Abstract
Aim. The present study was designed to investigate the cytotoxicity of a panel of 280 Korean medicinal plants belonging to 73 families and 198 species against human CCRF-CEM leukemia cells. Selected phytochemicals were investigated in more detail for their mode of action. Methods. The resazurin assay was used to determine cytotoxicity of the plant extracts. Microarray-based mRNA expression profiling, COMPARE, and hierarchical cluster analyses were applied to identify which genes correlate with sensitivity or resistance to selected phytochemicals of the Korean plants. Results. The results of the resazurin assay showed that cytotoxicity extracts tested at 10 μg/mL from 13 samples inhibited proliferation more than 50% (IC50 < 10 μg/mL) and the most active plants are Sedum middendorffianum (15.33%) and Lycoris radiata (17.61%). Out of 13 selected phytochemicals from these plants, hopeaphenol and deoxynarciclasine were the most cytotoxic ones. Genes from various functional groups (transcriptional or translational regulation, signal transduction, cellular proliferation, intracellular trafficking, RNA metabolism, endoplasmic/sarcoplasmic reticulum function, etc.) were significantly correlated with response of tumor cell lines to these two compounds. Conclusion. The results provide evidence on the possible use of selected Korean medicinal plants and chemical constituents derived from them for the treatment of tumors.
1. Introduction
Since ancient times, humans have derived many benefits from medicinal plants. A variety of different medicinal plants have traditionally been used in Asian cultures as medicinal plants to treat cancers [1]. The pharmacological screening of plants is an important mean for the discovery of new, safe, and effective drugs. Over 50,000 plants, possess therapeutic virtues in the world, and about 80% of human use herbal medicines at least once in their life [2, 3]. Medicinal plants through the multiplicities of their chemical constituents are important for the discovery of new substances active against tumors and other cancers. Screenings of medicinal plants used as anticancer drugs have provided modern medicine with effective cytotoxic pharmaceuticals. More than 60% of the approved anticancer drugs in the United State of America (from 1983 to 1994) were from natural origin [4, 5].
Traditional Korean medicine is widely used in Korea and is the primary health care system for more than 20% of the population [6, 7]. As previously emphasized in a regional demographic survey, about 30–40% of the Korean population had used complementary and alternative medicine (including traditional Korean medicine) within a 5-year period [6, 8].
In the pharmacopoeia of many countries worldwide including Korea, there is still a serious lack of information on the use of medicinal plants in the treatment of cancer. However, it has been recommended that ethnopharmacological usages such as for immune and skin disorders, inflammatory, infectious, parasitic, and viral diseases should be taken into account when selecting plants used to treat cancer, since these reflect disease states bearing relevance to cancer or cancer-like symptoms [9, 10]. In general, leukemia cells are often more sensitive to cytotoxic drugs than adherent cancer cells, making them a suitable tool for primary bioactivity screenings. Hence, the present work was designed to investigate the cytotoxicity of a panel of 280 Korean medicinal plants against human CCRF-CEM leukemia cells. Based on the bioactivity of the extracts, selected phytochemicals of active plants were analyzed in more detail. Genes determining sensitivity or resistance to selected compounds were identified by microarray-based mRNA expression profiles and hierarchical cluster analyses in a panel of tumor cell lines of the National Cancer Institute, USA.
2. Material and Methods
2.1. Plant Material and Extraction
Medicinal plants used in the present work were collected at different localities of the Republic of Korea and provided by Prof. Ik-Soo Lee (College of Pharmacy, Chonnam National University, Kwangju, South Korea). The plants were identified at the National herbarium, where voucher specimens were deposited under the references numbers (see Supplementary material available online at http://dx.doi.org/10.1155/2013/341724). The extraction of the air-dried and powdered plant material was conducted using methanol (HPLC grade) with either ASE 300 (Dionex) or a sonicator (Branson Ultrasonics) at 50°C. The extracts were then conserved at 4°C until further use.
2.2. Cell Culture
Human CCRF-CEM leukemia cells were obtained from Professor Axel Sauerbrey (University of Jena, Jena, Germany). Cells were maintained in RPMI 1640 containing 100 units/mL penicillin and 100 mg/mL streptomycin and supplemented with heat-inactivated 10% fetal bovine serum (FBS), in a humidified environment at 37°C with 5% CO2. Doxorubicin ≥ 98.0% (Sigma-Aldrich, Schnelldorf, Germany) was used as a positive (cytotoxic) control.
2.3. Resazurin Cell Growth Inhibition Assay
Alamar Blue or Resazurin (Promega, Mannheim, Germany) reduction assay [11] was used to assess the cytotoxicity of the studied samples. The assay tests cellular viability and mitochondrial function. Briefly, aliquots of 5 × 104 cells/mL were seeded in 96-well plates, and extracts were added immediately. After 24 h incubation, 20 μL resazurin 0.01% w/v solution was added to each well and the plates were incubated at 37°C for 1-2 h. Fluorescence was measured on an automated 96-well Infinite M2000 Pro plate reader (Tecan, Crailsheim, Germany) using an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Doxorubicin was used as positive control. The concentration of DMSO was kept at or below 0.1% in all experiments. Each assay was done at least three times, with three replicates each. All samples were tested at a single concentration of 10 μg/mL.
2.4. COMPARE and Hierarchical Cluster Analysis of mRNA Microarray Data
The mRNA microarray hybridization of the NCI cell line panel has been described [12, 13] and the date has been deposited at the NCI website (http://dtp.nci.nih.gov/). For hierarchical cluster analysis, objects were classified by calculation of distances according to the closeness of between-individual distances by means of hierarchical cluster analysis. All objects were assembled into a cluster tree (dendrogram). The merging of objects with similar features leads to the formation of a cluster, where the length of the branch indicates the degree of relation. The distance of a subordinate cluster to a superior cluster represents a criterion for the closeness of clusters as well as for the affiliation of single objects to clusters. Thus, objects with tightly related features appear together, while the separation in the cluster tree increases with progressive dissimilarity. Previously, cluster models have been validated for gene expression profiling and for approaching molecular pharmacology of cancer [12–14]. Hierarchical cluster analyses applying the WARD method were done with the WinSTAT program (Kalmia, Cambridge, MA, USA). Missing values were automatically omitted by the program, and the closeness of two joined objects was calculated by the number of data points they contained. In order to calculate distances between all variables included in the analysis, the program automatically standardizes the variables by transforming the data with a mean = 0 and a variance = 1. For COMPARE analysis, the mRNA expression values of genes of interest and IC50 values for selected phytochemicals of the NCI cell lines were selected from the NCI database (http://dtp.nci.nih.gov/). The mRNA expression has been determined by microarray analyses as reported [12]. COMPARE analyses were performed by a web-based algorithm (http://dtp.nci.nih.gov/) to produce rank-ordered lists of genes expressed in the NCI cell lines. The methodology has been described previously in detail [15]. Briefly, every gene of the NCI microarray database was ranked for similarity of its mRNA expression to the IC50 values for the corresponding compound. Scale indices of correlations coefficients (R-values) were created. Pearson's correlation coefficients with positive algebraic signs indicate that greater mRNA expression in cell lines correlate with enhanced drug resistance, whereas coefficients with negative algebraic signs indicate that greater mRNA expression in cell lines was associated with drug sensitivity. Pearson's correlation test and χ 2 test were implemented into the WinSTAT Program (Kalmia). The one-way ANOVA at 95% confidence level was used for statistical analysis.
3. Results
3.1. Cytotoxic Activity
In the present work, we screened 280 plant extracts derived from traditional Korean medicine belonging to 73 plant families and 198 species (see Supplementary Table) for their cytotoxicity against human CCRF-CEM leukemia cells.
The results of the cytotoxicity assay (Figure 1; Supplementary Table) indicated that is among the 280 studied plant extracts, 58 promoted the growth (% growth > 100%) of CCRF-CEM cells, while the remaining samples showed various extents of growth inhibition. Out of these extracts, 207 extracts showed no or a weak inhibition of cancer cell proliferation (<50%) at the tested concentration of 10 μg/mL. However, 13 samples induced the proliferation of less 50% cells (IC50 < 10 μg/mL). These samples included Sedum middendorffianum whole plant (15.33%), Lycoris radiata underground parts (17.61%), Camellia japonica fruits (22.28%), Myrica rubra stem-stem bark (27.61%), Lycoris radiata leaves (27.73%), Vitis flexuosa aerial parts (29.32%), Sedum takesimense whole plant (32.39%), Sorbaria sorbifolia var. stellipila stems (34.98%), Isodon japonicus whole plant (44.15%), Sageretia theezans leaves and stems (46.35%), Rubus corchorifolius aerial part (46.81%), Alnus japonica stem-stem bark (48.43%), and Meliosma oldhamii stem-stem bark (49.94%) (Figure 1). The traditional used and reported bioactivities are compiled in Table 1.
