompson NP. A randomized controlled trial of calcium with vitamin D, alone or in combination with intravenous pamidronate, for the treatment of low bone mineral density associated with Crohn's disease. Aliment Pharmacol Ther 18: 1121–1127, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Benchimol EI, Ward LM, Gallagher JC, Rauch F, Barrowman N, Warren J, Beedle S, Mack DR. Effect of calcium and vitamin D supplementation on bone mineral density in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 45: 538–545, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Blum AM, Metwali A, Elliott DE, Berg DJ, Weinstock JV. CD4+ T cells from IL-10-deficient mice transfer susceptibility to NSAID-induced Rag colitis. Am J Physiol Gastrointest Liver Physiol 287: G320–G325, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25: 1468–1486, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 130: 2648–2652, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Civitelli R, Armamento-Villareal R, Napoli N. Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int 20: 843–851, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Ding S, Schoenmakers I, Jones K, Koulman A, Prentice A, Volmer DA. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Anal Bioanal Chem 398: 779–789, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dresner-Pollak R, Ackerman Z, Eliakim R, Karban A, Chowers Y, Fidder HH. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genet Test 8: 417–420, 2004 [DOI] [PubMed] [Google Scholar]
- 13.El-Hodhod MA, Hamdy AM, Abbas AA, Moftah SG, Ramadan AA. Fibroblast growth factor 23 contributes to diminished bone mineral density in childhood inflammatory bowel disease. BMC Gastroenterol 12: 44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, Kaneko I, Haussler MR, Kerr Whitfield G. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun 414: 557–562, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 8: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 17: 2386–2392, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology 117: 310–318, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghishan F, Kiela P. Metabolic bone disease in inflammatory bowel disease. Practical Gastroenterol XXXVI: 2012 [Google Scholar]
- 19.Ghishan FK, Kiela PR. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol 300: G191–G201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc 71: 50–61, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Holick MF. Evidence-based D-bate on health benefits of vitamin D revisited. Dermatoendocrinology 4: 183–190, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick MF. Vitamin D deficiency. N Engl J Med 357: 266–281, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Kido S, Inoue D, Hiura K, Javier W, Ito Y, Matsumoto T. Expression of RANK is dependent upon differentiation into the macrophage/osteoclast lineage: induction by 1α,25-dihydroxyvitamin D3 and TPA in a human myelomonocytic cell line, HL60. Bone 32: 621–629, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kiela PR, Midura AJ, Kuscuoglu N, Jolad SD, Solyom AM, Besselsen DG, Timmermann BN, Ghishan FK. Effects of Boswellia serrata in mouse models of chemically induced colitis. Am J Physiol Gastrointest Liver Physiol 288: G798–G808, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Yamaori S, Tanabe T, Johnson CH, Krausz KW, Kato S, Gonzalez FJ. Implication of intestinal VDR deficiency in inflammatory bowel disease. Biochim Biophys Acta 1830: 2118–2128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK. 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289: G1036–G1042, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kuro-o M. Klotho in health and disease. Curr Opin Nephrol Hypertens 21: 362–368, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Lee SK, Kalinowski J, Jastrzebski S, Lorenzo JA. 1,25(OH)2 vitamin D3-stimulated osteoclast formation in spleen-osteoblast cocultures is mediated in part by enhanced IL-1α and receptor activator of NF-κB ligand production in osteoblasts. J Immunol 169: 2374–2380, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol 2: 308–315, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Matsue M, Kageyama R, Denhardt DT, Noda M. Helix-loop-helix-type transcription factor (HES-1) is expressed in osteoblastic cells, suppressed by 1,25(OH)2 vitamin D3, and modulates 1,25(OH)2 vitamin D3 enhancement of osteopontin gene expression. Bone 20: 329–334, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Medhora MM, Teitelbaum S, Chappel J, Alvarez J, Mimura H, Ross FP, Hruska K. 1α,25-Dihydroxyvitamin D3 up-regulates expression of the osteoclast integrin αvβ3. J Biol Chem 268: 1456–1461, 1993 [PubMed] [Google Scholar]
- 34.Razzaque MS. FGF23, klotho and vitamin D interactions: what have we learned from in vivo mouse genetics studies? Adv Exp Med Biol 728: 84–91, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 22: 659–661, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1α,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280: 2543–2549, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Sanders KM, Stuart AL, Williamson EJ, Jacka FN, Dodd S, Nicholson G, Berk M. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. Br J Psychiatry 198: 357–364, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Silva I, Branco JC. Rank/Rankl/opg: literature review. Acta Reumatol Port 36: 209–218, 2011 [PubMed] [Google Scholar]
- 39.Silvennoinen J. Relationships between vitamin D, parathyroid hormone and bone mineral density in inflammatory bowel disease. J Intern Med 239: 131–137, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Silvennoinen JA, Karttunen TJ, Niemela SE, Manelius JJ, Lehtola JK. A controlled study of bone mineral density in patients with inflammatory bowel disease. Gut 37: 71–76, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn's disease susceptibility. Gut 47: 211–214, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology 46: 1852–1857, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Thearle M, Horlick M, Bilezikian JP, Levy J, Gertner JM, Levine LS, Harbison M, Berdon W, Oberfield SE. Osteoporosis: an unusual presentation of childhood Crohn's disease. J Clin Endocrinol Metab 85: 2122–2126, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol 17: 2393–2403, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Uchida M, Shima M, Chikazu D, Fujieda A, Obara K, Suzuki H, Nagai Y, Yamato H, Kawaguchi H. Transcriptional induction of matrix metalloproteinase-13 (collagenase-3) by 1α,25-dihydroxyvitamin D3 in mouse osteoblastic MC3T3-E1 cells. J Bone Miner Res 16: 221–230, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Vogelsang H, Ferenci P, Resch H, Kiss A, Gangl A. Prevention of bone mineral loss in patients with Crohn's disease by long-term oral vitamin D supplementation. Eur J Gastroenterol Hepatol 7: 609–614, 1995 [PubMed] [Google Scholar]
- 47.Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, Kubo N, Muguruma K, Yamada N, Yashiro M, Sawada T, Nakata B, Ohira M, Hirakawa K. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep 22: 1021–1025, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Xue LN, Xu KQ, Zhang W, Wang Q, Wu J, Wang XY. Associations between vitamin D receptor polymorphisms and susceptibility to ulcerative colitis and Crohn's disease: a meta-analysis. Inflamm Bowel Dis 19: 54–60, 2012 [DOI] [PubMed] [Google Scholar]