Figure 1.
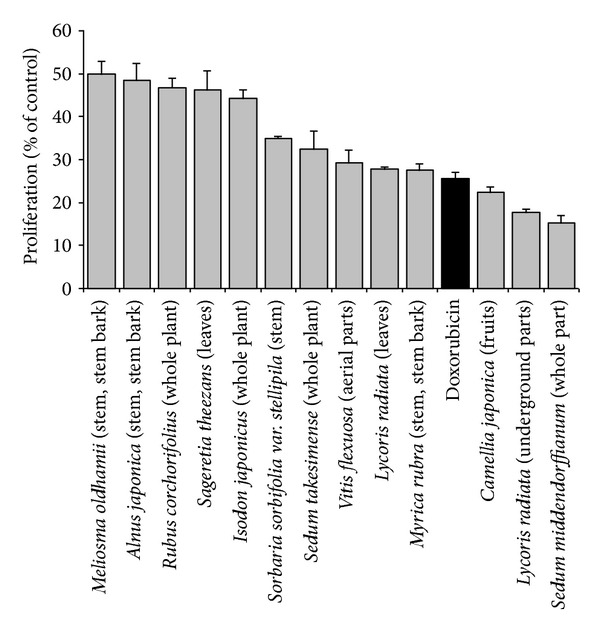
Cytotoxicity of the 13 most active samples from selected Korean medicinal plants extract (at 10 μg/mL) and doxorubicin (1 μM) on CCRF-CEM leukemia cells. Data with different superscript letters are significantly different (P < 0.05). (See Supplementary Data for the overview of the cytotoxicity of all 280 tested extracts. Are shown mean values ± SD of each five measurements.) The established anticancer drug, doxorubicin was used as positive control (black bar).
Table 1.
Korean plants with cytotoxic activity.
| Plants (and family) | Traditional uses | Part used | Previously reported activity of the plant | Reported chemical constituents |
|---|---|---|---|---|
| Alnus japonica Steudel (Betulaceae) | In oriental traditional medicine as remedies for fever, hemorrhage, diarrhea, and alcoholism [43] | Stems-stem bark | Hepatoprotective and antioxidant activities [44], antiviral activity against the influenza virus [45] | 1,7-Bis-(3,4-dihydroxyphenyl)-3-hydroxyheptane-5-O-β-D-xylopyranoside; 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-O-β-D-glucopyranosyl(1→3)-β-D-xylopyranoside; 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-O-β-D-apiofuranosyl(1→6)-β-D-glucopyranoside; 1,7-bis-(3,4-dihydroxyphenyl)-heptane-5-O-β-D-glucopyranoside; 1,7-bis-(3,4-dihydroxyphenyl)-5-hydroxylheptane; 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-one-5-O-β-D-glucopyranoside; oregonin; hirsutanonol; hirsutenone; platyphylloside [44]; tannins (alnusjaponins A and B); 5-O-galloyl-(−)-shikimic acid, 2,3-(S)-hexahydroxydiphenoyl-D-glucose, 4,6-di-O-galloyl-D-glucose, 1,4-di-O-galloyl-β-D-glucose, 4,6-(S)-valoneoyl-D-glucose; strictinin; gemin D; pedunculagin; praecoxin A; flosin A; stachyurin; casuarinin [46], lupeol; betulin; betulinic aldehyde; 3-acetoxybetulinic aldehyde, β-sitosterol [45] |
|
| ||||
| Camellia japonica L. (Theaceae) | Cosmetic protectant to keep the skin and hair healthy and as a soothing agent [47] | Fruits | Antibacterial activity [48], inhibitor of human immunodeficiency virus type 1 protease [49], Epstein-Barr virus inhibitor [50], antimetastasis activity [51], antioxidant activity [52, 53], inhibitor of human type I procollagen production [54], and antiallergic responses [55], anti-inflammatory [47] | 3β,18β-dihydroxy-28-norolean-12-en-16-one; 18β-hydroxy-28-norolean-12-ene-3,16-dione; camelliagenin A, B, and C [56], camellenodiol 3-O-β-D-galactopyranosyl(1→2)[β-D-xylopyranosyl(1→2)-β-D-galactopyranosyl(1→3)]-β-D-glucuronopyranoside; camellenodiol 3-O-4′′-O-acetyl-β-D-galactopyranosyl(1→2)[β-D-xylopyranosyl(1→2)-β-D-galactopyranosyl(1→3)]-β-D-glucuronopyranoside; camellenodiol 3-O-(β-D-galactopyranosyl(1→2)[β-D-xylopyranosyl(1→2)-β-D-galactopyranosyl(1→3)]-6′-methoxy-β-D-glucuronopyranoside; maragenin II 3-O-(β-D-galactopyranosyl(1→2)[β-D-xylopyranosyl(1→2)-β-D-galactopyranosyl(1→3)]-6′-methoxy-β-D-glucuronopyranoside, camellioside A; camellioside B [57] |
|
| ||||
| Isodon japonicus (Burman f.) H. Hara (Labiatae) | Antibacterial, anti-inflammation, and anthelmintic [58] | Whole plant | Cytotoxicity on K562 human leukemia cells and immunomodulatory activity [16], antibacterial activity for plant constituents [59] | Isadonol; epinodosin; sodoponin; epinodosinol [60, 61]; epinodosin; oridonin; taihangjaponicain A; lushanrubescensin J; bisjaponins A and B [58]; isodonal, trichodonin; nodosin; enmein; oridonin; enmein-3-acetate [59] |
|
| ||||
| Lycoris radiata (L'Her.) Herbert (Amaryllidaceae) | Laryngeal trouble, furuncle, carbuncle, suppurative wounds [62] | Leaves, underground parts | Cytotoxicity against B16F10 melanoma cells [17] | Different types of alkaloids (crinine-type; galanthamine-type; lycorine-type homolycorine-type; tazettine-type; narciclasine-type; and lycorine-type alkaloids); trisphaeridine; galanthine; bicolorine; 11-hydroxyvittatine; 8-O-demethymaritidine;O-demethylgalanthamine; O-demethyllycoramine [63] |
|
| ||||
| Meliosma oldhamii Miq. ex. Maxim. (Sabiaceae) | Liver ailments [64] | Stems-stem bark | Low Cholinesterase inhibition (12–19% at 5 mg/mL) [65], moderate alpha glucosidase activity [64] | — |
|
| ||||
| Myrica rubra Sieb. and Zucc. (Myricaceae) | Diarrhea; gastroenteritis in China [66] | Stems-stem bark | Antioxidant activity [67]; anti-influenza virus activity [68] | Taraxerone; taraxerol; myricadiol; sitosterol; 28-hydroxy-D-friedoolean-14-en-3-one (); myricanol 5-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside; myricanol 5-O-α-L-arabinofuranosyl-(1→6)-β-D-glucopyranoside; isomyricanone [69]; cyanidin-3-O-glucoside; myricetin; quercetin-3-O-rutinoside [67] |
|
| ||||
| Rubus corchorifolius L. f. (Rosaceae) | Stomachache, diarrhea, and dysentery [18] | Whole plant | Antioxidant activity of essential oil [70] | Ent-kauran-3β, 16β, 17, 19-tetrol; ent-2-carbonyl-16β-hydroxy-kauran-17β-D-glucoside [18]; rubusin A; quercetin; kaempferol [25] |
|
| ||||
| Sageretia theezans (L.) Brongn (Rhamnaceae) | Tea materials [71] | Leaves, Stems | Antioxidant activity [72] | 7-O-methylmearnsitrin; myricetrin, kaempferol 3-O-α-L-rhamnopyranoside, europetin 3-O-α-L-rhamnoside, and 7-O-methyl quercetin 3-O-alpha-L-rhamnopyranoside; 7-O-methylmearnsetin 3-O-rhamnoside [71, 72] |
|
| ||||
| Sedum middendorffianum Maxim. (Crassulaceae) | — | Whole plant | — | kaempferol; quercetin; myricetin; arbutin [24] |
|
| ||||
| Sedum takesimense Nakai (Crassulaceae) | — | Whole plant | Antioxidant activities [26] | Ferulic acid; caffeic acid; gallic acid; methyl gallate; myricetin; quercetin; luteolin; rhodalin; rhodalidin; luteolin-7-O-β-D-glucoside; arbutin; 1-(4-hydroxyphenyl)-2-(3,5-dihydroxyphenyl)-2-hydroxyethanone; gossypetin-8-O-β-D-xylopyranoside; 2,6-di-O-galloylarbutin [26] |
|
| ||||
| Sorbaria sorbifolia (L.) A. Br. var. stellipila MAX. (Rosaceae) | — | Stems | Antioxidant activities, cytotoxicity [73, 74] | Sutherlandin-5-trans-p-coumarate; cardiosdiospermin-5-(4-hydroxy) benzoate [75]; noreugenin; wogonin; 5,7,3′,4′-tetrahydroxy-3-methoxyflavone; protocatechuic acid; benzoic acid; emodin; daucosterol [76]; 5,2′,4′-trihydroxy-6,7,5′-trimethoxyflavone; succinic acid; p-hydroxybenzoic acid [77] |
|
| ||||
| Vitis flexuosa Thunb. (Vitaceae) | — | Aerial parts | — | Flexuosol A; gnetin A; (+)-epsilon-viniferin; vitisin A; hopeaphenol [78] |
(—): not reported; the complete list of the tested plants is available in supplementary material.
3.2. Cytotoxicity of Phytochemicals Derived from Koran Medicinal Plants
We searched the literature on the chemical constituents of the cytotoxic Korean plants (Table 1). Subsequently, we mined the NCI database for these compounds. Thirteen compounds with average log10IC50 values over the entire NCI cell line panel below −4.0 M are depicted in Figure 2. Hopeaphenol and deoxynarciclasine were the most cytotoxic compounds with log10IC50 values of 0.346 × 10−6 M (=0.346 μM) and 0.233 × 10−5 M (=2.33 μM), respectively.
Figure 2.
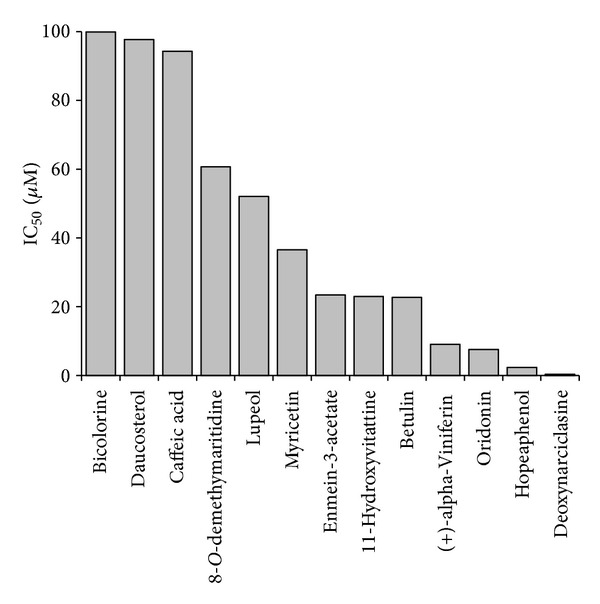
Mean log10IC50 values for selected phytochemicals derived from Korean medicinal plants for tumor cell lines from the NCI cell line panel.
If the average IC50 values over the entire range of cell lines were diversified regarding their tumor types, colon cancer and melanoma cell lines were most sensitive towards hopeaphenol, whereas leukemia and kidney cancer cell lines were most resistant (Figure 3(a)). The cell lines reacted in a different manner towards deoxynarciclasine. Leukemia and colon cancer were most sensitive towards this compound, whereas breast cancer and prostate cancer cell lines were most resistant (Figure 3(b)).
Figure 3.
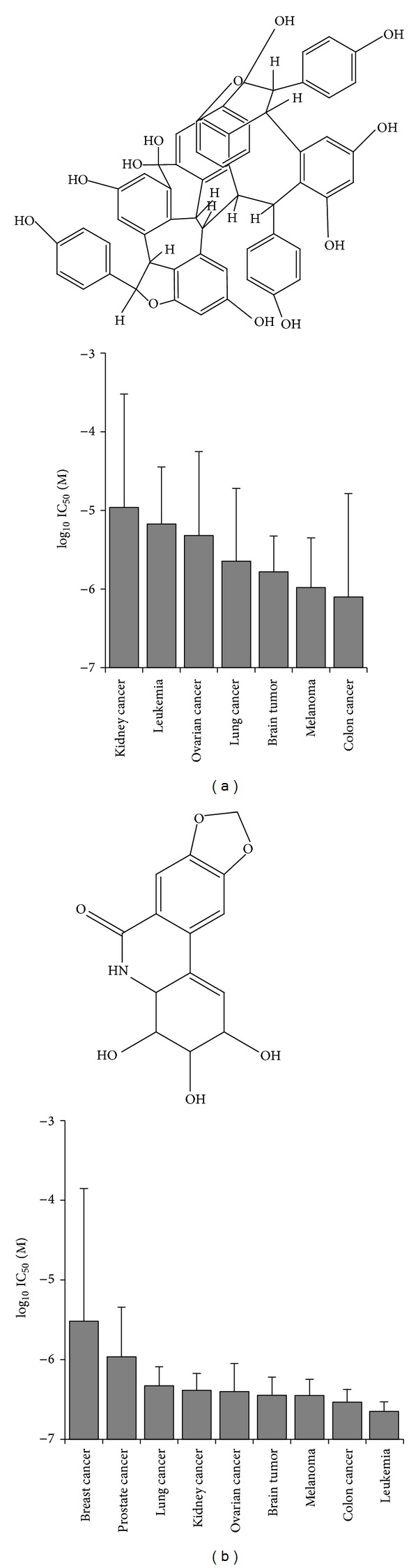
Cytotoxic activity of hopeaphenol (a) and deoxynarciclasine (b) towards cell lines of different tumor types (mean ± SD).
3.3. Cross-Resistance of the NCI Cell Line Panel towards Hopeaphenol and Deoxynarciclasine
The log10IC50 values of hopeaphenol and deoxynarciclasine were correlated with clinically well-established anticancer drugs. The cell line panel showed statistically significant correlations between deoxynarciclasine and doxorubicin, daunorubicin, vincristine, paclitaxel, cisplatin, melphalan and carmustin. By contrast, cross-resistance was not found between hopeaphenol and these standard drugs, indicating that hopeaphenol might be useful for the treatment of otherwise drug-resistant tumors (Table 2). Interestingly, an inverse correlation was found between the log10IC50 values for hopeaphenol and the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Such a relationship was not found between deoxynarciclasine and erlotinib (Table 2).
Table 2.
Cross-resistance profile of a panel of cell lines towards hopeaphenol and deoxynarciclasine determined by correlating the IC50 values by Pearson's correlation test.
| Hopeaphenol | Deoxynarciclasine | |
|---|---|---|
| Doxorubicin | ||
| R-value | <0.30 | 0.340 |
| P-value | >0.05 | 0.010 |
| Daunorubicin | ||
| R-value | <0.30 | 0.430 |
| P-value | >0.05 | 0.001 |
| Vincristine | ||
| R-value | <0.30 | 0.331 |
| P-value | >0.05 | 0.012 |
| Paclitaxel | ||
| R-value | <0.30 | 0.330 |
| P-value | >0.05 | 0.012 |
| Cisplatin | ||
| R-value | <0.30 | 0.300 |
| P-value | >0.05 | 0.020 |
| Melphalan | ||
| R-value | <0.30 | 0.388 |
| P-value | >0.05 | 0.004 |
| Carmustin | ||
| R-value | <0.30 | 0.394 |
| P-value | >0.05 | 0.003 |
| Erlotinib | ||
| R-value | −0.353 | >−0.30 |
| P-value | 0.004 | >0.05 |
Pearson's rank correlation test.
3.4. COMPARE and Hierarchical Cluster Analyses of mRNA Microarray Data
We further investigated the microarray-based transcriptomic mRNA expression by COMPARE analyses to test whether the responses of the tumor cells lines to hopeaphenol and deoxynarciclasine were associated with specific gene expression profiles. For this reason, we mined the transcriptome-wide mRNA expression database of the NCI and correlated the expression data with the IC50 values for both compounds. This represents a hypothesis-generating bioinformatical approach, which allows the identification of novel putative molecular determinants of cellular response for hopeaphenol and deoxynarciclasine. The scale rankings of genes obtained by COMPARE computation were subjected to Pearson's rank correlation tests. The thresholds for correlation coefficients were R > 0.50 for direct correlations and R < −0.50 for inverse correlations. The genes fulfilling these criteria are shown in Table 3 (for hopeaphenol) and Table 4 (for deoxynarciclasine). These genes were from diverse functional groups for hopeaphenol (Table 3). For deoxynarciclasine, genes were found involved in transcription (ILF2, BCLAF1, MATR3, PSPC1, ZBTB11, CNOT8, IKZF5, SF1, EBP, and MYBL1), RNA metabolism (HNRNPA1, LARS, SFRS1, FARSA, NUDT21, DDX39, and SERBP1), translation (GOT1, NGDN), cellular proliferation (CWF19L1, MKI67, HSPA9, MLF1IP, and DCBLD2), intracellular trafficking (OPTN, SNX6, and RAB11FIP5), endoplasmic/sarcoplasmic reticulum function (SLN, DNAJC10, LMAN2L, SEC24D, and KDELR2), signal transduction (FRAT2, CHUK, GNA12, and LPP), and others.
Table 3.
Genes identified by standard or reverse COMPARE analyses, whose mRNA expression in a panel of 60 cell lines correlated with IC50 values for hopeaphenol.
| Pearson's correlation coefficient | Experimental ID | Gene symbol | Name | Function |
|---|---|---|---|---|
| 0.552 | GC63503 | HMGN4 | High mobility group nucleosomal binding domain 4 | Binds nucleosomal DNA |
| 0.527 | GC190712 | UAP1 | UDP-N-acetylglucosamine pyrophosphorylase 1 | Nucleotidyltransferase |
| 0.521 | GC45602 | TBC1D2 | TBC1 domain family, member 2 | GTPase activator |
| 0.521 | GC188142 | ERBB2IP | Erbb2 interacting protein | Receptor adapter, structural constituent of cytoskeleton |
| 0.515 | GC26884 | PRPS1 | Phosphoribosyl pyrophosphate synthetase 1 | Involved in nucleotide synthesis |
| 0.513 | GC38343 | CNNTAL1 | Unknown | |
| −0.591 | GC97260 | MANBA | Mannosidase, beta A, lysosomal | Exoglycosidase |
| −0.574 | GC73531 | FGF9 | Fibroblast growth factor 9 (glia-activating factor) | Cell growth and differentiation during development |
| −0.534 | GC55495 | CYP7B1 | Cytochrome P450, family 7, subfamily B, polypeptide 1 | Monooxygenase |
| −0.516 | GC94617 | GABRA3 | Gamma-aminobutyric acid (GABA) A receptor, alpha 3 | Neurotransmission |
| −0.515 | GC184017 | HES1 | Hairy and enhancer of split 1, (Drosophila) | Transcriptional repressor |
Information on gene functions was taken from the OMIM database, NCI, USA, (http://www.ncbi.nlm.nih.gov/Omim/) and from the GeneCard database of the Weizmann Institute of Science, Rehovot, Israel (http://bioinfo.weizmann.ac.il/cards/index.html).
Table 4.
Genes identified by standard or reverse COMPARE analyses, whose mRNA expression in a panel of 60 cell lines correlated with IC50 values for deoxynarciclasine.
| Pearson's correlation coefficient | Experimental ID | Gene symbol | Name | Function |
|---|---|---|---|---|
| 0.572 | GC74997 | SNAP25 | Synaptosomal-associated protein, 25 kDa | Regulation of neurotransmitter release |
| 0.56 | GC32186 | OPTN | Optineurin | Maintenance of membrane trafficking |
| 0.557 | GC52658 | FAM116B | Family with sequence similarity 116, member B | Unknown |
| 0.531 | GC187393 | ZDHHC7 | Zinc finger, DHHCtype containing 7 | Palmitoyltransferase |
| 0.529 | GC12575 | CLEC9A | C-type lectin domain family 9, member A | Endocytic receptor for necrotic cells |
| 0.528 | GC188718 | SNX6 | Sorting nexin 6 | Involved in intracellular trafficking |
| 0.528 | GC154565 | RAB11FIP5 | RAB11 family interacting protein 5 (class I) | Involved in protein trafficking |
| 0.527 | GC18484 | NTAN 1 | N-terminal asparagine amidase | Ubiquitin-dependent turnover of intracellular proteins |
| 0.526 | GC16433 | DNAJC10 | DnaJ (Hsp40) homolog, subfamily C, member 10 | Endoplasmic reticulum cochaperone |
| 0.524 | GC10009 | PCOLCE2 | Procollagen C-endopeptidase enhancer 2 | Binds to procollagens |
| 0.524 | GC45803 | LMAN2L | Lectin, mannose-binding 2-like | Regulation of export from the endoplasmic reticulum |
| 0.524 | GC82947 | NGDN | Neuroguidin, EIF4E binding protein | Involved in the translational repression |
| 0.519 | GC75800 | SEC24D | SEC24 family, member D (S. cerevisiae) | Transport of ER-derived vesicles |
| 0.517 | GC90440 | KDELR2 | KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 2 | Retention of luminal endoplasmic reticulum proteins |
| 0.514 | GC173264 | GNA12 | Guanine nucleotide binding protein (G protein) α12 | Transducer in transmembrane signaling |
| 0.512 | GC170473 | MYBL1 | V-Myb myeloblastosis viral oncogene homolog (avian)-like 1 | Transcriptional activator |
| 0.512 | GC175225 | LPP | LIM domain containing preferred translocation partner in lipoma | Signal transduction from cell adhesion sites to the nucleus |
| 0.511 | GC14006 | ITGB5 | Integrin, β5 | Receptor for fibronectin |
| 0.509 | GC150035 | DCBLD2 | Discoidin, CUB, and LCCL domain containing 2 | Involved in tumor progression |
| 0.508 | GC73833 | NCEH1 | Neutral cholesterol ester hydrolase 1 | Promotes tumor cell migration |
| 0.507 | GC177466 | AHNAK | AHNAK nucleoprotein | Involved in neuronal cell differentiation |
| 0.502 | GC40315 | ADAL | Adenosine deaminaselike | Putative nucleoside deaminase |
| 0.502 | GC28174 | MPG | N-methylpurine-DNA glycosylase | Hydrolysis of alkylated DNA |
| 0.501 | GC18079 | CR1 | Complement component (3b/4b) receptor 1 (Knops blood group) | Mediates cellular binding of particles and immune complexes |
| −0.658 | GC18354 | ILF2 | Interleukin enhancer binding factor 2, 45 kDa | Transcription |
| −0.608 | GC44240 | NDUFA2 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 2, 8 kDa | Part of mitochondrial membrane respiratory chain |
| −0.605 | GC65788 | HNRNPA1 | Heterogeneous nuclear ribonucleoprotein A1 | Involved in RNA replication |
| −0.589 | GC43237 | CWF19L1 | CWF19-like 1, cell cycle control (S. pombe) | Cell cycle control |
| −0.572 | GC174320 | BCLAF1 | BCL2-associated transcription factor 1 | Death-promoting transcriptional repressor |
| −0.572 | GC40779 | LARS | Leucyl-tRNA synthetase | Editing of tRNA |
| −0.567 | GC151509 | FRAT2 | Frequently rearranged in advanced T-cell lymphomas 2 | Wnt signaling regulator |
| −0.561 | GC175610 | IK | IK cytokine, downregulator of HLA II | May bind to chromatin |
| −0.555 | GC30213 | SLN | Sarcolipin | Sarcoplasmic reticulum proteolipid |
| −0.553 | GC162737 | ANKHD2 | Unknown | |
| −0.552 | GC17532 | SFRS28 | Unknown | |
| −0.55 | GC185046 | SFRS1 | Serine/arginine-rich splicing factor 1 | Splicing regulator |
| −0.55 | GC37292 | FARSA | Phenylalanyl-tRNA synthetase, α subunit | Phenylalanine-tRNA ligase |
| −0.548 | GC32318 | MKI67 | Antigen identified by monoclonal antibody Ki-67 | Cell proliferation |
| −0.548 | GC149101 | MATR3 | Matrin 3 | Regulator of transcription |
| −0.547 | GC150688 | PSPC1 | Paraspeckle component 1 | Regulator of androgen receptor-mediated gene transcription |
| −0.546 | GC80581 | ZBTB11 | Zinc finger and BTB domain containing 11 | Regulator of transcription |
| −0.546 | GC52470 | MLF1IP | MLF1 interacting protein | Involved in mitotic progression |
| −0.545 | GC81490 | NUDT21 | Nudix (nucleoside diphosphate linked moiety X)-type motif 21 | Involved in pre-mRNA 3′-processing |
| −0.543 | GC33504 | DDX39 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 39A | Involved in pre-mRNA splicing |
| −0.543 | GC35760 | NDUGB8 | Unknown | |
| −0.539 | GC36766 | CHUK | Conserved helix-loop-helix ubiquitous kinase | Involved in NF-kappa-B signaling |
| −0.538 | GC33463 | POLD1 | Polymerase (DNA directed), δ1, catalytic subunit | DNA synthesis |
| −0.537 | GC83208 | SERBP1 | SERPINE1 mRNA binding protein 1 | Regulation of mRNA stability |
| −0.536 | GC153558 | CNOT8 | CCR4-NOT transcription complex, subunit 8 | Transcription factor |
| −0.535 | GC17771 | GOT1 | Glutamic-oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1) | Involved in amino acid metabolism |
| −0.535 | GC43091 | NUDCD2 | NudC domain containing 2 | Unknown |
| −0.534 | GC31949 | HSPA9 | Heat shock 70 kDa protein 9 (mortalin) | Control of cell proliferation and cellular aging |
| −0.533 | GC91403 | IKZF5 | IKAROS family zinc finger 5 (Pegasus) | Transcriptional repressor |
| −0.533 | GC31641 | SF1 | Splicing factor 1 | Transcriptional repressor |
| −0.532 | GC161453 | KDM2B | Lysine (K)-specific demethylase 2B | Histone demethylase |
| −0.53 | GC35520 | EBP | CCAAT/enhancer binding protein (C/EBP), gamma | Transcription factor |
| −0.53 | GC10226 | ABCB7 | ATP-binding cassette, sub-family B (MDR/TAP), member 7 | Heme transport |
Information on gene functions was taken from the OMIM database, NCI, USA, (http://www.ncbi.nlm.nih.gov/Omim/) and from the GeneCard database of the Weizmann Institute of Science, Rehovot, Israel (http://bioinfo.weizmann.ac.il/cards/index.html).
As a next step, the genes identified by COMPARE analyses and Pearson's rank correlation tests were subjected to hierarchical cluster analyses. Only the mRNA expression levels but not the IC50 values of the compounds for the cell line panel were used for cluster analyses. Four cluster branches were found in the hopeaphenol-related dendrogram (Figure 4) and three clusters were obtained in the deoxynarcilasine-related cluster analysis (Figure 5). Remarkably, the distribution of cell lines sensitive or resistance to both drugs considerably varied between the different clusters of the dendrograms. Since the IC50 values of the compounds were not included into the cluster analysis, we could address the question whether the gene expression profiles alone are sufficient to predict sensitivity or resistance of cell lines to the compounds.
Figure 4.
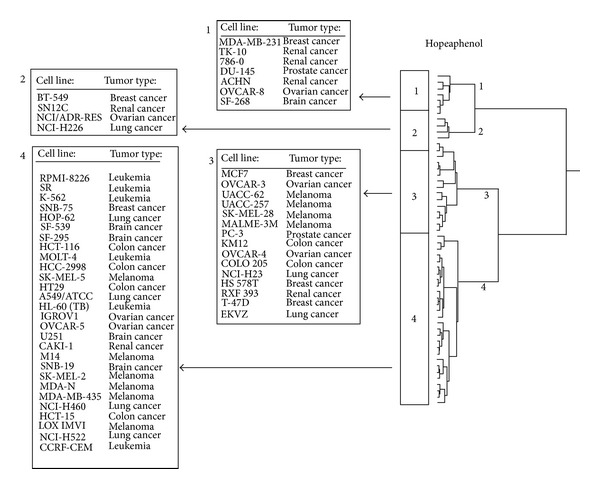
Dendrograms obtained by hierarchical cluster analysis of microarray-based gene expressions for hopeaphenol of the panel of NCI cell lines. The dendrograms were obtained by clustering using the WARD method.
Figure 5.
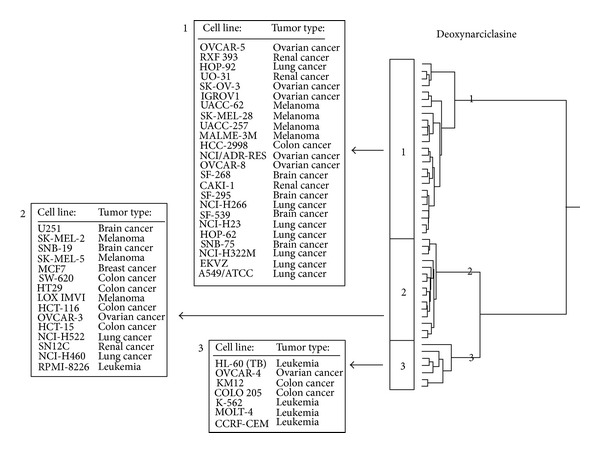
Dendrograms obtained by hierarchical cluster analysis of microarray-based gene expressions for deoxynarciclasine of the panel of NCI cell lines. The dendrograms were obtained by clustering using the WARD method.
As shown in Table 5, the distributions of cell lines sensitive or resistant to hopeaphenol and deoxynarciclasine, respectively, were significantly different between the clusters of the dendrograms, indicating that the expression of these genes was not only responsible for the branching of the dendrograms, but also predicted sensitivity or resistance of these cell lines towards these compounds.
Table 5.
Separation of clusters of 60 cancer cell lines obtained by hierarchical cluster analysis for hopeaphenol and deoxynarciclasine. The log10 IC50 median values (M) of each compound were used as cut-off values to define cell lines as being sensitive or resistant. P > 0.05 was considered as not significant (χ 2 test).
| Sensitive | Resistant | χ 2 test | |
|---|---|---|---|
| Hopeaphenol | |||
| Partition* | <−5.80 | >−5.80 | |
| Cluster 1 | 0 | 7 | |
| Cluster 2 | 0 | 4 | |
| Cluster 3 | 15 | 0 | |
| Cluster 4 | 12 | 16 | P = 4.49 × 10−6 |
|
| |||
| Deoxynarciclasine | |||
| Partition* | <−6.40 | >−6.40 | |
| Cluster 1 | 3 | 22 | |
| Cluster 2 | 13 | 2 | |
| Cluster 3 | 7 | 0 | P = 1.76 × 10−6 |
*log10 IC50 (M).
4. Discussion
4.1. Cytotoxic Activity
The cytotoxicity observed in Isodon. japonicus in this work is in concordance with previous reports. In effect, the cytotoxic effect of I. japonicus extract was reported against five human cancer cell lines (IC50 values below 10 μg/mL on stomach MKN-45, breast MCF-7, leukemia K562, colon HT29, and lung A549 cell lines) with leukemia K562 (IC50 : 2.70 μg/mL) being the most sensitive [16]. Also, the ethanol extract of Lycors radiata exhibited significant antiproliferative effect against B16F10 melanoma cells and induced apoptosis through the activation of p38 and AP-1 pathway [17]. The present report, therefore, provides evidence on the activity of L. radiata not only against cell lines derived from solid tumors but also derived from the hematopoietic system, that is, CCRF-CEM leukemia cells. Sorbaria sorbifolia was cytotoxic towards HepG-2 cells via induction of apoptosis and cell cycle arrest [18], and evidence of this activity towards CCRF-CEM leukemia cells is provided herein.
In the US NCI plant screening program, a crude extract is generally considered to have in vitro cytotoxic activity, if the IC50 value following incubation between 48 and 72 h is less than 20 μg/mL [19]. In this study, we reduced the cutoff point to 10 μg/mL. All extracts were tested at this concentration and only samples with an inhibitory effect >50% were considered to have highly promising activities against leukemia cells. Under this condition, 13 samples from 12 medicinal plants (Figure 1) were identified as promising anticancer products and should be screened for more cancer cell lines. It is noteworthy that only Lycoris radiata exhibited a significant (<50% growth proliferation) activity with both aerial and subterraneal parts, suggesting that different parts of the plant should be considered when screening the cytotoxicity of medicinal plants.
To the best of our knowledge, the cytotoxic effect of several active plants (Table 1) against leukemia cells was reported here for the first time. Nevertheless, some of the analyzed plants contain compounds with known cytotoxicity against cancer cells. In fact, the diarylheptanoids [1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-O-β- d-glucopyranosyl(1→3)-β-d-xylopyranoside; 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-O-β- d-apiofuranosyl(1→6)-β-d-glucopyranoside; 1,7-bis-(3,4-dihydroxyphenyl)-heptane-5-O-β- d-glucopyranoside, 1,7-bis-(3,4-dihydroxyphenyl)-5-hydroxyheptane; 1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-one-5-O-β-d-glucopyranoside; oregonin; hirsutanonol; hirsutenone; 1,7-bis-(3,4-dihydroxyphenyl)-5-hydroxyheptane-3-O-β- d-xylopyranoside and platyphylloside], isolated from the bark of A. japonica, showed cytotoxic activities on human, B16 mouse melanoma cells and SNU-1 gastric cancer cell lines with IC50 values varying from 17.02 to 55.47 μM [20]. Interestingly, the extract of the stem bark of this plant exhibited significant antiproliferative activity on leukemia CCRF-CEM cells (Figure 1), inducing less than 25% growth at 10 μg/mL. In addition, Kim et al. [21] reported the presence of two well-known cytotoxic compounds, betulin and lupeol [22] in the extract of this plant. Apoptosis induction via Fas-mediated pathway was also reported in human MCF-7 breast adenocarcinoma cells by prodelphinidin B-2 3,3′-di-O-gallate, a constituent of M. rubra [23]. The presence of such cytotoxic compounds could explain the good activity of the crude extract of A. japonica and M. rubra. Similarly, the presence of cytotoxic compounds such as quercetin or ferulic acid [22], in R. corchorifolius and S. middendorffianum extracts [24, 25] and Sedum takesimense [26] could also provide some explanation on their antiproliferative potentials. Nonetheless, it is interesting to know that the activity does not only depend on the presence of a cytotoxic substance in a plant, but also their quantities and possible interaction with other plant constituents. For example, cytotoxic compounds such as incanone {known to be active on HL60 leukemia cells (IC50 value of 6 µM) [25])} or verbascoside [27] {active on human HEP-2 larynx epidermoid carcinoma, human RD rhabdomyosarcoma and human MCF-7 breast adenocarcinoma cell lines [28]} were reported in Caryopteris incana. But extract from Caryopteris incana did not show significant activities against CCRF-CEM leukemia cells in the present study.
We screened the NCI database of the Developmental Therapeutics Program of the NCI for the constituents of our panel of Korean medicinal plants. The two most cytotoxic compounds were hopeaphenol and deoxynarciclasine. Both compounds have been previously reported to be cytotoxic [29–32]. While hopeaphenol's activity has been demonstrated in mouse tumors in vitro and in vivo [31, 32], the present investigation shows that this compounds also active against human tumor cells. To the best of our knowledge, the mechanisms of action of these two compounds have not been investigated in the past and are addressed in our analysis for the first time.
4.2. COMPARE and Hierarchical Cluster Analyses of mRNA Microarray Data
To gain insight into possible modes of action of both phytochemicals, we investigated gene expression profiles of the NCI cell line panel. By microarray-based gene expression and COMPARE analyses, we correlated the IC50 values for both compounds of 60 tumor cell lines with transcriptomic mRNA expression levels of this cell line panel [12]. This approach has been successfully used to unravel the mode of action of novel compounds [33]. Cluster and COMPARE analyses are also useful for comparing gene expression profiles with IC50 values for investigational drugs to identify candidate genes for drug resistance [34, 35] and to identify prognostic expression profiles in clinical oncology [36, 37].
Eleven genes passing the correlation thresholds of R > 0.5 and R < −0.5 were identified to significantly correlate with sensitivity or resistance to hopeaphenol. Except for fibroblast growth factor 9 [38], none of them have been associated with response of tumor cells to cytostatic drugs. This point of view is supported by the fact that the NCI cell lines did not exert cross-resistance between hopeaphenol and anticancer drugs, such as doxorubicin, vincristine, cisplatin, and others.
The genes correlating with the response of tumor cells to deoxynarciclasine were also not known to confer drug resistance as of yet, but many of them belong to functional groups, which are associated with response to chemotherapy. For example, transcriptional regulation, signal transduction, and cell proliferation are well-known processes influencing the success of cancer chemotherapy [39–42]. This might also explain why the NCI cell line panel exhibited cross-resistance between deoxynarciclasine and standard chemotherapy. On the other hand, genes involved in cellular trafficking or endoplasmic/sarcoplasmic reticulum functions have not been recognized as possible mechanisms of drug resistance of tumors. This finding merits more detailed investigations in the future.
4.3. Conclusion
The present work provides evidence that some plants derived from Korean medicine could be useful in the treatment of leukemia and supports the advanced investigation of the most active plants extracts. It also provides first pharmacological data on the cytotoxicity of some plants, such as Adenophora racemosa, Cinnamomum japonicum, Eurya japonica, Sedum middendorffianum, and Vitis flexuosa. The identification of cytotoxic phytochemicals from these plants, for example, hopeaphenol, and deoxynarciclasine, and the investigation of their possible molecular modes of action may foster the development of novel treatment strategies against otherwise drug-resistant and refractory tumors.
Supplementary Material
Extracts of medicinal plants used in traditional Korean medicine investigated for cytotoxic activity towards cancer cells.
Conflict of Interests
No potential conflict of interests was disclosed.
Authors' Contribution
Victor Kuete and Ean-Jeong Seo contributed equally to this paper.
Acknowledgments
The authors are thankful to Mrs. Christine Köppel for her technical assistance performing the cytotoxicity assays and Mrs. Ilona Zirbs for her secretarial support.
References
- 1.Lee S, Lillehoj HS, Chun H, et al. In vitro treatment of chicken peripheral blood lymphocytes, macrophages, and tumor cells with extracts of Korean medicinal plants. Nutrition Research. 2007;27(6):362–366. doi: 10.1016/j.nutres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Bhakuni DS, Bittner M, Marticorena C, et al. Screening of Chilean plants for antimicrobial activity. Lloydia. 1974;37(4):621–632. [PubMed] [Google Scholar]
- 3.Farnsworth NR, Akerele O, Bingel AS. Medicinal plants in therapy. Bulletin of the World Health Organization. 1985;63(6):965–981. [PMC free article] [PubMed] [Google Scholar]
- 4.Stévigny C, Bailly C, Quetin-Leclercq J. Cytotoxic and antitumor potentialities of aporphinoid alkaloids. Current Medicinal Chemistry. 2005;5(2):173–182. doi: 10.2174/1568011053174864. [DOI] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. Journal of Natural Products. 2007;70(3):461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 6.Cheong YS, Park EW, Yoo SM, et al. Use of traditional medicine and folk remedies in hypertensive patients: based on Cheonan practice-based research network. Journal of the Korean Academy of Family Medicine. 1998;19(2):141–149. [Google Scholar]
- 7.Hong CD. Complementary and alternative medicine in Korea: current status and future prospects. Journal of Alternative and Complementary Medicine. 2001;7(supplement 1):S33–S40. doi: 10.1089/107555301753393788. [DOI] [PubMed] [Google Scholar]
- 8.Hong MS, Chun KH, Song HJ, Park IW. Attitudes towards complementary and alternative medicine in Suwon city. Korean Journal of Preventive Medicine. 1999;32(2):162–169. [Google Scholar]
- 9.Cordell GA, Beecher CWW, Pezzuto JM. Can ethnopharmacology contribute to the development of new anticancer drugs? Journal of Ethnopharmacology. 1991;32(1–3):117–133. doi: 10.1016/0378-8741(91)90110-y. [DOI] [PubMed] [Google Scholar]
- 10.Popoca J, Aguilar A, Alonso D, Villarreal ML. Cytotoxic activity of selected plants used as antitumorals in Mexican traditional medicine. Journal of Ethnopharmacology. 1998;59(3):173–177. doi: 10.1016/s0378-8741(97)00110-4. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry. 2000;267(17):5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 12.Scherf U, Ross DT, Waltham M, et al. A gene expression database for the molecular pharmacology of cancer. Nature Genetics. 2000;24(3):236–244. doi: 10.1038/73439. [DOI] [PubMed] [Google Scholar]
- 13.Amundson SA, Do KT, Vinikoor LC, et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Research. 2008;68(2):415–424. doi: 10.1158/0008-5472.CAN-07-2120. [DOI] [PubMed] [Google Scholar]
- 14.Efferth T, Fabry U, Osieka R. Apoptosis and resistance to daunorubicin in human leukemic cells. Leukemia. 1997;11(7):1180–1186. doi: 10.1038/sj.leu.2400669. [DOI] [PubMed] [Google Scholar]
- 15.Wosikowski K, Schuurhuis D, Johnson K, et al. Identification of epidermal growth factor receptor and c-erbB2 pathway inhibitors by correlation with gene expression patterns. Journal of the National Cancer Institute. 1997;89(20):1505–1515. doi: 10.1093/jnci/89.20.1505. [DOI] [PubMed] [Google Scholar]
- 16.Hwang YJ, Kim J, Park DS, Hwang KA. Study on the immunomodulation effect of Isodon japonicus extract via splenocyte function and NK anti-tumor activity. International Journal of Molecular Sciences. 2012;13(4):4880–4888. doi: 10.3390/ijms13044880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son M, Kim A, Lee J, et al. Ethanol extract of Lycoris radiata induces cell death in B16F10 melanoma via p38-mediated AP-1 activation. Oncology Reports. 2010;24(2):473–478. doi: 10.3892/or_00000881. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XW, Cui CX, Chen LY. Inhibition of Sorbaria sorbifolia on proliferarion of hepatoma HepG-2 cell line. Journal of Chinese Medical Materials. 2007;30(6):681–684. [PubMed] [Google Scholar]
- 19.Boik J. Natural Compounds in Cancer Therapy. Princeton, Minn, USA: Oregon Medical Press; 2001. [Google Scholar]
- 20.Choi SE, Kim KH, Kwon JH, Kim SB, Kim HW, Lee MW. Cytotoxic activities of diarylheptanoids from Alnus japonica . Archives of Pharmacal Research. 2008;31(10):1287–1289. doi: 10.1007/s12272-001-2108-z. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Kim KH, Yeom SH, et al. New diarylheptanoid from the barks of Alnus japonica steudel. Chinese Chemical Letters. 2005;16(10):1337–1340. [Google Scholar]
- 22.Kuete V, Efferth T. Pharmacogenomics of Cameroonian traditional herbal medicine for cancer therapy. Journal of Ethnopharmacology. 2011;137(1):752–766. doi: 10.1016/j.jep.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Kuo PL, Hsu YL, Lin TC, Lin LT, Lin CC. Induction of apoptosis in human breast adenocarcinoma MCF-7 cells by prodelphinidin B-2 3,3′-di-O-gallate from Myrica rubra via Fas-mediated pathway. Journal of Pharmacy and Pharmacology. 2004;56(11):1399–1406. doi: 10.1211/0022357044625. [DOI] [PubMed] [Google Scholar]
- 24.Shnyakina GP, Zapesochnaya GG. Flavonols and phenolic compounds of Sedum middendorffianum . Chemistry of Natural Compounds. 1975;9(5):645–648. [Google Scholar]
- 25.Sun ZL, Zhang Y, Wan AH, Zhang XL, Feng J. A new active compound against kidney deficiency from the fruits of Rubus corchorifolius . Journal of Asian Natural Products Research. 2011;13(1):68–74. doi: 10.1080/10286020.2010.541156. [DOI] [PubMed] [Google Scholar]
- 26.Thuong PT, Kang HJ, Na M, et al. Anti-oxidant constituents from Sedum takesimense. Phytochemistry. 2007;68(19):2432–2438. doi: 10.1016/j.phytochem.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Harput US, Genc Y, Saracoglu I. Cytotoxic and antioxidative activities of Plantago lagopus L. and characterization of its bioactive compounds. Food and Chemical Toxicology. 2012;50(5):1554–1559. doi: 10.1016/j.fct.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Paper DH, Vogl H, Franz G, Hoffmann R. Defined carrageenan derivatives as angiogenesis inhibitors. Macromolecular Symposia. 1995;99(1):219–225. [Google Scholar]
- 29.Pettit GR, Gaddamidi V, Herald DL, et al. Antineoplastic agents, 120. Pancratium littorale. Journal of Natural Products. 1986;49(6):995–1002. doi: 10.1021/np50048a005. [DOI] [PubMed] [Google Scholar]
- 30.Pettit GR, Pettit GR, III, Backhaus RA, Boyd MR, Meerow AW. Antineoplastic agents, 256. Cell growth inhibitory isocarbostyrils from Hymenocallis. Journal of Natural Products. 1993;56(10):1682–1687. doi: 10.1021/np50100a004. [DOI] [PubMed] [Google Scholar]
- 31.Mishima S, Matsumoto K, Futamura Y, et al. Antitumor effect of stilbenoids from Vateria indica against allografted sarcoma S-180 in animal model. Journal of Experimental Therapeutics and Oncology. 2003;3(5):283–288. doi: 10.1111/j.1533-869x.2003.01102.x. [DOI] [PubMed] [Google Scholar]
- 32.Sahidin S, Hakim EH, Juliawaty LD, et al. Cytotoxic properties of oligostilbenoids from the tree barks of Hopea dryobalanoides. Zeitschrift fur Naturforschung C. 2005;60(9-10):723–727. doi: 10.1515/znc-2005-9-1011. [DOI] [PubMed] [Google Scholar]
- 33.Leteurtre F, Kohlhagen G, Paull KD, Pommier Y. Topoisomerase II inhibition and cytotoxicity of the anthrapyrazoles DuP 937 and DuP 941 (Losoxantrone) in the National Cancer Institute preclinical antitumor drug discovery screen. Journal of the National Cancer Institute. 1994;86(16):1239–1244. doi: 10.1093/jnci/86.16.1239. [DOI] [PubMed] [Google Scholar]
- 34.Efferth T, Olbrich A, Bauer R. mRNA expression profiles for the response of human tumor cell lines to the antimalarial drugs artesunate, arteether, and artemether. Biochemical Pharmacology. 2002;64(4):617–623. doi: 10.1016/s0006-2952(02)01221-2. [DOI] [PubMed] [Google Scholar]
- 35.Efferth T, Gebhart E, Ross DD, Sauerbrey A. Identification of gene expression profiles predicting tumor cell response to L-alanosine. Biochemical Pharmacology. 2003;66(4):613–621. doi: 10.1016/s0006-2952(03)00341-1. [DOI] [PubMed] [Google Scholar]
- 36.Volm M, Koomägi R, Mattern J, Efferth T. Expression profile of genes in non-small cell lung carcinomas from long-term surviving patients. Clinical Cancer Research. 2002;8(6):1843–1848. [PubMed] [Google Scholar]
- 37.Volm M, Koomägi R, Mattern J, Efferth T. Protein expression profiles indicative for drug resistance of non-small cell lung cancer. British Journal of Cancer. 2002;87(3):251–257. doi: 10.1038/sj.bjc.6600463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fillmore CM, Gupta PB, Rudnick JA, et al. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21737–21742. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burton TR, Kashour T, Wright JA, Amara FM. Cellular signaling pathways affect the function of ribonucleotide reductase mRNA binding proteins: mRNA stabilization, drug resistance, and malignancy (Review) International Journal of Oncology. 2003;22(1):21–31. [PubMed] [Google Scholar]
- 40.Efferth T, Konkimalla VB, Wang Y, et al. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clinical Cancer Research. 2008;14(8):2405–2412. doi: 10.1158/1078-0432.CCR-07-4525. [DOI] [PubMed] [Google Scholar]
- 41.Wu K, Bonavida B. The activated NF-κB-snail-RKIP circuitry in cancer regulates both the metastatic cascade and resistance to apoptosis by cytotoxic drugs. Critical Reviews in Immunology. 2009;29(3):241–254. doi: 10.1615/critrevimmunol.v29.i3.40. [DOI] [PubMed] [Google Scholar]
- 42.Mahajan K, Mahajan NP. PI3K-independent AKT activation in cancers: a treasure trove for novel therapeutics. Journal of Cell Physiology. 2012;227(9):3178–3184. doi: 10.1002/jcp.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SL. Korea Folk Medicine. Seoul, Republic of Korea: Seoul National University Publishing Center Press; 1996. [Google Scholar]
- 44.Kim ST, Kim JD, Ahn SH, Ahn GS, Lee YI, Jeong YS. Hepatoprotective and antioxidant effects of Alnus japonica extracts on acetaminophen-induced hepatotoxicity in rats. Phytotherapy Research. 2004;18(12):971–975. doi: 10.1002/ptr.1540. [DOI] [PubMed] [Google Scholar]
- 45.Tung NH, Kwon H, Kim J, Ra JC, Kim JA, Kim YH. An anti-influenza component of the bark of Alnus japonica . Archives of Pharmacal Research. 2010;33(3):363–367. doi: 10.1007/s12272-010-0303-5. [DOI] [PubMed] [Google Scholar]
- 46.Lee MW, Tanaka T, Nonaka GI, Nishioka I. Dimeric ellagitannins from Alnus japonica . Phytochemistry. 1992;31(8):2835–2839. [Google Scholar]
- 47.Kim S, Jung E, Shin S, et al. Anti-inflammatory activity of Camellia japonica oil. BMB Reports. 2012;45(3):177–182. doi: 10.5483/BMBRep.2012.45.3.177. [DOI] [PubMed] [Google Scholar]
- 48.Kim KY, Davidson PM, Chung HJ. Antibacterial activity in extracts of Camellia japonica L. petals and its application to a model food system. Journal of Food Protection. 2001;64(8):1255–1260. doi: 10.4315/0362-028x-64.8.1255. [DOI] [PubMed] [Google Scholar]
- 49.Park JC, Hur JM, Park JG, et al. Inhibitory effects of Korean medicinal plants and camelliatannin H from Camellia japonica on human immunodeficiency virus type 1 protease. Phytotherapy Research. 2002;16(5):422–426. doi: 10.1002/ptr.919. [DOI] [PubMed] [Google Scholar]
- 50.Akihisa T, Tokuda H, Ukiya M, et al. 3-Epicabraleahydroxylactone and other triterpenoids from camellia oil and their inhibitory effects on epstein-barr virus activation. Chemical and Pharmaceutical Bulletin. 2004;52(1):153–156. doi: 10.1248/cpb.52.153. [DOI] [PubMed] [Google Scholar]
- 51.Miura D, Kida Y, Nojima H. Camellia oil and its distillate fractions effectively inhibit the spontaneous metastasis of mouse melanoma BL6 cells. FEBS Letters. 2007;581(13):2541–2548. doi: 10.1016/j.febslet.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 52.Onodera K, Hanashiro K, Yasumoto T. Camellianoside, a novel antioxidant glycoside from the leaves of Camellia japonica . Bioscience, Biotechnology and Biochemistry. 2006;70(8):1995–1998. doi: 10.1271/bbb.60112. [DOI] [PubMed] [Google Scholar]
- 53.Piao MJ, Yoo ES, Koh YS, et al. Antioxidant effects of the ethanol extract from flower of Camellia japonica via scavenging of reactive oxygen species and induction of antioxidant enzymes. International Journal of Molecular Science. 2011;12(4):2618–2630. doi: 10.3390/ijms12042618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung E, Lee J, Baek J, et al. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. Journal of Ethnopharmacology. 2007;112(1):127–131. doi: 10.1016/j.jep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Lee J-H, Kim J-W, Ko N-Y, et al. Camellia japonica suppresses immunoglobulin E-mediated allergic response by the inhibition of Syk kinase activation in mast cells. Clinical and Experimental Allergy. 2008;38(5):794–804. doi: 10.1111/j.1365-2222.2008.02936.x. [DOI] [PubMed] [Google Scholar]
- 56.Itokawa H, Nakajima H, Ikuta A, Iitaka Y. Two triterpenes from the flowers of Camellia japonica . Phytochemistry. 1981;20(11):2539–2542. [Google Scholar]
- 57.Thao NTP, Hung TM, Cuong TD, et al. 28-Nor-oleanane-type triterpene saponins from Camellia japonica and their inhibitory activity on LPS-induced NO production in macrophage RAW264.7 cells. Bioorganic and Medicinal Chemistry Letters. 2010;20(24):7435–7439. doi: 10.1016/j.bmcl.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Jiangsu New Medical College. The Chinese Medicine Dictionary. Shanghai, China: Shanghai People's Publishing House; 1977. [Google Scholar]
- 59.Kubo I, Kamikawa T, Kubota T. Studies on constituents of Isodon japonicus Hara. The structures and absolute stereochemistry of isodonal, trichodonin and epinodosin. Tetrahedron. 1974;30(5):615–622. [Google Scholar]
- 60.Kubota T, Kubo I. A new bitter principle of Isodon japonicus hara. Tetrahedron Letters. 1967;8(38):3781–3784. [Google Scholar]
- 61.Fujita E, Fujita T, Taoka M, Katayama H, Shibuya M. The structure and absolute configuration of sodoponin and epinodosinol, new minor diterpenoids of Isodon japonicus . Tetrahedron Letters. 1970;11(6):421–424. [Google Scholar]
- 62.Wang L, Zhang XQ, Yin ZQ, Wang Y, Ye W. Two new amaryllidaceae alkaloids from the bulbs of Lycoris radiata . Chemical and Pharmaceutical Bulletin. 2009;57(6):610–611. doi: 10.1248/cpb.57.610. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Yin ZQ, Cai Y, Zhang X, Yao X, Ye W. Amaryllidaceae alkaloids from the bulbs of Lycoris radiata . Biochemical Systematics and Ecology. 2010;38(3):444–446. [Google Scholar]
- 64.Sancheti S, Sancheti S, Lee S, Lee J, Seo S. Screening of korean medicinal plant extracts for α-glucosidase inhibitory activities. Iranian Journal of Pharmaceutical Research. 2011;10(2):261–264. [PMC free article] [PubMed] [Google Scholar]
- 65.Sancheti S, Sancheti S, Um B-H, Seo S-Y. 1,2,3,4,6-penta-O-galloyl-β-d-glucose: a cholinesterase inhibitor from Terminalia chebula . South African Journal of Botany. 2010;76(2):285–288. [Google Scholar]
- 66.Chen ZL. The history of bayberries. Journal of Fruit Science. 1996;13:59–61. [Google Scholar]
- 67.Bao J, Cai Y, Sun M, Wang G, Corke H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese Bayberry (Myrica rubra) extracts and their color properties and stability. Journal of Agricultural and Food Chemistry. 2005;53(6):2327–2332. doi: 10.1021/jf048312z. [DOI] [PubMed] [Google Scholar]
- 68.Mochida K. Anti-influenza virus activity of Myrica rubra leaf ethanol extract evaluated using Madino-Darby canine kidney (MDCK) cells. Bioscience, Biotechnology and Biochemistry. 2008;72(11):3018–3020. doi: 10.1271/bbb.80330. [DOI] [PubMed] [Google Scholar]
- 69.Sakurai N, Yaguchi Y, Hirakawa T, Nagai M, Inoue T. Two myricanol glycosides from Myrica rubra and revision of the structure of isomyricanone. Phytochemistry. 1991;30(9):3077–3079. [Google Scholar]
- 70.Daayf F, Schmitt A, Bélanger RR. Evidence of phytoalexins in cucumber leaves infected with powdery mildew following treatment with leaf extracts of Reynoutria sachalinensis. Plant Physiology. 1997;113(3):719–727. doi: 10.1104/pp.113.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung SK, Kim YC, Takaya Y, Terashima K, Niwa M. Novel flavonol glycoside, 7-O-methyl mearnsitrin, from Sageretia theezans and its antioxidant effect. Journal of Agricultural and Food Chemistry. 2004;52(15):4664–4668. doi: 10.1021/jf049526j. [DOI] [PubMed] [Google Scholar]
- 72.Chung SK, Chen C-YO, Blumberg JB. Flavonoid-rich fraction from Sageretia theezans leaves scavenges reactive oxygen radical species and increases the resistance of low-density lipoprotein to oxidation. Journal of Medicinal Food. 2009;12(6):1310–1315. doi: 10.1089/jmf.2008.1309. [DOI] [PubMed] [Google Scholar]
- 73.Bae H, Kim R, Kim Y, et al. Effects of Schisandra chinensis Baillon (Schizandraceae) on lipopolysaccharide induced lung inflammation in mice. Journal of Ethnopharmacology. 2012;142(1):41–47. doi: 10.1016/j.jep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 74.Zhang M, Cao Y, Du FL, Ou-Yang W, Yuan Y. Isolation and identification of two new diterpenoid from Rubus corchorifolius L. f. Yaoxue Xuebao. 2007;42(11):1155–1158. [PubMed] [Google Scholar]
- 75.Kim DK, Zee OP. A new cyanogenic glycoside from Sorbaria sorbifolia var. stellipila . Chemical and Pharmaceutical Bulletin. 2000;48(11):1766–1767. doi: 10.1248/cpb.48.1766. [DOI] [PubMed] [Google Scholar]
- 76.Li X, Wu L, Zang X, Zheng J. Studies on chemical constituents of Sorbaria sorbifolia . Zhongguo Zhongyao Zazhi. 2002;27(11):842–843. [PubMed] [Google Scholar]
- 77.Zhang X, Zhang Y, Guan L, Quan Y, Sun Q. Study on extraction and isolation of active constituents from Sorbaria sorbifolia and antitumor effect of the constituents in vivo . Journal of Chinese Medicinal Materials. 2004;27(1):36–38. [PubMed] [Google Scholar]
- 78.Li W, Li B, Chen Y. Flexuosol A, a new tetrastilbene from Vitis flexuosa . Journal of Natural Products. 1998;61(5):646–647. doi: 10.1021/np970457v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extracts of medicinal plants used in traditional Korean medicine investigated for cytotoxic activity towards cancer cells.


